Oral Intake of Inosine 5′-Monophosphate in Mice Promotes the Absorption of Exogenous Fatty Acids and Their Conversion into Triglycerides though Enhancing the Phosphorylation of Adenosine 5′-Monophosphate-Activated Protein Kinase in the Liver, Leading to Lipohyperplasia
Abstract
:1. Introduction
2. Results
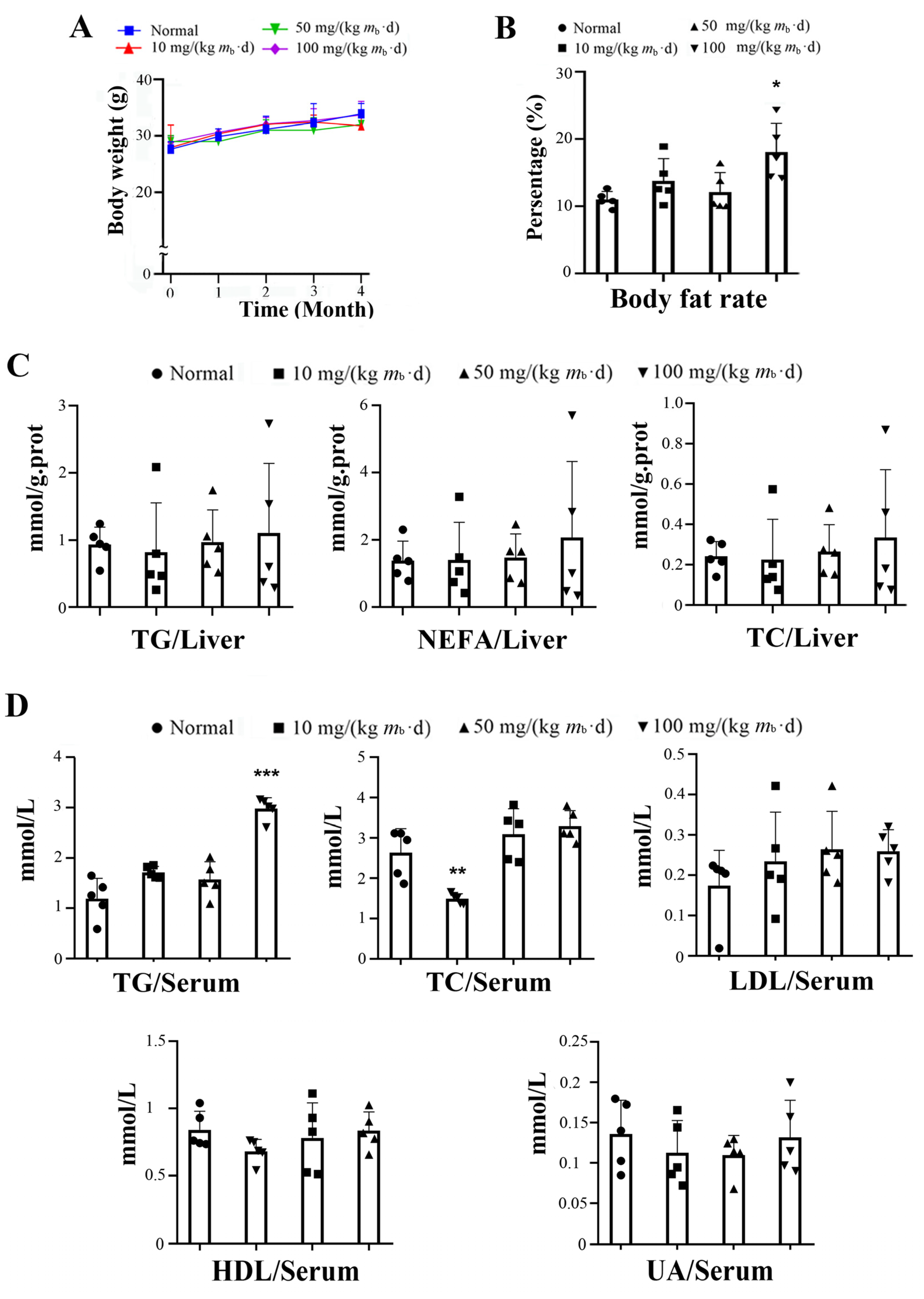
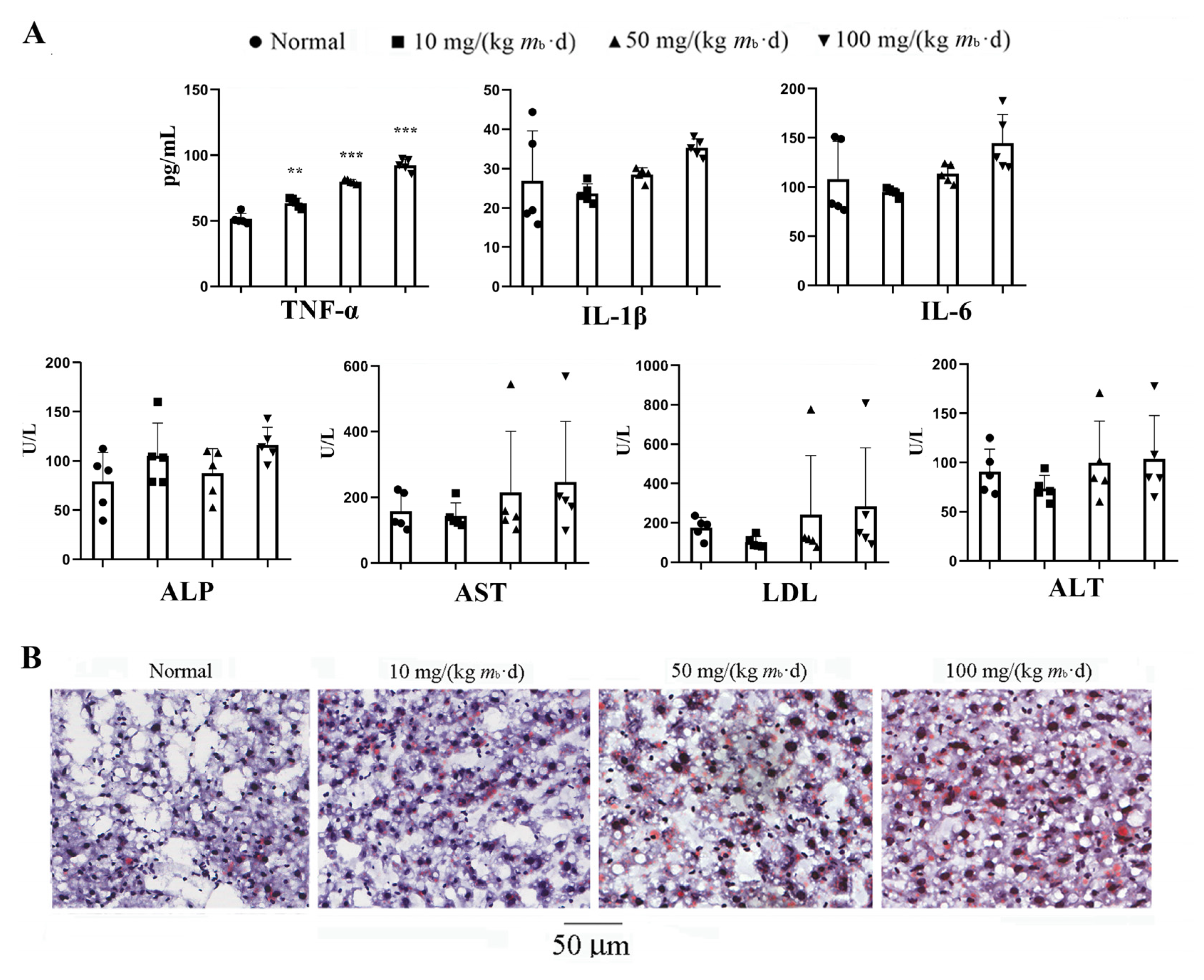
2.1. Intake of IMP in C57BL/6J Mice Induces Lipohyperplasia and Inflammation
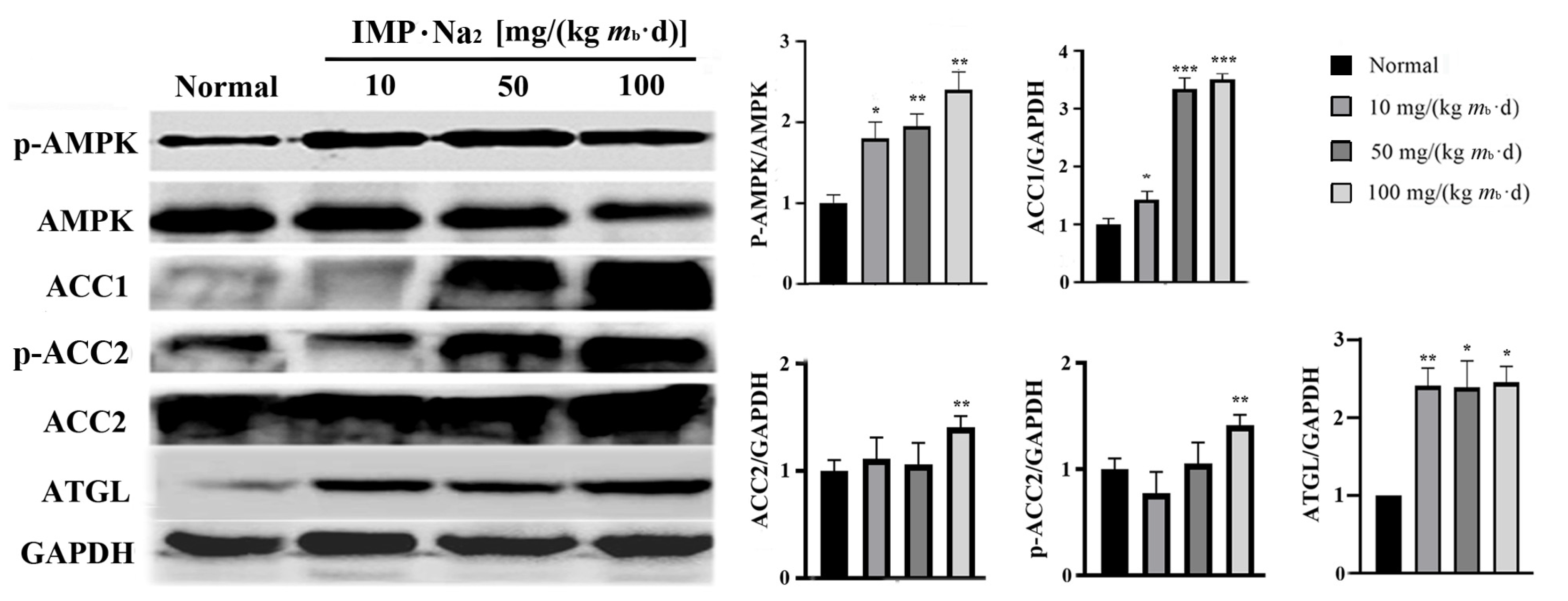
2.2. Adenosine 5′-Monophosphate-Activated Protein Kinase (AMPK) Is the Unique Target for IMP-Regulated TG Metabolism
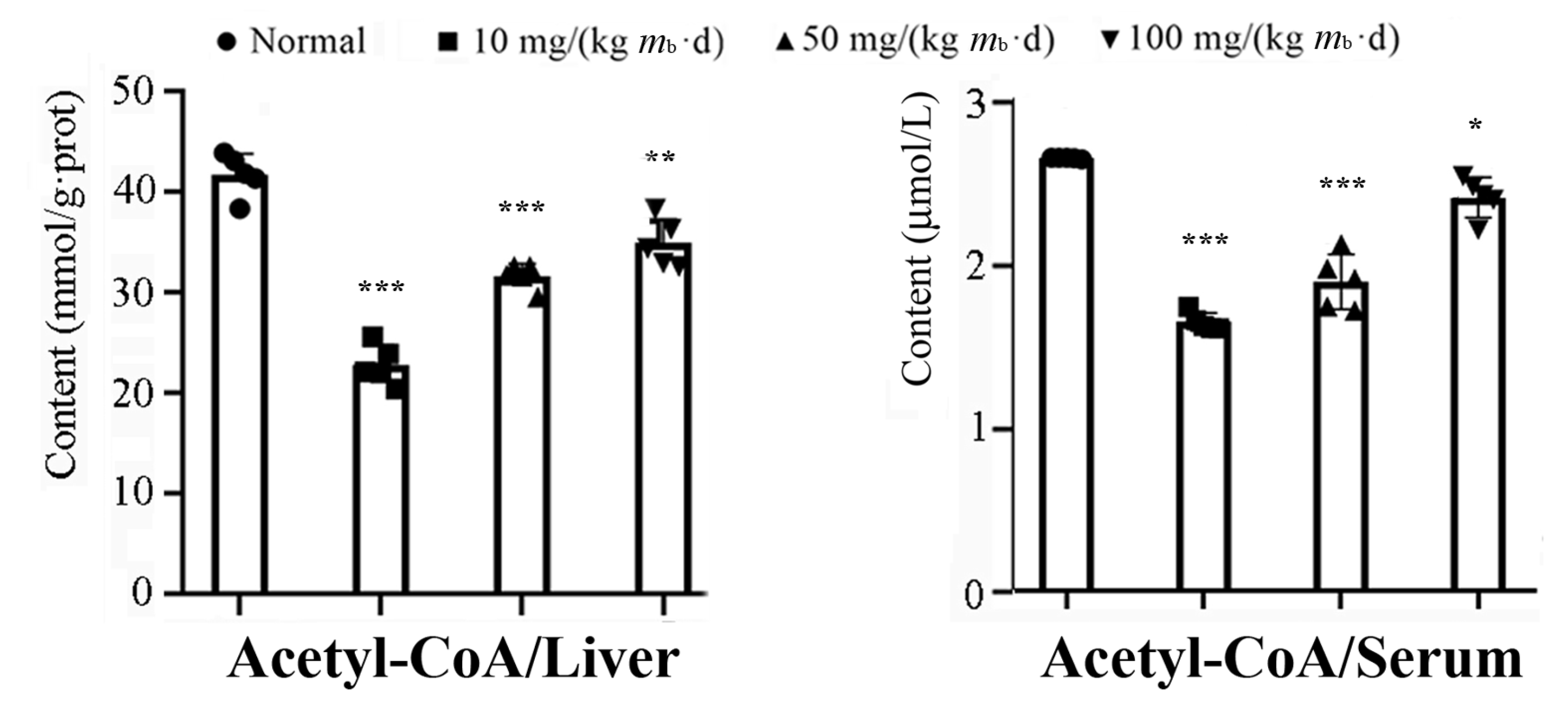
2.3. Intake of IMP in Healthy Mice Promoted Acetyl-CoA Regeneration though Enhancing Phosphorylation of AMPK
2.4. IMP Reduced Activity of Complement System to Induce Inflammation
3. Discussion
4. Materials and Methods
4.1. Mice Feeding, IMP Treatment, and Physiological Data Determination
4.2. Western Blotting Analysis
4.3. In Vitro Assay of Lipid-Lowering Effect of IMP with and without AMPK Inhibitor
4.4. TMT-Based Proteomics Analyses
4.5. Bioinformatics Analyses
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaudhari, N.; Roper, S.D. Molecular and physiological evidence for glutamate (umami) taste transduction via a G protein-coupled receptor. Ann. N. Y. Acad. Sci. 1998, 855, 398–406. [Google Scholar] [CrossRef]
- Kurihara, K.; Kashiwayanagi, M. Physiological studies on umami taste. J. Nutr. 2000, 130, 931S–934S. [Google Scholar] [CrossRef] [PubMed]
- Kinnamon, S.C. Umami taste transduction mechanisms. Am. J. Clin. Nutr. 2009, 90, 753S–755S. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Amaro, R.; Jiménez, A.; Santos, M.A.; Revuelta, J.L. Biotechnological production of feed nucleotides by microbial strain improvement. Process Biochem. 2013, 48, 1263–1270. [Google Scholar] [CrossRef]
- Kojima, K. Safety evaluation of disodium 5′-inosinate, disodium 5′-guanylate and disodium 5′-ribonucleotide. Toxicology 1974, 2, 185–206. [Google Scholar] [CrossRef]
- Gooding, J.R.; Jensen, M.V.; Dai, X.; Wenner, B.R.; Lu, D.; Arumugam, R.; Ferdaoussi, M.; MacDonald, P.E.; Newgard, C.B. Adenylosuccinate Is an Insulin Secretagogue Derived from Glucose-Induced Purine Metabolism. Cell Rep. 2015, 13, 157–167. [Google Scholar] [CrossRef]
- Becker, M.A. Summary of the 2015 Purine and Pyrimidine Society/Purine Metabolic Patients Association H. Anne Simmonds Memorial Lecture. Nucleosides Nucleotides Nucleic Acids 2016, 35, 502–506. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J.; Xu, S.; Wang, Y.; Zhang, B.; Cheng, Z.; Xu, Y.; Xie, Y. The freshener inosine 5′-monophosphate disodium aggravates lipid metabolic disorder in elderly db/db mice. Food Sci. 2021, 42, 150–158. [Google Scholar] [CrossRef]
- Guo, W.; Ge, X.; Lu, J.; Xu, X.; Gao, J.; Wang, Q.; Song, C.; Zhang, Q.; Yu, C. Diet and Risk of Non-Alcoholic Fatty Liver Disease, Cirrhosis, and Liver Cancer: A Large Prospective Cohort Study in UK Biobank. Nutrients 2022, 14, 5535. [Google Scholar] [CrossRef]
- Islam, M.S. Animal models of diabetic neuropathy: Progress since 1960s. J. Diabetes Res. 2013, 2013, 149452. [Google Scholar] [CrossRef]
- Liu, W.; Yu, Z.; Wang, Z.; Waubant, E.L.; Zhai, S.; Benet, L.Z. Using an animal model to predict the effective human dose for oral multiple sclerosis drugs. Clin. Transl. Sci. 2023, 16, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Huang, Z.; Feng, Y.; Tang, A.; Liu, L. Body surface area-based equivalent dose calculation in tree shrew. Sci. Prog. 2021, 104, 368504211016935. [Google Scholar] [CrossRef] [PubMed]
- Andres-Hernando, A.; Cicerchi, C.; Kuwabara, M.; Orlicky, D.J.; Sanchez-Lozada, L.G.; Nakagawa, T.; Johnson, R.J.; Lanaspa, M.A. Umami-induced obesity and metabolic syndrome is mediated by nucleotide degradation and uric acid generation. Nat. Metab. 2021, 3, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Zarrinpar, A.; Chaix, A.; Panda, S. Daily Eating Patterns and Their Impact on Health and Disease. Trends Endocrinol. Metab. 2016, 27, 69–83. [Google Scholar] [CrossRef]
- Mattson, M.P.; Allison, D.B.; Fontana, L.; Harvie, M.; Longo, V.D.; Malaisse, W.J.; Mosley, M.; Notterpek, L.; Ravussin, E.; Scheer, F.A.; et al. Meal frequency and timing in health and disease. Proc. Natl. Acad. Sci. USA 2014, 111, 16647–16653. [Google Scholar] [CrossRef]
- Jiang, J.X.; Tian, K.L.; Chen, H.S.; Zhu, P.F.; Wang, Z.G. Plasma cytokines and endotoxin levels in patients with severe injury and their relationship with organ damage. Injury 1997, 28, 509–513. [Google Scholar] [CrossRef]
- De Conti, A.; Tryndyak, V.; Willett, R.A.; Borowa-Mazgaj, B.; Watson, A.; Patton, R.; Khare, S.; Muskhelishvili, L.; Olson, G.R.; Avigan, M.I.; et al. Characterization of the variability in the extent of nonalcoholic fatty liver induced by a high-fat diet in the genetically diverse Collaborative Cross mouse model. FASEB J. 2020, 34, 7773–7785. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Cheng, Z.; Zhao, X.; Guo, P.; Cai, D.; Xie, Y. Adenylosuccinate, a metabolite in purine salvage pathway, binds to AMP-activated protein kinase (AMPK) γ subunit and enhances the metabolic efficiency of lipid. Chin. Med. Biotechnol. 2020, 15, 240–248. [Google Scholar] [CrossRef]
- Dasgupta, B.; Seibel, W. Compound C/Dorsomorphin: Its Use and Misuse as an AMPK Inhibitor. Methods Mol. Biol. 2018, 1732, 195–202. [Google Scholar] [CrossRef]
- Tobert, J.A. Lovastatin and beyond: The history of the HMG-CoA reductase inhibitors. Nat. Rev. Drug Discov. 2003, 2, 517–526. [Google Scholar] [CrossRef]
- Hunkeler, M.; Hagmann, A.; Stuttfeld, E.; Chami, M.; Guri, Y.; Stahlberg, H.; Maier, T. Structural basis for regulation of human acetyl-CoA carboxylase. Nature 2018, 558, 470–474. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, W.; Li, S.; Guo, D.; He, J.; Wang, Y. Acetyl-CoA Carboxylases and Diseases. Front. Oncol. 2022, 12, 836058. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.B. New appreciation for an old pathway: The Lands Cycle moves into new arenas in health and disease. Biochem. Soc. Trans. 2022, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Xu, X.; Xiang, X.; Li, Y.; Zhao, Z.; Li, J.; Gao, S.; Liu, Q.; Mai, K.; Ai, Q. High Fat Activates O-GlcNAcylation and Affects AMPK/ACC Pathway to Regulate Lipid Metabolism. Nutrients 2021, 13, 1740. [Google Scholar] [CrossRef]
- Patel, R.; Santoro, A.; Hofer, P.; Tan, D.; Oberer, M.; Nelson, A.T.; Konduri, S.; Siegel, D.; Zechner, R.; Saghatelian, A.; et al. ATGL is a biosynthetic enzyme for fatty acid esters of hydroxy fatty acids. Nature 2022, 606, 968–975. [Google Scholar] [CrossRef]
- Tripathy, S.; Lytle, K.A.; Stevens, R.D.; Bain, J.R.; Newgard, C.B.; Greenberg, A.S.; Huang, L.S.; Jump, D.B. Fatty acid elongase-5 (Elovl5) regulates hepatic triglyceride catabolism in obese C57BL/6J mice. J. Lipid Res. 2014, 55, 1448–1464. [Google Scholar] [CrossRef]
- Aleksander, S.A.; Balhoff, J.; Carbon, S.; Cherry, J.M.; Drabkin, H.J.; Ebert, D.; Feuermann, M.; Gaudet, P.; Harris, N.L.; Hill, D.P.; et al. The Gene Ontology knowledgebase in 2023. Genetics 2023, 224, iyad031. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Kawashima, M. KEGG mapping tools for uncovering hidden features in biological data. Protein Sci. 2022, 31, 47–53. [Google Scholar] [CrossRef]
- Santiesteban-Lores, L.E.; Carneiro, M.C.; Isaac, L.; Bavia, L. Complement System in Alcohol-Associated Liver Disease. Immunol. Lett. 2021, 236, 37–50. [Google Scholar] [CrossRef]
- Vignesh, P.; Rawat, A.; Sharma, M.; Singh, S. Complement in autoimmune diseases. Clin. Chim. Acta 2017, 465, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.; McCallin, T.; Martinez, J.; Chacko, S.; Yusuf, S. Hyperlipidemia. Pediatr. Rev. 2020, 41, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Chanoine, P.; Spector, N.D. Hyperlipidemia, eating disorders, and smoking cessation. Curr. Opin. Pediatr. 2008, 20, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Mintoff, D.; Agius, R.; Benhadou, F.; Das, A.; Frew, J.W.; Pace, N.P. Obesity and Hidradenitis Suppurativa: Targeting meta-inflammation for therapeutic gain? Clin. Exp. Dermatol. 2023, 48, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, R.; Li, M.; Ou, W.; Zhang, X.; Yang, W.; Huang, X.; Chai, H.; Wang, Q. TMT proteomics analysis of cerebrospinal fluid from patients with cerebral venous sinus thrombosis. J. Proteom. 2023, 275, 104820. [Google Scholar] [CrossRef] [PubMed]



| Accession No. | Symbol | Description | Ratio | p-Value (n = 3) |
|---|---|---|---|---|
| Q9EQ21 | HEPC | Hepcidin | 1.20 | 0.0257 |
| P52840 | ST1A1 | Sulfotransferase 1A1 | 1.26 | 0.0331 |
| Q9QXM0 | ABHD2 | Mono-acylglycerol lipase ABHD2 | 1.30 | 0.0353 |
| Q8BHI7 | ELOV5 | Elongation of very long chain fatty acids protein 5 | 1.24 | 0.0436 |
| Accession No. | Symbol | Description | Ratio | p-Value (n = 3) |
|---|---|---|---|---|
| Q8K182 | C8A | Complement component C8 α chain | 0.79 | 0.0024 |
| A6X935 | ITIH4 | Inter α-trypsin inhibitor, heavy chain 4 | 0.78 | 0.0049 |
| P06684 | C5 | Complement C5 | 0.81 | 0.0069 |
| P06909 | CFAH | Complement factor H | 0.82 | 0.0081 |
| P06683 | C9 | Complement component C9 | 0.78 | 0.0091 |
| Q61646 | HPT | Haptoglobin | 0.60 | 0.0114 |
| Q9ET22 | DPP2 | Dipeptidyl peptidase 2 | 0.81 | 0.0129 |
| Q8K558 | TRML1 | Trem-like transcript 1 protein | 0.81 | 0.0143 |
| Q8VCG4 | C8G | Complement component C8 γ chain | 0.81 | 0.0170 |
| Q9D1M7 | FKB11 | Peptidyl-prolyl cis-trans isomerase FKBP11 | 0.78 | 0.0230 |
| P58044 | IDI1 | Isopentenyl-diphosphate Delta-isomerase 1 | 0.81 | 0.0264 |
| P01029 | C4B | Complement C4-B | 0.81 | 0.0320 |
| P53798 | FDFT | Squalene synthase | 0.72 | 0.0320 |
| Q61704 | ITIH3 | Inter-α-trypsin inhibitor heavy chain H3 | 0.81 | 0.0341 |
| O35988 | SDC4 | Syndecan-4 | 0.77 | 0.0346 |
| Q91X72 | HEMO | Hemopexin | 0.70 | 0.0356 |
| P33146 | CAD15 | Cadherin-15 | 0.78 | 0.0372 |
| Q8R2E9 | ERO1B | endoplasmic reticulum oxidoreductase 1-like protein β | 0.79 | 0.0391 |
| Q9D964 | GATM | Glycine amidinotransferase, mitochondrial | 0.81 | 0.0408 |
| Q6P5D3 | DHX57 | Putative ATP-dependent RNA helicase DHX57 | 0.79 | 0.0434 |
| P01642 | KV5A9 | Ig κ chain V-V region L7 (Fragment) | 0.78 | 0.0440 |
| Q9R112 | SQOR | Sulfide:quinone oxidoreductase, mitochondrial | 0.80 | 0.0465 |
| P63168 | DYL1 | Dynein light chain 1, cytoplasmic | 0.82 | 0.0485 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Xu, Y.; Liu, J.; Wu, C.; Zhao, X.; Zhou, L.; Xie, Y. Oral Intake of Inosine 5′-Monophosphate in Mice Promotes the Absorption of Exogenous Fatty Acids and Their Conversion into Triglycerides though Enhancing the Phosphorylation of Adenosine 5′-Monophosphate-Activated Protein Kinase in the Liver, Leading to Lipohyperplasia. Int. J. Mol. Sci. 2023, 24, 14588. https://doi.org/10.3390/ijms241914588
Zhang B, Xu Y, Liu J, Wu C, Zhao X, Zhou L, Xie Y. Oral Intake of Inosine 5′-Monophosphate in Mice Promotes the Absorption of Exogenous Fatty Acids and Their Conversion into Triglycerides though Enhancing the Phosphorylation of Adenosine 5′-Monophosphate-Activated Protein Kinase in the Liver, Leading to Lipohyperplasia. International Journal of Molecular Sciences. 2023; 24(19):14588. https://doi.org/10.3390/ijms241914588
Chicago/Turabian StyleZhang, Bin, Yang Xu, Jinyan Liu, Chongming Wu, Xiaohong Zhao, Lidong Zhou, and Yong Xie. 2023. "Oral Intake of Inosine 5′-Monophosphate in Mice Promotes the Absorption of Exogenous Fatty Acids and Their Conversion into Triglycerides though Enhancing the Phosphorylation of Adenosine 5′-Monophosphate-Activated Protein Kinase in the Liver, Leading to Lipohyperplasia" International Journal of Molecular Sciences 24, no. 19: 14588. https://doi.org/10.3390/ijms241914588
APA StyleZhang, B., Xu, Y., Liu, J., Wu, C., Zhao, X., Zhou, L., & Xie, Y. (2023). Oral Intake of Inosine 5′-Monophosphate in Mice Promotes the Absorption of Exogenous Fatty Acids and Their Conversion into Triglycerides though Enhancing the Phosphorylation of Adenosine 5′-Monophosphate-Activated Protein Kinase in the Liver, Leading to Lipohyperplasia. International Journal of Molecular Sciences, 24(19), 14588. https://doi.org/10.3390/ijms241914588





