Autosomal Dominant Polycystic Kidney Disease: Is There a Role for Autophagy?
Abstract
1. Autosomal-Dominant Polycystic Kidney Disease
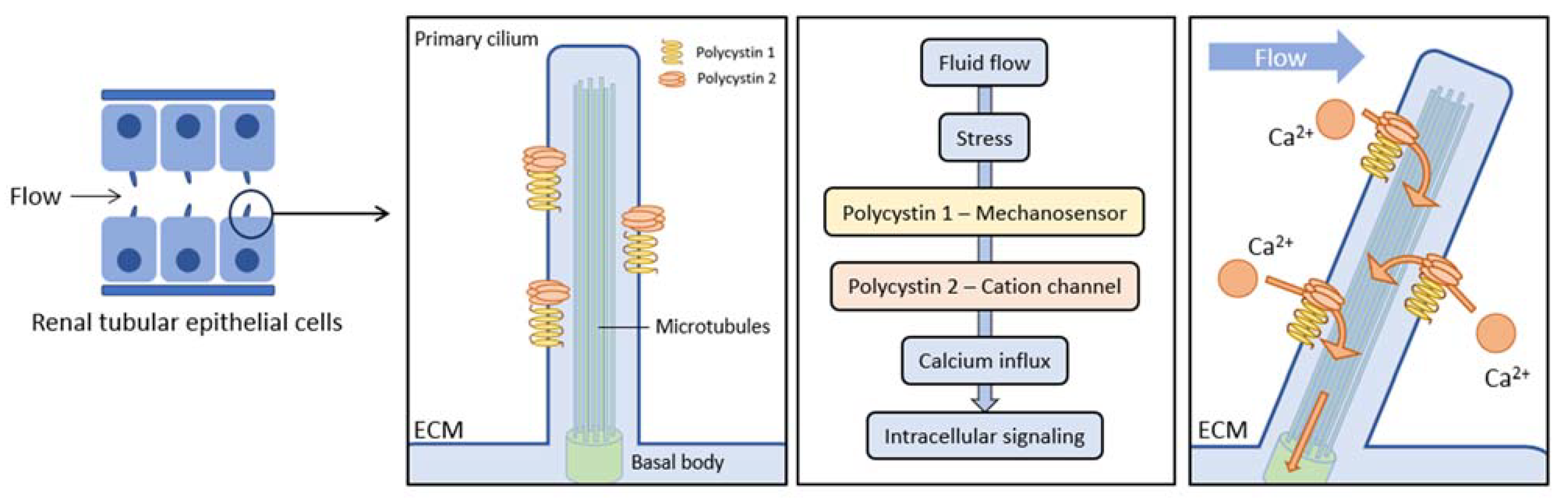
1.1. ADPKD Pathogenesis
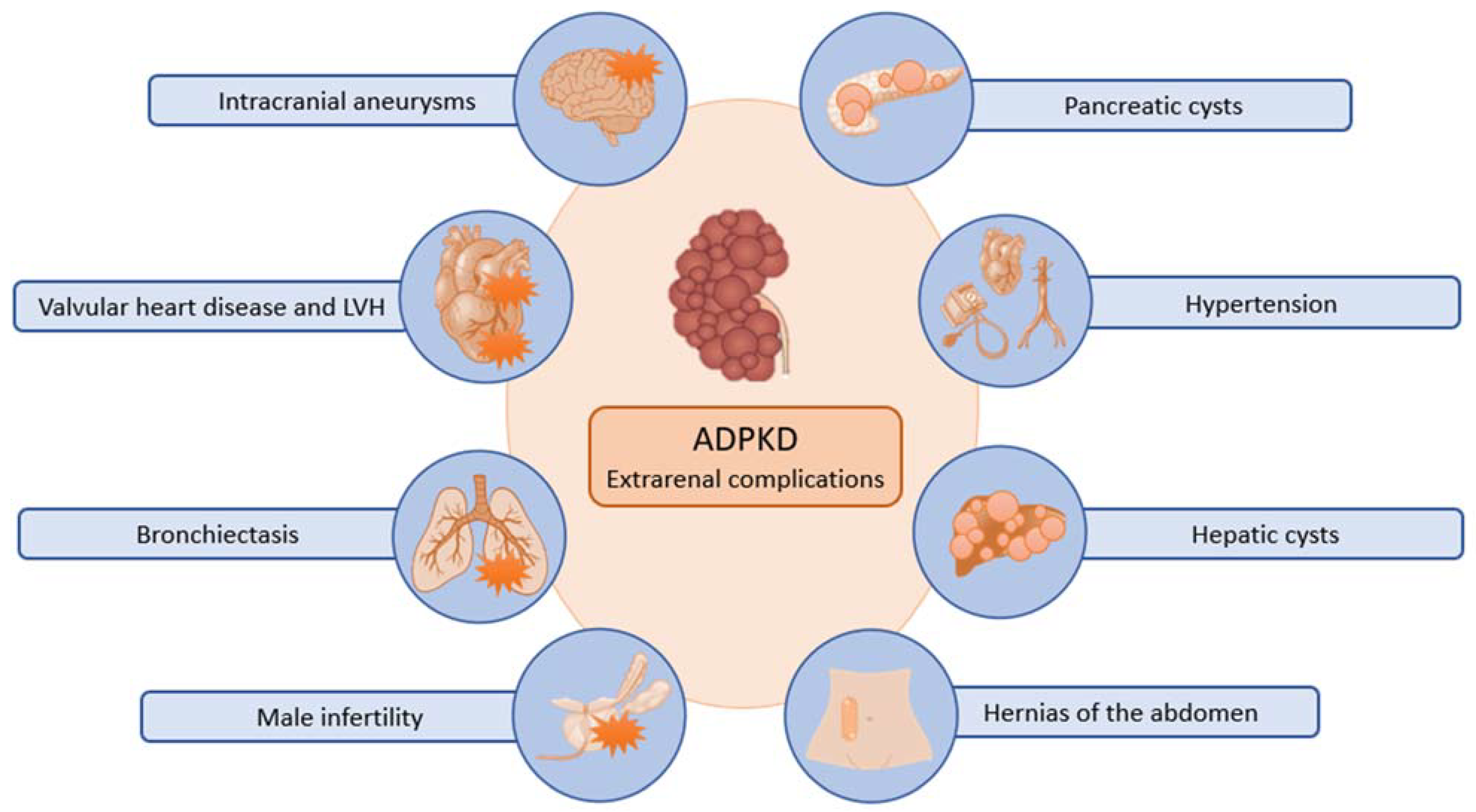
1.2. ADPKD Clinical Presentation and Outcome
1.3. ADPKD Treatment
2. Autophagy and ADPKD
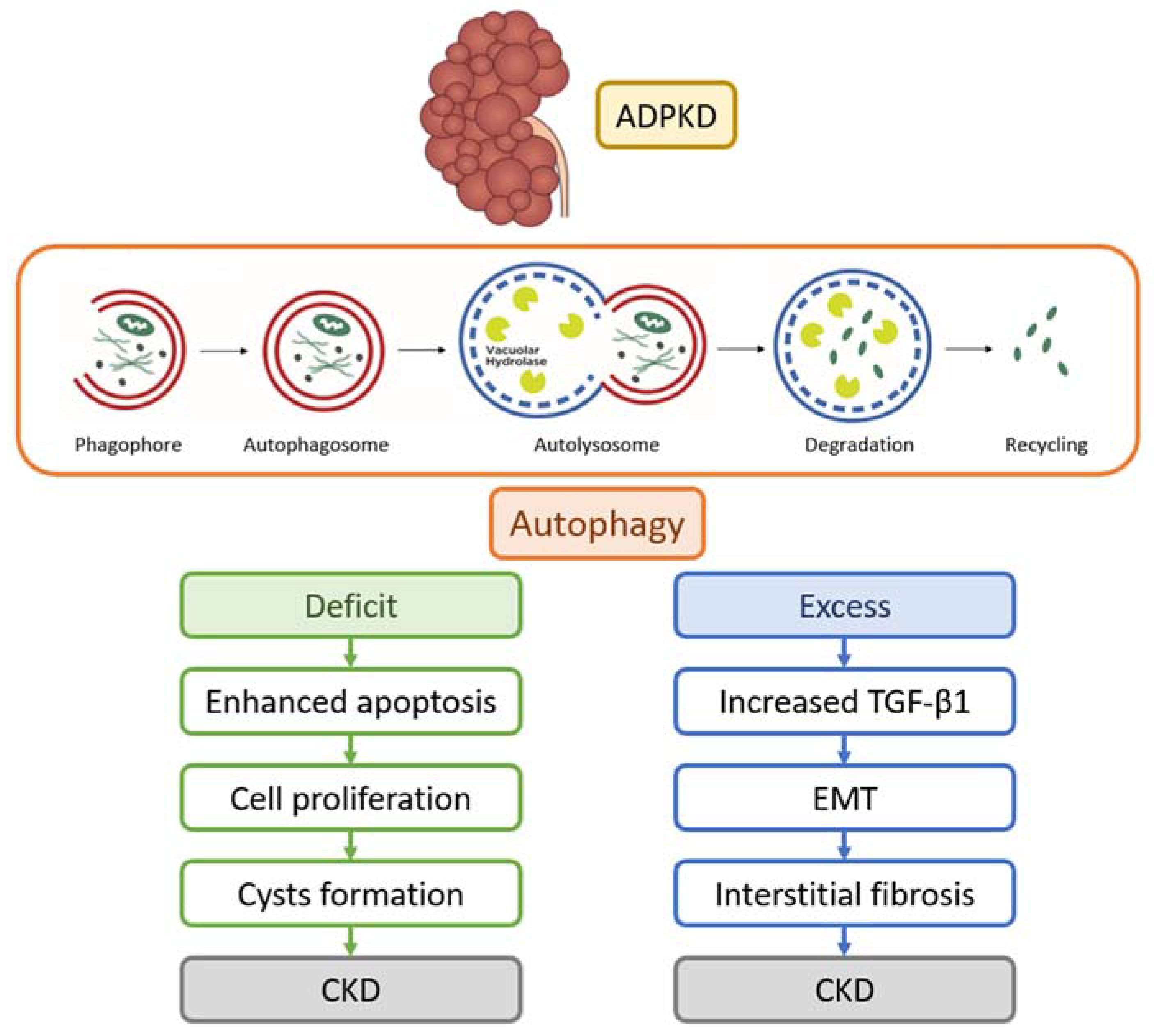
2.1. Autophagy
2.2. Autophagy in PKD Models
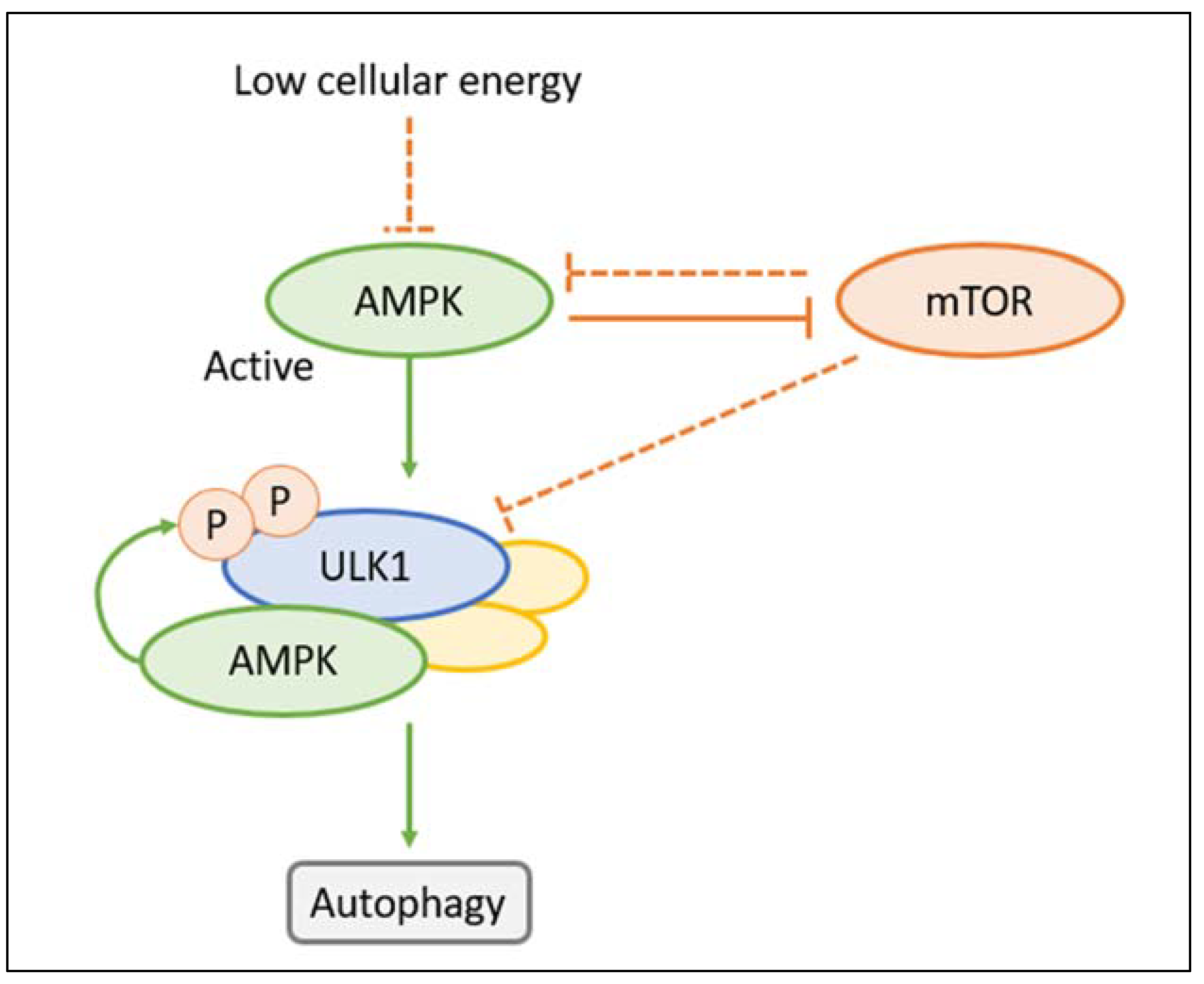
2.3. Autophagy Regulators in ADPKD
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gabow, P.A. Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 1993, 329, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Ward, C.J.; Peral, B.; Aspinwall, R.; Clark, K.; San Millán, J.L.; Gamble, V.; Harris, P.C. The Polycystic Kidney Disease 1 (PKD1) Gene Encodes a Novel Protein with Multiple Cell Recognition Domains. Nat. Genet. 1995, 10, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Burn, T.C.; Connors, T.D.; Dackowski, W.R.; Petry, L.R.; Van Raay, T.J.; Millholland, J.M.; Venet, M.; Miller, G.; Hakim, R.M.; Landes, G.M. Analysis of the Genomic Sequence for the Autosomal Dominant Polycystic Kidney Disease (PKD1) Gene Predicts the Presence of a Leucine-Rich Repeat. The American PKD1 Consortium (APKD1 Consortium). Hum. Mol. Genet. 1995, 4, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Cornec-Le Gall, E.; Audrézet, M.-P.; Renaudineau, E.; Hourmant, M.; Charasse, C.; Michez, E.; Frouget, T.; Vigneau, C.; Dantal, J.; Siohan, P.; et al. PKD2-Related Autosomal Dominant Polycystic Kidney Disease: Prevalence, Clinical Presentation, Mutation Spectrum, and Prognosis. Am. J. Kidney Dis. 2017, 70, 476–485. [Google Scholar] [CrossRef]
- Harris, P.C.; Torres, V.E. Polycystic Kidney Disease. Annu. Rev. Med. 2009, 60, 321–337. [Google Scholar] [CrossRef]
- Ferreira, F.M.; Watanabe, E.H.; Onuchic, L.F. Polycystins and Molecular Basis of Autosomal Dominant Polycystic Kidney Disease. In Polycystic Kidney Disease; Li, X., Ed.; Codon Publications: Brisbane, Australia, 2015; ISBN 978-0-9944381-0-2. [Google Scholar]
- Nigro, E.A.; Boletta, A. Role of the Polycystins as Mechanosensors of Extracellular Stiffness. Am. J. Physiol. Renal Physiol. 2021, 320, F693–F705. [Google Scholar] [CrossRef]
- Santos, A.G.; da Rocha, G.O.; de Andrade, J.B. Occurrence of the Potent Mutagens 2- Nitrobenzanthrone and 3-Nitrobenzanthrone in Fine Airborne Particles. Sci. Rep. 2019, 9, 1. [Google Scholar] [CrossRef]
- Huan, Y.; van Adelsberg, J. Polycystin-1, the PKD1 Gene Product, Is in a Complex Containing E-Cadherin and the Catenins. J. Clin. Investig. 1999, 104, 1459–1468. [Google Scholar] [CrossRef]
- Cantero, M.D.R.; Cantiello, H.F. Polycystin-2 (TRPP2): Ion Channel Properties and Regulation. Gene 2022, 827, 146313. [Google Scholar] [CrossRef]
- Su, Q.; Hu, F.; Ge, X.; Lei, J.; Yu, S.; Wang, T.; Zhou, Q.; Mei, C.; Shi, Y. Structure of the Human PKD1-PKD2 Complex. Science 2018, 361, eaat9819. [Google Scholar] [CrossRef]
- Lanktree, M.B.; Haghighi, A.; di Bari, I.; Song, X.; Pei, Y. Insights into Autosomal Dominant Polycystic Kidney Disease from Genetic Studies. Clin. J. Am. Soc. Nephrol. 2021, 16, 790. [Google Scholar] [CrossRef] [PubMed]
- Roitbak, T.; Ward, C.J.; Harris, P.C.; Bacallao, R.; Ness, S.A.; Wandinger-Ness, A. A Polycystin-1 Multiprotein Complex Is Disrupted in Polycystic Kidney Disease Cells. Mol. Biol. Cell 2004, 15, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Yoder, B.K.; Hou, X.; Guay-Woodford, L.M. The Polycystic Kidney Disease Proteins, Polycystin-1, Polycystin-2, Polaris, and Cystin, Are Co-Localized in Renal Cilia. J. Am. Soc. Nephrol. 2002, 13, 2508–2516. [Google Scholar] [CrossRef]
- Legué, E.; Liem, K.F. Tulp3 Is a Ciliary Trafficking Gene That Regulates Polycystic Kidney Disease. Curr. Biol. 2019, 29, 803–812.e5. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Harris, P.C. Autosomal Dominant Polycystic Kidney Disease: The Last 3 Years. Kidney Int. 2009, 76, 149–168. [Google Scholar] [CrossRef]
- Devuyst, O.; Torres, V.E. Osmoregulation, Vasopressin, and cAMP Signaling in Autosomal Dominant Polycystic Kidney Disease. Curr. Opin. Nephrol. Hypertens. 2013, 22, 459–470. [Google Scholar] [CrossRef]
- Putnam, W.C.; Swenson, S.M.; Reif, G.A.; Wallace, D.P.; Helmkamp, G.M.; Grantham, J.J. Identification of a Forskolin-like Molecule in Human Renal Cysts. J. Am. Soc. Nephrol. 2007, 18, 934–943. [Google Scholar] [CrossRef]
- Andries, A.; Daenen, K.; Jouret, F.; Bammens, B.; Mekahli, D.; Van Schepdael, A. Oxidative Stress in Autosomal Dominant Polycystic Kidney Disease: Player and/or Early Predictor for Disease Progression? Pediatr. Nephrol. 2019, 34, 993–1008. [Google Scholar] [CrossRef]
- Nowak, K.L.; Wang, W.; Farmer-Bailey, H.; Gitomer, B.; Malaczewski, M.; Klawitter, J.; Jovanovich, A.; Chonchol, M. Vascular Dysfunction, Oxidative Stress, and Inflammation in Autosomal Dominant Polycystic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2018, 13, 1493–1501. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Chertow, G.M.; Devarajan, P.; Levin, A.; Andreoli, S.P.; Bangalore, S.; Warady, B.A. Chronic Inflammation in Chronic Kidney Disease Progression: Role of Nrf2. Kidney Int. Rep. 2021, 6, 1775–1787. [Google Scholar] [CrossRef]
- Podrini, C.; Cassina, L.; Boletta, A. Metabolic Reprogramming and the Role of Mitochondria in Polycystic Kidney Disease. Cell. Signal. 2020, 67, 109495. [Google Scholar] [CrossRef] [PubMed]
- Pagliarini, R.; Podrini, C. Metabolic Reprogramming and Reconstruction: Integration of Experimental and Computational Studies to Set the Path Forward in ADPKD. Front. Med. 2021, 8, 740087. [Google Scholar] [CrossRef] [PubMed]
- Rowe, I.; Chiaravalli, M.; Mannella, V.; Ulisse, V.; Quilici, G.; Pema, M.; Song, X.W.; Xu, H.; Mari, S.; Qian, F.; et al. Defective Glucose Metabolism in Polycystic Kidney Disease Identifies a New Therapeutic Strategy. Nat. Med. 2013, 19, 488–493. [Google Scholar] [CrossRef]
- Pei, Y. A “Two-Hit” Model of Cystogenesis in Autosomal Dominant Polycystic Kidney Disease? Trends Mol. Med. 2001, 7, 151–156. [Google Scholar] [CrossRef]
- Qian, F.; Watnick, T.J.; Onuchic, L.F.; Germino, G.G. The Molecular Basis of Focal Cyst Formation in Human Autosomal Dominant Polycystic Kidney Disease Type I. Cell 1996, 87, 979–987. [Google Scholar] [CrossRef]
- Watnick, T.J.; Torres, V.E.; Gandolph, M.A.; Qian, F.; Onuchic, L.F.; Klinger, K.W.; Landes, G.; Germino, G.G. Somatic Mutation in Individual Liver Cysts Supports a Two-Hit Model of Cystogenesis in Autosomal Dominant Polycystic Kidney Disease. Mol. Cell 1998, 2, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Piontek, K.; Menezes, L.F.; Garcia-Gonzalez, M.A.; Huso, D.L.; Germino, G.G. A Critical Developmental Switch Defines the Kinetics of Kidney Cyst Formation after Loss of Pkd1. Nat. Med. 2007, 13, 1490–1495. [Google Scholar] [CrossRef]
- Lantinga-van Leeuwen, I.S.; Leonhard, W.N.; van der Wal, A.; Breuning, M.H.; de Heer, E.; Peters, D.J.M. Kidney-Specific Inactivation of the Pkd1 Gene Induces Rapid Cyst Formation in Developing Kidneys and a Slow Onset of Disease in Adult Mice. Hum. Mol. Genet. 2007, 16, 3188–3196. [Google Scholar] [CrossRef]
- Takakura, A.; Contrino, L.; Zhou, X.; Bonventre, J.V.; Sun, Y.; Humphreys, B.D.; Zhou, J. Renal Injury Is a Third Hit Promoting Rapid Development of Adult Polycystic Kidney Disease. Hum. Mol. Genet. 2009, 18, 2523–2531. [Google Scholar] [CrossRef]
- Chapman, A.B.; Stepniakowski, K.; Rahbari-Oskoui, F. Hypertension in Autosomal Dominant Polycystic Kidney Disease. Adv. Chronic Kidney Dis. 2010, 17, 153–163. [Google Scholar] [CrossRef]
- Sanchis, I.M.; Shukoor, S.; Irazabal, M.V.; Madsen, C.D.; Chebib, F.T.; Hogan, M.C.; El-Zoghby, Z.; Harris, P.C.; Huston, J.; Brown, R.D.; et al. Presymptomatic Screening for Intracranial Aneurysms in Patients with Autosomal Dominant Polycystic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2019, 14, 1151–1160. [Google Scholar] [CrossRef]
- Halvorson, C.R.; Bremmer, M.S.; Jacobs, S.C. Polycystic Kidney Disease: Inheritance, Pathophysiology, Prognosis, and Treatment. Int. J. Nephrol. Renovasc. Dis. 2010, 3, 69–83. [Google Scholar] [CrossRef]
- Luciano, R.L.; Dahl, N.K. Extra-Renal Manifestations of Autosomal Dominant Polycystic Kidney Disease (ADPKD): Considerations for Routine Screening and Management. Nephrol. Dial. Transplant. 2014, 29, 247–254. [Google Scholar] [CrossRef]
- Chebib, F.T.; Torres, V.E. Recent Advances in the Management of Autosomal Dominant Polycystic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2018, 13, 1765–1776. [Google Scholar] [CrossRef]
- Nowak, K.L.; Steele, C.; Gitomer, B.; Wang, W.; Ouyang, J.; Chonchol, M.B. Overweight and Obesity and Progression of ADPKD. Clin. J. Am. Soc. Nephrol. 2021, 16, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Abebe, K.Z.; Schrier, R.W.; Perrone, R.D.; Chapman, A.B.; Yu, A.S.; Braun, W.E.; Steinman, T.I.; Brosnahan, G.; Hogan, M.C.; et al. Dietary Salt Restriction Is Beneficial to the Management of Autosomal Dominant Polycystic Kidney Disease. Kidney Int. 2017, 91, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Bankir, L.; Grantham, J.J. A Case for Water in the Treatment of Polycystic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Abebe, K.Z.; Perrone, R.D.; Torres, V.E.; Braun, W.E.; Steinman, T.I.; Winklhofer, F.T.; Brosnahan, G.; Czarnecki, P.G.; Hogan, M.C.; et al. Blood Pressure in Early Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2014, 371, 2255–2266. [Google Scholar] [CrossRef]
- Torres, V.E.; Abebe, K.Z.; Chapman, A.B.; Schrier, R.W.; Braun, W.E.; Steinman, T.I.; Winklhofer, F.T.; Brosnahan, G.; Czarnecki, P.G.; Hogan, M.C.; et al. Angiotensin Blockade in Late Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2014, 371, 2267–2276. [Google Scholar] [CrossRef]
- Ponticelli, C.; Podestà, M.A.; Moroni, G. Hyperuricemia as a Trigger of Immune Response in Hypertension and Chronic Kidney Disease. Kidney Int. 2020, 98, 1149–1159. [Google Scholar] [CrossRef]
- Brosnahan, G.M.; You, Z.; Wang, W.; Gitomer, B.Y.; Chonchol, M. Serum Uric Acid and Progression of Autosomal Dominant Polycystic Kidney Disease: Results from the HALT PKD Trials. Curr. Hypertens. Rev. 2021, 17, 228–237. [Google Scholar] [CrossRef]
- Suwabe, T. Cyst Infection in Autosomal Dominant Polycystic Kidney Disease: Our Experience at Toranomon Hospital and Future Issues. Clin. Exp. Nephrol. 2020, 24, 748–761. [Google Scholar] [CrossRef]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Grantham, J.J.; Higashihara, E.; Perrone, R.D.; Krasa, H.B.; Ouyang, J.; Czerwiec, F.S.; et al. Tolvaptan in Patients with Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2012, 367, 2407–2418. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Perrone, R.D.; Dandurand, A.; Ouyang, J.; Czerwiec, F.S.; Blais, J.D. TEMPO 4:4 Trial Investigators Multicenter, Open-Label, Extension Trial to Evaluate the Long-Term Efficacy and Safety of Early versus Delayed Treatment with Tolvaptan in Autosomal Dominant Polycystic Kidney Disease: The TEMPO 4:4 Trial. Nephrol. Dial. Transplant. 2018, 33, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.E.; Chapman, A.B.; Devuyst, O.; Gansevoort, R.T.; Perrone, R.D.; Koch, G.; Ouyang, J.; McQuade, R.D.; Blais, J.D.; Czerwiec, F.S.; et al. Tolvaptan in Later-Stage Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2017, 377, 1930–1942. [Google Scholar] [CrossRef] [PubMed]
- Gansevoort, R.T.; Arici, M.; Benzing, T.; Birn, H.; Capasso, G.; Covic, A.; Devuyst, O.; Drechsler, C.; Eckardt, K.-U.; Emma, F.; et al. Recommendations for the Use of Tolvaptan in Autosomal Dominant Polycystic Kidney Disease: A Position Statement on Behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrol. Dial. Transplant. 2016, 31, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.C.; Masyuk, T.V.; Page, L.; Holmes, D.R.; Li, X.; Bergstralh, E.J.; Irazabal, M.V.; Kim, B.; King, B.F.; Glockner, J.F.; et al. Somatostatin Analog Therapy for Severe Polycystic Liver Disease: Results after 2 Years. Nephrol. Dial. Transplant. 2012, 27, 3532–3539. [Google Scholar] [CrossRef]
- Caroli, A.; Perico, N.; Perna, A.; Antiga, L.; Brambilla, P.; Pisani, A.; Visciano, B.; Imbriaco, M.; Messa, P.; Cerutti, R.; et al. Effect of Longacting Somatostatin Analogue on Kidney and Cyst Growth in Autosomal Dominant Polycystic Kidney Disease (ALADIN): A Randomised, Placebo-Controlled, Multicentre Trial. Lancet 2013, 382, 1485–1495. [Google Scholar] [CrossRef]
- Perico, N.; Ruggenenti, P.; Perna, A.; Caroli, A.; Trillini, M.; Sironi, S.; Pisani, A.; Riccio, E.; Imbriaco, M.; Dugo, M.; et al. Octreotide-LAR in Later-Stage Autosomal Dominant Polycystic Kidney Disease (ALADIN 2): A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. PLoS Med. 2019, 16, e1002777. [Google Scholar] [CrossRef]
- Trillini, M.; Caroli, A.; Perico, N.; Remuzzi, A.; Brambilla, P.; Villa, G.; Perna, A.; Peracchi, T.; Rubis, N.; Martinetti, D.; et al. Effects of Octreotide-Long-Acting Release Added-on Tolvaptan in Patients with Autosomal Dominant Polycystic Kidney Disease: Pilot, Randomized, Placebo-Controlled, Cross-Over Trial. Clin. J. Am. Soc. Nephrol. 2023, 18, 223–233. [Google Scholar] [CrossRef]
- Chiaravalli, M.; Rowe, I.; Mannella, V.; Quilici, G.; Canu, T.; Bianchi, V.; Gurgone, A.; Antunes, S.; D’Adamo, P.; Esposito, A.; et al. 2-Deoxy-d-Glucose Ameliorates PKD Progression. J. Am. Soc. Nephrol. 2016, 27, 1958–1969. [Google Scholar] [CrossRef] [PubMed]
- Boletta, A. Reversing Polycystic Kidney Disease. Nat. Genet. 2021, 53, 1623–1624. [Google Scholar] [CrossRef] [PubMed]
- Magistroni, R.; Boletta, A. Defective Glycolysis and the Use of 2-Deoxy-d-Glucose in Polycystic Kidney Disease: From Animal Models to Humans. J. Nephrol. 2017, 30, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. Autophagy: Process and Function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef]
- Boletta, A. Emerging Evidence of a Link between the Polycystins and the mTOR Pathways. Pathogenetics 2009, 2, 6. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR Regulate Autophagy through Direct Phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Nazio, F.; Strappazzon, F.; Antonioli, M.; Bielli, P.; Cianfanelli, V.; Bordi, M.; Gretzmeier, C.; Dengjel, J.; Piacentini, M.; Fimia, G.M.; et al. mTOR Inhibits Autophagy by Controlling ULK1 Ubiquitylation, Self-Association and Function through AMBRA1 and TRAF6. Nat. Cell. Biol. 2013, 15, 406–416. [Google Scholar] [CrossRef]
- Holczer, M.; Hajdú, B.; Lőrincz, T.; Szarka, A.; Bánhegyi, G.; Kapuy, O. A Double Negative Feedback Loop between mTORC1 and AMPK Kinases Guarantees Precise Autophagy Induction upon Cellular Stress. Int. J. Mol. Sci. 2019, 20, 5543. [Google Scholar] [CrossRef]
- Li, J.; Hochstrasser, M. Selective Microautophagy of Proteasomes Is Initiated by ESCRT-0 and Is Promoted by Proteasome Ubiquitylation. J. Cell. Sci. 2022, 135, jcs259393. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Bao, J. Microautophagy: Lesser-Known Self-Eating. Cell. Mol. Life Sci. 2012, 69, 1125–1136. [Google Scholar] [CrossRef]
- Schäfer, J.A.; Schuck, S. ESCRTing Endoplasmic Reticulum to Microautophagic Degradation. Autophagy 2020, 16, 763–764. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Cuervo, A.M. The Coming of Age of Chaperone-Mediated Autophagy. Nat. Rev. Mol. Cell. Biol. 2018, 19, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Liu, J.; Kang, R.; Klionsky, D.J.; Kroemer, G.; Tang, D. Ferroptosis Is a Type of Autophagy-Dependent Cell Death. Semin. Cancer Biol. 2020, 66, 89–100. [Google Scholar] [CrossRef]
- Peintner, L.; Venkatraman, A.; Waeldin, A.; Hofherr, A.; Busch, T.; Voronov, A.; Viau, A.; Kuehn, E.W.; Köttgen, M.; Borner, C. Loss of PKD1/Polycystin-1 Impairs Lysosomal Activity in a CAPN (Calpain)-Dependent Manner. Autophagy 2021, 17, 2384–2400. [Google Scholar] [CrossRef] [PubMed]
- Peña-Oyarzun, D.; Rodriguez-Peña, M.; Burgos-Bravo, F.; Vergara, A.; Kretschmar, C.; Sotomayor-Flores, C.; Ramirez-Sarmiento, C.A.; De Smedt, H.; Reyes, M.; Perez, W.; et al. PKD2/Polycystin-2 Induces Autophagy by Forming a Complex with BECN1. Autophagy 2021, 17, 1714–1728. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.L.; Edelstein, C.L. Apoptosis and Autophagy in Polycystic Kidney Disease (PKD). Cell. Signal. 2020, 68, 109518. [Google Scholar] [CrossRef]
- Criollo, A.; Altamirano, F.; Pedrozo, Z.; Schiattarella, G.G.; Li, D.L.; Rivera-Mejías, P.; Sotomayor-Flores, C.; Parra, V.; Villalobos, E.; Battiprolu, P.K.; et al. Polycystin-2-Dependent Control of Cardiomyocyte Autophagy. J. Mol. Cell. Cardiol. 2018, 118, 110–121. [Google Scholar] [CrossRef]
- Decuypere, J.-P.; Ceulemans, L.J.; Agostinis, P.; Monbaliu, D.; Naesens, M.; Pirenne, J.; Jochmans, I. Autophagy and the Kidney: Implications for Ischemia-Reperfusion Injury and Therapy. Am. J. Kidney Dis. 2015, 66, 699–709. [Google Scholar] [CrossRef]
- Yuajit, C.; Muanprasat, C.; Homvisasevongsa, S.; Chatsudthipong, V. Steviol Stabilizes Polycystin 1 Expression and Promotes Lysosomal Degradation of CFTR and β-Catenin Proteins in Renal Epithelial Cells. Biomed. Pharmacother. 2017, 94, 820–826. [Google Scholar] [CrossRef]
- Pampliega, O.; Orhon, I.; Patel, B.; Sridhar, S.; Díaz-Carretero, A.; Beau, I.; Codogno, P.; Satir, B.H.; Satir, P.; Cuervo, A.M. Functional Interaction between Autophagy and Ciliogenesis. Nature 2013, 502, 194–200. [Google Scholar] [CrossRef]
- Tang, Z.; Lin, M.G.; Stowe, T.R.; Chen, S.; Zhu, M.; Stearns, T.; Franco, B.; Zhong, Q. Autophagy Promotes Primary Ciliogenesis by Removing OFD1 from Centriolar Satellites. Nature 2013, 502, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Livingston, M.J.; Su, Y.; Dong, Z. Reciprocal Regulation of Cilia and Autophagy via the MTOR and Proteasome Pathways. Autophagy 2015, 11, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Tran Nguyen Truc, L.; Matsuda, S.; Takenouchi, A.; Tran Thuy Huong, Q.; Kotani, Y.; Miyazaki, T.; Kanda, H.; Yoshizawa, K.; Tsukaguchi, H. Mechanism of Cystogenesis by Cd79a-Driven, Conditional mTOR Activation in Developing Mouse Nephrons. Sci. Rep. 2023, 13, 508. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Sieben, C.J.; Xu, X.; Harris, P.C.; Lin, X. Autophagy Activators Suppress Cystogenesis in an Autosomal Dominant Polycystic Kidney Disease Model. Hum. Mol. Genet. 2017, 26, 158–172. [Google Scholar] [CrossRef]
- Holditch, S.J.; Brown, C.N.; Atwood, D.J.; Lombardi, A.M.; Nguyen, K.N.; Toll, H.W.; Hopp, K.; Edelstein, C.L. A Study of Sirolimus and mTOR Kinase Inhibitor in a Hypomorphic Pkd1 Mouse Model of Autosomal Dominant Polycystic Kidney Disease. Am. J. Physiol. Renal Physiol. 2019, 317, F187–F196. [Google Scholar] [CrossRef]
- Stayner, C.; Shields, J.; Slobbe, L.; Shillingford, J.M.; Weimbs, T.; Eccles, M.R. Rapamycin-Mediated Suppression of Renal Cyst Expansion in Del34 Pkd1-/- Mutant Mouse Embryos: An Investigation of the Feasibility of Renal Cyst Prevention in the Foetus. Nephrology 2012, 17, 739–747. [Google Scholar] [CrossRef]
- Li, A.; Fan, S.; Xu, Y.; Meng, J.; Shen, X.; Mao, J.; Zhang, L.; Zhang, X.; Moeckel, G.; Wu, D.; et al. Rapamycin Treatment Dose-Dependently Improves the Cystic Kidney in a New ADPKD Mouse Model via the mTORC1 and Cell-Cycle-Associated CDK1/Cyclin Axis. J. Cell. Mol. Med. 2017, 21, 1619–1635. [Google Scholar] [CrossRef]
- Choi, M.E. Autophagy in Kidney Disease. Annu. Rev. Physiol. 2020, 82, 297–322. [Google Scholar] [CrossRef]
- de Stephanis, L.; Bonon, A.; Varani, K.; Lanza, G.; Gafà, R.; Pinton, P.; Pema, M.; Somlo, S.; Boletta, A.; Aguiari, G. Double Inhibition of cAMP and mTOR Signalling May Potentiate the Reduction of Cell Growth in ADPKD Cells. Clin. Exp. Nephrol. 2017, 21, 203–211. [Google Scholar] [CrossRef][Green Version]
- Tanaka, Y.; Watari, M.; Saito, T.; Morishita, Y.; Ishibashi, K. Enhanced Autophagy in Polycystic Kidneys of AQP11 Null Mice. Int. J. Mol. Sci. 2016, 17, 1993. [Google Scholar] [CrossRef]
- Lee, E.J.; Ko, J.Y.; Oh, S.; Jun, J.; Mun, H.; Lim, C.J.; Seo, S.; Ko, H.W.; Kim, H.; Oh, Y.K.; et al. Autophagy Induction Promotes Renal Cyst Growth in Polycystic Kidney Disease. EBioMedicine 2020, 60, 102986. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dong, Z. Is Autophagy the Culprit of Cystogenesis in Polycystic Kidney Disease? EBioMedicine 2020, 61, 103043. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, Y.; Xue, H.; Luan, Z.; Liu, B.; Ren, J. Triptolide, A Potential Autophagy Modulator. Chin. J. Integr. Med. 2019, 25, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ahmadi, Z.; Farkhondeh, T.; Samarghandian, S. Modulatory Effects of Statins on the Autophagy: A Therapeutic Perspective. J. Cell. Physiol. 2020, 235, 3157–3168. [Google Scholar] [CrossRef]
- Jeong, H.-S.; Choi, H.Y.; Lee, E.-R.; Kim, J.-H.; Jeon, K.; Lee, H.-J.; Cho, S.-G. Involvement of Caspase-9 in Autophagy-Mediated Cell Survival Pathway. Biochim. Biophys. Acta 2011, 1813, 80–90. [Google Scholar] [CrossRef]
- Ozfiliz-Kilbas, P.; Sarikaya, B.; Obakan-Yerlikaya, P.; Coker-Gurkan, A.; Arisan, E.D.; Temizci, B.; Palavan-Unsal, N. Cyclin-Dependent Kinase Inhibitors, Roscovitine and Purvalanol, Induce Apoptosis and Autophagy Related to Unfolded Protein Response in HeLa Cervical Cancer Cells. Mol. Biol. Rep. 2018, 45, 815–828. [Google Scholar] [CrossRef]
- Yuajit, C.; Muanprasat, C.; Gallagher, A.-R.; Fedeles, S.V.; Kittayaruksakul, S.; Homvisasevongsa, S.; Somlo, S.; Chatsudthipong, V. Steviol Retards Renal Cyst Growth through Reduction of CFTR Expression and Inhibition of Epithelial Cell Proliferation in a Mouse Model of Polycystic Kidney Disease. Biochem. Pharmacol. 2014, 88, 412–421. [Google Scholar] [CrossRef]
- Zehender, A.; Li, Y.-N.; Lin, N.-Y.; Stefanica, A.; Nüchel, J.; Chen, C.-W.; Hsu, H.-H.; Zhu, H.; Ding, X.; Huang, J.; et al. TGFβ Promotes Fibrosis by MYST1-Dependent Epigenetic Regulation of Autophagy. Nat. Commun. 2021, 12, 4404. [Google Scholar] [CrossRef]
- Ghavami, S.; Cunnington, R.H.; Gupta, S.; Yeganeh, B.; Filomeno, K.L.; Freed, D.H.; Chen, S.; Klonisch, T.; Halayko, A.J.; Ambrose, E.; et al. Autophagy Is a Regulator of TGF-Β1-Induced Fibrogenesis in Primary Human Atrial Myofibroblasts. Cell Death Dis. 2015, 6, e1696. [Google Scholar] [CrossRef]
- Jung, M.; Choi, H.; Mun, J.Y. The Autophagy Research in Electron Microscopy. Appl. Microsc. 2019, 49, 11. [Google Scholar] [CrossRef]
- Mizushima, N.; Yamamoto, A.; Matsui, M.; Yoshimori, T.; Ohsumi, Y. In Vivo Analysis of Autophagy in Response to Nutrient Starvation Using Transgenic Mice Expressing a Fluorescent Autophagosome Marker. Mol. Biol. Cell. 2004, 15, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Vakifahmetoglu-Norberg, H.; Xia, H.; Yuan, J. Pharmacologic Agents Targeting Autophagy. J. Clin. Investig. 2015, 125, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Walz, G.; Budde, K.; Mannaa, M.; Nürnberger, J.; Wanner, C.; Sommerer, C.; Kunzendorf, U.; Banas, B.; Hörl, W.H.; Obermüller, N.; et al. Everolimus in Patients with Autosomal Dominant Polycystic Kidney Disease. N. Engl. J. Med. 2010, 363, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Dai, B.; Mei, C. Long-Term Treatment with Mammalian Target of Rapamycin Inhibitor Does Not Benefit Patients with Autosomal Dominant Polycystic Kidney Disease: A Meta-Analysis. Nephron. Clin. Pract. 2013, 124, 10–16. [Google Scholar] [CrossRef]
- Rangan, G.K. Sirolimus-Associated Proteinuria and Renal Dysfunction. Drug Saf. 2006, 29, 1153–1161. [Google Scholar] [CrossRef]
- Ko, H.T.; Yin, J.L.; Wyburn, K.; Wu, H.; Eris, J.M.; Hambly, B.D.; Chadban, S.J. Sirolimus Reduces Vasculopathy but Exacerbates Proteinuria in Association with Inhibition of VEGF and VEGFR in a Rat Kidney Model of Chronic Allograft Dysfunction. Nephrol. Dial. Transplant. 2013, 28, 327–336. [Google Scholar] [CrossRef][Green Version]
- Kandula, P.; Fridell, J.; Taber, T.E.; Sharfuddin, A.; Yaqub, M.S.; Phillips, C.L.; Chen, J.; Mujtaba, M. Impact of Tacrolimus-Sirolimus Maintenance Immunosuppression on Proteinuria and Kidney Function in Pancreas Transplant Alone Recipients. Transplantation 2012, 94, 940–946. [Google Scholar] [CrossRef]
- Krishnan, S.; Shrestha, Y.; Jayatunga, D.P.W.; Rea, S.; Martins, R.; Bharadwaj, P. Activate or Inhibit? Implications of Autophagy Modulation as a Therapeutic Strategy for Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 6739. [Google Scholar] [CrossRef]
- Carullo, N.; Zicarelli, M.T.; Casarella, A.; Nicotera, R.; Castagna, A.; Urso, A.; Presta, P.; Andreucci, M.; Russo, E.; Bolignano, D.; et al. Retarding Progression of Chronic Kidney Disease in Autosomal Dominant Polycystic Kidney Disease with Metformin and Other Therapies: An Update of New Insights. Int. J. Gen. Med. 2021, 14, 5993–6000. [Google Scholar] [CrossRef]
- Richards, T.; Modarage, K.; Malik, S.A.; Goggolidou, P. The Cellular Pathways and Potential Therapeutics of Polycystic Kidney Disease. Biochem. Soc. Trans. 2021, 49, 1171–1188. [Google Scholar] [CrossRef]
- Pastor-Soler, N.M.; Li, H.; Pham, J.; Rivera, D.; Ho, P.-Y.; Mancino, V.; Saitta, B.; Hallows, K.R. Metformin Improves Relevant Disease Parameters in an Autosomal Dominant Polycystic Kidney Disease Mouse Model. Am. J. Physiol. Renal. Physiol. 2022, 322, F27–F41. [Google Scholar] [CrossRef] [PubMed]
- Bharath, L.P.; Agrawal, M.; McCambridge, G.; Nicholas, D.A.; Hasturk, H.; Liu, J.; Jiang, K.; Liu, R.; Guo, Z.; Deeney, J.; et al. Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell Metab. 2020, 32, 44–55.e6. [Google Scholar] [CrossRef] [PubMed]
- Perrone, R.D.; Abebe, K.Z.; Watnick, T.J.; Althouse, A.D.; Hallows, K.R.; Lalama, C.M.; Miskulin, D.C.; Seliger, S.L.; Tao, C.; Harris, P.C.; et al. Primary Results of the Randomized Trial of Metformin Administration in Polycystic Kidney Disease (TAME PKD). Kidney Int. 2021, 100, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Kramers, B.J.; Koorevaar, I.W.; van Gastel, M.D.A.; van Goor, H.; Hallows, K.R.; Heerspink, H.L.; Li, H.; Leonhard, W.N.; Peters, D.J.M.; Qiu, J.; et al. Effects of Hydrochlorothiazide and Metformin on Aquaresis and Nephroprotection by a Vasopressin V2 Receptor Antagonist in ADPKD: A Randomized Crossover Trial. Clin. J. Am. Soc. Nephrol. 2022, 17, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Sun, J. Vitamin D, Vitamin D Receptor, and Macroautophagy in Inflammation and Infection. Discov. Med. 2011, 11, 325–335. [Google Scholar] [PubMed]
- Bhutia, S.K. Vitamin D in Autophagy Signaling for Health and Diseases: Insights on Potential Mechanisms and Future Perspectives. J. Nutr. Biochem. 2022, 99, 108841. [Google Scholar] [CrossRef]
- Vendramini, L.C.; Dalboni, M.A.; de Carvalho, J.T.G., Jr.; Batista, M.C.; Nishiura, J.L.; Heilberg, I.P. Association of Vitamin D Levels With Kidney Volume in Autosomal Dominant Polycystic Kidney Disease (ADPKD). Front. Med. 2019, 6, 112. [Google Scholar] [CrossRef]
- Chebib, F.T.; Perrone, R.D.; Chapman, A.B.; Dahl, N.K.; Harris, P.C.; Mrug, M.; Mustafa, R.A.; Rastogi, A.; Watnick, T.; Yu, A.S.L.; et al. A Practical Guide for Treatment of Rapidly Progressive ADPKD with Tolvaptan. J. Am. Soc. Nephrol. 2018, 29, 2458–2470. [Google Scholar] [CrossRef]
- Lian, X.; Wu, X.; Li, Z.; Zhang, Y.; Song, K.; Cai, G.; Li, Q.; Lin, S.; Chen, X.; Bai, X.-Y. The Combination of Metformin and 2-Deoxyglucose Significantly Inhibits Cyst Formation in Miniature Pigs with Polycystic Kidney Disease. Br. J. Pharmacol. 2019, 176, 711–724. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponticelli, C.; Moroni, G.; Reggiani, F. Autosomal Dominant Polycystic Kidney Disease: Is There a Role for Autophagy? Int. J. Mol. Sci. 2023, 24, 14666. https://doi.org/10.3390/ijms241914666
Ponticelli C, Moroni G, Reggiani F. Autosomal Dominant Polycystic Kidney Disease: Is There a Role for Autophagy? International Journal of Molecular Sciences. 2023; 24(19):14666. https://doi.org/10.3390/ijms241914666
Chicago/Turabian StylePonticelli, Claudio, Gabriella Moroni, and Francesco Reggiani. 2023. "Autosomal Dominant Polycystic Kidney Disease: Is There a Role for Autophagy?" International Journal of Molecular Sciences 24, no. 19: 14666. https://doi.org/10.3390/ijms241914666
APA StylePonticelli, C., Moroni, G., & Reggiani, F. (2023). Autosomal Dominant Polycystic Kidney Disease: Is There a Role for Autophagy? International Journal of Molecular Sciences, 24(19), 14666. https://doi.org/10.3390/ijms241914666




