Abstract
Obesity has become a major health problem worldwide, and increasing evidence supports the importance of microRNAs (miRNAs) in its pathogenesis. Recently, we found that miR-383-5p_1 is highly expressed in the perirenal fat of high-fat-fed rabbits, but it is not yet known whether miR-383-5p is involved in lipid metabolism. Here, we used transcriptome sequencing technology to screen 1642 known differentially expressed genes between miR-383-5p mimic groups and miR-383-5p negative control groups. Gene Ontology Resource (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) were enriched in the pathway related to lipid metabolism, and glycine biosynthesis, the NOD receptor signal pathway and nonalcoholic fatty liver were significantly enriched. Afterwards, our research results indicated that miR-383-5p can promote the proliferation and differentiation of rabbit preadipocytes, and there is a direct targeting relationship with RAD51AP1. Mechanistically, miR-383-5p directly interacts with the lipid metabolism and participates in adipogenesis and lipid accumulation by targeting RAD51AP1. In conclusion, our data highlight a physiological role for miRNA in lipid metabolism and suggest the miR-383-5p/RAD51AP1 axis may represent a potential mechanism for controlling lipid accumulation in obesity.
1. Introduction
Obesity is now considered to be an epidemic, spreading rapidly not only in developed countries but also in developing countries [1,2]. Obesity has a positive and significant impact on the prevalence of all chronic diseases, especially hypertension, hyperlipidemia, and diabetes [3,4]. In general, obesity is a chronic disease that is difficult to control, often interrupted by treatment failure, and has become a huge public health problem [5]. Obesity is mainly caused by excessive accumulation of lipids in adipose tissue, including the increase in the volume and number of adipocytes [6,7]. Observational studies on humans have shown that patients with high visceral fat have a higher risk of serious complications [8]. For example, excessive perirenal fat will increase the risk of hypertension and coronary heart disease [9]. miRNAs are small noncoding RNAs that typically inhibit the translation and stability of mRNAs, controlling genes involved in cellular processes such as inflammation, cell-cycle regulation, stress response, differentiation, apoptosis, and migration [10,11]. At the molecular level, Zhong et al. [12] found that Genipin increased the expression levels of miR-142a-5p, which bound to 3′untranslated region of Srebp-1c, an important regulator of lipogenesis, which thus led to the inhibition of lipogenesis. miR-125a-5p should be considered as a regulator of glycolipid metabolism in T2DM, which can inhibit hepatic lipogenesis and gluconeogenesis and elevate glycogen synthesis by targeting STAT3 [13]. Ni et al. [14] revealed the significant role of miR-802/AMPK axis in hepatic lipid metabolism and identified the therapeutic potential of Sjp40 in treating obesity-related fatty liver. A growing body of recent research indicates that miRNAs are important in the pathogenesis of abdominal obesity, hypertension, coronary heart disease, atherosclerosis, and diabetes [15].
A large number of studies have shown that miR-383-5p can directly target the 3′-untranslated region of some pro-tumor genes to weaken cancer-related processes, including cell proliferation, cell development and the glycolysis pathway [16,17,18]. miR-383-5p is used as a tumor inhibitor in many types of cancer, including breast cancer, gastric cancer, and liver cancer [19,20,21]. Nowadays, miR-383-5p has become a promising therapeutic agent for treating related cancers. In terms of lipid metabolism, global transcriptomics analysis of Huh7 human hepatoma cells overexpressing miR-383-5p revealed enrichment of lipid and cholesterol metabolic processes; miR-383-5p has potential therapeutic effects in regulating liver lipid metabolism [22]. The above studies suggested that miR-383-5p may be involved in lipid metabolism processes, but the specific mechanism remains unclear. Rabbits have a similar lipid metabolism pathway to humans, making them an ideal model for studying human obesity [23]. Therefore, we investigated the role of miR-383-5p in rabbit preadipocyte proliferation and differentiation by overexpressing or inhibiting miR-383-5p.
2. Results
2.1. miR-383-5p_1 Is Associated with Adipose Development
In our previous research, we found that rabbits fed with a high-fat diet had a higher body weight than those fed with a standard normal diet. In addition, the total weight of perirenal adipose tissue in rabbits fed with a high-fat diet was also higher [24]. So, we selected three heavier 35-day-old rabbits (heavy rabbits) from the high-fat feeding group and three lighter 35-day-old rabbits (light rabbits) from the standard feeding group to verify whether miR-383-5p_1 is related to adipose development. Triglyceride (TG) and cholesterol (TC) are markers of lipid metabolism and were significantly enhanced in the heavy rabbits (Figure 1A,B). After the qRT-PCR experiment, it was found that the expression of miR-383-5p_1 in heavy rabbits was significantly higher than that in light rabbits (Figure 1C). Afterwards, we also validated the relative expression of miR-383-5p_1 in other tissues. The results showed that miR-383-5p_1 had a higher expression in adipose tissues of light and heavy rabbits, and there was a significant difference compared to other tissues (Figure 1D). These data suggested that miR-383-5p may be related to adipose development.
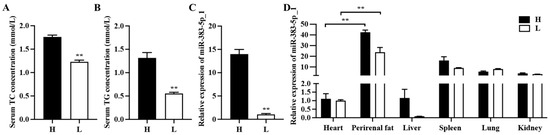
Figure 1.
miR-383-5p_1 is associated with adipose development. (A,B) TG and TC content in light (L) and heavy (H) rabbits. (C) The expression of miR-383-5p_1 in perirenal adipose tissue of light and heavy rabbits. (D) Expression of miR-383-5p_1 in light and heavy rabbit tissues. Data are expressed as means ± SEM. ** p < 0.01.
2.2. Analysis of Differentially Expressed Genes
To further understand the function of miR-383-5p, we sent the miR-383-5p mimic group and miR-383-5p NC group to the company for transcriptome sequencing. Six cDNA libraries were constructed from preadipocytes. As shown in Table S1, 262508156 clean readings were obtained. The levels of Q20 in the miR-383-5p mimic group and the NC group were equivalent (percentage of readings with Phred quality value > 20), ranging from 97.18% to 97.62%. The GC content of libraries ranged from 52.73% to 53.91%, with an average content of 53.31%. The comparative analysis showed that the average comparison rate of samples was 89.39%. Therefore, all libraries were of high quality and could be used for further analysis. String Tie was used to assemble new transcripts, and Pfam, SUPERFAMILY, GO, and KEGG were used to annotate them. A total of 64 unknown genes, 39 novel lncRNAs and 1514 novel protein-coding genes were obtained. A total of 1642 (721 up-regulated and 921 down-regulated) known differentially expressed genes were screened from the sequencing library using DESeq2 (Table S2). Values of log2 (fold change) and -log10 (p-value) were used to construct volcano figures for differentially expressed genes (Figure 2A). Three differentially expressed genes were randomly selected for qRT-PCR validation, and the results showed a similar trend to that of the sequencing (Figure 2B). In addition, we used the ENCORI website to identify 102 candidate genes out of 921 down-regulated genes that have potential binding sites with miR-383-5p (Figure 2C). Then, three highly expressed genes were selected from 102 candidate genes for qRT-PCR validation, including NUF2, CNIH4, and RAD51AP1. The results were consistent with the sequencing results (Figure 2D). Finally, the GO analysis results showed that 763 GO items were enriched (400 biological processes (BP), 107 cell composition (CC), and 256 molecular functions (MF)) (Figure 2E) (Table S3). The main biological processes involved protein catabolism, organic nitrogen compound biosynthesis, and lipid biosynthesis, especially some GO terms related to glucose metabolism. KEGG analysis showed that a total of 305 pathways were enriched, among which oxidative phosphorylation, non-alcoholic fatty liver disease and NOD receptor signal pathways were significantly enriched (Figure 2F) (Table S4).
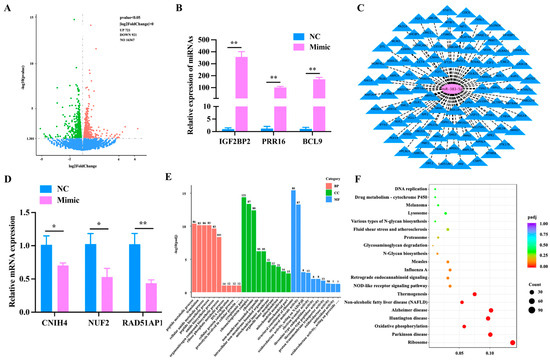
Figure 2.
Analysis of differentially expressed genes. (A) The volcano plot was constructed for the differentially expressed genes based on log2 (fold change) and −log10 (p-value). (B) Validation of differentially expressed genes. (C) The intersection target genes of miR-383-5p predicted by miRanda, TargetScan, and RNAhybrid software. (D) CNIH4, NUF2, and RAD51AP1 levels after transfecting with the miR-383-5p mimic and NC 24 h. (E) GO analysis of the differentially expressed genes were performed, imaging only those terms that were significantly enriched in the BP, CC, and MF categories. (F) KEGG analysis of the differentially expressed genes only listed significant enrichment pathways. The data are presented as means ± SEM. * p < 0.05; ** p < 0.01.
2.3. miR-383-5p Promotes Rabbit Preadipocyte Proliferation
To explore the role of miR-383-5p in the proliferation of rabbit preadipocytes, miR-383-5p mimic, miR-383-5p inhibitor, miR-383-5p negative control (NC) and miR-383-5p inhibitor negative control (INC) were transfected into cells. As shown in Figure 3A, the relative expression levels of miR-383-5p in the mimic (inhibitor) group were significantly higher (lower) than those in the NC (INC) group, indicating that transfection analogs successfully increased (decreased) the expression levels of miR-383-5p. As shown in Figure 3B,C, the CCK-8 results showed a significant increase in absorbance in the miR-383-5p mimic group at 0, 24, 48 and 72 h after transfection, while the miR-383-5p inhibition group showed the opposite trend. The positive cell rate in the miR-383-5p mimic group was significantly higher than that in the NC group. The positive cell rate in the miR-383-5p inhibitor group was significantly lower than that in the INC group (Figure 3D,E). Moreover, the results of WB showed that compared with the NC group, the expression of CDK2 and CDK4 in the miR-383-5p mimic group increased, and the expression in the miR-383-5p inhibitor group decreased (Figure 3F,G) (Table S5). The comprehensive results showed that miR-383-5p has a promotional effect on the proliferation of rabbit preadipocytes.
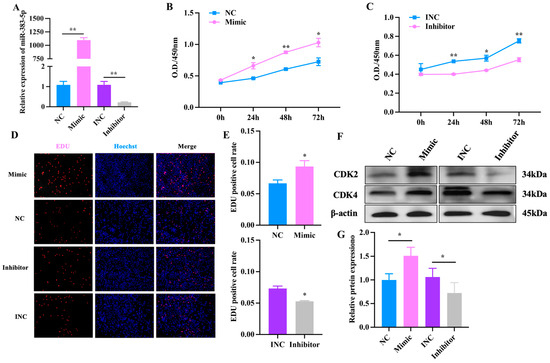
Figure 3.
miR-383-5p promotes rabbit preadipocyte proliferation. (A) Transfection efficiency detection of miR-383-5p mimic and inhibitor (n = 9).(B,C) The absorbance of preadipocytes at 0, 24, 48, and 72 h after transfection with the miR-383-5p mimic, NC, miR-383-5p inhibitor, and INC (n = 6). (D) The picture of the EDU proliferation assay for preadipocytes transfected with the miR-383-5p mimic, NC, miR-383-5p inhibitor, and INC. Red fluorescence represents the EDU-positive cells, and blue fluorescence represents the Hoechst-stained cells. (E) The percent of EDU-positive cells. EDUpositive cells rate = EDU-positive cells/Hoechst-stained cells × 100% (n = 3). (F,G) CDK2 and CDK4 protein levels during preadipocyte proliferation after transfecting with NC, miR-383-5p mimic, INC, and miR-383-5p inhibitor (n = 3). The data are presented as means ± SEM. * p < 0.05; ** p < 0.01.
2.4. miR-383-5p Promotes Rabbit Preadipocyte Differentiation
To investigate the function of miR-383-5p in the differentiation of rabbit preadipocytes, the fourth generation of preadipocytes were cultured in cell culture plates and differentiated with the above method when the density reached 80%. We first conducted an Oil Red O staining experiment; the results showed that lipid droplets rapidly increased during the differentiation of preadipocytes (Figure 4A). Meanwhile, the expression of miR-383-5p reached its peak on 6 d, revealing a possible regulatory role of miR-383-5p in preadipocyte differentiation (Figure 4B). Afterwards, Oil Red O staining showed that the lipid deposition was higher in the mimic group than the NC group, but the lipid deposition was lower in the inhibitor group than the INC group after 6 d of transfection miR-383-5p mimic, miR-383-5p inhibitor, NC, and INC (Figure 4C,D). The qRT-PCR results showed an obvious and highly significant increase of genes in the mimic group, including PPARγ, FABP4, and CEBP/α; in contrast, these genes were expressed at lower levels in the inhibitor group (Figure 4E,F). As shown in Figure 4G,H, the results of WB showed that, compared with the NC group, the expression of SREBP1 and FABP4 in the miR-383-5p mimic group increased, and the expression in the miR-383-5p inhibitor group decreased (Table S6). Therefore, we concluded that miR-383-5p plays a positive role in rabbit preadipocyte differentiation.
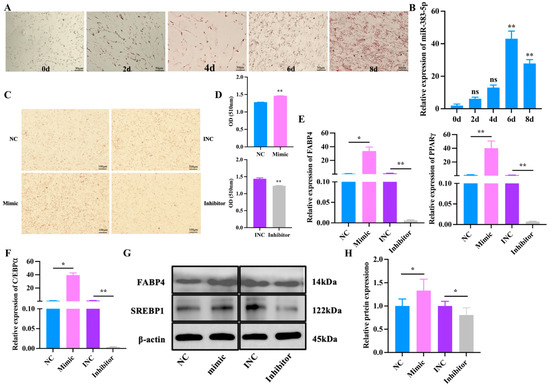
Figure 4.
miR-383-5p promotes rabbit preadipocyte differentiation. (A) Oil Red O staining of lipid droplets at 0, 2, 4, 6, and 8 d of differentiation. (B) The relative expression levels of miR-383-5p during preadipocyte differentiation progress (n = 9). (C,D) Oil Red O staining of lipid droplets after 6 d of transfection and quantitative results of Oil Red O staining (n = 9). (E,F) The relative expression levels of PPARγ, FABP4, and C/EBPα in rabbit preadipocytes induced differentiation at 6 d after transfecting with NC, miR-383-5p mimic, INC, and miR-383-5p inhibitor (n = 9). (G,H) SREBP1 and FABP4 protein levels during preadipocyte differentiation after transfecting with NC, miR-383-5p mimic, INC, and miR-383-5p inhibitor (n = 3). The data are presented as means ± SEM. * p < 0.05; ** p < 0.01.
2.5. Effects of miR-383-5p on Rabbit Preadipocytes Treated with Palmitic Acid
In order to explore the effect of miR-383-5p on palmitic acid (PA)-treated rabbit preadipocytes, we first constructed a model of PA-treated rabbit preadipocytes. After Oil Red O staining and triglyceride determination, we found that the lipid deposition in the PA group was significantly higher than in the blank group (CON) group (Figure 5A,B). The qRT-PCR results showed a significant increase in the expression of miR-383-5p in the PA group (Figure 5C). Moreover, Oil Red O staining showed that the lipid deposition was higher in the miR-383-5p mimic group than in the NC group, but the lipid deposition was lower in the miR-383-5p inhibitor group than in the INC group after 8 d of transfection (Figure 5D,E). TG results showed that the miR-383-5p mimic group had higher levels than the NC group and the miR-383-5p inhibitor group had lower levels than the INC group (Figure 5F). The expression of hypertrophy marker genes ACC, FAS and SCD were detected by qRT-PCR. ACC, FAS, and SCD were significantly increased in the miR-383-5p mimic group but decreased in the miR-383-5p inhibitor group (Figure 5H). These data suggested that miR-383-5p plays a positive role in rabbit preadipocytes treated with PA.
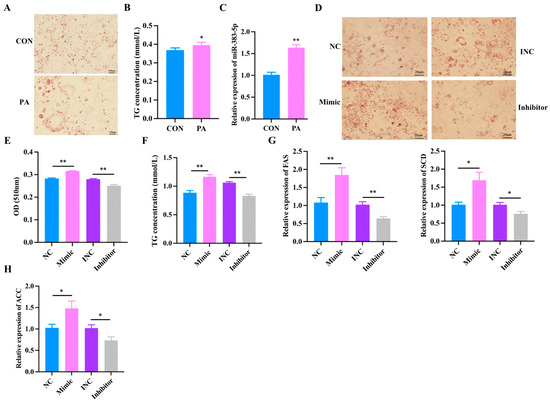
Figure 5.
Effects of miR-383-5p on rabbit preadipocytes treated with palmitic acid. (A) Oil Red O staining of lipid droplets after 2 d of PA treatment. (B) TG content after 2 d of PA treatment. (C) Relative expression of miR-383-5 after 2 d of PA treatment. (D,E) Oil Red O staining and quantitative results of lipid droplets 8 d after transfection. (F) TG content in rabbit preadipocytes induced differentiation at 6 d after transfecting and 2 d after treatment with PA (n = 3). (G,H) The relative expression levels of ACC, FAS, and SCD in rabbit preadipocytes induced differentiation at 6 d after transfecting with NC, miR-383-5p mimic, INC, and miR-383-5p inhibitor, and 2 d after treatment with PA (n = 9). The data are presented as means ± SEM. * p < 0.05; ** p < 0.01.
2.6. miR-383-5p Regulates the Proliferation and Differentiation of Rabbit Preadipocytes by Targeting RAD51AP1
To further explore the potential underlying mechanism of miR-383-5p regulation of adipogenesis, miRanda was used to predict potential target genes of miR-383-5p. Among the 921 downregulated genes, there are a total of 102 candidate genes, including the 3′UTR of RAD51AP1 (Figure 6A). The results of qRT-PCR experiments showed that RAD51AP1 was significantly down-regulated in the miR-383-5p mimic group (Figure 2C). The WB experiment verified the result (Figure 6B). The luciferase reporter assay revealed that the luciferase activity was highly suppressed in the group containing the wild-type 3′UTR of RAD51AP1 mRNA but not significantly changed in the mutant group (Figure 6C). These observations imply that RAD51AP1 was a direct miR-383-5p target. To reveal the function of RAD51AP1 on rabbit preadipocytes, si-RAD51AP1 and si-RAD51AP1-NC were transfected into cells. As shown in Figure 6D,E, the WB results showed that in the si-RAD51AP1-NC group, the expression of PCNA and CDK2 increased at the protein levels but decreased in the si-RAD51AP1 group. Moreover, the EDU proliferation assay revealed that the number of positive cells was significantly higher in the si-RAD51AP1-NC group than the si-RAD51AP1 group. (Figure 6F,G). These data suggested that si-RAD51AP1 played an inhibitory role in rabbit preadipocyte proliferation. Oil Red O staining showed that the lipid deposition was higher in the si-RAD51AP1 group than in the si-RAD51AP1-NC group after 6 d of transfection (Figure 6H,I). The WB results showed that in the si-RAD51AP1 group, the expression of PPARγ and C/EBPα increased at the protein level (Figure 6J,K). These data suggested that si-RAD51AP1 played a positive role in rabbit preadipocyte differentiation.
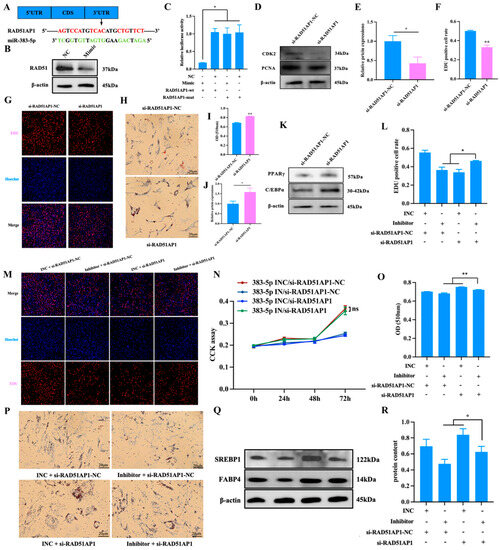
Figure 6.
miR-383-5p regulates the proliferation and differentiation of rabbit preadipocytes by targeting RAD51AP1. (A) The predicted binding site of gene RAD51AP1 with miR-383-5p. (B) After transfection with miR-383-5p mimic and NC, the expression of RAD51 was determined by WB. (C) Luciferase assays were performed by co-transfection of RAD51AP1 WT and mutant plasmids with miR-383-5p mimic and NC, respectively, in 293T cells, and the WT + NC group was used as the control group (n = 3). (D,E) CDK2 and PCNA protein levels during preadipocyte proliferation after transfecting with si-RAD51AP1 and si-RAD51AP1-NC (n = 3). (F) The percent of EDU-positive cells. EDU-positive cell rate = EDU-positive cells/Hoechst-stained cells × 100% (n = 3). (G) The picture of the EDU proliferation assay for preadipocytes transfected with the si-RAD51AP1 and si-RAD51AP1-NC. Red fluorescence represents the EDU-positive cells, and blue fluorescence represents the Hoechst-stained cells. (H,I) Oil Red O staining and quantitative results of lipid droplets 6 d after transfection. (J,K) PPARγ and C/EBPα protein levels during preadipocytes differentiation after transfecting with si-RAD51AP1 and si-RAD51AP1-NC (n = 3). (L) The percent of EDU-positive cells. EDU-positive cell rate = EDU-positive cells/Hoechst-stained cells × 100% (n = 3). (M) The picture of the EDU proliferation assay for preadipocytes transfected with the miR-383-5p INC/si-RAD51AP1-NC, miR-383-5p inhibitor/si-RAD51AP1-NC, miR-383-5p INC/si-RAD51AP1, and miR-383-5p inhibitor/si-RAD51AP1. Red fluorescence represents the EDU-positive cells, and blue fluorescence represents the Hoechst-stained cells. (N) The absorbance of preadipocytes at 0, 24, 48, and 72 h after transfection with the miR-383-5p INC/si-RAD51AP1-NC, miR-383-5p inhibitor/si-RAD51AP1-NC, miR-383-5p INC/si-RAD51AP1, and miR-383-5p inhibitor/si-RAD51AP1 (n = 6). (O) Quantitative results of Oil Red O staining (n = 5). (P) Oil Red O staining of lipid droplets after 6 d of transfection. (Q,R) SREBP1 and FABP4 protein levels during preadipocyte differentiation after transfecting with miR-383-5p INC/si-RAD51AP1-NC, miR-383-5p inhibitor/si-RAD51AP1-NC, miR-383-5p INC/si-RAD51AP1, and miR-383-5p inhibitor/si-RAD51AP1 (n = 3). The data were presented as means ± SEM. * p < 0.05; ** p < 0.01.
Finally, we also investigated whether miR-383-5p can regulate the proliferation and differentiation of rabbit preadipocytes by targeting RAD51AP1. miR-383-5p INC/si-RAD51AP1-NC, miR-383-5p inhibitor/si-RAD51AP1-NC, miR-383-5p INC/si-RAD51AP1, and miR-383-5p inhibitor/si-RAD51AP1 were co-transfected into rabbit preadipocytes, respectively. The EDU proliferation assay revealed that the number of positive cells was significantly higher in the miR-383-5p inhibitor/si-RAD51AP1 group than in the miR-383-5p inhibitor/si-RAD51AP1-NC and miR-383-5p INC/si-RAD51AP1 group (Figure 6L,M). As shown in Figure 6N, the CCK-8 results showed a significant increase in absorbance in the miR-383-5p inhibitor/si-RAD51AP1 group at 0, 24, 48 and 72 h after transfection. Oil Red O staining showed that the lipid deposition was higher in the miR-383-5p INC/si-RAD51AP1 group than in the miR-383-5p inhibitor/si-RAD51AP1 group, but the lipid deposition was lower in the miR-383-5p inhibitor/si-RAD51AP1-NC group than in the miR-383-5p inhibitor/si-RAD51AP1 after 6 d of transfection (Figure 6O,P). The WB results showed the same trend (Figure 6Q,R) (Table S7). The above results indicate that miR-383-5p may play a potential role in adipogenesis and development by targeting RAD51AP1.
3. Discussion
In higher vertebrates, adipose tissue is an organ that performs a lot of significant physiological functions [25]. Excess adipose tissue in the body can cause many organs and systems to be in a pathological state [26]. Adipocytes can undergo a transformation and change their structure and metabolism. The transition is driven by the concerted control of endogenous regulators, including miRNAs [27]. With the improvement in people’s living standards, more and more people are becoming obese, and this has reached epidemic level [28]. However, the prevention and treatment of obesity has not yet been successful. Here, we found that miR-383-5p is highly expressed in the perirenal fat of high-fat-fed rabbits, but it is not yet known whether miR-383-5p is involved in lipid metabolism. Subsequently, we conduct a detailed study of the proliferation and differentiation of rabbit preadipocytes using miR-383-5p to better understand the importance of miRNAs in lipid metabolism and to attempt to reveal the molecular regulatory mechanisms related to miRNAs in obesity treatment.
The same miRNA has different expressions in different tissues, and many miRNAs also have different expressions in adipose tissue, often with co-expression [29]. In our study, we found that miR-383-5p_1 had a high expression level in adipose tissue, which was significantly different from that in other tissues, and the expression level in heavy rabbits was significantly higher than that in light rabbits. These data revealed that miR-383-5p may play an important role in lipid metabolism. In adipose tissue, both increased adipocyte size and/or increased adipocyte number characterize the increased fat mass associated with obesity. The number of adipocytes largely determines the process of adipogenesis in body fat depots [30]. The proliferation of adipocyte precursors contributes to the development of obesity in mammals [31]. Therefore, we studied the effect of miR-383-5p on the proliferation and differentiation of rabbit preadipocytes. At the stage of preadipocyte proliferation, miR-383-5p plays a positive role. We found that CDK2 and CDK4 proteins were highly expressed in the miR-383-5p mimic group. It is worth noting that cyclin-dependent kinase 2 (CDK2) and CDK4 are key cell-cycle regulators [32]. CDK2 can phosphorylate many substrates to drive progression through the cell cycle [33]. Moreover, CDK4 deficiency has been found to affect cell proliferation in human malignant tumor cells [34]. At the stage of preadipocyte differentiation, miR-383-5p plays a positive role in preadipocyte differentiation. PPARγ, FABP4, C/EBPα, and SREBP1 are widely accepted as the essential transcription factors of preadipocyte differentiation [35]. The upregulation of miR-383-5p increased the expression of adipogenic marker genes FABP4, PPARγ, and C/EBPα, whereas the downregulation of miR-383-5p using the synthetic inhibitor markedly reduced the formation of neutral lipid droplets and suppressed the expression of marker genes at both the FABP4, PPARγ, and C/EBPα mRNAs and protein levels.
Research has shown that hyperplasia of adipose tissue occurs only in certain periods of life [36]. Adipocyte hypertrophy is the main process connected with obesity in adults [37]. The concentration of TG in cells is positively correlated with the increase in adipocyte area, which can better reflect the degree of adipocyte hypertrophy [30]. PA was chosen as our stimulus because it is the most common saturated fatty acid in the human body and is elevated in individuals with obesity due to lipolysis [38]. Secondly, PA is the physiological component of TG in adipocytes [39]. Finally, PA is noteworthy for its strong effects on gene expression, and can induce the differentiation of preadipocytes [40,41]. In the current study, we cultured rabbit preadipocytes with conditional medium derived from differentiated adipocytes incubated with PA. ACC, FAS, and SCD are widely accepted as the specific genes for fatty acid synthesis [42]. The upregulation of miR-383-5p increased the expression of marker genes ACC, FAS, and SCD, whereas the downregulation of miR-383-5p using the synthetic inhibitor markedly reduced the formation of neutral lipid droplets and suppressed the expression of marker genes at both the SCD mRNA and protein levels.
A total of 1642 known differentially expressed genes were screened from the sequencing library using DESeq2. GO and KEGG enriched the pathways related to lipid metabolism. Therefore, miR-383-5p may be important regulators in adipogenesis. In addition, bioinformatic predictions show that there are 102 candidate genes for miR-383-5p, including CNIH4, NUF2, and RAD51AP1. CNIH4 is a novel biomarker associated with poor prognosis and cell proliferation in low-grade glioma patients [43]. NUF2 plays an important role in tumorigenesis and is upregulated in multiple human cancers, including serous adenocarcinoma, liver cancer, and colorectal cancer [44]. Moreover, a large number of studies have shown that RAD51AP1 plays a key role in the development of cancer cells. Allison et al. [45] unraveled the role of RAD51AP1, a protein involved in homologous recombination in genotoxic carcinogen (azoxymethane, AOM)-induced colorectal cancer. RAD51AP1 might accelerate the progression of ovarian cancer by the TGF-β/Smad signaling pathway [46]. RAD51AP1 silencing significantly inhibited cell proliferation and invasion in esophageal squamous cell carcinoma (ESCC), thereby highlighting its potential as a novel target for ESCC treatment [47]. However, there is limited research on the regulation of adipogenesis by RAD51AP1. Among this research, RAD51AP1 was found in prenatal testosterone-induced visceral adipose tissue of female sheep and was highly expressed in the adipose-derived stem cells, which may be involved in lipid metabolism processes [48,49]. In our research, RAD51AP1 was a direct miR-383-5p target. RAD51AP1 may provide a new therapeutic target for metabolic diseases caused by obesity, which can help us better understand fat production and metabolism.
4. Materials and Methods
4.1. Ethics Statement
All experiments in the present study involving animals were performed under the direction of the Institutional Animal Care and Use Committee from the College of Animal Science and Technology, Sichuan Agricultural University, China. (Certification No. SYXK2019-187).
4.2. Animals and Cell Collection
A newly born Tianfu black rabbit was used for sampling. The experimental rabbit was rubbed all over with alcohol and sacrificed humanely to reduce suffering. The primary preadipocytes were collected in the perirenal area of Tianfu black rabbits using the method described earlier [50]. The tissue samples of 35-day-old rabbits were obtained from experiments conducted by Wang et al. [24]. Briefly, 24 female Tianfu black rabbits were raised in the rabbit farm of Sichuan Agricultural University and randomly divided into two groups. Twelve rabbits were fed a standard diet (SON) (light rabbits), twelve rabbits were fed a high-fat diet (HFD: 10% lard was added to SON) (heavy rabbits); the feed was supplied three times a day, and free drinking water was used. After four weeks, the rabbits were slaughtered, and tissue samples were collected. At the same time, body weight, TG, TC, and body fat rate were measured as indicators to distinguish between light and heavy rabbits.
4.3. Bioinformatic Analysis of Sequencing Data
The sample RNA was prepared, and strict quality control was carried out on the RNA sample, mainly through the Agilent 2100 bioanalyzer (Agilent technologies, Santa Clara, CA, USA). The first cDNA strand was synthesized in M-MuLV reverse transcriptase system, the second cDNA strand was synthesized in dNTPs and DNA polymerase I, poly (A) tails were added, and sequencing connectors were connected to generate 250–300 BP cDNA, and PCR-amplified cDNA to build the cDNA libraries using the NEBNext® UltraTM RNA Library Prep Kit for Illumina® (New England Biolabs, Ipswich, MA, USA). After passing the library inspection, the different libraries were pooled according to the effective concentration and target offline-data volume requirements, and then Illumina sequencing was performed. Differential expression analysis was performed using the DESeq2 with p ≤ 0.05.
4.4. Establish the Hypertrophic Cell Model
A total of 4 mmol PA (Sigma, Shanghai, China) was added to 0.1 mol/L NaOH (50 mL) for 30 min at 70 °C and then mixed with 10% bovine serum albumin (BSA, Sigma, Shanghai, China) to obtain the 1 mmol/L PA mother liquor. The mother liquor was diluted with the serum-free medium and filtered to obtain a 0.3 mmol/L PA working solution. For the CON group, the mother liquor was replaced with the same volume of 10% BSA and mixed with the serum-free medium. After transfection, PA and CON reagents were added, and samples were collected 2 d later.
4.5. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
Total RNA from the samples was extracted using the Total RNA Extraction Kit (Solarbio, Beijing, China), following the guidelines of the manufacturer. Reverse transcription of mRNA and miRNA was performed using the RT EasyTM II (With gDNase) (FOREGENE, Chengdu, China) and the Mir-XTM miRNA First-Strand Synthesis Kit (Takara, Dalian, China), respectively. Then, qRT-PCR was performed in triplicate using the NovoStart®SYBR qPCR SuperMix plus (Novoprotein, Shanghai, China) on a CFX96 instrument (Bio-Rad, Hercules, CA, USA), and the relative levels of mRNAs and miRNAs were calculated using the 2−ΔΔCt method. U6 and GAPDH were used as the internal reference for mRNA quantification. The sequences of primers are shown in Table S8 and were synthesized by Sangon Biotech (Shanghai, China).
4.6. Cell Culture and Induced Differentiation
Preadipocytes were maintained in a growth medium (GM) containing 10% fetal bovine serum (FBS, Gibco, CA, USA) in an incubator (Thermo Fisher Scientific, San Jose, CA, USA) at 37 °C and 5% CO2 environment, and the medium was changed every 24 h. When the cell density reached 70–80%, the induced differentiation medium (5% FBS, 1 μmol/L dexamethasone (DEX; Solarbio, Beijing, China), 0.5 mmol/L 3-isobutyl-1-methylxanthine (IBMX; Solarbio, Beijing, China) and 10 ug/mL insulin (Solarbio, Beijing, China)) was used to induce differentiation for 3 d to observe the lipid droplet formation of preadipocytes. Then, the medium was replaced with a maintenance medium containing 5% FBS and 10 ug/mL insulin for 3 d. Subsequently, the medium was replaced with GM to obtain mature adipocytes.
4.7. Transfection
In the proliferation assay, miR-383-5p mimic, miR-383-5p inhibitor, NC, INC, si-RAD51AP1, and si-RAD51AP1 were transfected using lipoMax (Sudgen, Nanjing, China) when the cell density reached 50–60%. After 6 h, the medium was changed to GM. In the differentiation assay, when the cell density reached 80% LipoMax was used for transfection. After 6 h, the medium was changed to the induction differentiation medium. RNA oligo was synthesized by Sangon Biotech (Shanghai, China). The miRNA mimic is chemically synthesized mature double-stranded miRNA that enhances endogenous miRNA function. This includes a sequence that is consistent with the target miRNA mature body sequence and a sequence that is complementary to the miRNA mature body sequence. The miRNA inhibitor is a chemically synthesized mature complementary single-stranded miRNA designed with methoxy modification. The inhibitor specifically targeting miRNA in cells can efficiently inhibit the activity of endogenous miRNA in organisms and conduct miRNA functional deficiency research. NC and INC are negative controls, respectively. The detailed RNA oligo sequences are shown in Table S9.
4.8. Measurement of Triglycerides and Cholesterol
The blood sample was centrifuged at 4 °C for 5 min and the serum was transferred to a clean pipe. According to the standard guides, the serum concentration of TG and cholesterol TC were respectively tested using the commercial kit based on enzymatic colorimetry (Jiancheng, Nanjing, China). Similarly, preadipocytes were inoculated in the 6-well plates (NEST Biotechnology, Wuxi, China), and transfected with the miR-383-5p mimic, NC, miR-383-5p inhibitor, and INC when the cell density was about 70%. After inducing differentiation, cells were collected, broken up, and tested for TG content with the above kit.
4.9. Western Blotting
Total protein from the cell was collected using the ProteinExt®Mammalian Total Protein Extraction Kit (TransGen Biotech, Beijing, China), following the manufacturer’s protocol. The concentration of the protein was measured using the Bradford protein assay kit (Novoprotein, Shanghai, China). The protein was resolved on 10% SDS-PAGE and then transferred to a PVDF membrane, followed by sealing of the sealing fluid. The membranes were incubated with the corresponding primary antibodies for 8 h at 4 °C and subsequently incubated with the secondary anti-bodies (Goat Anti-Rabbit IgG H&L (HRP), Zen bioscience, Chengdu, China) for 2 h. Then the bands were washed three times, and the hypersensitive chromogenic solution was developed using chemiluminescence in Touch Imager Pro, e-BLOT Life Science (Shanghai) Co., Ltd. (Shanghai, China). The primary antibodies PCNA, CDK2, and CDK4 were purchased from Zen bioscience (Chengdu, China). The primary antibodies PPARγ, CEBP/α, FABP4, SREBP1, and RAD51 were purchased from the Abclonal (Wuhan, China).
4.10. Cell Counting Kit 8 (CCK-8) Assay
Preadipocytes were inoculated in the 96-well plates (NEST Biotechnology, Wuxi, China) and transfected with the miR-383-5p mimic, NC, miR-383-5p inhibitor, and INC when the cell density was about 60%. According to manufacturer’s instructions, 10 μL CCK-8 was added to each well at 0, 24, 48 and 72 h after transfection, and the cells were cultured at 37 °C for 2 h in 5% CO2 incubator. The absorbance at 450 nm was measured by Thermo ScientificTM Varioskan LUX (Thermo Scientific, Waltham, MA, USA). The absorbance was drawn and analyzed using GraphPad Prism 9 (GraphPad Software Inc., La Jolla, CA, USA).
4.11. EDU Proliferation Assay
Preadipocytes were inoculated in 24-well plates. After 24 h of transfection, the cells were cultured in a complete medium containing 50 μL 5-ethynyl-2′-deoxyuridine (EDU, RiboBio, Guangzhou, China) for 2 h and fixed and dyed according to manufacturer’s instructions. The nucleus and positive cells were stained with Hoechst and EDU, respectively, and photographed using an inverted fluorescence microscope (Olympus, Tokyo, Japan) at the same visual field conditions. Images were analyzed using image-pro plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
4.12. Oil Red O Staining
Preadipocytes were inoculated in the 35 mm cell culture dishes (NEST Biotechnology, Wuxi, China). The cells were washed two times with phosphate-buffered saline (PBS) and fixed in 10% paraformaldehyde for 30 min. Subsequently, Oil Red O (NJBI, Nanjing, China) was mixed with deionized water at a rate of 3:2 and then added to the stained cell for 25 min. Finally, the cells were washed with PBS. Images were collected under an inverted microscope. Then, 2 mL of isopropyl alcohol was added into the cell culture dishes, and the cells were fully lysed and then transferred into the 96-well plate. The absorbance at 510 nm was measured by Thermo ScientificTM Varioskan LUX. The absorbance was drawn and analyzed using GraphPad Prism 9 (GraphPad Software Inc., La Jolla, CA, USA). Moreover, the count of lipid droplets was measured using image-pro plus 6.0 software (Media Cybernetics).
4.13. Dual-Luciferase Reporter Assay
The MiRanda database (https://www.microRNA.org; accessed on 1 March 2022) was used to predict miR-383-5p-related target genes. The potential binding site between miRNA and mRNA was predicted using the RNA22 website (https://cm.jefferson.edu/rna22/Interactive/; accessed on 15 January 2023), based on the sequence information. According to the results, RAD51AP1 was selected as the possible target gene of miR-383-5p. Luciferase reporter plasmids (wild-type (WT) and mutant (MUT) of target sequence) were constructed by Tsingke Biotechnology Co., Ltd. (Beijing, China). Next, 293T cells were seeded into 24-well plates (NEST Biotechnology, Wuxi, China). The miR-383-5p mimic or NC was co-transfected with specific WT or mutant plasmids using the lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, USA) when the cell density reached 70%. Then, luciferase activities were measured using the Duo-Lite TM Luciferase Assay System (Vazyme, Nanjing, China) after Aa24 h.
4.14. Statistical Analysis
All data were analyzed using SPSS 20.0 statistical software; they were consistent with normal distribution and presented as means ± SEM. Student’s t-test and one-way analyses were used to analyze the significance of differences between the groups (* p < 0.05; ** p < 0.01).
5. Conclusions
Our research results indicate that miR-383-5p can promote the proliferation and differentiation of rabbit preadipocytes, and there is a direct targeting relationship with RAD51AP1. This reveals a new molecular mechanism of miR-383-5p regulating adipogenesis, which can provide theoretical guidance and new ideas for the treatment of metabolic diseases such as obesity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241814025/s1.
Author Contributions
J.W., W.S., X.J. and S.L. conceived and designed the study; X.Z., Z.L., T.T., G.C., S.X., K.Z. and Z.K. collected data and conducted the research; M.W. and J.S. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
Our study was funded by the key research and development project of Sichuan Province: high quality and characteristic rabbit breeding materials and method innovation and new variety breeding (2021YFYZ0033).
Institutional Review Board Statement
All experiments in the present study involving animals were performed under the direction of the Institutional Animal Care and Use Committee from the College of Animal Science and Technology, Sichuan Agricultural University, China. (Certification No. SYXK2019-187; Approval time: 29 January 2019).
Data Availability Statement
The raw data is publicly available on NCBI portal at Sequence Read Archive (SRA) BioProject ID: PRJNA904507. RNA-Seq data Submission ID: SUB12315011.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lavie, C.J.; Alpert, M.A.; Arena, R.; Mehra, M.R.; Milani, R.V.; Ventura, H.O. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013, 1, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.M.; Loftus, E.V. Obesity in inflammatory bowel disease: A review of its role in the pathogenesis, natural history, and treatment of IBD. Saudi J. Gastroenterol. 2021, 27, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Chade, A.R.; Hall, J.E. Role of the Renal Microcirculation in Progression of Chronic Kidney Injury in Obesity. Am. J. Nephrol. 2016, 44, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Dettoni, R.; Bahamondes, C.; Yevenes, C.; Cespedes, C.; Espinosa, J. The effect of obesity on chronic diseases in USA: A flexible copula approach. Sci. Rep. 2023, 13, 1831. [Google Scholar] [CrossRef]
- Stabouli, S.; Erdine, S.; Suurorg, L.; Jankauskienė, A.; Lurbe, E. Obesity and Eating Disorders in Children and Adolescents: The Bidirectional Link. Nutrients 2021, 13, 4321. [Google Scholar] [CrossRef]
- Marcelin, G.; Silveira AL, M.; Martins, L.B.; Ferreira, A.V.; Clément, K. Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. J. Clin. Investig. 2019, 129, 4032–4040. [Google Scholar] [CrossRef]
- Rouault, C.; Marcelin, G.; Adriouch, S.; Rose, C.; Genser, L.; Ambrosini, M.; Bichet, J.C.; Zhang, Y.; Marquet, F.; Aron-Wisnewsky, J.; et al. Senescence-associated β-galactosidase in subcutaneous adipose tissue associates with altered glycaemic status and truncal fat in severe obesity. Diabetologia 2021, 64, 240–254. [Google Scholar] [CrossRef]
- Rosqvist, F.; Iggman, D.; Kullberg, J.; Cedernaes, J.; Johansson, H.E.; Larsson, A.; Johansson, L.; Ahlström, H.; Arner, P.; Dahlman, I.; et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes 2014, 63, 2356–2368. [Google Scholar] [CrossRef]
- Liu, B.X.; Sun, W.; Kong, X.Q. Perirenal Fat: A Unique Fat Pad and Potential Target for Cardiovascular Disease. Angiology 2019, 70, 584–593. [Google Scholar] [CrossRef]
- Chen, L.; Heikkinen, L.; Wang, C.; Yang, Y.; Sun, H.; Wong, G. Trends in the development of miRNA bioinformatics tools. Brief. Bioinform. 2019, 20, 1836–1852. [Google Scholar] [CrossRef]
- Hill, M.; Tran, N. miRNA interplay: Mechanisms and consequences in cancer. Dis. Model. Mech. 2021, 14, dmm047662. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Chen, K.; Feng, M.; Shao, W.; Wu, J.; Chen, K.; Liang, T.; Liu, C. Genipin alleviates high-fat diet-induced hyperlipidemia and hepatic lipid accumulation in mice via miR-142a-5p/SREBP-1c axis. FEBS J. 2018, 285, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Yin, L.; Qi, Y.; Sun, H.; Sun, P.; Xu, M.; Tang, Z.; Peng, J. miR-125a-5p ameliorates hepatic glycolipid metabolism disorder in type 2 diabetes mellitus through targeting of STAT3. Theranostics 2018, 8, 5593–5609. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Xu, Z.; Li, C.; Zhu, Y.; Liu, R.; Zhang, F.; Chang, H.; Li, M.; Sheng, L.; Li, Z.; et al. Therapeutic inhibition of miR-802 protects against obesity through AMPK-mediated regulation of hepatic lipid metabolism. Theranostics 2021, 11, 1079–1099. [Google Scholar] [CrossRef]
- Voruganti, V.S. Precision Nutrition: Recent Advances in Obesity. Physiology 2023, 38, 42–50. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, H.; Zeng, B.; Gou, X.; Gou, X.; Ren, K.; Yuan, F. miR-383-5p serves as a tumor suppressor in bladder cancer by suppressing PI3K/AKT signaling pathway. Cancer Biomark. 2023, 37, 121–131. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, Y.; Liu, J.; Cai, Y.; Zhang, M.; Fang, X. LINC01128 expedites cervical cancer progression by regulating miR-383-5p/SFN axis. BMC Cancer 2019, 19, 1157. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Chen, L.; Wei, K.; Liu, Y.; Han, Y.; Xia, X. Circ_0003611 regulates apoptosis and oxidative stress injury of Alzheimer’s disease via miR-383-5p/KIF1B axis. Metab. Brain Dis. 2022, 37, 2915–2924. [Google Scholar] [CrossRef]
- Dastmalchi, N.; Azarbarzin, S.; Safaralizadeh, R.; Khojasteh, S.M.B.; Shadbad, M.A.; Amini, M.; Baghbanzadeh, A.; Asl, E.R.; Baghbani, E.; Lotfinejad, P.; et al. The combined therapy of miR-383-5p restoration and paclitaxel for treating MDA-MB-231 breast cancer. Med. Oncol. 2021, 39, 9. [Google Scholar] [CrossRef]
- Wei, C.; Gao, J.J. Downregulated miR-383-5p contributes to the proliferation and migration of gastric cancer cells and is associated with poor prognosis. PeerJ 2019, 7, e7882. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Fei, X.; Chen, X.; Chen, Y. Biostatistics mining associated method identifies AKR1B10 enhancing hepatocellular carcinoma cell growth and degenerated by miR-383-5p. Sci. Rep. 2018, 8, 11094. [Google Scholar] [CrossRef]
- Ahmed, N.; Ahmed, N.; Pezacki, J.P. miR-383 Regulates Hepatic Lipid Homeostasis and Response to Dengue Virus Infection. ACS Infect. Dis. 2022, 8, 928–941. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, M.; Shao, J.; Liu, Z.; Fu, C.; Chen, G.; Zhao, K.; Li, H.; Sun, W.; Jia, X.; et al. Combined analysis of differentially expressed lncRNAs and miRNAs in liver tissues of high-fat fed rabbits by transcriptome sequencing. Front. Genet. 2022, 13, 1000574. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shao, J.; Li, Y.; Elzo, M.A.; Jia, X.; Lai, S. Genome-wide identification and characterization of perirenal adipose tissue microRNAs in rabbits fed a high-fat diet. Biosci. Rep. 2021, 41, BSR20204297. [Google Scholar] [CrossRef] [PubMed]
- Murawska-Ciałowicz, E. Adipose tissue-morphological and biochemical characteristic of different depots. Postepy Hig. Med. Dosw. 2017, 71, 466–484. [Google Scholar] [CrossRef]
- White, U.; Ravussin, E. Dynamics of adipose tissue turnover in human metabolic health and disease. Diabetologia 2019, 62, 17–23. [Google Scholar] [CrossRef]
- Tsiloulis, T.; Watt, M.J. Exercise and the Regulation of Adipose Tissue Metabolism. Prog. Mol. Biol. Transl. Sci. 2015, 135, 175–201. [Google Scholar]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Shao, J.; Pan, T.; Wang, J.; Tang, T.; Li, Y.; Jia, X.; Lai, S. MiR-208b Regulates Rabbit Preadipocyte Proliferation and Differentiation. Genes 2021, 12, 890. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Lazar, M.A. New developments in adipogenesis. Trends Endocrinol. Metab. 2009, 20, 107–114. [Google Scholar] [CrossRef]
- Della-Fera, M.A.; Qian, H.; Baile, C.A. Adipocyte apoptosis in the regulation of body fat mass by leptin. Diabetes Obes. Metab. 2001, 3, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, I.M.; Hemming, M.L.; Lundberg, M.Z.; Serrata, M.P.; Goldaracena, I.; Liu, N.; Yin, P.; Paulo, J.A.; Gygi, S.P.; George, S.; et al. Concurrent inhibition of CDK2 adds to the anti-tumour activity of CDK4/6 inhibition in GIST. Br. J. Cancer 2022, 127, 2072–2085. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Moser, J.; Hoffman, T.E.; Watts, L.P.; Watts, L.P.; Min, M.; Musteanu, M.; Rong, Y.; Ryland, C., III; Nangia, V.; et al. Rapid adaptation to CDK2 inhibition exposes intrinsic cell-cycle plasticity. Cell 2023, 186, 2628–2643. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Bergholz, J.S.; Zhao, J.J. Targeting CDK4 and CDK6 in cancer. Nat. Rev. Cancer 2022, 22, 356–372. [Google Scholar] [CrossRef]
- Luo, M.; Wang, L.; Xiao, C.; Zhou, M.; Li, M.; Li, H. miR136 regulates proliferation and differentiation of small tail han sheep preadipocytes. Adipocyte 2023, 12, 2173966. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Caslin, H.L.; Cottam, M.A.; Piñon, J.M.; Boney, L.Y.; Hasty, A.H. Weight cycling induces innate immune memory in adipose tissue macrophages. Front. Immunol. 2022, 13, 984859. [Google Scholar] [CrossRef]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vázquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef]
- Choi, S.H.; Park, S.K.; Johnson, B.J.; Chung, K.Y.; Choi, C.W.; Kim, K.H.; Kim, W.Y.; Smith, B. AMPKα, C/EBPβ, CPT1β, GPR43, PPARγ, and SCD Gene Expression in Single- and Co-cultured Bovine Satellite Cells and Intramuscular Preadipocytes Treated with Palmitic, Stearic, Oleic, and Linoleic Acid. Asian-Australas J. Anim. Sci. 2015, 28, 411–419. [Google Scholar] [CrossRef]
- John, C.M.; Arockiasamy, S. Inhibition of palmitic acid induced adipogenesis by natural polyphenols in 3T3-L1 adipocytes. Vitro Cell. Dev. Biol. Anim. 2022, 58, 396–407. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.; Liang, C.; Bao, P.; Ding, X.; Chu, M.; Jia, C.; Guo, X.; Yan, P. MicroRNA-200a regulates adipocyte differentiation in the domestic yak Bos grunniens. Gene 2018, 650, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Sun, G.; Zhu, H.; Guo, Y.; Xu, F.; Hu, G.; Huang, K.; Guo, H. CNIH4: A novel biomarker connected with poor prognosis and cell proliferation in patients with lower-grade glioma. Am. J. Cancer Res. 2023, 13, 2135–2154. [Google Scholar]
- Sun, J.; Chen, J.; Wang, Z.; Deng, Y.; Liu, L.; Liu, X. Expression of NUF2 in breast cancer and its clinical significance. Nan Fang Yi Ke Da Xue Xue Bao 2019, 39, 591–597. [Google Scholar] [PubMed]
- Bridges, A.E.; Ramachandran, S.; Tamizhmani, K.; Parwal, U.; Lester, A.; Rajpurohit, P.; Morera, D.S.; Hasanali, S.L.; Arjunan, P.; Jedeja, R.N.; et al. RAD51AP1 Loss Attenuates Colorectal Cancer Stem Cell Renewal and Sensitizes to Chemotherapy. Mol. Cancer Res. 2021, 19, 1486–1497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Gao, Y.; Chen, Q.; Li, J.; Ren, M.; Zhao, X.; Yue, W. RAD51AP1 promotes progression of ovarian cancer via TGF-β/Smad signalling pathway. J. Cell. Mol. Med. 2021, 25, 1927–1938. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Ma, C.C.; Ai, K.X. Knockdown of RAD51AP1 suppressed cell proliferation and invasion in esophageal squamous cell carcinoma. Discov. Oncol. 2022, 13, 101. [Google Scholar] [CrossRef]
- Ishikura, S.; Nagai, M.; Tsunoda, T.; Nishi, K.; Tanaka, Y.; Koyanagi, M.; Shirasawa, S. The transcriptional regulator Zfat is essential for maintenance and differentiation of the adipocytes. J. Cell. Biochem. 2021, 122, 626–638. [Google Scholar] [CrossRef]
- Dou, J.; Puttabyatappa, M.; Padmanabhan, V.; Bakulski, K.M. Developmental programming: Adipose depot-specific transcriptional regulation by prenatal testosterone excess in a sheep model of PCOS. Mol. Cell. Endocrinol. 2021, 523, 111137. [Google Scholar] [CrossRef]
- Luo, G.; Hu, S.; Lai, T.; Wang, J.; Wang, L.; Lai, S. MiR-9-5p promotes rabbit preadipocyte differentiation by suppressing leptin gene expression. Lipids Health Dis. 2020, 19, 126. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).