Effects of Exogenous Application of Glycine Betaine Treatment on ‘Huangguoggan’ Fruit during Postharvest Storage
Abstract
:1. Introduction
2. Results
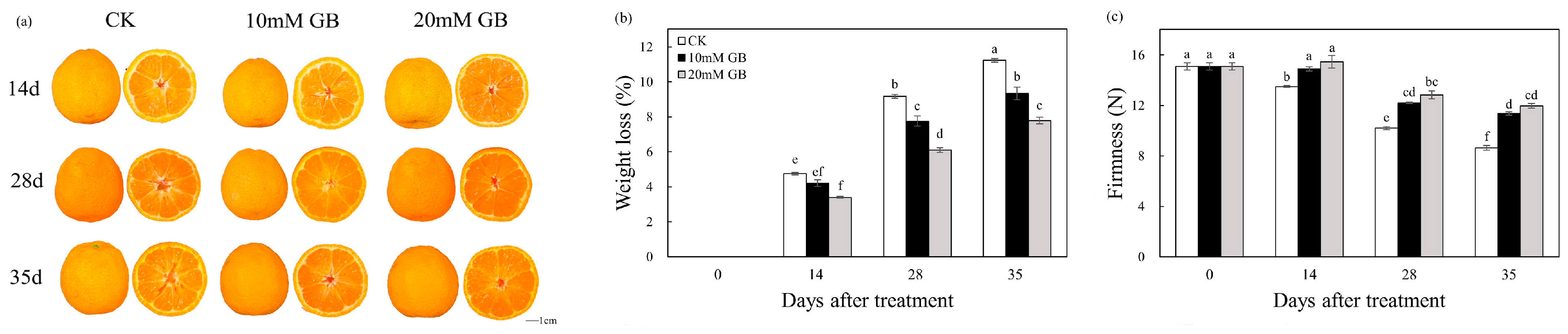
2.1. Phenotype, Weight Loss, and Firmness
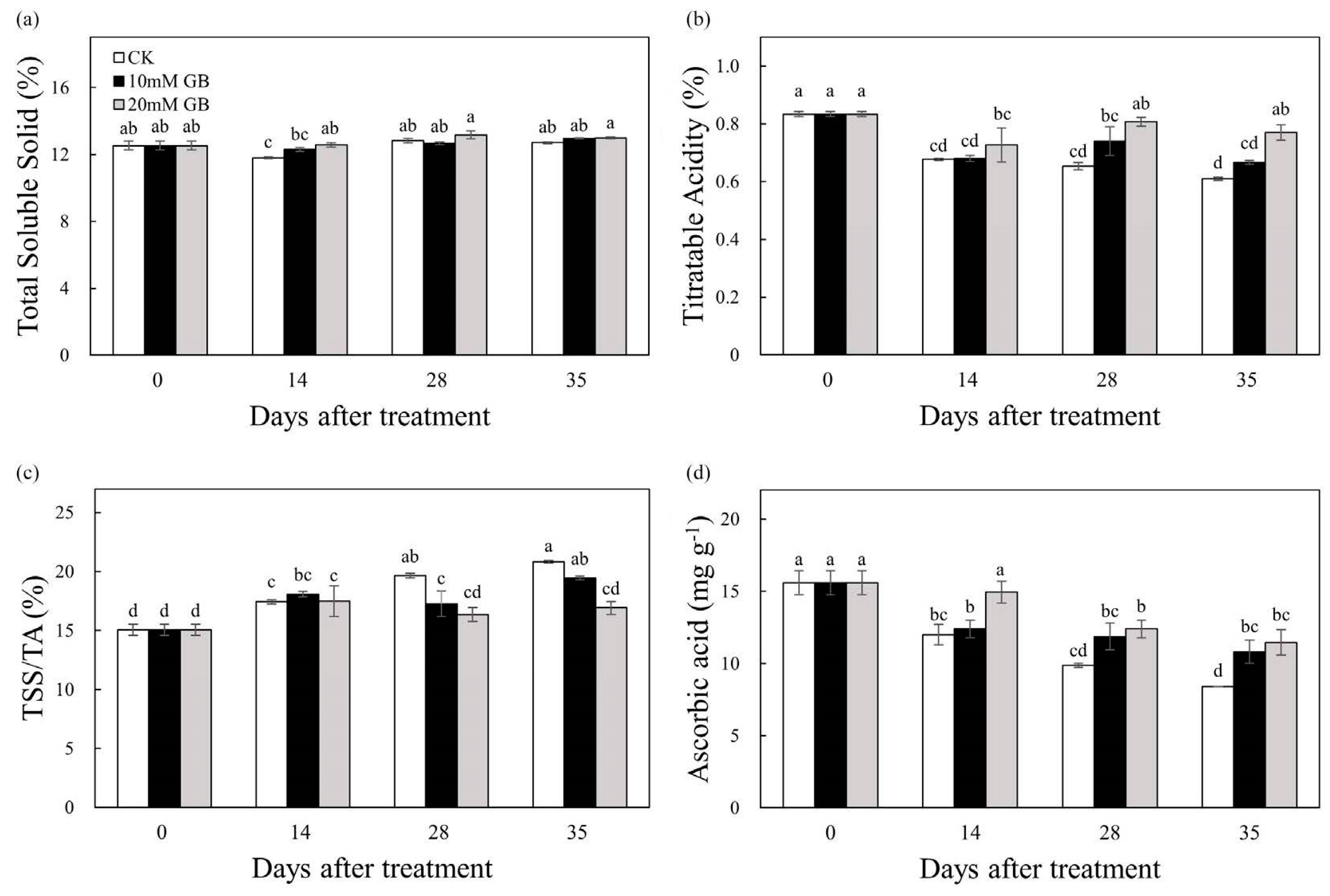
2.2. Total Soluble Solid, Titratable Acid, and Ascorbic Acid
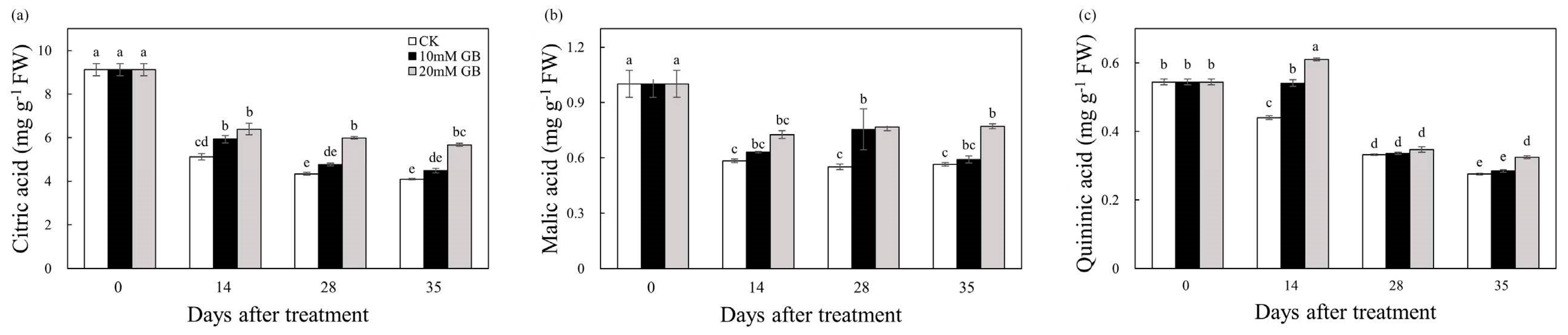
2.3. Organic Acids
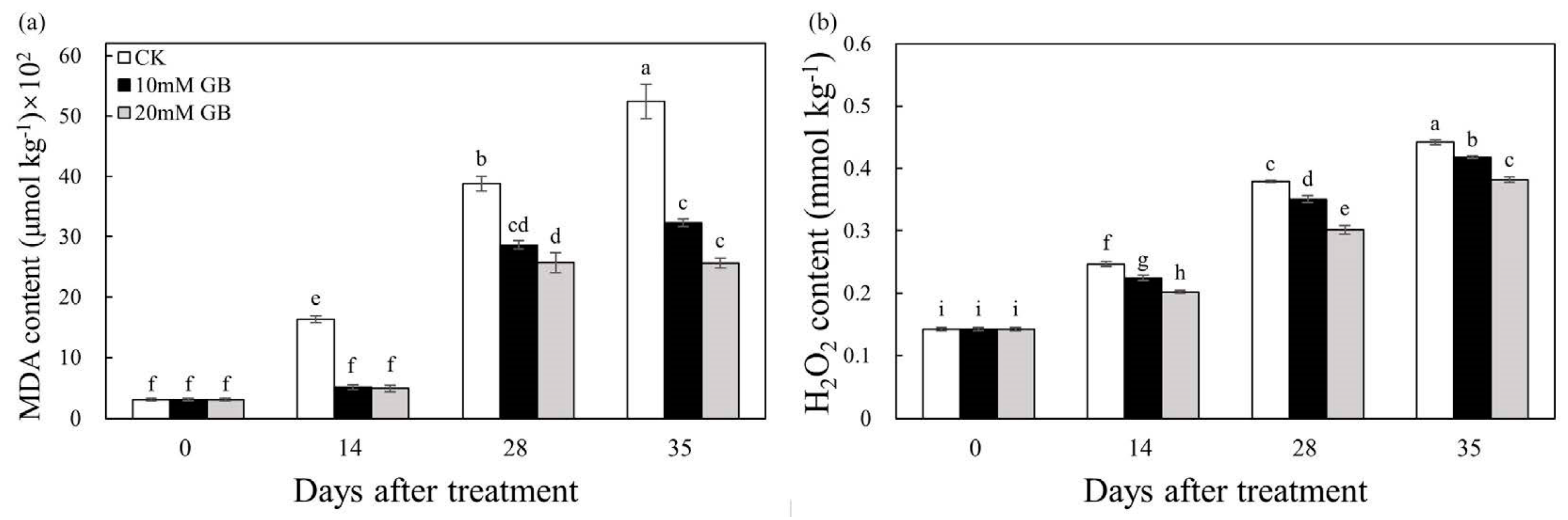
2.4. MDA and H2O2
2.5. Total Phenolic Content, Total Flavonoid Content, and Antioxidant Enzyme Activity
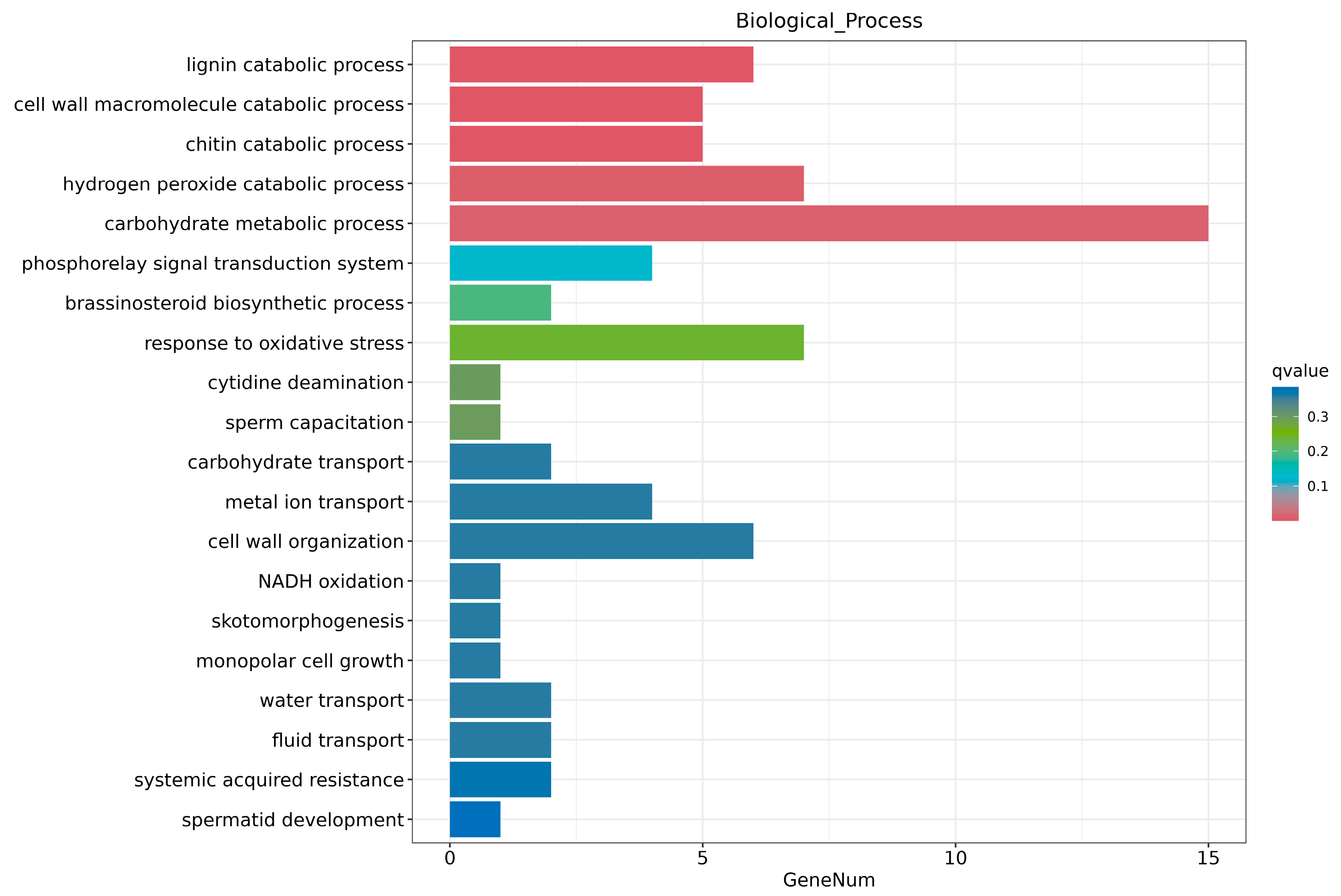
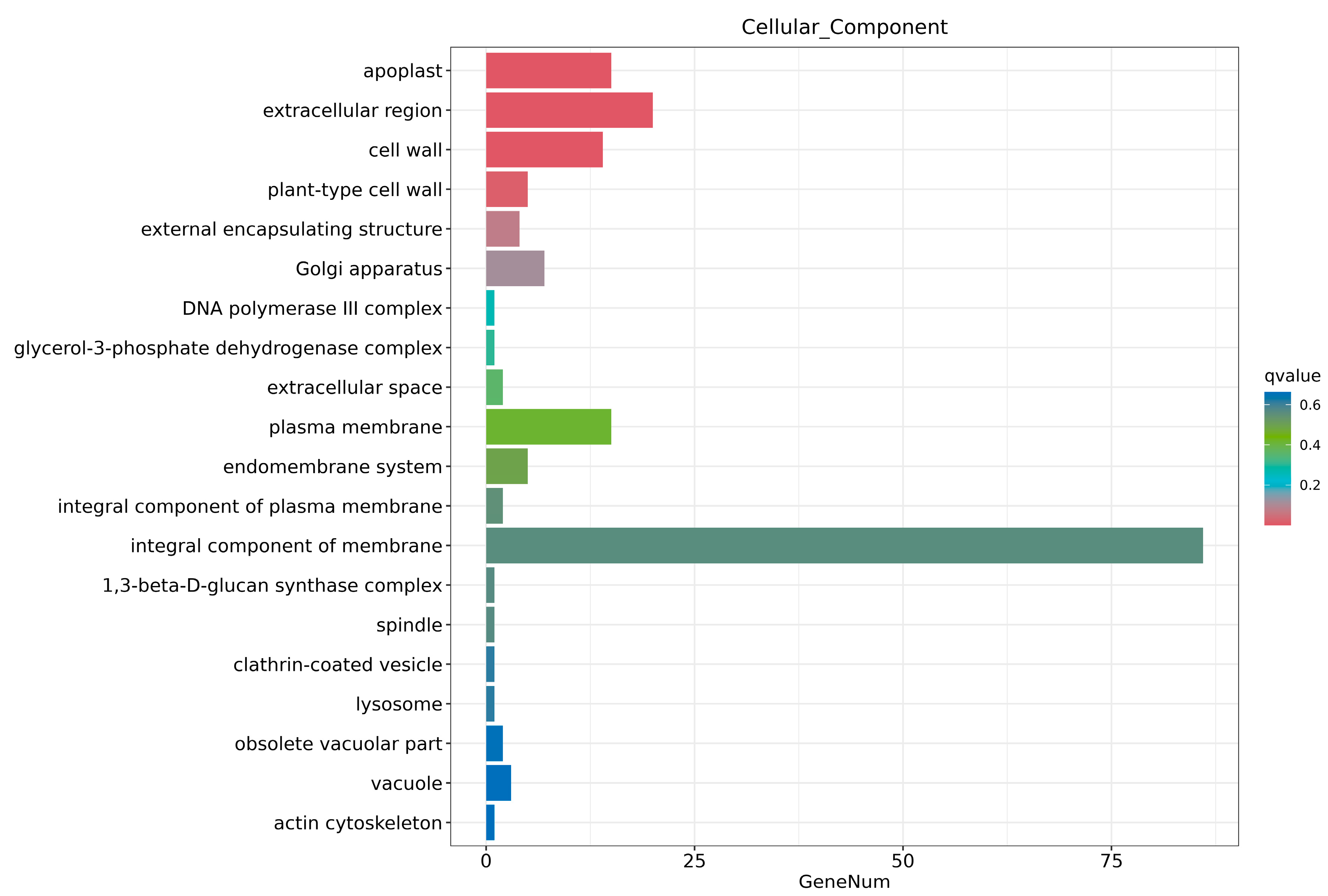
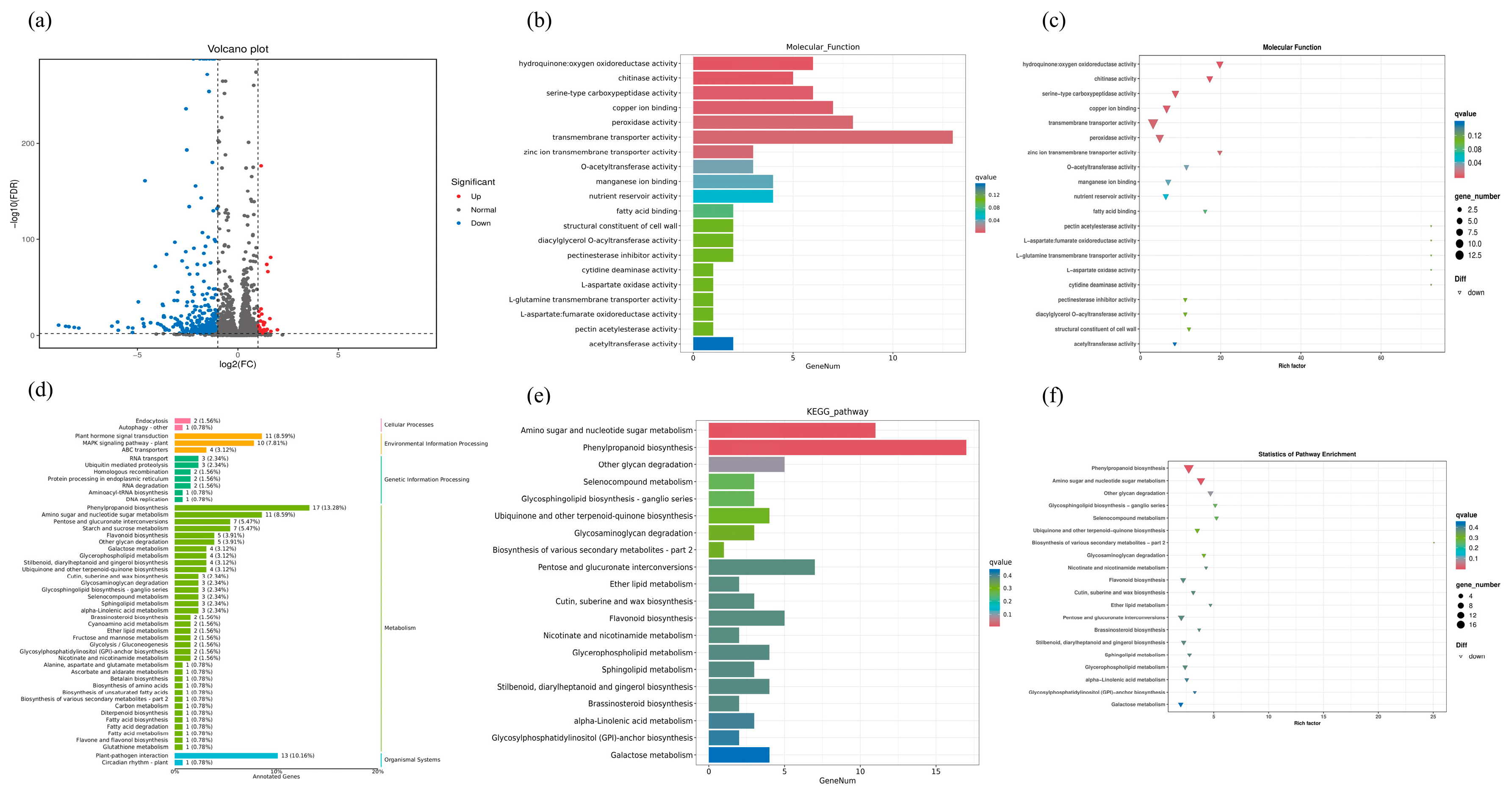
2.6. Transcriptome Analysis and Enrichment of DEGs
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatment
4.2. Weight Loss and Firmness
4.3. Total Soluble Solid, Titratable Acidity, and Ascorbic Acid
4.4. Organic Acids Detection
4.5. Total Phenolic and Total Flavonoids Contents and Antioxidant Enzymes
4.6. Malondialdehyde (MDA) and Hydrogen Peroxide (H2O2) Content
4.7. Transcriptome Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Agarwal, P.; Sebghatollahi, Z.; Kamal, M.; Dhyani, A.; Shrivastava, A.; Singh, K.K.; Sinha, M.; Mahato, N.; Mishra, A.K.; Baek, K.H. Citrus Essential Oils in Aromatherapy: Therapeutic Effects and Mechanisms. Antioxidants 2022, 11, 2374. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T. Misunderstanding with Regards Citrus Classification and Nomeclature. Bull. Univ. Osaka Prefect. Ser. B Agric. Biol. 1969, 21, 139–145. [Google Scholar]
- Strano, M.C.; Altieri, G.; Allegra, M.; Di Renzo, G.C.; Paterna, G.; Matera, A.; Genovese, F. Postharvest Technologies of Fresh Citrus Fruit: Advances and Recent Developments for the Loss Reduction during Handling and Storage. Horticulturae 2022, 8, 612. [Google Scholar] [CrossRef]
- Sun, X.; Baldwin, E.; Bai, J. Applications of Gaseous Chlorine Dioxide on Postharvest Handling and Storage of Fruits and Vegetables—A Review. Food Control 2019, 95, 18–26. [Google Scholar] [CrossRef]
- Strano, M.C.; Timpanaro, N.; Allegra, M.; Foti, P.; Pangallo, S.; Romeo, F.V. Effect of Ozonated Water Combined with Sodium Bicarbonate on Microbial Load and Shelf Life of Cold Stored Clementine (Citrus clementina Hort. ex Tan.). Sci. Hortic. 2021, 276, 109775. [Google Scholar] [CrossRef]
- Palou, L.; Ali, A.; Fallik, E.; Romanazzi, G. GRAS, Plant- and animal-derived Compounds as Alternatives to Conventional Fungicides for the Control of Postharvest Diseases of Fresh Horticultural Produce. Postharvest Biol. Technol. 2016, 122, 41–52. [Google Scholar] [CrossRef]
- Khalid, M.; Rehman, H.M.; Ahmed, N.; Nawaz, S.; Saleem, F.; Ahmad, S.; Uzair, M.; Rana, I.A.; Atif, R.M.; Zaman, Q.U.; et al. Using Exogenous Melatonin, Glutathione, Proline, and Glycine Betaine Treatments to Combat Abiotic Stresses in Crops. Int. J. Mol. Sci. 2022, 23, 12913. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Lin, X.; Wei, Q.; Yang, X.; Zhang, Y.; Chen, J. Melatonin Treatment Delays Postharvest Senescence and Maintains the Organoleptic Quality of ‘Newhall’ Navel Orange (Citrus sinensis (L.) Osbeck) by Inhibiting Respiration and Enhancing Antioxidant Capacity. Sci. Hortic. 2021, 286, 110236. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.P.; Yang, Q.Z.; Zhao, Q.F. Exogenous Melatonin Delays Postharvest Fruit Senescence and Maintains the Quality of Sweet Cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef]
- Chen, X.Q.; Song, Z.L.; Pang, Q.Q.; Lv, J.H.; Yu, L.H.; Li, T.; Fang, J.G.; Jia, H.F. Effect of Postharvest Application of Jasmonic Acid on Shelf-life Quality of Strawberries. Eur. J. Hortic. Sci. 2022, 87, 1–13. [Google Scholar] [CrossRef]
- Habibi, F.; Valero, D.; Serrano, M.; Guillen, F. Exogenous Application of Glycine Betaine Maintains Bioactive Compounds, Antioxidant Activity, and Physicochemical Attributes of Blood Orange Fruit During Prolonged Cold Storage. Front. Nutr. 2022, 9, 873915. [Google Scholar] [CrossRef]
- Razavi, F.; Mahmoudi, R.; Rabiei, V.; Aghdam, M.S.; Soleimani, A. Glycine Betaine Treatment Attenuates Chilling Injury and Maintains Nutritional Quality of Hawthorn Fruit during Storage at Low Temperature. Sci. Hortic. 2018, 233, 188–194. [Google Scholar] [CrossRef]
- Awad, M.A.; Al-Qurashi, A.D.; El-Dengawy, E.-R.F.A.; Elsayed, M.I. Quality and Biochemical Changes of ‘Hindi-Besennara’ Mangoes during Shelf Life as Affected by Chitosan, Trans-resveratrol and Glycine Betaine Postharvest Dipping. Sci. Hortic. 2017, 217, 156–163. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Chen, C.; Zhang, S.; Zhao, X.; Wu, C.; Kou, X.; Xue, Z. Glycine Betaine Inhibits Postharvest Softening and Quality Decline of Winter Jujube Fruit by Regulating Energy and Antioxidant Metabolism. Food Chem. 2023, 410, 135445. [Google Scholar] [CrossRef]
- Sun, H.; Luo, M.; Zhou, X.; Zhou, Q.; Sun, Y.; Ge, W.; Wei, B.; Cheng, S.; Ji, S. Exogenous Glycine Betaine Treatment Alleviates Low Temperature-induced Pericarp Browning of ‘Nanguo’ Pears by Regulating Antioxidant Enzymes and Proline Metabolism. Food Chem. 2020, 306, 125626. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Lv, M.; He, H.S.; Jiang, Y.G.; Yang, J.Y.; Ji, S.J. Glycine Betaine Alleviated Peel Browning in Cold-stored ‘Nanguo’ Pears during Shelf Life by Regulating Phenylpropanoid and Soluble Sugar Metabolisms. Sci. Hortic. 2020, 262, 109100. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, P.; Huang, Y.; Shan, T.; Wang, L.; Li, Y.; Zheng, Y. Effect of Hot Water Combined with Glycine Betaine Alleviates Chilling Injury in Cold-stored Loquat Fruit. Postharvest Biol. Technol. 2016, 118, 141–147. [Google Scholar] [CrossRef]
- Shan, T.; Jin, P.; Zhang, Y.; Huang, Y.; Wang, X.; Zheng, Y. Exogenous Glycine Betaine Treatment Enhances Chilling Tolerance of Peach Fruit during Cold Storage. Postharvest Biol. Technol. 2016, 114, 104–110. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Razavi, F.; Rabiei, V.; Gohari, G.; Palou, L. Application of Glycine Betaine Coated Chitosan Nanoparticles Alleviate Chilling Injury and Maintain Quality of Plum (Prunus domestica L.) Fruit. Int. J. Biol. Macromol. 2022, 207, 965–977. [Google Scholar] [CrossRef]
- Liao, L.; Dong, T.; Qiu, X.; Rong, Y.; Sun, G.; Wang, Z.; Zhu, J. Antioxidant Enzyme Activity and Growth Responses of Huangguogan Citrus Cultivar to Nitrogen Supplementation. Biosci. Biotechnol. Biochem. 2019, 83, 1924–1936. [Google Scholar] [CrossRef]
- Bi, X.Y.; Liao, L.; Deng, L.J.; Jin, Z.H.; Huang, Z.H.; Sun, G.C.; Xiong, B.; Wang, Z.H. Combined Transcriptome and Metabolome Analyses Reveal Candidate Genes Involved in Tangor (Citrus reticulata × Citrus sinensis) Fruit Development and Quality Formation. Int. J. Mol. Sci. 2022, 23, 5457. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Guillen, F.; Castillo, S.; Serrano, M.; Valero, D. Changes in Bioactive Compounds, Antioxidant Activity, and Nutritional Quality of Blood Orange Cultivars at Different Storage Temperatures. Antioxidants 2020, 9, 1016. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A. Vacuum Infiltration of Putrescine Enhances Bioactive Compounds and Maintains Quality of Blood Orange during Cold Storage. Food Chem. 2017, 227, 1–8. [Google Scholar] [CrossRef]
- Xu, F.; Yang, Z.F.; Chen, X.H.; Jin, P.; Wang, X.L.; Zheng, Y.H. 6-Benzylaminopurine Delays Senescence and Enhances Health-Promoting Compounds of Harvested Broccoli. J. Agric. Food Chem. 2012, 60, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Cercos, M.; Soler, G.; Iglesias, D.J.; Gadea, J.; Forment, J.; Talon, M. Global Analysis of Gene Expression during Development and Ripening of Citrus Fruit Flesh. A Proposed Mechanism for Citric Acid Utilization. Plant Mol. Biol. 2006, 62, 513–527. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, T.; Zuo, J.; Gao, L.; Fan, L. Amelioration of Postharvest Chilling Injury in Sweet Pepper by Glycine Betaine. Postharvest. Postharvest Biol. Technol. 2016, 116, 129–130. [Google Scholar] [CrossRef]
- Li, M.; Zhi, H.; Dong, Y. Influence of Preharvest and Postharvest Applications of Glycine Betaine on Fruit Quality Attributes and Storage Disorders of ‘Lapins’ and ‘Regina’ Cherries. Hortscience 2019, 54, 1540–1545. [Google Scholar] [CrossRef]
- Chen, L.L.; Shan, W.; Cai, D.L.; Chen, J.Y.; Lu, W.J.; Su, X.G.; Kuang, J.F. Postharvest Application of Glycine Betaine Ameliorates Chilling Injury in Cold-stored Banana Fruit by Enhancing Antioxidant System. Sci. Hortic. 2021, 287, 110264. [Google Scholar] [CrossRef]
- Ma, X.Q.; Li, N.; Guo, J.; Yang, L.Q.; Hao, C.X.; Li, Y.; Gentile, A.; Lu, X.P.; Ma, X.F.; Deng, Z.N. Involvement of CsPH8 in Citrate Accumulation Directly Related to Fruit Storage Performance of ‘Bingtang’ Sweet Orange Mutants. Postharvest Biol. Technol. 2020, 170, 111316. [Google Scholar] [CrossRef]
- Nunes, C.; Emond, J.P. Relationship Between Weight Loss and Visual Quality of Fruits and Vegetables. Proc. Fla. State Hortic. Soc. 2007, 120, 235–245. [Google Scholar]
- Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z.; Opara, U.L. Postharvest Factors Affecting Vitamin C Content of Citrus Fruits: A Review. Sci. Hortic. 2017, 218, 95–104. [Google Scholar] [CrossRef]
- Acharyya, A.; Shin, D.; Troxler, T.; Gai, F. Can glycine betaine denature proteins? Phys. Chem. Chem. Phys. 2020, 22, 7794–7802. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Dell’Aversana, E.; Carillo, P. Spatial and Temporal Profile of Glycine Betaine Accumulation in Plants Under Abiotic Stresses. Front. Plant Sci. 2019, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, H.; Jiang, H.; Xu, Y.; Cao, J.; Jiang, W. Multiple 1-MCP Treatment More Effectively Alleviated Postharvest Nectarine Chilling Injury than Conventional One-time 1-MCP Treatment by Regulating ROS and Energy Metabolism. Food Chem. 2020, 330, 127256. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Xu, T.; Farooq, S.U.; Jin, P.; Zheng, Y. Glycine Betaine Treatment Alleviates Chilling Injury in Zucchini Fruit (Cucurbita pepo L.) by Modulating Antioxidant Enzymes and Membrane Fatty Acid Metabolism. Postharvest Biol. Technol. 2018, 144, 20–28. [Google Scholar] [CrossRef]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative Stress and Antioxidant Metabolism under Adverse Environmental Conditions: A Review. Bot. Rev. 2020, 87, 421–466. [Google Scholar] [CrossRef]
- Habibi, F.; Guillén, F.; Serrano, M.; Valero, D. Postharvest Treatment with Glycine Betaine Enhances Chilling Tolerance of Blood Orange Fruit by Increasing Antioxidant Defence Systems and Osmoregulation during Cold Storage. Sci. Hortic. 2022, 305, 111352. [Google Scholar] [CrossRef]
- Meitha, K.; Pramesti, Y.; Suhandono, S. Reactive Oxygen Species and Antioxidants in Postharvest Vegetables and Fruits. Int. J. Food. Sci. 2020, 2020, 8817778. [Google Scholar] [CrossRef]
- Slesak, I.; Libik, M.; Karpinska, B.; Karpinski, S.; Miszalski, Z. The Role of Hydrogen Peroxide in Regulation of Plant Metabolism and Cellular Signalling in Response to Environmental Stresses. Acta Biochim. Pol. 2007, 54, 39–50. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, L.; Yang, H.; Wang, A. Effect of Exogenous Glycine Betaine on Qualities of Button Mushrooms (Agaricus bisporus) during Postharvest Storage. Eur. Food Res. Technol. 2014, 240, 41–48. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.J.; Chen, J.B.; Cao, J.P.; Li, X.; Sun, C.D. Citrus Flavonoids and Their Antioxidant Evaluation. Crit. Rev. Food Sci. Nutr. 2022, 62, 3833–3854. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S. Identification of Phenolic Compositions and the Antioxidant Capacity of Mandarin Juices and Wines. J. Food Sci. Technol. 2014, 51, 1094–1101. [Google Scholar] [CrossRef]
- Wang, T.; Xiong, B.; Zheng, Z.; Qin, Z.; Deng, L.; Zheng, W.; Zhang, M.; Sun, G.; He, S.; Wang, J.; et al. Natural Variation Confers ‘Aiyuan 38’ Citrus Mutant a New Color and Unique Flavor. Int. J. Mol. Sci. 2023, 24, 8816. [Google Scholar] [CrossRef] [PubMed]
- Sdiri, S.; Bermejo, A.; Aleza, P.; Navarro, P.; Salvador, A. Phenolic Composition, Organic Acids, Sugars, Vitamin C and Antioxidant Activity in the Juice of Two New Triploid Late-season Mandarins. Food Res. Int. 2012, 49, 462–468. [Google Scholar] [CrossRef]
- Baswal, A.K.; Dhaliwal, H.S.; Gill, K.S.; Ozturk, B. Impact of Modified Atmospheric Packaging Films on Health-Promoting Compounds in Cold-Stored ‘Kinnow’ Mandarin (Citrus nobilis Lour × C. deliciosa Tenora) Fruit. Erwerbs-Obstbau 2022, 65, 899–904. [Google Scholar] [CrossRef]
- Deng, L.; Wang, T.; Hu, J.; Yang, X.; Yao, Y.; Jin, Z.; Huang, Z.; Sun, G.; Xiong, B.; Liao, L.; et al. Effects of Pollen Sources on Fruit Set and Fruit Characteristics of ‘Fengtangli’ Plum (Prunus salicina Lindl.) Based on Microscopic and Transcriptomic Analysis. Int. J. Mol. Sci. 2022, 23, 12959. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Wang, T.; Liu, M.; Xu, X.; Wang, J.; Sun, G.; He, S.; Liao, L.; Xiong, B.; Wang, X.; et al. Effects of Exogenous Application of Glycine Betaine Treatment on ‘Huangguoggan’ Fruit during Postharvest Storage. Int. J. Mol. Sci. 2023, 24, 14316. https://doi.org/10.3390/ijms241814316
Zheng Z, Wang T, Liu M, Xu X, Wang J, Sun G, He S, Liao L, Xiong B, Wang X, et al. Effects of Exogenous Application of Glycine Betaine Treatment on ‘Huangguoggan’ Fruit during Postharvest Storage. International Journal of Molecular Sciences. 2023; 24(18):14316. https://doi.org/10.3390/ijms241814316
Chicago/Turabian StyleZheng, Zhendong, Tie Wang, Miaoyi Liu, Xiaozhu Xu, Jun Wang, Guochao Sun, Siya He, Ling Liao, Bo Xiong, Xun Wang, and et al. 2023. "Effects of Exogenous Application of Glycine Betaine Treatment on ‘Huangguoggan’ Fruit during Postharvest Storage" International Journal of Molecular Sciences 24, no. 18: 14316. https://doi.org/10.3390/ijms241814316
APA StyleZheng, Z., Wang, T., Liu, M., Xu, X., Wang, J., Sun, G., He, S., Liao, L., Xiong, B., Wang, X., He, J., Wang, Z., & Zhang, M. (2023). Effects of Exogenous Application of Glycine Betaine Treatment on ‘Huangguoggan’ Fruit during Postharvest Storage. International Journal of Molecular Sciences, 24(18), 14316. https://doi.org/10.3390/ijms241814316





