Alkylresorcinols as New Modulators of the Metabolic Activity of the Gut Microbiota
Abstract
:1. Introduction
2. Results
2.1. Estimation of AR Content in Germ-Free Mouse Faeces after FMT
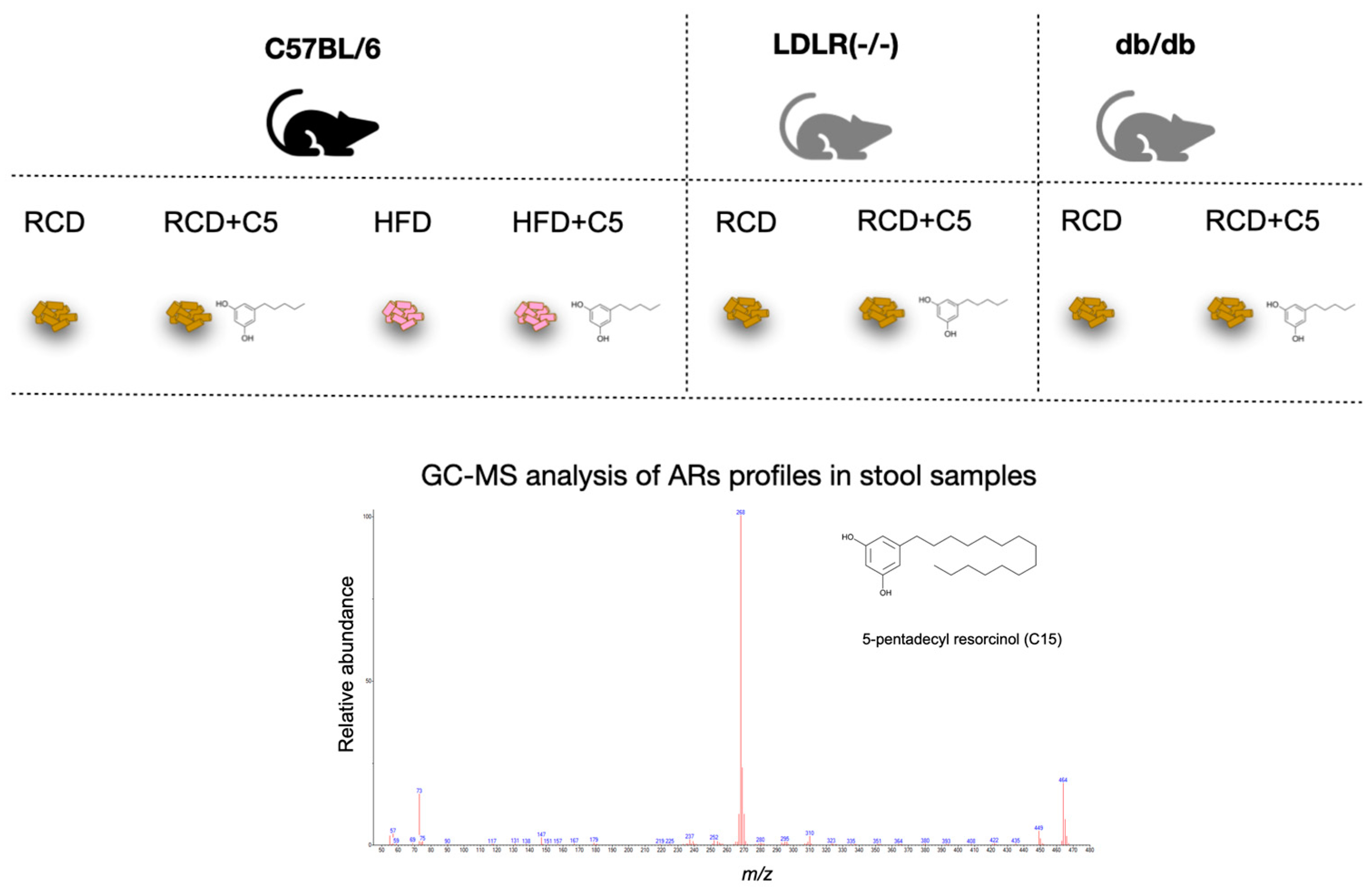
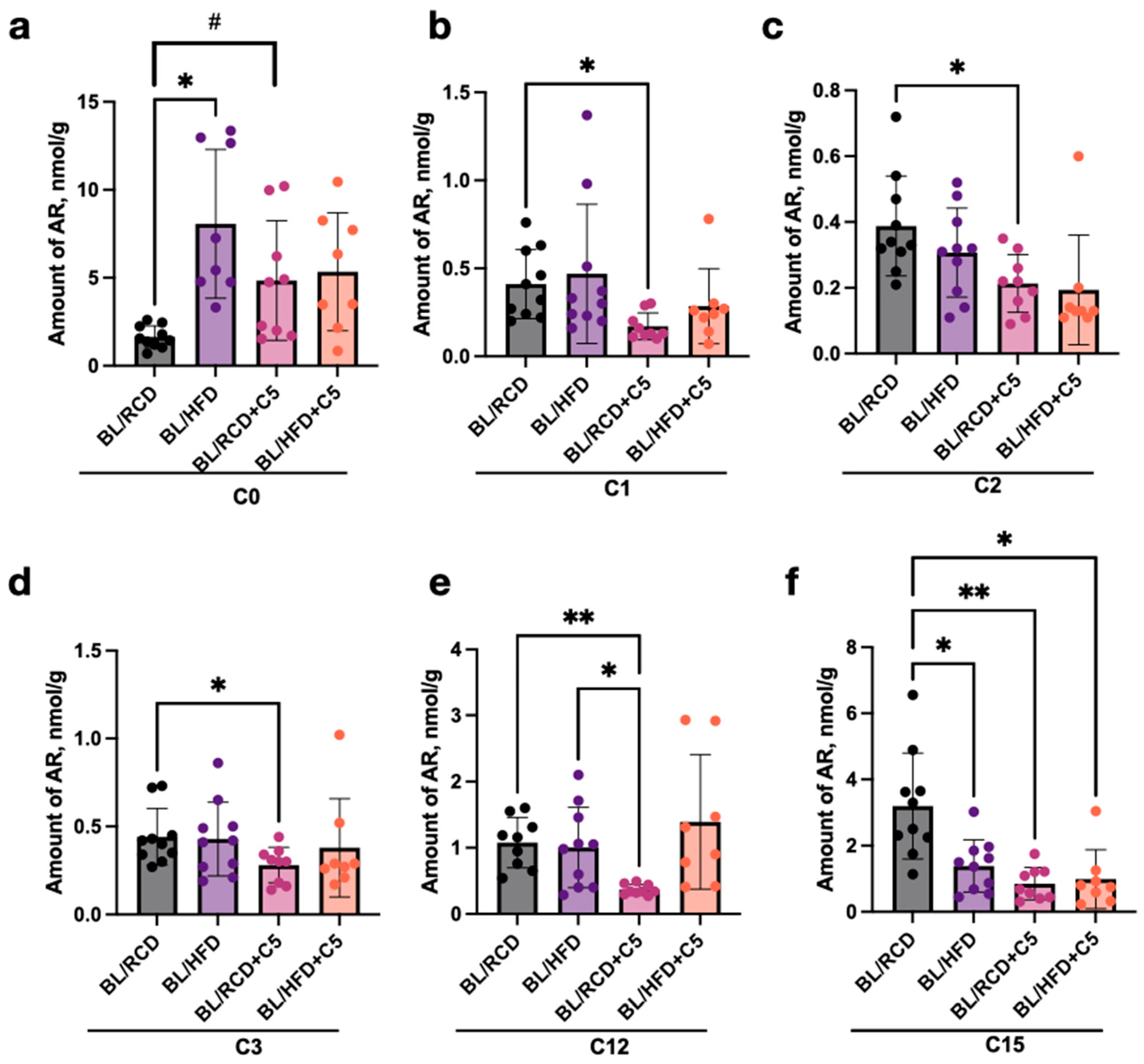
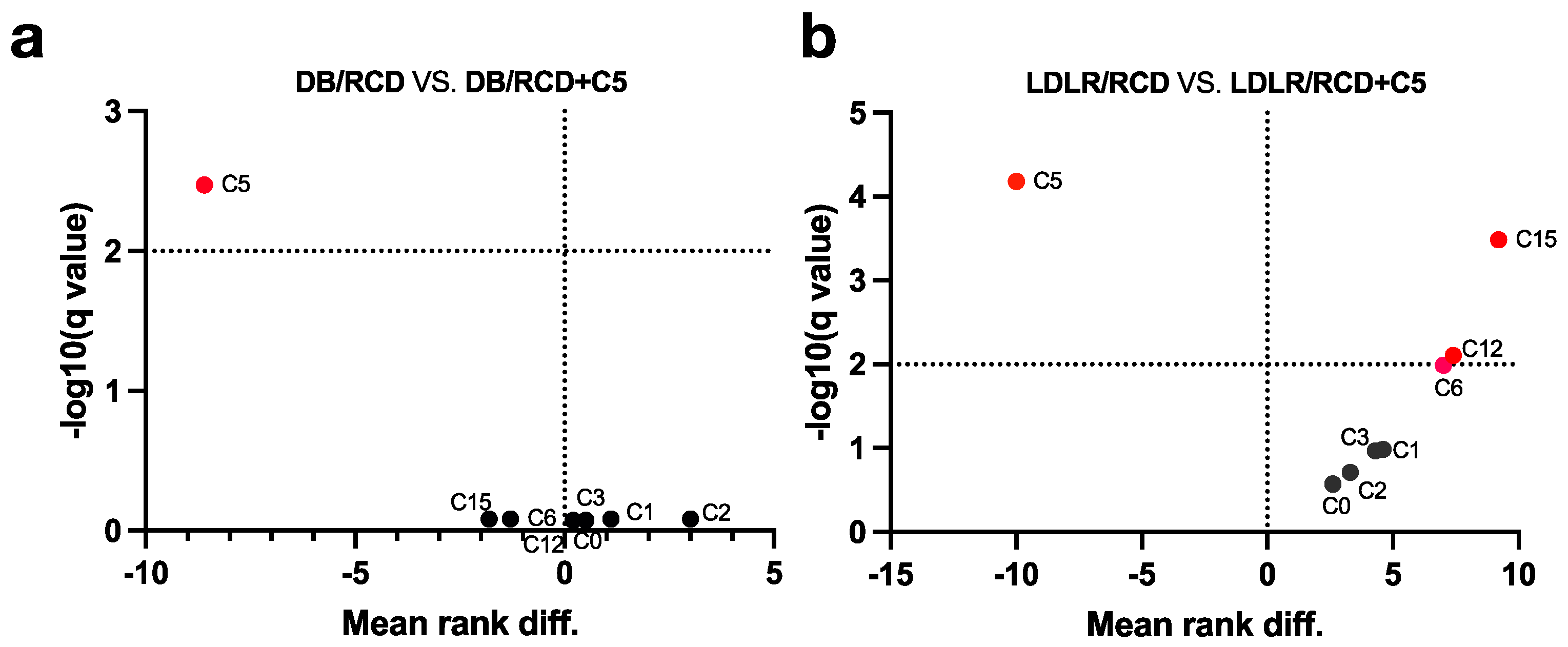
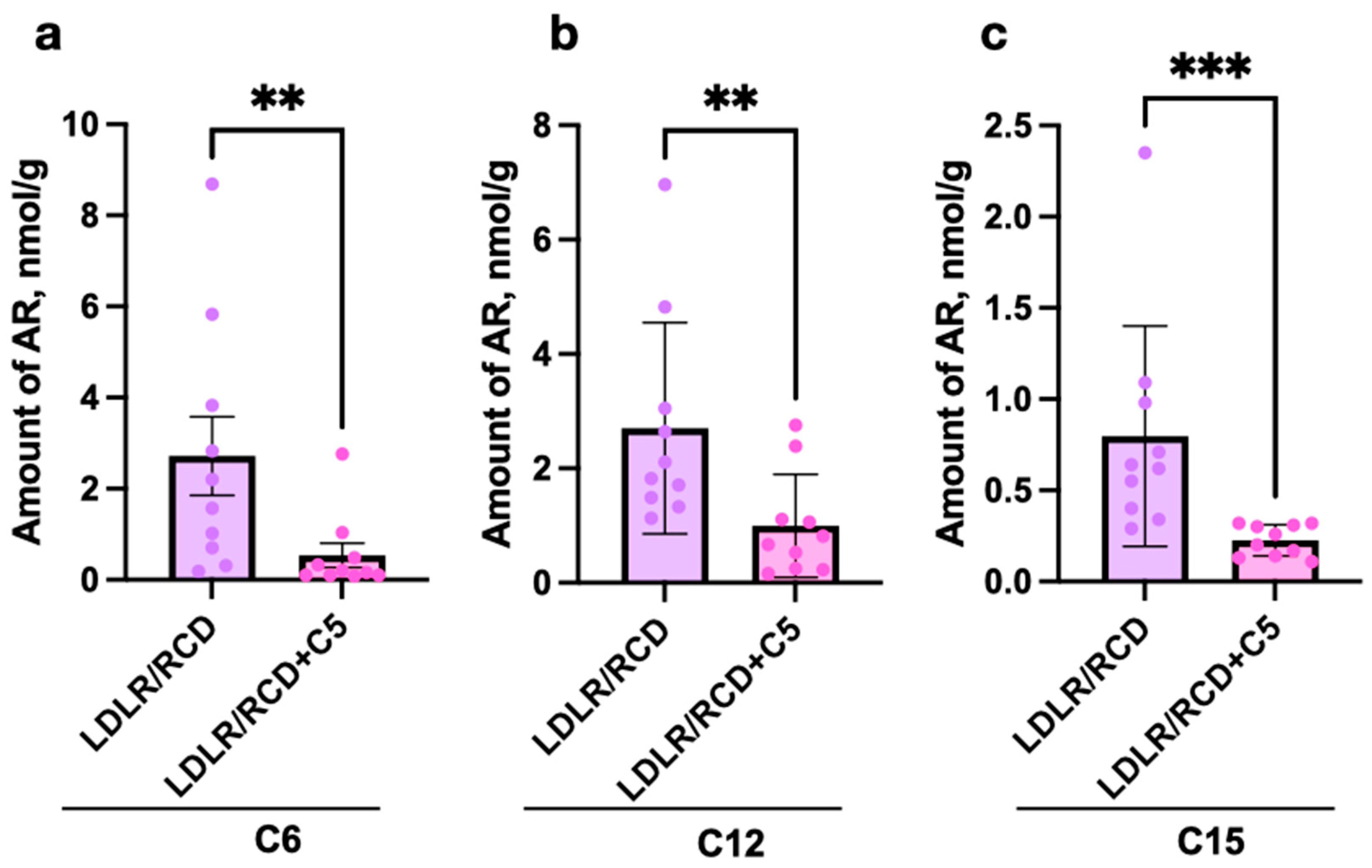
2.2. Estimation of AR Content in C57BL/6 and Db/Db and Ldlr (−/−) Mice’s Faeces Depending on Diet Content
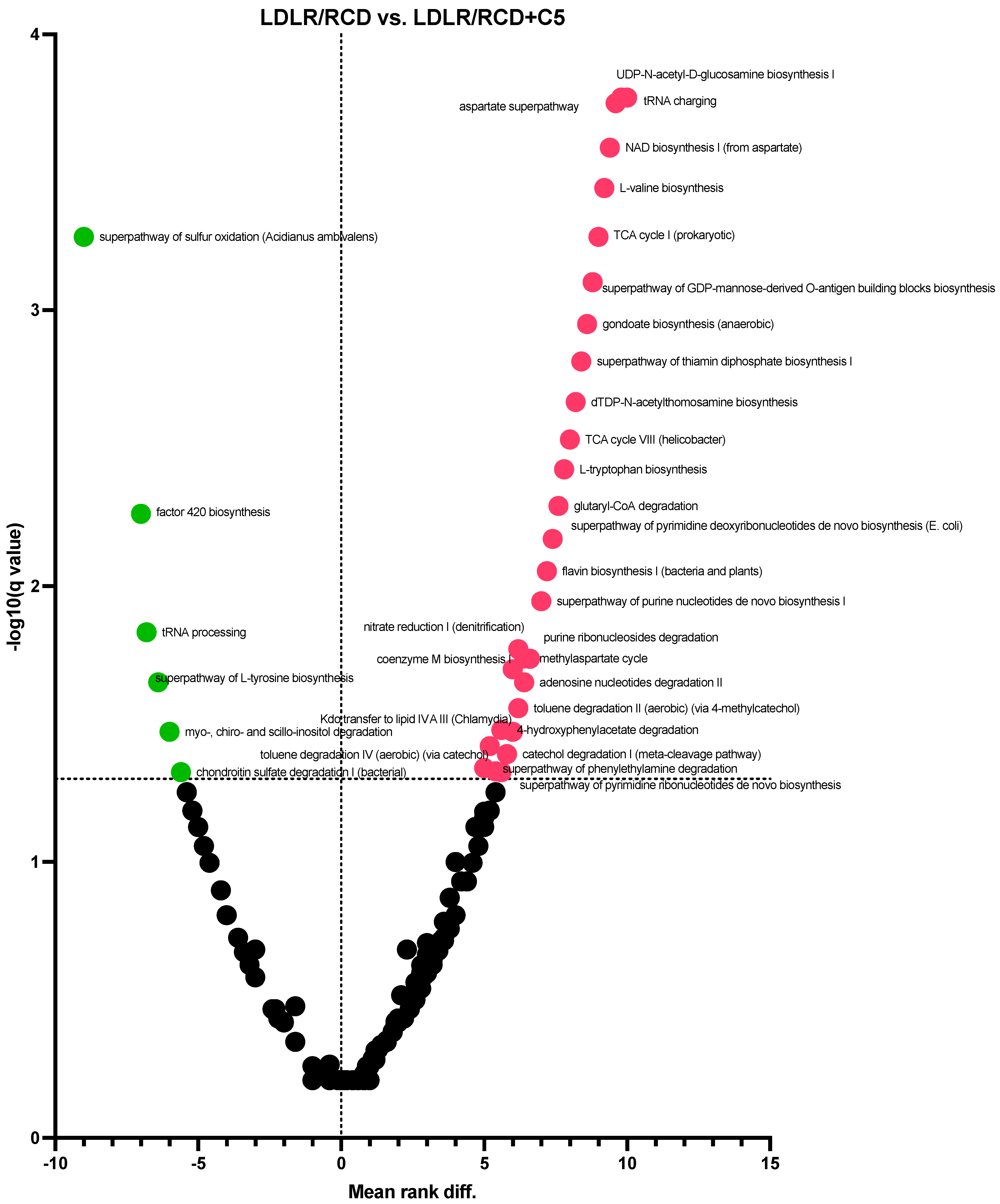
2.3. Prediction of Gut Microbiota Metabolic Activity
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Study Design
4.2. Faecal Microbiota Transplantation
4.3. Quantitative Analysis of ARs
4.4. High-Throughput Sequencing Analysis and Reconstruction of Gut Microbiota Metabolic Activity
4.5. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Zabolotneva, A.A.; Shatova, O.P.; Sadova, A.A.; Shestopalov, A.V.; Roumiantsev, S.A. An Overview of Alkylresorcinols Biological Properties and Effects. J. Nutr. Metab. 2022, 2022, 4667607. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Ogata, H.; Goto, S. Type III Polyketide Synthases: Functional Classification and Phylogenomics. Chem. Bio. Chem. 2017, 18, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, D.W.; Kim, S.-G.; Lee, S.K. 4-Hexylresorcinol-Induced Protein Expression Changes in Human Umbilical Cord Vein Endothelial Cells as Determined by Immunoprecipitation High-Performance Liquid Chromatography. PLoS ONE 2020, 15, e0243975. [Google Scholar] [CrossRef] [PubMed]
- Funabashi, M.; Funa, N.; Horinouchi, S. Phenolic Lipids Synthesized by Type III Polyketide Synthase Confer Penicillin Resistance on Streptomyces Griseus. J. Biol. Chem. 2008, 283, 13983–13991. [Google Scholar] [CrossRef]
- Ridley, C.P.; Lee, H.Y.; Khosla, C. Evolution of Polyketide Synthases in Bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 4595–4600. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.B.; Izumikawa, M.; Bowman, M.E.; Udwary, D.W.; Ferrer, J.-L.; Moore, B.S.; Noel, J.P. Crystal Structure of a Bacterial Type III Polyketide Synthase and Enzymatic Control of Reactive Polyketide Intermediates. J. Biol. Chem. 2004, 279, 45162–45174. [Google Scholar] [CrossRef]
- Anand, A.; Verma, P.; Singh, A.K.; Kaushik, S.; Pandey, R.; Shi, C.; Kaur, H.; Chawla, M.; Elechalawar, C.K.; Kumar, D.; et al. Polyketide Quinones Are Alternate Intermediate Electron Carriers during Mycobacterial Respiration in Oxygen-Deficient Niches. Mol. Cell 2015, 60, 637–650. [Google Scholar] [CrossRef]
- Stasiuk, M.; Kozubek, A. Biological Activity of Phenolic Lipids. Cell. Mol. Life Sci. 2010, 67, 841–860. [Google Scholar] [CrossRef]
- Kozubek, A.; Tyman, J.H.P. Resorcinolic Lipids, the Natural Non-Isoprenoid Phenolic Amphiphiles and Their Biological Activity. Chem. Rev. 1999, 99, 1–26. [Google Scholar] [CrossRef]
- Aura, A.-M. Microbial Metabolism of Dietary Phenolic Compounds in the Colon. Phytochem. Rev. 2008, 7, 407–429. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of Dietary Compounds, Especially Polyphenols, with the Intestinal Microbiota: A Review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Lampe, J.; Chang, J. Interindividual Differences in Phytochemical Metabolism and Disposition. Semin. Cancer Biol. 2007, 17, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Rechner, A. Colonic Metabolism of Dietary Polyphenols: Influence of Structure on Microbial Fermentation Products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Nikolaev, Y.A.; Tutel’yan, A.; Loiko, N.G.; Buck, J.; Sidorenko, S.V.; Lazareva, I.; Gostev, V.; Manzen’yuk, O.Y.; Shemyakin, I.G.; Abramovich, R.A.; et al. The Use of 4-Hexylresorcinol as Antibiotic Adjuvant. PLoS ONE 2020, 15, e0239147. [Google Scholar] [CrossRef]
- Bukharin, O.; Perunova, N.B.; El’-Registan, G.I.; Nikolaev, I.A.; Iavnova, S.V.; Molostov, E.V.; Kirillov, D.A. Influence of Chemical Analogue of Extracellular Microbial Autoregulators on Antilysozyme Activity of Bacteria. Zh Mikrobiol. Epidemiol. Immunobiol. 2007, 6, 3–6. [Google Scholar]
- Oishi, K.; Yamamoto, S.; Itoh, N.; Nakao, R.; Yasumoto, Y.; Tanaka, K.; Kikuchi, Y.; Fukudome, S.; Okita, K.; Takano-Ishikawa, Y. Wheat Alkylresorcinols Suppress High-Fat, High-Sucrose Diet-Induced Obesity and Glucose Intolerance by Increasing Insulin Sensitivity and Cholesterol Excretion in Male Mice. J. Nutr. 2015, 145, 199–206. [Google Scholar] [CrossRef]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of Polyphenols and Polyphenol-Rich Dietary Sources on Gut Microbiota Composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef]
- Cascio, M.G.; Bisogno, T.; Palazzo, E.; Thomas, A.; van der Stelt, M.; Brizzi, A.; de Novellis, V.; Marabese, I.; Ross, R.; van de Doelen, T.; et al. In vitro and in vivo Pharmacology of Synthetic Olivetol- or Resorcinol-Derived Cannabinoid Receptor Ligands. Br. J. Pharmacol. 2006, 149, 431–440. [Google Scholar] [CrossRef]
- Piomelli, D. The Molecular Logic of Endocannabinoid Signalling. Nat. Rev. Neurosci. 2003, 4, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, T. Recent Development of CB2 Selective and Peripheral CB1/CB2 Cannabinoid Receptor Ligands. Curr. Med. Chem. 2013, 21, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Cuddihey, H.; MacNaughton, W.K.; Sharkey, K.A. Role of the Endocannabinoid System in the Regulation of Intestinal Homeostasis. Cell Mol. Gastroenterol. Hepatol. 2022, 14, 947–963. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, S.; Pechereau, F.; Leblanc, N.; Boubertakh, B.; Houde, A.; Martin, C.; Flamand, N.; Silvestri, C.; Raymond, F.; Di Marzo, V.; et al. Rapid and Concomitant Gut Microbiota and Endocannabinoidome Response to Diet-Induced Obesity in Mice. mSystems 2019, 4, 1110–1128. [Google Scholar] [CrossRef]
- Mehrpouya-Bahrami, P.; Chitrala, K.N.; Ganewatta, M.S.; Tang, C.; Murphy, E.A.; Enos, R.T.; Velazquez, K.T.; McCellan, J.; Nagarkatti, M.; Nagarkatti, P. Blockade of CB1 Cannabinoid Receptor Alters Gut Microbiota and Attenuates Inflammation and Diet-Induced Obesity. Sci. Rep. 2017, 7, 15645. [Google Scholar] [CrossRef]
- Tahir, M.N.; Shahbazi, F.; Rondeau-Gagné, S.; Trant, J.F. The Biosynthesis of the Cannabinoids. J. Cannabis Res. 2021, 3, 7. [Google Scholar] [CrossRef]
- Zagzoog, A.; Cabecinha, A.; Abramovici, H.; Laprairie, R.B. Modulation of Type 1 Cannabinoid Receptor Activity by Cannabinoid By-Products from Cannabis Sativa and Non-Cannabis Phytomolecules. Front. Pharmacol. 2022, 13, 956030. [Google Scholar] [CrossRef]
- James, J. Carberry Composition of Olivetol and Method of Use to Reduce or Inhibit the Effects of Tetrahydrocannabinol in the Human Body. 2017. Available online: https://patents.google.com/patent/WO2017091755A1/en (accessed on 12 September 2023).
- Yang, X.; Zhao, Z.; Zhao, C.; Li, Y.; El-kott, A.F.; Bani-Fwaz, M.Z. Anti-Breast Adenocarcinoma and Anti-Urease Anti-Tyrosinase Properties of 5-Pentylresorcinol as Natural Compound with Molecular Docking Studies. J Oleo Sci 2022, 71, ess22024. [Google Scholar] [CrossRef]
- Imai, S.; Ohama, M.; Suzuki, M.; Katayanagi, Y.; Kayashima, Y.; Tezuka, H. Research Article Short Alkyl Chain-Length Resorcinol Olivetol Protects Against Obesity with Mitochondrial Activation by Pgc-1α Deacetylation. Jpn. J. Food Chem. Safety 2023, 30, 68–81. [Google Scholar] [CrossRef]
- Yu, F.; Han, W.; Zhan, G.; Li, S.; Jiang, X.; Wang, L.; Xiang, S.; Zhu, B.; Yang, L.; Luo, A.; et al. Abnormal Gut Microbiota Composition Contributes to the Development of Type 2 Diabetes Mellitus in Db/Db Mice. Aging 2019, 11, 10454–10467. [Google Scholar] [CrossRef]
- Suriano, F.; Vieira-Silva, S.; Falony, G.; Roumain, M.; Paquot, A.; Pelicaen, R.; Régnier, M.; Delzenne, N.M.; Raes, J.; Muccioli, G.G.; et al. Novel Insights into the Genetically Obese (Ob/Ob) and Diabetic (Db/Db) Mice: Two Sides of the Same Coin. Microbiome 2021, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Pontarollo, G.; Kiouptsi, K.; Reinhardt, C. A Holobiont View on Thrombosis: Unravelling the Microbiota’s Influence on Arterial Thrombus Growth. Microb. Cell 2020, 7, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, E.; Kloosterhuis, N.; Dekker, D.; Koster, M.; Gijbels, M.; Van Der Velden, S.; de Bruin, A.; de Winther, M.; Westerterp, M.; Van De Sluis, B.; et al. Gut Microbiota Dysbiosis Augments Atherosclerosis in LDLR−/− Mice. Atherosclerosis 2017, 263, e97. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Rejman, J.; Kozubek, A. Long-Chain Orcinol Homologs from Cereal Bran Are Effective Inhibitors of Glycerophosphate Dehydrogenase. Cell. Mol. Biol. Lett. 1997, 2, 411–419. [Google Scholar]
- Shestopalov, A.V.; Gaponov, A.M.; Zabolotneva, A.A.; Appolonova, S.A.; Markin, P.A.; Borisenko, O.V.; Tutelyan, A.V.; Rumyantsev, A.G.; Teplyakova, E.D.; Shin, V.F.; et al. Alkylresorcinols: New Potential Bioregulators in the Superorganism System (Human–Microbiota). Biol. Bull. 2022, 49, 150–159. [Google Scholar] [CrossRef]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front Nutr. 2019, 6, 188. [Google Scholar] [CrossRef]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]
- Horikawa, K.; Hashimoto, C.; Kikuchi, Y.; Makita, M.; Oishi, K. Wheat Alkylresorcinol Increases Fecal Lipid Excretion and Suppresses Feed Efficiency in Mice Depending on Time of Supplementation. Nutrition 2022, 103–104, 111796. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clément, K. Metabolism and Metabolic Disorders and the Microbiome: The Intestinal Microbiota Associated With Obesity, Lipid Metabolism, and Metabolic Health—Pathophysiology and Therapeutic Strategies. Gastroenterology 2021, 160, 573–599. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]


| Superpathway of Sulfur Oxidation (Acidianus Ambivalens) | Reductive Acetyl Coenzyme A Pathway | Factor 420 Biosynthesis | Superpathway of Haem Biosynthesis from Uroporphyrinogen-III/Haem Biosynthesis II (Anaerobic) | Catechol Degradation | Thiamine Biosynthesis/Salvage | Methanogenesis from Acetate | Adenosylcobalamine Synthesis/Salvage | L-Histidine Degradation I | Mannan Degradation | Synthesis of Fatty Acids (Palmitate, Oleate, Stearate, etc.) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BL/RCD vs. BL/HFD |  |  |  |  |  |  |  |  |  |  | |
| BL/RCD vs. BL/RCD + C5 |  |  |  |  |  | ||||||
| BL/HFD vs. BL/HFD + C5 | 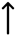 | 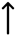 | |||||||||
| DB/RCD vs. DB/RCD + C5 |  |  |  |  | |||||||
| LDLR/RCD vs. LDLR/RCD + C5 | 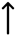 |  | 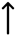 |  |  |  |  |  |  |
| Group of Animals | Number of Animals | Material for Transplantation | Route of Administration | Microbiota Donor |
|---|---|---|---|---|
| 1 (control) | 10 | 0.9% NaCl solution | Intragastric | N/A |
| 2 | 10 | Faecal microbiota sample from Donor 1 | Intragastric | Donor 1. Male, 42 yo |
| 3 | 10 | Faecal microbiota sample from Donor 2 | Intragastric | Donor 2. Male, 36 yo |
| 4 | 10 | Faecal microbiota sample from Donor 3 | Intragastric | Donor 3. Female, 28 yo |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabolotneva, A.A.; Gaponov, A.M.; Roumiantsev, S.A.; Vasiliev, I.Y.; Grigoryeva, T.V.; Kit, O.I.; Zlatnik, E.Y.; Maksimov, A.Y.; Goncharova, A.S.; Novikova, I.A.; et al. Alkylresorcinols as New Modulators of the Metabolic Activity of the Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 14206. https://doi.org/10.3390/ijms241814206
Zabolotneva AA, Gaponov AM, Roumiantsev SA, Vasiliev IY, Grigoryeva TV, Kit OI, Zlatnik EY, Maksimov AY, Goncharova AS, Novikova IA, et al. Alkylresorcinols as New Modulators of the Metabolic Activity of the Gut Microbiota. International Journal of Molecular Sciences. 2023; 24(18):14206. https://doi.org/10.3390/ijms241814206
Chicago/Turabian StyleZabolotneva, Anastasia A., Andrei M. Gaponov, Sergey A. Roumiantsev, Ilya Yu. Vasiliev, Tatiana V. Grigoryeva, Oleg I. Kit, Elena Yu. Zlatnik, Aleksey Yu. Maksimov, Anna S. Goncharova, Inna A. Novikova, and et al. 2023. "Alkylresorcinols as New Modulators of the Metabolic Activity of the Gut Microbiota" International Journal of Molecular Sciences 24, no. 18: 14206. https://doi.org/10.3390/ijms241814206
APA StyleZabolotneva, A. A., Gaponov, A. M., Roumiantsev, S. A., Vasiliev, I. Y., Grigoryeva, T. V., Kit, O. I., Zlatnik, E. Y., Maksimov, A. Y., Goncharova, A. S., Novikova, I. A., Appolonova, S. A., Markin, P. A., & Shestopalov, A. V. (2023). Alkylresorcinols as New Modulators of the Metabolic Activity of the Gut Microbiota. International Journal of Molecular Sciences, 24(18), 14206. https://doi.org/10.3390/ijms241814206





