Activation of 5-HT1A Receptors Normalizes the Overexpression of Presynaptic 5-HT1A Receptors and Alleviates Diabetic Neuropathic Pain
Abstract
1. Introduction
2. Results
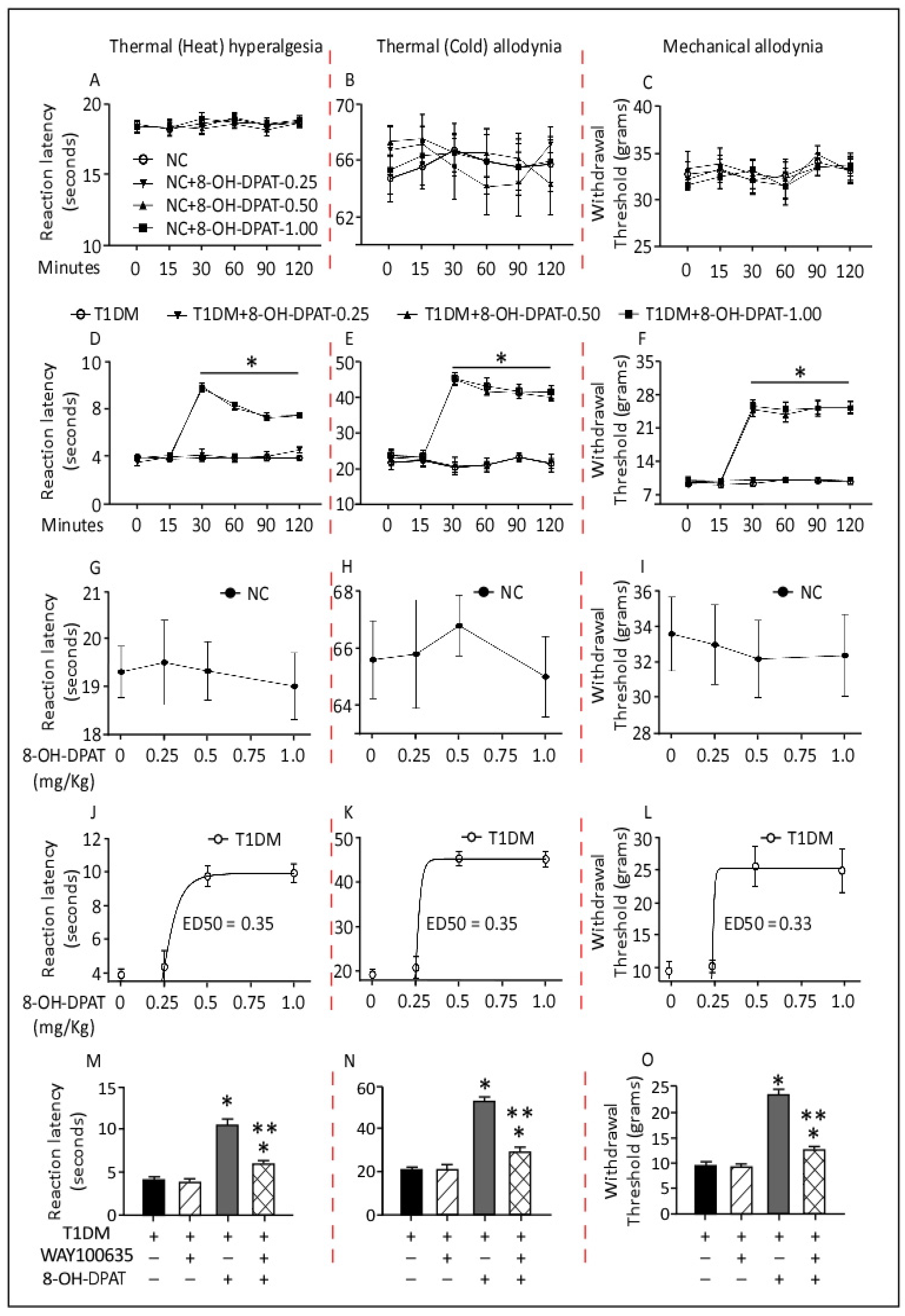
2.1. Time– and Dose–Response Curves of the Antinociceptive Effects of 8-Hydroxy-2-(dipropylamino)tetralin (8-OH-DPAT) in Control and Type 1 Diabetes Mellitus (T1DM) Rats
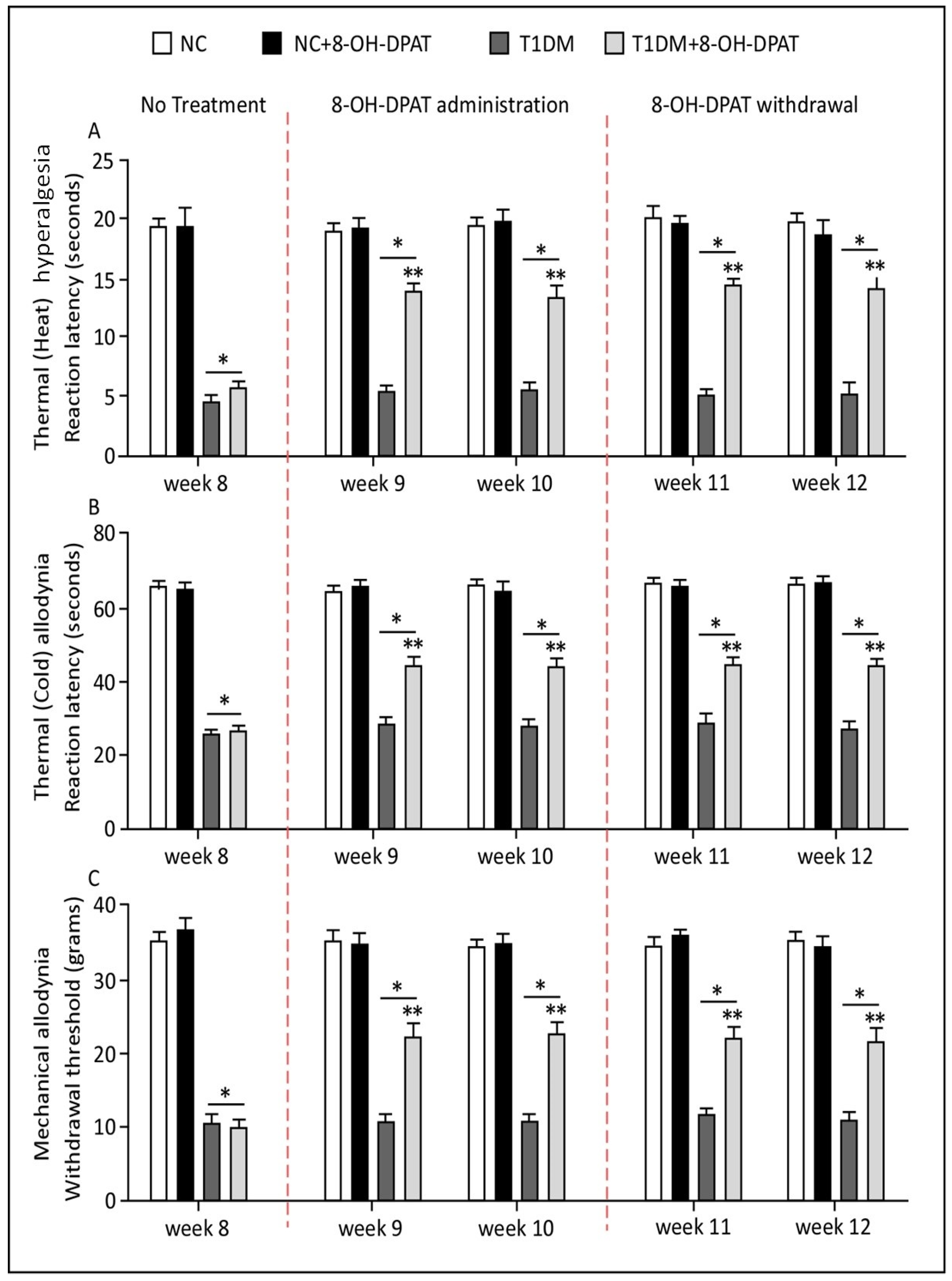
2.2. Persistent 8-OH-DPAT Antinociceptive Effects following Two Weeks of Withdrawal
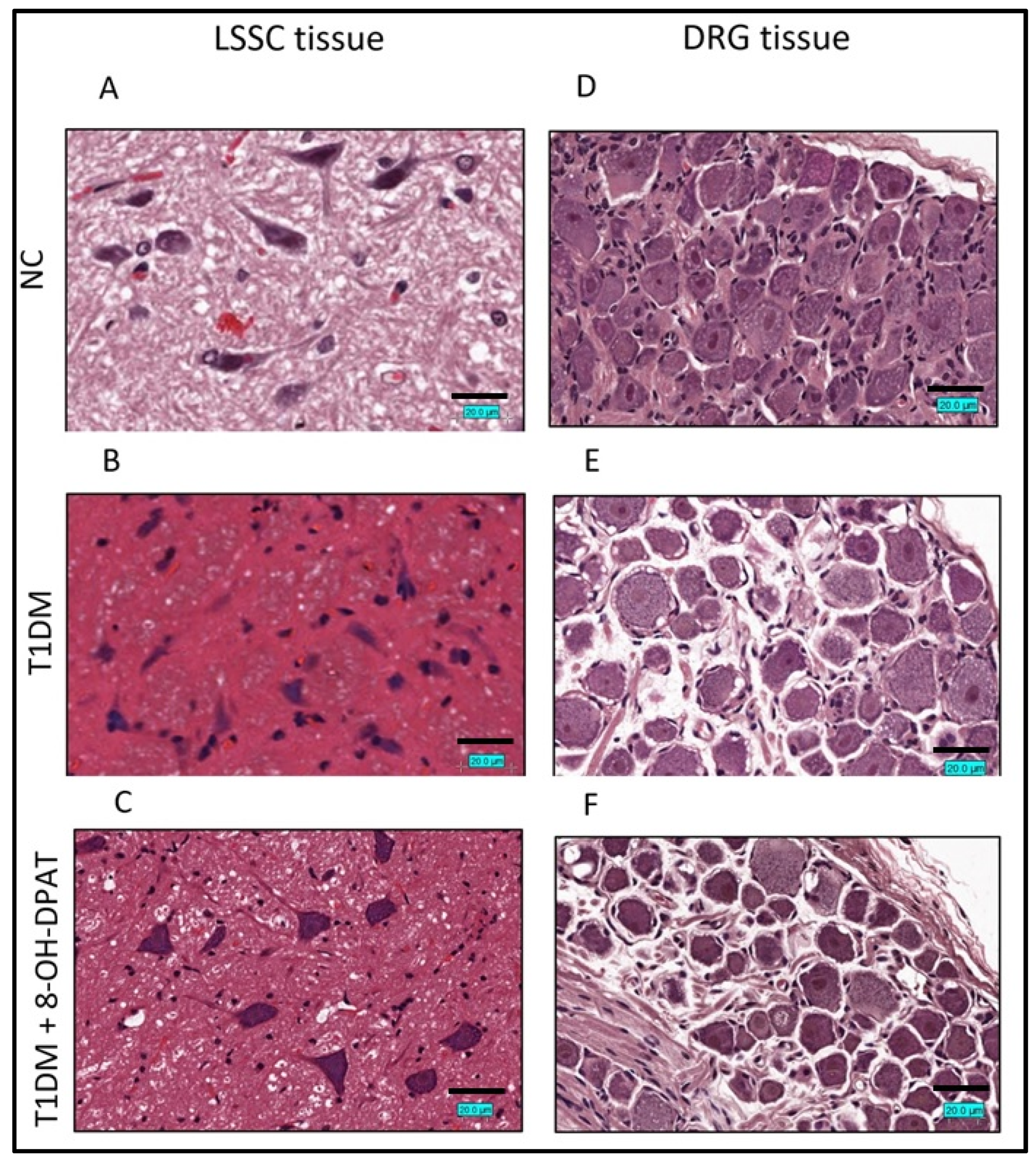
2.3. Chronically Administered 8-OH-DPAT Arrests T1DM-Induced Neuronal Degeneration
2.4. Chronically Administered 8-OH-DPAT Reverses T1DM-Induced Upregulation of 5-HT1AR in the RVM, LSSC and DRG
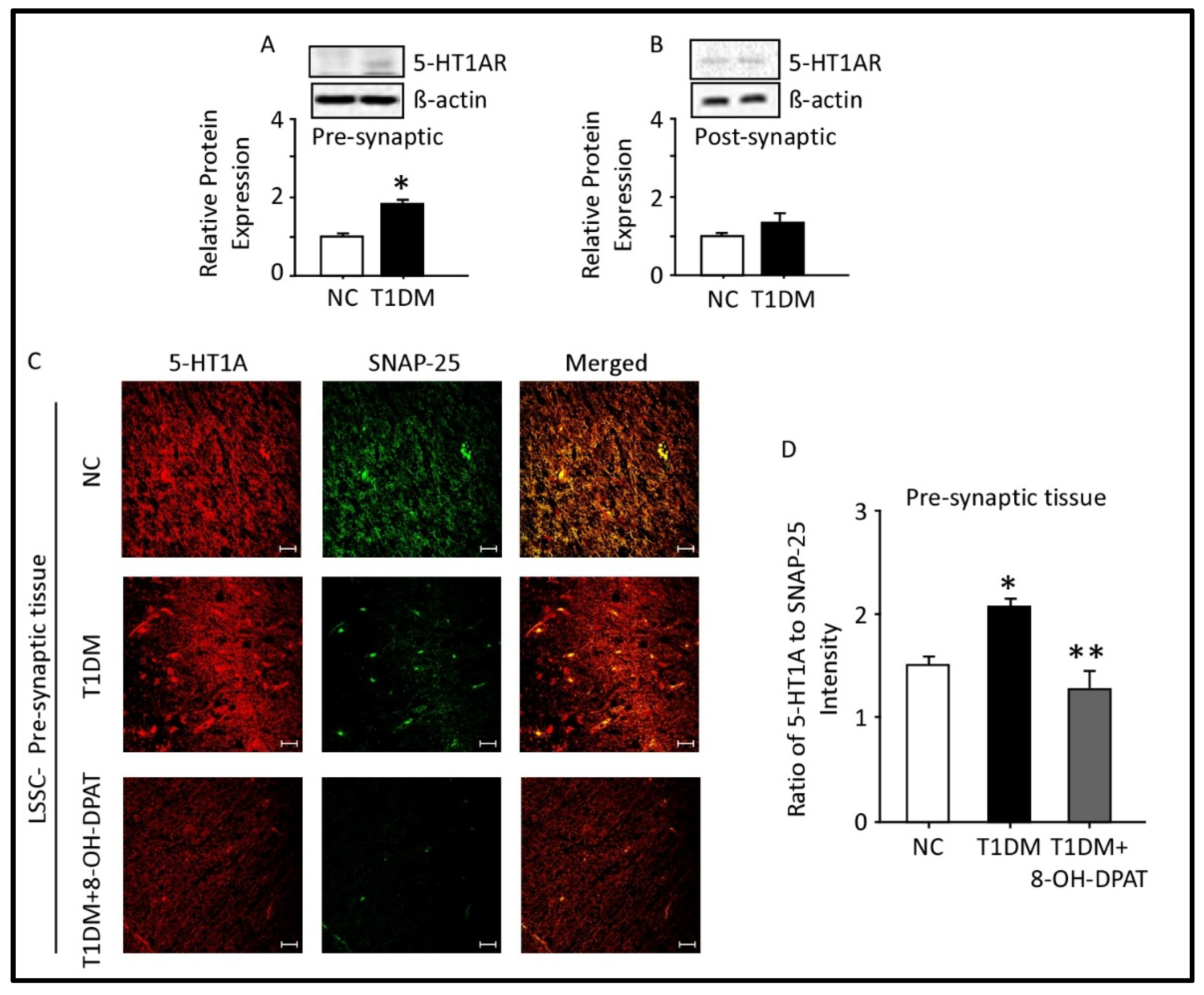
2.5. Expression of 5-HT1AR in the Presynaptic and Postsynaptic Fractions of the Spinal Cord Lumbar Region
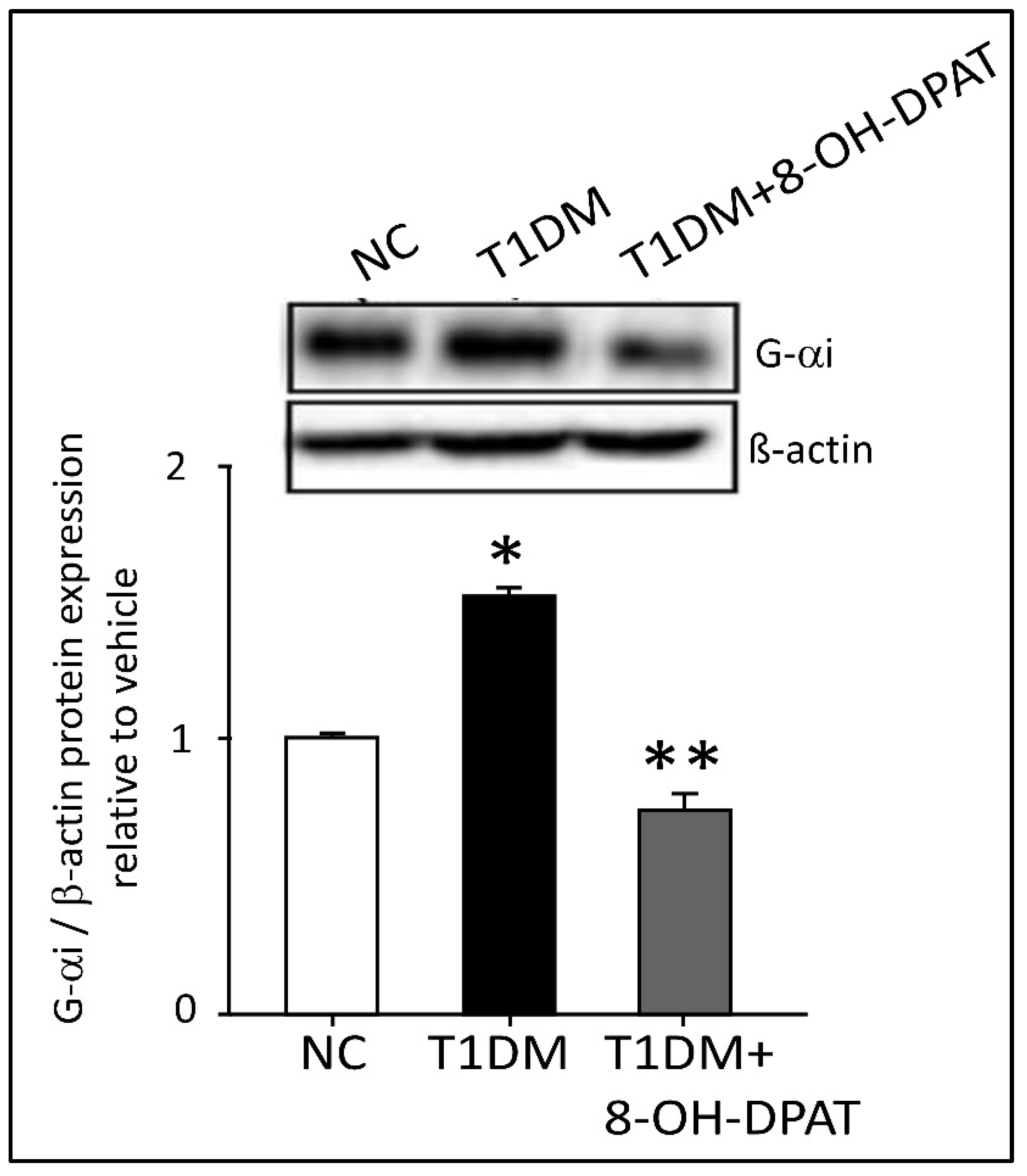
2.6. Chronically Administered 8-OH-DPAT Reversed T1DM-Induced Dysregulation of Neuronal Spinal 5-HT1AR Signaling
2.7. Chronically Administered 8-OH-DPAT Normalizes the Upregulated Kynurenine Pathway, but Not the Methoxyindole Pathway
2.8. Effects of Chronic 8-OH-DPAT Administration on the Rate of Release of Serotonin (5-Hydroxytryptamine, 5-HT) as Reflected by the 5-Hydroxyindole Acetic Acid (5-HIAA)/5-HT Ratio and on the Levels of Quinolinic Acid (QA) in the Spinal Cord Lumbar Region STZ-Induced T1DM
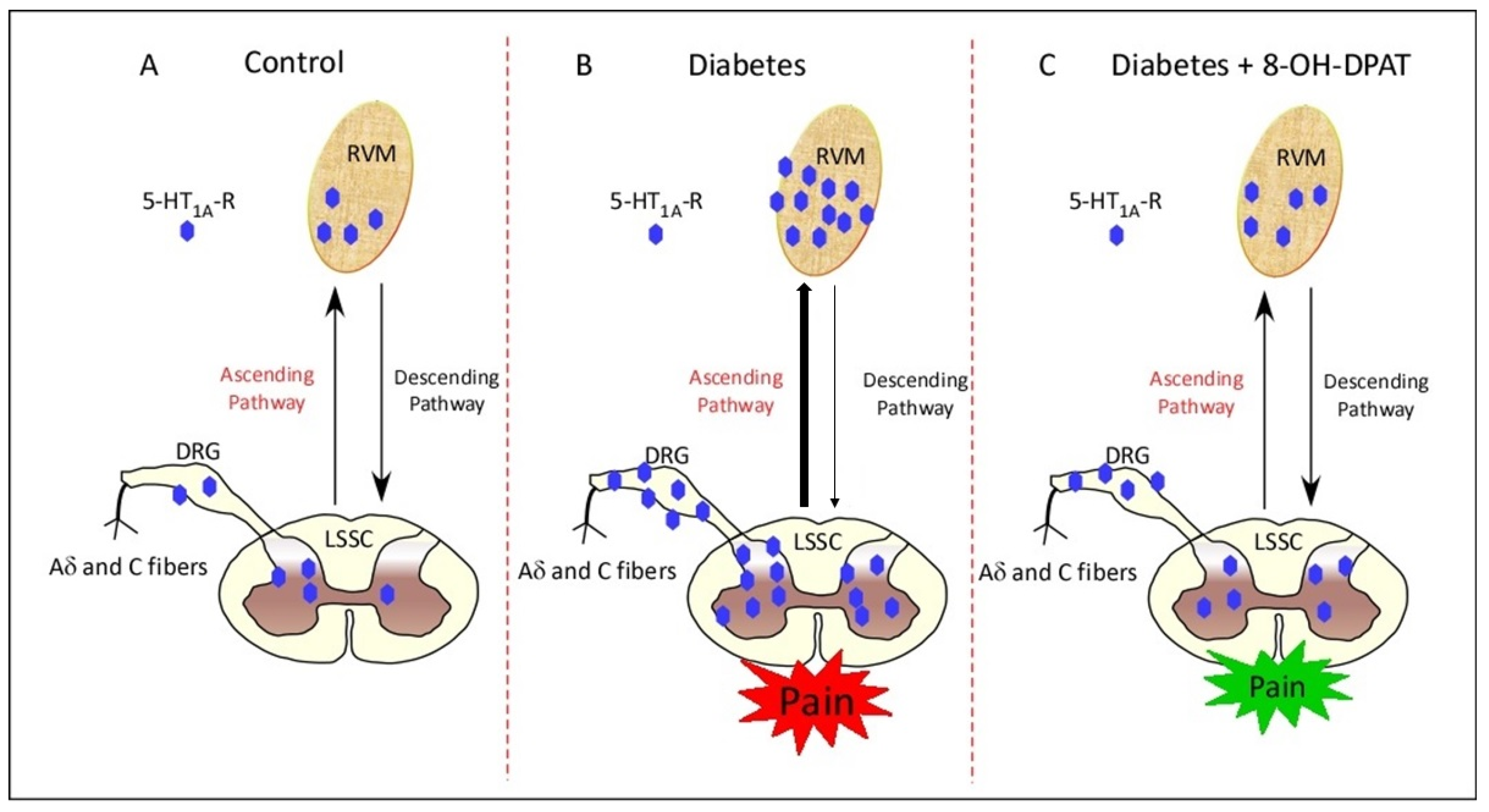
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Diabetes Induction
4.3. Drugs Administration
4.4. Nociceptive Testing
4.5. Tissue Isolation
4.6. Analysis of Gene Expression by qRT-PCR
4.7. Western Blot Analysis and Immunohistochemistry- and Immunofluorescence-Staining of Tissue Sections
4.8. Extraction of Synaptosome and Pre-Synaptic and Post-Synaptic Fractions Isolation from Spinal Lumbar Region
4.9. Levels of Spinal 5-HT and Its Metabolite, 5-HIAA, and QA Acid Using High-Performance Liquid Chromatography (HPLC) with Photodiode Array Detection (PDA)
4.10. Statistical Analyses
5. Conclusions
6. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hicks, C.W.; Selvin, E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Curr. Diabetes Rep. 2019, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef]
- Javed, S.; Alam, U.; Malik, R.A. Treating Diabetic Neuropathy: Present Strategies and Emerging Solutions. Rev. Diabet. Stud. 2015, 12, 63–83. [Google Scholar] [CrossRef] [PubMed]
- Jordan, L.M.; Kenshalo, D.R., Jr.; Martin, F.R.; Haber, L.H.; Willis, W.D. Depression of primate spinothalamic tract neurons by iontophoretic application of 5-hydroxytryptamine. Pain 1978, 5, 135–142. [Google Scholar] [CrossRef]
- Bardin, L. The complex role of serotonin and 5-HT receptors in chronic pain. Behav. Pharmacol. 2011, 22, 390–404. [Google Scholar] [CrossRef]
- Martin, S.L.; Power, A.; Boyle, Y.; Anderson, I.M.; Silverdale, M.A.; Jones, A.K.P. 5-HT modulation of pain perception in humans. Psychopharmacology 2017, 234, 2929–2939. [Google Scholar] [CrossRef]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Dogrul, A.; Ossipov, M.H.; Porreca, F. Differential mediation of descending pain facilitation and inhibition by spinal 5HT-3 and 5HT-7 receptors. Brain Res. 2009, 1280, 52–59. [Google Scholar] [CrossRef]
- Green, M.G.; Scarth, J.; Dickenson, A. An excitatory role for 5-HT in spinal inflammatory nociceptive transmission; state-dependent actions via dorsal horn 5-HT(3) receptors in the anaesthetized rat. Pain 2000, 89, 81–88. [Google Scholar] [CrossRef]
- Rahman, W.; Suzuki, R.; Rygh, L.J.; Dickenson, A.H. Descending serotonergic facilitation mediated through rat spinal 5HT3 receptors is unaltered following carrageenan inflammation. Neurosci. Lett. 2004, 361, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Haleem, D.J. Targeting Serotonin1A Receptors for Treating Chronic Pain and Depression. Curr. Neuropharmacol. 2019, 17, 1098–1108. [Google Scholar] [CrossRef]
- Jacobs, B.L.; Azmitia, E.C. Structure and function of the brain serotonin system. Physiol. Rev. 1992, 72, 165–229. [Google Scholar] [CrossRef]
- Doherty, M.D.; Pickel, V.M. Targeting of serotonin 1A receptors to dopaminergic neurons within the parabrachial subdivision of the ventral tegmental area in rat brain. J. Comp. Neurol. 2001, 433, 390–400. [Google Scholar] [CrossRef]
- Pompeiano, M.; Palacios, J.M.; Mengod, G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: Correlation with receptor binding. J. Neurosci. 1992, 12, 440–453. [Google Scholar] [CrossRef]
- Perrin, F.E.; Gerber, Y.N.; Teigell, M.; Lonjon, N.; Boniface, G.; Bauchet, L.; Rodriguez, J.J.; Hugnot, J.P.; Privat, A.M. Anatomical study of serotonergic innervation and 5-HT(1A) receptor in the human spinal cord. Cell Death Dis. 2011, 2, e218. [Google Scholar] [CrossRef] [PubMed]
- Otoshi, C.K.; Walwyn, W.M.; Tillakaratne, N.J.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Distribution and localization of 5-HT(1A) receptors in the rat lumbar spinal cord after transection and deafferentation. J. Neurotrauma 2009, 26, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Braz, J.M.; Basbaum, A.I. Genetically expressed transneuronal tracer reveals direct and indirect serotonergic descending control circuits. J. Comp. Neurol. 2008, 507, 1990–2003. [Google Scholar] [CrossRef]
- Seyrek, M.; Kahraman, S.; Deveci, M.S.; Yesilyurt, O.; Dogrul, A. Systemic cannabinoids produce CB(1)-mediated antinociception by activation of descending serotonergic pathways that act upon spinal 5-HT(7) and 5-HT(2A) receptors. Eur. J. Pharmacol. 2010, 649, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Gal, E.M.; Sherman, A.D. L-kynurenine: Its synthesis and possible regulatory function in brain. Neurochem. Res. 1980, 5, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Rubi, B.; Maechler, P. Minireview: New roles for peripheral dopamine on metabolic control and tumor growth: Let’s seek the balance. Endocrinology 2010, 151, 5570–5581. [Google Scholar] [CrossRef]
- Malek, Z.S.; Dardente, H.; Pevet, P.; Raison, S. Tissue-specific expression of tryptophan hydroxylase mRNAs in the rat midbrain: Anatomical evidence and daily profiles. Eur. J. Neurosci. 2005, 22, 895–901. [Google Scholar] [CrossRef]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Oxenkrug, G.F. Genetic and hormonal regulation of tryptophan kynurenine metabolism: Implications for vascular cognitive impairment, major depressive disorder, and aging. Ann. N. Y. Acad. Sci. 2007, 1122, 35–49. [Google Scholar] [CrossRef]
- Guidetti, P.; Amori, L.; Sapko, M.T.; Okuno, E.; Schwarcz, R. Mitochondrial aspartate aminotransferase: A third kynurenate-producing enzyme in the mammalian brain. J. Neurochem. 2007, 102, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, P.; Hoffman, G.E.; Melendez-Ferro, M.; Albuquerque, E.X.; Schwarcz, R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia 2007, 55, 78–92. [Google Scholar] [CrossRef]
- Bardin, L.; Tarayre, J.P.; Koek, W.; Colpaert, F.C. In the formalin model of tonic nociceptive pain, 8-OH-DPAT produces 5-HT1A receptor-mediated, behaviorally specific analgesia. Eur. J. Pharmacol. 2001, 421, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Munawar, N.; Nader, J.; Khadadah, N.H.; Al Madhoun, A.; Al-Ali, W.; Varghese, L.A.; Masocha, W.; Al-Mulla, F.; Bitar, M.S. Guanfacine Normalizes the Overexpression of Presynaptic alpha-2A Adrenoceptor Signaling and Ameliorates Neuropathic Pain in a Chronic Animal Model of Type 1 Diabetes. Pharmaceutics 2022, 14, 2146. [Google Scholar] [CrossRef] [PubMed]
- Pytliak, M.; Vargova, V.; Mechirova, V.; Felsoci, M. Serotonin receptors—From molecular biology to clinical applications. Physiol. Res. 2011, 60, 15–25. [Google Scholar] [CrossRef]
- Demeulemeester, H.; Feys, H.; Goris, I.; Zwaenepoel, I.; de Weerdt, W.; de Sutter, P.; Gybels, J.; Plets, C.; Nuttin, B. Effect of the serotonin agonist 8-OH-DPAT on the sensorimotor system of the rat. Pharmacol. Biochem. Behav. 2001, 70, 95–103. [Google Scholar] [CrossRef]
- Hu, B.; Doods, H.; Treede, R.D.; Ceci, A. Duloxetine and 8-OH-DPAT, but not fluoxetine, reduce depression-like behaviour in an animal model of chronic neuropathic pain. Neurosci. Lett. 2016, 619, 162–167. [Google Scholar] [CrossRef]
- Rodriguez, J.J.; Noristani, H.N.; Verkhratsky, A. The serotonergic system in ageing and Alzheimer’s disease. Prog. Neurobiol. 2012, 99, 15–41. [Google Scholar] [CrossRef]
- Schneider-Matyka, D.; Jurczak, A.; Szkup, M.; Samochowiec, A.; Grzywacz, A.; Wieder-Huszla, S.; Grochans, E. The influence of the serotonergic system on the personality and quality of life of postmenopausal women. Clin. Interv. Aging 2017, 12, 963–970. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Politis, M.; Niccolini, F. Serotonin in Parkinson’s disease. Behav. Brain Res. 2015, 277, 136–145. [Google Scholar] [CrossRef]
- Lalut, J.; Karila, D.; Dallemagne, P.; Rochais, C. Modulating 5-HT(4) and 5-HT(6) receptors in Alzheimer’s disease treatment. Future Med. Chem. 2017, 9, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Yohn, C.N.; Gergues, M.M.; Samuels, B.A. The role of 5-HT receptors in depression. Mol. Brain 2017, 10, 28. [Google Scholar] [CrossRef]
- Cai, X.; Gu, Z.; Zhong, P.; Ren, Y.; Yan, Z. Serotonin 5-HT1A receptors regulate AMPA receptor channels through inhibiting Ca2+/calmodulin-dependent kinase II in prefrontal cortical pyramidal neurons. J. Biol. Chem. 2002, 277, 36553–36562. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.M.; DeKosky, S.T. Monoamine neurons in aging and Alzheimer’s disease. J. Neural Transm. Gen. Sect. 1993, 91, 135–159. [Google Scholar] [CrossRef]
- Dillon, K.A.; Gross-Isseroff, R.; Israeli, M.; Biegon, A. Autoradiographic analysis of serotonin 5-HT1A receptor binding in the human brain postmortem: Effects of age and alcohol. Brain Res. 1991, 554, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Marcusson, J.; Oreland, L.; Winblad, B. Effect of age on human brain serotonin (S-1) binding sites. J. Neurochem. 1984, 43, 1699–1705. [Google Scholar] [CrossRef]
- Tauscher, J.; Verhoeff, N.P.; Christensen, B.K.; Hussey, D.; Meyer, J.H.; Kecojevic, A.; Javanmard, M.; Kasper, S.; Kapur, S. Serotonin 5-HT1A receptor binding potential declines with age as measured by [11C]WAY-100635 and PET. Neuropsychopharmacology 2001, 24, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Steinbusch, H.W.; Van Luijtelaar, M.G.; Dijkstra, H.; Nijssen, A.; Tonnaer, J.A. Aging and regenerative capacity of the rat serotonergic system. A morphological, neurochemical and behavioral analysis after transplantation of fetal raphe cells. Ann. N. Y. Acad. Sci. 1990, 600, 384–402; discussion 402–384. [Google Scholar] [CrossRef] [PubMed]
- Venero, J.L.; de la Roza, C.; Machado, A.; Cano, J. Age-related changes on monoamine turnover in hippocampus of rats. Brain Res. 1993, 631, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Birthelmer, A.; Lazaris, A.; Schweizer, T.; Jackisch, R.; Cassel, J.C. Presynaptic regulation of neurotransmitter release in the cortex of aged rats with differential memory impairments. Pharmacol. Biochem. Behav. 2003, 75, 147–162. [Google Scholar] [CrossRef]
- Birthelmer, A.; Stemmelin, J.; Jackisch, R.; Cassel, J.C. Presynaptic modulation of acetylcholine, noradrenaline, and serotonin release in the hippocampus of aged rats with various levels of memory impairments. Brain Res. Bull. 2003, 60, 283–296. [Google Scholar] [CrossRef]
- Gur, R.E.; Gur, R.C. Gender differences in aging: Cognition, emotions, and neuroimaging studies. Dialogues Clin. Neurosci. 2002, 4, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Pandaranandaka, J.; Poonyachoti, S.; Kalandakanond-Thongsong, S. Differential effects of exogenous and endogenous estrogen on anxiety as measured by elevated T-maze in relation to the serotonergic system. Behav. Brain Res. 2009, 198, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Thiblin, I.; Finn, A.; Ross, S.B.; Stenfors, C. Increased dopaminergic and 5-hydroxytryptaminergic activities in male rat brain following long-term treatment with anabolic androgenic steroids. Br. J. Pharmacol. 1999, 126, 1301–1306. [Google Scholar] [CrossRef]
- Harman, S.M.; Metter, E.J.; Tobin, J.D.; Pearson, J.; Blackman, M.R. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J. Clin. Endocrinol. Metab. 2001, 86, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Mohr, B.A.; Guay, A.T.; O’Donnell, A.B.; McKinlay, J.B. Normal, bound and nonbound testosterone levels in normally ageing men: Results from the Massachusetts Male Ageing Study. Clin. Endocrinol. 2005, 62, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Horstman, A.M.; Dillon, E.L.; Urban, R.J.; Sheffield-Moore, M. The role of androgens and estrogens on healthy aging and longevity. J. Gerontol. Biol. Sci. Med. Sci. 2012, 67, 1140–1152. [Google Scholar] [CrossRef]
- Gjerstad, J.; Tjolsen, A.; Hole, K. The effect of 5-HT1A receptor stimulation on nociceptive dorsal horn neurones in rats. Eur. J. Pharmacol. 1996, 318, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Mjellem-Joly, N.; Lund, A.; Berge, O.G.; Hole, K. Intrathecal co-administration of substance P and NMDA augments nociceptive responses in the formalin test. Pain 1992, 51, 195–198. [Google Scholar] [CrossRef]
- Fasmer, O.B.; Berge, O.G.; Post, C.; Hole, K. Effects of the putative 5-HT1A receptor agonist 8-OH-2-(di-n-propylamino)tetralin on nociceptive sensitivity in mice. Pharmacol. Biochem. Behav. 1986, 25, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Haleem, D.J.; Nawaz, S. Inhibition of Reinforcing, Hyperalgesic, and Motor Effects of Morphine by Buspirone in Rats. J. Pain. 2017, 18, 19–28. [Google Scholar] [CrossRef]
- Barnes, N.M.; Sharp, T. A review of central 5-HT receptors and their function. Neuropharmacology 1999, 38, 1083–1152. [Google Scholar] [CrossRef]
- Uys, M.M.; Shahid, M.; Harvey, B.H. Therapeutic Potential of Selectively Targeting the alpha(2C)-Adrenoceptor in Cognition, Depression, and Schizophrenia-New Developments and Future Perspective. Front. Psychiatry 2017, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Albert, P.R.; Vahid-Ansari, F. The 5-HT1A receptor: Signaling to behavior. Biochimie 2019, 161, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Zochodne, D.W. Sensory neurons with activated caspase-3 survive long-term experimental diabetes. Diabetes 2003, 52, 2363–2371. [Google Scholar] [CrossRef]
- Kishi, M.; Tanabe, J.; Schmelzer, J.D.; Low, P.A. Morphometry of dorsal root ganglion in chronic experimental diabetic neuropathy. Diabetes 2002, 51, 819–824. [Google Scholar] [CrossRef][Green Version]
- Kobayashi, M.; Chandrasekhar, A.; Cheng, C.; Martinez, J.A.; Ng, H.; de la Hoz, C.; Zochodne, D.W. Diabetic polyneuropathy, sensory neurons, nuclear structure and spliceosome alterations: A role for CWC22. Dis. Model. Mech. 2017, 10, 215–224. [Google Scholar] [CrossRef]
- Bellush, L.L.; Reid, S.G.; North, D. The functional significance of biochemical alterations in streptozotocin-induced diabetes. Physiol. Behav. 1991, 50, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Bitar, M.; Koulu, M.; Rapoport, S.I.; Linnoila, M. Diabetes-induced alteration in brain monoamine metabolism in rats. J. Pharmacol. Exp. Ther. 1986, 236, 432–437. [Google Scholar] [PubMed]
- Miyata, S.; Hirano, S.; Kamei, J. Diabetes attenuates the antidepressant-like effect mediated by the activation of 5-HT1A receptor in the mouse tail suspension test. Neuropsychopharmacology 2004, 29, 461–469. [Google Scholar] [CrossRef]
- Heisler, J.M.; O’Connor, J.C. Indoleamine 2,3-dioxygenase-dependent neurotoxic kynurenine metabolism mediates inflammation-induced deficit in recognition memory. Brain Behav. Immun. 2015, 50, 115–124. [Google Scholar] [CrossRef]
- Owe-Young, R.; Webster, N.L.; Mukhtar, M.; Pomerantz, R.J.; Smythe, G.; Walker, D.; Armati, P.J.; Crowe, S.M.; Brew, B.J. Kynurenine pathway metabolism in human blood-brain-barrier cells: Implications for immune tolerance and neurotoxicity. J. Neurochem. 2008, 105, 1346–1357. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Rohrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.C.; Elmer, G.I.; Bergeron, R.; Albuquerque, E.X.; Guidetti, P.; Wu, H.Q.; Schwarcz, R. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology 2010, 35, 1734–1742. [Google Scholar] [CrossRef]
- Ferreira, F.S.; Schmitz, F.; Marques, E.P.; Siebert, C.; Wyse, A.T.S. Intrastriatal Quinolinic Acid Administration Impairs Redox Homeostasis and Induces Inflammatory Changes: Prevention by Kynurenic Acid. Neurotox. Res. 2020, 38, 50–58. [Google Scholar] [CrossRef]
- Heyes, M.P.; Saito, K.; Crowley, J.S.; Davis, L.E.; Demitrack, M.A.; Der, M.; Dilling, L.A.; Elia, J.; Kruesi, M.J.; Lackner, A.; et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain 1992, 115 Pt 5, 1249–1273. [Google Scholar] [CrossRef]
- Adayev, T.; Ranasinghe, B.; Banerjee, P. Transmembrane signaling in the brain by serotonin, a key regulator of physiology and emotion. Biosci. Rep. 2005, 25, 363–385. [Google Scholar] [CrossRef]
- Hjorth, S.; Bengtsson, H.J.; Kullberg, A.; Carlzon, D.; Peilot, H.; Auerbach, S.B. Serotonin autoreceptor function and antidepressant drug action. J. Psychopharmacol. 2000, 14, 177–185. [Google Scholar] [CrossRef]
- Polter, A.M.; Li, X. 5-HT1A receptor-regulated signal transduction pathways in brain. Cell Signal 2010, 22, 1406–1412. [Google Scholar] [CrossRef]
- Riad, M.; Zimmer, L.; Rbah, L.; Watkins, K.C.; Hamon, M.; Descarries, L. Acute treatment with the antidepressant fluoxetine internalizes 5-HT1A autoreceptors and reduces the in vivo binding of the PET radioligand [18F]MPPF in the nucleus raphe dorsalis of rat. J. Neurosci. 2004, 24, 5420–5426. [Google Scholar] [CrossRef]
- Bitar, M.S.; Bajic, K.T.; Farook, T.; Thomas, M.I.; Pilcher, C.W. Spinal cord noradrenergic dynamics in diabetic and hypercortisolaemic states. Brain Res. 1999, 830, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Prow, M.R.; Martin, K.F.; Heal, D.J. 8-OH-DPAT-induced mydriasis in mice: A pharmacological characterisation. Eur. J. Pharmacol. 1996, 317, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Thangamani, D.; Edafiogho, I.O.; Masocha, W. The anticonvulsant enaminone E139 attenuates paclitaxel-induced neuropathic pain in rodents. Sci. World J. 2013, 2013, 240508. [Google Scholar] [CrossRef]
- Hatzis, P.; Al-Madhoon, A.S.; Jullig, M.; Petrakis, T.G.; Eriksson, S.; Talianidis, I. The intracellular localization of deoxycytidine kinase. J. Biol. Chem. 1998, 273, 30239–30243. [Google Scholar] [CrossRef] [PubMed]
- Masocha, W. Systemic lipopolysaccharide (LPS)-induced microglial activation results in different temporal reduction of CD200 and CD200 receptor gene expression in the brain. J. Neuroimmunol. 2009, 214, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, S.M.; Al Madhoun, A.; Nizam, R.; Melhem, M.; Cherian, P.; Al-Khairi, I.; Haddad, D.; Abu-Farha, M.; Abubaker, J.; Bitar, M.S.; et al. Increased Plasma Levels of Adenylate Cyclase 8 and cAMP Are Associated with Obesity and Type 2 Diabetes: Results from a Cross-Sectional Study. Biology 2020, 9, 244. [Google Scholar] [CrossRef]
- Al-Roub, A.; Al Madhoun, A.; Akhter, N.; Thomas, R.; Miranda, L.; Jacob, T.; Al-Ozairi, E.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. IL-1beta and TNFalpha Cooperativity in Regulating IL-6 Expression in Adipocytes Depends on CREB Binding and H3K14 Acetylation. Cells 2021, 10, 3228. [Google Scholar] [CrossRef]
- Gil-Recio, C.; Montori, S.; Al Demour, S.; Ababneh, M.A.; Ferres-Padro, E.; Marti, C.; Ferres-Amat, E.; Barajas, M.; Al Madhoun, A.; Atari, M. Chemically Defined Conditions Mediate an Efficient Induction of Dental Pulp Pluripotent-Like Stem Cells into Hepatocyte-Like Cells. Stem Cells Int. 2021, 2021, 5212852. [Google Scholar] [CrossRef]
- Al Madhoun, A.; Haddad, D.; Al Tarrah, M.; Jacob, S.; Al-Ali, W.; Nizam, R.; Miranda, L.; Al-Rashed, F.; Sindhu, S.; Ahmad, R.; et al. Microarray analysis reveals ONC201 mediated differential mechanisms of CHOP gene regulation in metastatic and nonmetastatic colorectal cancer cells. Sci. Rep. 2021, 11, 11893. [Google Scholar] [CrossRef]
- Akhter, N.; Wilson, A.; Thomas, R.; Al-Rashed, F.; Kochumon, S.; Al-Roub, A.; Arefanian, H.; Al-Madhoun, A.; Al-Mulla, F.; Ahmad, R.; et al. ROS/TNF-alpha Crosstalk Triggers the Expression of IL-8 and MCP-1 in Human Monocytic THP-1 Cells via the NF-kappaB and ERK1/2 Mediated Signaling. Int. J. Mol. Sci. 2021, 22, 519. [Google Scholar] [CrossRef] [PubMed]
- Kochumon, S.; Al Madhoun, A.; Al-Rashed, F.; Thomas, R.; Sindhu, S.; Al-Ozairi, E.; Al-Mulla, F.; Ahmad, R. Elevated adipose tissue associated IL-2 expression in obesity correlates with metabolic inflammation and insulin resistance. Sci. Rep. 2020, 10, 16364. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.P.; Morato, X.; Souza, C.; Pinhal, C.; Machado, N.J.; Canas, P.M.; Silva, H.B.; Stagljar, I.; Gandia, J.; Fernandez-Duenas, V.; et al. The role of parkinson’s disease-associated receptor GPR37 in the hippocampus: Functional interplay with the adenosinergic system. J. Neurochem. 2015, 134, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.J.; Jeon, J.H.; Oh, M.S.; Hong, S.P. Measuring levels of biogenic amines and their metabolites in rat brain tissue using high-performance liquid chromatography with photodiode array detection. Arch. Pharm. Res. 2016, 39, 59–65. [Google Scholar] [CrossRef]
- Al Madhoun, A.S.; Mehta, V.; Li, G.; Figeys, D.; Wiper-Bergeron, N.; Skerjanc, I.S. Skeletal myosin light chain kinase regulates skeletal myogenesis by phosphorylation of MEF2C. EMBO J. 2011, 30, 2477–2489. [Google Scholar] [CrossRef]


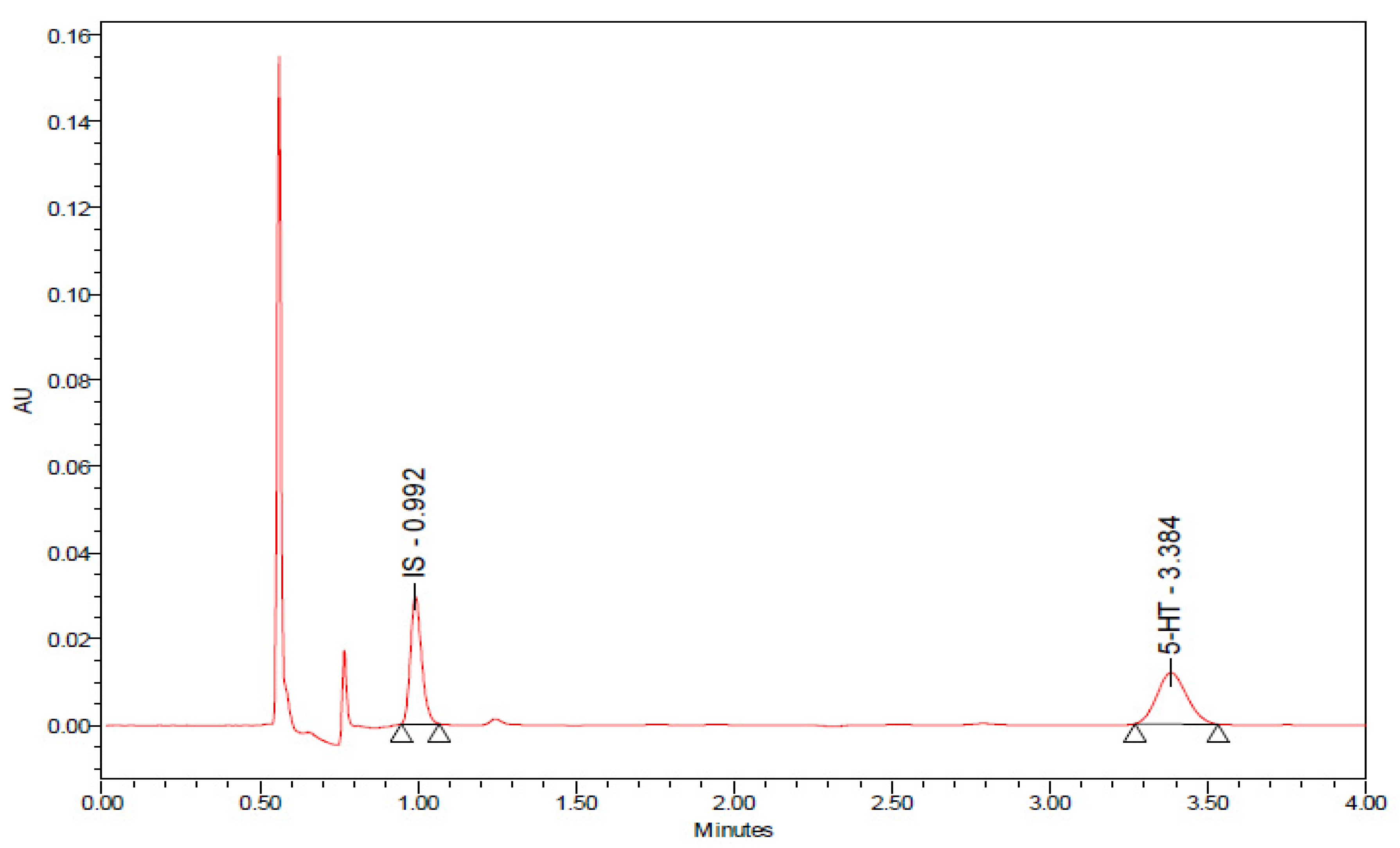
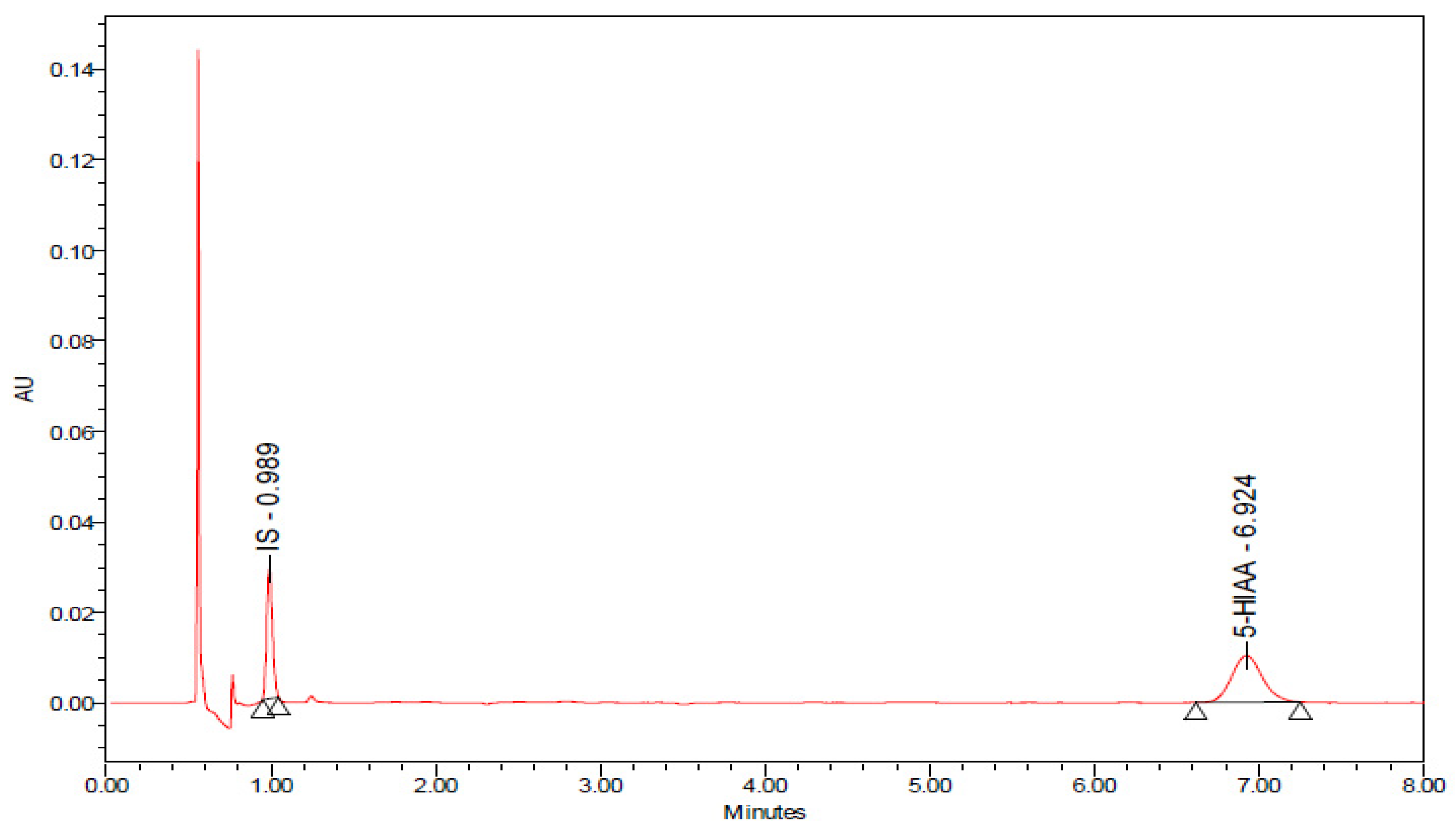
| Treatment | 5-HIAA (ng/mL) | 5-HT (ng/mL) | 5-HIAA/5-HT (ng/mL) |
|---|---|---|---|
| NC | 12.0 ± 0.302 | 14.1 ± 0.364 | 0.854 ± 0.03 |
| T1DM | 10.6 ± 0.484 * | 18.3 ± 0.510 * | 0.578 ± 0.03 * |
| T1DM + 8-OH-DPAT | 11.5 ± 0.06 | 16.7 ± 0.843 | 0.689 ± 0.03 ** |
| Treatment | QA (ng/mL) |
|---|---|
| NC | 147.4 ± 7.52 |
| T1DM | 373.9 ± 30.2 * |
| T1DM + 8-OH-DPAT | 88.5 ± 7.85 ** |
| T1DM | Effects of Chronic 8-OH-DPAT (0.5 mg/kg, i.p.) Administration |
|---|---|
| ↓ Pain threshold | ↑ Pain threshold |
| Neuronal degeneration (LSSC and DRG) | Improved neuronal degeneration (LSSC and DRG) |
| ↓ 5-HT1AR mRNA and protein expression (RVM, LSSC and DRG) | ↑ 5-HT1AR mRNA and protein expression (RVM, LSSC and DRG) |
| ↑ Presynaptic 5-HT1AR | ↓ Presynaptic 5-HT1AR |
| ↑ Gαi | ↓ Gαi |
| ↑ Tph-2 mRNA (RVM, LSSC and DRG) | No change in Tph-2 mRNA (RVM, LSSC and DRG) |
| ↑ Tdo/Ido-1/2 mRNA (LSSC) | ↓ Tdo/Ido-1/2 mRNA (LSSC) |
| ↓ 5-HIAA/5-HT (LSSC) | ↑ 5-HIAA/5-HT (LSSC) |
| ↑ QA (LSSC) | ↓ QA (LSSC) |
| Antibody (Vendor) | Dilution |
|---|---|
| 5-HT1A receptor (5-HT1AR) (Abcam, Cambridge, MA, USA) | 1:500, 1:100 |
| SNAP-25 (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) | 1:1000, 1:100 |
| PSD-95 (Novus Biologicals, Centennial CO, USA) | 1:2000, 1:100 |
| Secondary antibody, horseradish peroxidase (HRP) anti-rabbit IgG (Cell Signaling Technology, Danvers, MA, USA) | 1:3000 |
| Secondary antibody, HRP-linked anti-mouse IgG (Cell Signaling Technology, Inc., Danvers, MA, USA) | 1:3000 |
| AlexaFluor-488-labeled goat anti-mouse IgG antibody (Invitrogen, Carlsbad, CA, USA) | 1:100 |
| AlexaFluor-546 labeled goat anti-rabbit IgG antibody (Invitrogen, Carlsbad, CA, USA) | 1:100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munawar, N.; Bitar, M.S.; Masocha, W. Activation of 5-HT1A Receptors Normalizes the Overexpression of Presynaptic 5-HT1A Receptors and Alleviates Diabetic Neuropathic Pain. Int. J. Mol. Sci. 2023, 24, 14334. https://doi.org/10.3390/ijms241814334
Munawar N, Bitar MS, Masocha W. Activation of 5-HT1A Receptors Normalizes the Overexpression of Presynaptic 5-HT1A Receptors and Alleviates Diabetic Neuropathic Pain. International Journal of Molecular Sciences. 2023; 24(18):14334. https://doi.org/10.3390/ijms241814334
Chicago/Turabian StyleMunawar, Neha, Milad S. Bitar, and Willias Masocha. 2023. "Activation of 5-HT1A Receptors Normalizes the Overexpression of Presynaptic 5-HT1A Receptors and Alleviates Diabetic Neuropathic Pain" International Journal of Molecular Sciences 24, no. 18: 14334. https://doi.org/10.3390/ijms241814334
APA StyleMunawar, N., Bitar, M. S., & Masocha, W. (2023). Activation of 5-HT1A Receptors Normalizes the Overexpression of Presynaptic 5-HT1A Receptors and Alleviates Diabetic Neuropathic Pain. International Journal of Molecular Sciences, 24(18), 14334. https://doi.org/10.3390/ijms241814334





