BrrA02.LMI1 Encodes a Homeobox Protein That Affects Leaf Margin Development in Brassica rapa
Abstract
1. Introduction
2. Results
2.1. Candidate Gene Identification Underlying the qBrrLLA02 Locus
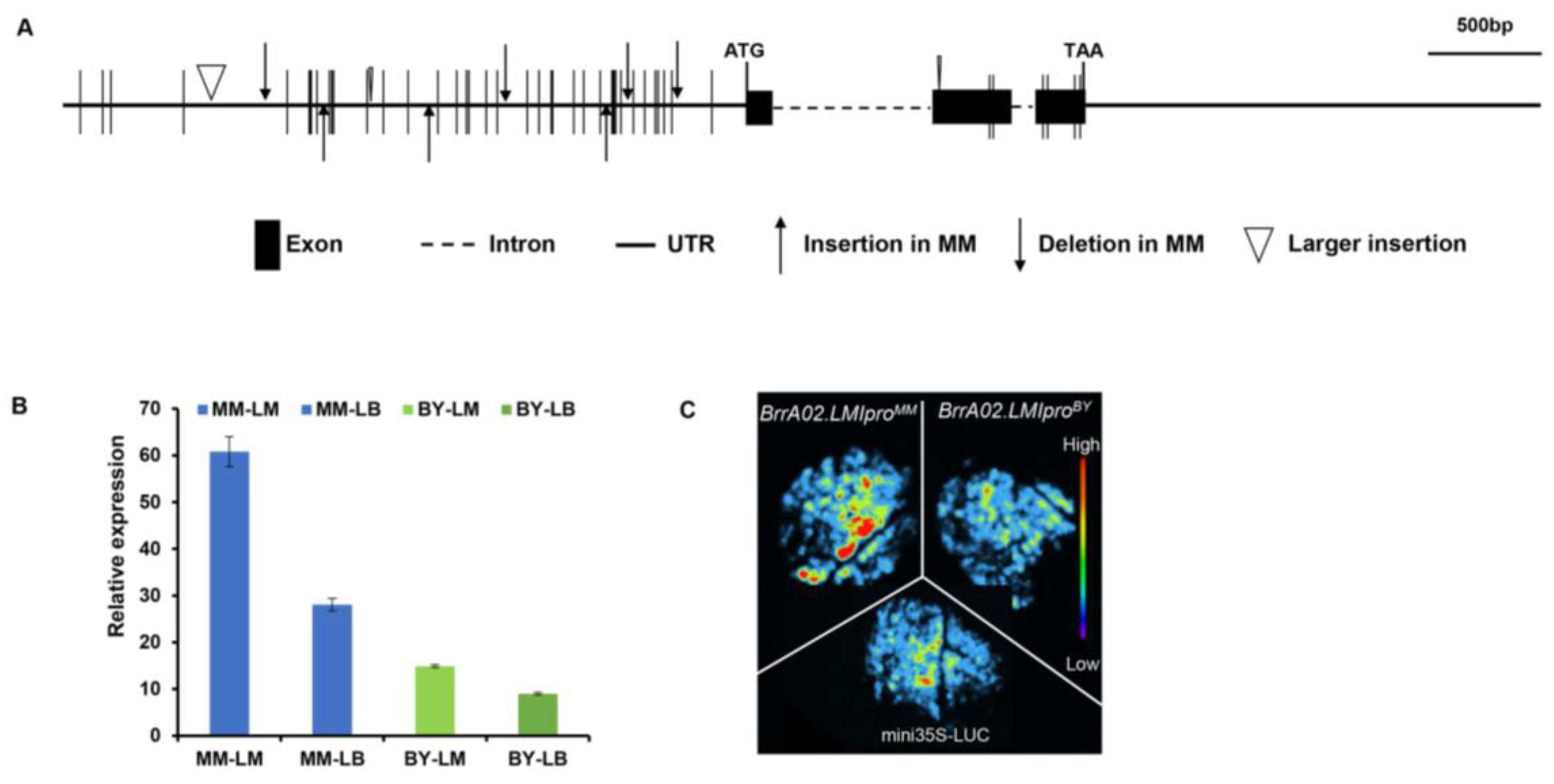
2.2. Genotyping Analysis of BrrA02.LMI1 in B. rapa
2.3. Tissue-Specific Expression Patterns of BrrA02.LMI1 in B. rapa
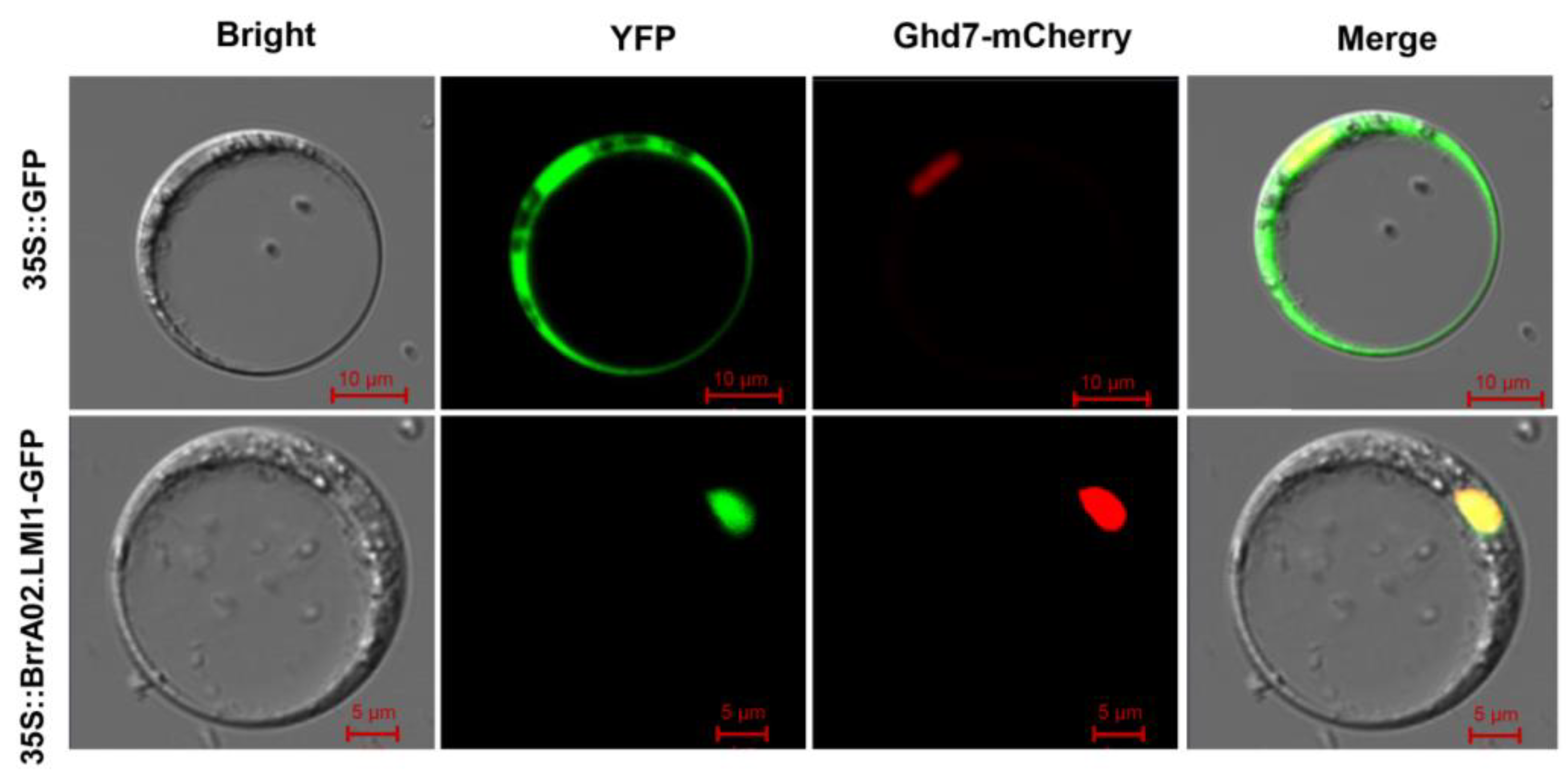
2.4. Subcellular Localization of BrrA02.LMI1
2.5. Function of BrrA02.LMI1 in Regulating Lobed Leaf Formation
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. RNA Extraction and Expression Analysis of BrrA02.LMI1
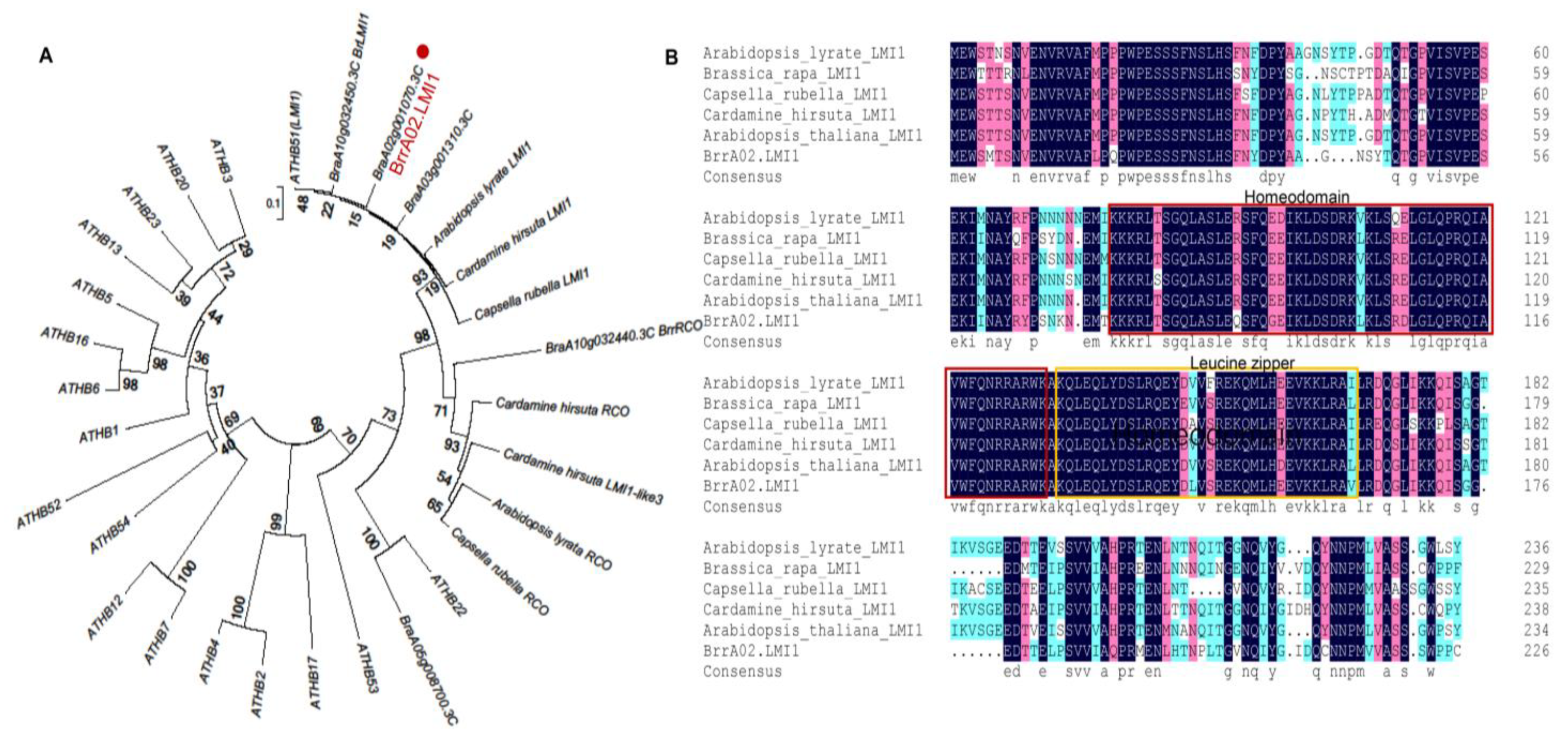
4.3. Phylogenetic Analysis of BrrA02.LMI1
4.4. Subcellular Localization of BrrA02.LMI1
4.5. GUS Staining
4.6. Functional Analysis of BrrA02.LMI1
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Liu, X.; Ji, X.; Zhang, L.; Liu, Y.; Lv, X.; Feng, H. Identification and validation of a major QTL controlling the presence/absence of leaf lobes in Brassica rapa L. Euphytica 2015, 205, 761–771. [Google Scholar] [CrossRef]
- Nikolov, L.A.; Runions, A.; Das Gupta, M.; Tsiantis, M. Leaf development and evolution. Curr. Top. Dev. Biol. 2019, 131, 109–139. [Google Scholar] [PubMed]
- Ren, J.; Liu, Z.; Du, J.; Fu, W.; Hou, A.; Feng, H. Fine-mapping of a gene for the lobed leaf, BoLl, in ornamental kale (Brassica oleracea L. var. acephala). Mol. Breed. 2019, 39, 40. [Google Scholar] [CrossRef]
- Peppe, D.J.; Royer, D.L.; Cariglino, B.; Oliver, S.Y.; Newman, S.; Leight, E.; Enikolopov, G.; Fernandez-Burgos, M.; Herrera, F.; Adams, J.M.; et al. Sensitivity of leaf size and shape to climate: Global patterns and paleoclimatic applications. New Phytol. 2011, 190, 724–739. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Hake, S. How a leaf gets its shape. Curr. Opin. Plant Biol. 2011, 14, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Dengler, N.G.; Tsukaya, H. Leaf morphogenesis in dicotyledons. Int. J. Plant Sci. 2001, 162, 459–464. [Google Scholar] [CrossRef]
- Hagemann, W.; Gleissberg, S. Organogenetic capacity of leaves: The significance of marginal blastozones in angiosperms. Plant Syst. Evol. 1996, 199, 121–152. [Google Scholar] [CrossRef]
- Sluis, A.; Hake, S. Organogenesis in plants: Initiation and elaboration of leaves. Trends Genet. 2015, 31, 300–306. [Google Scholar] [CrossRef]
- Sedivy, E.J.; Wu, F.; Hanzawa, Y. Soybean domestication: The origin, genetic architecture and molecular bases. New Phytol. 2017, 214, 539–553. [Google Scholar] [CrossRef]
- Satterlee, J.W.; Scanlon, M.J. Coordination of leaf development across developmental axes. Plants 2019, 8, 433. [Google Scholar] [CrossRef]
- Rowland, S.D.; Zumstein, K.; Nakayama, H.; Cheng, Z.; Flores, A.M.; Chitwood, D.H.; Maloof, J.N.; Sinha, N.R. Leaf shape is a predictor of fruit quality and cultivar performance in tomato. New Phytol. 2020, 226, 851–865. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zhang, Y.; Li, H.; Fu, X.; Shang, H.; Zou, C.; Friml, J.; Xiao, G. GhARF16-1 modulates leaf development by transcriptionally regulating the GhKNOX2-1 gene in cotton. Plant Biotechnol. J. 2021, 19, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Thomas, J.; Collins, G.; Timmermans, M.C. Direct repression of KNOX loci by the ASYMMETRIC LEAVES1 complex of Arabidopsis. Plant Cell 2008, 20, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Luo, F.; Hochholdinger, F. LOB domain proteins: Beyond lateral organ boundaries. Trends Plant Sci. 2016, 21, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Weir, I.; Lu, J.; Cook, H.; Causier, B.; Schwarz-Sommer, Z.; Davies, B. CUPULIFORMIS establishes lateral organ boundaries in Antirrhinum. Development 2004, 131, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Hasson, A.; Plessis, A.; Blein, T.; Adroher, B.; Grigg, S.; Tsiantis, M.; Boudaoud, A.; Damerval, C.; Laufs, P. Evolution and diverse roles of the CUP-SHAPED COTYLEDON genes in Arabidopsis leaf development. Plant Cell 2011, 23, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Blein, T.; Pulido, A.; Vialette-Guiraud, A.; Nikovics, K.; Morin, H.; Hay, A.; Johansen, I.; Tsiantis, M.; Laufs, P. A conserved molecular framework for compound leaf development. Science 2008, 322, 1835–1838. [Google Scholar] [CrossRef]
- Bilsborough, G.D.; Runions, A.; Barkoulas, M.; Jenkins, H.W.; Hasson, A.; Galinha, C.; Laufs, P.; Hay, A.; Prusinkiewicz, P.; Tsiantis, M. Model for the regulation of Arabidopsis thaliana leaf margin development. Proc. Natl. Acad. Sci. USA 2011, 108, 3424–3429. [Google Scholar] [CrossRef]
- Monniaux, M.; Mc Kim, S.M.; Cartolano, M.; Thevenon, E.; Parcy, F.; Tsiantis, M.; Hay, A. Conservation vs divergence in LEAFY and APETALA1 functions between Arabidopsis thaliana and Cardamine hirsuta. New Phytol. 2017, 216, 549–561. [Google Scholar] [CrossRef]
- Vlad, D.; Kierzkowski, D.; Rast, M.I.; Vuolo, F.; Ioio, R.D.; Galinha, C.; Gan, X.; Hajheidari, M.; Hay, A.; Smith, R.S.; et al. Leaf shape evolution through duplication, regulatory diversification, and loss of a homeobox gene. Science 2014, 343, 780–783. [Google Scholar] [CrossRef]
- Andres, R.J.; Coneva, V.; Frank, M.H.; Tuttle, J.R.; Samayoa, L.F.; Han, S.-W.; Kaur, B.; Zhu, L.; Fang, H.; Bowman, D.T.; et al. Modifications to a LATE MERISTEM IDENTITY1 gene are responsible for the major leaf shapes of upland cotton (Gossypium hirsutum L.). Proc. Natl. Acad. Sci. USA 2017, 114, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, M.; Cubas, P. TCP factors: New kids on the signaling block. Curr. Opin. Plant Biol. 2016, 33, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Challa, K.R.; Aggarwal, P.; Nath, U. Activation of YUCCA5 by the transcription factor TCP4 integrates developmental and environmental signals to promote hypocotyl elongation in Arabidopsis. Plant Cell 2016, 28, 2117–2130. [Google Scholar] [CrossRef] [PubMed]
- Kougioumoutzi, E.; Cartolano, M.; Canales, C.; Dupré, M.; Bramsiepe, J.; Vlad, D.; Rast, M.; Ioio, R.D.; Tattersall, A.; Schnittger, A.; et al. SIMPLE LEAF3 encodes a ribosome-associated protein required for leaflet development in Cardamine hirsuta. Plant J. 2013, 73, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Jost, M.; Taketa, S.; Mascher, M.; Himmelbach, A.; You, T.; Shahinnia, F.; Rutten, T.; Druka, A.; Schmutzer, T.; Steuernagel, B.; et al. A homolog of Blade-On-Petiole 1 and 2 (BOP1/2) controls internode length and homeotic changes of the barley inflorescence. Plant Physiol. 2016, 171, 1113–1127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Su, W.; Tao, R.; Zhang, W.; Chen, J.; Wu, P.; Yan, C.; Jia, Y.; Larkin, R.M.; Lavelle, D.; et al. RNA sequencing provides insights into the evolution of lettuce and the regulation of flavonoid biosynthesis. Nat. Commun. 2017, 8, 2264. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Byrne, M. SAW homeodomain transcription factors regulate initiation of leaf margin serrations. J. Exp. Bot. 2021, 72, 1738–1747. [Google Scholar] [CrossRef]
- Rubio-Somoza, I.; Zhou, C.-M.; Confraria, A.; Martinho, C.; von Born, P.; Baena-Gonzalez, E.; Wang, J.-W.; Weigel, D. Temporal control of leaf complexity by mi RNA-regulated licensing of protein complexes. Curr. Biol. 2014, 24, 2714–2719. [Google Scholar] [CrossRef]
- Chitwood, D.; Sinha, N. Plant development: Small RNAs and the metamorphosis of leaves. Curr. Biol. 2014, 24, R1087–R1089. [Google Scholar] [CrossRef][Green Version]
- Rast, M.I.; Simon, R. Arabidopsis JAGGED LATERAL ORGANS acts with ASYMMETRIC LEAVES2 to coordinate KNOX and PIN expression in shoot and root meristems. Plant Cell 2012, 24, 2917–2933. [Google Scholar] [CrossRef]
- Ding, L.; Yan, S.; Jiang, L.; Zhao, W.; Ning, K.; Zhao, J.; Liu, X.; Zhang, J.; Wang, Q.; Zhang, X. HANABA TARANU (HAN) bridges meristem and organ primordia boundaries through PINHEAD, JAGGED, BLADE-ON-PETIOLE2 and CYTOKININ OXIDASE 3 during flower development in Arabidopsis. PLoS Genet. 2015, 11, e1005479. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, H.; Yang, Q.; Meng, Q.; Han, S.; Nwafor, C.C.; Khan, M.H.U.; Fan, C.; Zhou, Y. Promoter variations in a homeobox gene, BnA10.LMI1, determine lobed leaves in rapeseed (Brassica napus L.). Theor. Appl. Genet. 2018, 131, 2699–2708. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhang, H.; Sun, Y.; Shen, X.; Amoo, O.; Wang, Y.; Fan, C.; Zhou, Y. BnA10.RCO, a homeobox gene, positively regulates leaf lobe formation in Brassica napus L. Theor. Appl. Genet. 2020, 133, 3333–3343. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, X.; Yang, X.R.; Zhu, P.F. Fine mapping and identification of the leaf shape gene BoFL in ornamental kale. Theor. Appl. Genet. 2020, 133, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Yang, X.; Zhong, M.; Li, X.; Zhu, P. BoALG10, an α-1,2 glycosyltransferase, plays an essential role in maintaining leaf margin shape in ornamental kale. Hortic. Res. 2022, 9, uhac137. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Su, T.; Li, H.; Wu, Y.; Wang, L.; Zhang, F.; Yu, S.; Wang, Z. BrrRCO, encoding a homeobox protein, is involved in leaf lobe development in Chinese cabbage. bioRxiv 2023. [Google Scholar] [CrossRef]
- Saddic, L.; Huvermann, B.; Bezhani, S.; Su, Y.; Winter, C.; Kwon, C.; Collum, R.; Wagner, D. The LEAFY target LMI1 is a meristem identity regulator and acts together with LEAFY to regulate expression of CAULIFLOWER. Development 2006, 133, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Pflieger, S.; Blanchet, S.; Camborde, L.; Drugeon, G.; Rousseau, A.; Noizet, M.; Planchais, S.; Planchais, S.; Jupin, T. Efficient virus-induced gene silencing in Arabidopsis using a ‘one-step’ TYMV-derived vector. Plant J. 2008, 56, 678–690. [Google Scholar] [CrossRef]
- Yu, J.; Yang, X.-D.; Wang, Q.; Gao, L.-W.; Yang, Y.; Xiao, D.; Liu, T.-K.; Li, Y.; Hou, X.-L.; Zhang, C.-W. Efficient virus-induced gene silencing in Brassica rapa using a turnip yellow mosaic virus vector. Biol. Plant. 2018, 62, 826–834. [Google Scholar] [CrossRef]
- Shopan, J.; Mou, H.P.; Zhang, L.L.; Zhang, C.T.; Ma, W.W.; Walsh, J.A.; Hu, Z.Y.; Yang, J.H.; Zhang, M.F. Eukaryotic translation initiation factor 2B-beta (eIF2B beta), a new class of plant virus resistance gene. Plant J. 2017, 90, 929–940. [Google Scholar] [CrossRef]
- Muntha, S.; Zhang, L.; Zhou, Y.; Zhao, X.; Hum, Z.; Yang, J.; Zhang, M. Phytochrome A signal transducton 1 and constans-like 13 coordinately orchestrate shoot branching and flowering in leafy Brassica Juncea. Plant Biotechnol. J. 2019, 17, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.; Garraway, J.; Cai, Q.; Zhou, Y.; Li, X.; Hu, Z.; Zhang, M.; Yang, J. The casein kinase 2 β subunit CK2B1 is required for swollen stem formation via cell cycle control in vegetable Brassica juncea. Plant J. 2020, 104, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Bo, K.; Duan, Y.; Qiu, X.; Zhang, M.; Shu, Q.; Sun, Y.; He, Y.; Shi, Y.; Weng, Y.; Wang, C. Promoter variation in a homeobox gene, CpDll, is associated with deeply lobed leaf in Cucurbita pepo L. Theor. Appl. Genet. 2022, 135, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Efroni, I.; Eshed, Y.; Lifschitz, E. Morphogenesis of Simple and Compound Leaves: A Critical Review. Plant Cell 2010, 22, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Floyd, S.; Bowman, J. Gene expression patterns in seed plant shoot meristems and leaves: Homoplasy or homology? J. Plant Res. 2010, 123, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Vuolo, F.; Kierzkowski, D.; Runions, A.; Hajheidari, M.; Mentink, R.A.; Das Gupta, M.; Zhang, Z.; Vlad, D.; Wang, Y.; Pecinka, A.; et al. Corrigendum: LMI1 homeodomain protein regulates organ proportions by spatial modulation of endoreduplication. Genes Dev. 2018, 33, 377. [Google Scholar] [CrossRef] [PubMed]
- Hofer, J.; Turner, L.; Moreau, C.; Ambrose, M.; Isaac, P.; Butcher, S.; Weller, J.; Dupin, A.; Dalmais, M.; Le Signor, C.; et al. Tendril-less regulates tendril formation in pea leaves. Plant Cell 2009, 21, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Dezar, C.A.; Gago, G.M.; González, D.H.; Chan, R.L. Hahb-4, a sunflower homeobox-leucine zipper gene, is a developmental regulator and confers drought tolerance to Arabidopsis thaliana plants. Transgenic Res. 2005, 14, 429–440. [Google Scholar] [CrossRef]
- Hanson, J.; Johannesson, H.; Engström, P. Sugar-dependent alterations in cotyledon and leaf development in transgenic plants expressing the HDZhdip gene ATHB13. Plant Mol. Biol. 2001, 45, 247–262. [Google Scholar] [CrossRef]
- Wang, Y.; Henriksson, E.; Söderman, E.; Henriksson, K.N.; Sundberg, E.; Engström, P. The Arabidopsis homeobox gene, ATHB16, regulates leaf development and the sensitivity to photoperiod in Arabidopsis. Dev. Biol. 2003, 264, 228–239. [Google Scholar] [CrossRef]
- Qi, J.; Yu, S.; Zhang, F.; Shen, X.; Zhao, X.; Yu, Y.; Zhang, D. Reference gene selection for real time quantitative polymerase chain reaction of mRNA transcript levels in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Plant Mol. Biol. Rep. 2010, 28, 597–604. [Google Scholar] [CrossRef]
- Niu, F.; Ji, C.; Liang, Z.; Guo, R.; Chen, Y.; Zeng, Y.; Jiang, L. ADP-ribosylation factor D1 modulates Golgi morphology, cell plate formation, and plant growth in Arabidopsis. Plant Physiol. 2022, 190, 1199–1213. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Wu, Y.; Han, X.; Li, H.; Wang, L.; Chen, B.; Yu, S.; Wang, Z. BrrA02.LMI1 Encodes a Homeobox Protein That Affects Leaf Margin Development in Brassica rapa. Int. J. Mol. Sci. 2023, 24, 14205. https://doi.org/10.3390/ijms241814205
Li P, Wu Y, Han X, Li H, Wang L, Chen B, Yu S, Wang Z. BrrA02.LMI1 Encodes a Homeobox Protein That Affects Leaf Margin Development in Brassica rapa. International Journal of Molecular Sciences. 2023; 24(18):14205. https://doi.org/10.3390/ijms241814205
Chicago/Turabian StyleLi, Pan, Yudi Wu, Xiangyang Han, Hui Li, Limin Wang, Bin Chen, Shuancang Yu, and Zheng Wang. 2023. "BrrA02.LMI1 Encodes a Homeobox Protein That Affects Leaf Margin Development in Brassica rapa" International Journal of Molecular Sciences 24, no. 18: 14205. https://doi.org/10.3390/ijms241814205
APA StyleLi, P., Wu, Y., Han, X., Li, H., Wang, L., Chen, B., Yu, S., & Wang, Z. (2023). BrrA02.LMI1 Encodes a Homeobox Protein That Affects Leaf Margin Development in Brassica rapa. International Journal of Molecular Sciences, 24(18), 14205. https://doi.org/10.3390/ijms241814205



