Cost-Efficient Detection of NTRK1/2/3 Gene Fusions: Single-Center Analysis of 8075 Tumor Samples
Abstract
:1. Introduction
2. Results
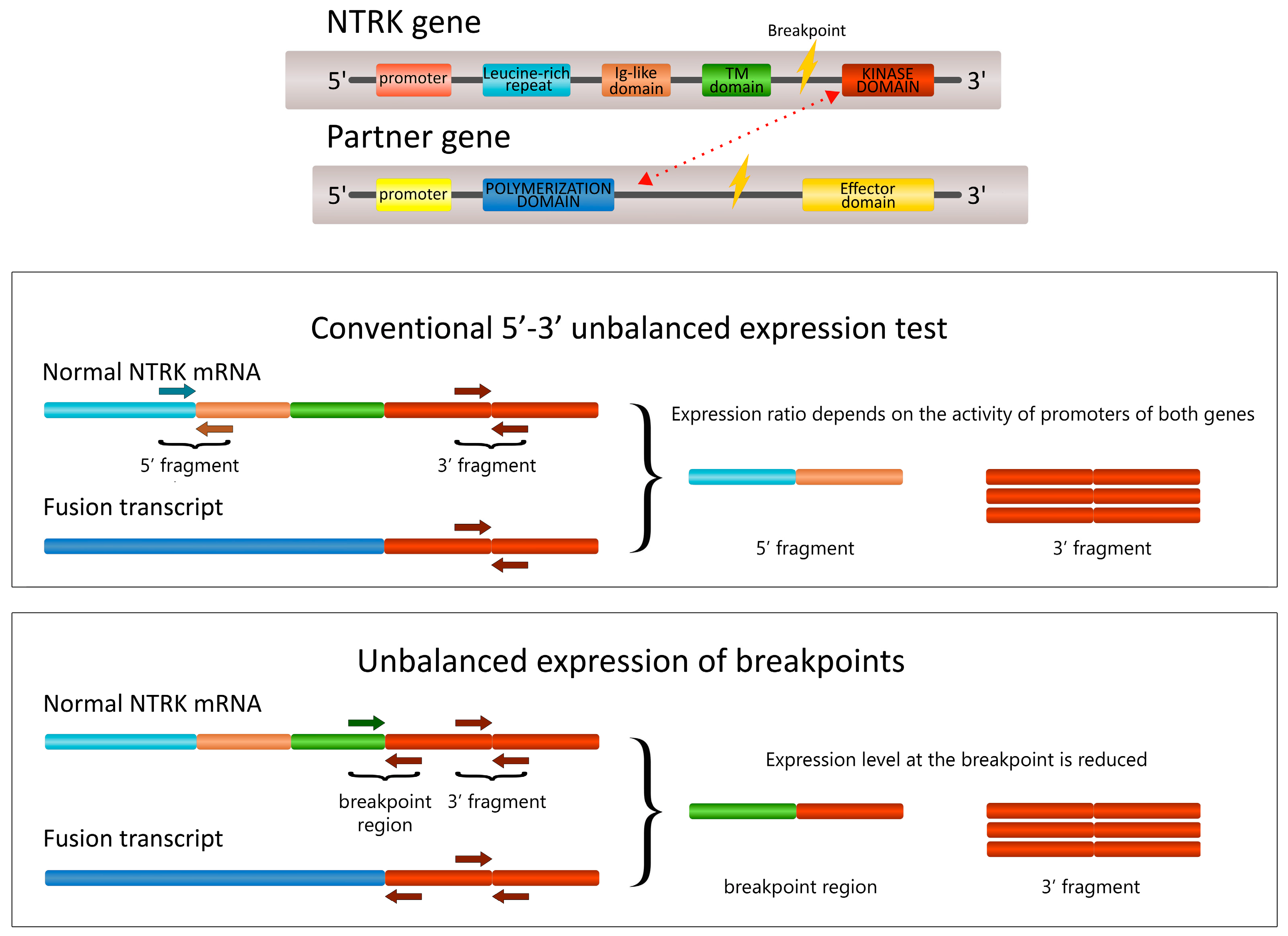
2.1. The Development of the 5′/3′-End NTRK Expression Test
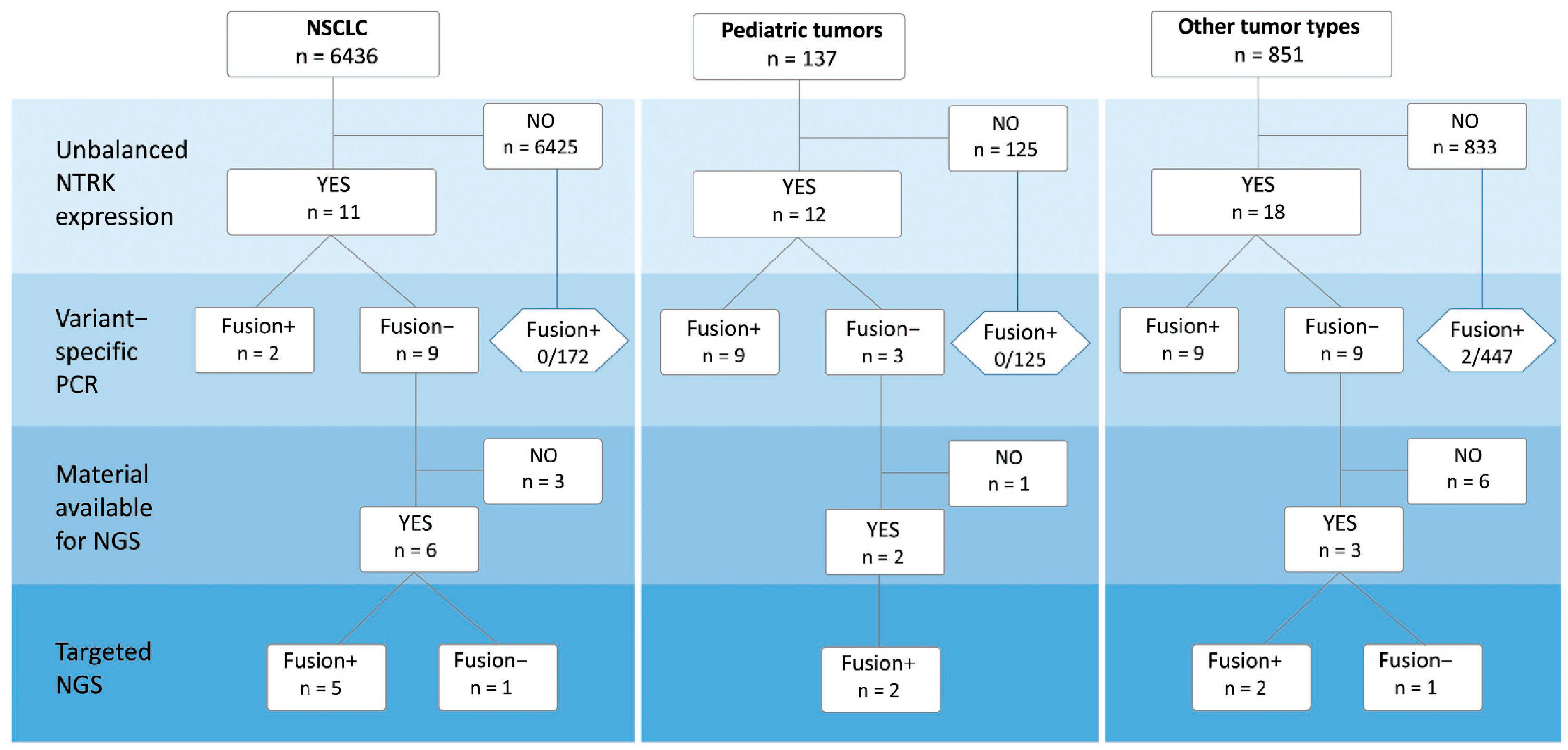
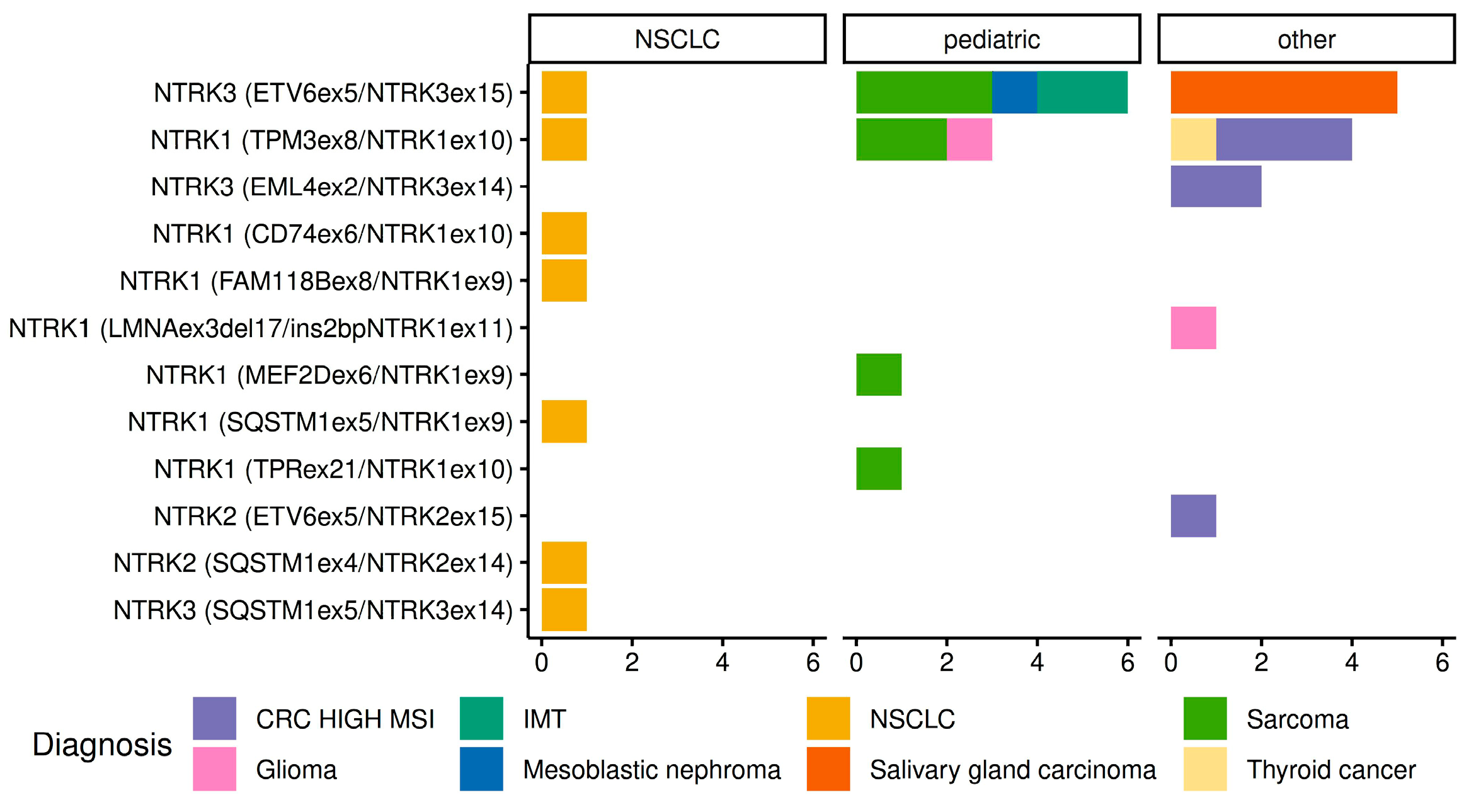
2.2. Detection of NTRK1/2/3 Rearrangements
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S. van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Solomon, J.P.; Hechtman, J.F. Detection of NTRK Fusions: Merits and Limitations of Current Diagnostic Platforms. Cancer Res. 2019, 79, 3163–3168. [Google Scholar] [CrossRef]
- Weiss, L.M.; Funari, V.A. NTRK fusions and Trk proteins: What are they and how to test for them. Hum. Pathol. 2021, 112, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Hechtman, J.F. NTRK insights: Best practices for pathologists. Mod. Pathol. 2022, 35, 298–305. [Google Scholar] [CrossRef]
- Marchetti, A.; Ferro, B.; Pasciuto, M.P.; Zampacorta, C.; Buttitta, F.; D’Angelo, E. NTRK gene fusions in solid tumors: Agnostic relevance, prevalence and diagnostic strategies. Pathologica 2022, 114, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Huygens, S.; Vellekoop, H.; Versteegh, M.; Santi, I.; Szilberhorn, L.; Zelei, T.; Nagy, B.; Tsiachristas, A.; Koleva-Kolarova, R.; Wordsworth, S.; et al. Cost-Effectiveness Analysis of Treating Patients With NTRK-Positive Cancer With the Histology-Independent Therapy Entrectinib. Value Health 2023, 26, 193–203. [Google Scholar] [CrossRef]
- Hondelink, L.M.; Schrader, A.M.R.; Asri Aghmuni, G.; Solleveld-Westerink, N.; Cleton-Jansen, A.M.; van Egmond, D.; Boot, A.; Ouahoud, S.; Khalifa, M.N.; Wai Lam, S.; et al. The sensitivity of pan-TRK immunohistochemistry in solid tumours: A meta-analysis. Eur. J. Cancer 2022, 173, 229–237. [Google Scholar] [CrossRef]
- Marchiò, C.; Scaltriti, M.; Ladanyi, M.; Iafrate, A.J.; Bibeau, F.; Dietel, M. ESMO recommendations on the standard methods to detect NTRK fusions in daily practice and clinical research. Ann. Oncol. 2019, 30, 1417–1427. [Google Scholar] [CrossRef]
- Solomon, J.P.; Benayed, R.; Hechtman, J.F.; Ladanyi, M. Identifying patients with NTRK fusion cancer. Ann. Oncol. 2019, 30 (Suppl. S8), viii16–viii22. [Google Scholar] [CrossRef]
- Park, H.J.; Baek, I.; Cheang, G.; Solomon, J.P.; Song, W. Comparison of RNA-Based Next-Generation Sequencing Assays for the Detection of NTRK Gene Fusions. J. Mol. Diagn. 2021, 23, 1443–1451. [Google Scholar] [CrossRef]
- Bormann Chung, C.; Lee, J.; Barritault, M.; Bringuier, P.P.; Xu, Z.; Huang, W.Y.; Beharry, A.; Castillo, J.; Christiansen, J.; Lin, J.C.; et al. Evaluating Targeted Next-Generation Sequencing Assays and Reference Materials for NTRK Fusion Detection. J. Mol. Diagn. 2022, 24, 18–32. [Google Scholar] [CrossRef]
- Koopman, B.; Kuijpers, C.C.H.J.; Groen, H.J.M.; Timens, W.; Schuuring, E.; Willems, S.M.; van Kempen, L.C. Detection of NTRK Fusions and TRK Expression and Performance of pan-TRK Immunohistochemistry in Routine Diagnostics: Results from a Nationwide Community-Based Cohort. Diagnostics 2022, 12, 668. [Google Scholar] [CrossRef]
- Stockley, T.L.; Lo, B.; Box, A.; Corredor, A.G.; DeCoteau, J.; Desmeules, P.; Feilotter, H.; Grafodatskaya, D.; Greer, W.; Hawkins, C.; et al. CANTRK: A Canadian Ring Study to Optimize Detection of NTRK Gene Fusions by Next-Generation RNA Sequencing. J. Mol. Diagn. 2023, 25, 68–174. [Google Scholar] [CrossRef]
- Wang, R.; Pan, Y.; Li, C.; Hu, H.; Zhang, Y.; Li, H.; Luo, X.; Zhang, J.; Fang, Z.; Li, Y.; et al. The use of quantitative real-time reverse transcriptase PCR for 5′ and 3′ portions of ALK transcripts to detect ALK rearrangements in lung cancers. Clin. Cancer Res. 2012, 18, 4725–4732. [Google Scholar] [CrossRef]
- Preobrazhenskaya, E.V.; Iyevleva, A.G.; Suleymanova, A.M.; Tiurin, V.I.; Mitiushkina, N.V.; Bizin, I.V.; Ivanstov, A.O.; Gorustovich, O.A.; Shelekhova, K.V.; Kachanov, D.Y.; et al. Gene rearrangements in consecutive series of pediatric inflammatory myofibroblastic tumors. Pediatr. Blood Cancer 2020, 67, e28220. [Google Scholar] [CrossRef] [PubMed]
- Mitiushkina, N.V.; Romanko, A.A.; Preobrazhenskaya, E.V.; Tiurin, V.I.; Ermachenkova, T.I.; Martianov, A.S.; Mulkidjan, R.S.; Sokolova, T.N.; Kholmatov, M.M.; Bizin, I.V.; et al. Comprehensive evaluation of the test for 5′-/3′-end mRNA unbalanced expression as a screening tool for ALK and ROS1 fusions in lung cancer. Cancer Med. 2022, 11, 3226–3237. [Google Scholar] [CrossRef]
- Tiurin, V.I.; Preobrazhenskaya, E.V.; Mitiushkina, N.V.; Romanko, A.A.; Anuskina, A.A.; Mulkidjan, R.S.; Saitova, E.S.; Krivosheyeva, E.A.; Kharitonova, E.D.; Shevyakov, M.P.; et al. Rapid and Cost-Efficient Detection of RET Rearrangements in a Large Consecutive Series of Lung Carcinomas. Int. J. Mol. Sci. 2023, 24, 10530. [Google Scholar] [CrossRef] [PubMed]
- Newton, Y.; Sedgewick, A.J.; Cisneros, L.; Golovato, J.; Johnson, M.; Szeto, C.W.; Rabizadeh, S.; Sanborn, J.Z.; Benz, S.C.; Vaske, C. Large scale, robust, and accurate whole transcriptome profiling from clinical formalin-fixed paraffin-embedded samples. Sci. Rep. 2020, 10, 17597. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, M.; Blay, S.; Liang, S.; Swanson, D.; Lerner-Ellis, J.; Dickson, B.; Wong, A.; Charames, G.S. Improving RNA Fusion Call Confidence and Reliability in Molecular Diagnostic Testing. J. Mol. Diagn. 2023, 25, 320–330. [Google Scholar] [CrossRef]
- Ramani, N.S.; Chen, H.; Broaddus, R.R.; Lazar, A.J.; Luthra, R.; Medeiros, L.J.; Patel, K.P.; Rashid, A.; Routbort, M.J.; Stewart, J.; et al. Utilization of cytology smears improves success rates of RNA-based next-generation sequencing gene fusion assays for clinically relevant predictive biomarkers. Cancer Cytopathol. 2021, 129, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Benayed, R.; Offin, M.; Mullaney, K.; Sukhadia, P.; Rios, K.; Desmeules, P.; Ptashkin, R.; Won, H.; Chang, J.; Halpenny, D.; et al. High Yield of RNA Sequencing for Targetable Kinase Fusions in Lung Adenocarcinomas with No Mitogenic Driver Alteration Detected by DNA Sequencing and Low Tumor Mutation Burden. Clin. Cancer Res. 2019, 25, 4712–4722. [Google Scholar] [CrossRef]
- Cohen, D.; Hondelink, L.M.; Solleveld-Westerink, N.; Uljee, S.M.; Ruano, D.; Cleton-Jansen, A.M.; von der Thüsen, J.H.; Ramai, S.R.S.; Postmus, P.E.; Graadt van Roggen, J.F.; et al. Optimizing Mutation and Fusion Detection in NSCLC by Sequential DNA and RNA Sequencing. J. Thorac. Oncol. 2020, 15, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Braunschweig, T.; Williams, R.; Guerrero, N.; Hoffmann, K.M.; Kwon, M.; Song, Y.K.; Libutti, S.K.; Hewitt, S.M. Factors in tissue handling and processing that impact RNA obtained from formalin-fixed, paraffin-embedded tissue. J. Histochem. Cytochem. 2008, 56, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Overbeck, T.R.; Reiffert, A.; Schmitz, K.; Rittmeyer, A.; Körber, W.; Hugo, S.; Schnalke, J.; Lukat, L.; Hugo, T.; Hinterthaner, M.; et al. NTRK Gene Fusions in Non-Small-Cell Lung Cancer: Real-World Screening Data of 1068 Unselected Patients. Cancers 2023, 15, 2966. [Google Scholar] [CrossRef]
- Solomon, J.P.; Linkov, I.; Rosado, A.; Mullaney, K.; Rosen, E.Y.; Frosina, D.; Jungbluth, A.A.; Zehir, A.; Benayed, R.; Drilon, A.; et al. NTRK fusion detection across multiple assays and 33,997 cases: Diagnostic implications and pitfalls. Mod. Pathol. 2020, 33, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Haberecker, M.; Töpfer, A.; Melega, F.; Moch, H.; Pauli, C. A systematic comparison of pan-Trk immunohistochemistry assays among multiple cancer types. Histopathology 2023, 82, 1003–1012. [Google Scholar] [CrossRef]
- Schraa, S.J.; Stelloo, E.; Laclé, M.M.; Swennenhuis, J.F.; Brosens, L.A.A.; Fijneman, R.J.A.; Feitsma, H.; Koopman, M.; de Leng, W.W.; Vink, G.R.; et al. Comparison of NTRK fusion detection methods in microsatellite-instability-high metastatic colorectal cancer. Virchows Arch. 2023, 482, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Hofman, V.; Heeke, S.; Bontoux, C.; Chalabreysse, L.; Barritault, M.; Bringuier, P.P.; Fenouil, T.; Benzerdjeb, N.; Begueret, H.; Merlio, J.P.; et al. Ultrafast Gene Fusion Assessment for Nonsquamous NSCLC. JTO Clin. Res. Rep. 2022, 4, 100457. [Google Scholar] [CrossRef]
- Sorber, L.; Van Dorst, B.; Bellon, E.; Zwaenepoel, K.; Lambin, S.; De Winne, K.; Lardon, F.; Pauwels, P.; Siozopoulou, V. NTRK Gene Fusion Detection in a Pan-Cancer Setting Using the Idylla GeneFusion Assay. J. Mol. Diagn. 2022, 24, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Guillard, M.; Caumont, C.; Marcorelles, P.; Merlio, J.P.; Cappellen, D.; Uguen, A. Performances of the Idylla GeneFusion Assay: Contribution to a rapid diagnosis of targetable gene fusions in tumour samples. J. Clin. Pathol. 2023, jcp-2023-208798. [Google Scholar] [CrossRef]
- Lira, M.E.; Choi, Y.L.; Lim, S.M.; Deng, S.; Huang, D.; Ozeck, M.; Han, J.; Jeong, J.Y.; Shim, H.S.; Cho, B.C.; et al. A single-tube multiplexed assay for detecting ALK, ROS1, and RET fusions in lung cancer. J. Mol. Diagn. 2014, 16, 229–243. [Google Scholar] [CrossRef]
- Heydt, C.; Wölwer, C.B.; Velazquez Camacho, O.; Wagener-Ryczek, S.; Pappesch, R.; Siemanowski, J.; Rehker, J.; Haller, F.; Agaimy, A.; Worm, K.; et al. Detection of gene fusions using targeted next-generation sequencing: A comparative evaluation. BMC Med. Genom. 2021, 14, 62. [Google Scholar] [CrossRef]
- O’Haire, S.; Franchini, F.; Kang, Y.J.; Steinberg, J.; Canfell, K.; Desai, J.; Fox, S.; IJzerman, M. Systematic review of NTRK 1/2/3 fusion prevalence pan-cancer and across solid tumours. Sci. Rep. 2023, 13, 4116. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, A.; Zhang, W.; Phillip Strauss, U.; Fellous, M.; Korei, M.; Keating, K. A systematic review and meta-analysis of neurotrophic tyrosine receptor kinase gene fusion frequencies in solid tumors. Ther. Adv. Med. Oncol. 2020, 12, 1758835920975613. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Kawazu, M.; Yamamoto, Y.; Ueno, T.; Kojima, S.; Nagae, G.; Abe, H.; Soda, M.; Oga, T.; Kohsaka, S.; et al. Fusion Kinases Identified by Genomic Analyses of Sporadic Microsatellite Instability-High Colorectal Cancers. Clin. Cancer Res. 2019, 25, 378–389. [Google Scholar] [CrossRef]
- Ukkola, I.; Nummela, P.; Kero, M.; Tammio, H.; Tuominen, J.; Kairisto, V.; Kallajoki, M.; Haglund, C.; Peltomäki, P.; Kytölä, S.; et al. Gene fusions and oncogenic mutations in MLH1 deficient and BRAFV600E wild-type colorectal cancers. Virchows Arch. 2022, 480, 807–817. [Google Scholar] [CrossRef]
- Wang, J.; Li, R.; Li, J.; Yi, Y.; Liu, X.; Chen, J.; Zhang, H.; Lu, J.; Li, C.; Wu, H.; et al. Comprehensive analysis of oncogenic fusions in mismatch repair deficient colorectal carcinomas by sequential DNA and RNA next generation sequencing. J. Transl. Med. 2021, 19, 433. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yao, F.; Xiang, C.; Zhao, J.; Shang, Z.; Guo, L.; Ding, W.; Ma, S.; Yu, A.; Shao, J.; et al. Identification of NTRK gene fusions in lung adenocarcinomas in the Chinese population. J. Pathol. Clin. Res. 2021, 7, 375–384. [Google Scholar] [CrossRef]
- Liu, F.; Wei, Y.; Zhang, H.; Jiang, J.; Zhang, P.; Chu, Q. NTRK Fusion in Non-Small Cell Lung Cancer: Diagnosis, Therapy, and TRK Inhibitor Resistance. Front. Oncol. 2022, 12, 864666. [Google Scholar] [CrossRef]
- Preobrazhenskaya, E.; Zagrebin, F.; Mulkidzhan, R.; Krivosheeva, E.; Saitova, E.; Raskin, G.; Bizin, I.; Imyanitov, E. Novel kinase-activating fusions in lung adenocarcinomas. In Proceedings of the 35st European Congress of Pathology, Dublin, Ireland, 9–13 September 2023. [Google Scholar]
- Zito Marino, F.; Buono, S.; Montella, M.; Giannatiempo, R.; Messina, F.; Casaretta, G.; Arpino, G.; Vita, G.; Fiorentino, F.; Insabato, L.; et al. NTRK gene aberrations in triple-negative breast cancer: Detection challenges using IHC, FISH, RT-PCR, and NGS. J. Pathol. Clin. Res. 2023, 9, 367–377. [Google Scholar] [CrossRef]
- Vingiani, A.; Lorenzini, D.; Conca, E.; Volpi, C.C.; Trupia, D.V.; Gloghini, A.; Perrone, F.; Tamborini, E.; Dagrada, G.P.; Agnelli, L.; et al. Pan-TRK immunohistochemistry as screening tool for NTRK fusions: A diagnostic workflow for the identification of positive patients in clinical practice. Cancer Biomark. 2023, in press. [Google Scholar] [CrossRef]
- Beresford, L.; Murphy, P.; Dias, S.; Claxton, L.; Walton, M.; Metcalf, R.; Schlecht, H.; Ottensmeier, C.; Pereira, M.; Hodgson, R. Appraising the Costs of Genomic Testing for Histology-Independent Technologies: An Illustrative Example for NTRK Fusions. Value Health 2022, 25, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Schlatter, R.P.; Matte, U.; Polanczyk, C.A.; Koehler-Santos, P.; Ashton-Prolla, P. Costs of genetic testing: Supporting Brazilian Public Policies for the incorporating of molecular diagnostic technologies. Genet. Mol. Biol. 2015, 38, 332–337. [Google Scholar] [CrossRef]
- Imyanitov, E.N.; Iyevleva, A.G.; Levchenko, E.V. Molecular testing and targeted therapy for non-small cell lung cancer: Current status and perspectives. Crit. Rev. Oncol. Hematol. 2021, 157, 103194. [Google Scholar] [CrossRef] [PubMed]
- Mitiushkina, N.V.; Kholmatov, M.M.; Tiurin, V.I.; Romanko, A.A.; Yatsuk, O.S.; Sokolova, T.N.; Ivantsov, A.O.; Kuligina, E.S.; Stepanov, I.A.; Belyaev, A.M.; et al. Comparative analysis of expression of mutant and wild-type alleles is essential for reliable PCR-based detection of MET exon 14 skipping. Biochimie 2019, 165, 267–274. [Google Scholar] [CrossRef] [PubMed]
| Tumor Type | Number of Cases with Confirmed NTRK Fusion |
|---|---|
| NSCLC | 7/6436 1 (0.1%) |
| Sarcoma | 7/143 2 (4.9%) |
| Glioma, neuroepithelial tumor | 2/124 (1.6%) |
| Salivary gland carcinoma | 5/93 3 (5.4%) |
| MSI-high CRC | 6/48 (12.5%) |
| IMT | 2/34 (5.9%) |
| Thyroid cancer | 1/26 (3.9%) |
| Mesoblastic nephroma | 1/4 (25.0%) |
| Other | 0/516 (0.0%) |
| ID | Tumor Type | Age | Gender | Fusion Type |
|---|---|---|---|---|
| NTRK1 | ||||
| 22240 | CRC, MSI-high | 60 | f | TPM3::NTRK1 (T8;N10) |
| 31589 | CRC, MSI-high | 64 | f | TPM3::NTRK1 (T8;N10) |
| 21693 | CRC, MSI-high | 85 | m | TPM3::NTRK1 (T8;N10) |
| 28114 | Glioblastoma | 62 | f | LMNA::NTRK1 (L3del17;ins2N11) |
| 27845 | Oligodendroglioma | 3 | f | TPM3::NTRK1 (T8;N10) |
| 15197 | NSCLC | 47 | f | CD74::NTRK1 (C6;N10) |
| 10580 | NSCLC | 64 | m | FAM118B::NTRK1 (F8;N9) |
| 12285 | NSCLC | 64 | f | SQSTM1::NTRK1 (S5;N9) |
| 14281 | NSCLC | 60 | f | TPM3::NTRK1 (T8;N10) |
| 14131 | Dermatofibrosarcoma protuberans | 6 | m | TPM3::NTRK1 (T8;N10) |
| 30476 | Sarcoma, NOS 1 | 1 | f | TPR::NTRK1 (T21;N10) |
| 16009 | Infantile fibrosarcoma | 2 | m | TPM3::NTRK1 (T8;N10) |
| 13669 | Malignant peripheral nerve sheath tumor | 0 | f | MEF2D::NTRK1 (M6;N9) |
| 353067 | Papillary thyroid cancer | 19 | f | TPM3::NTRK1 (T8;N10) |
| NTRK2 | ||||
| 29665 | CRC, MSI-high | 74 | m | ETV6::NTRK2 (E5;N15) |
| 13098 | NSCLC | 79 | m | SQSTM1::NTRK2 (S4;N14) |
| NTRK3 | ||||
| 13666 | Congenital mesoblastic nephroma | 0 | m | ETV6::NTRK3 (E5;N15) |
| 24631 | CRC, MSI-high | 68 | f | EML4::NTRK3 (E2;N14) |
| 33968 | CRC, MSI-high | 70 | f | EML4::NTRK3 (E2;N14) |
| 21856 | IMT | 3 | m | ETV6::NTRK3 (E5;N15) |
| 12193 | IMT | 17 | m | ETV6::NTRK3 (E5;N15) |
| 11479 | NSCLC | 58 | m | ETV6::NTRK3 (E5;N15) |
| 22443 | NSCLC | 44 | m | SQSTM1::NTRK3 (S5;N14) |
| 32662 | Salivary gland adenocarcinoma, NOS | 55 | f | ETV6::NTRK3 (E5;N15) |
| 33039 | Salivary gland adenocarcinoma, NOS | 32 | f | ETV6::NTRK3 (E5;N15) |
| 787 | Salivary duct carcinoma | 65 | f | ETV6::NTRK3 (E5;N15) |
| 798 | Salivary duct carcinoma | 49 | f | ETV6::NTRK3 (E5;N15) |
| 24388 | Salivary gland secretory carcinoma | 37 | f | ETV6::NTRK3 (E5;N15) |
| 12471 | Soft tissue sarcoma | 3 | m | ETV6::NTRK3 (E5;N15) |
| 16011 | Hemangioendothelioma | 4 | f | ETV6::NTRK3 (E5;N15) |
| 16396 | Infantile fibrosarcoma | 1 | m | ETV6::NTRK3 (E5;N15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanko, A.A.; Mulkidjan, R.S.; Tiurin, V.I.; Saitova, E.S.; Preobrazhenskaya, E.V.; Krivosheyeva, E.A.; Mitiushkina, N.V.; Shestakova, A.D.; Belogubova, E.V.; Ivantsov, A.O.; et al. Cost-Efficient Detection of NTRK1/2/3 Gene Fusions: Single-Center Analysis of 8075 Tumor Samples. Int. J. Mol. Sci. 2023, 24, 14203. https://doi.org/10.3390/ijms241814203
Romanko AA, Mulkidjan RS, Tiurin VI, Saitova ES, Preobrazhenskaya EV, Krivosheyeva EA, Mitiushkina NV, Shestakova AD, Belogubova EV, Ivantsov AO, et al. Cost-Efficient Detection of NTRK1/2/3 Gene Fusions: Single-Center Analysis of 8075 Tumor Samples. International Journal of Molecular Sciences. 2023; 24(18):14203. https://doi.org/10.3390/ijms241814203
Chicago/Turabian StyleRomanko, Aleksandr A., Rimma S. Mulkidjan, Vladislav I. Tiurin, Evgeniya S. Saitova, Elena V. Preobrazhenskaya, Elena A. Krivosheyeva, Natalia V. Mitiushkina, Anna D. Shestakova, Evgeniya V. Belogubova, Alexandr O. Ivantsov, and et al. 2023. "Cost-Efficient Detection of NTRK1/2/3 Gene Fusions: Single-Center Analysis of 8075 Tumor Samples" International Journal of Molecular Sciences 24, no. 18: 14203. https://doi.org/10.3390/ijms241814203
APA StyleRomanko, A. A., Mulkidjan, R. S., Tiurin, V. I., Saitova, E. S., Preobrazhenskaya, E. V., Krivosheyeva, E. A., Mitiushkina, N. V., Shestakova, A. D., Belogubova, E. V., Ivantsov, A. O., Iyevleva, A. G., & Imyanitov, E. N. (2023). Cost-Efficient Detection of NTRK1/2/3 Gene Fusions: Single-Center Analysis of 8075 Tumor Samples. International Journal of Molecular Sciences, 24(18), 14203. https://doi.org/10.3390/ijms241814203







