Effects of Vitamin E on the Gut Microbiome in Ageing and Its Relationship with Age-Related Diseases: A Review of the Current Literature
Abstract
:1. Introduction
2. Role of the Gut Microbiota on Ageing and Age-Related Disease
| Type of Age-Related Disease | Type of Study | Aim of Study | Study Design and Sequencing Method | Main Findings | Reference |
|---|---|---|---|---|---|
| Alzheimer’s Disease | Animal | To elucidate the effects of Ganmaidazao on AD by exploring the potential mechanism and establish the brain-gut microbiota axis to analyze the connection between gut microbiota, metabolites and AD | 8-week-old adult male Sprague Dawley rats (6 control & 6 AD model) 16S rDNA sequencing | Proteobacteria ↑ | [32] |
| Human | To characterize the gut microbiota of amnestic mild cognitive impairment (aMCI) and AD patients | 32 healthy control, 32 aMCI patients and 33 AD patients 16S rRNA sequencing | Firmicutes ↓ (Clostridiaceae, Lachnospiraceae, Ruminococcaceae) Proteobacteria ↑ (Gammaproteobateria) | [33] | |
| To explore the possible biomarkers before the onset of dementia and the alteration in the gut microbiota before the onset of AD and in the stage of mild cognitive impairment (MCI) | 30 normal control, 30 MCI patients and 30 AD patients 16S rRNA sequencing | α-diversity ↓ Parabacteroides ↓ Paraprevotella ↓ Alistipes ↓ Sutterella ↓ Haemophilus ↓ Bacteroides↓ Butyricimonas ↓ Prevotella ↓ Alloprevotella ↓ Succinivibrio ↓ Bifidobacterium ↑ Lactobacillus ↑ Acinetobacter ↑ Akkermansia ↑ Streptococcus ↑ Dorea ↑ Blautia ↑ LPS ↑ IL-1 ↑ TNF-α ↑ Intestinal permeability ↑ | [35] | ||
| To examine the combination of taxonomic, functional gut microbiome and clinical data to differentiate amyloid-positive AD patients and cognitively healthy elderly controls | 75 amyloid-positive AD patients shotgun metagenomic sequencing | Aliivibrio ↓ Propionibacterium ↓ Orrella ↓ Veillonella ↓ Mucinivorans ↓ Paenarthrobacter ↓ Plesiomonas ↓ Roseovarius ↓ Lactococcus ↓ Sulfuricella ↓ Moritella ↑ Parabacteroides ↑ Basfia ↑ Arsenophonus ↑ Acidothermus ↑ Aureimonas ↑ Candidatus Arthromitus ↑ Asaia ↑ | [34] | ||
| Parkinson’s Disease | Animal | To examine the protective effects of osteocalcin on PD and whether the underlying mechanism is due to the alteration in the gut microbiota | Male C57BL/6 J mice (12 control and 12 PD mice model) 16S rRNA sequencing | Bacteroidetes ↓ Rikenellaceae ↓ Erysipelotrichaceae ↓ Firmicutes ↑ Lachnospiraceae ↑ Clostridiales ↑ Propionate ↓ | [30] |
| Human | To compare the identify the associations of the gut microbiota, gut barrier permeability, SCFAs and inflammation in PD patients and controls and to understand how these factors are connected to PD clinical symptoms | 56 control subjects and 55 PD patients 16S rRNA sequencing | Prevotella ↓ Firmicutes ↑ Butyrate & propionate ↓ IL-1α ↑ IL-1β ↑ IL-8 ↑ CRP ↑ | [26] | |
| To evaluate the effects of gut microbiota alterations and cytokines on PD patients | (1) 77 control subjects and 80 PD patients (2) 120 control subjects and 120 PD patients 16S rRNA sequencing | Prevotella ↓ Parabacteroides ↑ Verrucomicrobia ↑ Enterococcus ↑ Akkermansia ↑ Veillonella ↑ Butyricimonas ↑ Mucispirillum ↑ Odoribacter ↑ Bilophila ↑ Lactobacillus ↑ | [25] | ||
| To investigate the possible functional consequences due to alterations in the gut microbiota of PD patients in comparison to healthy controls | 248 healthy control and 206 PD patients 16S rRNA sequencing | Roseburia intestinalis ↓ Turicibacter sanguinis ↓ Ruminococcus bromii ↓ Ruminococcus torques ↓ Akkermansia ↑ Lactobacillaceae ↑ Christensenella ↑ Lactobacillus ↑ | [28] | ||
| Type 2 Diabetes Mellitus | Human | To evaluate whether clinical biomarkers along with the gut microbiota composition may enhance the prediction of new cases of T2DM in coronary heart disease patients | 1002 patients with coronary heart disease 16S rRNA sequencing | Prevotellaceae ↑ Carnobacteriaceae ↑ Veillonellaceae ↑ Streptococcaceae ↑ Actinomycetaceae ↑ Oxalobacteraceae ↑ | [37] |
| To study the connections between the composition of the gut microbiome and insulin resistance as well as T2DM in an extensive population-based setting, taking a variety of sociodemographic and lifestyle variables into account | 4671 participants 16S rRNA sequencing | Intestinibacter ↑ Clostridiaceae 1 ↑ Clostridium sensu stricto 1↑ Peptostreptococcaceae ↑ Romboutsia ↑ | [36] | ||
| Irritable Bowel Syndrome | Animal | To examine the effects of five diarrhea-predominant irritable bowel syndrome (IBS-D) rat models on the BGM axis | 5 7-week-old Wistar rats 16S rRNA sequencing | Bacteroidetes ↓ Firmicutes ↑ | [38] |
| Human | To examine the colonic melatonin levels and microbiota profiles in IBS-D patients | 28 healthy controls and 32 IBS-D patients 16S rRNA sequencing | Bacteroidetes ↓ Firmicutes ↑ | [39] | |
| To investigate the changes in gut microbiome in IBS patients, followed by the efficacy, side effects and changes in gut microbiome with FMT treatment | 11 men and 6 women with IBS 16S rRNA sequencing | α-diversity ↓ Prevotella ↓ Faecalibacterium ↓ Sutterella ↓ Akkermansia ↓ Bifidobacterium ↓ | [31] |
3. Role of Vitamin E in Ageing and Age-Related Disease
4. Effect of Vitamin E on Gut Microbiome and Age-Related Disease
| Type of Age-Related Disease | Type of Study | Study Design | Effects of Vitamin E | Reference |
|---|---|---|---|---|
| Type 2 diabetes mellitus | Animal | 48 male C57BL/6 J mice aged weeks (4 groups (n = 12 per group): low fat diet (LFD, 5% of energy from fat), high fat diet (HFD, 58% of energy from fat), HFD supplemented with 800 mg tocotrienol/kg diet (AT) and HFD supplemented with 200 mg metformin/kg diet (MET)) | Verrucomicrobia ↑ IL-6 ↓ | [69] |
| 58 male C57BL/6J mice of aged 5 weeks (5 group: low fat diet (LFD, 5% of energy from fat), high fat diet (HFD, 58% of energy from fat), HFD + 400 mg tocotrienol/kg diet, HFD + 1600 mg tocotrienol/kg diet and HFD + 200 mg metformin/kg diet) | IL-2 ↓ IL-23 ↓ IFN ↓ | [70] | ||
| Inflammatory bowel disease | Animal | 5–6-week-old male Balb/c mice (4 groups: healthy control fed with AIN93G diet (n = 6), mice treated with DSS (n = 10), DSS-treated mice + 0.05% αT supplement (n = 10) and DSS-treated mice + 0.05% γTmT supplement (n = 10)) | IL-6 ↓ LBP ↓ Lachnospiraceae UCG006 ↑ Roseburia ↑ | [58] |
| Colorectal cancer | Animal | 6–7-week-old male Balb/c mice (all mice were injected with AOM and distributed randomly into 3 groups: AIN-93G control group, δTE supplemented group and δTE-13′-COOh group) | IL-1β ↓ GM-CSF↓ MCP-1 ↓ Firmicutes: Bacteroidetes ↓ Roseburia ↑ Eubacterium coprostanoligenes ↑ Clostridiales vadinBB60↓ Streptococcaceae ↑ Lactococcus ↑ Parabacteroides goldsteinii CL02T12C30 ↑ | [74] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Zhang, X.; Li, R. Recent insights into the cellular and molecular determinants of aging. J. Cell Sci. 2018, 131, jcs210831. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.M.; Garbarino, V.R.; Pollet, E.; Palavicini, J.P.; Kellogg, D.L., Jr.; Kraig, E.; Orr, M.E. Biological aging processes underlying cognitive decline and neurodegenerative disease. J. Clin. Investig. 2022, 132, e158453. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.F.L.; Schumacher, B. Principles of the Molecular and Cellular Mechanisms of Aging. J. Investig. Dermatol. 2021, 141, 951–960. [Google Scholar] [CrossRef]
- Azam, S.; Haque, M.E.; Balakrishnan, R.; Kim, I.S.; Choi, D.K. The Ageing Brain: Molecular and Cellular Basis of Neurodegeneration. Front. Cell Dev. Biol. 2021, 9, 683459. [Google Scholar] [CrossRef]
- Alsegiani, A.S.; Shah, Z.A. The influence of gut microbiota alteration on age-related neuroinflammation and cognitive decline. Neural Regen. Res. 2022, 17, 2407–2412. [Google Scholar]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Wang, L. Role and Mechanism of Gut Microbiota in Human Disease. Front. Cell Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef]
- Yan, H.; Qin, Q.; Yan, S.; Chen, J.; Yang, Y.; Li, T.; Gao, X.; Ding, S. Comparison of The Gut Microbiota In Different Age Groups In China. Front. Cell Infect. Microbiol. 2022, 12, 877914. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, D.Y.; Kang, H.J.; Kang, J.H.; Cho, M.G.; Jang, H.W.; Kim, B.K.; Hur, S.J. Differences in the gut microbiota between young and elderly persons in Korea. Nutr. Res. 2021, 87, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Gualtieri, P.; De Lorenzo, A. Diet, Nutrition and Chronic Degenerative Diseases. Nutrients 2021, 13, 1372. [Google Scholar] [CrossRef]
- Zhu, S.; Jiang, Y.; Xu, K.; Cui, M.; Ye, W.; Zhao, G.; Jin, L.; Chen, X. The progress of gut microbiome research related to brain disorders. J. Neuroinflammation 2020, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Ragonnaud, E.; Biragyn, A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun. Ageing 2021, 18, 2. [Google Scholar] [CrossRef]
- Longo, V.D.; Antebi, A.; Bartke, A.; Barzilai, N.; Brown-Borg, H.M.; Caruso, C.; Curiel, T.J.; de Cabo, R.; Franceschi, C.; Gems, D.; et al. Interventions to Slow Aging in Humans: Are We Ready? Aging Cell 2015, 14, 497–510. [Google Scholar] [CrossRef]
- Sánchez Y Sánchez de la Barquera, B.; Martinez Carrillo, B.E.; Aguirre Garrido, J.F.; Martinez Mendez, R.; Benitez Arciniega, A.D.; Valdes Ramos, R.; Soto Pina, A.E. Emerging Evidence on the Use of Probiotics and Prebiotics to Improve the Gut Microbiota of Older Adults with Frailty Syndrome: A Narrative Review. J. Nutr. Health Aging 2022, 26, 926–935. [Google Scholar] [CrossRef]
- Tsai, Y.L.; Lin, T.L.; Chang, C.J.; Wu, T.R.; Lai, W.F.; Lu, C.C.; Lai, H.C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Z.; Cai, J. Fecal microbiota transplantation: Emerging applications in autoimmune diseases. J. Autoimmun. 2023, in press. [Google Scholar] [CrossRef]
- Jamal, R.; Messaoudene, M.; de Figuieredo, M.; Routy, B. Future indications and clinical management for fecal microbiota transplantation (FMT) in immuno-oncology. Semin. Immunol. 2023, 67, 101754. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. Sci. Politics Nutr. 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Chen, C.-C.; Chiang, H.-L.; Liou, J.-M.; Chang, C.-M.; Lu, T.-P.; Chuang, E.Y.; Tai, Y.-C.; Cheng, C.; Lin, H.-Y.; et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflamm. 2019, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; Chang, J.; Rudi, K.; Paulin, L.; Hertzberg, V.; Auvinen, P.; Tansey, M.G.; Scheperjans, F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 2021, 16, 6. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Baldini, F.; Hertel, J.; Sandt, E.; Thinnes, C.C.; Neuberger-Castillo, L.; Pavelka, L.; Betsou, F.; Krüger, R.; Thiele, I. Parkinson’s disease-associated alterations of the gut microbiome predict disease-relevant changes in metabolic functions. BMC Biol. 2020, 18, 62. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, H.; Zhan, L.; Lu, X.; Zhang, L. Dynamic Development of Fecal Microbiome During the Progression of Diabetes Mellitus in Zucker Diabetic Fatty Rats. Front. Microbiol. 2019, 10, 232. [Google Scholar] [CrossRef]
- Hou, Y.-F.; Shan, C.; Zhuang, S.-Y.; Zhuang, Q.-Q.; Ghosh, A.; Zhu, K.-C.; Kong, X.-K.; Wang, S.-M.; Gong, Y.-L.; Yang, Y.-Y.; et al. Gut microbiota-derived propionate mediates the neuroprotective effect of osteocalcin in a mouse model of Parkinson’s disease. Microbiome 2021, 9, 34. [Google Scholar] [CrossRef]
- Hamazaki, M.; Sawada, T.; Yamamura, T.; Maeda, K.; Mizutani, Y.; Ishikawa, E.; Furune, S.; Yamamoto, K.; Ishikawa, T.; Kakushima, N.; et al. Fecal microbiota transplantation in the treatment of irritable bowel syndrome: A single-center prospective study in Japan. BMC Gastroenterol. 2022, 22, 342. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Shan, X.; Yan, Y.; Zhao, T.; Sun, Y.; Hao, W.; Wang, Z.; Chang, Y.; Xie, Y.; Wei, B. Ganmaidazao decoction alleviated cognitive impairment on Alzheimer’s disease rats by regulating gut microbiota and their corresponding metabolites. Arab. J. Chem. 2023, 16, 104688. [Google Scholar] [CrossRef]
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K.; et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 2019, 80, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Laske, C.; Müller, S.; Preische, O.; Ruschil, V.; Munk, M.H.J.; Honold, I.; Peter, S.; Schoppmeier, U.; Willmann, M. Signature of Alzheimer’s Disease in Intestinal Microbiome: Results from the AlzBiom Study. Front. Neurosci. 2022, 16, 792996. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; He, Y.; Ma, J.; Huang, P.; Du, J.; Cao, L.; Wang, Y.; Xiao, Q.; Tang, H.; Chen, S. Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimers Dement. 2019, 15, 1357–1366. [Google Scholar] [CrossRef]
- Chen, Z.; Radjabzadeh, D.; Chen, L.; Kurilshikov, A.; Kavousi, M.; Ahmadizar, F.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J.; et al. Association of Insulin Resistance and Type 2 Diabetes with Gut Microbial Diversity: A Microbiome-Wide Analysis From Population Studies. JAMA Netw. Open 2021, 4, e2118811. [Google Scholar] [CrossRef]
- Vals-Delgado, C.; Alcala-Diaz, J.F.; Molina-Abril, H.; Roncero-Ramos, I.; Caspers, M.P.M.; Schuren, F.H.J.; Van den Broek, T.J.; Luque, R.; Perez-Martinez, P.; Katsiki, N.; et al. An altered microbiota pattern precedes Type 2 diabetes mellitus development: From the CORDIOPREV study. J. Adv. Res. 2022, 35, 99–108. [Google Scholar] [CrossRef]
- Wu, H.; Zhan, K.; Rao, K.; Zheng, H.; Qin, S.; Tang, X.; Huang, S. Comparison of five diarrhea-predominant irritable bowel syndrome (IBS-D) rat models in the brain-gut-microbiota axis. Biomed. Pharmacother. 2022, 149, 112811. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, S.; Liu, Z.; Wei, H.; Zhang, L.; He, M.; Pei, F.; Zhang, J.; Sun, Q.; Duan, L. Increased Expression of Colonic Mucosal Melatonin in Patients with Irritable Bowel Syndrome Correlated with Gut Dysbiosis. Genom. Proteom. Bioinform. 2020, 18, 708–720. [Google Scholar] [CrossRef]
- Ciarcià, G.; Bianchi, S.; Tomasello, B.; Acquaviva, R.; Malfa, G.A.; Naletova, I.; La Mantia, A.; Di Giacomo, C. Vitamin E and Non-Communicable Diseases: A Review. Biomedicines 2022, 10, 2473. [Google Scholar] [CrossRef]
- Szymańska, R.; Nowicka, B.; Trela, A.; Kruk, J. Vitamin E: Structure and forms. In Molecular Nutrition; Academic Press: Cambridge, MA, USA, 2020; pp. 67–90. [Google Scholar]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and Tocotrienols-Bioactive Dietary Compounds; What Is Certain, What Is Doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.A.; Varatharajan, R.; Muthuraman, A. Palm Oil Derived Tocotrienol-Rich Fraction Attenuates Vascular Dementia in Type 2 Diabetic Rats. Int. J. Mol. Sci. 2022, 23, 13531. [Google Scholar] [CrossRef] [PubMed]
- Dallner, G.; Bentinger, M.; Hussain, S.; Sinha, I.; Yang, J.; Schwank-Xu, C.; Zheng, X.; Swiezewska, E.; Brismar, K.; Valladolid-Acebes, I.; et al. Dehydro-Tocotrienol-β Counteracts Oxidative-Stress-Induced Diabetes Complications in db/db Mice. Antioxidants 2021, 10, 1070. [Google Scholar] [CrossRef] [PubMed]
- Mahjabeen, W.; Khan, D.A.; Mirza, S.A.; Pervez, M.A. Effects of delta-tocotrienol supplementation on Glycemic Control, oxidative stress, inflammatory biomarkers and miRNA expression in type 2 diabetes mellitus: A randomized control trial. Phytother. Res. 2021, 35, 3968–3976. [Google Scholar] [CrossRef]
- Bergin, P.; Leggett, A.; Cardwell, C.R.; Woodside, J.V.; Thakkinstian, A.; Maxwell, A.P.; McKay, G.J. The effects of vitamin E supplementation on malondialdehyde as a biomarker of oxidative stress in haemodialysis patients: A systematic review and meta-analysis. BMC Nephrol. 2021, 22, 126. [Google Scholar] [CrossRef] [PubMed]
- Shawki, H.A.; Elzehery, R.; Shahin, M.; Abo-Hashem, E.M.; Youssef, M.M. Evaluation of some oxidative markers in diabetes and diabetic retinopathy. Diabetol. Int. 2021, 12, 108–117. [Google Scholar] [CrossRef]
- Rao, Y.L.; Ganaraja, B.; Marathe, A.; Manjrekar, P.A.; Joy, T.; Ullal, S.; Pai, M.M.; Murlimanju, B.V. Comparison of malondialdehyde levels and superoxide dismutase activity in resveratrol and resveratrol/donepezil combination treatment groups in Alzheimer’s disease induced rat model. 3 Biotech. 2021, 11, 329. [Google Scholar] [CrossRef]
- Tan, G.C.J.; Tan, S.M.Q.; Phang, S.C.W.; Ng, Y.T.; Ng, E.Y.; Ahmad, B.; Palamisamy, U.D.M.; Kadir, K.A. Tocotrienol-rich vitamin E improves diabetic nephropathy and persists 6-9 months after washout: A phase IIa randomized controlled trial. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819895462. [Google Scholar] [CrossRef]
- Foudah, A.I.; Devi, S.; Alam, A.; Salkini, M.A.; Ross, S.A. Anticholinergic effect of resveratrol with vitamin E on scopolamine-induced Alzheimer’s disease in rats: Mechanistic approach to prevent inflammation. Front. Pharmacol. 2023, 14, 1115721. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, Q. Vitamin E δ-tocotrienol inhibits TNF-α-stimulated NF-κB activation by up-regulation of anti-inflammatory A20 via modulation of sphingolipid including elevation of intracellular dihydroceramides. J. Nutr. Biochem. 2019, 64, 101–109. [Google Scholar] [CrossRef]
- Ibrahim, N.F.; Hamezah, H.S.; Yanagisawa, D.; Tsuji, M.; Kiuchi, Y.; Ono, K.; Tooyama, I. The effect of α-tocopherol, α- and γ-tocotrienols on amyloid-β aggregation and disaggregation in vitro. Biochem. Biophys. Rep. 2021, 28, 101131. [Google Scholar] [CrossRef] [PubMed]
- Juárez Olguín, H.; Calderón Guzmán, D.; Hernández García, E.; Barragán Mejía, G. The Role of Dopamine and Its Dysfunction as a Consequence of Oxidative Stress. Oxid. Med. Cell Longev. 2016, 2016, 9730467. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zou, Y.; Wang, L. Neurotransmitters in Prevention and Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 3841. [Google Scholar] [CrossRef] [PubMed]
- Nobili, A.; Latagliata, E.C.; Viscomi, M.T.; Cavallucci, V.; Cutuli, D.; Giacovazzo, G.; Krashia, P.; Rizzo, F.R.; Marino, R.; Federici, M.; et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat. Commun. 2017, 8, 14727. [Google Scholar] [CrossRef]
- Nam, E.; Derrick, J.S.; Lee, S.; Kang, J.; Han, J.; Lee, S.J.C.; Chung, S.W.; Lim, M.H. Regulatory Activities of Dopamine and Its Derivatives toward Metal-Free and Metal-Induced Amyloid-β Aggregation, Oxidative Stress, and Inflammation in Alzheimer’s Disease. ACS Chem. Neurosci. 2018, 9, 2655–2666. [Google Scholar] [CrossRef]
- Fan, X.; Yin, J.; Yin, J.; Weng, X.; Ding, R. Comparison of the anti-inflammatory effects of vitamin E and vitamin D on a rat model of dextran sulfate sodium-induced ulcerative colitis. Exp. Ther. Med. 2023, 25, 98. [Google Scholar] [CrossRef]
- Liu, K.Y.; Nakatsu, C.H.; Jones-Hall, Y.; Kozik, A.; Jiang, Q. Vitamin E alpha- and gamma-tocopherol mitigate colitis, protect intestinal barrier function and modulate the gut microbiota in mice. Free Radic. Biol. Med. 2021, 163, 180–189. [Google Scholar] [CrossRef]
- Kurnik-Lucka, M.; Pasieka, P.; Laczak, P.; Wojnarski, M.; Jurczyk, M.; Gil, K. Gastrointestinal Dopamine in Inflammatory Bowel Diseases: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 12932. [Google Scholar] [CrossRef]
- Feng, Y.; Lu, Y. Immunomodulatory Effects of Dopamine in Inflammatory Diseases. Front. Immunol. 2021, 12, 663102. [Google Scholar] [CrossRef]
- Ugalde, V.; Contreras, F.; Prado, C.; Chovar, O.; Espinoza, A.; Pacheco, R. Dopaminergic signalling limits suppressive activity and gut homing of regulatory T cells upon intestinal inflammation. Mucosal Immunol. 2021, 14, 652–666. [Google Scholar] [CrossRef]
- Iqbal, A.; Anwar, F.; Saleem, U.; Khan, S.S.; Karim, A.; Ahmad, B.; Gul, M.; Iqbal, Z.; Ismail, T. Inhibition of Oxidative Stress and the NF-κB Pathway by a Vitamin E Derivative: Pharmacological Approach against Parkinson’s Disease. ACS Omega 2022, 7, 45088–45095. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Ramdas, P.; Radhakrishnan, A.K.; Kutty, M.K.; Haleagrahara, N. Tocotrienols Ameliorate Neurodegeneration and Motor Deficits in the 6-OHDA-Induced Rat Model of Parkinsonism: Behavioural and Immunohistochemistry Analysis. Nutrients 2021, 13, 1583. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, S.; Kim, S.; Lee, J.; Ha, J.; Oh, H.; Lee, Y.; Kim, Y.; Yoon, Y. Vitamin E (α-tocopherol) consumption influences gut microbiota composition. Int. J. Food Sci. Nutr. 2020, 71, 221–225. [Google Scholar] [CrossRef]
- Pham, V.T.; Fehlbaum, S.; Seifert, N.; Richard, N.; Bruins, M.J.; Sybesma, W.; Rehman, A.; Steinert, R.E. Effects of colon-targeted vitamins on the composition and metabolic activity of the human gut microbiome- a pilot study. Gut Microbes 2021, 13, 1875774. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Lin, J.; Isnard, S.; Fombuena, B.; Peng, X.; Marette, A.; Routy, B.; Messaoudene, M.; Chen, Y.; Routy, J.P. The Bacterium Akkermansia muciniphila: A Sentinel for Gut Permeability and Its Relevance to HIV-Related Inflammation. Front. Immunol. 2020, 11, 645. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Ye, K.; Li, M.; Ying, J.; Wang, H.; Han, J.; Shi, L.; Xiao, J.; Shen, Y.; Feng, X.; et al. Xylitol enhances synthesis of propionate in the colon via cross-feeding of gut microbiota. Microbiome 2021, 9, 62. [Google Scholar] [CrossRef]
- Parsaei, M.; Sarafraz, N.; Moaddab, S.Y.; Ebrahimzadeh Leylabadlo, H. The importance of Faecalibacterium prausnitzii in human health and diseases. New Microbes New Infect. 2021, 43, 100928. [Google Scholar] [CrossRef]
- Chung, E.; Elmassry, M.M.; Kottapalli, P.; Kottapalli, K.R.; Kaur, G.; Dufour, J.M.; Wright, K.; Ramalingam, L.; Moustaid-Moussa, N.; Wang, R.; et al. Metabolic benefits of annatto-extracted tocotrienol on glucose homeostasis, inflammation, and gut microbiome. Nutr. Res. 2020, 77, 97–107. [Google Scholar] [CrossRef]
- Shen, C.-L.; Kaur, G.; Wanders, D.; Sharma, S.; Tomison, M.D.; Ramalingam, L.; Chung, E.; Moustaid-Moussa, N.; Mo, H.; Dufour, J.M. Annatto-extracted tocotrienols improve glucose homeostasis and bone properties in high-fat diet-induced type 2 diabetic mice by decreasing the inflammatory response. Sci. Rep. 2018, 8, 11377. [Google Scholar] [CrossRef]
- Pang, K.L.; Chin, K.Y. The Role of Tocotrienol in Protecting Against Metabolic Diseases. Molecules 2019, 24, 923. [Google Scholar] [CrossRef]
- González-Bosch, C.; Boorman, E.; Zunszain, P.A.; Mann, G.E. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biol. 2021, 47, 102165. [Google Scholar] [CrossRef] [PubMed]
- Saw, T.Y.; Malik, N.A.; Lim, K.P.; Teo, C.W.L.; Wong, E.S.M.; Kong, S.C.; Fong, C.W.; Petkov, J.; Yap, W.N. Oral Supplementation of Tocotrienol-Rich Fraction Alleviates Severity of Ulcerative Colitis in Mice. J. Nutr. Sci. Vitaminol. 2019, 65, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, Y.; Im, S.; Nakatsu, C.; Jones-Hall, Y.; Jiang, Q. Vitamin E delta-tocotrienol and metabolite 13′-carboxychromanol inhibit colitis-associated colon tumorigenesis and modulate gut microbiota in mice. J. Nutr. Biochem. 2021, 89, 108567. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. gamma-Tocotrienol suppresses growth and sensitises human colorectal tumours to capecitabine in a nude mouse xenograft model by down-regulating multiple molecules. Br. J. Cancer 2016, 115, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Zhang, J.-S.; Zhang, S.-J.; Li, Q.; Liu, Y.-H.; He, N.; Zhang, J.; Zhou, P.-H.; Li, M.; Guan, T.; et al. Tocotrienol-Rich Fraction (TRF) Suppresses the Growth of Human Colon Cancer Xenografts in Balb/C Nude Mice by the Wnt Pathway. PLoS ONE 2015, 10, 916848. [Google Scholar]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A Beneficial Gut Organism from the Discoveries in Genus and Species. Front. Cell Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef]
- Wu, T.R.; Lin, C.S.; Chang, C.J.; Lin, T.L.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Lu, C.C.; Young, J.D.; Lai, H.C. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 2019, 68, 248–262. [Google Scholar] [CrossRef]
- Lai, C.H.; Lin, T.L.; Huang, M.Z.; Li, S.W.; Wu, H.Y.; Chiu, Y.F.; Yang, C.Y.; Chiu, C.H.; Lai, H.C. Gut Commensal Parabacteroides goldsteinii MTS01 Alters Gut Microbiota Composition and Reduces Cholesterol to Mitigate Helicobacter pylori-Induced Pathogenesis. Front. Immunol. 2022, 13, 916848. [Google Scholar] [CrossRef]
- Park, B.H.; Kim, I.S.; Park, J.K.; Zhi, Z.; Lee, H.M.; Kwon, O.W.; Lee, B.C. Probiotic effect of Lactococcus lactis subsp. cremoris RPG-HL-0136 on intestinal mucosal immunity in mice. Appl. Biol. Chem. 2021, 64, 93. [Google Scholar] [CrossRef]
- Asbaghi, O.; Nazarian, B.; Yousefi, M.; Anjom-Shoae, J.; Rasekhi, H.; Sadeghi, O. Effect of vitamin E intake on glycemic control and insulin resistance in diabetic patients: An updated systematic review and meta-analysis of randomized controlled trials. Nutr. J. 2023, 22, 10. [Google Scholar] [CrossRef]
- Browne, D.; McGuinness, B.; Woodside, J.V.; McKay, G.J. Vitamin E and Alzheimer’s disease: What do we know so far? Clin. Interv. Aging 2019, 14, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
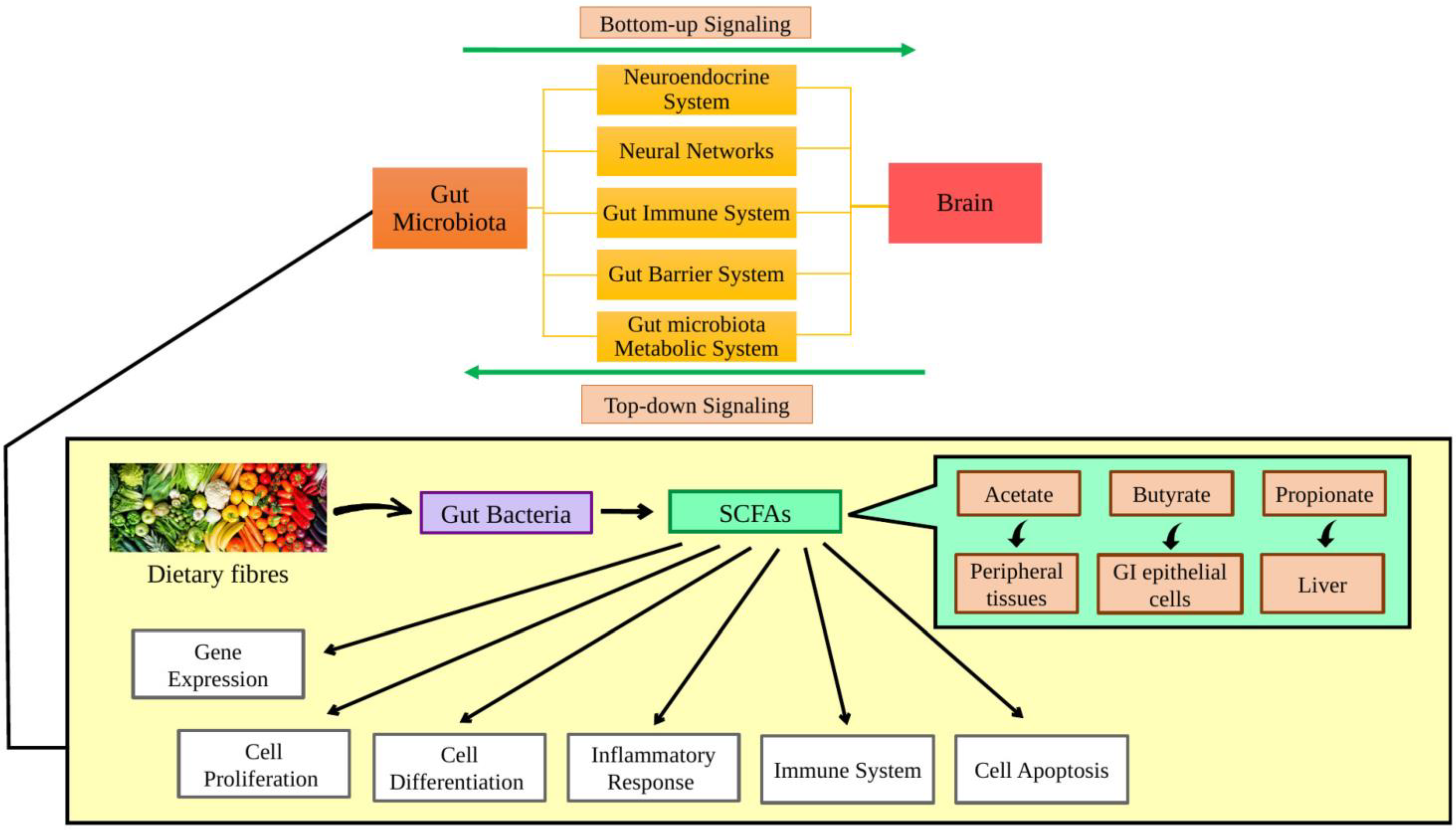
 inhibition,
inhibition,  : increased;
: increased;  : decreased.
: decreased.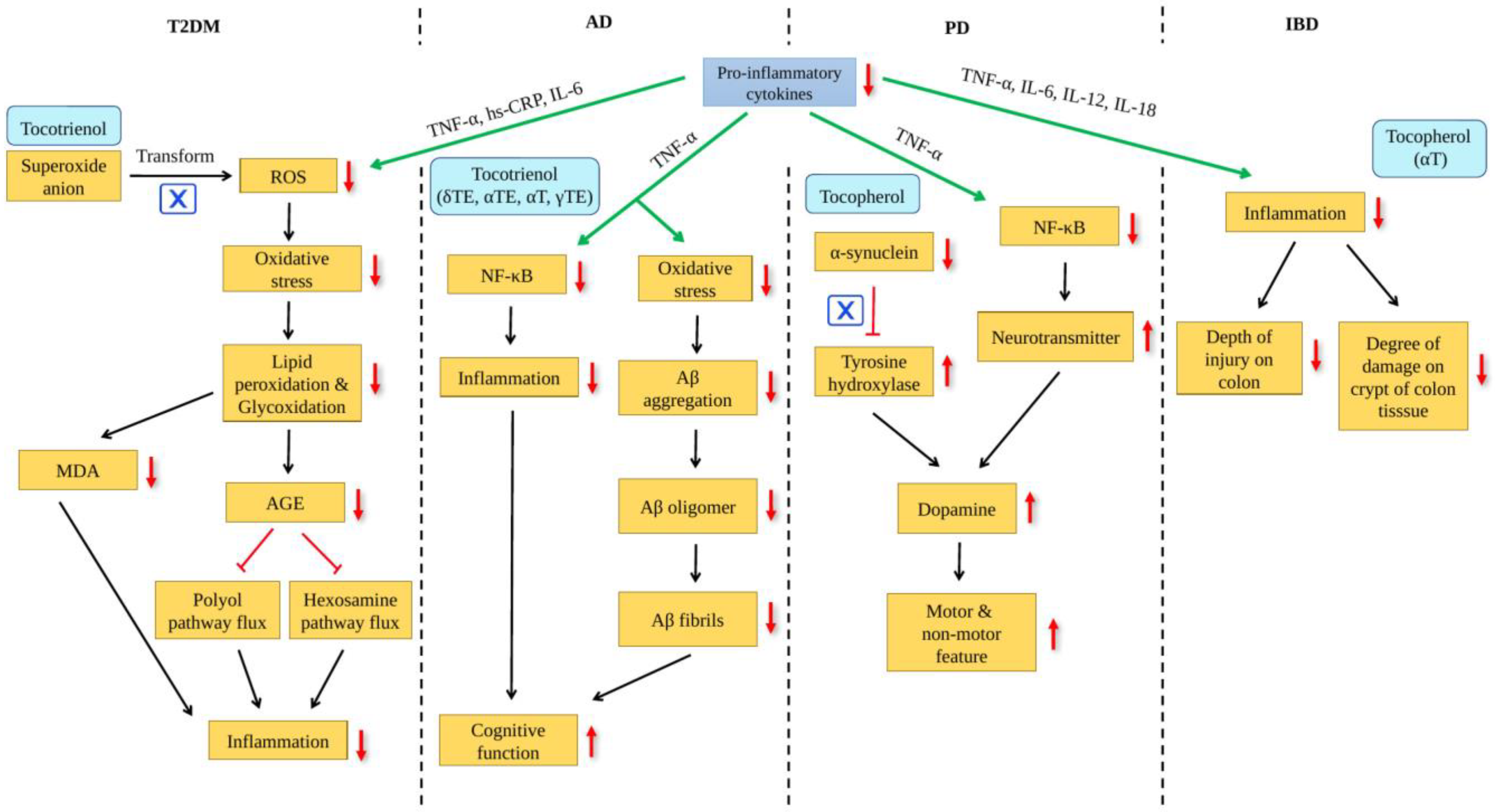
 : increased,
: increased,  : decreased.
: decreased.
 : increased,
: increased,  : decreased.
: decreased.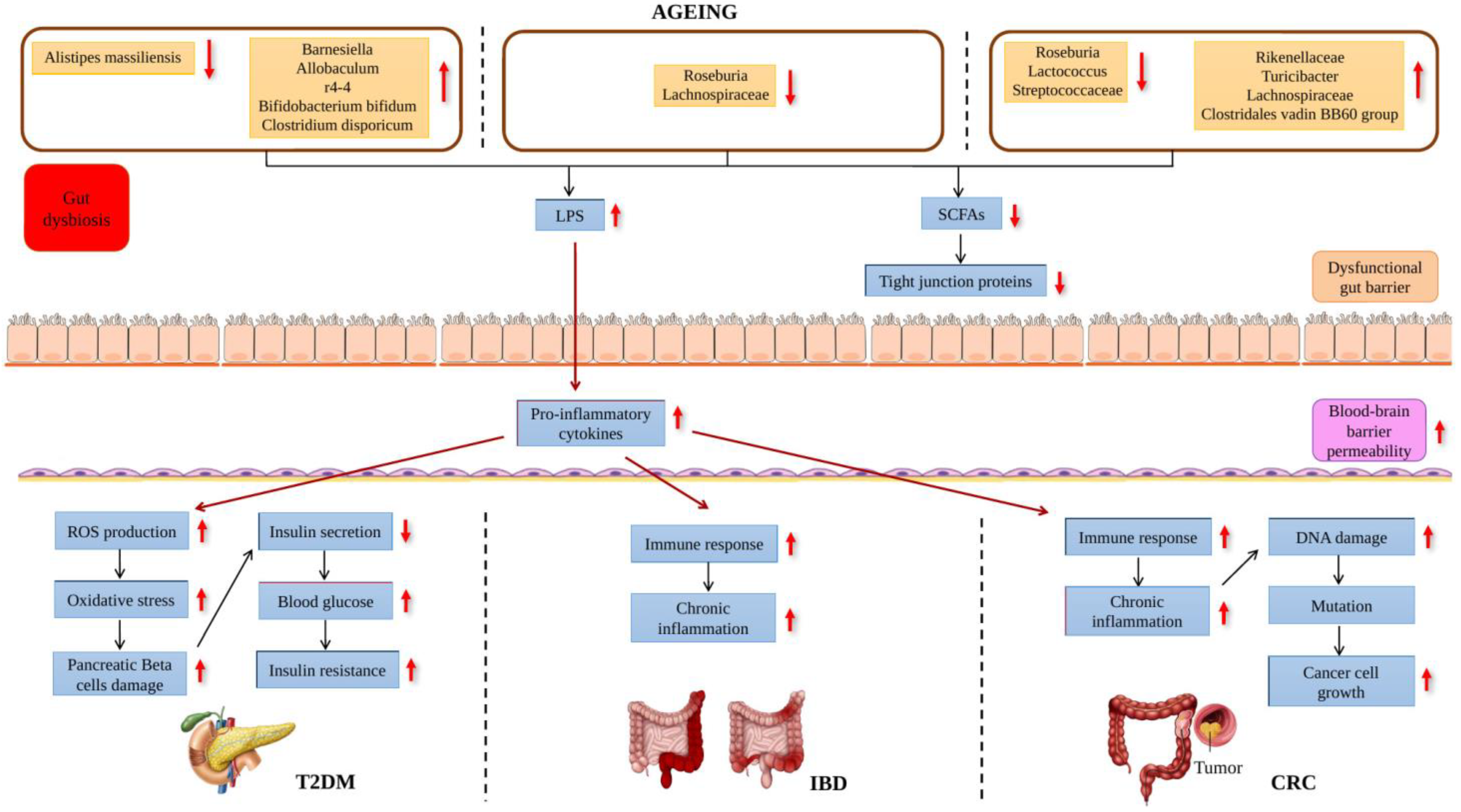
 : increased,
: increased,  : decreased.
: decreased.
 : increased,
: increased,  : decreased.
: decreased.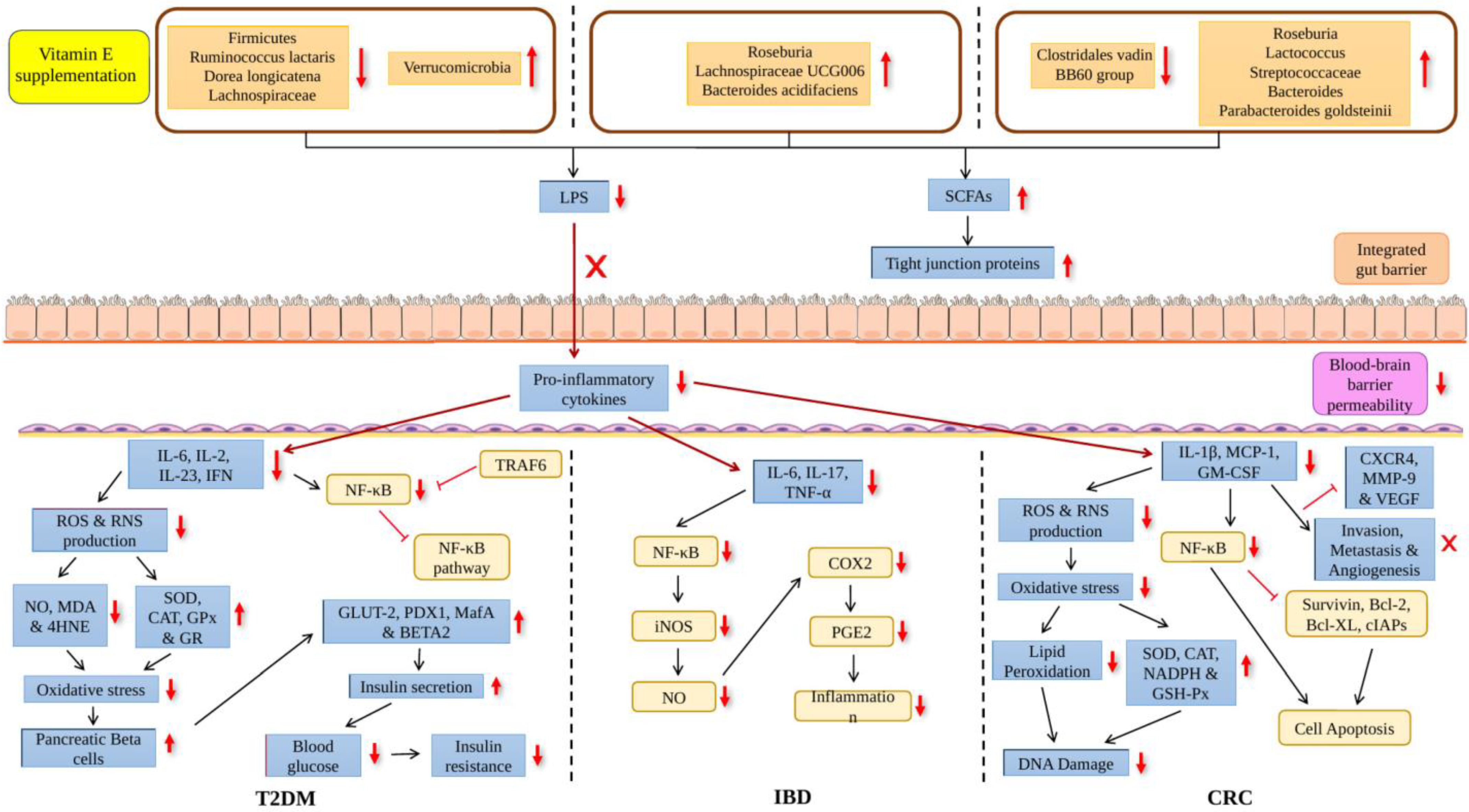
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gothandapani, D.; Makpol, S. Effects of Vitamin E on the Gut Microbiome in Ageing and Its Relationship with Age-Related Diseases: A Review of the Current Literature. Int. J. Mol. Sci. 2023, 24, 14667. https://doi.org/10.3390/ijms241914667
Gothandapani D, Makpol S. Effects of Vitamin E on the Gut Microbiome in Ageing and Its Relationship with Age-Related Diseases: A Review of the Current Literature. International Journal of Molecular Sciences. 2023; 24(19):14667. https://doi.org/10.3390/ijms241914667
Chicago/Turabian StyleGothandapani, Dashine, and Suzana Makpol. 2023. "Effects of Vitamin E on the Gut Microbiome in Ageing and Its Relationship with Age-Related Diseases: A Review of the Current Literature" International Journal of Molecular Sciences 24, no. 19: 14667. https://doi.org/10.3390/ijms241914667
APA StyleGothandapani, D., & Makpol, S. (2023). Effects of Vitamin E on the Gut Microbiome in Ageing and Its Relationship with Age-Related Diseases: A Review of the Current Literature. International Journal of Molecular Sciences, 24(19), 14667. https://doi.org/10.3390/ijms241914667







