Remodeling of Cardiomyocytes: Study of Morphological Cellular Changes Preceding Symptomatic Ischemic Heart Failure
Abstract
1. Introduction
2. Results
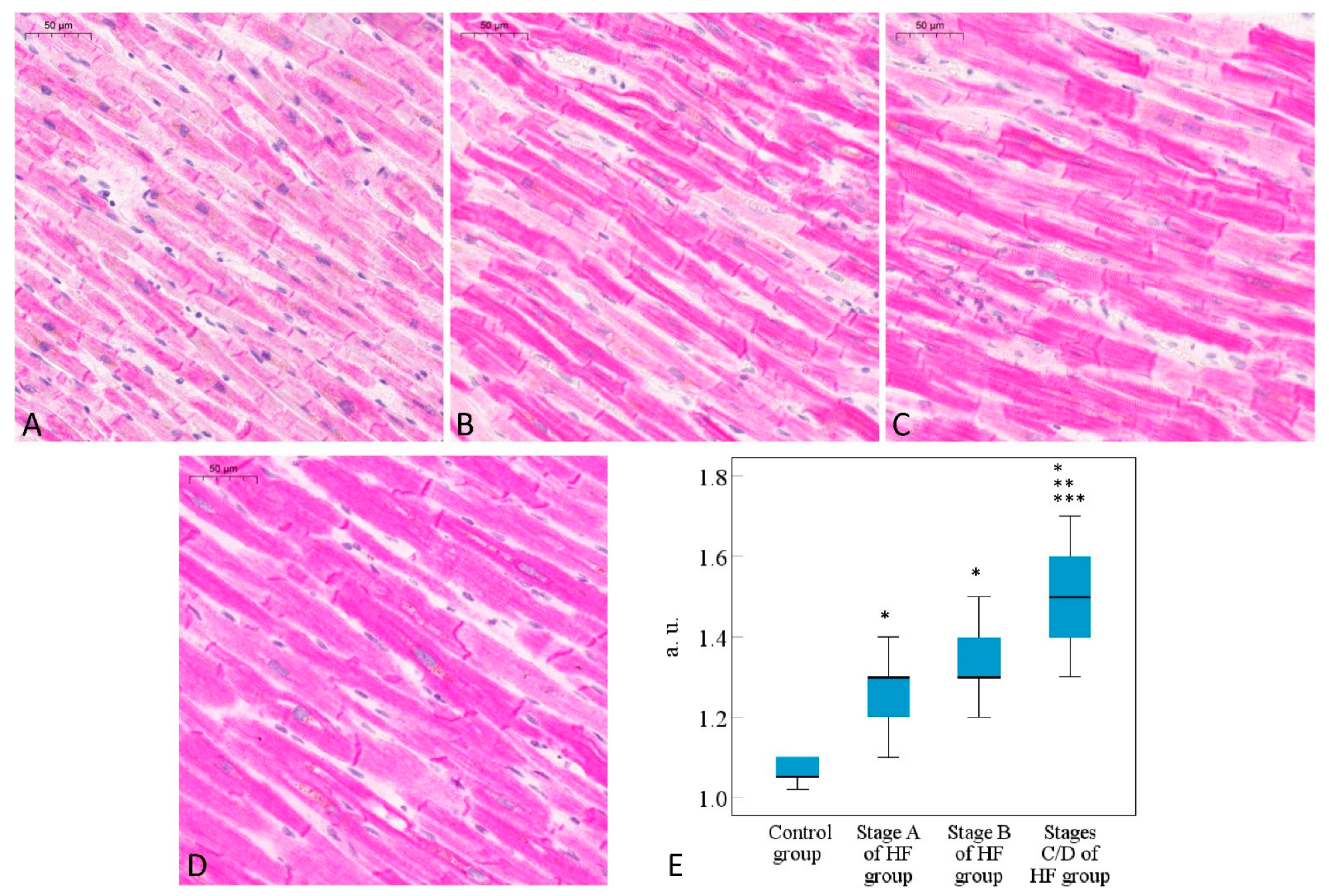
2.1. Histomorphometric Analysis of Cardiomyocytes
2.2. Immunohistochemical Analysis of Desmin Expression in Cardiomyocytes
3. Discussion
4. Materials and Methods
4.1. Study Design and Groups
- Stage A of HF (At risk for HF group)—patients who died suddenly within 1 h after the first clinical symptoms of myocardial infarction (MI) in the witnessed cases or within 24 h of last being seen alive in the unwitnessed cases [44,45]; no previous symptoms of HF were reported, no scars after MI were detected during the morphological tissue inspection, the acute ischemic injuries were up to 6 h [46], HF was diagnosed as being A stage according to the American College of Cardiology (ACC)/American Heart Association (AHA) classification [43], and an extensive morphological examination of the heart was performed during this postmortem procedure (n = 26);
- Stage B of HF (Pre-HF group)—patients who died suddenly due to the cardiovascular complications associated with the ischemic heart injury within 1 h after the first clinical symptoms in the witnessed cases or within 24 h of last being seen alive in the unwitnessed cases [44,45]; no previous symptoms of HF were reported, but a scar after MI was detected in the postmortem morphological inspection of the heart, HF was classified as B stage according to the ACC/AHA classification [43], and the extensive morphological examination of the heart was performed during this postmortem procedure (n = 25);
- Stages C/D of HF (Symptomatic/Advanced HF group)—patients who were diagnosed with symptomatic ischemic HF classified as C or D stage according to ACC/AHA classification [43], a heart transplantation procedure was performed for them, and an extensive morphological examination of surgical material after the failing heart procedure was carried out (n = 33).
4.2. Histomorphometric Analysis of Cardiomyocytes
4.3. Semi-Quantitative Immunohistochemical Analysis of Desmin Expression in Cardiomyocytes
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tromp, J.; Ferreira, J.P.; Janwanishstaporn, S.; Shah, M.; Greenberg, B.; Zannad, F.; Lam, C.S. Heart failure around the world. Eur. J. Heart Fail. 2019, 21, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Stretti, L.; Zippo, D.; Coats, A.J.; Anker, M.S.; von Haehling, S.; Metra, M.; Tomasoni, D. A year in heart failure: An update of recent findings. ESC Heart Fail. 2021, 8, 4370–4393. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Kaku, H.; Matsushima, S.; Tohyama, T.; Enzan, N.; Funakoshi, K.; Sumita, Y.; Nakai, M.; Nishimura, K.; Miyamoto, Y.; et al. Clinical characteristics and outcomes of hospitalized patients with heart failure from the large-scale Japanese registry of acute decompensated heart failure (JROADHF). Circ. J. 2021, 85, 1438–1450. [Google Scholar] [CrossRef]
- Seferović, P.M.; Jankowska, E.; Coats, A.J.; Maggioni, A.P.; Lopatin, Y.; Milinković, I.; Polovina, M.; Lainščak, M.; Timmis, A.; Huculeci, R.; et al. The heart failure association atlas: Rationale, objectives, and methods. Eur. J. Heart Fail. 2020, 22, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Frantz, S.; Hundertmark, M.J.; Schulz-Menger, J.; Bengel, F.M.; Bauersachs, J. Left ventricular remodelling post-myocardial infarction: Pathophysiology, imaging, and novel therapies. Eur. Heart J. 2022, 43, 2549–2561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bolli, R.; Garry, D.J.; Marbán, E.; Menasché, P.; Zimmermann, W.-H.; Kamp, T.J.; Wu, J.C.; Dzau, V.J. Basic and translational research in cardiac repair and regeneration. JACC 2021, 78, 2092–2105. [Google Scholar] [CrossRef]
- De Ponti, F.F.; Scott, C.L. In matters of the heart, (cellular) communication is key. Immunity 2021, 54, 1906–1908. [Google Scholar] [CrossRef]
- Nomura, S.; Satoh, M.; Fujita, T.; Higo, T.; Sumida, T.; Ko, T.; Yamaguchi, Y.; Tobita, T.; Naito, A.T.; Ito, M.; et al. Cardiomyocyte gene programs encoding morphological and functional signatures in cardiac hypertrophy and failure. Nat. Commun. 2018, 9, 4435. [Google Scholar] [CrossRef]
- Kologrivova, I.; Shtatolkina, M.; Suslova, T.; Ryabov, V. Cells of the immune system in cardiac remodeling: Main players in resolution of inflammation and repair after myocardial infarction. Front. Immunol. 2021, 12, 664457. [Google Scholar] [CrossRef]
- Brasoveanu, A.-M.; Mogoanta, L.; Malaescu, G.D.; Predescu, O.I.; Cotoi, B.-V.; Chen, F.I. Hypertensive cardiomyopathy—Histopathological and immunohistochemical aspects. Rom. J. Morphol. Embryol. 2019, 60, 487–494. [Google Scholar]
- Guichard, J.L.; Rogowski, M.; Agnetti, G.; Fu, L.; Powell, P.; Wei, C.-C.; Collawn, J.; Dell’Italia, L.J. Desmin loss and mitochondrial damage precede left ventricular systolic failure in volume overload heart failure. Am. J. Physiol. 2017, 313, H32–H45. [Google Scholar] [CrossRef] [PubMed]
- Hein, S.; Kostin, S.; Heling, A.; Maeno, Y.; Schaper, J. The role of the cytoskeleton in heart failure. Cardiovasc. Res. 2000, 45, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Tsikitis, M.; Galata, M.; Mavroidis, M.; Psarras, S.; Capetanaki, Y. Intermediate filaments in cardiomyopathy. Biophys. Rev. 2018, 10, 1007–1031. [Google Scholar] [CrossRef]
- Mewton, N.; Croisille, P.; Revel, D.; Weber, O.; Higgins, C.; Saeed, M. Left ventricular postmyocardial infarction remodeling studied by combining MR-Tagging with delayed MR Contrast Enhancement. Investig. Radiol. 2008, 43, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, A.M. How to improve the overall quality of cardiac morphometric data. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H9–H14. [Google Scholar] [CrossRef][Green Version]
- Chen, C.Y.; Caporizzo, M.A.; Bedi, K.; Vite, A.; Bogush, A.I.; Robison, P.; Heffler, J.G.; Salomon, A.K.; Kelly, N.A.; Babu, A.; et al. Supression of detyrosinated microtubules improves cardiomyocyte function in human heart failure. Nat. Med. 2018, 24, 1225–1233. [Google Scholar] [CrossRef]
- Tracy, E.; Rowe, G.; LeBlanc, A.J. Cardiac tissue remodeling in healthy aging: The road to pathology. Am. J. Physiol. Cell Physiol. 2020, 319, C166–C182. [Google Scholar] [CrossRef]
- Kehat, I.; Molkentin, J.D. Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation 2010, 122, 2727–2735. [Google Scholar] [CrossRef]
- Nicol, R.L.; Frey, N.; Pearson, G.; Cobb, M.; Richardson, J.; Olson, E.N. Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. EMBO J. 2001, 11, 2757–2767. [Google Scholar] [CrossRef]
- Mühlfeld, C.; Rajces, A.; Manninger, M.; Alogna, A.; Wierich, M.C.; Scherr, D.; Post, H.; Schipke, J. A transmural gradient of myocardial remodeling in early-stage heart failure with preserved ejection fraction in the pig. J. Anat. 2020, 236, 531–539. [Google Scholar] [CrossRef]
- Vigliano, C.A.; Cabeza Meckert, P.M.; Diez, M.; Favaloro, L.E.; Cortés, C.; Fazzi, L.; Favaloro, R.R.; Laguens, R.P. Cardiomyocyte hypertrophy, oncosis, and autophagic vacuolization predict mortality in idiopathic dilated cardiomyopathy with advanced heart failure. J. Am. Coll. Cardiol. 2011, 57, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, J.; Bosmans, H.; Maes, A.; Suetens, P.; Marchal, G.; Rademakers, F.E. Remote myocardial dysfunction after acute anterior myocardial infarction: Impact of left ventricular shape on regional function. J. Am. Coll. Cardiol. 2000, 35, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Onodera, T.; Said, S.; Gerdes, A.M. Correlation of myocyte lengthening to chamber dilation in the spontaneously hypertensive heart failure (SHHF) rat. JMCC 1998, 30, 2175–2181. [Google Scholar] [CrossRef] [PubMed]
- Janczewski, A.M.; Kadokami, T.; Lemster, B.; Frye, C.S.; McTiernan, C.F.; Feldman, A. Morphological and functional changes in cardiac myocytes from mice overexpressing TNFα. Am. J. Physiol. Heart Circ. Physiol. 2002, 284, H960–H969. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, A.K.; Yu, C.-M.; Lam, W.W.; Yip, G.W.; Fung, W.-H.; So, N.M.; Wang, M.; Sanderson, J.E. Left ventricular systolic asynchrony after acute myocardial infarction in patients with narrow QRS complexes. Am. Heart J. 2005, 149, 497–503. [Google Scholar] [CrossRef]
- Tsuda, T. Clinical assessment of ventricular wall stress in understanding compensatory hypertrophic response and maladaptive ventricular remodeling. J. Cardiovasc Dev. Dis. 2021, 8, 122. [Google Scholar] [CrossRef]
- Canty, J.M., Jr. Myocardial injury, troponin release, and cardiomyocyte death in brief ischemia, failure, and ventricular remodeling. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H1–H5. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Moroni, F.; Montone, R.A.; Azzalini, L.; Sanna, T.; Abbate, A. Ischemic cardiomyopathy and heart failure after acute myocardial infarction. Curr. Cardiol. Rep. 2022, 24, 1505–1515. [Google Scholar] [CrossRef]
- Schwinger, R.H.G. Pathophysiology of heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef]
- Tsutsui, H.; Ishihara, K.; Cooper, G., IV. Cytoskeletal role in the contractile dysfunction of hypertrophied myocardium. Science 1993, 260, 682–687. [Google Scholar] [CrossRef]
- Tagawa, H.; Koide, M.; Sato, H.; Zile, M.R.; Carabello, B.A.; Cooper, G., IV. Cytoskeletal role in the transition from compensated to decompensated hypertrophy during adult canine left ventricular pressure overloading. Circ. Res. 1998, 82, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Tsutsui, H.; Yamamoto, S.; Takahashi, M.; Imanaka-Yoshida, K.; Yoshida, T.; Urabe, Y.; Sugimachi, M.; Takeshita, A. Role of microtubules in myocyte contractile dysfunction during cardiac hypertrophy in rat. Am. J. Physiol. 1996, 271 Pt 2, H1978–H1987. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.R.; Kadioglu, H.; Patel, K.; Carrier, L.; Agnetti, G. Is desmin propensity to aggregate part of its protective function? Cells 2020, 9, 491. [Google Scholar] [CrossRef] [PubMed]
- Milner, D.J.; Taffet, G.E.; Wang, X.; Pham, T.; Tamura, T.; Hartley, C.; Gerdes, M.A.; Capetanaki, Y. The absence of desmin leads to cardiomyocyte hypertrophy and cardiac dilation with compromised systolic function. J. Mol. Cell. Cardiol. 1999, 31, 2063–2076. [Google Scholar] [CrossRef]
- Heffler, J.; Shah, P.P.; Robison, P.; Phyo, S.; Veliz, K.; Uchida, K.; Bogush, A.; Rhoades, J.; Jain, R.; Prosser, B.L. A balance between intermediate filaments and microtubules maintains nuclear architecture in the cardiomyocyte. Circ. Res. 2020, 126, e10–e26. [Google Scholar] [CrossRef]
- Corbett, J.M.; Why, H.J.; Wheeler, C.H.; Richardson, P.J.; Archard, L.C.; Yacoub, M.H.; Dunn, M.J. Cardiac protein abnormalities in dilated cardiomyopathy detected by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis 2005, 19, 2031–2042. [Google Scholar] [CrossRef]
- Pawlak, A.; Gil, R.J.; Kulawik, T.; Pronicki, M.; Karkucińska-Więckowska, A.; Szymańska-Dębińska, T.; Gil, K.; Lagwinski, N.; Czarnowska, E. Type of desmin expression in cardiomyocytes—A good marker of heart failure development in idiopathic dilated cardiomyopathy. J. Intern. Med. 2012, 272, 287–297. [Google Scholar] [CrossRef]
- Bouvet, M.; Dubois-Deruy, E.; Turkieh, A.; Mulder, P.; Peugnet, V.; Chwastyniak, M.; Beseme, O.; Dechaumes, A.; Amouyel, P.; Richard, V.; et al. Desmin aggrephagy in rat and human ischemic heart failure through PKCς and GSK3β as upstream signalling pathways. Cell Death Discov. 2021, 7, 153. [Google Scholar] [CrossRef]
- Brodehl, A.; Gaertner-Rommel, A.; Milting, H. Molecular insights into cardiomyopathies associated with desmin (DES) mutations. Biophys. Rev. 2018, 10, 983–1006. [Google Scholar] [CrossRef]
- Osborn, M.; Goebel, H.H. The cytoplasmic bodies in a congenital myopathy can be stained with antibodies to desmin, the muscle-specific intermediate filament protein. Acta Neuropath. 1983, 62, 149–152. [Google Scholar] [CrossRef]
- del Monte, F.; Agnetti, G. Protein post-translational modifications and misfolding: New concepts in heart failure. Proteomics 2014, 8, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Leone, O.; Veinot, J.P.; Angelini, A.; Baandrup, U.T.; Basso, C.; Berry, G.; Bruneval, P.; Burke, M.; Butany, J.; Calabrese, F.; et al. 2011 Consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc. Pathol. 2012, 21, 245–274. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- WHO Scientific Group on Sudden Cardiac Death & World Health Organization. Sudden Cardiac Death: Report of a WHO Scientific Group [Meeting Held in Geneva from 24 to 27 October 1984]; World Health Organization: Geneva, Switzerland, 1985; Available online: https://apps.who.int/iris/handle/10665/39554 (accessed on 8 August 2023).
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Michaud, K.; Basso, C.; D’amati, G.; Giordano, C.; Kholová, I.; Preston, S.D.; Rizzo, S.; Sabatasso, S.; Sheppard, M.N.; Vink, A.; et al. Diagnosis of myocardial infarction at autopsy: AECVP reappraisal in the light of the current clinical classification. Virchows Arch. 2019, 476, 179–194. [Google Scholar] [CrossRef]
| Parameter | Control Group | Stage A of the HF Group | Stage B of the HF Group | Stages C/D of the HF Group |
|---|---|---|---|---|
| Number of the representative cardiomyocytes | 1929 | 2080 | 1992 | 2637 |
| Mean length (SE), µm | 61.82 (0.34) | 72.23 * (0.32) | 78.86 *,** (0.33) | 103.28 *,**,*** (0.29) |
| 95% CI of length, µm | 61.16–62.47 | 71.59–72.86 | 78.21–79.50 | 102.71–103.83 |
| Mean diameter (SE), µm | 11.73 (0.06) | 14.34 * (0.05) | 15.19 *,** (0.05) | 18.92 *,**,*** (0.05) |
| 95% CI of diameter, µm | 11.62–11.84 | 14.23–14.45 | 15.08–15.30 | 18.83–19.02 |
| Mean volume (SE), µm3 | 7271 (201) | 12,320 * (193) | 15,170 *,** (197) | 31,433 *,**,*** (172) |
| 95% CI of volume, µm3 | 6877–7666 | 11,941–12,699 | 14,783–15,557 | 31,096–31,769 |
| Mean cellular length–diameter ratio (SE) | 5.392 (0.025) | 5.137 * (0.024) | 5.301 #,** (0.025) | 5.583 *,**,*** (0.022) |
| 95% CI of cellular length–diameter ratio | 5.343–5.442 | 5.090–5.185 | 5.252–5.349 | 5.541–5.625 |
| Control Group | Stage A of the HF Group | Stage B of the HF Group | Stages C/D of the HF Group | |
|---|---|---|---|---|
| Number of cases | 25 | 26 | 25 | 33 |
| Mean age (SD), years | 50.5 (8.7) | 54.4 (8.6) | 54.4 (7.4) | 56.8 (7.5) |
| Sex | Male | Male | Male | Male |
| Cardiovascular disease of ischemic origin | No | Yes | Yes | Yes |
| Previous clinical symptoms of HF | No | No | No | Yes |
| Stage of HF according to ACC/AHA * | Not applied | At-Risk for HF | Pre-HF | Symptomatic HF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuprytė, M.; Lesauskaitė, V.; Keturakis, V.; Bunevičienė, V.; Utkienė, L.; Jusienė, L.; Pangonytė, D. Remodeling of Cardiomyocytes: Study of Morphological Cellular Changes Preceding Symptomatic Ischemic Heart Failure. Int. J. Mol. Sci. 2023, 24, 14557. https://doi.org/10.3390/ijms241914557
Kuprytė M, Lesauskaitė V, Keturakis V, Bunevičienė V, Utkienė L, Jusienė L, Pangonytė D. Remodeling of Cardiomyocytes: Study of Morphological Cellular Changes Preceding Symptomatic Ischemic Heart Failure. International Journal of Molecular Sciences. 2023; 24(19):14557. https://doi.org/10.3390/ijms241914557
Chicago/Turabian StyleKuprytė, Milda, Vaiva Lesauskaitė, Vytenis Keturakis, Vitalija Bunevičienė, Lina Utkienė, Lina Jusienė, and Dalia Pangonytė. 2023. "Remodeling of Cardiomyocytes: Study of Morphological Cellular Changes Preceding Symptomatic Ischemic Heart Failure" International Journal of Molecular Sciences 24, no. 19: 14557. https://doi.org/10.3390/ijms241914557
APA StyleKuprytė, M., Lesauskaitė, V., Keturakis, V., Bunevičienė, V., Utkienė, L., Jusienė, L., & Pangonytė, D. (2023). Remodeling of Cardiomyocytes: Study of Morphological Cellular Changes Preceding Symptomatic Ischemic Heart Failure. International Journal of Molecular Sciences, 24(19), 14557. https://doi.org/10.3390/ijms241914557






