Cold Exposure Regulates Hepatic Glycogen and Lipid Metabolism in Newborn Goats
Abstract
1. Introduction
2. Results
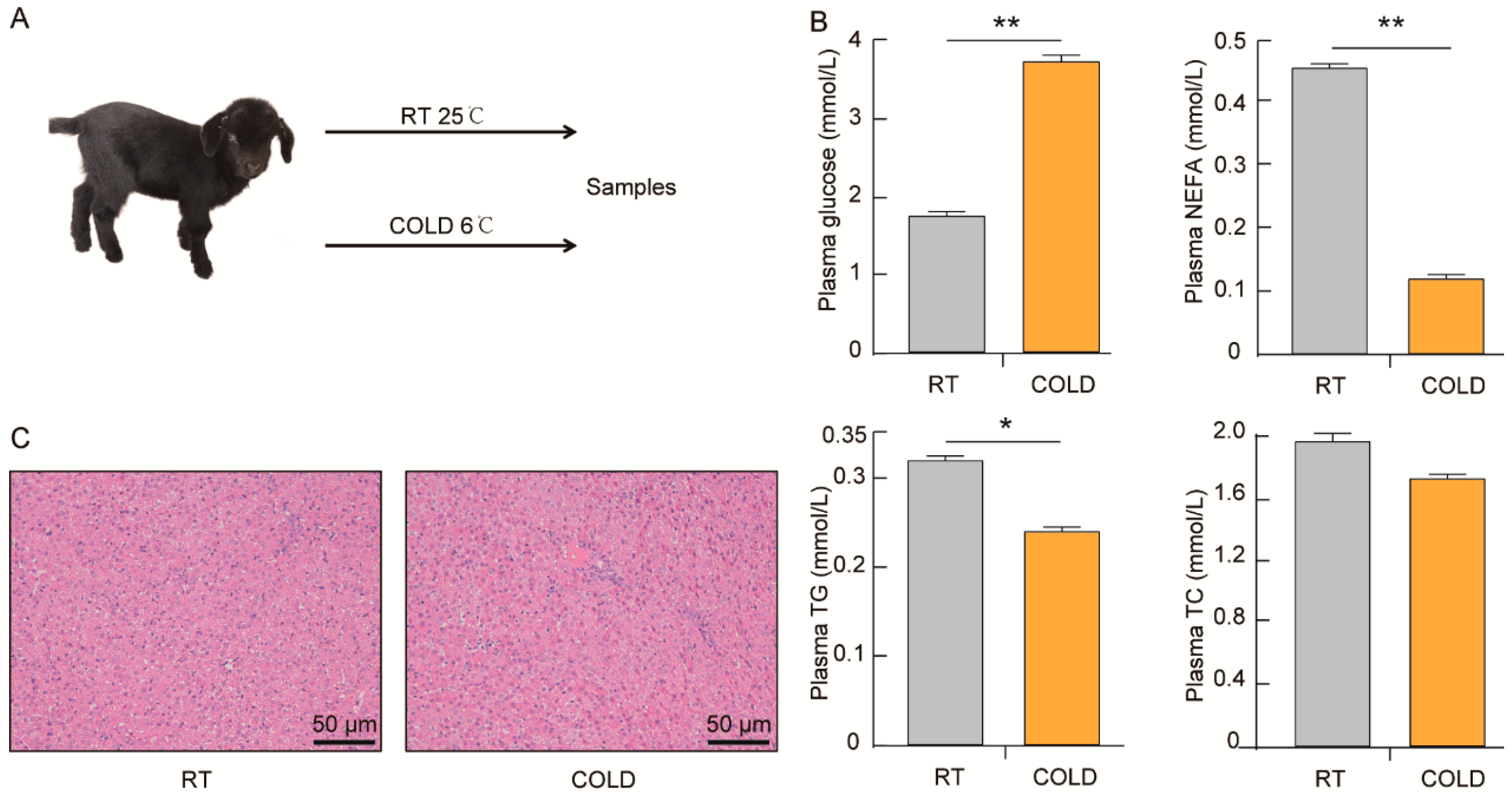
2.1. Effects of Cold Exposure on Plasma Biochemical of Newborn Goats
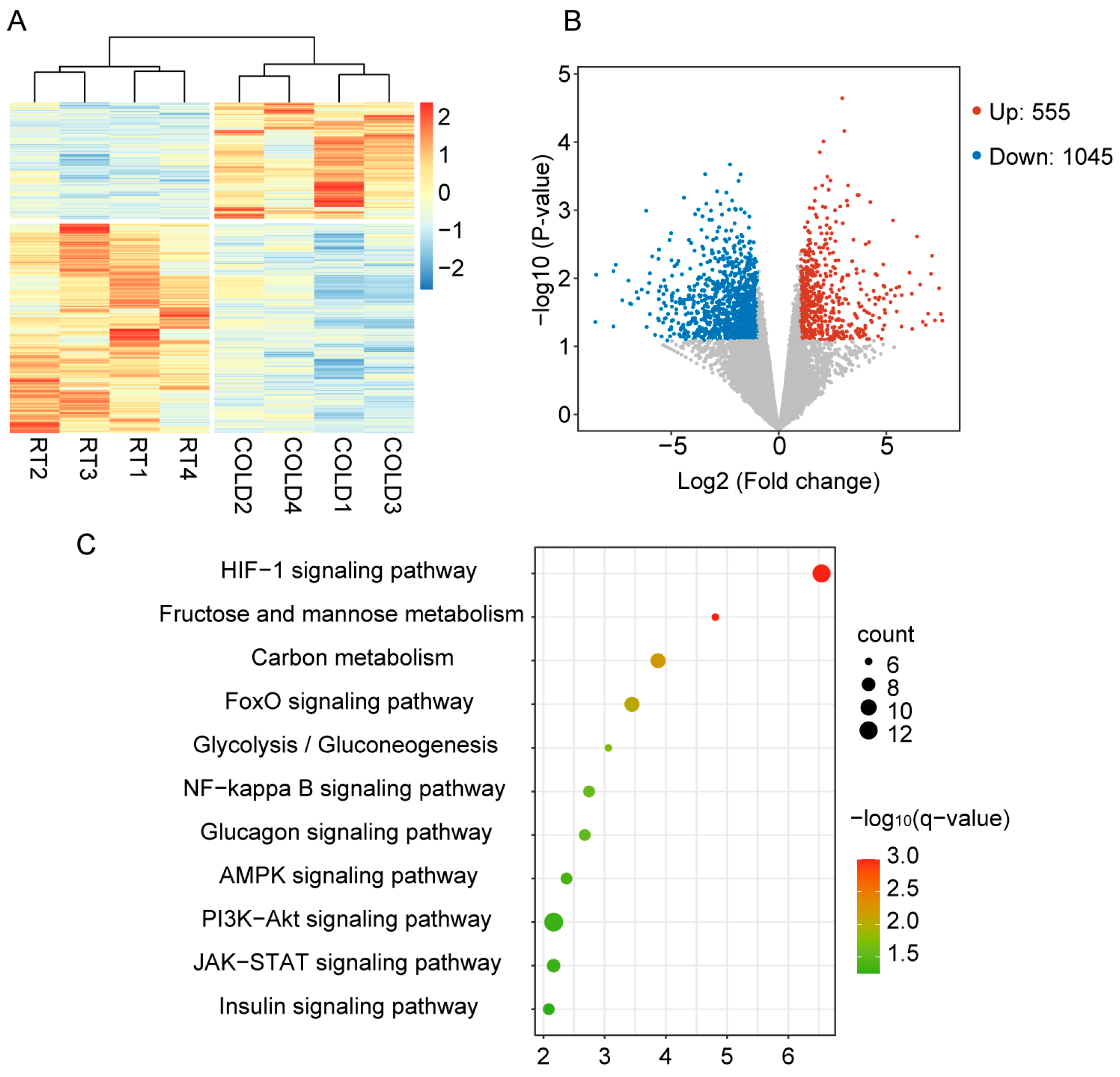
2.2. Cold Exposure Changed the Gene Expression Pattern in Liver of Newborn Goats
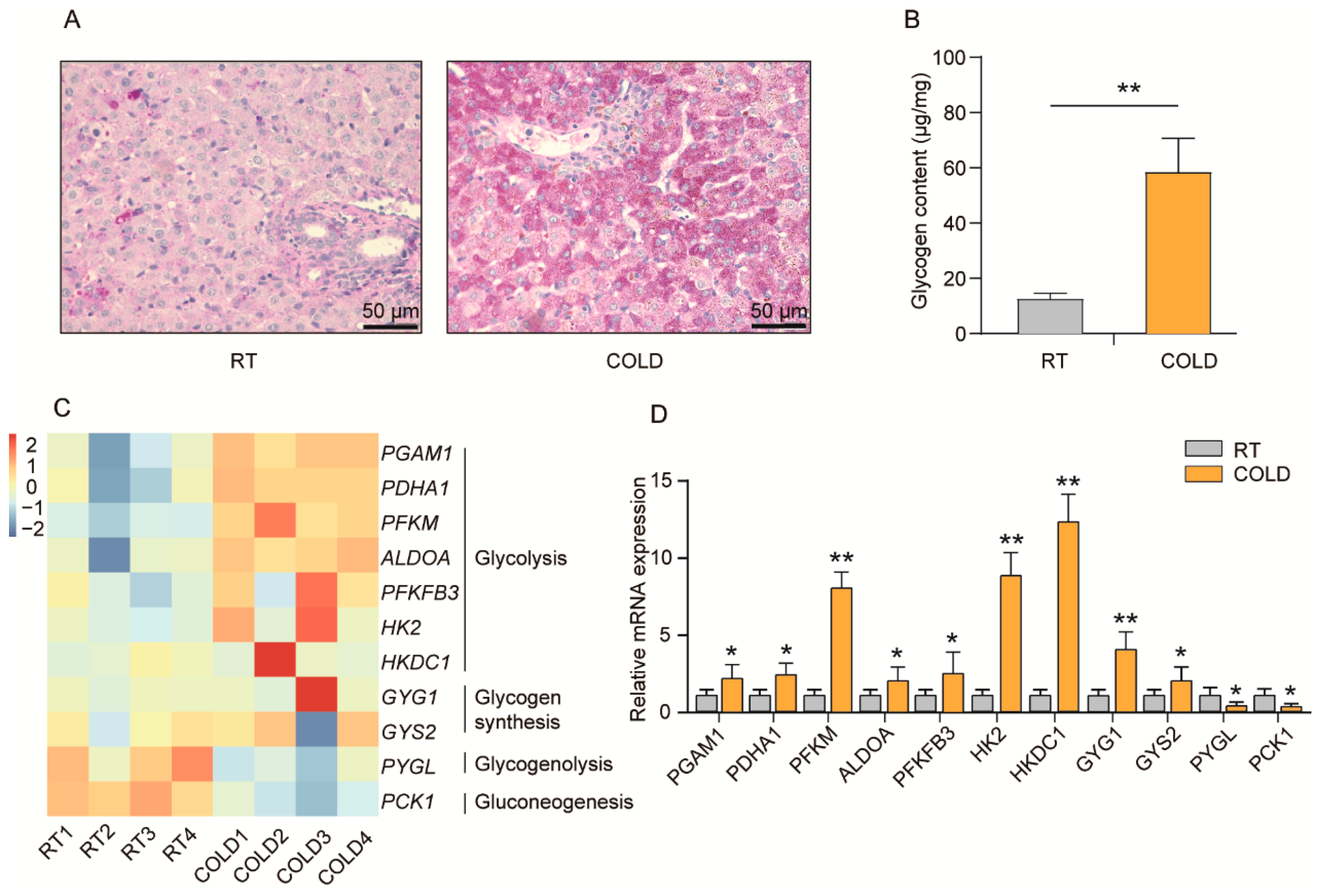
2.3. Cold Exposure Regulates Glycogen Metabolism in Liver of Newborn Goats
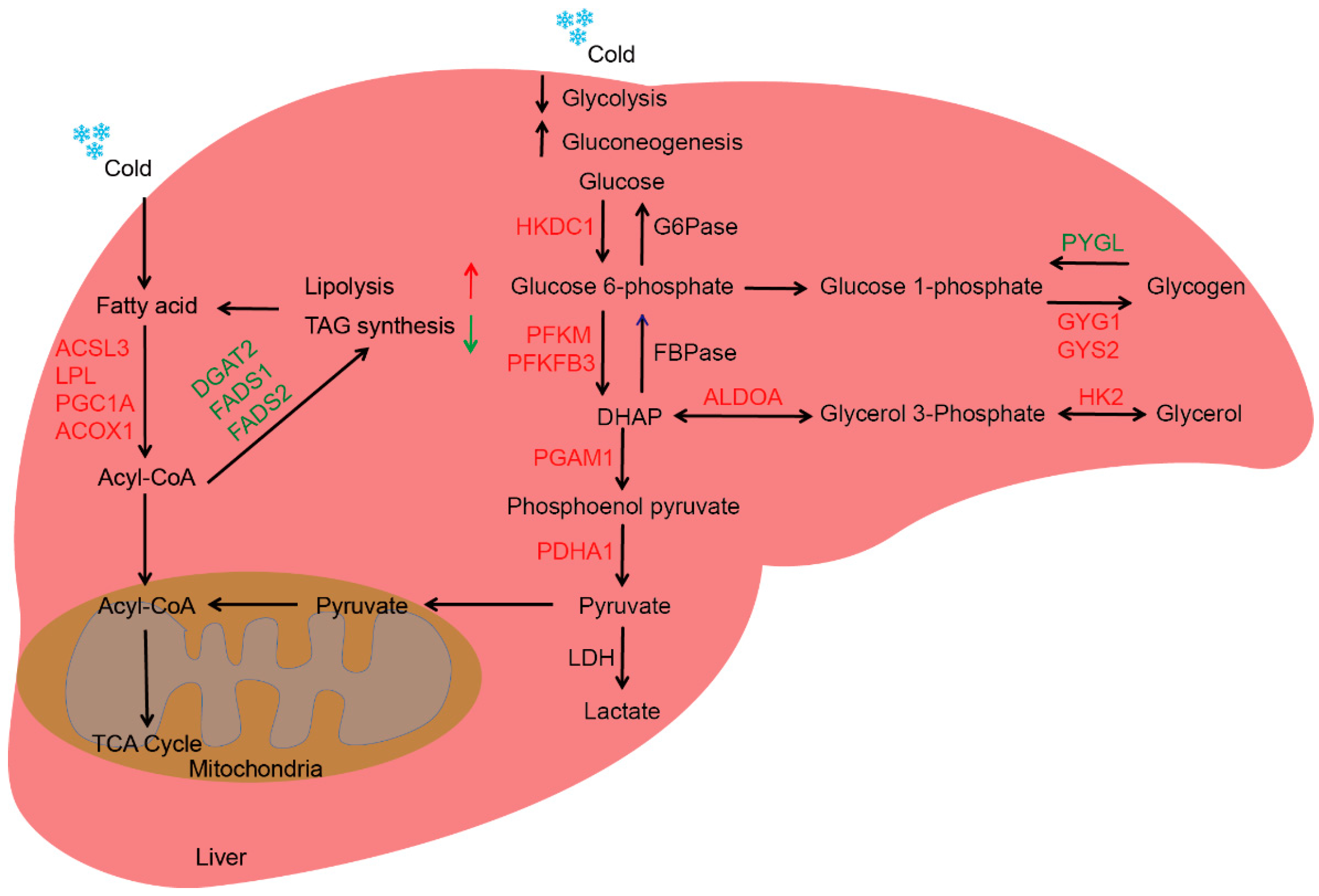
2.4. Cold Exposure Regulates Lipid Metabolism in Liver of Newborn Goats
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals and Sample Collection
4.3. Plasma Biochemical Analysis
4.4. Hematoxylin-Eosin (H&E), Periodic Acid Schiff (PAS), and Oil Red O Staining
4.5. Glycogen, Triglyceride (TG), and Total Cholesterol (TC) Analysis
4.6. Quantitative Real-Time PCR
4.7. RNA Library Construction, and Sequencing
4.8. RNA-Seq Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gavahian, M.; Chu, Y.H.; Jo, C. Prospective Applications of Cold Plasma for Processing Poultry Products: Benefits, Effects on Quality Attributes, and Limitations. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1292–1309. [Google Scholar] [CrossRef] [PubMed]
- Slee, J. The effects of breed, birthcoat and body weight on the cold resistance of newborn lambs. Anim. Sci. 1978, 27, 43–49. [Google Scholar] [CrossRef]
- Doubek, J.; Losrkov, S.; Fleischer, P.; Mal, G.; Skivnek, M. Metabolic and hormonal profiles of potentiated cold stress in lambs during early postnatal period. Czech J. Anim. Sci. 2003, 48, 403–411. [Google Scholar]
- Shi, L.; Xu, Y.; Jin, X.; Wang, Z.; Mao, C.; Guo, S.; Yan, S.; Shi, B. Influence of Cold Environments on Growth, Antioxidant Status, Immunity and Expression of Related Genes in Lambs. Animals 2022, 12, 2535. [Google Scholar] [CrossRef]
- Zimmermann, B.; Diebold, G.; Galbraith, J.; Whitmore, W.; Okamoto, M.; Robinson, J.; Young, B.; Murdoch, G.; Mosenthin, R.; Christopherson, R. Effect of aminophylline on metabolic and thermoregulatory responses during hypothermia associated with cold exposure in lambs. Can. J. Anim. Sci. 2003, 83, 739–748. [Google Scholar] [CrossRef]
- Venditti, P.; Pamplona, R.; Ayala, V.; De Rosa, R.; Caldarone, G.; Di Meo, S. Differential effects of experimental and cold-induced hyperthyroidism on factors inducing rat liver oxidative damage. J. Exp. Biol. 2006, 209 Pt 5, 817–825. [Google Scholar] [CrossRef]
- Sahin, E.; Gümüşlü, S. Cold-stress-induced modulation of antioxidant defence: Role of stressed conditions in tissue injury followed by protein oxidation and lipid peroxidation. Int. J. Biometeorol. 2004, 48, 165–171. [Google Scholar] [CrossRef]
- Qin, J.; Mai, Y.; Li, Y.; Jiang, Z.; Gao, Y. Effect of mild hypothermia preconditioning against low temperature (4 °C) induced rat liver cell injury in vitro. PLoS ONE 2017, 12, e0176652. [Google Scholar] [CrossRef]
- Bartelt, A.; John, C.; Schaltenberg, N.; Berbée, J.F.P.; Worthmann, A.; Cherradi, M.L.; Schlein, C.; Piepenburg, J.; Boon, M.R.; Rinninger, F.; et al. Thermogenic adipocytes promote HDL turnover and reverse cholesterol transport. Nat. Commun. 2017, 8, 15010. [Google Scholar] [CrossRef]
- Ghafoory, S.; Breitkopf-Heinlein, K.; Li, Q.; Scholl, C.; Dooley, S.; Wölfl, S. Zonation of nitrogen and glucose metabolism gene expression upon acute liver damage in mouse. PLoS ONE 2013, 8, e78262. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Pérez-Felpete, N.; Fernández-Fernández, C.; Donapetry-García, C.; Pazos-García, C. Liver glucose metabolism in humans. Biosci. Rep. 2016, 36, e00416. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, Z.; Liu, P.; Zhang, S. Comparative proteomic study of liver lipid droplets and mitochondria in mice housed at different temperatures. FEBS Lett. 2019, 593, 2118–2138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, B.; Hu, Y.; Liu, P.; Lian, S.; Lv, H.; Yang, Y.; Ji, H.; Yang, H.; Liu, J.; et al. O-GlcNAc/Akt pathway regulates glucose metabolism and reduces apoptosis in liver of piglets with acute cold stress. Cryobiology 2021, 100, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tang, J.; Zhang, R.; Zhan, S.; Zhong, T.; Guo, J.; Wang, Y.; Cao, J.; Li, L.; Zhang, H.; et al. Cold exposure induces lipid dynamics and thermogenesis in brown adipose tissue of goats. BMC Genom. 2022, 23, 528. [Google Scholar] [CrossRef] [PubMed]
- Labbé, S.M.; Caron, A.; Bakan, I.; Laplante, M.; Carpentier, A.C.; Lecomte, R.; Richard, D. In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. Faseb J. 2015, 29, 2046–2058. [Google Scholar] [CrossRef]
- Coloma-García, W.; Mehaba, N.; Such, X.; Caja, G.; Salama, A. Effects of Cold Exposure on Some Physiological, Productive, and Metabolic Variables in Lactating Dairy Goats. Animals 2020, 10, 2383. [Google Scholar] [CrossRef]
- Thompson, G.E.; Manson, W.; Clarke, P.L.; Bell, A.W. Acute cold exposure and the metabolism of glucose and some of its precursors in the liver of the fed and fasted sheep. Exp. Physiol. 1978, 63, 189. [Google Scholar] [CrossRef]
- Grefhorst, A.; van den Beukel, J.C.; Dijk, W.; Steenbergen, J.; Voortman, G.J.; Leeuwenburgh, S.; Visser, T.J.; Kersten, S.; Friesema, E.C.; Themmen, A.P. Multiple effects of cold exposure on livers of male mice. J. Endocrinol. 2018, 238, 91–106. [Google Scholar] [CrossRef]
- Mizoe, A.; Fujioka, H.; Kamohara, Y.; Watanabe, Y.; Azuma, T.; Kanematsu, T. The significance of measuring the serum total bile acids levels during orthotopic liver transplantation. Hepato-Gastroenterol. 1999, 46, 2454–2459. [Google Scholar]
- Qin, B.; Sun, W.Y.; Xia, H.Z.; Li, Y.L.; Zhang, Z.W.; Xu, S.W. Effects of Cold Stress on mRNA Level of Uncoupling Protein 2 in Liver of Chicks. Pak. Vet. J. 2014, 34, 309–313. [Google Scholar]
- Wei, X.; Jia, R.; Yang, Z.; Jiang, J.; Huang, J.; Yan, J.; Luo, X. NAD(+)/sirtuin metabolism is enhanced in response to cold-induced changes in lipid metabolism in mouse liver. FEBS Lett. 2020, 594, 1711–1725. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yao, R.; Shi, H.; Liu, Y.; Lian, S.; Yang, Y.; Yang, H.; Li, S. Effects of Cold-inducible RNA-binding Protein (CIRP) on Liver Glycolysis during Acute Cold Exposure in C57BL/6 Mice. Int. J. Mol. Sci. 2019, 20, 1470. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ji, H.; Guo, J.; Chen, Y.; Li, W.; Wang, S.; Zhen, L. Non-targeted Metabolomics Analysis Based on LC-MS to Assess the Effects of Different Cold Exposure Times on Piglets. Front. Physiol. 2022, 13, 853995. [Google Scholar] [CrossRef] [PubMed]
- Smith, T. A Mammalian hexokinases and their abnormal expression in cancer. Br. J. Biomed. Sci. 2000, 57, 170–178. [Google Scholar]
- Pusec, C.M.; De Jesus, A.; Khan, M.W.; Terry, A.R.; Ludvik, A.E.; Xu, K.; Giancola, N.; Pervaiz, H.; Daviau Smith, E.; Ding, X.; et al. Hepatic HKDC1 Expression Contributes to Liver Metabolism. Endocrinology 2019, 160, 313–330. [Google Scholar] [CrossRef]
- Ausina, P.; Da Silva, D.; Majerowicz, D.; Zancan, P.; Sola-Penna, M. Insulin specifically regulates expression of liver and muscle phosphofructokinase isoforms. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 103, 228–233. [Google Scholar] [CrossRef]
- Botchlett, R.; Li, H.; Guo, X.; Qi, T.; Zhao, J.; Zheng, J.; Woo, S.L.; Pei, Y.; Liu, M.; Hu, X.; et al. Glucose and Palmitate Differentially Regulate PFKFB3/iPFK2 and Inflammatory Responses in Mouse Intestinal Epithelial Cells. Sci. Rep. 2016, 6, 28963. [Google Scholar] [CrossRef][Green Version]
- Burwinkel, B.; Shiomi, S.; Al Zaben, A.; Kilimann, M.W. Liver glycogenosis due to phosphorylase kinase deficiency: PHKG2 gene structure and mutations associated with cirrhosis. Hum. Mol. Genet. 1998, 7, 149–154. [Google Scholar] [CrossRef]
- Fanin, M.; Torella, A.; Savarese, M.; Nigro, V.; Angelini, C. GYG1 gene mutations in a family with polyglucosan body myopathy. Neurol. Genet. 2015, 1, e21. [Google Scholar] [CrossRef]
- Zhong, H.; Zhou, Y.; Zhang, H.; Xiao, W. DNA methylation pattern is associated with elevated expression of DGAT2 in hybrid tilapia. Aquac. Nutr. 2021, 27, 1750–1760. [Google Scholar] [CrossRef]
- Xiong, L.; Pei, J.; Wu, X.; Bao, P.; Guo, X.; Yan, P. Explaining Unsaturated Fatty Acids (UFAs), Especially Polyunsaturated Fatty Acid (PUFA) Content in Subcutaneous Fat of Yaks of Different Sex by Differential Proteome Analysis. Genes 2022, 13, 790. [Google Scholar] [CrossRef]
- Osman, R.H.; Liu, L.; Xia, L.; Zhao, X.; Wang, Q.; Sun, X.; Zhang, Y.; Yang, B.; Zheng, Y.; Gong, D.; et al. Fads1 and 2 are promoted to meet instant need for long-chain polyunsaturated fatty acids in goose fatty liver. Mol. Cell. Biochem. 2016, 418, 103–117. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, H.H.; Wang, J.W.; Lv, J.; Li, L.; Pan, Z.X. Molecular cloning of the goose ACSL3 and ACSL5 coding domain sequences and their expression characteristics during goose fatty liver development. Mol. Biol. Rep. 2014, 41, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Cao, A.; Dong, B.; Liu, J. Reduction of serum free fatty acids and triglycerides by liver-targeted expression of long chain acyl-CoA synthetase 3. Int. J. Mol. Med. 2011, 27, 655–662. [Google Scholar] [PubMed]
- Li, Y.X.; Han, T.T.; Liu, Y.; Zheng, S.; Zhang, Y.; Liu, W.; Hu, Y.M. Insulin resistance caused by lipotoxicity is related to oxidative stress and endoplasmic reticulum stress in LPL gene knockout heterozygous mice. Atherosclerosis 2015, 239, 276–282. [Google Scholar] [CrossRef]
- Teratani, T.; Tomita, K.; Furuhashi, H.; Sugihara, N.; Higashiyama, M.; Nishikawa, M.; Irie, R.; Takajo, T.; Wada, A.; Horiuchi, K.; et al. Lipoprotein Lipase Up-regulation in Hepatic Stellate Cells Exacerbates Liver Fibrosis in Nonalcoholic Steatohepatitis in Mice. Hepatol. Commun. 2019, 3, 1098–1112. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, D.; Zhou, T.; Wang, Y.; Wang, L. Cold Exposure Regulates Hepatic Glycogen and Lipid Metabolism in Newborn Goats. Int. J. Mol. Sci. 2023, 24, 14330. https://doi.org/10.3390/ijms241814330
Su D, Zhou T, Wang Y, Wang L. Cold Exposure Regulates Hepatic Glycogen and Lipid Metabolism in Newborn Goats. International Journal of Molecular Sciences. 2023; 24(18):14330. https://doi.org/10.3390/ijms241814330
Chicago/Turabian StyleSu, Duo, Tianhui Zhou, Yan Wang, and Linjie Wang. 2023. "Cold Exposure Regulates Hepatic Glycogen and Lipid Metabolism in Newborn Goats" International Journal of Molecular Sciences 24, no. 18: 14330. https://doi.org/10.3390/ijms241814330
APA StyleSu, D., Zhou, T., Wang, Y., & Wang, L. (2023). Cold Exposure Regulates Hepatic Glycogen and Lipid Metabolism in Newborn Goats. International Journal of Molecular Sciences, 24(18), 14330. https://doi.org/10.3390/ijms241814330





