Enterobacter Strain IPPBiotE33 Displays a Synergistic Effect with Bacillus thuringiensis Bt185
Abstract
1. Introduction
2. Results
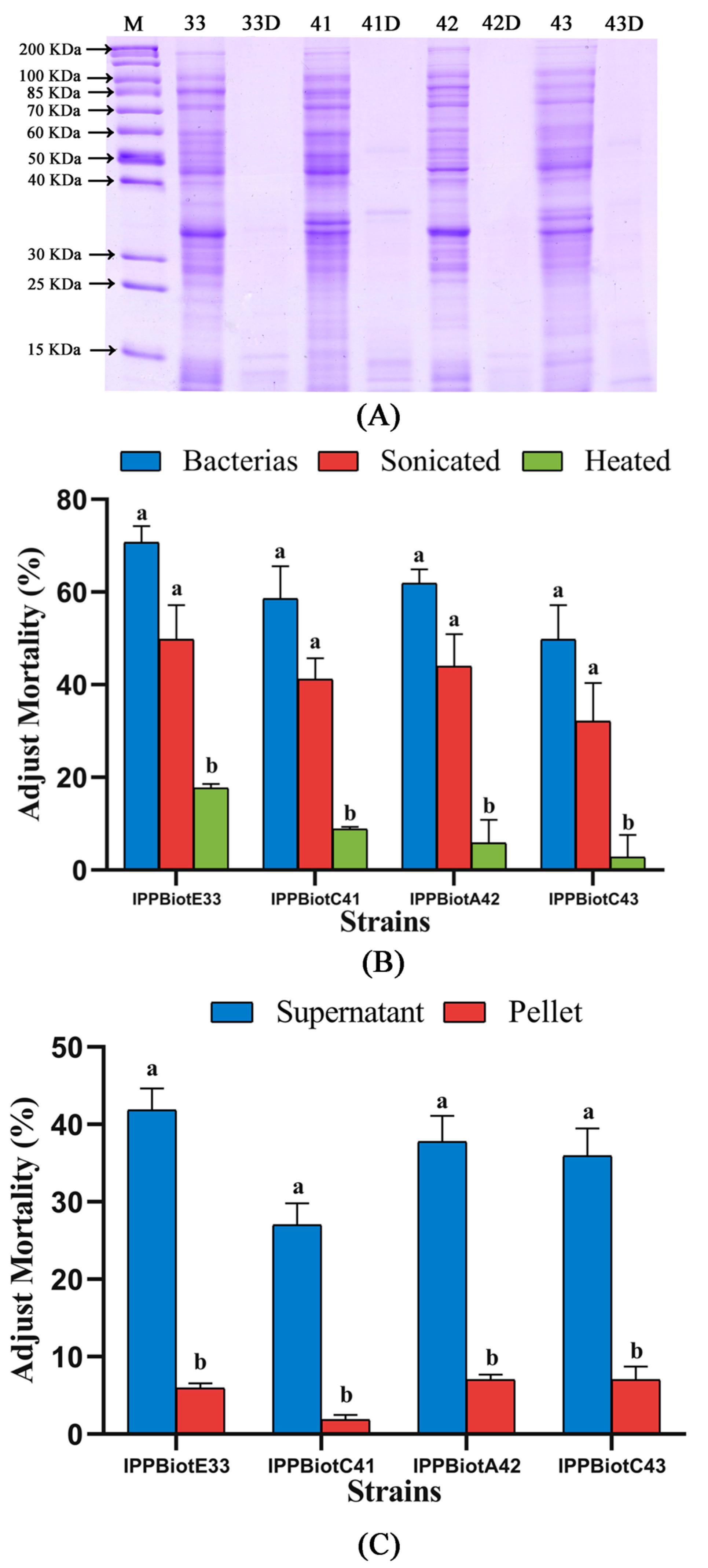
2.1. The Strains Showed Specific Toxicity against Coleopteran Insects
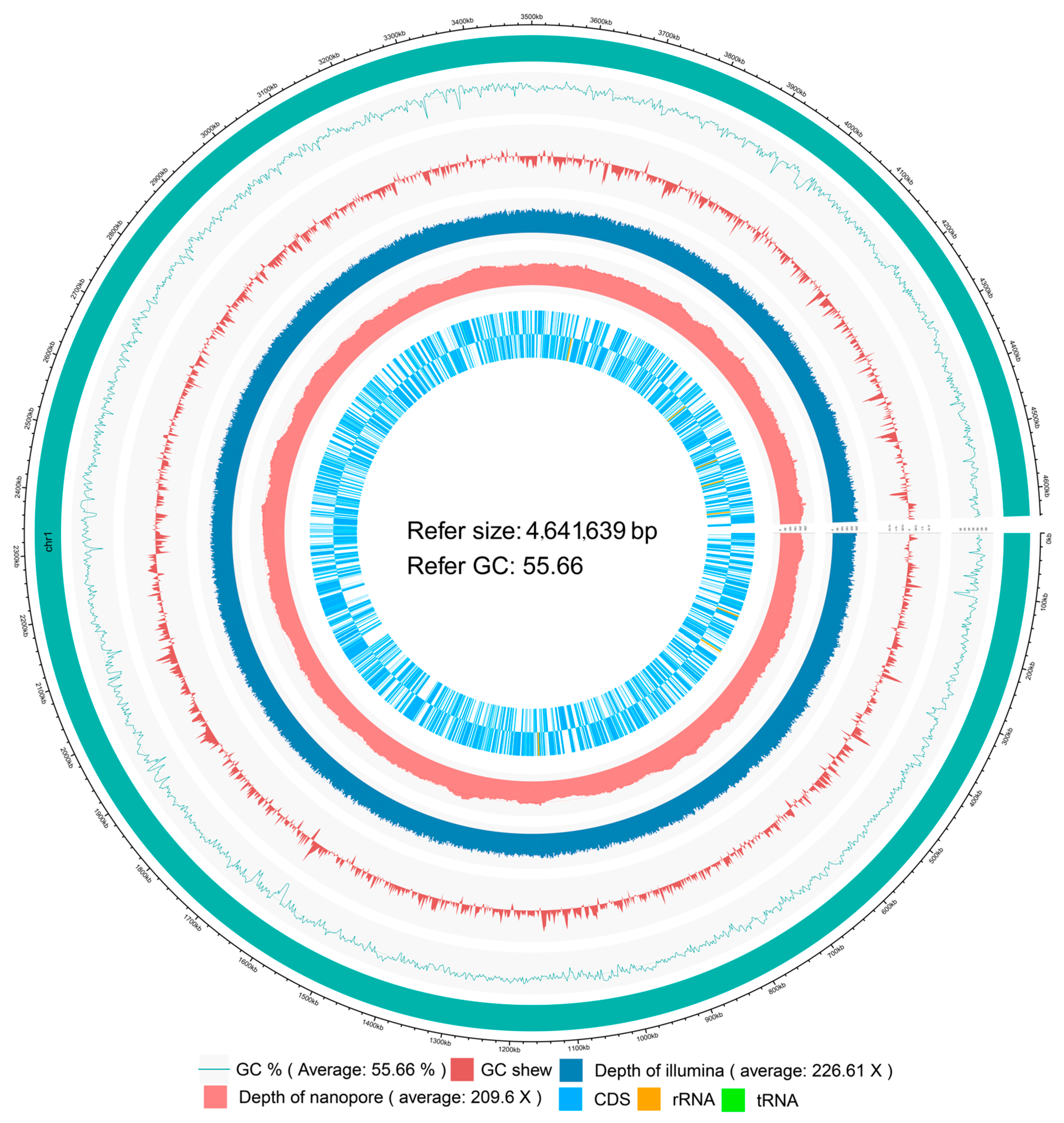
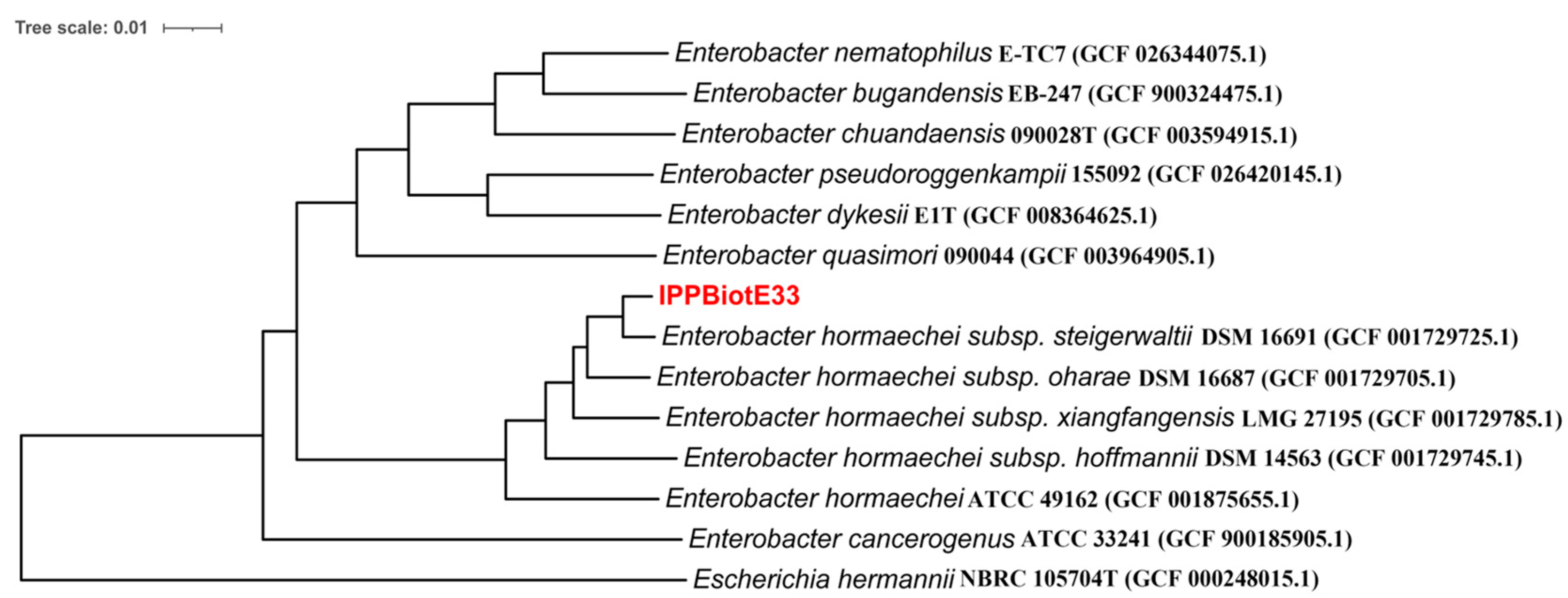
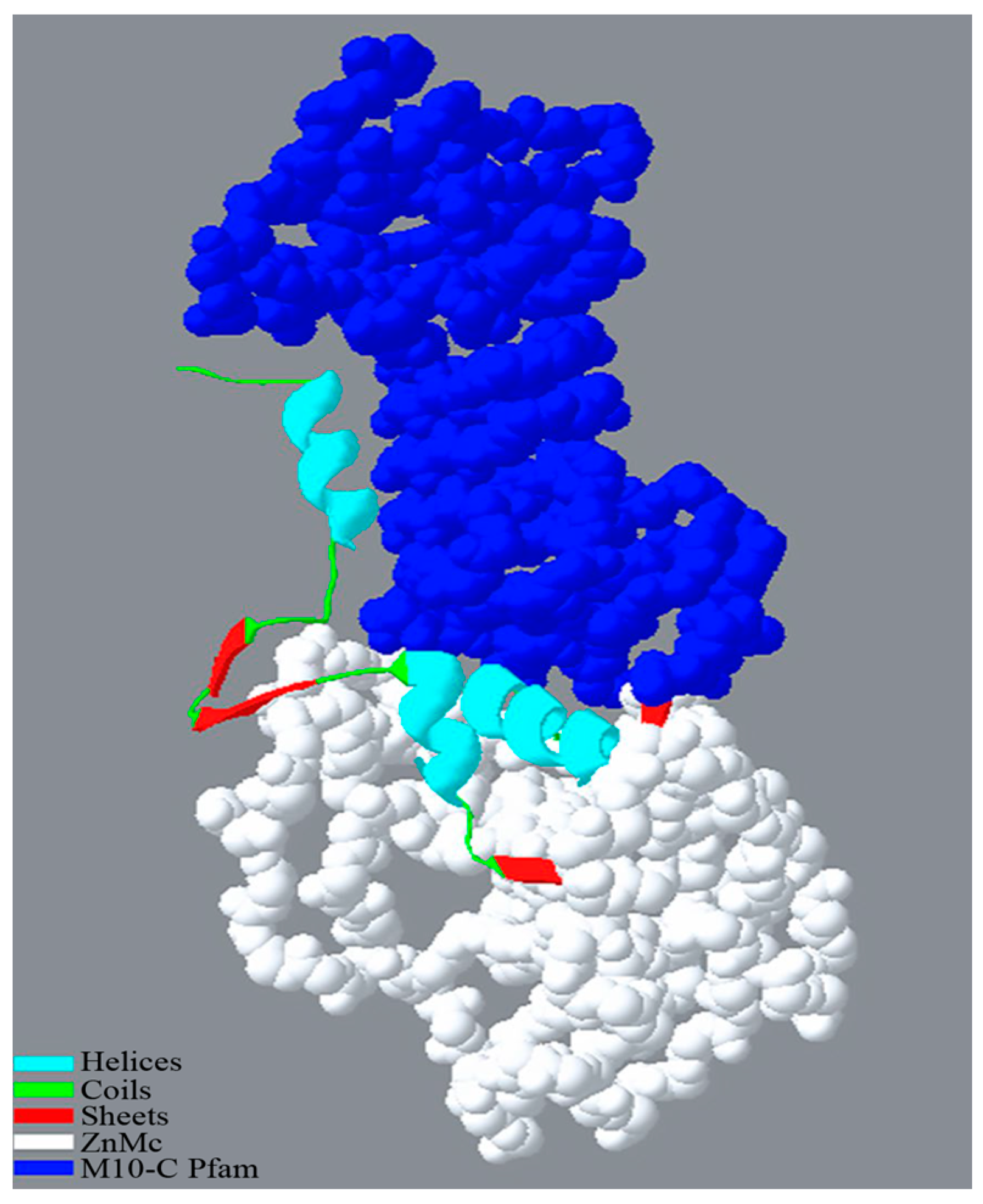
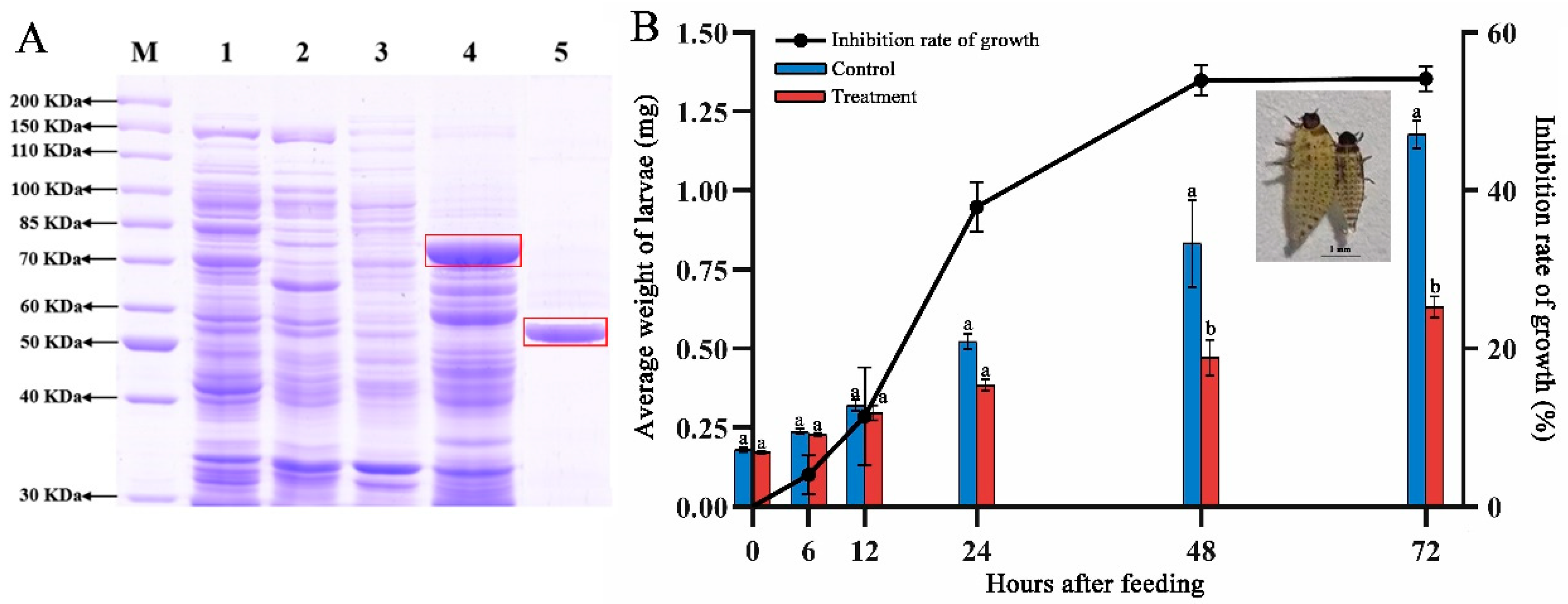
2.2. Analysis of the Whole Genome and Insecticidal Protein for IPPBiotE33
2.3. The Synergistic Effect of IPPBiotE33 and Bt185
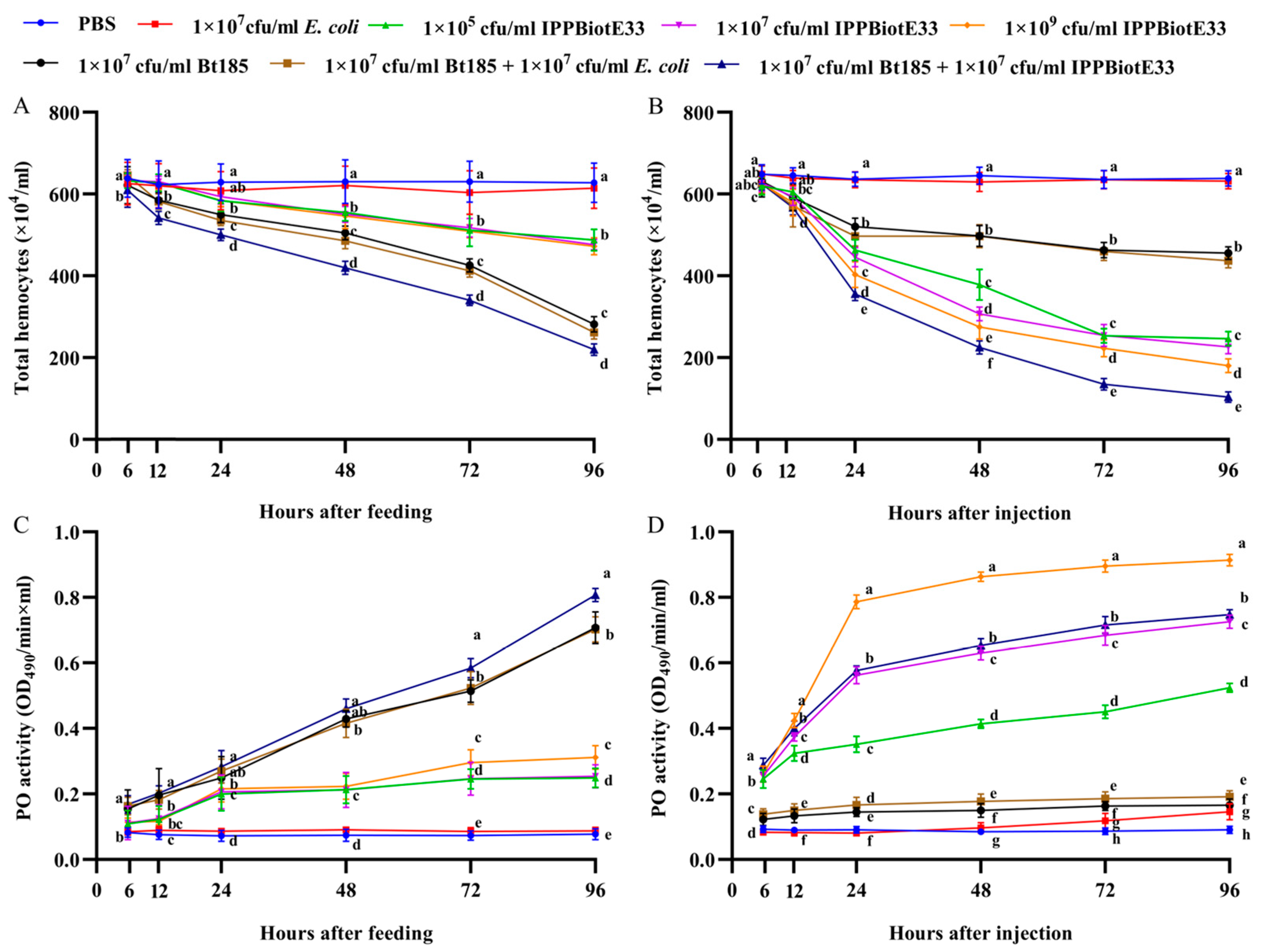
2.4. Immunosuppressive Activity of IPPBiotE33 in H. parallela
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Insect Rearing and Bioassays
4.3. Genomic DNA Extraction and Sequencing
4.4. Discovery and Purification of Insecticidal Protein 03673
4.5. Synergistic Analysis
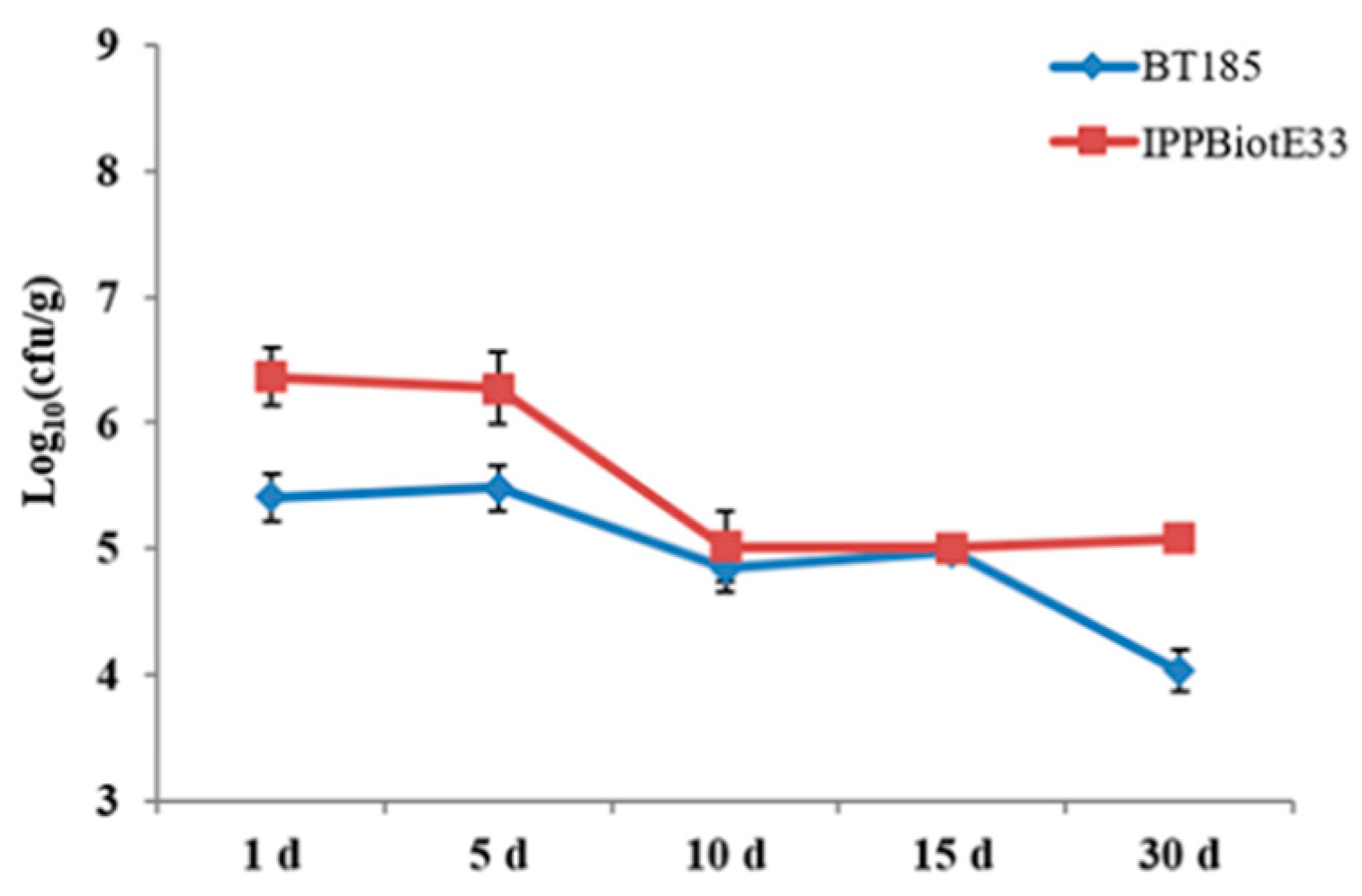
4.6. Pot Experiment and the Evaluation of Colonization Abilities
4.7. Estimation of Hemocyte Concentration
4.8. Phenol Oxidase Activity in Insect Hemolymph
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deans, C.; Krischik, V. The current state and future potential of microbial control of scarab pests. Appl. Sci. 2023, 13, 766. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, J.; Huang, D.; Gao, J.; Song, F. Characterization of Bacillus thuringiensis strain Bt185 toxic to the asian cockchafer: Holotrichia parallela. Curr. Microbiol. 2006, 53, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Sun, X. The Effects of Holotrichia parallela larva damage on rhizosphere bacterial community of peanut and preliminary exploration of its mechanism. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2019. [Google Scholar]
- Zhang, H.; Teng, X.; Luo, Q.; Sheng, Z.; Guo, X.; Wang, G.; Li, W.; Yuan, G. Flight and walking performance of dark black chafer beetle Holotrichia parallela (Coleoptera: Scarabaeidae) in the presence of known hosts and attractive nonhost plants. J. Insect Sci. 2019, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, W.; Fei, D.; Yang, T.; Wang, S. Field control effect of several common granules on corn grub. J. Anhui Agric. Sci. 2022, 50, 140–142. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Cao, J.; Shi, R.; Hu, T. Efficacy evaluation of five insecticides on grubs in peanut field. J. Shanxi Agric. Sci 2019, 47, 1640–1642. [Google Scholar]
- Wang, X.P.; Ge, F.; Xue, F.S. Host plant mediation of diapause induction in the cabbage beetle, Colaphellus bowringi baly (Coleoptera: Chrysomelidae). Insect Sci. 2006, 13, 189–193. [Google Scholar] [CrossRef]
- Oliver, J.B.; Reding, M.E.; Youssef, N.N.; Klein, M.G.; Bishop, B.L.; Lewis, P.A. Surface-applied insecticide treatments for quarantine control of japanese beetle, Popillia japonica newman (Coleoptera: Scarabaeidae), larvae in field-grown nursery plants. Pest Manag. Sci. 2009, 65, 381–390. [Google Scholar] [CrossRef]
- Guo, W.; Yan, X.; Zhao, G.; Han, R. Increased efficacy of entomopathogenic nematode-insecticide combinations against Holotrichia oblita (Coleoptera: Scarabaeidae). J. Econ. Entomol. 2017, 110, 41–51. [Google Scholar] [CrossRef]
- Beegle, C.C.; Yamamoto, T. Invitation paper (C.P. Alexander Fund): History of Bacillus thuringiensis berliner research and development. Can. Ent. 1992, 124, 587–616. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Song, F.; Wu, J.; Feng, S.; Huang, D. Engineered Bacillus thuringiensis G033A with broad insecticidal activity against lepidopteran and coleopteran pests. Appl. Microbiol. Biotechnol. 2006, 72, 924–930. [Google Scholar] [CrossRef]
- Wang, K.; Shu, C.; Zhang, J. Effective bacterial insecticidal proteins against coleopteran pests: A review. Arch. Insect Biochem. Physiol. 2019, 102, e21558. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Zhang, Y.; Shu, C.; Crickmore, N.; Wang, Q.; Du, L.; Song, F.; Zhang, J. Genomic sequencing identifies novel Bacillus thuringiensis Vip1/Vip2 binary and Cry8 toxins that have high toxicity to Scarabaeoidea larvae. Appl. Microbiol. Biotechnol. 2015, 99, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Theunis, W.; Aloali’i, I. Susceptibility of the taro beetle, Papuana uninodis (Coleoptera, Scarabaeidae) to two new Bacillus popilliae isolates from Papuana spp. J. Invertebr. Pathol. 1999, 73, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Rippere, K.E.; Tran, M.T.; Yousten, A.A.; Hilu, K.H.; Klein, M.G. Bacillus popilliae and Bacillus lentimorbus, bacteria causing milky disease in japanese beetles and related scarab larvae. Int. J. Syst. Bacteriol. 1998, 48, 395–402. [Google Scholar] [CrossRef]
- Prasanna, L.; Eijsink, V.G.H.; Meadow, R.; Gaseidnes, S. A novel strain of Brevibacillus laterosporus produces chitinases that contribute to its biocontrol potential. Appl. Microbiol. Biotechnol. 2013, 97, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Shankhu, P.Y.; Mathur, C.; Mandal, A.; Sagar, D.; Somvanshi, V.S.; Dutta, T.K. Txp40, a protein from Photorhabdus akhurstii, conferred potent insecticidal activity against the larvae of Helicoverpa armigera, Spodoptera litura and S. exigua. Pest Manag. Sci. 2020, 76, 2004–2014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, X.; Li, Y.; Ding, X.; Yang, Q.; Sun, Y.; Yu, Z.; Xia, L.; Hu, S. Xaxab-like binary toxin from Photorhabdus luminescens exhibits both insecticidal activity and cytotoxicity. FEMS Microbiol. Lett. 2014, 350, 48–56. [Google Scholar] [CrossRef][Green Version]
- Landsberg, M.J.; Jones, S.A.; Rothnagel, R.; Busby, J.N.; Marshall, S.D.G.; Simpson, R.M.; Lott, J.S.; Hankamer, B.; Hurst, M.R.H. 3D structure of the Yersinia entomophaga toxin complex and implications for insecticidal activity. Proc. Natl. Acad. Sci. USA 2011, 108, 20544–20549. [Google Scholar] [CrossRef]
- Péchy-Tarr, M.; Bruck, D.J.; Maurhofer, M.; Fischer, E.; Vogne, C.; Henkels, M.D.; Donahue, K.M.; Grunder, J.; Loper, J.E.; Keel, C. Molecular analysis of a novel gene cluster encoding an insect toxin in plant-associated strains of Pseudomonas fluorescens. Environ. Microbiol. 2008, 10, 2368–2386. [Google Scholar] [CrossRef]
- Yang, G.; Dowling, A.J.; Gerike, U.; Ffrench-Constant, R.H.; Waterfield, N.R. Photorhabdus virulence cassettes confer injectable insecticidal activity against the wax moth. J. Bacteriol. 2006, 188, 2254–2261. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, J.; Li, T.; Liu, S.; Song, P.; Nangong, Z.; Wang, Q. Pirab protein from Xenorhabdus nematophila Hb310 exhibits a binary toxin with insecticidal activity and cytotoxicity in Galleria mellonella. J. Invertebr. Pathol. 2017, 148, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Berini, F.; Katz, C.; Gruzdev, N.; Casartelli, M.; Tettamanti, G.; Marinelli, F. Microbial and viral chitinases: Attractive biopesticides for integrated pest management. Biotechnol. Adv. 2018, 36, 818–838. [Google Scholar] [CrossRef] [PubMed]
- Suganthi, M.; Senthilkumar, P.; Arvinth, S.; Chandrashekara, K.N. Chitinase from Pseudomonas fluorescens and its insecticidal activity against Helopeltis theivora. J. Gen. Appl. Microbiol. 2017, 63, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiang, H.; Cheng, Q.; Chen, J.; Wu, G.; Kumar, A.; Sun, M.; Liu, Z. Enhanced nematicidal potential of the chitinase Pachi from Pseudomonas aeruginosa in association with Cry21Aa. Sci. Rep. 2015, 5, 14395. [Google Scholar] [CrossRef] [PubMed]
- Hurst, M.R.H.; Beattie, A.; Jones, S.A.; Laugraud, A.; van Koten, C.; Harper, L. Serratia proteamaculans strain Agr96x encodes an antifeeding prophage (Tailocin) with activity against grass grub (Costelytra giveni) and manuka beetle (Pyronota species) larvae. Appl. Environ. Microbiol. 2018, 84, e02739-17. [Google Scholar] [CrossRef]
- Asano, S.; Suzuki, K.; Horit, H.; Watanabet, T. Synergistic effects of supernatants from Serratia marcescens culture on larvicidal activity of Bacillus thuringiensis CrylC toxin against common cutworm, Spodoptera litura. J. Pestic. Sci. 1999, 24, 44–48. [Google Scholar] [CrossRef]
- Virgillito, C.; Manica, M.; Marini, G.; Rosà, R.; Della Torre, A.; Martini, S.; Drago, A.; Baseggio, A.; Caputo, B. Evaluation of Bacillus thuringiensis subsp. israelensis and Bacillus sphaericus combination against Culex pipiens in highly vegetated ditches. J. Am. Mosq. Control Assoc. 2022, 38, 40–45. [Google Scholar] [CrossRef]
- Jin, F.; Xu, X.; Fu, D.; Li, S. Glutamicibacter halophytocola PxG15 with Synergistic Cry1Ac Insecticidal Activity and Application of Glutamicibacter halophytocola PxG15. 2022. Available online: http://www.soopat.com/Patent/202111556647 (accessed on 10 January 2023).
- Xu, W.; Sun, X.; Mi, L.; Wang, L.; Gu, Z.; Wang, M.; Shu, C.; Bai, X.; Zhang, J.; Geng, L. Plants recruit insecticidal bacteria to defend against herbivore attacks. Unpublished work. 2023. [Google Scholar]
- Tao, K.; Yu, X.; Liu, Y.; Shi, G.; Liu, S.; Hou, T. Cloning, expression, and purification of insecticidal protein Pr596 from locust pathogen Serratia marcescens HR-3. Curr. Microbiol. 2007, 55, 228–233. [Google Scholar] [CrossRef]
- Champion, O.L.; Wagley, S.; Titball, R.W. Galleria mellonella as a model host for microbiological and toxin research. Virulence 2016, 7, 840–845. [Google Scholar] [CrossRef]
- Whitten, M.M.A.; Coates, C.J. Re-evaluation of insect melanogenesis research: Views from the dark side. Pigm. Cell Melanoma Res. 2017, 30, 386–401. [Google Scholar] [CrossRef]
- Fen, S.; Wang, R.; Wang, J.; DU, L.; Huang, D. Evaluation of control effect of Bacillus thuringiensis strain HBF-1 against larvae of Scarabaeoidae. Acta Phytophy. Sin. 2006, 38, 417–422. (In Chinese) [Google Scholar]
- Shu, C.; Yan, G.; Wang, R.; Zhang, J.; Feng, S.; Huang, D.; Song, F. Characterization of a novel Cry8 gene specific to melolonthidae pests: Holotrichia oblita and Holotrichia parallela. Appl. Microbiol. Biotechnol. 2009, 84, 701–707. [Google Scholar] [CrossRef]
- Althoff, E.R.; Rice, K.B. Japanese beetle (Coleoptera: Scarabaeidae) invasion of north america: History, ecology, and management. J. Integr. Pest Manag. 2022, 13, 2. [Google Scholar] [CrossRef]
- Redmond, C.; Potter, D. Lack of efficacy of in-vivo-produced and putatively in-vitro-produced Bacillus-popilliae against field populations of japanese-beetle (Coleoptera, Scarabaeidae) grubs in Kentucky. J. Econ. Entomol. 1995, 88, 846–854. [Google Scholar] [CrossRef]
- Villalobos, F.J.; Goh, K.M.; Saville, D.J.; Chapman, R.B. Interactions among soil organic matter, levels of the indigenous entomopathogenic bacterium Serratia entomophila in soil, amber disease and the feeding activity of the scarab larva of Costelytra zealandica: A microcosm approach. Appl. Soil Ecol. 1997, 5, 231–246. [Google Scholar] [CrossRef]
- Townsend, R.J.; Ferguson, C.M.; Proffitt, J.; Slay, M.W.A.; Swaminathan, J.; Day, S.; Gerard, E.; O’Callaghan, M.; Johnson, V.W.; Jackson, T.A. Establishment of Serratia Entomophila after Application of a New Formulation for Grass Grub Control. In Proceedings of the New Zealand Plant Protection, 57th Annual Meeting of the New-Zealand-Plant-Protection-Society, Hamilton, New Zealand, 10 August 2004; Zydenbos, S.M., Ed.; New Zealand Plant Protection Society: Rotorua, New Zealand, 2004; Volume 57, pp. 310–313. [Google Scholar]
- Hurst, M.R.H.; Becher, S.A.; Young, S.D.; Nelson, T.L.; Glare, T.R. Yersinia entomophaga sp. nov., isolated from the new zealand grass grub Costelytra zealandica. Int. J. Syst. Evol. Microbiol. 2011, 61, 844–849. [Google Scholar] [CrossRef]
- Mansfield, S.; Wilson, M.J.; Gerard, P.J.; Wilson, D.J.; Swaminathan, J.; Wright, D.A.; van Koten, C.; Hurst, M.R. Potential for a biopesticide bait to control black beetle, Heteronychus arator (Coleoptera: Scarabaeidae). Pest Manag. Sci. 2020, 76, 4150–4158. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Liu, H.; Zhang, F.; Wang, J.; Liu, Q.; Zhao, W. Bacillus megaterium Zlp-21 and Its Applications 2022. Available online: http://www.soopat.com/Patent/202210954204 (accessed on 1 January 2023).
- Lee, S.A.; Kim, Y.; Kim, J.M.; Chu, B.; Joa, J.-H.; Sang, M.K.; Song, J.; Weon, H.-Y. A Preliminary examination of bacterial, archaeal, and fungal communities inhabiting different rhizocompartments of tomato plants under real-world environments. Sci. Rep. 2019, 9, 9300. [Google Scholar] [CrossRef]
- Shu, C.; Yan, G.; Huang, S.; Geng, Y.; Soberon, M.; Bravo, A.; Geng, L.; Zhang, J. Characterization of two novel Bacillus thuringiensis Cry8 toxins reveal differential specificity of protoxins or activated toxins against chrysomeloidea coleopteran superfamily. Toxins 2020, 12, 642. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, K.; Shu, C.; Zhang, J. Analysis of functional genes of Bacillus thuringiensis isolates against white grubs by comparative genomics. Chin. J. Biol. Control. 2018, 34, 693–700. (In Chinese) [Google Scholar] [CrossRef]
- Morishita, M.; Mon, H.; Lee, J.M.; Kusakabe, T.; Cassal, M.C.; Yasunaga-Aoki, C.; Iiyama, K. Toxin complex is a major virulence determinant of Enterobacter Sp. 532 against the silkworm, Bombyx mori. J. Insect Biotechnol. Sericol. 2019, 88, 1021–1025. [Google Scholar] [CrossRef]
- Morishita, M.; Masuda, A.; Mon, H.; Lee, J.M.; Kusakabe, T.; Tashiro, K.; Yasunaga-Aoki, C.; Iiyama, K. Identification of an insecticidal toxin produced by Enterobacter sp. strain 532 isolated from diseased Bombyx mori silkworms. FEMS Microbiol. Lett. 2019, 366, fny295. [Google Scholar] [CrossRef] [PubMed]
- Hang, F.; Liu, P.; Mu, H.; Wang, Q.; Liu, Z.; Chen, W. Advance in recent research on bacterial metalloproteases. Ind. Microbiol. 2015, 45, 53–59. (In Chinese) [Google Scholar]
- Lee, S.A.; Jang, S.H.; Kim, B.H.; Shibata, T.; Yoo, J.; Jung, Y.; Kawabata, S.; Lee, B.L. Insecticidal activity of the metalloprotease AprA occurs through suppression of host cellular and humoral immunity. Dev. Comp. Immunol. 2018, 81, 116–126. [Google Scholar] [CrossRef]
- Yesilyurt, A.; Muratoglu, H.; Demirbag, Z.; Nalcacioglu, R. Chilo iridescent virus encodes two functional metalloproteases. Arch. Virol. 2019, 164, 657–665. [Google Scholar] [CrossRef]
- Diab, M.R.; Ahmed, M.M.; Hussein, E.H.A.; Ahmed, M.A. Isolation of the astacin-like metalloprotease coding gene (Astl) and assessment of its insecticidal activity towards Spodoptera littoralis and Sitophilus oryzae. Jordan J. Biol. Sci. 2021, 14, 677–685. [Google Scholar] [CrossRef]
- Lu, W.; Cai, J.; Chen, Y. Research Progress on Bacillus thuringiensis chitinase. Microbiol. China 2007, 34, 143–147. (In Chinese) [Google Scholar] [CrossRef]
- Broderick, N.A.; Goodman, R.M.; Raffa, K.F.; Handelsman, J. Synergy between Zwittermicin A and Bacillus thuringiensis subsp kurstaki against gypsy moth (Lepidoptera: Lymantriidae). Environ. Entomol. 2000, 29, 101–107. [Google Scholar] [CrossRef]
- Wang, M.; Geng, L.; Xue, B.; Wang, Z.; Xu, W.; Shu, C.; Zhang, J. Structure characteristics and function of a novel extracellular polysaccharide from Bacillus thuringiensis strain 4D19. Int. J. Biol. Macromol. 2021, 189, 956–964. [Google Scholar] [CrossRef]
- Raymond, B.; Johnston, P.R.; Wright, D.J.; Ellis, R.J.; Crickmore, N.; Bonsall, M.B. A mid-gut microbiota is not required for the pathogenicity of Bacillus thuringiensis to diamondback moth larvae. Environ. Microbiol. 2009, 11, 2556–2563. [Google Scholar] [CrossRef]
- Buisson, C.; Gohar, M.; Huillet, E.; Nielsen-LeRoux, C. Bacillus thuringiensis spores and vegetative bacteria: Infection capacity and role of the virulence regulon PlcR following intrahaemocoel injection of Galleria mellonella. Insects 2019, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.L.; Stepien, T.A.; Blum, J.E.; Holt, J.F.; Labbe, N.H.; Rush, J.S.; Raffa, K.F.; Handelsman, J. From commensal to pathogen: Translocation of Enterococcus faecalis from the midgut to the hemocoel of Manduca sexta. mBio 2011, 2, e00065-11. [Google Scholar] [CrossRef] [PubMed]
- Caccia, S.; Di Lelio, I.; La Storia, A.; Marinelli, A.; Varricchio, P.; Franzetti, E.; Banyuls, N.; Tettamanti, G.; Casartelli, M.; Giordana, B. Midgut microbiota and host immunocompetence underlie Bacillus thuringiensis killing mechanism. Proc. Natl. Acad. Sci. USA 2016, 113, 9486–9491. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, D.; Wu, H.; Ji, Y.; Liu, Z.; Guo, X.; Guo, W.; Bi, Y. Bt GS57 interaction with gut microbiota accelerates Spodoptera exigua mortality. Front. Microbiol. 2022, 13, 835227. [Google Scholar] [CrossRef]
- Gao, Y.; Jurat-Fuentes, J.L.; Oppert, B.; Fabrick, J.A.; Liu, C.; Gao, J.; Lei, Z. Increased toxicity of Bacillus thuringiensis Cry3Aa against Crioceris quatuordecimpunctata, Phaedon brassicae and Colaphellus bowringi by a Tenebrio molitor cadherin fragment. Pest. Manag. Sci. 2011, 67, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y. Discovery and Activity Analysis of Novel Insecticidal Genes from Bacillus thuringiensis. Ph.D. Thesis, Northeast Agricultural University, Harbin, China, 14 June 2019. [Google Scholar]
- Tel-Zur, N.; Abbo, S.; Myslabodski, D.; Mizrahi, Y. Modified ctab procedure for DNA isolation from epiphytic cacti of the genera Hylocereus and Selenicereus (Cactaceae). Plant Mol. Biol. Rep. 1999, 17, 249–254. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. Circlize implements and enhances circular visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zeng, H.M.; Lin, H.F.; Yang, X.F.; Liu, Z.; Guo, L.H.; Yuan, J.J.; Qiu, D.W. An insecticidal protein from Xenorhabdus budapestensis that results in prophenoloxidase activation in the wax moth, Galleria mellonella. J. Invertebr. Pathol. 2012, 110, 60–67. [Google Scholar] [CrossRef]
- Wadley, F.M. Experimental Statistics in Entomology; Graduate School Press: Washington, DC, USA, 1967; pp. 80–85. [Google Scholar]
- Wu, G.; Yi, Y. Haemocoel injection of PirA1B1 to Glleria mellonella larvae leads to disruption of the haemocyte immune functions. Sci. Rep. 2016, 6, 34996. [Google Scholar] [CrossRef] [PubMed]
- Finney, D.J. Probit Analysis; Cambridge University Press: London, UK, 1971; p. 333. [Google Scholar]


| Strains | The LC50 Values (×108 CFU/g) (95% Confidence Interval) | |||
|---|---|---|---|---|
| C. bowringi | H. oblita | A. corpulenta | P. brevitarsis | |
| IPPBiotE33 | 5.18 (1.04–19.80) | 3.71 (1.23–7.73) | 8.40 (2.12–18.78) | 0.19 (0.02–0.53) |
| IPPBiotC41 | — | 83.31 (25.33–191.62) | 18.12 (2.86–52.96) | 0.78 (0.12–1.89) |
| IPPBiotA42 | — | 351.16 (113.52–919.17) | 10.43 (1.97–23.43) | 1.16 (0.27–2.87) |
| IPPBiotC43 | — | 416.28 (114.84–911.42) | 29.64 (2.99–85.74) | 1.82 (0.30–4.41) |
| Strains | Corrected Mortality (Mean ± SD) (%) | |||||
|---|---|---|---|---|---|---|
| Third-Instar Larvae | First-Instar Larvae | |||||
| H. parallela a | H. oblita a | A. corpulenta a | P. brevitarsis a | H. armigera ab | A. ypsilon b | |
| IPPBiotE33 | 68.87 ± 3.72 | 59.33 ± 2.01 | 61.76 ± 3.27 | 44.10 ± 3.57 | 21.91 ± 3.32 | 13.10 ± 2.72 |
| IPPBiotC41 | 48.50 ± 1.70 | 34.57 ± 4.13 | 35.29 ± 2.51 | 28.43 ± 4.31 | 13.90 ± 9.01 | 11.90 ± 2.42 |
| IPPBiotA42 | 54.33 ± 4.15 | 21.60 ± 4.10 | 50.00 ± 4.21 | 20.57 ± 3.70 | 14.60 ± 12.52 | 4.76 ± 1.14 |
| IPPBiotC43 | 42.60 ± 1.51 | 23.53 ± 2.60 | 40.12 ± 1.94 | 19.63 ± 2.65 | 22.14 ± 7.12 | 10.71 ± 4.71 |
| Genomic Contents | Number |
|---|---|
| Plasmid | 1 |
| Gene | 4562 |
| CDS | 4305 |
| tRNA | 85 |
| rRNA | 25 |
| Strains | LC50 Values (107 CFU/g) (95% Confidence Interval) | Synergic Ratio (SR) |
|---|---|---|
| IPPBiotE33 | 8.81 (1.53–22.40) | 3.66 |
| Bt185 | 6.59 (1.44–14.14) | |
| IPPBiotE33:Bt185 = 1:1 | 2.06 (0.38–4.63) |
| Data (Mean ± SD) | PBS Buffer | IPPBiotE33 | Bt185 | IPPBiotE33 + Bt185 |
|---|---|---|---|---|
| Changes in root length (cm) | 0.878 ± 0.630 b | 3.38 ± 0.597 a | 4.307 ± 0.519 a | 5.577 ± 0.814 a |
| Changes in shoot height (cm) | 0.971 ± 0.667 d | 3.961 ± 1.285 c | 4.096 ± 1.910 b | 4.265 ± 2.180 a |
| Changes in fresh weight (g) | 1.518 ± 0.305 c | 4.105 ± 0.351 b | 5.041 ± 1.948 b | 8.486 ± 1.710 a |
| Changes in dry weight (g) | 0.502 ± 0.519 c | 1.387 ± 0.490 b | 1.484 ± 1.375 ab | 2.063 ± 0.717 a |
| Weight reduction rate of larvae (%) | — | 21.83 ± 41.57 b | 34.85 ± 24.24 b | 52.86 ± 18.28 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, L.; Gu, Z.; Li, Y.; Xu, W.; Shu, C.; Zhang, J.; Bai, X.; Geng, L. Enterobacter Strain IPPBiotE33 Displays a Synergistic Effect with Bacillus thuringiensis Bt185. Int. J. Mol. Sci. 2023, 24, 14193. https://doi.org/10.3390/ijms241814193
Mi L, Gu Z, Li Y, Xu W, Shu C, Zhang J, Bai X, Geng L. Enterobacter Strain IPPBiotE33 Displays a Synergistic Effect with Bacillus thuringiensis Bt185. International Journal of Molecular Sciences. 2023; 24(18):14193. https://doi.org/10.3390/ijms241814193
Chicago/Turabian StyleMi, Liang, Ziqiong Gu, Ying Li, Wenyue Xu, Changlong Shu, Jie Zhang, Xi Bai, and Lili Geng. 2023. "Enterobacter Strain IPPBiotE33 Displays a Synergistic Effect with Bacillus thuringiensis Bt185" International Journal of Molecular Sciences 24, no. 18: 14193. https://doi.org/10.3390/ijms241814193
APA StyleMi, L., Gu, Z., Li, Y., Xu, W., Shu, C., Zhang, J., Bai, X., & Geng, L. (2023). Enterobacter Strain IPPBiotE33 Displays a Synergistic Effect with Bacillus thuringiensis Bt185. International Journal of Molecular Sciences, 24(18), 14193. https://doi.org/10.3390/ijms241814193







