Oncogene-Induced Senescence Is a Crucial Antitumor Defense Mechanism of Human Endometrial Stromal Cells
Abstract
1. Introduction
2. Results
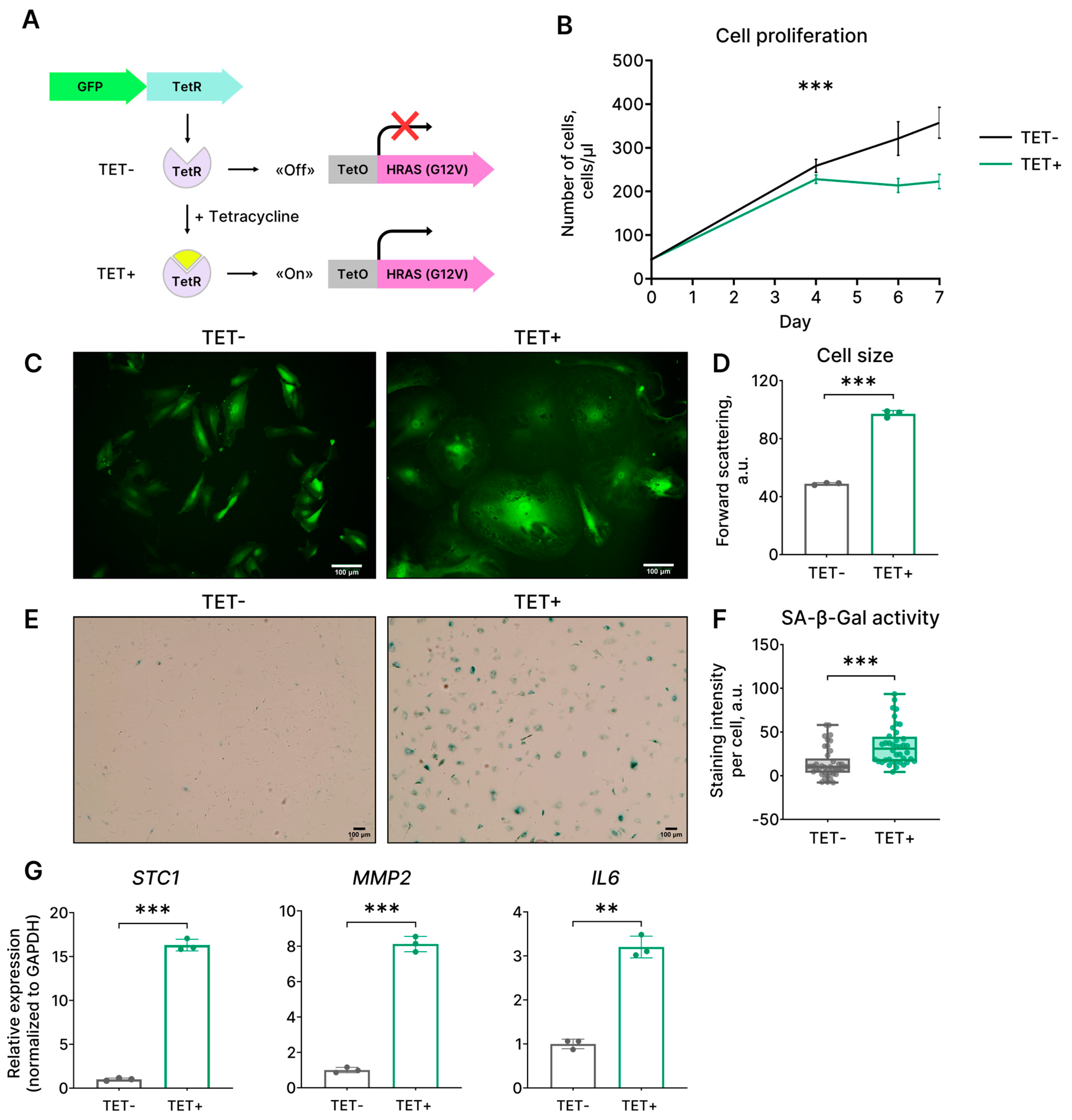
2.1. Expression of HRASG12V Results in a Senescence-Like Phenotype in EnSCs
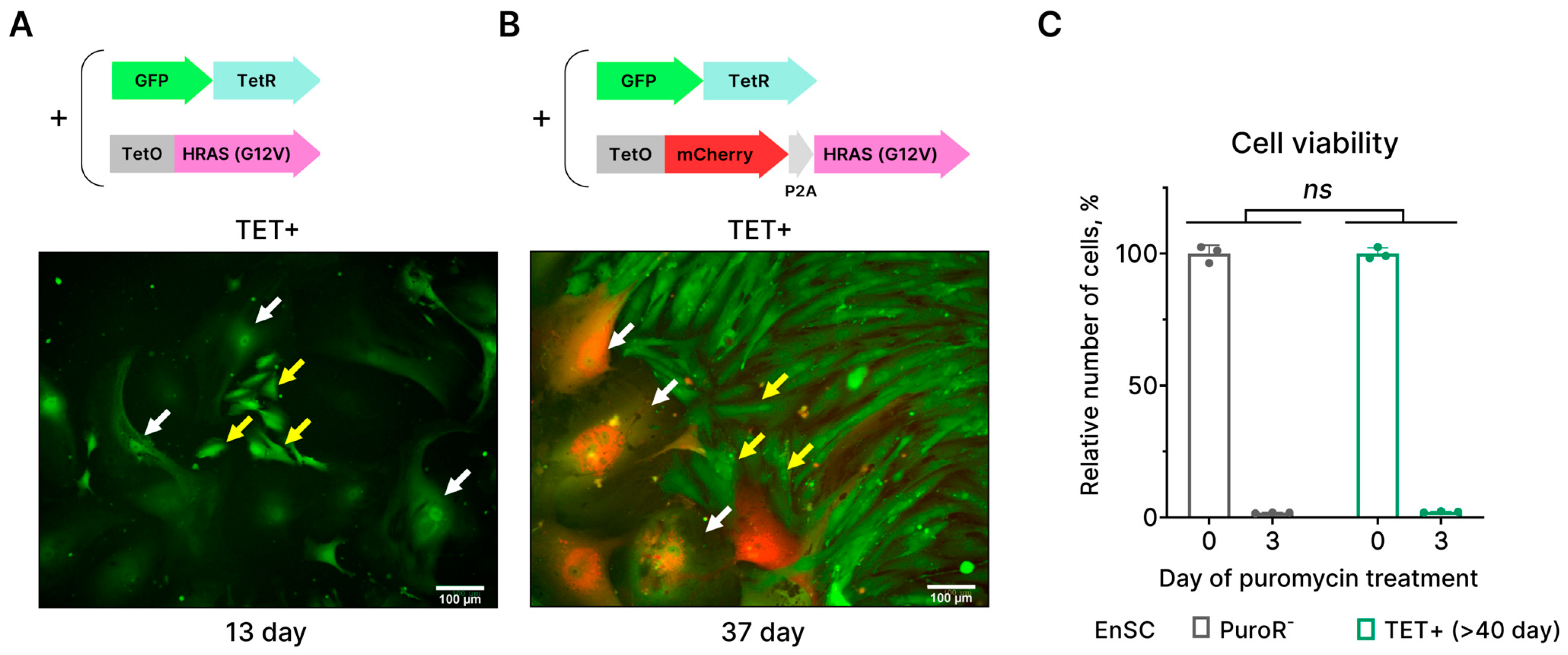
2.2. EnSCs Expressing HRASG12V Are Unable to Bypass Senescence
2.3. Estrogen Supplementation Does Not Prevent Proliferation Loss in HRASG12V-Expressing EnSCs
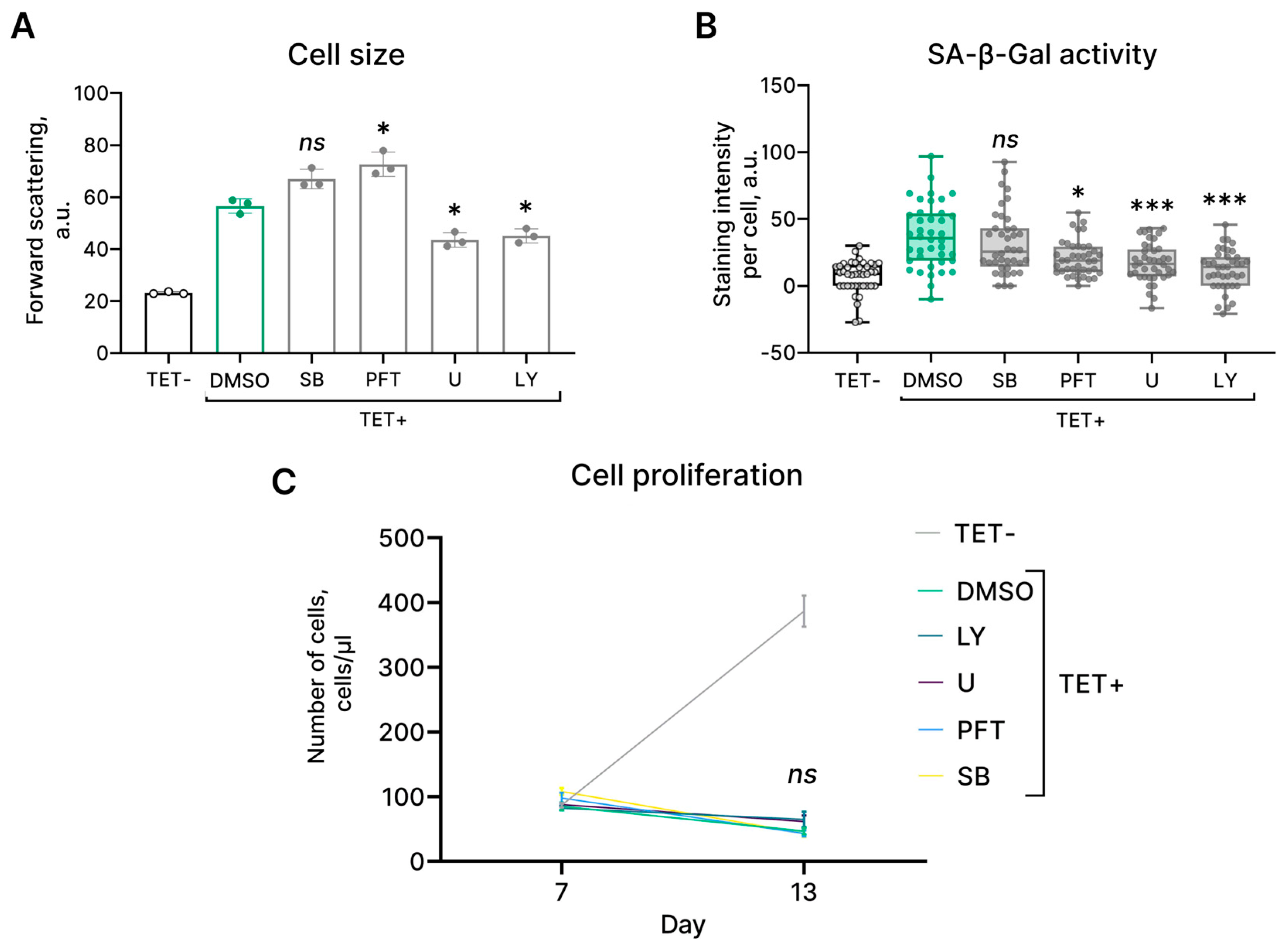
2.4. Molecular Mechanisms Regulating HRASG12V-Induced Senescence in EnSCs
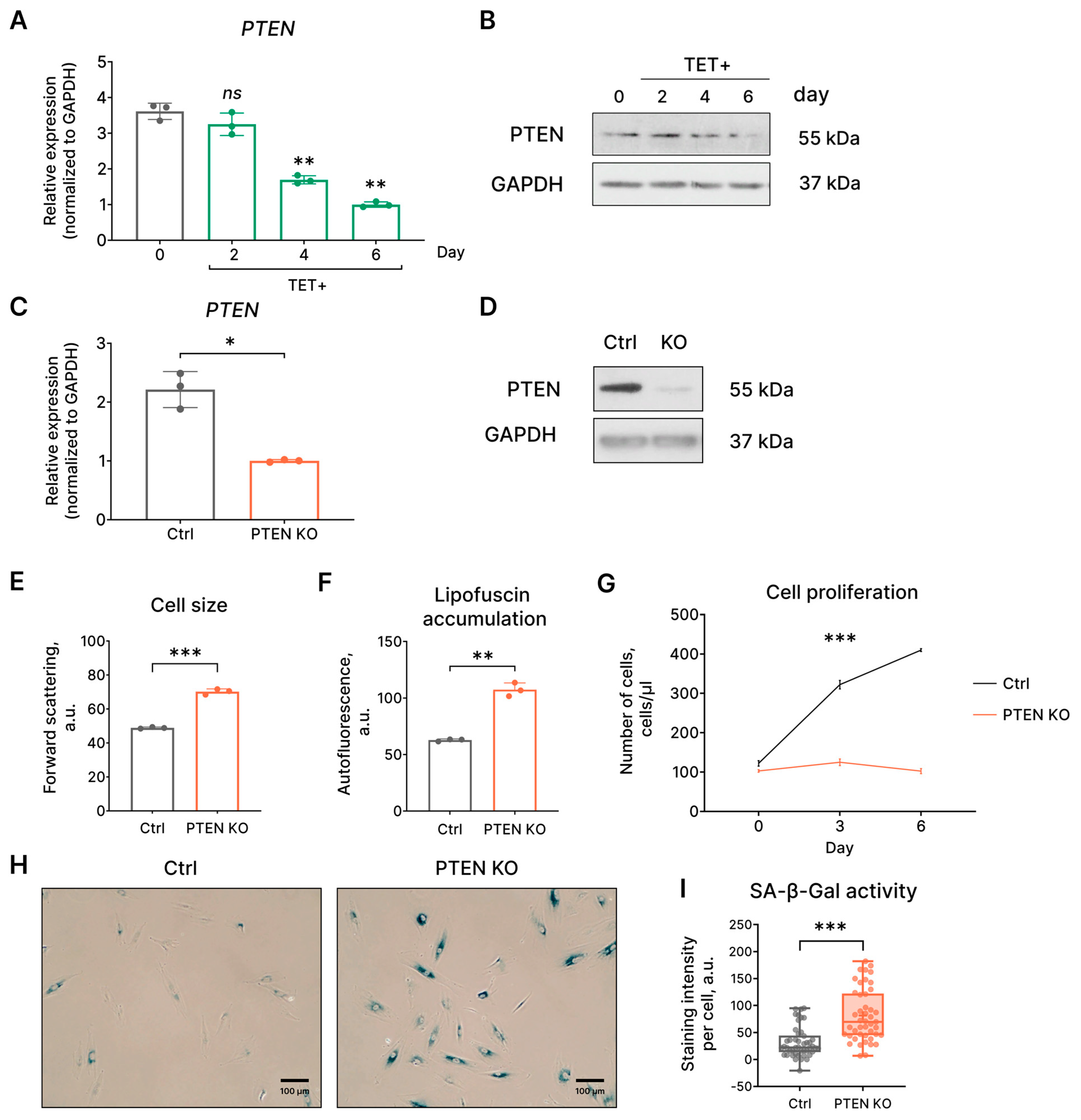
2.5. PTEN Loss-Induced Senescence Is a Backup Molecular Mechanism to Prevent the Transformation of HRASG12V-Expressing EnSCs
3. Discussion
4. Materials and Methods
4.1. EnSC Culture Conditions
4.2. Molecular Cloning
4.3. Lentiviral Transduction and Tetracycline Treatment
4.4. Cells Treatment Conditions
4.5. Flow Cytometry
4.6. SA-β-Gal Staining
4.7. Western Blotting
4.8. Immunofluorescence
4.9. RNA Extraction, Reverse Transcription, and Real-Time PCR
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EnSCs | human MSCs derived from endometrium |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DDR | DNA damage response |
| HSD | honestly significant difference |
| OIS | oncogene-induced senescence |
| SASP | senescence-associated secretory phenotype |
| SA-β-Gal | senescence-associated β-Galactosidase |
References
- Owusu-Akyaw, A.; Krishnamoorthy, K.; Goldsmith, L.T.; Morelli, S.S. The role of mesenchymal-epithelial transition in endometrial function. Hum. Reprod. Update 2019, 25, 114–133. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.W.R. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.; Leongamornlert, D.; Coorens, T.H.H.; Sanders, M.A.; Ellis, P.; Dentro, S.C.; Dawson, K.J.; Butler, T.; Rahbari, R.; Mitchell, T.J.; et al. The mutational landscape of normal human endometrial epithelium. Nature 2020, 580, 640–646. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Nakaoka, H.; Suda, K.; Yoshihara, K.; Ishiguro, T.; Yachida, N.; Saito, K.; Ueda, H.; Sugino, K.; Mori, Y.; et al. Spatiotemporal dynamics of clonal selection and diversification in normal endometrial epithelium. Nat. Commun. 2022, 13, 943. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, D.; Sung, W.J.; Hong, J. High-Grade Endometrial Stromal Sarcoma: Molecular Alterations and Potential Immunotherapeutic Strategies. Front. Immunol. 2022, 13, 837004. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Liang, J.; Zhang, Y.; Jiang, N.; Xu, Y.; Qiu, M.; Wang, Y.; Zhao, B.; Chen, X. Single-cell transcriptomic analysis highlights origin and pathological process of human endometrioid endometrial carcinoma. Nat. Commun. 2022, 13, 6300. [Google Scholar] [CrossRef]
- Campisi, J.; Dimri, G.; Hara, E. Handbook of the Biology of Aging; Academic Press: New York, NY, USA, 1996. [Google Scholar]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular senescence: The good, the bad and the unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef]
- Serrano, M.; Lin, A.W.; McCurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997, 88, 593–602. [Google Scholar] [CrossRef]
- Zhu, H.; Blake, S.; Kusuma, F.K.; Pearson, R.B.; Kang, J.; Chan, K.T. Oncogene-induced senescence: From biology to therapy. Mech. Ageing Dev. 2020, 187, 111229. [Google Scholar] [CrossRef]
- Collado, M.; Serrano, M. Senescence in tumours: Evidence from mice and humans. Nat. Rev. Cancer 2010, 10, 51–57. [Google Scholar] [CrossRef]
- Saab, R. Senescence and pre-malignancy: How do tumors progress? Semin. Cancer Biol. 2011, 21, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Komseli, E.S.; Pateras, I.S.; Krejsgaard, T.; Stawiski, K.; Rizou, S.V.; Polyzos, A.; Roumelioti, F.M.; Chiourea, M.; Mourkioti, I.; Paparouna, E.; et al. A prototypical non-malignant epithelial model to study genome dynamics and concurrently monitor micro-RNAs and proteins in situ during oncogene-induced senescence. BMC Genom. 2018, 19, 37, Erratum in BMC Genom. 2022, 22, 327. [Google Scholar] [CrossRef]
- Martínez-Zamudio, R.I.; Stefa, A.; Nabuco Leva Ferreira Freitas, J.A.; Vasilopoulos, T.; Simpson, M.; Doré, G.; Roux, P.F.; Galan, M.A.; Chokshi, R.J.; Bischof, O.; et al. Escape from oncogene-induced senescence is controlled by POU2F2 and memorized by chromatin scars. Cell Genom. 2023, 3, 100293. [Google Scholar] [CrossRef] [PubMed]
- Borodkina, A.; Shatrova, A.; Abushik, P.; Nikolsky, N.; Burova, E. Interaction between ROS dependent DNA damage, mitochondria and p38 MAPK underlies senescence of human adult stem cells. Aging 2014, 6, 481–495. [Google Scholar] [CrossRef]
- Alekseenko, L.L.; Zemelko, V.I.; Domnina, A.P.; Lyublinskaya, O.G.; Zenin, V.V.; Pugovkina, N.A.; Kozhukharova, I.V.; Borodkina, A.V.; Grinchuk, T.M.; Fridlyanskaya, I.I.; et al. Sublethal heat shock induces premature senescence rather than apoptosis in human mesenchymal stem cells. Cell Stress Chaperones 2014, 19, 355–366. [Google Scholar] [CrossRef]
- Deryabin, P.; Borodkina, A. Reduced Efficiency of DNA Repair and Antioxidant Defense Promotes the Accumulation of DNA Damage During Cell Senescence. Cell Tissue Biol. 2021, 15, 532–543. [Google Scholar] [CrossRef]
- Deryabin, P.; Griukova, A.; Shatrova, A.; Petukhov, A.; Nikolsky, N.; Borodkina, A. Optimization of lentiviral transduction parameters and its application for CRISPR-based secretome modification of human endometrial mesenchymal stem cells. Cell Cycle 2019, 18, 742–758. [Google Scholar] [CrossRef]
- Kohsaka, S.; Sasai, K.; Takahashi, K.; Akagi, T.; Tanino, M.; Kimura, T.; Nishihara, H.; Tanaka, S. A population of BJ fibroblasts escaped from Ras-induced senescence susceptible to transformation. Biochem. Biophys. Res. Commun. 2011, 410, 878–884. [Google Scholar] [CrossRef][Green Version]
- Imanishi, T.; Hano, T.; Nishio, I. Estrogen reduces endothelial progenitor cell senescence through augmentation of telomerase activity. J. Hypertens. 2005, 23, 1699–1706. [Google Scholar] [CrossRef]
- Breu, A.; Sprinzing, B.; Merkl, M.; Bechmann, V.; Kujat, R.; Jenei-Lanzl, Z.; Prantl, L.; Angele, P. Estrogen reduces cellular aging in human mesenchymal stem cells and chondrocytes. J. Orthop. Res. 2011, 29, 1563–1571. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ikeda, Y.; Miyauchi, T.; Uchikado, Y.; Akasaki, Y.; Ohishi, M. Estrogen-SIRT1 Axis Plays a Pivotal Role in Protecting Arteries Against Menopause-Induced Senescence and Atherosclerosis. J. Atheroscler. Thromb. 2020, 27, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, K.M.; Burikhanov, R.; Goswami, A.; Rangnekar, V.M. Suppression of PTEN expression is essential for antiapoptosis and cellular transformation by oncogenic Ras. Cancer Res. 2007, 67, 10343–10350. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Fumagalli, M.; Cicalese, A.; Piccinin, S.; Gasparini, P.; Luise, C.; Schurra, C.; Garre’, M.; Nuciforo, P.G.; Bensimon, A.; et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 2006, 444, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Deryabin, P.I.; Borodkina, A.V.; Nikolsky, N.N.; Burova, E.B. The relationship between p53/p21/Rb and MAPK signaling pathways in human endometrium-derived stem cells under oxidative stress. Cell Tissue Biol. 2016, 10, 185–193. [Google Scholar] [CrossRef]
- Borodkina, A.V.; Shatrova, A.N.; Deryabin, P.I.; Grukova, A.A.; Nikolsky, N.N.; Burova, E.B. Tetraploidization or autophagy: The ultimate fate of senescent human endometrial stem cells under ATM or p53 inhibition. Cell Cycle 2016, 15, 117–127. [Google Scholar] [CrossRef]
- Grukova, A.A.; Shatrova, A.N.; Deryabin, P.I.; Borodkina, A.V.; Knyazev, N.A.; Nikolsky, N.N.; Burova, E.B. Modulation of senescence phenotype of human endometrial stem cells under inhibition of mtor and map-kinase signaling pathways. Tsitologiia 2017, 59, 410–420. [Google Scholar]
- Bianchi-Smiraglia, A.; Nikiforov, M.A. Controversial aspects of oncogene-induced senescence. Cell Cycle 2012, 11, 4147–4151. [Google Scholar] [CrossRef]
- Gorgoulis, V.G.; Halazonetis, T.D. Oncogene-induced senescence: The bright and dark side of the response. Curr. Opin. Cell Biol. 2010, 22, 816–827. [Google Scholar] [CrossRef]
- Harada, H.; Nakagawa, H.; Oyama, K.; Takaoka, M.; Andl, C.D.; Jacobmeier, B.; Von Werder, A.; Enders, G.H.; Opitz, O.G.; Rustgi, A.K. Telomerase induces immortalization of human esophageal keratinocytes without p16INK4a inactivation. Mol. Cancer Res. 2003, 1, 729–738. [Google Scholar]
- Cipriano, R.; Kan, C.E.; Graham, J.; Danielpour, D.; Stampfer, M.; Jackson, J.W. TGF-beta signaling engages an ATM-CHK2-p53-independent RAS-induced senescence and prevents malignant transformation in human mammary epithelial cells. Proc. Natl. Acad. Sci. USA 2011, 108, 8668–8673. [Google Scholar] [CrossRef]
- Drayton, S.; Rowe, J.; Jones, R.; Vatcheva, R.; Cuthbert-Heavens, D.; Marshall, J.; Fried, M.; Peters, G. Tumor suppressor p16INK4a determines sensitivity of human cells to transformation by cooperating cellular oncogenes. Cancer Cell 2003, 4, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Nikiforov, M.A. Pathways of oncogene-induced senescence in human melanocytic cells. Cell Cycle 2010, 9, 2782–2788. [Google Scholar] [CrossRef] [PubMed]
- Brandt, L.P.; Albers, J.; Hejhal, T.; Catalno, A.; Wild, P.J.; Frew, I.J. Oncogenic HrasG12V expression plus knockdown of Cdkn2a using ecotropic lentiviral vectors induces high-grade endometrial stromal sarcoma. PLoS ONE 2017, 12, e0186102. [Google Scholar] [CrossRef] [PubMed]
- Parisotto, M.; Grelet, E.; El Bizri, R.; Metzger, D. Senescence controls prostatic neoplasia driven by Pten loss. Mol. Cell. Oncol. 2018, 6, 1511205. [Google Scholar] [CrossRef]
- Jung, S.H.; Hwang, H.J.; Kang, D.; Park, H.A.; Lee, H.C.; Jeong, D.; Lee, K.; Park, H.J.; Ko, Y.-G.; Lee, J.-S. mTOR kinase leads to PTEN-loss-induced cellular senescence by phosphorylating p53. Oncogene 2019, 38, 1639–1650. [Google Scholar] [CrossRef]
- Gupta, S.; Panda, P.K.; Silveira, D.A.; Ahuja, R.; Hashimoto, R.F. Quadra-Stable Dynamics of p53 and PTEN in the DNA Damage Response. Cells 2023, 12, 1085. [Google Scholar] [CrossRef]
- Taheri, M.; Ghafouri-Fard, S.; Najafi, S.; Kallenbach, J.; Keramatfar, E.; Roozbahani, G.A.; Horestani, M.H.; Hussen, B.M.; Baniahmad, A. Hormonal regulation of telomerase activity and hTERT expression in steroid-regulated tissues and cancer. Cancer Cell Int. 2022, 22, 258. [Google Scholar] [CrossRef]
- Bayne, S.; Li, H.; Jones, M.E.; Pinto, A.R.; Van Sinderen, M.; Drummond, A.; Simpson, E.R.; Liu, J.-P. Estrogen deficiency reversibly induces telomere shortening in mouse granulosa cells and ovarian aging in vivo. Protein Cell 2011, 2, 333–346. [Google Scholar] [CrossRef]
- Zemelko, V.I.; Grinchuk, T.M.; Domnina, A.P.; Artsybasheva, I.V.; Zenin, V.V.; Kirsanov, A.A.; Bichevaia, N.K.; Korsak, V.S.; Nikolsky, N.N. Multipotent mesenchymal stem cells of desquamated endometrium: Isolation, characterization, and application as a feeder layer for maintenance of human embryonic stem cells. Cell Tisssue Biol. 2012, 6, 1–11. [Google Scholar] [CrossRef]
- Ye, M.; Huang, X.; Wu, Q.; Liu, F. Senescent Stromal Cells in the Tumor Microenvironment: Victims or Accomplices? Cancers 2003, 15, 1927. [Google Scholar] [CrossRef]
- Yoon, G.; Koh, C.W.; Yoon, N.; Kim, J.-Y.; Kim, H.-S. Stromal p16 expression is significantly increased in endometrial carcinoma. Oncotarget 2016, 8, 4826–4836. [Google Scholar] [CrossRef] [PubMed]


| # | Oligonucleotide | Sequence | Tm, °C |
|---|---|---|---|
| 1 | mCherry forward | 5′-ATATTTGGATCCGGTCCGATCCACCGGTCGC-3′ | 63.0 |
| 2 | mCherry reverse | 5′-CGTCATCGCTCCAGAAGGCCCGGGATTCTCCTCC-3′ | 63.0 |
| 3 | HRASG12V forward | 5′-GGGCCTTCTGGAGCGATGACGGAATATAAGCTGGTGG-3′ | 64.0 |
| 4 | HRASG12V reverse | 5′-GTCGAGCGGCCGCCACTGTG-3′ | 64.0 |
| 5 | PTEN sgRNA forward | 5′-CACCGAAACAAAAGGAGATATCAAG-3′ | - |
| 6 | PTEN sgRNA reverse | 5′-AAACCTTGATATCTCCTTTTGTTTC-3′ | - |
| # | Oligonucleotide | Sequence | Tm, °C |
|---|---|---|---|
| 1 | GAPDH forward | 5′-GAGGTCAATGAAGGGGTCAT-3′ | 57.0 |
| 2 | GAPDH reverse | 5′-AGTCAACGGATTTGGTCGTA-3′ | 57.0 |
| 3 | PTEN forward | 5′-TTGAAGACCATAACCCACCA-3′ | 58.0 |
| 4 | PTEN reverse | 5′-CACATAGCGCCTCTGACTG-3′ | 58.0 |
| 5 | STC1 forward | 5′-TGAGGCGGAGCAGAATGACT-3′ | 59.5 |
| 6 | STC1 reverse | 5′-CAGGTGGAGTTTTCCAGGCAT-3′ | 59.5 |
| 7 | IL6 forward | 5′-ATGTAGCCGCCCCACACA-3′ | 58.0 |
| 8 | IL6 reverse | 5′-CCAGTGCCTCTTTGCTGCTT-3′ | 58.0 |
| 9 | MMP2 forward | 5′-AGATCTTCTTCTTCAAGGACCGGTT-3′ | 59.5 |
| 10 | MMP2 reverse | 5′-GGCTGGTCAGTGGCTTGGGGTA-3′ | 59.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toropov, A.L.; Deryabin, P.I.; Shatrova, A.N.; Borodkina, A.V. Oncogene-Induced Senescence Is a Crucial Antitumor Defense Mechanism of Human Endometrial Stromal Cells. Int. J. Mol. Sci. 2023, 24, 14089. https://doi.org/10.3390/ijms241814089
Toropov AL, Deryabin PI, Shatrova AN, Borodkina AV. Oncogene-Induced Senescence Is a Crucial Antitumor Defense Mechanism of Human Endometrial Stromal Cells. International Journal of Molecular Sciences. 2023; 24(18):14089. https://doi.org/10.3390/ijms241814089
Chicago/Turabian StyleToropov, Artem L., Pavel I. Deryabin, Alla N. Shatrova, and Aleksandra V. Borodkina. 2023. "Oncogene-Induced Senescence Is a Crucial Antitumor Defense Mechanism of Human Endometrial Stromal Cells" International Journal of Molecular Sciences 24, no. 18: 14089. https://doi.org/10.3390/ijms241814089
APA StyleToropov, A. L., Deryabin, P. I., Shatrova, A. N., & Borodkina, A. V. (2023). Oncogene-Induced Senescence Is a Crucial Antitumor Defense Mechanism of Human Endometrial Stromal Cells. International Journal of Molecular Sciences, 24(18), 14089. https://doi.org/10.3390/ijms241814089






