Uremic Toxin-Induced Exosome-like Extracellular Vesicles Contain Enhanced Levels of Sulfated Glycosaminoglycans which Facilitate the Interaction with Very Small Superparamagnetic Iron Oxide Particles
Abstract
:1. Introduction
2. Results
2.1. EVs Derived from Endothelial Cells and Vascular Smooth Muscle Cells Reduce the T1-Relaxivity of VSOPs in a Concentration-Dependent Manner
2.2. EVs Derived from Cells Treated with Uremic Toxins Show a Stronger Interaction with VSOPs in T1 Relaxivity Measurements Compared to Control
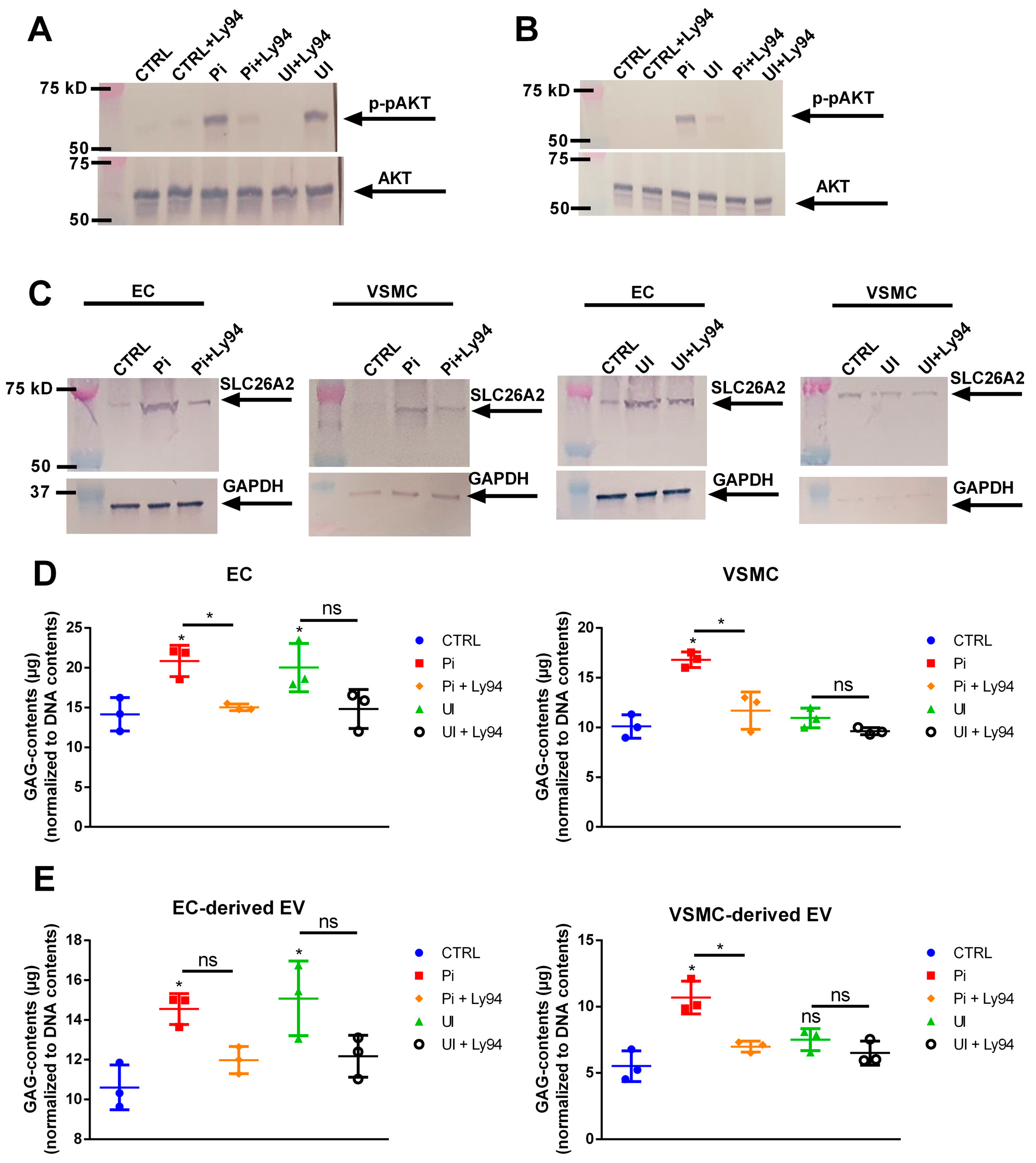
2.3. Treatment of ECs and VSMCs with Pi or UI Alter the Gene Expression Profile of Proteins Being Involved in GAG Homeostasis and Enhances the Amount of GAG in Cells and Isolated EVs
2.4. Indirect Modulation of GAG Contents in EVs Affects the Interaction between VSOPs and EVs in T1 Relaxivity Measurements
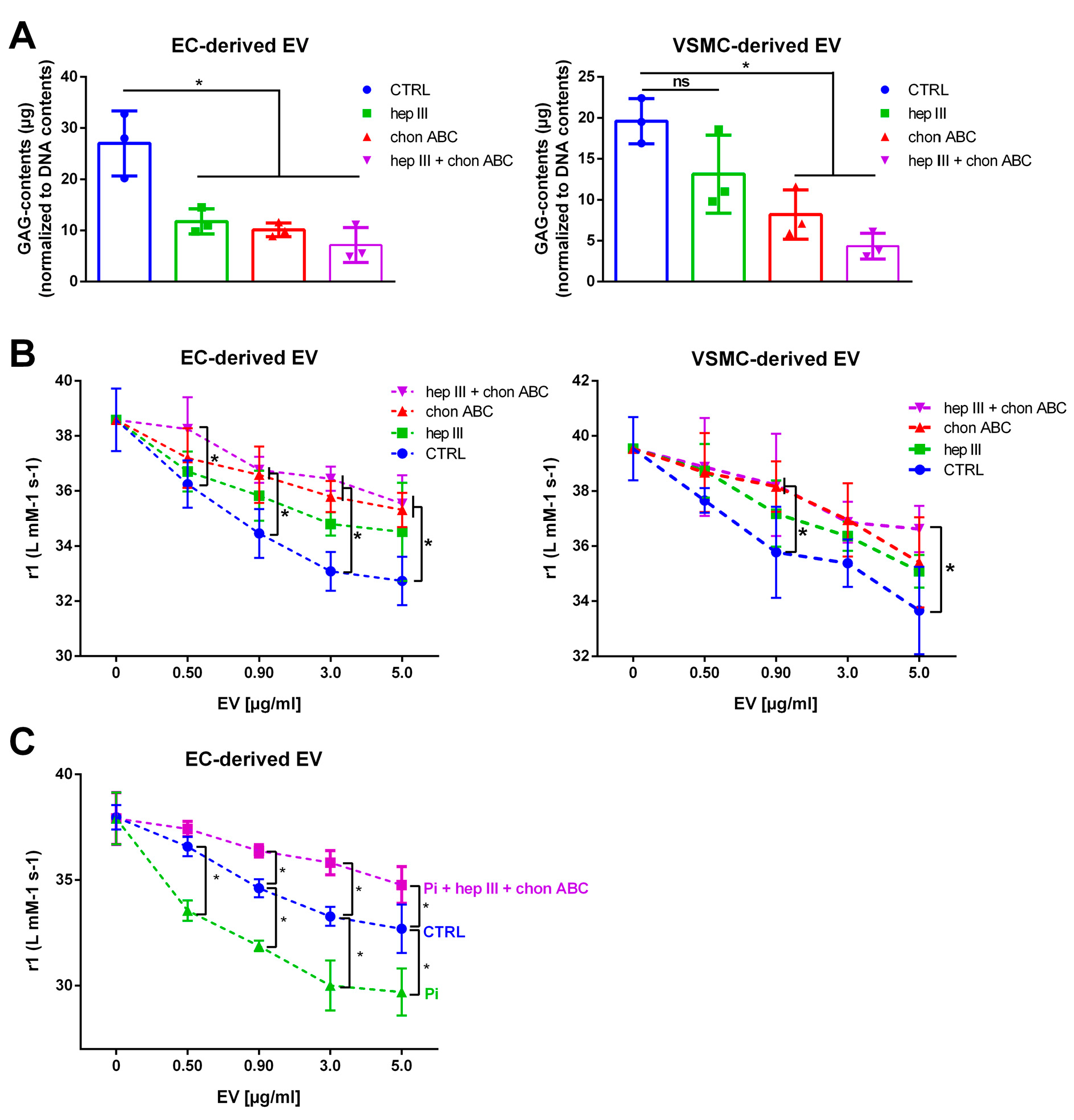
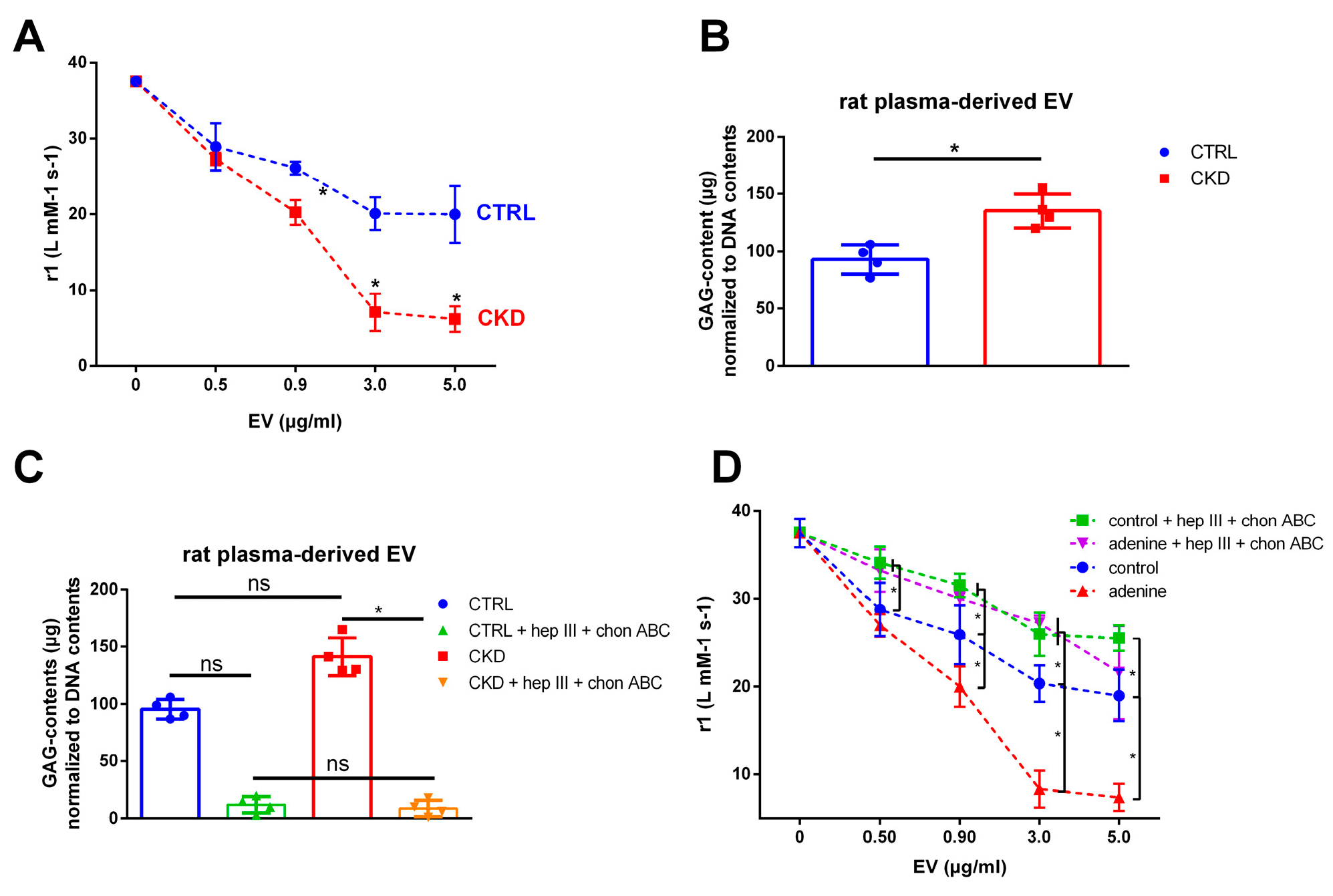
2.5. Treatment of EVs with GAG Lyases Attenuates the Interaction between VSOPs and EVs in T1 Relaxivity Measurements
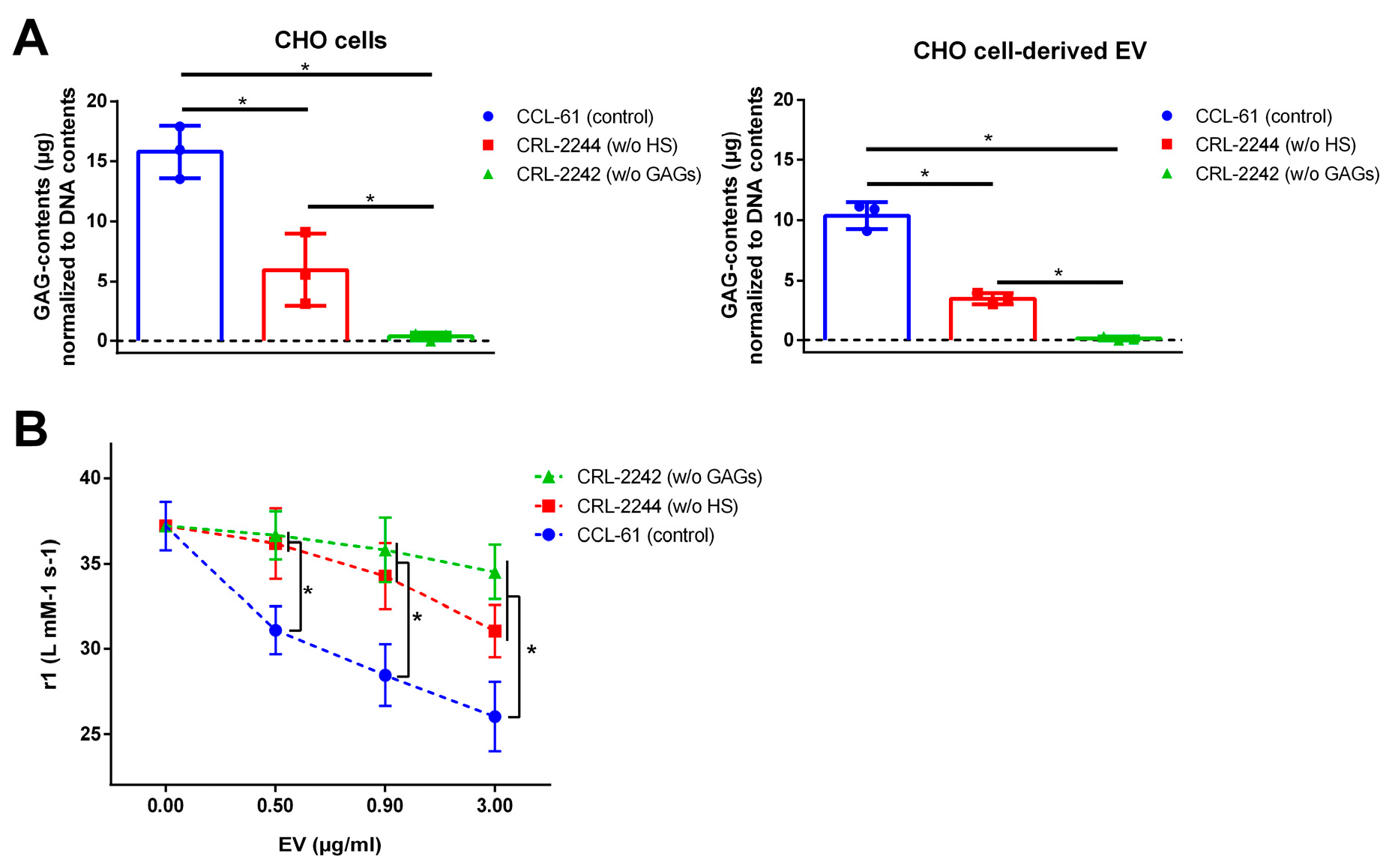
2.6. EVs from GAG-Depleted Cells Show a Decreased Interaction with VSOPs in T1 Relaxation Studies
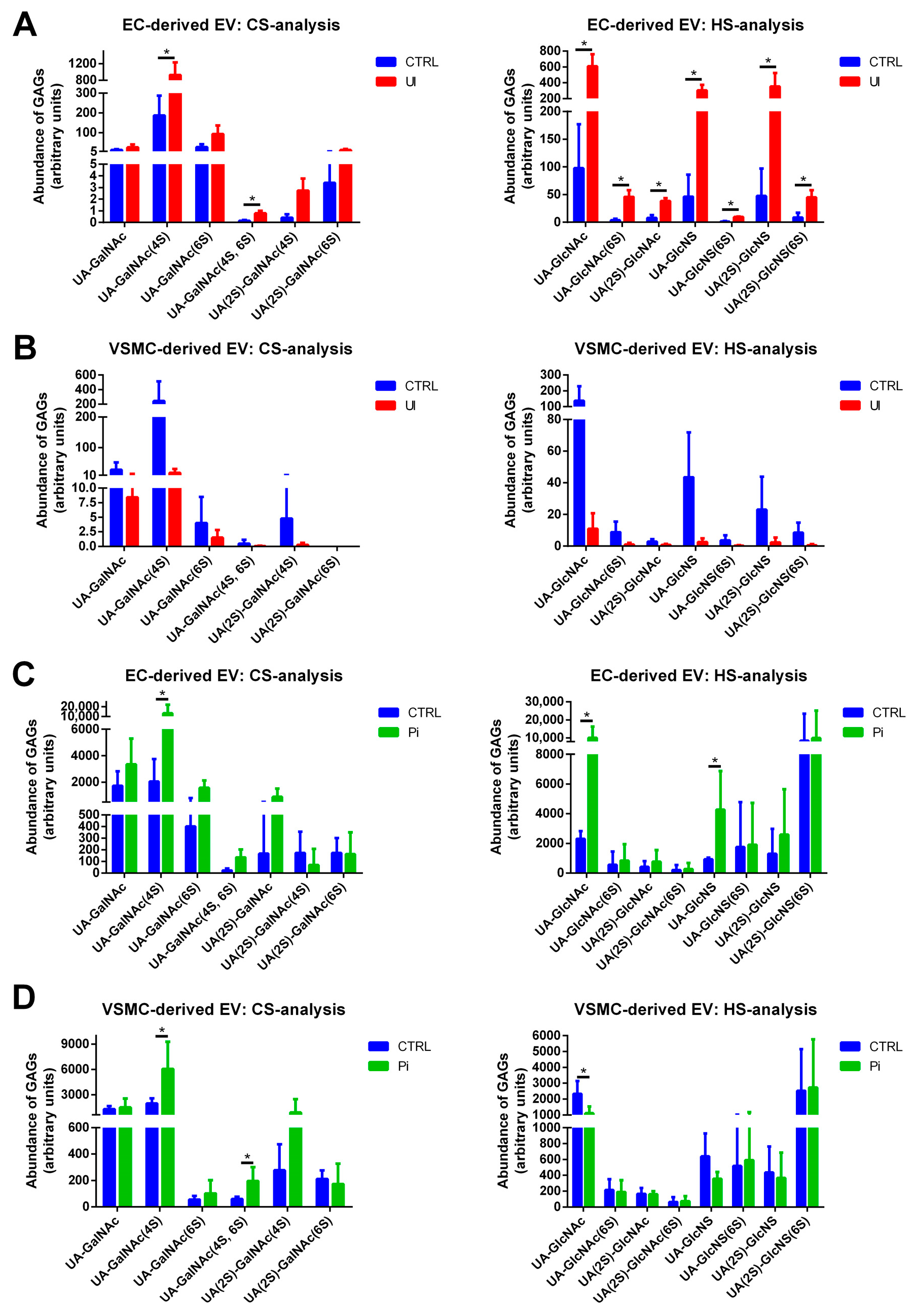
2.7. EVs Isolated from Pi- and UI-Treated ECs and VSMCs Comprise Treatment-Dependent Differences in HS and CS Contents including the Degree of Sulfation
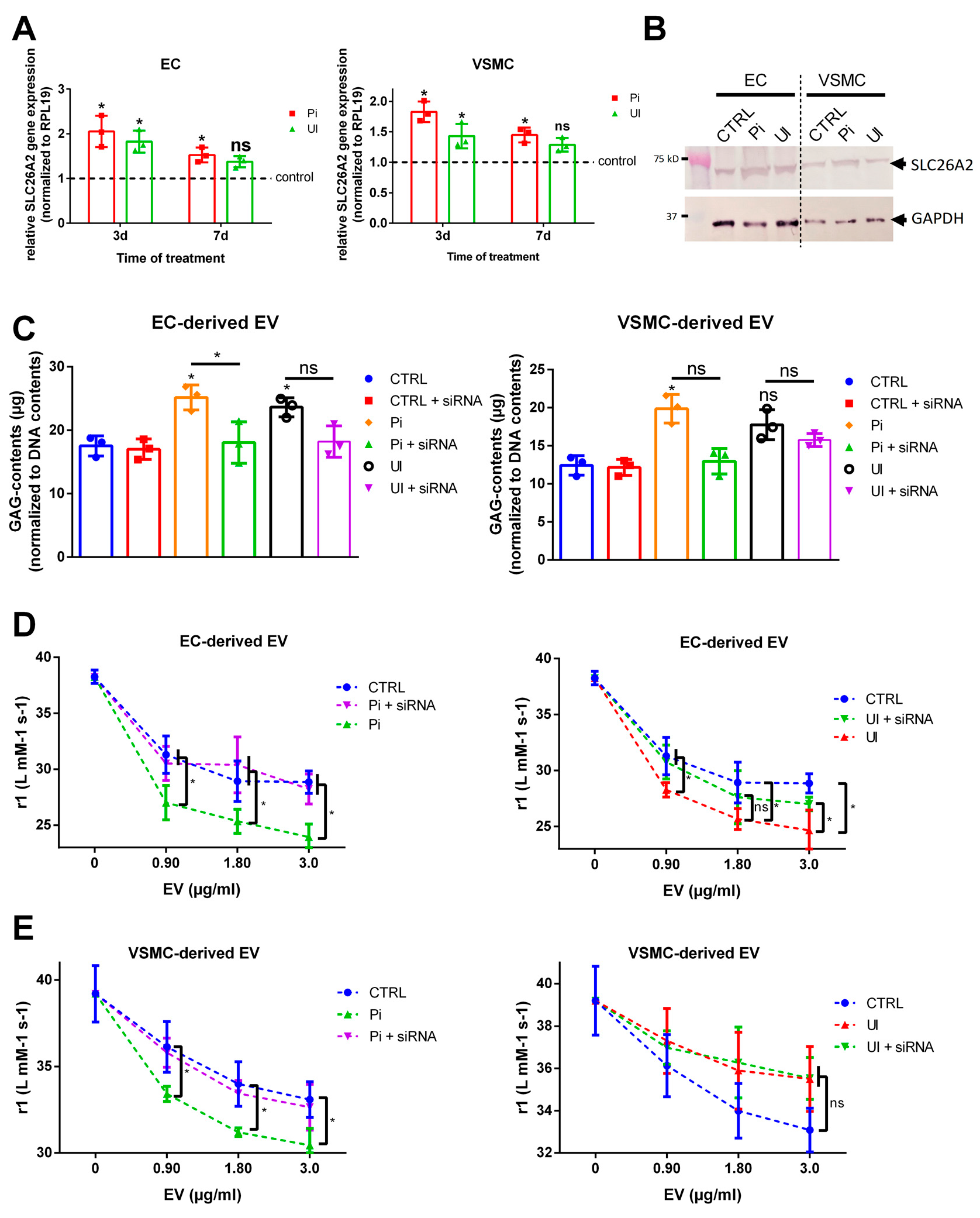
2.8. The Sulfate Transporter SLC26A2 Is Involved in Uremic Toxin-Induced Formation of GAG-Enriched EVs
2.9. Uremic Toxin-Induced Upregulation of SLC26A2 Involves PI3K/AKT Signaling
2.10. EVs from Plasma of Uremic Rats also Reduce T1 Relaxivity of VSOPs
3. Discussion
Limitations
4. Materials and Methods
4.1. Cell Culture
4.2. Treatment of Cells with Uremic Toxins
4.3. Isolation and Characterization of EVs
4.4. Synthesis of VSOP
4.5. T1 Relaxivity Measurements
4.6. Magnetic Particle Spectroscopy (MPS) Measurements
4.7. Determination of GAG Contents in Cells and EVs
4.8. Incubation of EVs with the Lyases Heparinase III and Chondroitinase ABC
4.9. Transfection of Cells with siRNA
4.10. Western Blot Analyses
4.11. Inhibition of AKT-Signaling in the Host Cells
4.12. Rat Model of Chronic Kidney Diseases (CKD)
4.13. Manipulation of GAG Contents in Host Cells
4.14. HS and CS/DS Disaccharide Analysis of EVs
4.15. PCR Measurements
4.16. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Six, I.; Flissi, N.; Lenglet, G.; Louvet, L.; Kamel, S.; Gallet, M.; Massy, Z.A.; Liabeuf, S. Uremic Toxins and Vascular Dysfunction. Toxins 2020, 12, 404. [Google Scholar] [CrossRef] [PubMed]
- Mizobuchi, M.; Towler, D.; Slatopolsky, E. Vascular calcification: The killer of patients with chronic kidney disease. J. Am. Soc. Nephrol. 2009, 20, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Giussani, M.; Triulzi, T.; Sozzi, G.; Tagliabue, E. Tumor Extracellular Matrix Remodeling: New Perspectives as a Circulating Tool in the Diagnosis and Prognosis of Solid Tumors. Cells 2019, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.E. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. Faseb. J. 1992, 6, 2639–2645. [Google Scholar] [CrossRef] [PubMed]
- Freise, C.; Querfeld, U.; Ludwig, A.; Hamm, B.; Schnorr, J.; Taupitz, M. Uraemic extracellular vesicles augment osteogenic transdifferentiation of vascular smooth muscle cells via enhanced AKT signalling and PiT-1 expression. J. Cell. Mol. Med. 2021, 25, 5602–5614. [Google Scholar] [CrossRef] [PubMed]
- Andrews, A.M.; Rizzo, V. Microparticle-Induced Activation of the Vascular Endothelium Requires Caveolin-1/Caveolae. PLoS ONE 2016, 11, e0149272. [Google Scholar] [CrossRef]
- Burger, D.; Montezano, A.C.; Nishigaki, N.; He, Y.; Carter, A.; Touyz, R.M. Endothelial microparticle formation by angiotensin II is mediated via Ang II receptor type I/NADPH oxidase/ Rho kinase pathways targeted to lipid rafts. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1898–1907. [Google Scholar] [CrossRef]
- Chen, N.X.; Kiattisunthorn, K.; O’Neill, K.D.; Chen, X.; Moorthi, R.N.; Gattone, V.H., 2nd; Allen, M.R.; Moe, S.M. Decreased microRNA is involved in the vascular remodeling abnormalities in chronic kidney disease (CKD). PLoS ONE 2013, 8, e64558. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Chauhan, S.; Danielson, S.; Clements, V.; Edwards, N.; Ostrand-Rosenberg, S.; Fenselau, C. Surface Glycoproteins of Exosomes Shed by Myeloid-Derived Suppressor Cells Contribute to Function. J. Proteome Res. 2017, 16, 238–246. [Google Scholar] [CrossRef]
- Muncie, J.M.; Weaver, V.M. The Physical and Biochemical Properties of the Extracellular Matrix Regulate Cell Fate. Curr. Top. Dev. Biol. 2018, 130, 1–37. [Google Scholar] [CrossRef]
- Golusda, L.; Kühl, A.A.; Lehmann, M.; Dahlke, K.; Mueller, S.; Boehm-Sturm, P.; Saatz, J.; Traub, H.; Schnorr, J.; Freise, C.; et al. Visualization of Inflammation in Experimental Colitis by Magnetic Resonance Imaging Using Very Small Superparamagnetic Iron Oxide Particles. Front. Physiol. 2022, 13, 862212. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Schnorr, J.; Ludwig, A.; Stangl, V.; Ebert, M.; Hamm, B.; Taupitz, M. Contrast-enhanced MR imaging of atherosclerosis using citrate-coated superparamagnetic iron oxide nanoparticles: Calcifying microvesicles as imaging target for plaque characterization. Int. J. Nanomed. 2013, 8, 767–779. [Google Scholar] [CrossRef]
- Berndt, D.; Millward, J.M.; Schnorr, J.; Taupitz, M.; Stangl, V.; Paul, F.; Wagner, S.; Wuerfel, J.T.; Sack, I.; Ludwig, A.; et al. Inflammation-induced brain endothelial activation leads to uptake of electrostatically stabilized iron oxide nanoparticles via sulfated glycosaminoglycans. Nanomedicine 2017, 13, 1411–1421. [Google Scholar] [CrossRef]
- Bloembergen, N.; Morgan, L.O. Proton Relaxation Times in Paramagnetic Solutions. Effects of Electron Spin Relaxation. J. Chem. Phys. 1961, 34, 842–850. [Google Scholar] [CrossRef]
- Caravan, P.; Cloutier, N.J.; Greenfield, M.T.; McDermid, S.A.; Dunham, S.U.; Bulte, J.W.; Amedio, J.C., Jr.; Looby, R.J.; Supkowski, R.M.; Horrocks, W.D., Jr.; et al. The interaction of MS-325 with human serum albumin and its effect on proton relaxation rates. J. Am. Chem. Soc. 2002, 124, 3152–3162. [Google Scholar] [CrossRef]
- Stetz, M.A.; Caro, J.A.; Kotaru, S.; Yao, X.; Marques, B.S.; Valentine, K.G.; Wand, A.J. Characterization of Internal Protein Dynamics and Conformational Entropy by NMR Relaxation. Methods Enzymol. 2019, 615, 237–284. [Google Scholar] [CrossRef] [PubMed]
- Togayachi, A.; Sato, T.; Narimatsu, H. Comprehensive enzymatic characterization of glycosyltransferases with a beta3GT or beta4GT motif. Methods Enzymol. 2006, 416, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Qasba, P.K.; Ramakrishnan, B.; Boeggeman, E. Structure and function of beta -1,4-galactosyltransferase. Curr. Drug Targets 2008, 9, 292–309. [Google Scholar] [CrossRef]
- Ohtake, S.; Ito, Y.; Fukuta, M.; Habuchi, O. Human N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase cDNA is related to human B cell recombination activating gene-associated gene. J. Biol. Chem. 2001, 276, 43894–43900. [Google Scholar] [CrossRef] [PubMed]
- Izumikawa, T.; Koike, T.; Shiozawa, S.; Sugahara, K.; Tamura, J.; Kitagawa, H. Identification of chondroitin sulfate glucuronyltransferase as chondroitin synthase-3 involved in chondroitin polymerization: Chondroitin polymerization is achieved by multiple enzyme complexes consisting of chondroitin synthase family members. J. Biol. Chem. 2008, 283, 11396–11406. [Google Scholar] [CrossRef]
- Sugahara, K.; Kitagawa, H. Recent advances in the study of the biosynthesis and functions of sulfated glycosaminoglycans. Curr. Opin. Struct. Biol. 2000, 10, 518–527. [Google Scholar] [CrossRef]
- Weigel, P.H.; Hascall, V.C.; Tammi, M. Hyaluronan synthases. J. Biol. Chem. 1997, 272, 13997–14000. [Google Scholar] [CrossRef]
- Norflus, F.; Yamanaka, S.; Proia, R.L. Promoters for the human beta-hexosaminidase genes, HEXA and HEXB. DNA Cell Biol. 1996, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Fiddler, J.L.; Clarke, S.L. Evaluation of candidate reference genes for quantitative real-time PCR analysis in a male rat model of dietary iron deficiency. Genes Nutr. 2021, 16, 17. [Google Scholar] [CrossRef]
- Park, M.; Ohana, E.; Choi, S.Y.; Lee, M.S.; Park, J.H.; Muallem, S. Multiple roles of the SO4(2−)/Cl−/OH− exchanger protein Slc26a2 in chondrocyte functions. J. Biol. Chem. 2014, 289, 1993–2001. [Google Scholar] [CrossRef]
- Götting, C.; Kuhn, J.; Zahn, R.; Brinkmann, T.; Kleesiek, K. Molecular Cloning and Expression of Human UDP-d-Xylose:Proteoglycan Core Protein β-d-Xylosyltransferase and its First Isoform XT-II. J. Mol. Biol. 2000, 304, 517–528. [Google Scholar] [CrossRef]
- Afroz, R.; Cao, Y.; Rostam, M.A.; Ta, H.; Xu, S.; Zheng, W.; Osman, N.; Kamato, D.; Little, P.J. Signalling pathways regulating galactosaminoglycan synthesis and structure in vascular smooth muscle: Implications for lipoprotein binding and atherosclerosis. Pharmacol. Ther. 2018, 187, 88–97. [Google Scholar] [CrossRef]
- Ramakrishnan, B.; Boeggeman, E.; Ramasamy, V.; Qasba, P.K. Structure and catalytic cycle of beta-1,4-galactosyltransferase. Curr. Opin. Struct. Biol. 2004, 14, 593–600. [Google Scholar] [CrossRef]
- Piotrowska, E.; Jakóbkiewicz-Banecka, J.; Barańska, S.; Tylki-Szymańska, A.; Czartoryska, B.; Wegrzyn, A.; Wegrzyn, G. Genistein-mediated inhibition of glycosaminoglycan synthesis as a basis for gene expression-targeted isoflavone therapy for mucopolysaccharidoses. Eur. J. Hum. Genet. 2006, 14, 846–852. [Google Scholar] [CrossRef]
- Ernst, S.; Langer, R.; Cooney, C.L.; Sasisekharan, R. Enzymatic degradation of glycosaminoglycans. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 387–444. [Google Scholar] [CrossRef]
- Taupitz, M.; Stolzenburg, N.; Ebert, M.; Schnorr, J.; Hauptmann, R.; Kratz, H.; Hamm, B.; Wagner, S. Gadolinium-containing magnetic resonance contrast media: Investigation on the possible transchelation of Gd³⁺ to the glycosaminoglycan heparin. Contrast Media Mol. Imaging 2013, 8, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.; Poller, W.C.; Westphal, K.; Minkwitz, S.; Lättig-Tünnemann, G.; Metzkow, S.; Stangl, K.; Baumann, G.; Taupitz, M.; Wagner, S.; et al. Rapid binding of electrostatically stabilized iron oxide nanoparticles to THP-1 monocytic cells via interaction with glycosaminoglycans. Basic. Res. Cardiol. 2013, 108, 328. [Google Scholar] [CrossRef]
- Mastarone, D.J.; Harrison, V.S.; Eckermann, A.L.; Parigi, G.; Luchinat, C.; Meade, T.J. A modular system for the synthesis of multiplexed magnetic resonance probes. J. Am. Chem. Soc. 2011, 133, 5329–5337. [Google Scholar] [CrossRef] [PubMed]
- Poller, W.C.; Löwa, N.; Wiekhorst, F.; Taupitz, M.; Wagner, S.; Möller, K.; Baumann, G.; Stangl, V.; Trahms, L.; Ludwig, A. Magnetic Particle Spectroscopy Reveals Dynamic Changes in the Magnetic Behavior of Very Small Superparamagnetic Iron Oxide Nanoparticles During Cellular Uptake and Enables Determination of Cell-Labeling Efficacy. J. Biomed. Nanotechnol. 2016, 12, 337–346. [Google Scholar] [CrossRef]
- Chacko, B.K.; Scott, D.W.; Chandler, R.T.; Patel, R.P. Endothelial surface N-glycans mediate monocyte adhesion and are targets for anti-inflammatory effects of peroxisome proliferator-activated receptor γ ligands. J. Biol. Chem. 2011, 286, 38738–38747. [Google Scholar] [CrossRef]
- Mohamed, R.; Dayati, P.; Mehr, R.N.; Kamato, D.; Seif, F.; Babaahmadi-Rezaei, H.; Little, P.J. Transforming growth factor-β1 mediated CHST11 and CHSY1 mRNA expression is ROS dependent in vascular smooth muscle cells. J. Cell Commun. Signal 2019, 13, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Piva, M.B.; Suarez, E.R.; Melo, C.M.; Cavalheiro, R.P.; Nader, H.B.; Pinhal, M.A. Glycosaminoglycans affect heparanase location in CHO cell lines. Glycobiology 2015, 25, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Brézillon, S.; Untereiner, V.; Mohamed, H.T.; Hodin, J.; Chatron-Colliet, A.; Maquart, F.X.; Sockalingum, G.D. Probing glycosaminoglycan spectral signatures in live cells and their conditioned media by Raman microspectroscopy. Analyst 2017, 142, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Theocharis, D.A. High-performance capillary electrophoretic analysis of hyaluronan and galactosaminoglycan-disaccharides in gastrointestinal carcinomas. Differential disaccharide composition as a possible tool-indicator for malignancies. Biomed. Chromatogr. 2002, 16, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Pothacharoen, P.; Siriaunkgul, S.; Ong-Chai, S.; Supabandhu, J.; Kumja, P.; Wanaphirak, C.; Sugahara, K.; Hardingham, T.; Kongtawelert, P. Raised serum chondroitin sulfate epitope level in ovarian epithelial cancer. J. Biochem. 2006, 140, 517–524. [Google Scholar] [CrossRef]
- ten Dam, G.B.; van de Westerlo, E.M.; Purushothaman, A.; Stan, R.V.; Bulten, J.; Sweep, F.C.; Massuger, L.F.; Sugahara, K.; van Kuppevelt, T.H. Antibody GD3G7 selected against embryonic glycosaminoglycans defines chondroitin sulfate-E domains highly up-regulated in ovarian cancer and involved in vascular endothelial growth factor binding. Am. J. Pathol. 2007, 171, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Krog, M.; Ejerblad, S.; Johansson, H. The aortic content of glycosaminoglycans, hydroxyproline and calcium in experimental uraemia with special reference to parathyroidectomy and vitamin-D treatment. Scand. J. Urol. Nephrol. 1984, 18, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Corsi, A.; Riminucci, M.; Fisher, L.W.; Bianco, P. Achondrogenesis type IB: Agenesis of cartilage interterritorial matrix as the link between gene defect and pathological skeletal phenotype. Arch. Pathol. Lab. Med. 2001, 125, 1375–1378. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.A.; Markovich, D. Pathogenetics of the human SLC26 transporters. Curr. Med. Chem. 2005, 12, 385–396. [Google Scholar] [CrossRef]
- de Sousa Mesquita, A.P.; de Araújo Lopes, S.; Pernambuco Filho, P.C.A.; Nader, H.B.; Lopes, C.C. Acquisition of anoikis resistance promotes alterations in the Ras/ERK and PI3K/Akt signaling pathways and matrix remodeling in endothelial cells. Apoptosis 2017, 22, 1116–1137. [Google Scholar] [CrossRef]
- Toyoda, S.; Shin, J.; Fukuhara, A.; Otsuki, M.; Shimomura, I. Transforming growth factor β1 signaling links extracellular matrix remodeling to intracellular lipogenesis upon physiological feeding events. J. Biol. Chem. 2022, 298, 101748. [Google Scholar] [CrossRef]
- Li, X.G.; Wang, Y.B. SRPK1 gene silencing promotes vascular smooth muscle cell proliferation and vascular remodeling via inhibition of the PI3K/Akt signaling pathway in a rat model of intracranial aneurysms. CNS Neurosci. Ther. 2019, 25, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Yu, K.N.; Lee, Y.S.; An, G.H.; Beck, G.R., Jr.; Colburn, N.H.; Lee, K.H.; Cho, M.H. Elevated inorganic phosphate stimulates Akt-ERK1/2-Mnk1 signaling in human lung cells. Am. J. Respir. Cell Mol. Biol. 2006, 35, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Xu, C.X.; Lim, H.T.; Park, S.J.; Shin, J.Y.; Chung, Y.S.; Park, S.C.; Chang, S.H.; Youn, H.J.; Lee, K.H.; et al. High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling. Am. J. Respir. Crit. Care Med. 2009, 179, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Lindner, M.; Laporte, A.; Elomaa, L.; Lee-Thedieck, C.; Olmer, R.; Weinhart, M. Flow-induced glycocalyx formation and cell alignment of HUVECs compared to iPSC-derived ECs for tissue engineering applications. Front. Cell Dev. Biol. 2022, 10, 953062. [Google Scholar] [CrossRef]
- Grigor’eva, A.E.; Dyrkheeva, N.S.; Bryzgunova, O.E.; Tamkovich, S.N.; Chelobanov, B.P.; Ryabchikova, E.I. Contamination of exosome preparations, isolated from biological fluids. Biomed. Khim 2017, 63, 91–96. [Google Scholar] [CrossRef] [PubMed]
- D’Apolito, M.; Du, X.; Pisanelli, D.; Pettoello-Mantovani, M.; Campanozzi, A.; Giacco, F.; Maffione, A.B.; Colia, A.L.; Brownlee, M.; Giardino, I. Urea-induced ROS cause endothelial dysfunction in chronic renal failure. Atherosclerosis 2015, 239, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef]
- de Schellenberger, A.A.; Hauptmann, R.; Millward, J.M.; Schellenberger, E.; Kobayashi, Y.; Taupitz, M.; Infante-Duarte, C.; Schnorr, J.; Wagner, S. Synthesis of europium-doped VSOP, customized enhancer solution and improved microscopy fluorescence methodology for unambiguous histological detection. J. Nanobiotechnol. 2017, 15, 71. [Google Scholar] [CrossRef]
- Farhat, Y. PicoGreen Cell Proliferation Assay Protocol. Available online: http://protocol-place.com (accessed on 25 July 2022).



| Gene | Function | Reference |
|---|---|---|
| B3GNT2 | Galactosyltransferase which catalyzes the chain elongation process in GAG synthesis | [19] |
| B4GALT1 | Involved in the synthesis and elongation of GAG chains | [20] |
| CHST15 | Encodes for a 6-O-sulfotransferase being involved in the formation of sulfated GalNAc4S6S residues of CS/DS | [21] |
| CHSY1 | Involved in the synthesis and elongation of GAG chains | [22] |
| EXT1 | Involved in the synthesis of HS-chains | [23] |
| HAS1 | Hyaluronan synthase responsible for the synthesis of hyaluronic acid | [24] |
| HEX A | Involved in the hydrolysis of GAG components | [25] |
| HEX B | Involved in the hydrolysis of GAG components | [25] |
| RPL19 | Housekeeping gene | [26] |
| SLC26A2 | Sulfate transporter which regulates sulfation of GAGs | [27] |
| XYLT2 | Glycosyltransferase that initiates biosynthesis of GAG chains in proteoglycans including CS, HS and DS | [28] |
| Target | Dilution | Company | Product Number |
|---|---|---|---|
| CD9 | 1:1000 | StemcellTM Technologies (Köln, Germany) | #100-0211 |
| CD63 | 1:1000 | StemcellTM Technologies | #100-0211 |
| CD81 | 1:1000 | StemcellTM Technologies | #100-0211 |
| CHST15 | 1:1000 | Thermo Fisher | #100440 |
| GAPDH | 1:2000 | Thermo Fisher | #MA5-15738 |
| p-p-AKT | 1:1000 | Cell Signaling (Danvers, MA, USA) | #2965S |
| p-AKT | 1:1000 | Cell Signaling | #4691S |
| SLC26A2 | 1:1000 | Thermo Fisher | #PA576918 |
| Gene | Full Name | Assay-ID (Rat) | Assay-ID (Human) |
|---|---|---|---|
| B3GNT2 | UDP-GlcNAc:BetaGal Beta-1,3-N-Acetylglucosaminyl-transferase 2 | Rn02112835_s1 | Hs01935859_s1 |
| B4GALT1 | UDP-Gal:betaGlcNAc beta 1,4-galactosyltransferase, polypeptide 1 | Rn01764643_m1 | Hs00155245_m1 |
| CHST15 | Carbohydrate (N-acetylgalactosamine 4-sulfate 6-O) sulfotransferase | Rn00597859_m1 | Hs01031067_m1 |
| CHSY1 | Chondroitin sulfate synthase 1 | Rn01478125_m1 | Hs00208704_m1 |
| EXT1 | Exostosin Glycosyltransferase 1 | Rn00468764_m1 | Hs00609162_m1 |
| HAS1 | Hyaluronan synthase 1 | Rn01455687_g1 | HS04398914_m1 |
| HEX A | Hexosaminidase A | Rn01422539_m1 | Hs00942655_m1 |
| HEX B | Hexosaminidase B | Rn01493909_m1 | Hs01077594_m1 |
| RPL19 | Ribosomal Protein L19 | Rn00821265_g1 | Hs02338565_gH |
| SLC26A2 | Solute Carrier Family 26 Member 2 | Rn00589156_m1 | Hs00164423_m1 |
| XYLT2 | Xylosyltransferase 2 | Rn00574186_m1 | Hs01048792_m1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freise, C.; Zappe, A.; Löwa, N.; Schnorr, J.; Pagel, K.; Wiekhorst, F.; Taupitz, M. Uremic Toxin-Induced Exosome-like Extracellular Vesicles Contain Enhanced Levels of Sulfated Glycosaminoglycans which Facilitate the Interaction with Very Small Superparamagnetic Iron Oxide Particles. Int. J. Mol. Sci. 2023, 24, 14253. https://doi.org/10.3390/ijms241814253
Freise C, Zappe A, Löwa N, Schnorr J, Pagel K, Wiekhorst F, Taupitz M. Uremic Toxin-Induced Exosome-like Extracellular Vesicles Contain Enhanced Levels of Sulfated Glycosaminoglycans which Facilitate the Interaction with Very Small Superparamagnetic Iron Oxide Particles. International Journal of Molecular Sciences. 2023; 24(18):14253. https://doi.org/10.3390/ijms241814253
Chicago/Turabian StyleFreise, Christian, Andreas Zappe, Norbert Löwa, Jörg Schnorr, Kevin Pagel, Frank Wiekhorst, and Matthias Taupitz. 2023. "Uremic Toxin-Induced Exosome-like Extracellular Vesicles Contain Enhanced Levels of Sulfated Glycosaminoglycans which Facilitate the Interaction with Very Small Superparamagnetic Iron Oxide Particles" International Journal of Molecular Sciences 24, no. 18: 14253. https://doi.org/10.3390/ijms241814253
APA StyleFreise, C., Zappe, A., Löwa, N., Schnorr, J., Pagel, K., Wiekhorst, F., & Taupitz, M. (2023). Uremic Toxin-Induced Exosome-like Extracellular Vesicles Contain Enhanced Levels of Sulfated Glycosaminoglycans which Facilitate the Interaction with Very Small Superparamagnetic Iron Oxide Particles. International Journal of Molecular Sciences, 24(18), 14253. https://doi.org/10.3390/ijms241814253






