Methods of Formation of Protective Inhibited Polymer Films on Tungsten
Abstract
:1. Introduction
2. Results
2.1. Determination of Optimal Modes of Film Formation by CPD
2.2. Film Structure
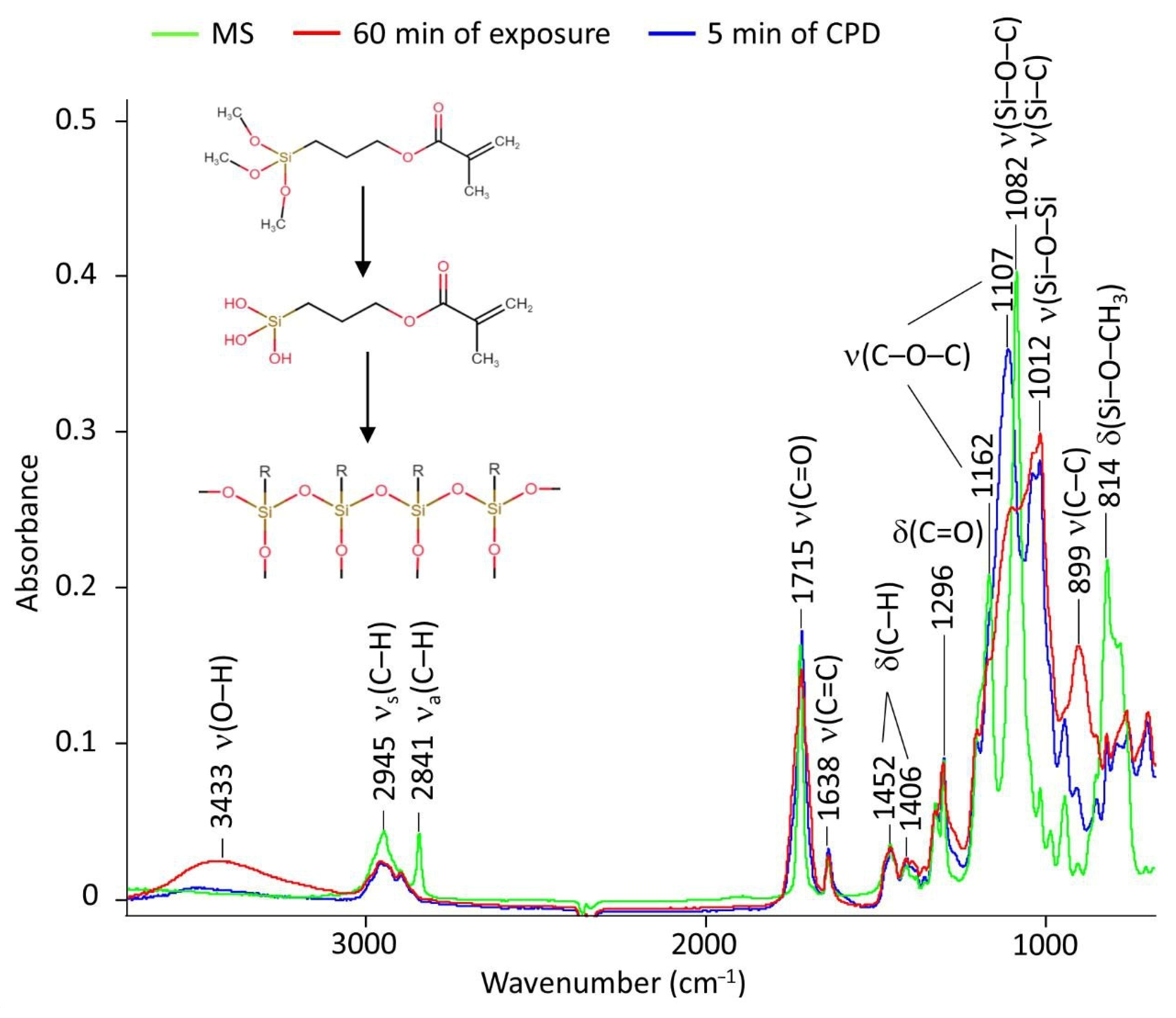
2.2.1. The Molecular Behavior
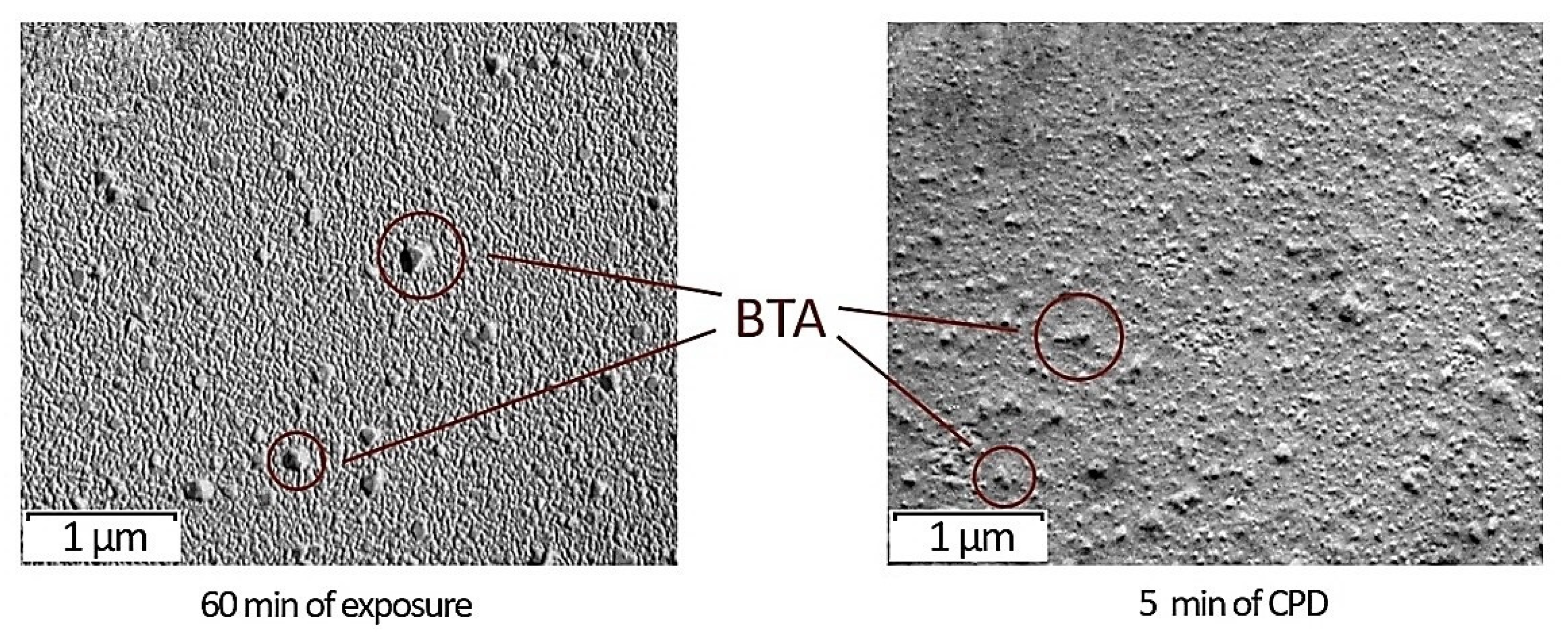
2.2.2. Permolecular Structure of Films
2.3. Phase Transitions
2.4. Energy Characteristics of the Obtained Films
2.5. Study of the Adhesive Properties of Films
2.6. Evaluation of the Barrier Properties of Films Formed on Tungsten Depending on the Method of Film Formation
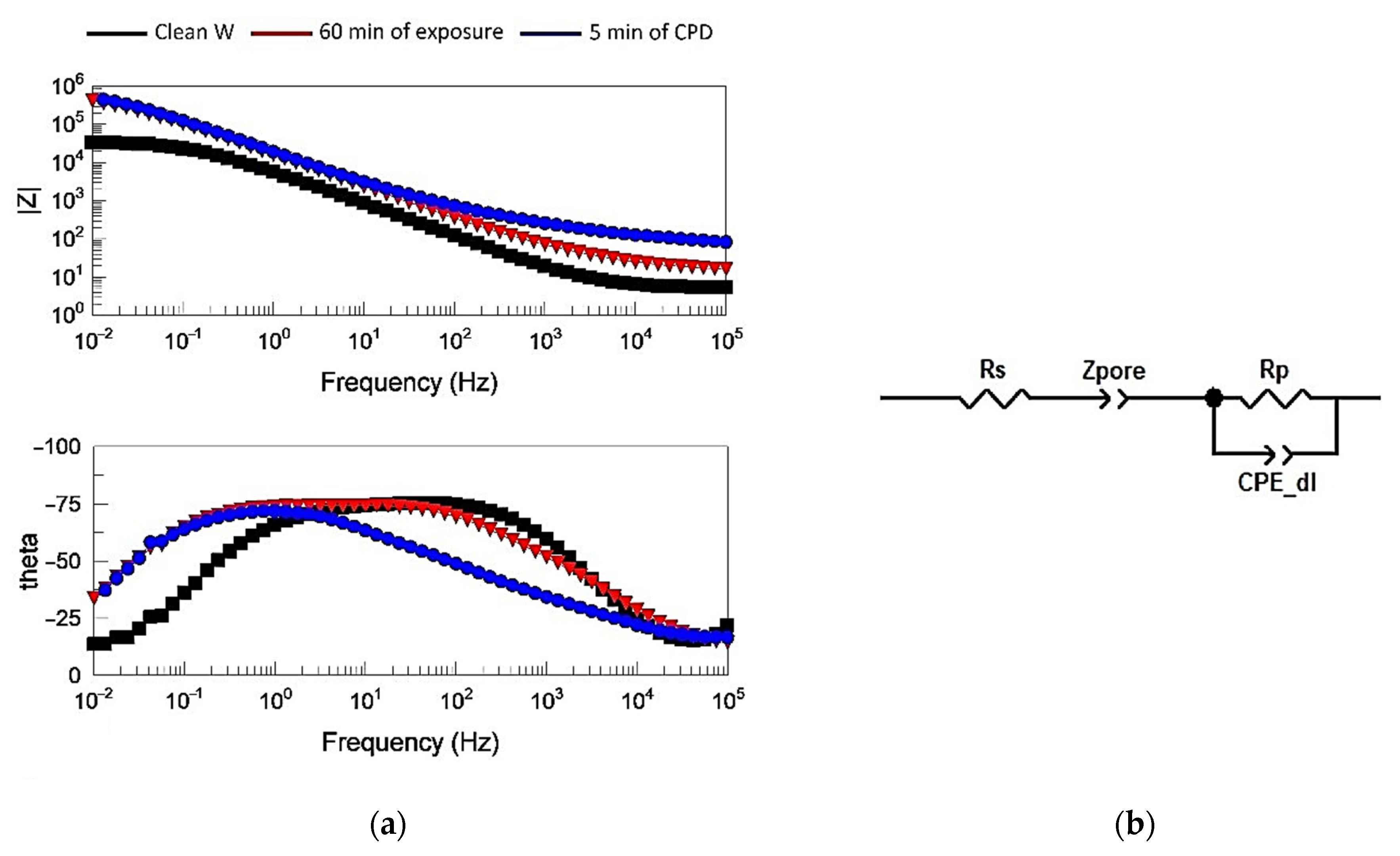
2.6.1. Electrochemical Behavior of Films
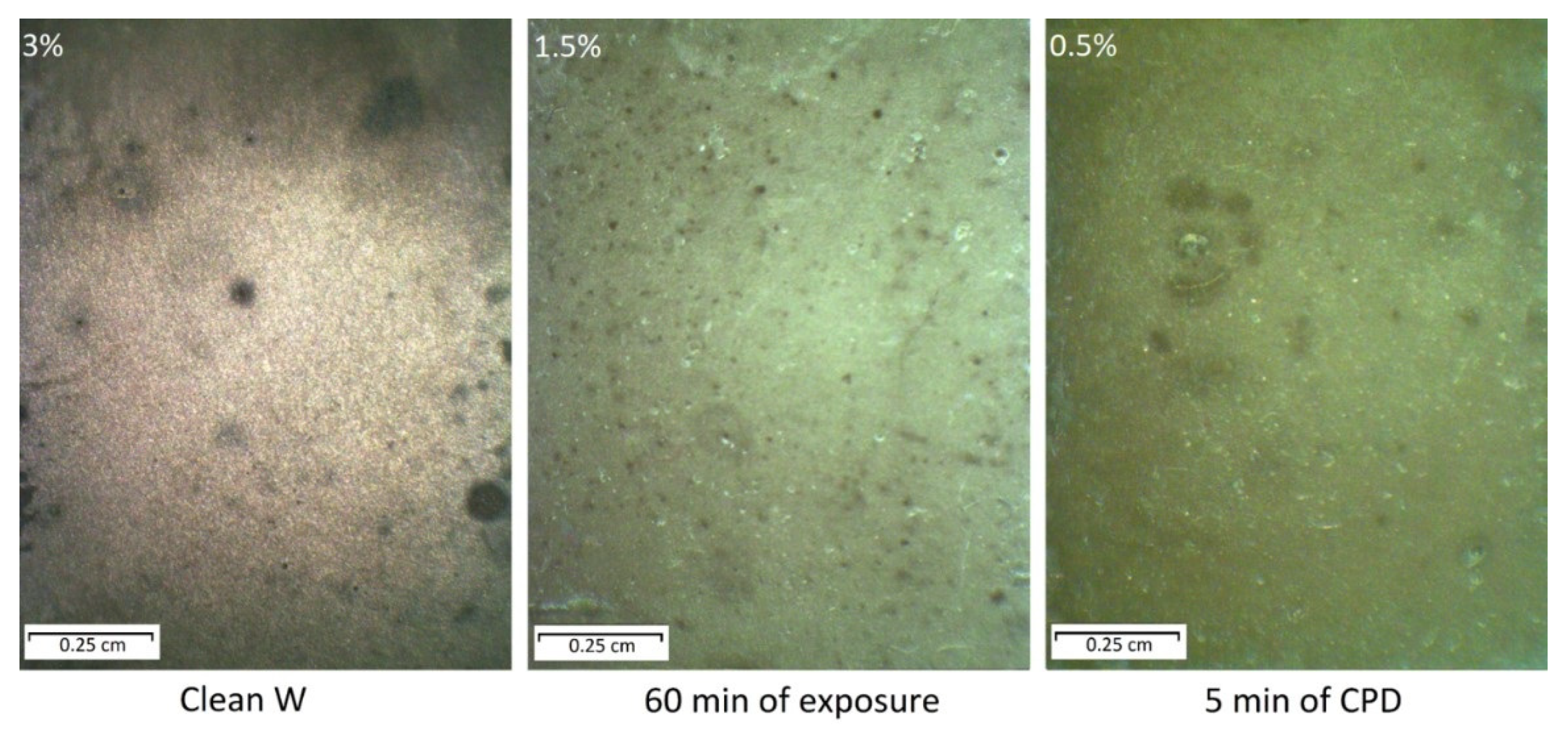
2.6.2. Corrosion Resistance of Films
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Substrate Materials
4.1.2. Composition of the INFOR Aqueous Solution
- −
- methacryloxypropyltrimetoxysilane—MS (Eksport-import, Moscow, Russia) (Figure 12). This organosilane has a long chain and also has a methacrylic group in its structure. The concentration of MS was 0.1 M.
- −
- 1,2,3-benzotriazole—BTA (Henan GP Chemicals Co., Ltd., Zhengzhou, China) and hydroxyethylidene diphosphonic acid—HEDP (Prime Chemical Group, Moscow, Russia) (Figure 12). They were used as adsorption-type corrosion inhibitors. BTA and HEDP contents were constant at 0.01 M and 0.02 M, respectively.
4.2. Preparation of the Aqueous Solution
4.3. Techniques for Forming Films on Tungsten Surfaces
4.3.1. Prolonged Exposure of the Metal Sample in the INFOR Aqueous Solution
4.3.2. Cataphoretic Deposition (CPD)
4.4. Scanning Electron Microscopy (SEM)
4.5. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
4.6. Transmission Electron Microscopy (TEM)
4.7. Differential Scanning Calorimetry (DSC)
4.8. Surface Free Energy Definition
4.9. Adhesion Tests
4.10. Protective Properties of Films
4.10.1. Electrochemical Investigations
4.10.2. Corrosion Tests
5. Conclusions
- Using scanning electron microscopy, the optimal modes of obtaining continuous and defect-free inhibited polymer films on the tungsten surface by cataphoresis deposition were determined: the duration of the CPD was 5 min, and the value of current density applied to the sample was 0.5 A/dm2.
- By means of transmission electron microscopy and differential scanning calorimetry, it was established that in the case of 60 min sample soaking in modifying solution, crystal structures of BTA came to the surface that led to the creation of a denser chemical bond grid.
- The adhesion tests showed that both in the case of 60 min exposure of a sample in the INFOR solution and for the CPD method, the adhesion strength of the film to the metal substrate was not lower than 330 N/m.
- The use of impedance spectroscopy and corrosion studies demonstrated a better efficiency of films formed on tungsten by CPD.
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anik, M.; Osseo-Asare, K. Effect of pH on the anodic behavior of tungsten. J. Electrochem. Soc. 2002, 149, B224. [Google Scholar] [CrossRef]
- Lillard, R.S.; Kanner, G.S.; Butt, D.P. The nature of oxide films on tungsten in acidic and alkaline solutions. J. Electrochem. Soc. 1998, 145, 2718–2725. [Google Scholar] [CrossRef]
- Dushik, V.V.; Lakhotkin, Y.V.; Kuzmin, V.P.; Rozhanskii, N.V. The corrosion behavior of hard W–C system chemical vapor deposition layers in HCl and H2S aqueous solutions. Prot. Met. Phys. Chem. Surf. 2016, 52, 1153–1156. [Google Scholar] [CrossRef]
- Dushik, V.V.; Lakhotkin, Y.V.; Kuzmin, V.P.; Rybkina, T.V.; Rozhanskii, N.V.; Rychkov, B.A. The corrosion and electrochemical behavior of tungsten-based CVD coatings in alkaline aqueous solutions. Prot. Met. Phys. Chem. Surf. 2018, 54, 1315–1319. [Google Scholar] [CrossRef]
- Child, T.F.; van Ooij, W.J. Application of silane technology to prevent corrosion of metals and improve paint adhesion. Trans. IMF 1999, 77, 64–70. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Adhesion through Silane Coupling Agents. J. Adhes. 1970, 2, 184–201. [Google Scholar] [CrossRef]
- Aramaki, K. Protection of iron corrosion by ultrathin two-dimensional polymer films of an alkanethiol monolayer modified with alkylethoxysilanes. Corros. Sci. 1999, 41, 1715–1730. [Google Scholar] [CrossRef]
- Aramaki, K. Prevention of iron corrosion at scratched surfaces in NaCl solutions by thin organosiloxane polymer films containing octylthiopropionate. Corros. Sci. 2000, 42, 2023–2036. [Google Scholar] [CrossRef]
- Subramanian, V.; van Ooij, W.J. Effect of the amine functional group on corrosion rate of iron coated with films of organofunctional silanes. Corrosion 1998, 54, 204–215. [Google Scholar] [CrossRef]
- Kuznetsov, Y.I. Organic corrosion inhibitors: Where are we now? A review. Part IV. Passivation and the role of mono- and diphosphonates. Int. J. Corros. Scale Inhib. 2017, 6, 384–427. [Google Scholar] [CrossRef]
- Hart, E. (Ed.) Corrosion Inhibitors: Principles, Mechanisms and Applications; Nova Science Publishers Incorporated: Hauppauge, NY, USA, 2016; p. 173. [Google Scholar]
- Gladkikh, N.; Petrunin, M.; Maksaeva, L.; Yurasova, T. Adsorption of organosilanes on the surface of aluminium and the formation of organosilane films to protect it from corrosion. Materials 2021, 14, 5757. [Google Scholar] [CrossRef] [PubMed]
- Gladkikh, N.; Makarychev, Y.; Maleeva, M.; Petrunin, M.; Maksaeva, L.; Rybkina, A.; Marshakov, A.; Kuznetsov, Y. Synthesis of thin organic layers containing silane coupling agents and azole on the surface of mild steel. Synergism of inhibitors for corrosion protection of underground pipelines. Prog. Org. Coat. 2019, 132, 481–489. [Google Scholar] [CrossRef]
- Gladkikh, N.; Makarychev, Y.; Petrunin, M.; Maleeva, M.; Maksaeva, L.; Marshakov, A. Synergistic effect of silanes and azole for enhanced corrosion protection of carbon steel by polymeric coatings. Prog. Org. Coat. 2020, 138, 105386. [Google Scholar] [CrossRef]
- Gladkikh, N.; Makarychev, Y.; Chirkunov, A.; Shapagin, A.; Petrunin, M.; Maksaeva, L.; Maleeva, M.; Yurasova, T.; Marshakov, A. Formation of polymer-like anticorrosive films based on organosilanes with benzotriazole, carboxylic and phosphonic acids. Protection of copper and steel against atmospheric corrosion. Prog. Org. Coat. 2020, 141, 105544. [Google Scholar] [CrossRef]
- Moriguchi, K.; Utagava, S. Silane: Chemistry, Applications, and Performance; Nova Science Publishers Incorporated: New York, NY, USA, 2013; p. 176. [Google Scholar]
- Plueddenmann, E.P. Silane Coupling Agents, 2nd ed.; Plenum Press: New York, NY, USA, 1991; pp. 79–152. [Google Scholar]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1534. [Google Scholar] [CrossRef] [PubMed]
- Van der Biest, O.O.; Vandeperre, L.J. Electrophoretic deposition of materials. Annu. Rev. Mater. Sci. 1999, 29, 327–352. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Dickerson, J.H. Electrophoretic deposition: Fundamentals and applications. J. Phys. Chem. B 2013, 117, 1501. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Babaei, N.; Yeganeh, H.; Gharibi, R. Anticorrosive and self-healing waterborne poly(urethane-triazole) coatings made through a combination of click polymerization and cathodic electrophoretic deposition. Eur. Polym. J. 2019, 112, 636–647. [Google Scholar] [CrossRef]
- Chng, E.; Watson, A.; Suresh, V.; Fujiwara, T.; Bumgardner, J.; Gopalakrishnan, R. Adhesion of electrosprayed chitosan coatings using silane surface chemistry. Thin Solid Films 2019, 692, 137454. [Google Scholar] [CrossRef]
- Abele, L.; Jäger, A.K.; Schulz, W.; Ruck, S.; Riegel, H.; Sörgel, T.; Albrecht, J. Superoleophobic surfaces via functionalization of electrophoretic deposited SiO2 spheres on smart aluminum substrates. Appl. Surf. Sci. 2019, 490, 56–60. [Google Scholar] [CrossRef]
- Wua, L.; Zhang, J.T.; Hua, J.; Zhang, J.Q. Improved corrosion performance of electrophoretic coatings by silane addition. Corros. Sci. 2012, 56, 58–66. [Google Scholar] [CrossRef]
- Zhu, R.; Zhang, J.; Chang, C.; Gao, S.; Ni, N. Effect of silane and zirconia on the thermal property of cathodic electrophoretic coating on AZ31 magnesium alloy. J. Magnes. Alloy. 2013, 1, 235–241. [Google Scholar] [CrossRef]
- Castro, Y.; Aparicio, M.; Moreno, R.; Duran, A. Silica-zirconia sol–gel coatings obtained by different synthesis routes. J. Sol-Gel Sci. Technol. 2005, 35, 41–50. [Google Scholar] [CrossRef]
- Castro, Y.; Ferrari, B.; Moreno, R.; Duraґn, A. Coatings produced by electrophoretic deposition from nano-particulate silica sol–gel suspensions. Surf. Coat. Technol. 2004, 182, 199–203. [Google Scholar] [CrossRef]
- Bautista, Y.; Gozalbo, A.; Mestre, S.; Sanz, V. Improvement in char strength with an open cage silsesquioxane flame retardant. Materials 2017, 10, 567. [Google Scholar] [CrossRef]
- Pajkossy, T. Impedance spectroscopy at interfaces of metals and aqueous solutions—Surface roughness, CPE and related issues. Solid State Ion. 2005, 176, 1997–2003. [Google Scholar] [CrossRef]
- Bisquert, J. Influence of the boundaries in the impedance of porous film electrodes. PCCP 2000, 2, 4185–4192. [Google Scholar] [CrossRef]
- Petrunin, M.; Maksaeva, L.; Gladkikh, N.; Makarychev, Y.; Maleeva, M.; Yurasova, T.; Nazarov, A. Thin benzotriazole films for inhibition of carbon steel corrosion in neutral electrolytes. Coatings 2020, 10, 362. [Google Scholar] [CrossRef]
- Kuznetsov, Y.I.; Kazansky, L.P. Physicochemical aspects by azoles as corrosion inhibitors. Russ. Chem. Rev. 2008, 77, 219–232. [Google Scholar] [CrossRef]
- Gladkikh, N.A.; Dushik, V.V.; Shaporenkov, A.A.; Shapagin, A.V.; Makarychev, Y.B.; Gordeev, A.V.; Marshakov, A.I. Water Suspension Containing Organosilan, Corrosion Inhibitor and Polycondensation Promoter and Method for Producing Protective Films on Surface of Tungsten and Coatings on Its Basis from Water Suspension Containing Organosilan, Corrosion Inhibitor and Polycondensation Promoter. Patent RU2744336C1, 5 March 2021. [Google Scholar]
- Shapagina, N.A.; Dushik, V.V. Application of electrophoretic deposition as an advanced technique of inhibited polymer films formation on metals from environmentally safe aqueous solutions of inhibited formulations. Materials 2022, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Lakhotkin, Y.V.; Dushik, V.V.; Kuz’min, V.P.; Rozhanskii, N.V. Nanostructured hard coatings: The key to safe operation of equipment in extreme conditions. Prot. Met. Phys. Chem. Surf. 2015, 51, 1165–1169. [Google Scholar] [CrossRef]
- Osterholtz, F.D.; Pohl, E.R. Kinetics of the hydrolysis and condensation of organofunctional alkoxysilanes—A review. J. Adhes. Sci. Technol. 1992, 6, 127–149. [Google Scholar] [CrossRef]
- Shapagin, A.V.; Budylin, N.Y.; Chalykh, A.E.; Solodilov, V.I.; Korokhin, R.A.; Poteryaev, A.A. Phase equilibrium, morphology, and physico-mechanics in epoxy–thermoplastic mixtures with upper and lower critical solution temperatures. Polymers 2020, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Shapagin, A.V.; Gladkikh, N.A.; Poteryaev, A.A.; Stepanenko, V.Y.; Nikulova, U.V.; Khasbiullin, R.R. Epoxyorganosilane finishing compositions for fibrous fillers of thermosetting and thermoplastic binders. Polymers 2021, 14, 59. [Google Scholar] [CrossRef]
- GOST 9.054–75; Unified System of Corrosion and Ageing Protection. Anticorrosive Oils, Greases and Inhibited Film-Forming Petroleum Compounds. Accelerated Test Methods of Protective Property. IPK Publishing Standards: Moscow, Russia, 2006; p. 11.
- ASTM D 610-08; Standard Test Method for Evaluating Degree of Rusting on Painted Steel Surfaces. ASTM International: West Conshohocken, PA, USA, 2019; p. 7.









| Sample | γO, mJ/m2 | γP, mJ/m2 | γD, mJ/m2 |
|---|---|---|---|
| 60 min of exposure | 40.23 | 4.70 | 37.53 |
| 5 min of CPD | 37.20 | 5.11 | 32.09 |
| Difference Δ | 5.03 | 0.41 | 5.44 |
| Materials | Rs, Ohm | Z-T_pore, sP/Ohm | Z-P_pore | Rp, Ohm | CPE-T_dl, sP/Ohm | CPE-P_dl | Z, % |
|---|---|---|---|---|---|---|---|
| Clean W | 6.9 ± 1.1 | – | – | (3.1 ± 1.1) × 104 | (4.3 ± 1.5) × 10−5 | 0.84 ± 0.02 | – |
| 60 min of exposure | 10.2 ± 2.8 | (2.1 ± 0.7) × 10−3 | 0.42 ± 0.06 | (3.6 ± 1.4) × 105 | (1.6 ± 0.2) × 10−5 | 0.92 ± 0.05 | 91.41 |
| 5 min of CPD | 12.8 ± 3.2 | (4.9 ± 1.8) × 10−5 | 0.47 ± 0.09 | (6.1 ± 2.8) × 105 | (1.4 ± 0.6) × 10−5 | 0.95 ± 0.05 | 96.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shapagina, N.A.; Shapagin, A.V.; Dushik, V.V.; Shaporenkov, A.A.; Nikulova, U.V.; Stepanenko, V.Y.; Matveev, V.V.; Klyuev, A.L.; Loginov, B.A. Methods of Formation of Protective Inhibited Polymer Films on Tungsten. Int. J. Mol. Sci. 2023, 24, 14412. https://doi.org/10.3390/ijms241914412
Shapagina NA, Shapagin AV, Dushik VV, Shaporenkov AA, Nikulova UV, Stepanenko VY, Matveev VV, Klyuev AL, Loginov BA. Methods of Formation of Protective Inhibited Polymer Films on Tungsten. International Journal of Molecular Sciences. 2023; 24(19):14412. https://doi.org/10.3390/ijms241914412
Chicago/Turabian StyleShapagina, Natalia A., Alexey V. Shapagin, Vladimir V. Dushik, Andrey A. Shaporenkov, Uliana V. Nikulova, Valentina Yu. Stepanenko, Vladimir V. Matveev, Alexey L. Klyuev, and Boris A. Loginov. 2023. "Methods of Formation of Protective Inhibited Polymer Films on Tungsten" International Journal of Molecular Sciences 24, no. 19: 14412. https://doi.org/10.3390/ijms241914412
APA StyleShapagina, N. A., Shapagin, A. V., Dushik, V. V., Shaporenkov, A. A., Nikulova, U. V., Stepanenko, V. Y., Matveev, V. V., Klyuev, A. L., & Loginov, B. A. (2023). Methods of Formation of Protective Inhibited Polymer Films on Tungsten. International Journal of Molecular Sciences, 24(19), 14412. https://doi.org/10.3390/ijms241914412









