Immunohistochemical Identification and Assessment of the Location of Immunoproteasome Subunits LMP2 and LMP7 in Acquired Cholesteatoma
Abstract
1. Introduction
- Stage I: Cholesteatoma localized in the primary site (the site of cholesteatoma origin, i.e., the attic (for pars flaccida cholesteatoma); the tympanic cavity (for pars tensa cholesteatoma).
- Stage II: Cholesteatoma involving two or more sites.
- Stage III: Cholesteatoma with extracranial complications or pathologic conditions including facial palsy, labyrinthine fistula with conditions at risk of labyrinthitis, postauricular abscesses or fistula, zygomatic abscesses, and neck abscesses [1].
2. Results
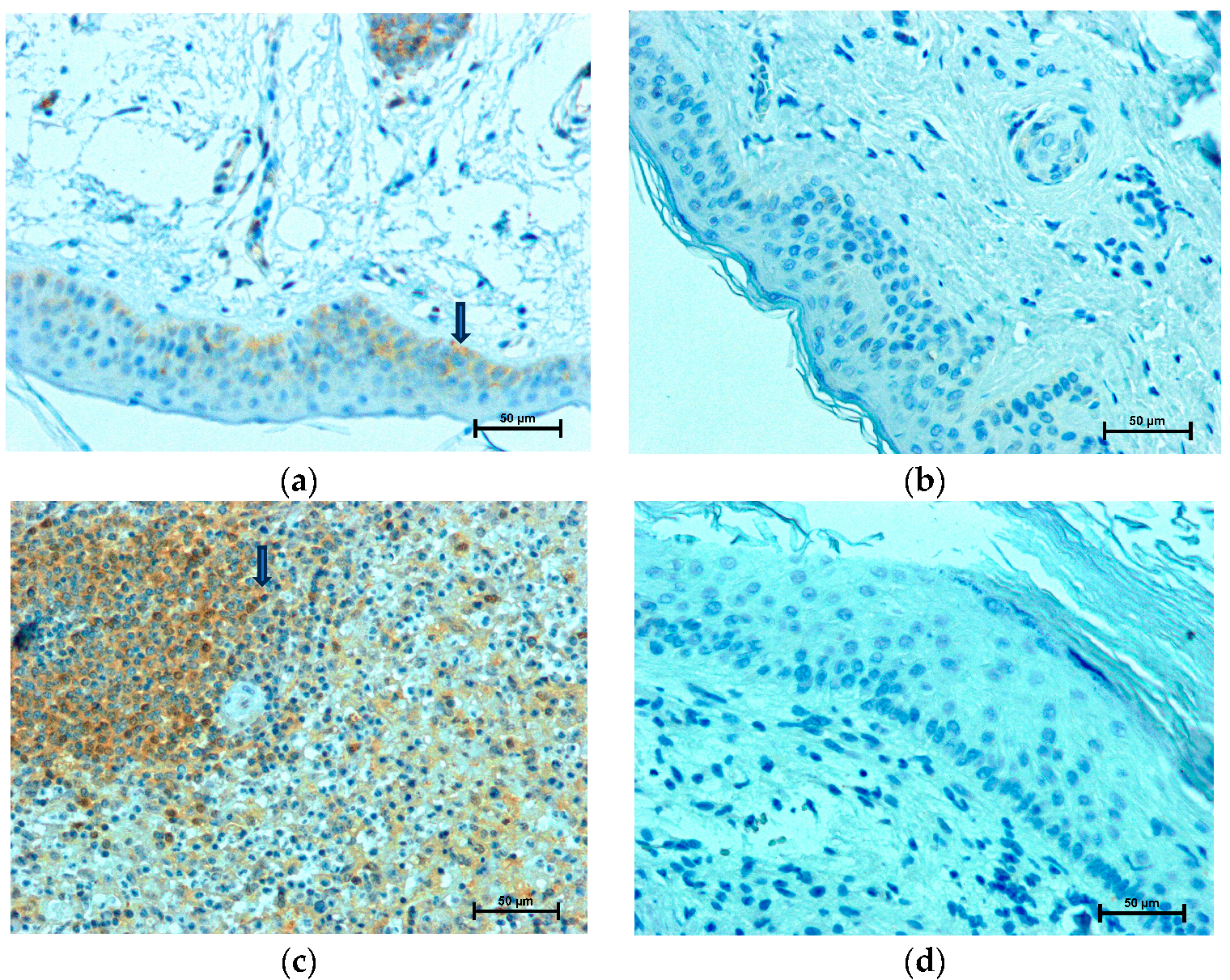
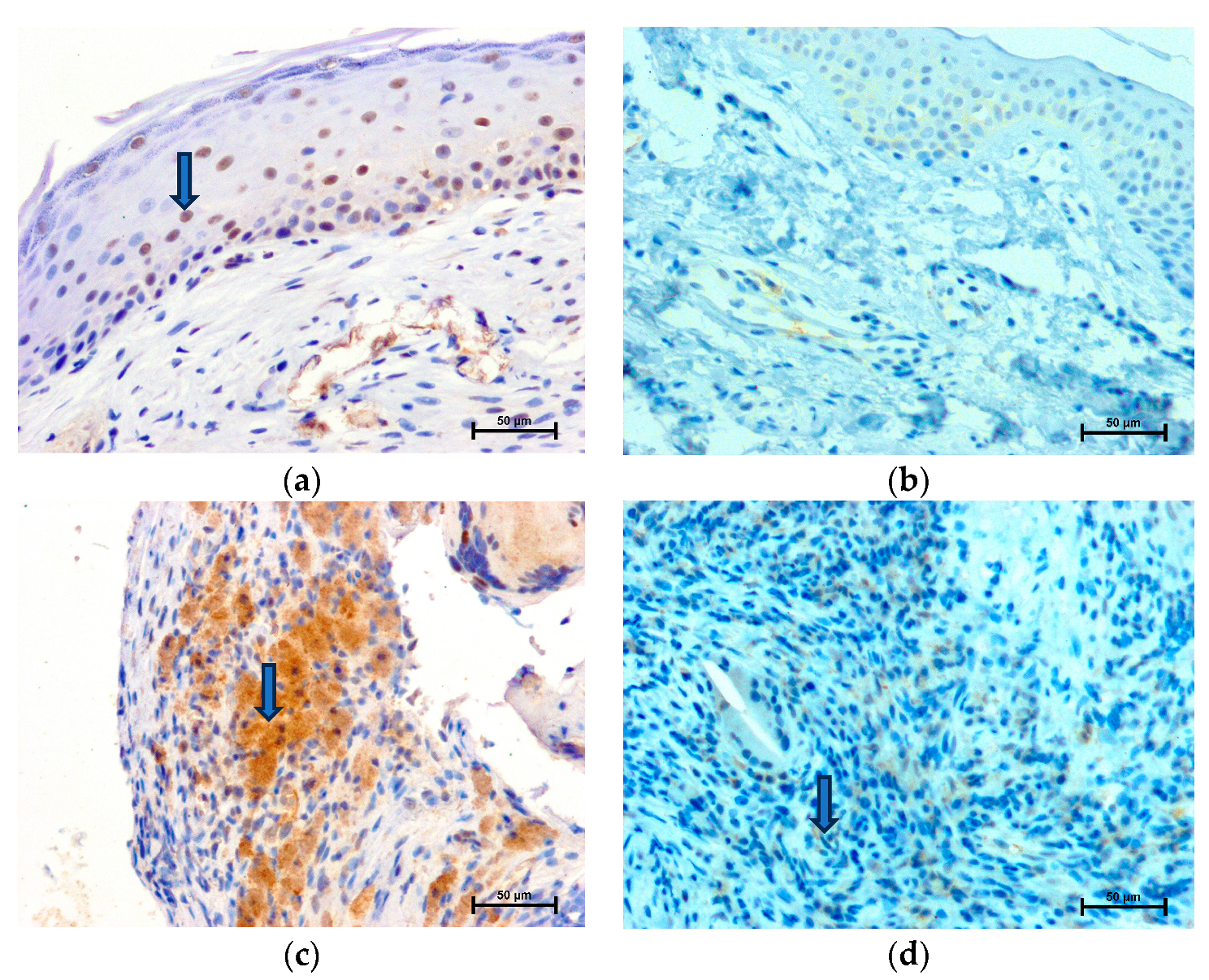
2.1. LMP2 Expression
2.2. LMP7 Expression
3. Materials and Methods
3.1. Study Samples
3.2. Immunohistochemistry
3.3. Quantitative Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yung, M.; Tono, T.; Olszewska, E.; Yamamoto, Y.; Sudhoff, H.; Sakagami, M.; Mulder, J.; Kojima, H.; İncesulu, A.; Trabalzini, F.; et al. EAONO/JOS Joint Consensus Statements on the Definitions, Classification and Staging of Middle Ear Cholesteatoma. J. Int. Adv. Otol. 2017, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zanker, D.; Pang, K.; Oveissi, S.; Lu, C.; Faou, P.; Nowell, C.; Mbogo, G.W.; Carotta, S.; Quillici, C.; Karupiah, G.; et al. LMP2 immunoproteasome promotes lymphocyte survival by degrading apoptotic BH3-only proteins. Immunol. Cell Biol. 2018, 96, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Miller, Z.; Ao, L.; Kim, K.B.; Lee, W. Inhibitors of the immunoproteasome: Current status and future directions. Curr. Pharm. Des. 2013, 19, 4140–4151. [Google Scholar] [CrossRef] [PubMed]
- Wehenkel, M.; Ban, J.O.; Ho, Y.K.; Carmony, K.C.; Hong, J.T.; Kim, K.B. A selective inhibitor of the immunoproteasome subunit LMP2 induces apoptosis in PC-3 cells and suppresses tumour growth in nude mice. Br. J. Cancer 2012, 107, 53–62. [Google Scholar] [CrossRef]
- Muchamuel, T.; Basler, M.; Aujay, M.A.; Suzuki, E.; Kalim, K.W.; Lauer, C.; Sylvain, C.; Ring, E.R.; Shields, J.; Jiang, J.; et al. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nat. Med. 2009, 15, 781–787. [Google Scholar]
- Basler, M.; Kirk, C.J.; Groettrup, M. The immunoproteasome in antigen processing and other immunological functions. Curr. Opin. Immunol. 2013, 25, 74–80. [Google Scholar] [CrossRef]
- Ortega-Atienza, S.; Krawic, C.; Watts, L.; McCarthy, C.; Luczak, M.W.; Zhitkovich, A. 20S immunoproteasomes remove formaldehyde-damaged cytoplasmic proteins suppressing caspase-independent cell death. Sci. Rep. 2017, 7, 654. [Google Scholar] [CrossRef]
- Yang, Z.; Gagarin, D.; St Laurent, G., 3rd; Hammell, N.; Toma, I.; Hu, C.A.; Iwasa, A.; McCaffrey, T.A. Cardiovascular inflammation and lesion cell apoptosis: A novel connection via the interferon-inducible immunoproteasome. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1213–1219. [Google Scholar] [CrossRef]
- Seifert, U.; Bialy, L.P.; Ebstein, F.; Bech-Otschir, D.; Voigt, A.; Schröter, F.; Prozorovski, T.; Lange, N.; Steffen, J.; Rieger, M.; et al. Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell 2010, 142, 613–624. [Google Scholar] [CrossRef]
- Van Deventer, S.; Neefjes, J. The Immunoproteasome Cleans up after Inflammation. Cell 2010, 142, 517–518. [Google Scholar] [CrossRef][Green Version]
- Olszewska, E.; Chodynicki, S.; Chyczewski, L. Apoptosis in the pathogenesis ofcholesteatoma in adults. Eur. Arch. Otorhinolaryngol. 2006, 263, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Garça, M.F.; Turan, M.; Avşar, B.; Kalkan, F.; Demir, H.; Kozan, A.; Bozan, N. The evaluation of oxidative stress in the serum and tissue specimens of patients with chronic otitis media. Clin. Exp. Otorhinolaryngol. 2015, 8, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Szczepanski, M.J.; Luczak, M.; Olszewska, E.; Molinska-Glura, M.; Zagor, M.; Krzeski, A.; Skarzynski, H.; Misiak, J.; Dzaman, K.; Bilusiak, M.; et al. Molecular signaling of the HMGB1/RAGE axis contributes to cholesteatoma pathogenesis. J. Mol. Med. 2015, 93, 305–314. [Google Scholar] [CrossRef]
- Herman, G.E.; Elfont, E.A. The taming of immunohistochemistry: The new era of quality control. Biotech. Histochem. 1991, 66, 194–199. [Google Scholar] [CrossRef]
- Bhattarai, S.; Ghannam, K.; Krause, S.; Benveniste, O.; Marg, A.; de Bruin, G.; Xin, B.T.; Overkleeft, H.S.; Spuler, S.; Stenzel, W.; et al. The immunoproteasomes are key to regulate myokines and MHC class I expression in idiopathic inflammatory myopathies. J. Autoimmun. 2016, 75, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Bujía, J.; Holly, A.; Kim, C.; Scanady, N.; Kastenbauer, E. Expression of human intercellular adhesion molecules in middle ear cholesteatoma. Am. J. Otolaryngol. 1994, 15, 271–275. [Google Scholar] [CrossRef]
- Sastry, K.V.; Sharma, S.C.; Mann, S.B.; Ganguly, N.K.; Panda, N.K. Aural cholesteatoma: Role of tumor necrosis factor-alpha in bone destruction. Am. J. Otol. 1999, 20, 158–161. [Google Scholar]
- Akimoto, R.; Pawankar, R.; Yagi, T.; Baba, S. Acquired and congenital cholesteatoma: Determination of tumor necrosis factor-alpha, intercellular adhesion molecule-1, interleukin-1-alpha and lymphocyte functional antigen-1 in the inflammatory process. ORL J. Otorhinolaryngol. Relat. Spec. 2000, 62, 257–265. [Google Scholar] [CrossRef]
- Vitale, R.F.; de Andrade Quintanilha Ribeiro, F. The role of tumor necrosis factor-alpha (TNF-alpha) in bone 346 resorption present in middle ear cholesteatoma. Braz. J. Otorhinolaryngol. 2007, 73, 117–121. [Google Scholar] [CrossRef]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, R.; Peng, H.; Liu, H.; Wen, L.; Wu, T.; Yi, H.; Li, A.; Zhang, Z. MiR-451 suppresses the NF-kappaB-mediated proinflammatory molecules expression through inhibiting LMP7 in diabetic nephropathy. Mol. Cell Endocrinol. 2016, 433, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Qin, Z.B. Inflammation-induced miR-802 promotes cell proliferation in cholesteatoma. Biotechnol. Lett. 2014, 36, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.Y.; Yune, T.Y.; Lee, J.Y.; Yeo, S.G.; Park, M.S. Expression of CYLD and NF-kappaB in human cholesteatoma epithelium. Mediat. Inflamm. 2010, 2010, 796315. [Google Scholar] [CrossRef] [PubMed]
- Huisman, M.A.; De Heer, E.; Grote, J.J. Survival signaling and terminal differentiation in cholesteatoma epithelium. Acta Otolaryngol. 2007, 27, 424–429. [Google Scholar] [CrossRef]
- Khan, M.A.; Oubrahim, H.; Stadtman, E.R. Inhibition of apoptosis in acute promyelocytic leukemia cells leads to increases in levels of oxidized protein and LMP2 immunoproteasome. Proc. Natl. Acad. Sci. USA 2004, 101, 11560–11565. [Google Scholar] [CrossRef]
- Wang, H.X.; Wang, H.M.; Li, Q.L.; Lin, H.Y.; Qian, D.; Zhu, C. Expression of proteasome subunits low molecular mass polypeptide (LMP) 2 and LMP7 in the endometrium and placenta of rhesus monkey (Macaca mulatta) during early pregnancy. Biol. Reprod. 2004, 71, 1317–1324. [Google Scholar] [CrossRef]
- Wang, H.X.; Wang, H.M.; Lin, H.Y.; Yang, Q.; Zhang, H.; Tsang, B.K.; Zhu, C. Proteasome subunit LMP2 is required for matrix metalloproteinase-2 and -9 expression and activities in human invasive extravillous trophoblast cell line. J. Cell Physiol. 2006, 206, 616–623. [Google Scholar] [CrossRef]
- Suchozebrska-Jesionek, D.; Szymański, M.; Kurzepa, J.; Gołabek, W.; Stryjecka-Zimmer, M. Gelatinolytic activity of matrix metalloproteinases 2 and 9 in middle ear cholesteatoma. J. Otolaryngol. Head. Neck Surg. 2008, 37, 628–632. [Google Scholar]
- Jiang, H.; Si, Y.; Li, Z.; Huang, X.; Chen, S.; Zheng, Y.; Xu, G.; Chen, X.; Chen, Y.; Liu, Y.; et al. TREM-2 promotes acquired cholesteatoma-induced bone destruction by modulating TLR4 signaling pathway and osteoclasts activation. Sci. Rep. 2016, 6, 38761. [Google Scholar] [CrossRef]
| Gender | Age | Ossicular Chain Destruction | The EAONO/JOS Staging System |
|---|---|---|---|
| female | 31 | absent of maleus and incus with intact stapes | III (Labyrhintitis) |
| female | 28 | erosion of maleus and incus with intact stapes | I |
| female | 41 | erosion of maleus and incus with intact stapes | I |
| female | 59 | erosion of maleus and incus with intact stapes | I |
| male | 57 | erosion of maleus and incus with erosion of stapes | I |
| female | 69 | erosion of maleus and incus with intact stapes | III (Facial palsy) |
| male | 57 | erosion of maleus and incus with intact stapes | III (Labyrinthine fistula) |
| female | 56 | absent of incus with erosion of maleus and intact stapes | II |
| male | 27 | erosion of maleus and incus with intact stapes | II |
| female | 28 | erosion of maleus and incus with intact stapes | II |
| female | 57 | absent of incus with erosion of maleus and erosion of stapes | III (Labyrinthine fistula) |
| male | 74 | absent of maleus and incus with erosion of stapes | II |
| female | 66 | absent of maleus, incus and stapes | II |
| female | 56 | erosion of maleus, incus and stapes | II |
| male | 44 | erosion of maleus and incus with intact stapes | II |
| LMP2 Cytoplasm | LMP2 Nuclei | LMP7 Cytoplasm | LMP7 Nuclei | |
|---|---|---|---|---|
| Cholesteatoma Matrix | 161.9 ± 3.10 | No reaction | 210.9 ± 2.39 | 125.7 ± 3.21 |
| Control Skin Epidermis | No Reaction | No reaction | 222.4 ± 1.55 | No reaction |
| Cholesteatoma Perimatrix | 141.3 ± 2.42 | No reaction | 131.0 ± 2.71 | No reaction |
| Control Skin Dermis | No Reaction | No reaction | 181.6 ± 2.60 | No reaction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rutkowska, J.; Kasacka, I.; Rogowski, M.; Olszewska, E. Immunohistochemical Identification and Assessment of the Location of Immunoproteasome Subunits LMP2 and LMP7 in Acquired Cholesteatoma. Int. J. Mol. Sci. 2023, 24, 14137. https://doi.org/10.3390/ijms241814137
Rutkowska J, Kasacka I, Rogowski M, Olszewska E. Immunohistochemical Identification and Assessment of the Location of Immunoproteasome Subunits LMP2 and LMP7 in Acquired Cholesteatoma. International Journal of Molecular Sciences. 2023; 24(18):14137. https://doi.org/10.3390/ijms241814137
Chicago/Turabian StyleRutkowska, Justyna, Irena Kasacka, Marek Rogowski, and Ewa Olszewska. 2023. "Immunohistochemical Identification and Assessment of the Location of Immunoproteasome Subunits LMP2 and LMP7 in Acquired Cholesteatoma" International Journal of Molecular Sciences 24, no. 18: 14137. https://doi.org/10.3390/ijms241814137
APA StyleRutkowska, J., Kasacka, I., Rogowski, M., & Olszewska, E. (2023). Immunohistochemical Identification and Assessment of the Location of Immunoproteasome Subunits LMP2 and LMP7 in Acquired Cholesteatoma. International Journal of Molecular Sciences, 24(18), 14137. https://doi.org/10.3390/ijms241814137






