Effects of Benzo[a]pyrene on Human Sperm Functions: An In Vitro Study
Abstract
1. Introduction
2. Results
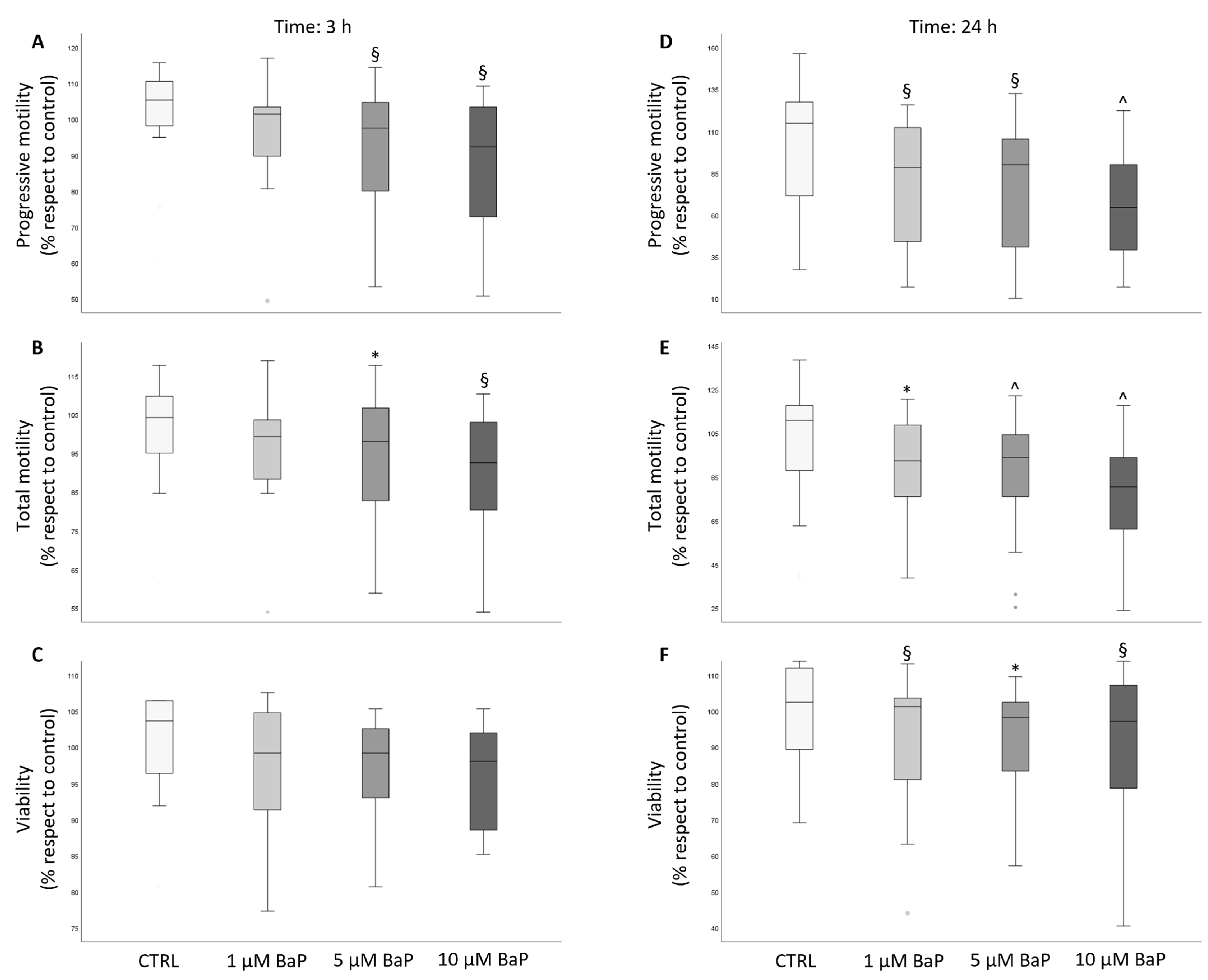
2.1. BaP Effect on Sperm Motility and Viability
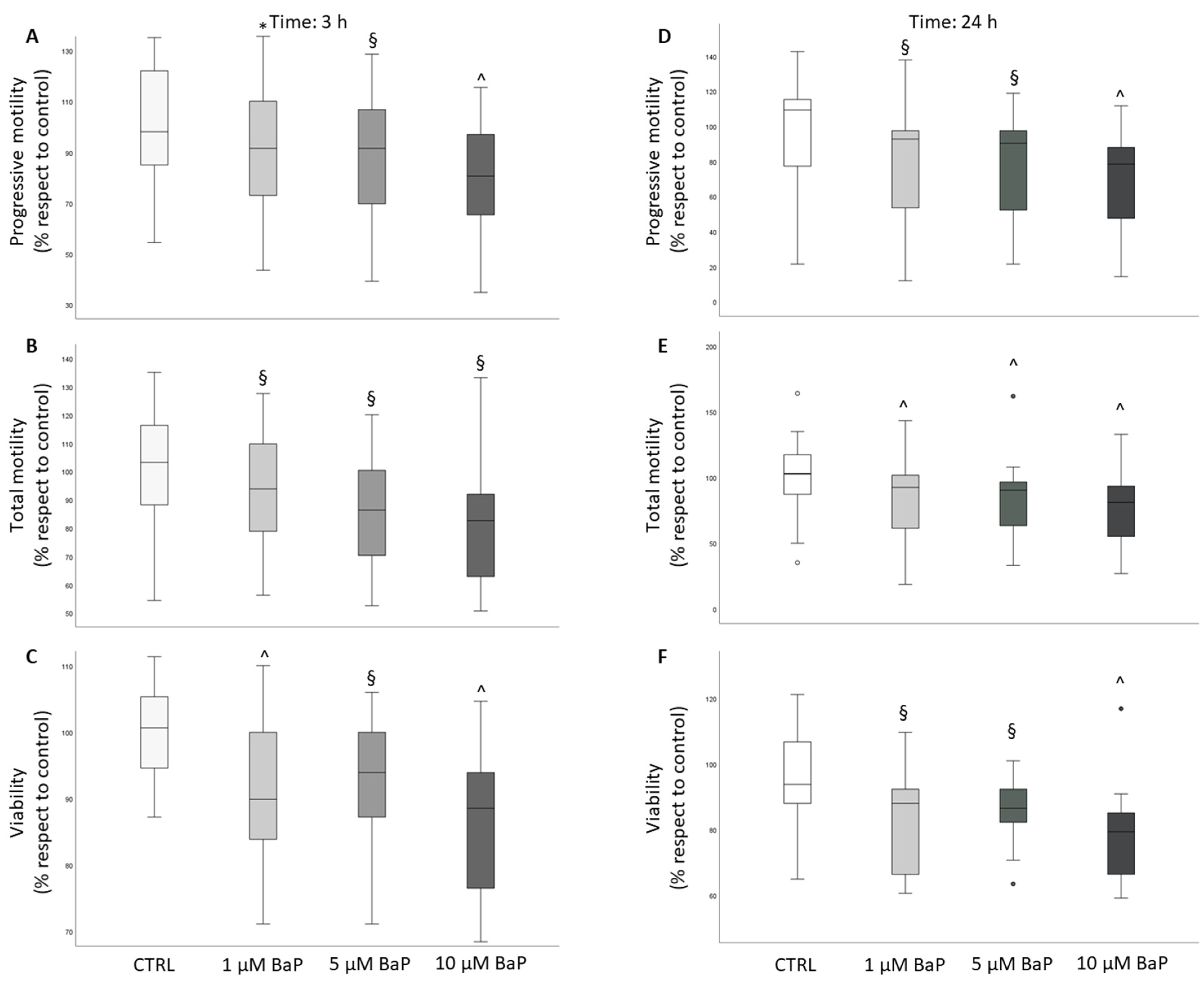
2.2. BaP effect on Sperm Ability to Penetrate a Viscous Medium
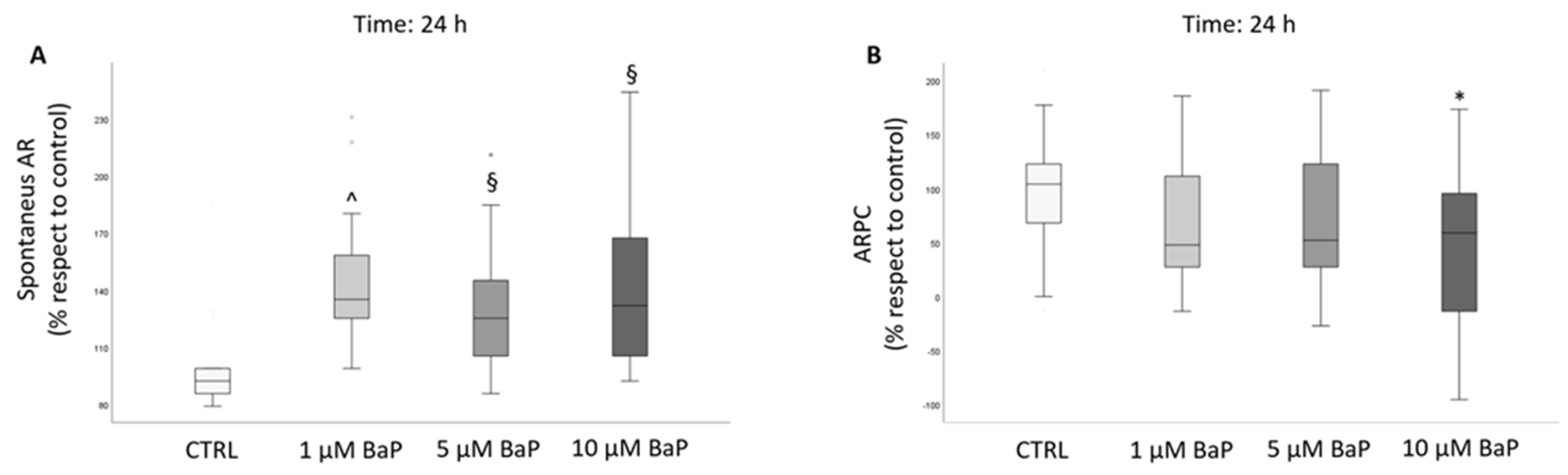
2.3. BaP Effect on Acrosome Reaction
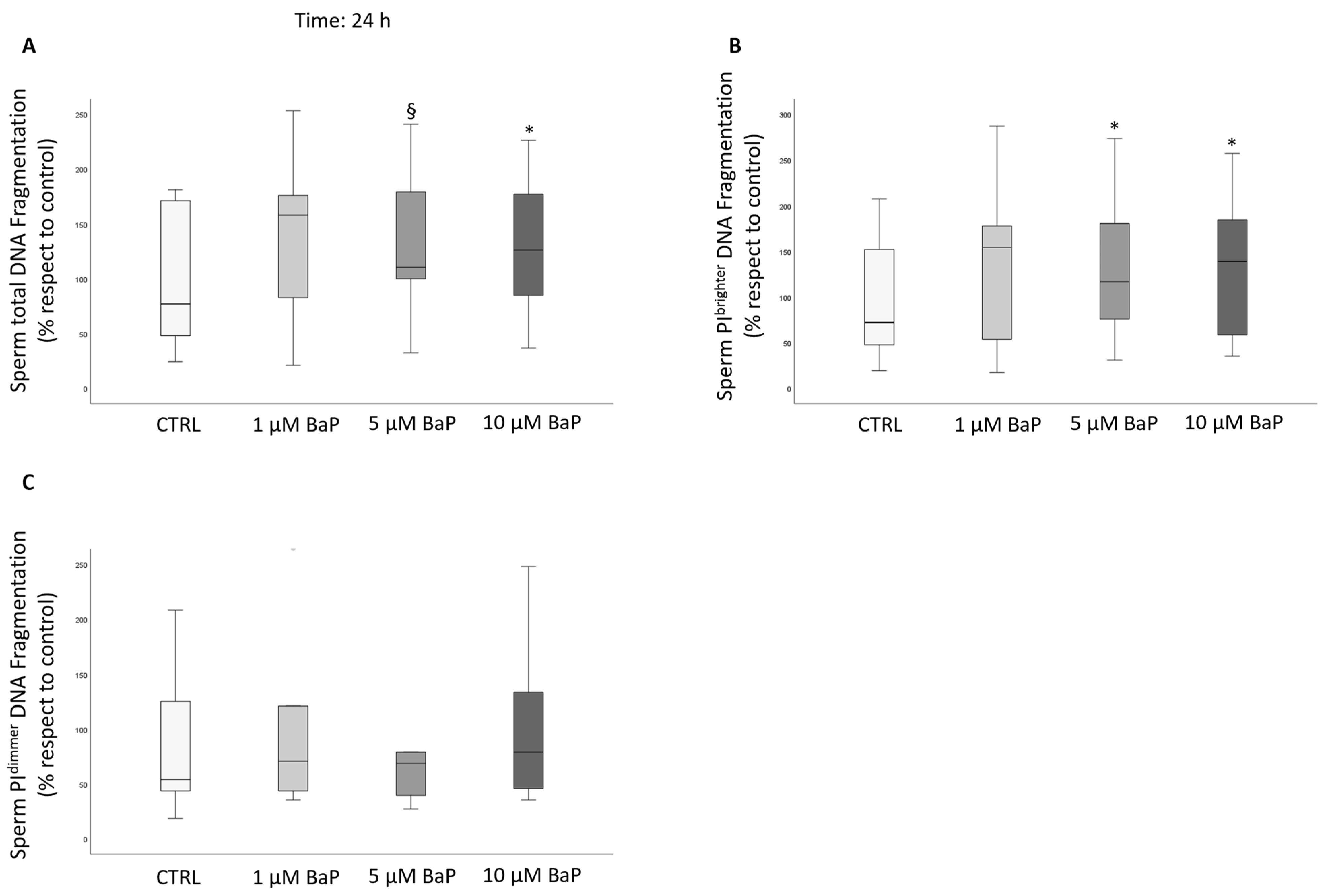
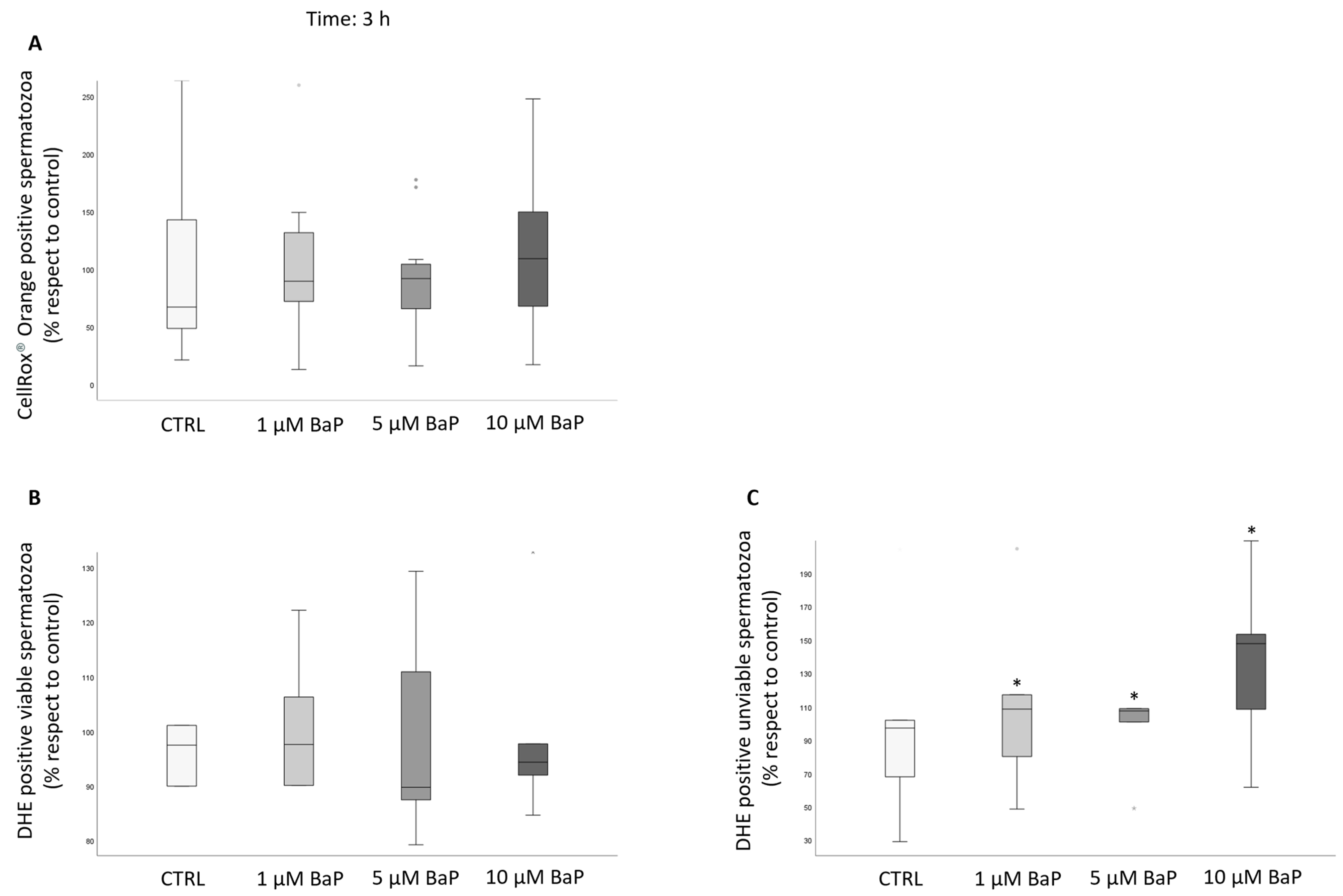
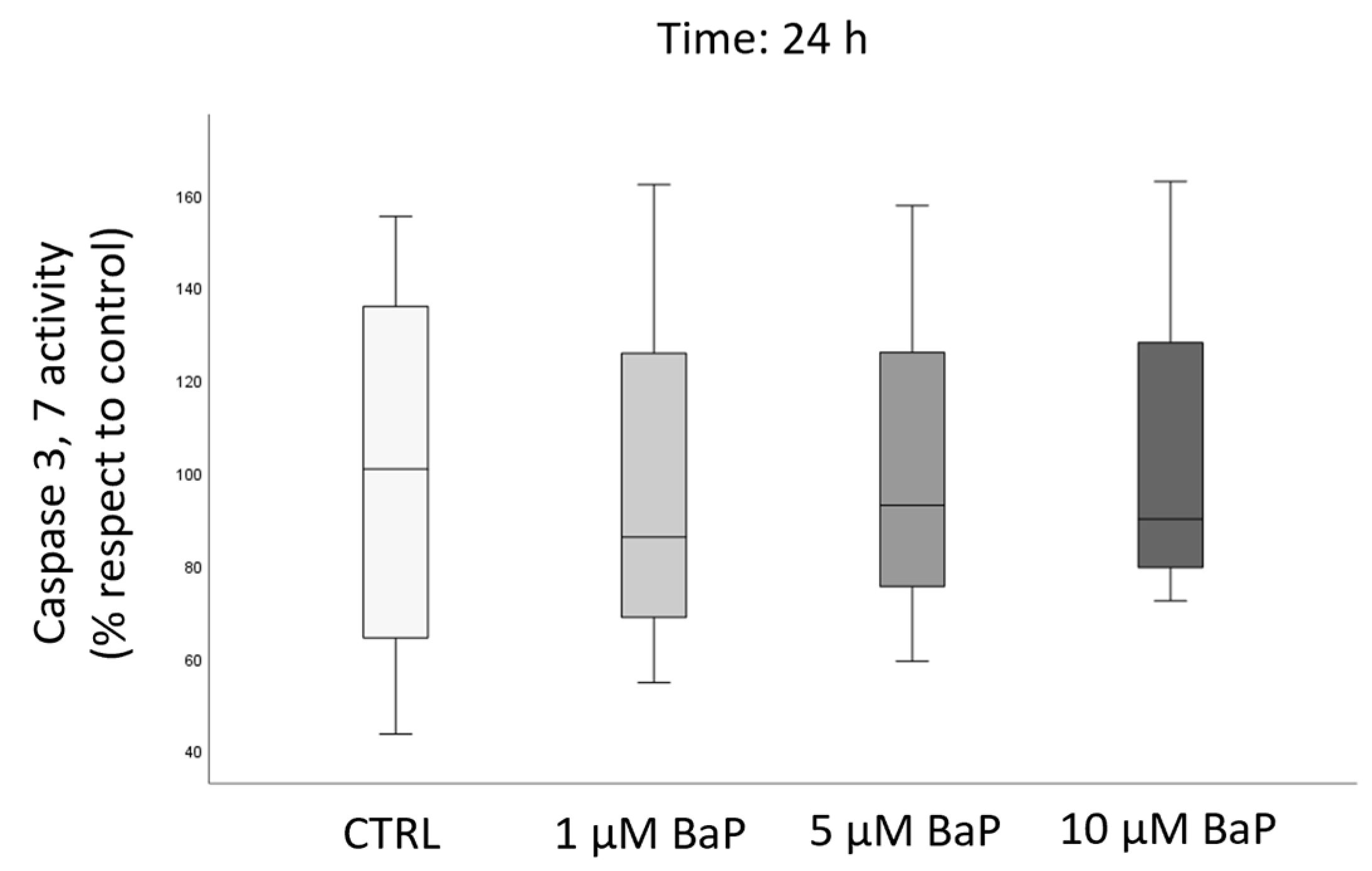
2.4. BaP Effect on DNA Fragmentation, Sperm Oxidative Status, and Apoptosis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Human Semen Samples
4.3. Assessment of Sperm Motility and Viability
4.4. Assessment of Caspase-3 and -7 Activity
4.5. Assessment of Sperm DNA Fragmentation (sDF)
4.6. Assessment of Oxidative Stress
4.7. Flow Cytometry Acquisition and Analysis
4.8. Assessment of Kinetic Parameters and Hyperactivation
4.9. Assessment of Acrosome Reaction (AR)
4.10. Assessment of Sperm Penetration in Artificial Viscous Medium
4.11. Statistical Analysis
4.12. Ethical Approval
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Pizzol, D.; Ferlin, A.; Garolla, A.; Lenzi, A.; Bertoldo, A.; Foresta, C. Genetic and molecular diagnostics of male infertility in the clinical practice. Front. Biosci. Landmark Ed. 2014, 19, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. Recent trends in male reproductive health problems. Asian J. Pharm. Clin. Res. 2014, 7, 1–5. [Google Scholar]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Jolles, M.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries. Hum. Reprod. Update 2023, 29, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, J.; Dziewirska, E.; Radwan, M.; Hanke, W. Air pollution from natural and anthropic sources and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 109. [Google Scholar] [CrossRef]
- Han, X.; Huang, Q. Environmental pollutants exposure and male reproductive toxicity: The role of epigenetic modifications. Toxicology 2021, 456, 152780. [Google Scholar] [CrossRef]
- Skakkebæk, N.E.; Lindahl-Jacobsen, R.; Levine, H.; Andersson, A.M.; Jørgensen, N.; Main, K.M.; Lidegaard, Ø.; Priskorn, L.; Holmboe, S.A.; Bräuner, E.V.; et al. Environmental factors in declining human fertility. Nat. Rev. Endocrinol. 2022, 18, 139–157. [Google Scholar] [CrossRef]
- Guerreiro, C.B.B.; Horálek, J.; de Leeuw, F.; Couvidat, F. Benzo(a)pyrene in Europe: Ambient air concentrations, population exposure and health effects. Environ. Pollut. 2016, 214, 657–667. [Google Scholar] [CrossRef]
- Mishra, N.; Ayoko, G.A.; Morawska, L. Atmospheric polycyclic aromatic hydrocarbons in the urban environment: Occurrence, toxicity and source apportionment. Environ. Pollut. 2016, 208, 110–117. [Google Scholar] [CrossRef]
- Boström, C.E.; Gerde, P.; Hanberg, A.; Jernström, B.; Johansson, C.; Kyrklund, T.; Rannug, A.; Törnqvist, M.; Victorin, K.; Westerholm, R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ. Health Perspect. 2002, 110 (Suppl. S3), 451–488. [Google Scholar] [CrossRef]
- Knafla, A.; Phillipps, K.A.; Brecher, R.W.; Petrovic, S.; Richardson, M. Development of a Dermal Cancer Slope Factor for Benzo[a]Pyrene. Regul. Toxicol. Pharmacol. 2006, 45, 159–168. [Google Scholar] [CrossRef]
- Payan, J.P.; Lafontaine, M.; Simon, P.; Marquet, F.; Champmartin-Gendre, C.; Beydon, D.; Wathier, L.; Ferrari, E. 3-Hydroxybenzo(a)pyrene as a biomarker of dermal exposure to benzo(a)pyrene. Arch. Toxicol. 2009, 83, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, B.; Mokra, K.; Michałowicz, J. Benzo[a]pyrene-Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity. Int. J. Mol. Sci. 2022, 23, 6348. [Google Scholar] [CrossRef] [PubMed]
- Revel, A.; Raanani, H.; Younglai, E.; Xu, J.; Han, R.; Savouret, J.F.; Casper, R.F. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects sperm from DNA damage and apoptosis caused by benzo(a)pyrene. Reprod. Toxicol. 2001, 15, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Inyang, F.; Ramesh, A.; Kopsombut, P.; Niaz, M.S.; Hood, D.B.; Nyanda, A.M.; Archibong, A.E. Disruption of testicular steroidogenesis and epididymal function by inhaled benzo(a)pyrene. Reprod. Toxicol. 2003, 17, 527–537. [Google Scholar] [CrossRef]
- Beal, M.A.; Meier, M.J.; Williams, A.; Rowan-Carroll, A.; Gagné, R.; Lindsay, S.J.; Fitzgerald, T.; Hurles, M.E.; Marchetti, F.; Yauk, C.L. Paternal exposure to benzo(a)pyrene induces genome-wide mutations in mouse offspring. Commun. Biol. 2019, 2, 228. [Google Scholar] [CrossRef]
- Jorge, B.C.; Reis, A.C.C.; Sterde, É.T.; Balin, P.D.S.; Scarano, W.R.; Hisano, H.; Arena, A.C. Exposure to benzo(a)pyrene from juvenile period to peripubertal impairs male reproductive parameters in adult rats. Chemosphere 2021, 263, 128016. [Google Scholar] [CrossRef]
- Guarnieri, G.; Becatti, M.; Comeglio, P.; Vignozzi, L.; Maggi, M.; Vannelli, G.B.; Morelli, A. Benzo[a]pyrene impairs the migratory pattern of human gonadotropin-releasing-hormone-secreting neuroblasts. Eur. J. Histochem. 2021, 65, 3282. [Google Scholar] [CrossRef]
- Guarnieri, G.; Becatti, M.; Squecco, R.; Comeglio, P.; Garella, R.; Tamburrino, L.; Marchiani, S.; Vignozzi, L.; Vannelli, G.B.; Maggi, M.; et al. Effects of benzo[a]pyrene on the reproductive axis: Impairment of kisspeptin signaling in human gonadotropin-releasing hormone primary neurons. Environ. Pollut. 2023, 317, 120766. [Google Scholar] [CrossRef]
- Hsu, P.C.; Chen, I.Y.; Pan, C.H.; Wu, K.Y.; Pan, M.H.; Chen, J.R.; Chen, C.J.; Chang-Chien, G.P.; Hsu, C.H.; Liu, C.S.; et al. Sperm DNA damage correlates with polycyclic aromatic hydrocarbons biomarker in coke-oven workers. Int. Arch. Occup. Environ. Health 2006, 79, 349–356. [Google Scholar] [CrossRef]
- Oliveri Conti, G.; Calogero, A.E.; Giacone, F.; Fore, M.; Barchitta, M.; Agodi, A.; Ferrante, M. B(a)P adduct levels and fertility: A cross-sectional study in a Sicilian population. Mol. Med. Rep. 2017, 15, 3398–3404. [Google Scholar] [CrossRef]
- Jeng, H.A.C.; Lin, W.Y.; Chao, M.R.; Lin, W.Y.; Pan, C.H. Semen quality and sperm DNA damage associa -revised-final-finalted with oxidative stress in relation to exposure to polycyclic aromatic hydrocarbons. J. Environ. Sci. Health A Tox Hazard Subst. Environ. Eng. 2018, 53, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Jeng, H.A.; Sikdar, S.; Pan, C.H.; Chao, M.R.; Chang-Chien, G.P.; Lin, W.Y. Mixture analysis on associations between semen quality and sperm DNA integrity and occupational exposure to polycyclic aromatic hydrocarbons. Arch. Environ. Occup. Health 2023, 78, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Nayak, J.; Jena, S.R.; Kumar, S.; Kar, S.; Dixit, A.; Samanta, L. Human sperm proteome reveals the effect of environmental borne seminal polyaromatic hydrocarbons exposome in etiology of idiopathic male factor infertility. Front. Cell Dev. Biol. 2023, 11, 1117155. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Troncoso, N.; Sanchez, F.; Garbarino, J.A.; Vanella, A. Propolis protects human spermatozoa from DNA damage caused by benzo[a]pyrene and exogenous reactive oxygen species. Life Sci. 2006, 78, 1401–1406. [Google Scholar] [CrossRef]
- Sipinen, V.; Laubenthal, J.; Baumgartner, A.; Cemeli, E.; Linschooten, J.O.; Godschalk, R.W.; Van Schooten, F.J.; Anderson, D.; Brunborg, G. In vitro evaluation of baseline and induced DNA damage in human sperm exposed to benzo[a]pyrene or its metabolite benzo[a]pyrene-7,8-diol-9,10-epoxide, using the comet assay. Mutagenesis 2010, 25, 417–425. [Google Scholar] [CrossRef]
- Alamo, A.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Giacone, F.; Calabrese, V.; La Vignera, S.; Calogero, A.E. Environment and Male Fertility: Effects of Benzo-α-Pyrene and Resveratrol on Human Sperm Function In Vitro. J. Clin. Med. 2019, 8, 561. [Google Scholar] [CrossRef]
- Cotena, M.; Auffan, M.; Tassistro, V.; Resseguier, N.; Rose, J.; Perrin, J. In Vitro Co-Exposure to CeO2 Nanomaterials from Diesel Engine Exhaust and Benzo(a)Pyrene Induces Additive DNA Damage in Sperm and Cumulus Cells but Not in Oocytes. Nanomaterials 2021, 11, 478. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Nandi, P.; Varghese, A.C.; Gutgutia, R.; Banerjee, S.; Bhattacharyya, A.K. The in vitro effect of benzo[a]pyrene on human sperm hyperactivation and acrosome reaction. Fertil. Steril. 2010, 94, 595–598. [Google Scholar] [CrossRef]
- Melikian, A.A.; Sun, P.; Prokopczyk, B.; El-Bayoumy, K.; Hoffmann, D.; Wang, X.; Waggoner, S. Identification of benzo[a]pyrene metabolites in cervical mucus and DNA adducts in cervical tissues in humans by gas chromatography-mass spectrometry. Cancer Lett. 1999, 146, 127–134. [Google Scholar] [CrossRef]
- Neal, M.S.; Zhu, J.; Foster, W.G. Quantification of benzo[a]pyrene and other PAHs in the serum and follicular fluid of smokers versus non-smokers. Reprod. Toxicol. 2008, 25, 100–106. [Google Scholar] [CrossRef]
- Rekhadevi, P.V.; Diggs, D.L.; Huderson, A.C.; Harris, K.L.; Archibong, A.E.; Ramesh, A. Metabolism of the environmental toxicant benzo(a)pyrene by subcellular fractions of human ovary. Hum. Exp. Toxicol. 2014, 33, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Muratori, M.; Tamburrino, L.; Marchiani, S.; Cambi, M.; Olivito, B.; Azzari, C.; Forti, G.; Baldi, E. Investigation on the Origin of Sperm DNA Fragmentation: Role of Apoptosis, Immaturity and Oxidative Stress. Mol. Med. 2015, 21, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Traini, G.; Tamburrino, L.; Vignozzi, L.; Baldi, E.; Marchiani, S. Is oxidative stress evaluated in viable human spermatozoa a marker of good semen quality? Front. Endocrinol. Lausanne 2022, 13, 1012416. [Google Scholar] [CrossRef] [PubMed]
- Ivic, A.; Onyeaka, H.; Girling, A.; Brewis, I.A.; Ola, B.; Hammadieh, N.; Papaioannou, S.; Barratt, C.L. Critical evaluation of methylcellulose as an alternative medium in sperm migration tests. Hum. Reprod. 2002, 17, 143–149. [Google Scholar] [CrossRef]
- Yang, P.; Wang, Y.X.; Sun, L.; Chen, Y.J.; Liu, C.; Huang, L.L.; Lu, W.Q.; Zeng, Q. Urinary metabolites of polycyclic aromatic hydrocarbons, sperm DNA damage and spermatozoa apoptosis. J. Hazard Mater. 2017, 329, 241–248. [Google Scholar] [CrossRef]
- Xu, Y.J.; Gao, H.L.; Liu, H.; Zhao, N.W.; Cheng, Q.; Zhang, F.R.; Ye, J.; Wang, A.Q.; Dou, Y.J.; Ma, B.; et al. Urinary levels of dimethoate, bisphenol A and benzo[a]pyrene in first-year students of Hohai University from different geographical regions. BMC Public Health. 2021, 21, 1692. [Google Scholar] [CrossRef]
- Zenzes, M.T.; Puy, L.A.; Bielecki, R. Immunodetection of benzo[a]pyrene adducts in ovarian cells of women exposed to cigarette smoke. Mol. Hum. Reprod. 1998, 4, 159–165. [Google Scholar] [CrossRef][Green Version]
- Neal, M.S.; Zhu, J.; Holloway, A.C.; Foster, W.G. Follicle growth is inhibited by benzo-[a]-pyrene, at concentrations representative of human exposure, in an isolated rat follicle culture assay. Hum. Reprod. 2007, 22, 961–967. [Google Scholar] [CrossRef][Green Version]
- Mattison, D.R. The effects of smoking on fertility from gametogenesis to implantation. Environ. Res. 1982, 28, 410–433. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gibb, Z.; Mitchell, L.A.; Lambourne, S.R.; Connaughton, H.S.; De Iuliis, G.N. Sperm motility is lost in vitro as a consequence of mitochondrial free radical production and the generation of electrophilic aldehydes but can be significantly rescued by the presence of nucleophilic thiols. Biol. Reprod. 2012, 87, 110. [Google Scholar] [CrossRef]
- Aitken, R.J.; Whiting, S.; De Iuliis, G.N.; McClymont, S.; Mitchell, L.A.; Baker, M.A. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J. Biol. Chem. 2012, 287, 33048–33060. [Google Scholar] [CrossRef] [PubMed]
- Marchiani, S.; Tamburrino, L.; Farnetani, G.; Muratori, M.; Vignozzi, L.; Baldi, E. Acute effects on human sperm exposed in vitro to cadmium chloride and diisobutyl phthalate. Reproduction 2019, 158, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Sadeu, J.C.; Foster, W.G. Effect of in vitro exposure to benzo[a]pyrene, a component of cigarette smoke, on folliculogenesis, steroidogenesis and oocyte nuclear maturation. Reprod. Toxicol. 2011, 31, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Mahé, C.; Zlotkowska, A.M.; Reynaud, K.; Tsikis, G.; Mermillod, P.; Druart, X.; Schoen, J.; Saint-Dizier, M. Sperm migration, selection, survival, and fertilizing ability in the mammalian oviduct†. Biol. Reprod. 2021, 105, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Fujiwara, E.; Kakiuchi, Y.; Okabe, M.; Satouh, Y.; Baba, S.A.; Chiba, K.; Hirohashi, N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl. Acad. Sci. USA 2011, 108, 4892–4896. [Google Scholar] [CrossRef]
- Tamburrino, L.; Marchiani, S.; Muratori, M.; Luconi, M.; Baldi, E. Progesterone, spermatozoa and reproduction: An updated review. Mol. Cell Endocrinol. 2020, 516, 110952. [Google Scholar] [CrossRef]
- Marinaro, J.A.; Schlegel, P.N. Sperm DNA Damage and Its Relevance in Fertility Treatment: A Review of Recent Literature and Current Practice Guidelines. Int. J. Mol. Sci. 2023, 24, 1446. [Google Scholar] [CrossRef]
- Muratori, M.; Marchiani, S.; Tamburrino, L.; Tocci, V.; Failli, P.; Forti, G.; Baldi, E. Nuclear staining identifies two populations of human sperm with different DNA fragmentation extent and relationship with semen parameters. Hum. Reprod. 2008, 23, 1035–1043. [Google Scholar] [CrossRef]
- Escada-Rebelo, S.; Mora, F.G.; Sousa, A.P.; Almeida-Santos, T.; Paiva, A.; Ramalho-Santos, J. Fluorescent probes for the detection of reactive oxygen species in human spermatozoa. Asian J. Androl. 2020, 22, 465–471. [Google Scholar] [CrossRef]
- de Lamirande, E.; Gagnon, C. Impact of reactive oxygen species on spermatozoa: A balancing act between beneficial and detrimental effects. Hum. Reprod. 1995, 10 (Suppl. S1), 15–21. [Google Scholar] [CrossRef]
- Ichikawa, T.; Oeda, T.; Ohmori, H.; Schill, W.B. Reactive oxygen species influence the acrosome reaction but not acrosin activity in human spermatozoa. Int. J. Androl. 1999, 22, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Rorke, E.A.; Sizemore, N.; Mukhtar, H.; Couch, L.H.; Howard, P.C. Polycyclic aromatic hydrocarbons enhance terminal cell death of human ectocervical cells. Int. J. Oncol. 1998, 13, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radical. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Baker, M.A.; Nixon, B. Are sperm capacitation and apoptosis the opposite ends of a continuum driven by oxidative stress? Asian J. Androl. 2015, 17, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B.; Chakraborty, S.; Ghosh, D.; Raha, S.; Sen, P.C.; Jana, K. Benzo(a)pyrene Induced p53 Mediated Male Germ Cell Apoptosis: Synergistic Protective Effects of Curcumin and Resveratrol. Front. Pharmacol. 2016, 7, 245. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Marchiani, S.; Tamburrino, L.; Olivito, B.; Betti, L.; Azzari, C.; Forti, G.; Baldi, E.; Muratori, M. Characterization and sorting of flow cytometric populations in human semen. Andrology 2014, 2, 394–401. [Google Scholar] [CrossRef]
- Mortimer, S.T.; Swan, M.A.; Mortimer, D. Effect of seminal plasma on capacitation and hyperactivation in human spermatozoa. Hum. Reprod. 1998, 13, 2139–2146. [Google Scholar] [CrossRef]
- Tamburrino, L.; Marchiani, S.; Minetti, F.; Forti, G.; Muratori, M.; Baldi, E. The CatSper calcium channel in human sperm: Relation with motility and involvement in progesterone-induced acrosome reaction. Hum. Reprod. 2014, 29, 418–428. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traini, G.; Tamburrino, L.; Ragosta, M.E.; Guarnieri, G.; Morelli, A.; Vignozzi, L.; Baldi, E.; Marchiani, S. Effects of Benzo[a]pyrene on Human Sperm Functions: An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 14411. https://doi.org/10.3390/ijms241914411
Traini G, Tamburrino L, Ragosta ME, Guarnieri G, Morelli A, Vignozzi L, Baldi E, Marchiani S. Effects of Benzo[a]pyrene on Human Sperm Functions: An In Vitro Study. International Journal of Molecular Sciences. 2023; 24(19):14411. https://doi.org/10.3390/ijms241914411
Chicago/Turabian StyleTraini, Giulia, Lara Tamburrino, Maria Emanuela Ragosta, Giulia Guarnieri, Annamaria Morelli, Linda Vignozzi, Elisabetta Baldi, and Sara Marchiani. 2023. "Effects of Benzo[a]pyrene on Human Sperm Functions: An In Vitro Study" International Journal of Molecular Sciences 24, no. 19: 14411. https://doi.org/10.3390/ijms241914411
APA StyleTraini, G., Tamburrino, L., Ragosta, M. E., Guarnieri, G., Morelli, A., Vignozzi, L., Baldi, E., & Marchiani, S. (2023). Effects of Benzo[a]pyrene on Human Sperm Functions: An In Vitro Study. International Journal of Molecular Sciences, 24(19), 14411. https://doi.org/10.3390/ijms241914411









