Decoding the IL-33/ST2 Axis: Its Impact on the Immune Landscape of Breast Cancer
Abstract
:1. Introduction
2. Unraveling the Enigma of Interleukin-33 in the Cancer Landscape
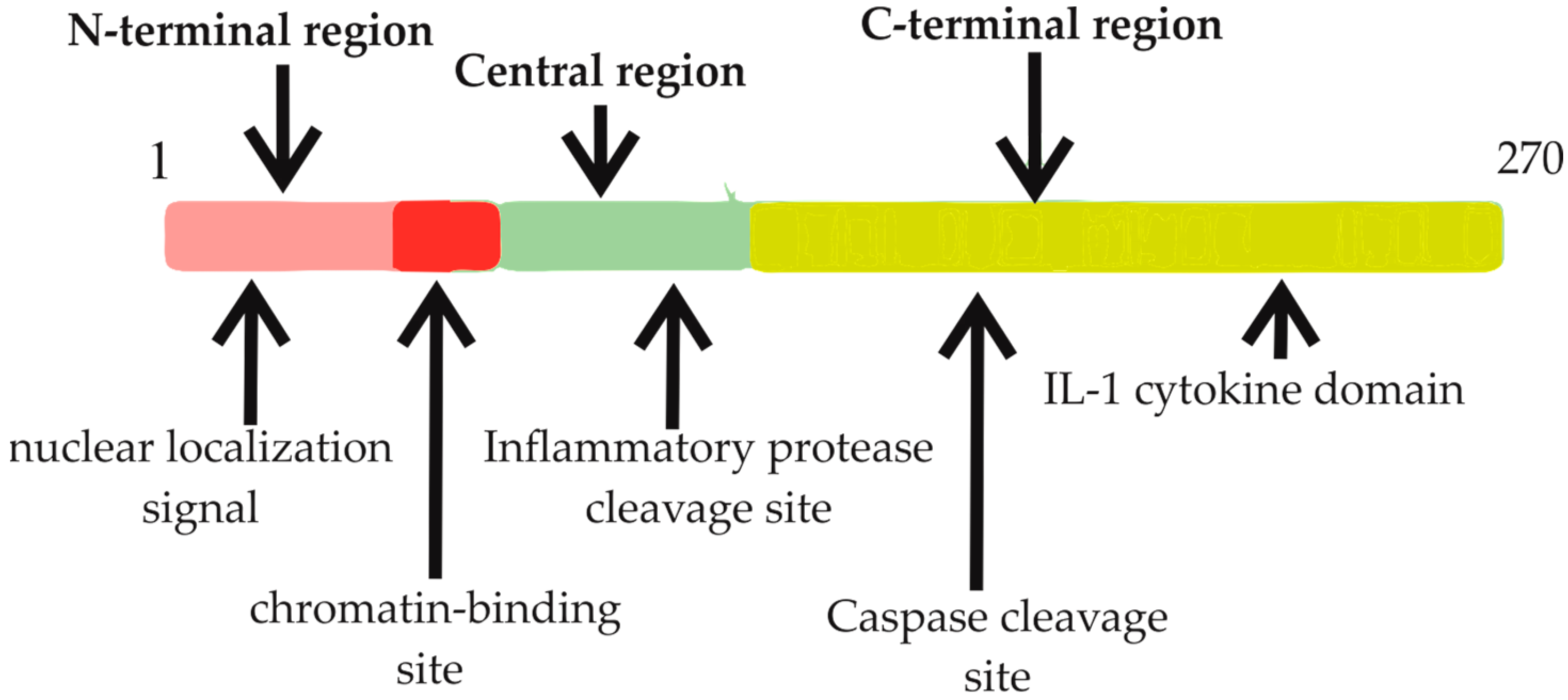
2.1. Comprehensive Understanding of Interleukin-33: Structure, Function, and Regulation
2.2. Characteristics and Functions of the IL-33 Receptor (ST2) Isoforms
2.3. The Intricate Dynamics of IL-33/ST2 Signaling
3. IL-33 and Its Implications in Human Breast Cancer
3.1. The Influence of IL-33 on ER-Positive Breast Cancer
3.2. IL-33 and Its Role in Triple-Negative Breast Cancer
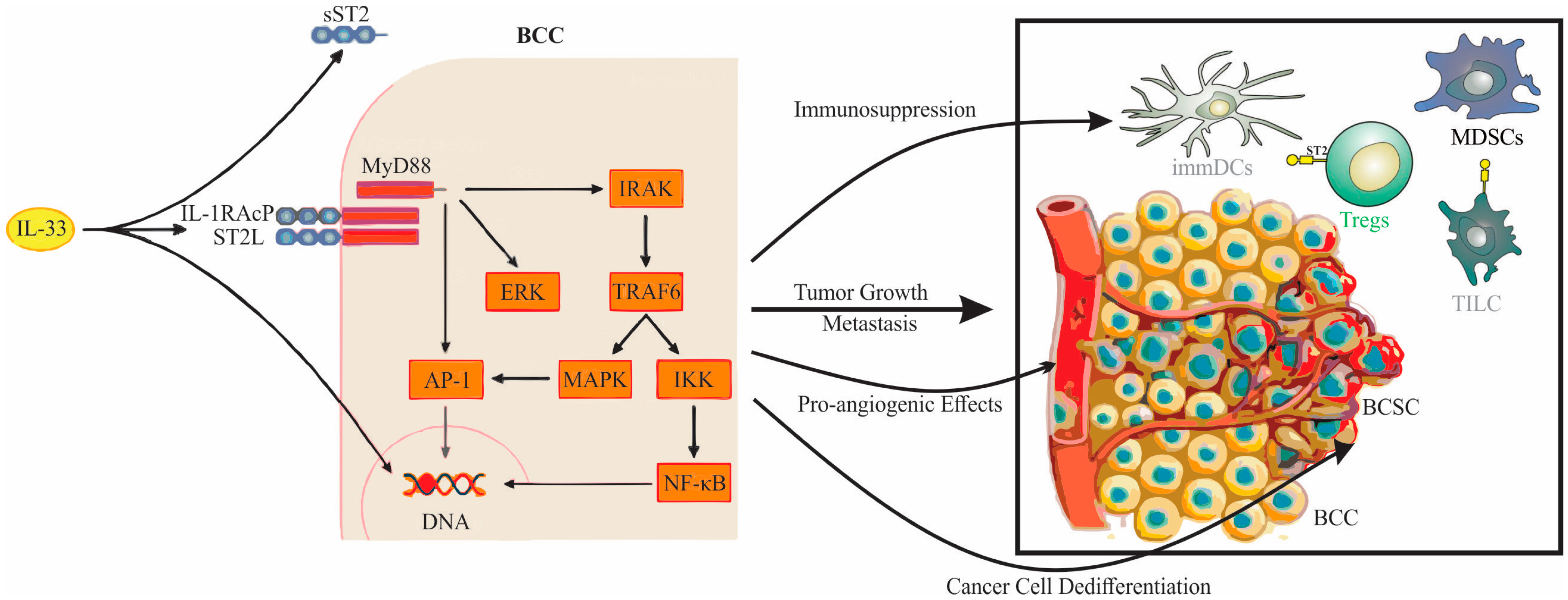
4. The Role of the IL-33/ST2 Axis in Breast Cancer Biology
IL-33/ST2 Axis as a Therapeutic Target
5. Future Perspectives in the IL-33/ST2 Axis and Breast Carcinoma Research
- Elucidating Mechanisms: Our current comprehension of the IL-33/ST2 axis in breast carcinoma, while extensive, is yet incomplete. It is imperative that future research dives deeper into the molecular pathways influenced by the IL-33/ST2 axis, particularly those that might govern therapeutic resistance or susceptibility.
- Therapeutic Targeting: The centrality of the IL-33/ST2 axis in shaping the immune response in breast cancer lends itself as an attractive target for therapy. Potential interventions could range from direct IL-33 inhibitors and neutralizing antibodies to antagonists that block ST2-mediated signaling, thereby neutralizing its pro-tumorigenic activities.
- Biomarker Potential: Given the differential expression of IL-33 and ST2 across breast carcinoma subtypes, there is potential to employ the IL-33/ST2 axis as a diagnostic or prognostic biomarker. Multi-center studies assessing IL-33 and ST2 levels could shed light on their role in disease trajectory, therapeutic responsiveness, or potential recurrence.
- Synergy with Current Therapies: The prospect of combining therapies targeting the IL-33/ST2 axis with existing immunotherapies holds promise. For instance, integrating IL-33 inhibition with immune checkpoint blockade might unveil unforeseen synergistic effects in tumor suppression.
- Role in Metastasis: The IL-33/ST2 axis’ potential role in driving metastatic tendencies, especially to organs such as the lungs, necessitates comprehensive exploration. Understanding how this axis participates in establishing metastatic niches could inform therapeutic strategies to counteract these processes.
- Tumor Microenvironment Modulation: The dynamic interplay between the IL-33/ST2 axis, the TME, and its constituent immune cells (like tumor-infiltrating lymphocytes, macrophages, and dendritic cells) needs detailed investigation. Understanding how the IL-33/ST2 axis modulates the TME can provide insights into strategies to render the TME unfavorable for tumor advancement.
- Patient Stratification: Given breast cancer’s heterogeneity, determining treatment strategies based on IL-33 and ST2 expression, or the resulting downstream effects, could lead to more personalized and effective therapeutic regimens.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies-An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Bhushan, A.; Gonsalves, A.; Menon, J.U. Current State of Breast Cancer Diagnosis, Treatment, and Theranostics. Pharmaceutics 2021, 13, 723. [Google Scholar] [CrossRef]
- Jin, X.; Mu, P. Targeting Breast Cancer Metastasis. Breast Cancer Basic Clin. Res. 2015, 9, 23–34. [Google Scholar] [CrossRef]
- Lüönd, F.; Tiede, S.; Christofori, G. Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression. Br. J. Cancer 2021, 125, 164–175. [Google Scholar] [CrossRef]
- Weigelt, B.; Reis-Filho, J.S. Histological and molecular types of breast cancer: Is there a unifying taxonomy? Nat. Rev. Clin. Oncol. 2009, 6, 718–730. [Google Scholar] [CrossRef]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Fumagalli, C.; Barberis, M. Breast Cancer Heterogeneity. Diagnostics 2021, 11, 1555. [Google Scholar] [CrossRef]
- Vasanthakumar, A.; Kallies, A. Interleukin (IL)-33 and the IL-1 Family of Cytokines-Regulators of Inflammation and Tissue Homeostasis. Cold Spring Harb. Perspect. Biol. 2019, 11, a028506. [Google Scholar] [CrossRef]
- Jiang, W.; Lian, J.; Yue, Y.; Zhang, Y. IL-33/ST2 as a potential target for tumor immunotherapy. Eur. J. Immunol. 2021, 51, 1943–1955. [Google Scholar] [CrossRef] [PubMed]
- Markovic, S.S.; Jovanovic, M.; Gajovic, N.; Jurisevic, M.; Arsenijevic, N.; Jovanovic, M.; Jovanovic, M.; Mijailovic, Z.; Lukic, S.; Zornic, N.; et al. IL 33 Correlates With COVID-19 Severity, Radiographic and Clinical Finding. Front. Med. 2021, 8, 749569. [Google Scholar] [CrossRef]
- Hollande, C.; Boussier, J.; Ziai, J.; Nozawa, T.; Bondet, V.; Phung, W.; Lu, B.; Duffy, D.; Paradis, V.; Mallet, V.; et al. Inhibition of the dipeptidyl peptidase DPP4 (CD26) reveals IL-33-dependent eosinophil-mediated control of tumor growth. Nat. Immunol. 2019, 20, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C.; Girard, J.-P. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol. Rev. 2018, 281, 154–168. [Google Scholar] [CrossRef]
- Zhou, Z.; Yan, F.; Liu, O. Interleukin (IL)-33: An orchestrator of immunity from host defence to tissue homeostasis. Clin. Transl. Immunol. 2020, 9, e1146. [Google Scholar] [CrossRef] [PubMed]
- Afferni, C.; Buccione, C.; Andreone, S.; Galdiero, M.R.; Varricchi, G.; Marone, G.; Mattei, F.; Schiavoni, G. The Pleiotropic Immunomodulatory Functions of IL-33 and Its Implications in Tumor Immunity. Front. Immunol. 2018, 9, 2601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, W.; Zeng, P.; Xu, J.; Diao, H. The Contradictory Role of Interleukin-33 in Immune Cells and Tumor Immunity. Cancer Manag. Res. 2020, 12, 7527–7537. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.-P. Interleukin-33 (IL-33): A critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine. Cytokine 2022, 156, 155891. [Google Scholar] [CrossRef]
- Yi, X.M.; Lian, H.; Li, S. Signaling and functions of interleukin-33 in immune regulation and diseases. Cell Insight 2022, 1, 100042. [Google Scholar] [CrossRef]
- Yeoh, W.J.; Vu, V.P.; Krebs, P. IL-33 biology in cancer: An update and future perspectives. Cytokine 2022, 157, 155961. [Google Scholar] [CrossRef]
- Gao, X.; Wang, X.; Yang, Q.; Zhao, X.; Wen, W.; Li, G.; Lu, J.; Qin, W.; Qi, Y.; Xie, F.; et al. Tumoral expression of IL-33 inhibits tumor growth and modifies the tumor microenvironment through CD8+ T and NK cells. J. Immunol. 2015, 194, 438–445. [Google Scholar] [CrossRef]
- Larsen, K.M.; Minaya, M.K.; Vaish, V.; Peña, M.M.O. The Role of IL-33/ST2 Pathway in Tumorigenesis. Int. J. Mol. Sci. 2018, 19, 2676. [Google Scholar] [CrossRef]
- Miller, A.M. Role of IL-33 in inflammation and disease. J. Inflamm. 2011, 8, 22. [Google Scholar] [CrossRef]
- Carriere, V.; Roussel, L.; Ortega, N.; Lacorre, D.A.; Americh, L.; Aguilar, L.; Bouche, G.; Girard, J.P. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Bossila, E.A.; Ma, X.; Zhao, C.; Zhao, Y. Dual Immune Regulatory Roles of Interleukin-33 in Pathological Conditions. Cells 2022, 11, 3237. [Google Scholar] [CrossRef]
- Di Salvo, E.; Casciaro, M.; Gangemi, S. IL-33 genetics and epigenetics in immune-related diseases. Clin. Mol. Allergy 2021, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C. IL-33, an Alarmin of the IL-1 Family Involved in Allergic and Non Allergic Inflammation: Focus on the Mechanisms of Regulation of Its Activity. Cells 2022, 11, 107. [Google Scholar] [CrossRef]
- Talabot-Ayer, D.; Lamacchia, C.; Gabay, C.; Palmer, G. Interleukin-33 is biologically active independently of caspase-1 cleavage. J. Biol. Chem. 2009, 284, 19420–19426. [Google Scholar] [CrossRef] [PubMed]
- Lüthi, A.U.; Cullen, S.P.; McNeela, E.A.; Duriez, P.J.; Afonina, I.S.; Sheridan, C.; Brumatti, G.; Taylor, R.C.; Kersse, K.; Vandenabeele, P.; et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity 2009, 31, 84–98. [Google Scholar] [CrossRef]
- Lefrançais, E.; Roga, S.; Gautier, V.; Gonzalez-de-Peredo, A.; Monsarrat, B.; Girard, J.P.; Cayrol, C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc. Natl. Acad. Sci. USA 2012, 109, 1673–1678. [Google Scholar] [CrossRef]
- Lefrançais, E.; Duval, A.; Mirey, E.; Roga, S.; Espinosa, E.; Cayrol, C.; Girard, J.P. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc. Natl. Acad. Sci. USA 2014, 111, 15502–15507. [Google Scholar] [CrossRef]
- Molofsky, A.B.; Savage, A.K.; Locksley, R.M. Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation. Immunity 2015, 42, 1005–1019. [Google Scholar] [CrossRef]
- Drake, L.Y.; Kita, H. IL-33: Biological properties, functions, and roles in airway disease. Immunol. Rev. 2017, 278, 173–184. [Google Scholar] [CrossRef]
- Dominguez, D.; Zhang, Y.; Zhang, B. IL-33 in Tumor Immunity: Nothing to Sneeze At. Crit. Rev. Immunol. 2018, 38, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Bae, S.; Jhun, H.; Lee, S.; Choi, J.; Kang, T.; Kwak, A.; Hong, K.; Kim, E.; Jo, S.; et al. Identification of constitutively active interleukin 33 (IL-33) splice variant. J. Biol. Chem. 2011, 286, 20078–20086. [Google Scholar] [CrossRef]
- Roussel, L.; Erard, M.; Cayrol, C.; Girard, J.P. Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A-H2B acidic pocket. EMBO Rep. 2008, 9, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Griesenauer, B.; Paczesny, S. The ST2/IL-33 Axis in Immune Cells during Inflammatory Diseases. Front. Immunol. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, R.; Lee, R.T. The IL-33/ST2 pathway: Therapeutic target and novel biomarker. Nat. Rev. Drug Discov. 2008, 7, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Lingel, A.; Weiss, T.M.; Niebuhr, M.; Pan, B.; Appleton, B.A.; Wiesmann, C.; Bazan, J.F.; Fairbrother, W.J. Structure of IL-33 and its interaction with the ST2 and IL-1RAcP receptors--insight into heterotrimeric IL-1 signaling complexes. Structure 2009, 17, 1398–1410. [Google Scholar] [CrossRef]
- Rao, X.; Hua, F.; Zhang, L.; Lin, Y.; Fang, P.; Chen, S.; Ying, J.; Wang, X. Dual roles of interleukin-33 in cognitive function by regulating central nervous system inflammation. J. Transl. Med. 2022, 20, 369. [Google Scholar] [CrossRef]
- Travers, J.; Rochman, M.; Miracle, C.E.; Habel, J.E.; Brusilovsky, M.; Caldwell, J.M.; Rymer, J.K.; Rothenberg, M.E. Chromatin regulates IL-33 release and extracellular cytokine activity. Nat. Commun. 2018, 9, 3244. [Google Scholar] [CrossRef]
- Funakoshi-Tago, M.; Tago, K.; Hayakawa, M.; Tominaga, S.; Ohshio, T.; Sonoda, Y.; Kasahara, T. TRAF6 is a critical signal transducer in IL-33 signaling pathway. Cell. Signal. 2008, 20, 1679–1686. [Google Scholar] [CrossRef]
- Milovanovic, M.; Volarevic, V.; Radosavljevic, G.; Jovanovic, I.; Pejnovic, N.; Arsenijevic, N.; Lukic, M.L. IL-33/ST2 axis in inflammation and immunopathology. Immunol. Res. 2012, 52, 89–99. [Google Scholar] [CrossRef]
- O’Donnell, C.; Mahmoud, A.; Keane, J.; Murphy, C.; White, D.; Carey, S.; O’Riordain, M.; Bennett, M.W.; Brint, E.; Houston, A. An antitumorigenic role for the IL-33 receptor, ST2L, in colon cancer. Br. J. Cancer 2016, 114, 37–43. [Google Scholar] [CrossRef]
- Smithgall, M.D.; Comeau, M.R.; Yoon, B.R.; Kaufman, D.; Armitage, R.; Smith, D.E. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int. Immunol. 2008, 20, 1019–1030. [Google Scholar] [CrossRef]
- Pisani, L.F.; Teani, I.; Vecchi, M.; Pastorelli, L. Interleukin-33: Friend or Foe in Gastrointestinal Tract Cancers? Cells 2023, 12, 1481. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shen, J.-X.; Hu, J.-L.; Huang, W.-H.; Zhang, G.-J. Significance of Interleukin-33 and Its Related Cytokines in Patients with Breast Cancers. Front. Immunol. 2014, 5, 141. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-P.; Ling, D.-Y.; Xie, Y.-H.; Wu, W.-X.; Li, J.-R.; Jiang, J.; Zheng, J.-L.; Fan, Y.-H.; Zhang, Y. The Association of Serum IL-33 and sST2 with Breast Cancer. Dis. Markers 2015, 2015, 516895. [Google Scholar] [CrossRef] [PubMed]
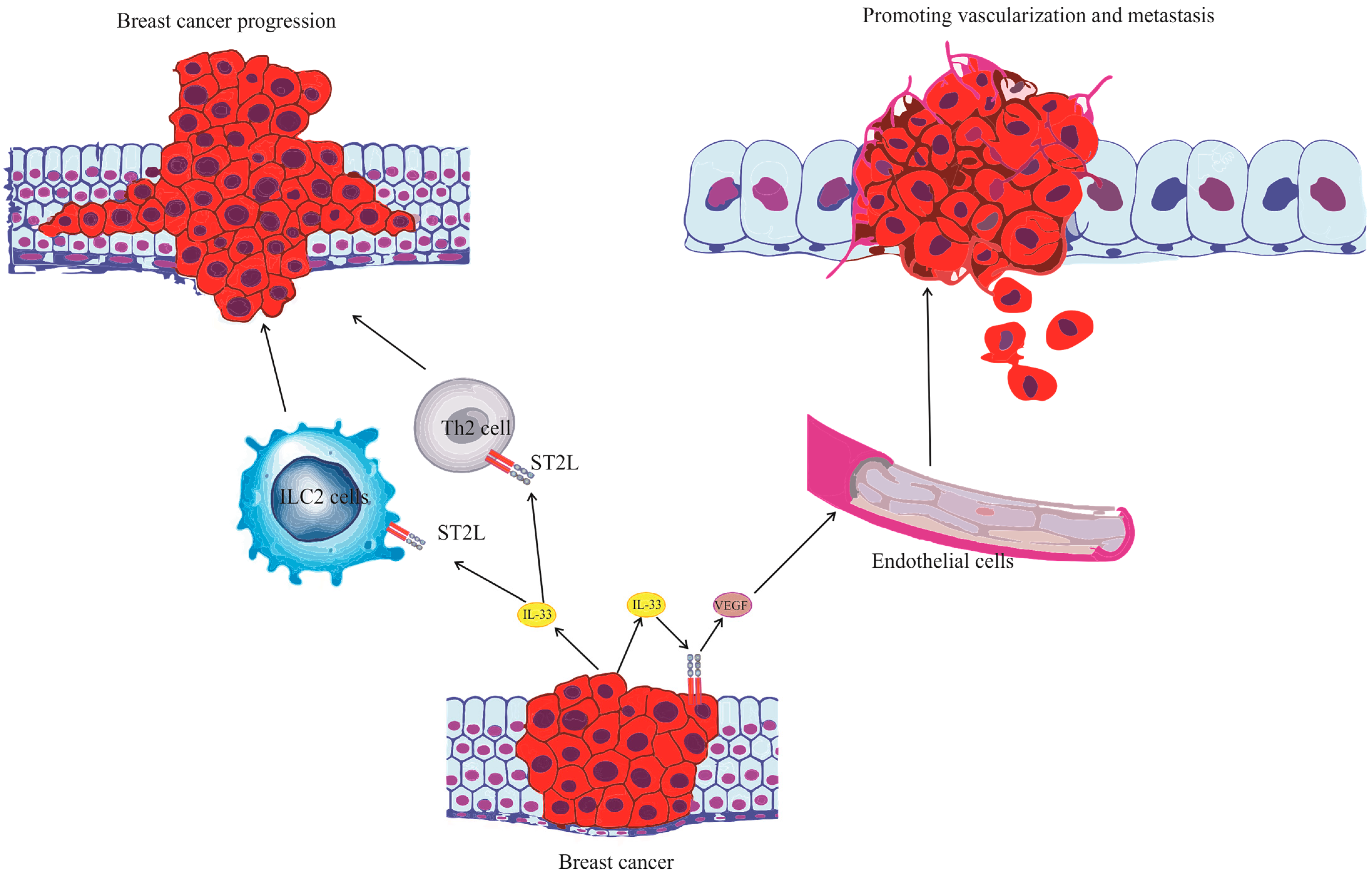
- Milosavljevic, M.Z.; Jovanovic, I.P.; Pejnovic, N.N.; Mitrovic, S.L.; Arsenijevic, N.N.; Simovic Markovic, B.J.; Lukic, M.L. Deletion of IL-33R attenuates VEGF expression and enhances necrosis in mammary carcinoma. Oncotarget 2016, 7, 18106–18115. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Minaee, K.; Farsinejad, A.R.; Nemati, M.; Khosravimashizi, A.; Daneshvar, H.; Mohammadi, M.M.; Sheikhi, A.; Ghaderi, A. Evaluation of the circulating levels of IL-12 and IL-33 in patients with breast cancer: Influences of the tumor stages and cytokine gene polymorphisms. Iran. J. Basic. Med. Sci. 2015, 18, 1189–1198. [Google Scholar]
- Yigitbasi, M.R.; Guntas, G.; Atak, T.; Sonmez, C.; Yalman, H.; Uzun, H. The Role of Interleukin-33 as an Inflammatory Marker in Differential Diagnosis of Idiopathic Granulomatous Mastitis and Breast Cancer. J. Investig. Surg. 2017, 30, 272–276. [Google Scholar] [CrossRef]
- Lu, D.P.; Zhou, X.Y.; Yao, L.T.; Liu, C.G.; Ma, W.; Jin, F.; Wu, Y.F. Serum soluble ST2 is associated with ER-positive breast cancer. BMC Cancer 2014, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Sun, J.; Wang, C.; Bu, X.; Liu, X.; Mao, Y.; Wang, H. IL-33 facilitates endocrine resistance of breast cancer by inducing cancer stem cell properties. Biochem. Biophys. Res. Commun. 2017, 485, 643–650. [Google Scholar] [CrossRef]
- Goda, N.; Nakashima, C.; Nagamine, I.; Otagaki, S. The Effect of Intratumoral Interrelation among FOXP3+ Regulatory T Cells on Treatment Response and Survival in Triple-Negative Breast Cancer. Cancers 2022, 14, 2138. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, I.P.; Pejnovic, N.N.; Radosavljevic, G.D.; Pantic, J.M.; Milovanovic, M.Z.; Arsenijevic, N.N.; Lukic, M.L. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int. J. Cancer 2014, 134, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Shani, O.; Vorobyov, T.; Monteran, L.; Lavie, D.; Cohen, N.; Raz, Y.; Tsarfaty, G.; Avivi, C.; Barshack, I.; Erez, N. Fibroblast-Derived IL33 Facilitates Breast Cancer Metastasis by Modifying the Immune Microenvironment and Driving Type 2 Immunity. Cancer Res. 2020, 80, 5317–5329. [Google Scholar] [CrossRef]
- Ito, A.; Akama, Y.; Satoh-Takayama, N.; Saito, K.; Kato, T.; Kawamoto, E.; Gaowa, A.; Park, E.J.; Takao, M.; Shimaoka, M. Possible Metastatic Stage-Dependent ILC2 Activation Induces Differential Functions of MDSCs through IL-13/IL-13Rα1 Signaling during the Progression of Breast Cancer Lung Metastasis. Cancers 2022, 14, 3267. [Google Scholar] [CrossRef]
- Dai, J.Z.; Yang, C.C.; Shueng, P.W.; Wang, Y.J.; Huang, C.S.; Chao, Y.C.; Chen, C.H.; Lin, C.W. Obesity-mediated upregulation of the YAP/IL33 signaling axis promotes aggressiveness and induces an immunosuppressive tumor microenvironment in breast cancer. J. Cell. Physiol. 2023, 238, 992–1005. [Google Scholar] [CrossRef]
- Xiao, P.; Wan, X.; Cui, B.; Liu, Y.; Qiu, C.; Rong, J.; Zheng, M.; Song, Y.; Chen, L.; He, J.; et al. Interleukin 33 in tumor microenvironment is crucial for the accumulation and function of myeloid-derived suppressor cells. Oncoimmunology 2016, 5, e1063772. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.W.; Sun, X.F.; Guo, X.H.; He, Y.N.; Cui, S.D.; Fan, Q.X. Tamoxifen-resistant breast cancer cells possess cancer stem-like cell properties. Chin. Med. J. 2013, 126, 3030–3034. [Google Scholar]
- Kim, J.Y.; Lim, S.C.; Kim, G.; Yun, H.J.; Ahn, S.G.; Choi, H.S. Interleukin-33/ST2 axis promotes epithelial cell transformation and breast tumorigenesis via upregulation of COT activity. Oncogene 2015, 34, 4928–4938. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, G.; Lim, S.C.; Choi, H.S. IL-33-Induced Transcriptional Activation of LPIN1 Accelerates Breast Tumorigenesis. Cancers 2021, 13, 2174. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, F.; Tay, L.W.R.; Boroda, S.; Nian, W.; Levental, K.R.; Levental, I.; Harris, T.E.; Chang, J.T.; Du, G. Lipin-1 regulation of phospholipid synthesis maintains endoplasmic reticulum homeostasis and is critical for triple-negative breast cancer cell survival. FASEB J. 2017, 31, 2893–2904. [Google Scholar] [CrossRef] [PubMed]
- Kudo-Saito, C.; Miyamoto, T.; Imazeki, H.; Shoji, H.; Aoki, K.; Boku, N. IL33 Is a Key Driver of Treatment Resistance of Cancer. Cancer Res. 2020, 80, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Long, A.; Dominguez, D.; Qin, L.; Chen, S.; Fan, J.; Zhang, M.; Fang, D.; Zhang, Y.; Kuzel, T.M.; Zhang, B. Type 2 Innate Lymphoid Cells Impede IL-33-Mediated Tumor Suppression. J. Immunol. 2018, 201, 3456–3464. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ji, Y.; Wang, H.; Zhang, H.; Zhou, H. IL-33 Promotes the Development of Colorectal Cancer Through Inducing Tumor-Infiltrating ST2L+ Regulatory T Cells in Mice. Technol. Cancer Res. Treat. 2018, 17, 1533033818780091. [Google Scholar] [CrossRef]
- Wang, K.; Shan, S.; Yang, Z.; Gu, X.; Wang, Y.; Wang, C.; Ren, T. IL-33 blockade suppresses tumor growth of human lung cancer through direct and indirect pathways in a preclinical model. Oncotarget 2017, 8, 68571–68582. [Google Scholar] [CrossRef]
- Jovanovic, I.; Radosavljevic, G.; Mitrovic, M.; Juranic, V.L.; McKenzie, A.N.; Arsenijevic, N.; Jonjic, S.; Lukic, M.L. ST2 deletion enhances innate and acquired immunity to murine mammary carcinoma. Eur. J. Immunol. 2011, 41, 1902–1912. [Google Scholar] [CrossRef]
- Lucarini, V.; Ziccheddu, G.; Macchia, I.; La Sorsa, V.; Peschiaroli, F.; Buccione, C.; Sistigu, A.; Sanchez, M.; Andreone, S.; D’Urso, M.T.; et al. IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology 2017, 6, e1317420. [Google Scholar] [CrossRef]
- Lim, H.X.; Choi, S.; Cho, D.; Kim, T.S. IL-33 inhibits the differentiation and immunosuppressive activity of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. Immunol. Cell Biol. 2017, 95, 99–107. [Google Scholar] [CrossRef]
- Dominguez, D.; Ye, C.; Geng, Z.; Chen, S.; Fan, J.; Qin, L.; Long, A.; Wang, L.; Zhang, Z.; Zhang, Y.; et al. Exogenous IL-33 Restores Dendritic Cell Activation and Maturation in Established Cancer. J. Immunol. 2017, 198, 1365–1375. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, Q.; Miao, Y.; Kang, W.; Tian, Z.; Xu, D.; Xiao, W.; Fang, F. Interleukin-33 activates and recruits natural killer cells to inhibit pulmonary metastatic cancer development. Int. J. Cancer 2020, 146, 1421–1434. [Google Scholar] [CrossRef]
- Chen, L.; Sun, R.; Xu, J.; Zhai, W.; Zhang, D.; Yang, M.; Yue, C.; Chen, Y.; Li, S.; Turnquist, H.; et al. Tumor-Derived IL33 Promotes Tissue-Resident CD8+ T Cells and Is Required for Checkpoint Blockade Tumor Immunotherapy. Cancer Immunol. Res. 2020, 8, 1381–1392. [Google Scholar] [CrossRef]
- Song, M.; Yang, J.; Di, M.; Hong, Y.; Pan, Q.; Du, Y.; Xiang, T.; Liu, J.; Tang, Y.; Wang, Q.; et al. Alarmin IL-33 orchestrates antitumoral T cell responses to enhance sensitivity to 5-fluorouracil in colorectal cancer. Theranostics 2023, 13, 1649–1668. [Google Scholar] [CrossRef]
- Blomberg, O.S.; Spagnuolo, L.; Garner, H.; Voorwerk, L.; Isaeva, O.I.; van Dyk, E.; Bakker, N.; Chalabi, M.; Klaver, C.; Duijst, M.; et al. IL-5-producing CD4+ T cells and eosinophils cooperate to enhance response to immune checkpoint blockade in breast cancer. Cancer Cell 2023, 41, 106–123.e10. [Google Scholar] [CrossRef]
- Zerdes, I.; Matikas, A.; Foukakis, T. The interplay between eosinophils and T cells in breast cancer immunotherapy. Mol. Oncol. 2023, 17, 545–547. [Google Scholar] [CrossRef]
- Jovanovic, M.Z.; Geller, D.A.; Gajovic, N.M.; Jurisevic, M.M.; Arsenijevic, N.N.; Jovanovic, M.M.; Supic, G.M.; Vojvodic, D.V.; Jovanovic, I.P. Dual blockage of PD-L/PD-1 and IL33/ST2 axes slows tumor growth and improves antitumor immunity by boosting NK cells. Life Sci. 2022, 289, 120214. [Google Scholar] [CrossRef]
| Role of IL-33 | Mechanism | Reference |
|---|---|---|
| Promotion of breast cancer proliferation | Stimulates the accumulation of immunosuppressive cell populations in the tumor microenvironment | [54] |
| Promotion of metastasis | Th2 immune response favoring secondary deposit formation, elicits IL-13 from innate lymphoid 2 cells | [54,55,56] |
| Induction of tumor tissue inflammation | Enhances neoangiogenesis, supports tumor vitality, reduces tumor necrosis | [48] |
| Promotion of mammary tumor growth | Upregulates VEGF expression in tumor cells, attenuates tumor necrosis | [48] |
| Induction of malignant cell stemness | Induces stemness, making treatment with classic therapeutics, such as estrogen receptor inhibitors, more challenging | [58] |
| Role of IL-33 | Mechanism | Reference |
|---|---|---|
| Association with endocrine resistance | Challenges some first-line therapeutic modalities | [52] |
| Dedifferentiation of malignant breast cancer cells | Activates cancer stem cell genes like ALDH1A3, OCT4, NANOG, and SOX2 | [59,60] |
| Acceleration of breast cancer progression | Signals molecules like COT to stimulate inflammation in the tumor microenvironment | [60] |
| Contribution to immunosuppression | Positive correlation with FOXP3 expression within the tumor microenvironment | [53] |
| Influence on breast cancer metabolism | Elevation of LPIN-1 expression, a molecule involved in phospholipid metabolism | [61,62] |
| Therapeutic Intervention | Mechanism |
|---|---|
| Blocking IL-33/ST2 Signaling | Inhibits the dedifferentiation of malignant breast cancer cells |
| Inhibition of IL-33 Expression | Targets cancer stem cells by reducing the activation of key stem cell genes |
| Regulation of IL-33 Induced Signaling Molecules | Decreases inflammation in the tumor microenvironment and prevents malignant transformation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanovic, B.; Gajovic, N.; Jurisevic, M.; Stojanovic, M.D.; Jovanovic, M.; Jovanovic, I.; Stojanovic, B.S.; Milosevic, B. Decoding the IL-33/ST2 Axis: Its Impact on the Immune Landscape of Breast Cancer. Int. J. Mol. Sci. 2023, 24, 14026. https://doi.org/10.3390/ijms241814026
Stojanovic B, Gajovic N, Jurisevic M, Stojanovic MD, Jovanovic M, Jovanovic I, Stojanovic BS, Milosevic B. Decoding the IL-33/ST2 Axis: Its Impact on the Immune Landscape of Breast Cancer. International Journal of Molecular Sciences. 2023; 24(18):14026. https://doi.org/10.3390/ijms241814026
Chicago/Turabian StyleStojanovic, Bojan, Nevena Gajovic, Milena Jurisevic, Milica Dimitrijevic Stojanovic, Marina Jovanovic, Ivan Jovanovic, Bojana S. Stojanovic, and Bojan Milosevic. 2023. "Decoding the IL-33/ST2 Axis: Its Impact on the Immune Landscape of Breast Cancer" International Journal of Molecular Sciences 24, no. 18: 14026. https://doi.org/10.3390/ijms241814026
APA StyleStojanovic, B., Gajovic, N., Jurisevic, M., Stojanovic, M. D., Jovanovic, M., Jovanovic, I., Stojanovic, B. S., & Milosevic, B. (2023). Decoding the IL-33/ST2 Axis: Its Impact on the Immune Landscape of Breast Cancer. International Journal of Molecular Sciences, 24(18), 14026. https://doi.org/10.3390/ijms241814026





