Femoral Structure and Biomechanical Characteristics in Sanfilippo Syndrome Type-B Mice
Abstract
1. Introduction
2. Results
2.1. Peripheral Quantitative Computed Tomography (pQCT) Analysis of Femurs
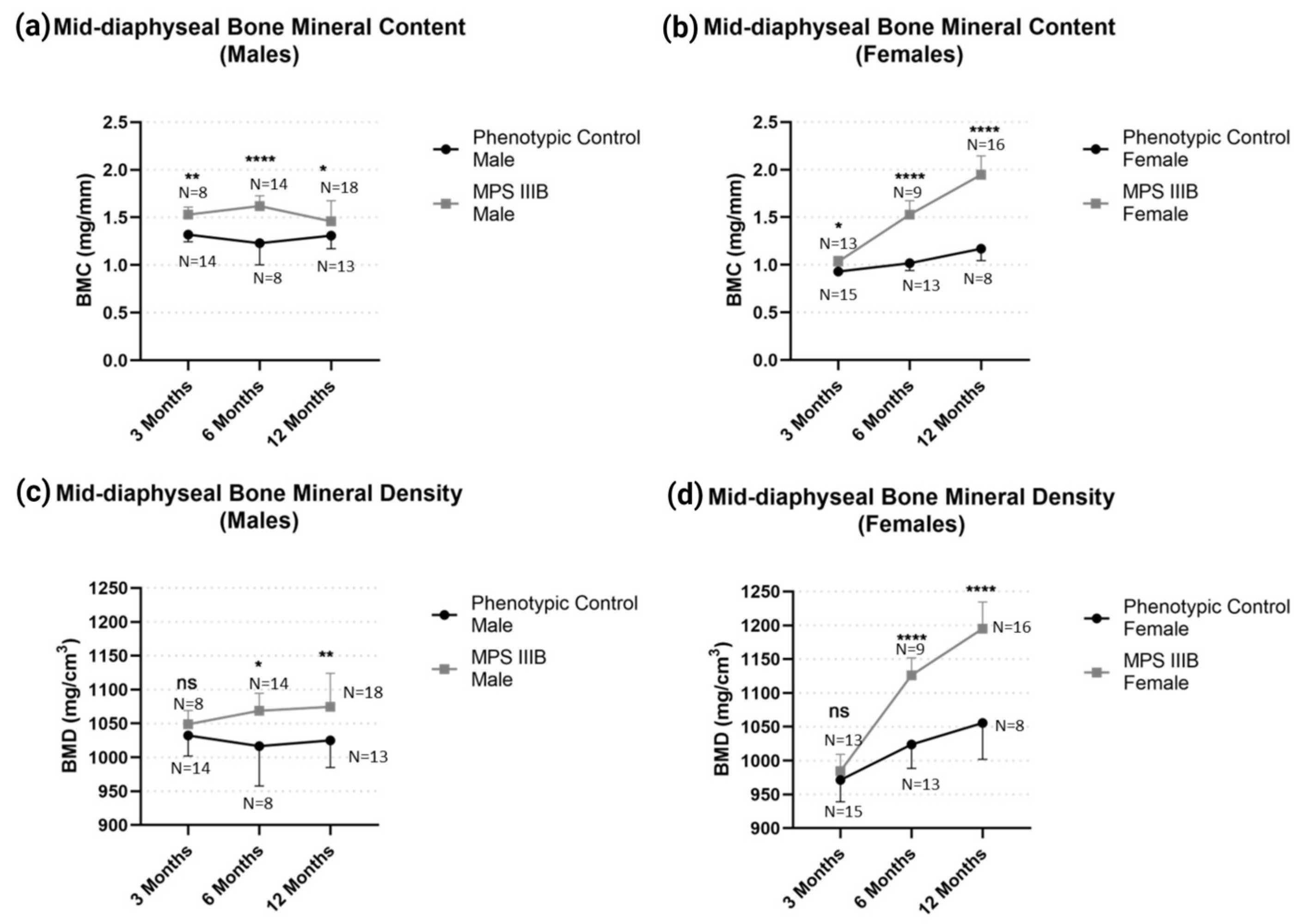
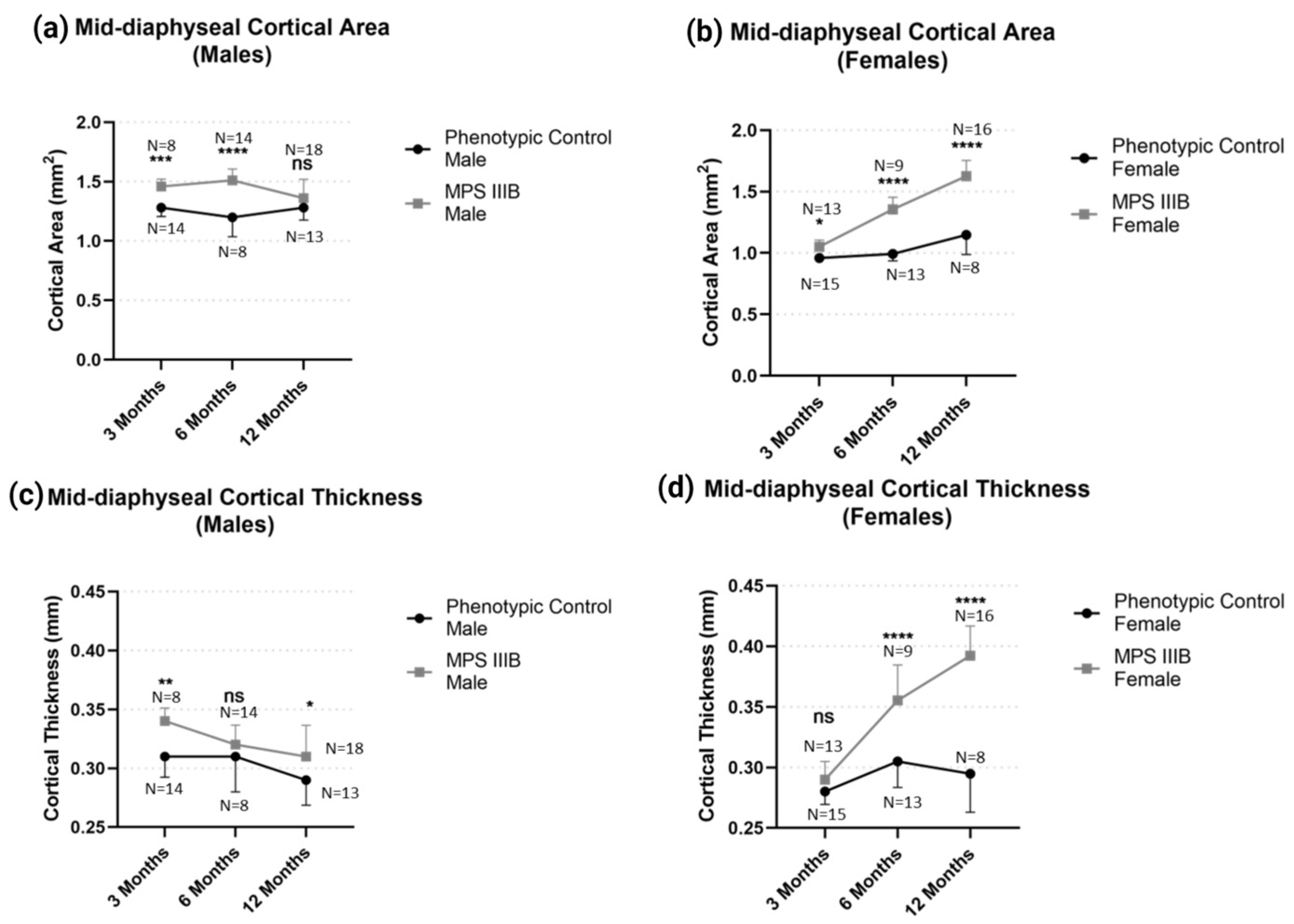
2.1.1. Mid-Diaphyseal qQCT
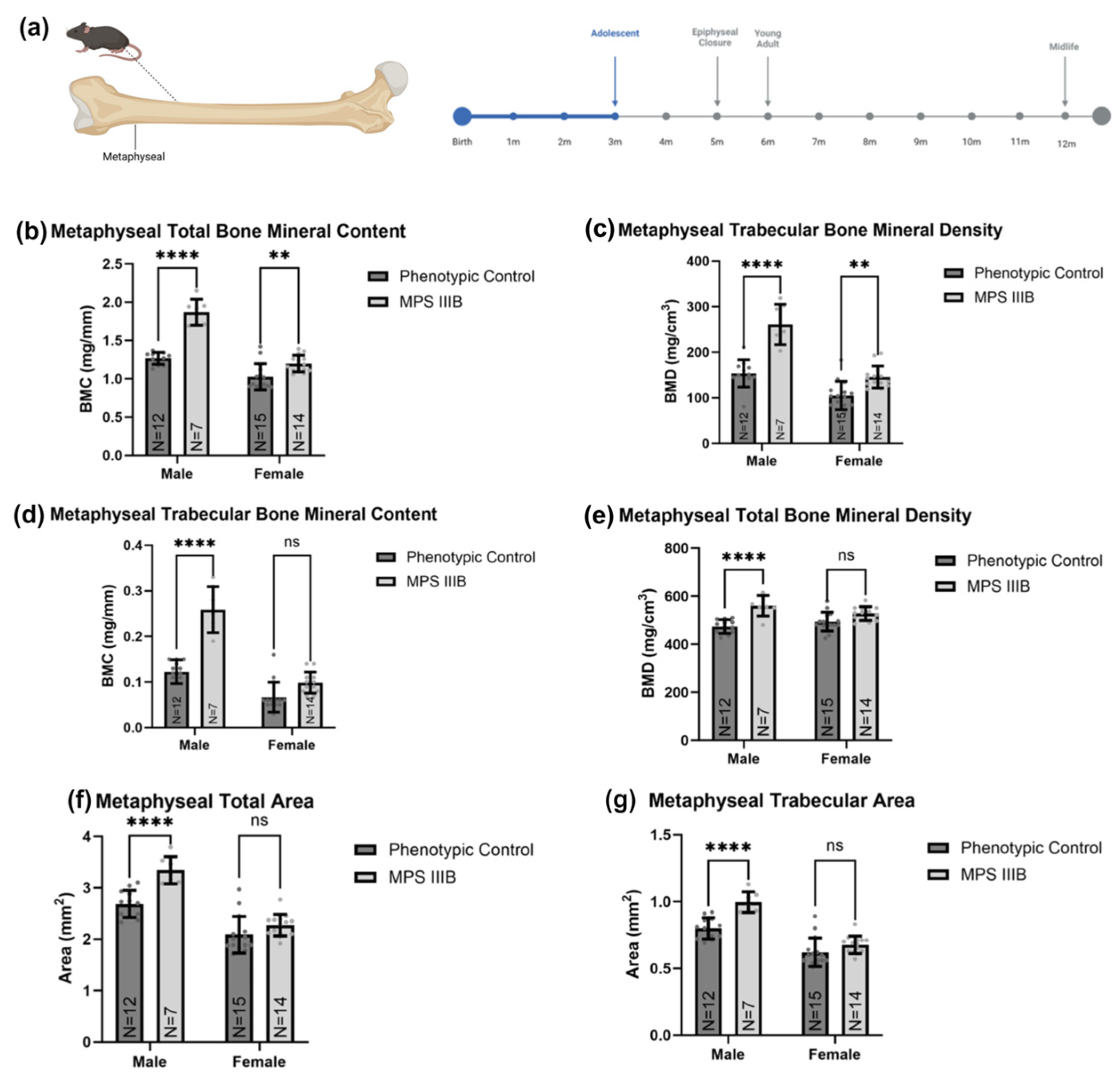
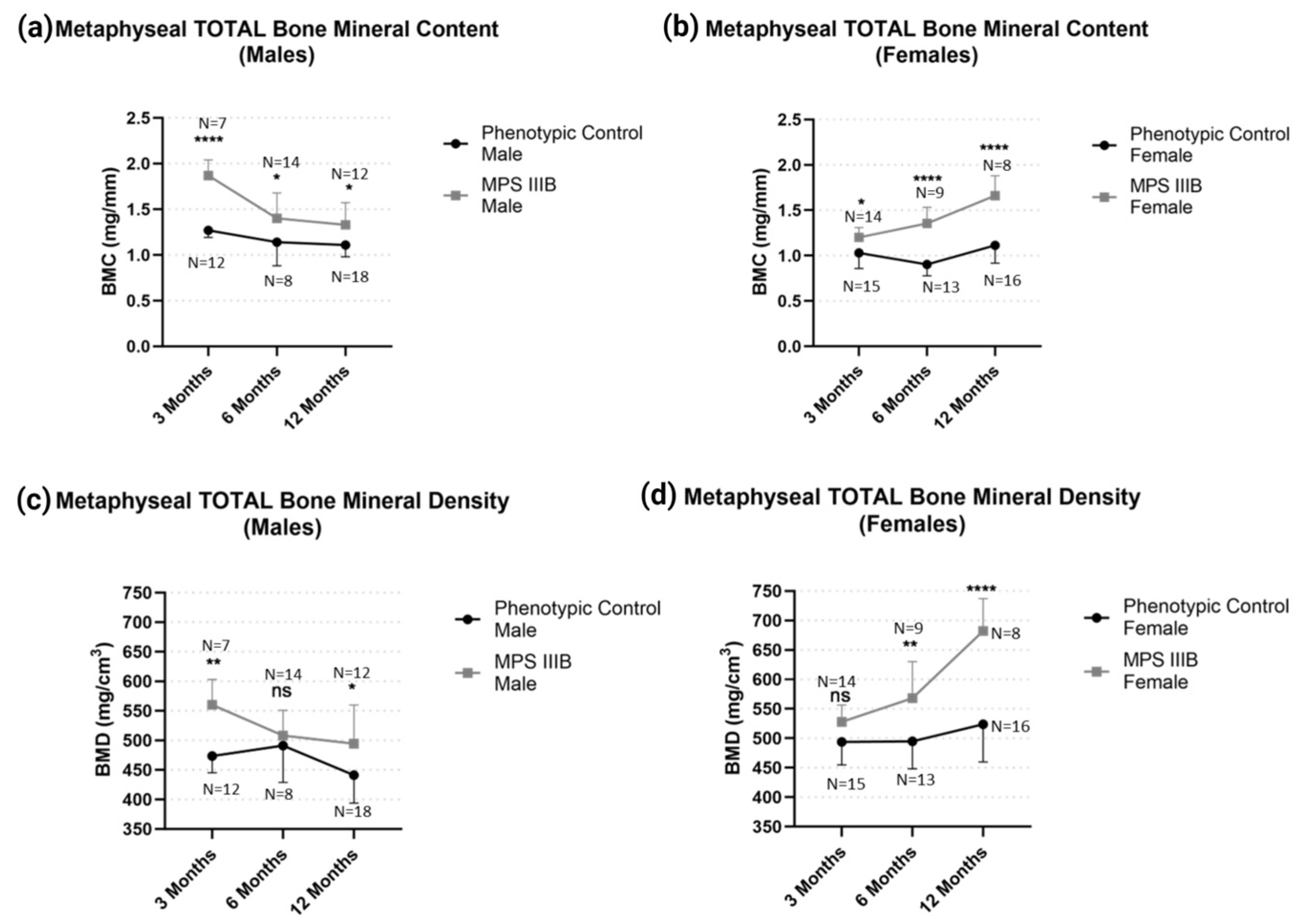
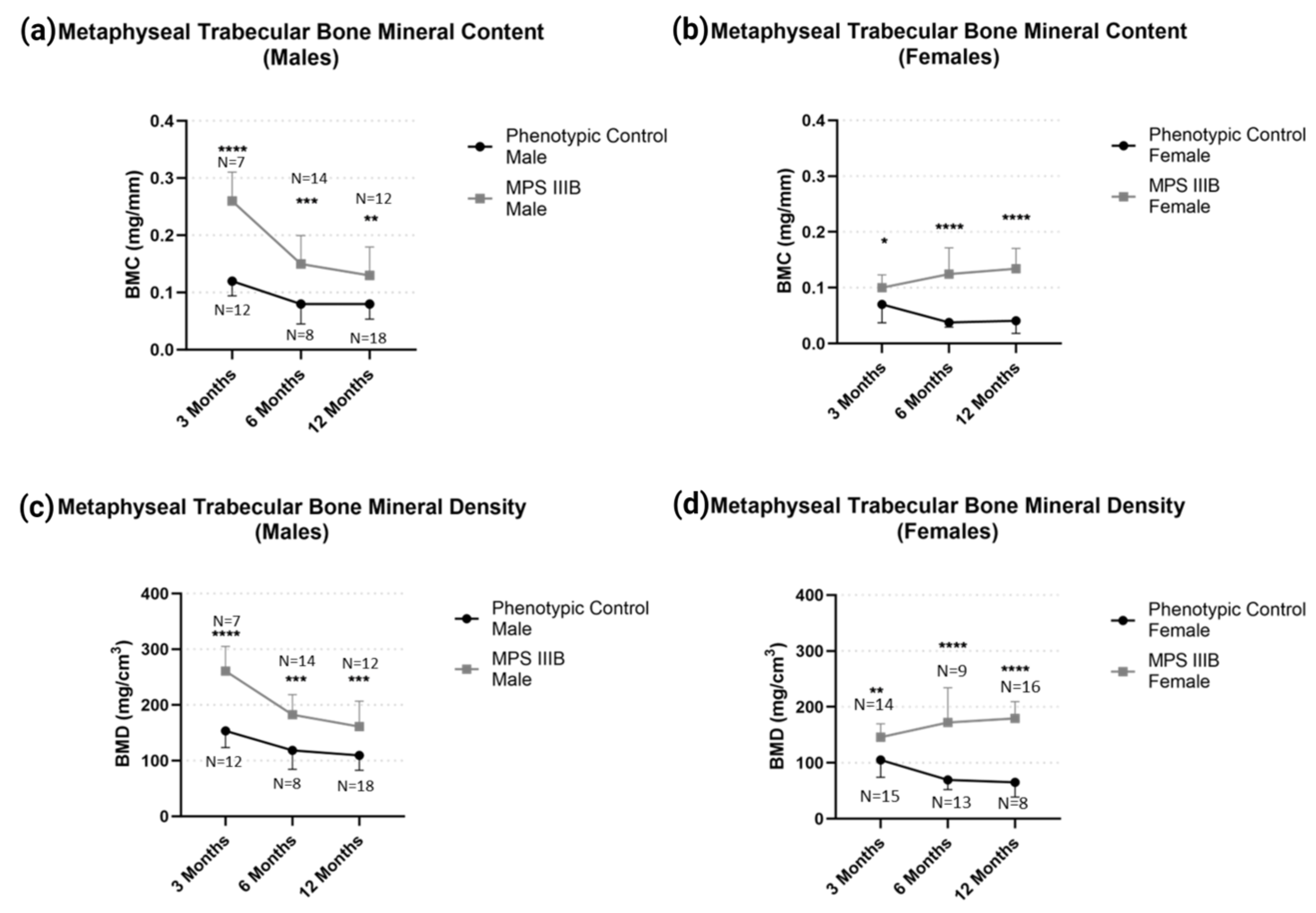
2.1.2. Metaphyseal pQCT
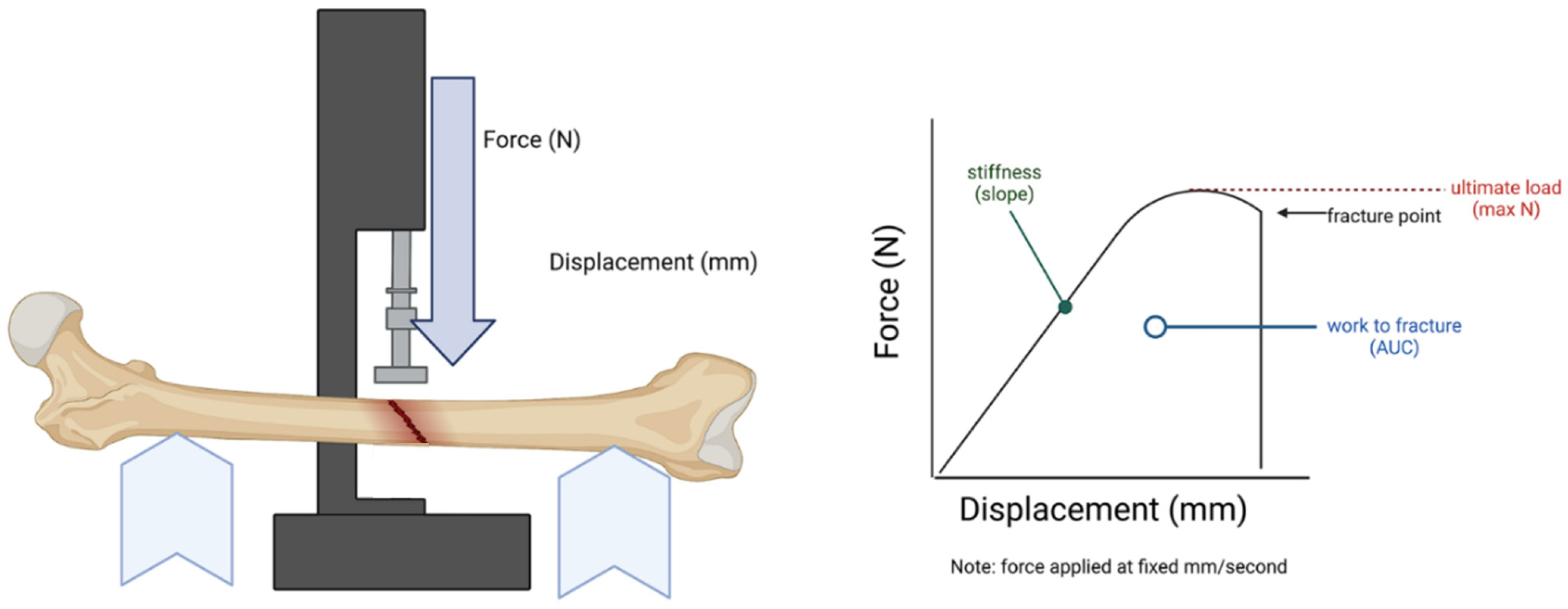
2.2. Biomechanical Analysis of Femurs
3. Discussion
3.1. Summary
3.2. Future Work
4. Materials and Methods
4.1. Sanfilippo Syndrome Type-B Mouse Colony
4.2. Euthanasia and Tissue Collection
4.3. Quantitative Computed Tomography (pQCT)
4.4. Biomechanical Strength Tests
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cimaz, R.; La Torre, F. Mucopolysaccharidoses. Curr. Rheumatol. Rep. 2014, 16, 389. [Google Scholar] [CrossRef]
- Griffin, L.S.; Gloster, T.M. The Enzymatic Degradation of Heparan Sulfate. Protein Pept. Lett. 2017, 24, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, E.; Muenzer, J. The Metabolic and Molecular Bases of Inherited Disease; McGraw Hill: New York, NY, USA, 2001; Volume 3. [Google Scholar]
- Kodaira, Y.; Nair, S.K.; Wrenshall, L.E.; Gilboa, E.; Platt, J.L. Phenotypic and functional maturation of dendritic cells mediated by heparan sulfate. J. Immunol. 2000, 165, 1599–1604. [Google Scholar] [CrossRef]
- Johnson, G.B.; Brunn, G.J.; Kodaira, Y.; Platt, J.L. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J. Immunol. 2002, 168, 5233–5239. [Google Scholar] [CrossRef] [PubMed]
- Simonaro, C.M.; D’Angelo, M.; E Haskins, M.; Schuchman, E.H. Joint and bone disease in mucopolysaccharidoses VI and VII: Identification of new therapeutic targets and biomarkers using animal models. Pediatr. Res. 2005, 57 Pt 1, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Simonaro, C.M.; Ge, Y.; Eliyahu, E.; He, X.; Jepsen, K.J.; Schuchman, E.H. Involvement of the Toll-like receptor 4 pathway and use of TNF-alpha antagonists for treatment of the mucopolysaccharidoses. Proc. Natl. Acad. Sci. USA 2010, 107, 222–227. [Google Scholar] [CrossRef]
- Ausseil, J.; Desmaris, N.; Bigou, S.; Attali, R.; Corbineau, S.; Vitry, S.; Parent, M.; Cheillan, D.; Fuller, M.; Maire, I.; et al. Early Neurodegeneration progresses independently of microglial activation by heparan sulfate in the brain of mucopolysaccharidosis IIIB mice. PLoS ONE 2008, 3, e2296. [Google Scholar] [CrossRef]
- Trudel, S.; Trécherel, E.; Gomila, C.; Peltier, M.; Aubignat, M.; Gubler, B.; Morlière, P.; Heard, J.-M.; Ausseil, J. Oxidative stress is independent of inflammation in the neurodegenerative Sanfilippo syndrome type B. J. Neurosci. Res. 2015, 93, 424–432. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Westermann, L.M.; Yorgan, T.A.; Stürznickel, J.; Ludwig, N.F.; Ammer, L.S.; Baranowsky, A.; Ahmadi, S.; Pourbarkhordariesfandabadi, E.; Breyer, S.R.; et al. Pathogenic variants in GNPTAB and GNPTG encoding distinct subunits of GlcNAc-1-phosphotransferase differentially impact bone resorption in patients with mucolipidosis type II and III. Genet. Med. 2021, 23, 2369–2377. [Google Scholar] [CrossRef]
- Rigante, D.; Caradonna, P. Secondary skeletal involvement in Sanfilippo syndrome. QJM 2004, 97, 205–209. [Google Scholar] [CrossRef]
- Kingma, S.D.; Jonckheere, A.I. MPS I: Early diagnosis, bone disease and treatment, where are we now? J. Inherit. Metab. Dis. 2021, 44, 1289–1310. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Breyer, S.; Löbel, U.; Yarar, S.; Stücker, R.; Ullrich, K.; Müller, I.; Muschol, N. Musculoskeletal manifestations in mucopolysaccharidosis type I (Hurler syndrome) following hematopoietic stem cell transplantation. Orphanet J. Rare Dis. 2016, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Rintz, E.; Herreño-Pachón, A.M.; Celik, B.; Nidhi, F.; Khan, S.; Benincore-Flórez, E.; Tomatsu, S. Bone Growth Induction in Mucopolysaccharidosis IVA Mouse. Int. J. Mol. Sci. 2023, 24, 9890. [Google Scholar] [CrossRef]
- Chen, S.J.; Li, Y.W.; Wang, T.R.; Hsu, J.C.Y. Bony changes in common mucopolysaccharidoses. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi 1996, 37, 178–184. [Google Scholar]
- Morishita, K.; Petty, R.E. Musculoskeletal manifestations of mucopolysaccharidoses. Rheumatology 2011, 50 (Suppl. S5), v19–v25. [Google Scholar] [CrossRef]
- Killedar, S.; DiRosario, J.; Divers, E.; Popovich, P.G.; McCarty, D.M.; Fu, H. Mucopolysaccharidosis IIIB, a lysosomal storage disease, triggers a pathogenic CNS autoimmune response. J. Neuroinflamm. 2010, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Shih, S.-C.; Chuang, C.-K.; Chen, M.-R.; Niu, D.-M.; Lin, S.-P. Assessment of bone mineral density by dual energy x-ray absorptiometry in patients with mucopolysaccharidoses. Orphanet J. Rare Dis. 2013, 8, 71. [Google Scholar] [CrossRef]
- Muschol, N.M.; Pape, D.; Kossow, K.; Ullrich, K.; Arash-Kaps, L.; Hennermann, J.B.; Stücker, R.; Breyer, S.R. Growth charts for patients with Sanfilippo syndrome (Mucopolysaccharidosis type III). Orphanet J. Rare Dis. 2019, 14, 93. [Google Scholar] [CrossRef]
- Muschol, N.; Giugliani, R.; Jones, S.A.; Muenzer, J.; Smith, N.J.C.; Whitley, C.B.; Donnell, M.; Drake, E.; Elvidge, K.; Melton, L.; et al. Sanfilippo syndrome: Consensus guidelines for clinical care. Orphanet J. Rare Dis. 2022, 17, 391. [Google Scholar] [CrossRef]
- White, K.K.; Karol, L.A.; White, D.R.; Hale, S. Musculoskeletal manifestations of Sanfilippo Syndrome (mucopolysaccharidosis type III). J. Pediatr. Orthop. 2011, 31, 594–598. [Google Scholar] [CrossRef]
- Devanney, S.C.; Gibney, J.M.; Le Prell, C.G.; Wronski, T.J.; Aguirre, J.; Mcdoom, I.; Heldermon, C.D. The beta-glucuronidase intracisternal A particle insertion model results in similar overall MPSVII phenotype as the single base deletion model when on the same C57BL/6J mouse background. Mol. Genet. Metab. Rep. 2021, 27, 100727. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Mano, H.; Yuasa, T.; Mori, Y.; Miyazawa, K.; Shiokawa, M.; Nakamaru, Y.; Hiroi, E.; Hiura, K.; Kameda, A.; et al. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J. Exp. Med. 1997, 186, 489–495. [Google Scholar] [CrossRef]
- Polgreen, L.E.; Vehe, R.K.; Rudser, K.; Kunin-Batson, A.; Utz, J.J.; Dickson, P.; Shapiro, E.; Whitley, C.B. Elevated TNF-α is associated with pain and physical disability in mucopolysaccharidosis types I, II, and VI. Mol. Genet. Metab. 2016, 117, 427–430. [Google Scholar] [CrossRef]
- Polgreen, L.E.; Kunin-Batson, A.; Rudser, K.; Vehe, R.K.; Utz, J.J.; Whitley, C.B.; Dickson, P. Pilot study of the safety and effect of adalimumab on pain, physical function, and musculoskeletal disease in mucopolysaccharidosis types I and II. Mol. Genet. Metab. Rep. 2017, 10, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Byers, S.; Casal, M.L.; Smith, L.J. Failures of Endochondral Ossification in the Mucopolysaccharidoses. Curr. Osteoporos. Rep. 2020, 18, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Ii, M.; Matsunaga, N.; Hazeki, K.; Nakamura, K.; Takashima, K.; Seya, T.; Hazeki, O.; Kitazaki, T.; Iizawa, Y. A Novel cyclohexene derivative, ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), Selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol. Pharmacol. 2006, 69, 1288–1295. [Google Scholar] [CrossRef]
- Kawamoto, T.; Ii, M.; Kitazaki, T.; Iizawa, Y.; Kimura, H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur. J. Pharmacol. 2008, 584, 40–48. [Google Scholar] [CrossRef]
- Matsunaga, N.; Tsuchimori, N.; Matsumoto, T.; Ii, M. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-Like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol. Pharmacol. 2011, 79, 34–41. [Google Scholar] [CrossRef]
- Feng, X.; Jiang, L.-J.; Xu, Z.-X.; Wu, M.-F.; Dong, G.-Q.; Zhang, L.-L.; Gao, J.-Y.; Feng, C.-X. Resatorvid protects against hypoxic-ischemic brain damage in neonatal rats. Neural Regen. Res. 2020, 15, 1316–1325. [Google Scholar] [CrossRef]
- Samarpita, S.; Kim, J.Y.; Rasool, M.K.; Kim, K.S. Investigation of toll-like receptor (TLR) 4 inhibitor TAK-242 as a new potential anti-rheumatoid arthritis drug. Arthritis Res. Ther. 2020, 22, 16. [Google Scholar] [CrossRef]
- Rice, T.W.; Wheeler, A.P.; Bernard, G.R.; Vincent, J.-L.; Angus, D.C.; Aikawa, N.; Demeyer, I.; Sainati, S.; Amlot, N.; Cao, C.; et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit. Care Med. 2010, 38, 1685–1694. [Google Scholar] [CrossRef]
- Hernández-Jiménez, M.; Martín-Vílchez, S.; Ochoa, D.; Mejía-Abril, G.; Román, M.; Camargo-Mamani, P.; Luquero-Bueno, S.; Jilma, B.; Moro, M.A.; Fernández, G.; et al. First-in-human phase I clinical trial of a TLR4-binding DNA aptamer, ApTOLL: Safety and pharmacokinetics in healthy volunteers. Mol. Ther. Nucleic Acids 2022, 28, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.-Y.; Lee, H.-J.; Woo, H.; Kang, R.-J.; Han, K.-M.; Park, H.; Lee, S.M.; Lee, J.-Y.; Jeong, Y.J.; Nam, H.-W.; et al. Dasatinib regulates LPS-induced microglial and astrocytic neuroinflammatory responses by inhibiting AKT/STAT3 signaling. J. Neuroinflammation 2019, 16, 190. [Google Scholar] [CrossRef]
- Heine, A.; Wolf, A.M.; Schlaweck, S.; Daecke, S.N.; Brossart, P.; Wolf, D. Pacritinib protects dendritic cells more efficiently than ruxolitinib. Exp. Hematol. 2021, 100, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.Z.; Qi, H.; Chidsey-Frink, K.L.; Crawford, D.T.; Thompson, D.D. Lasofoxifene (CP-336,156) protects against the age-related changes in bone mass, bone strength, and total serum cholesterol in intact aged male rats. J. Bone Miner. Res. 2001, 16, 765–773. [Google Scholar] [CrossRef]
- Castillo, E.J.; Croft, S.M.; Jiron, J.M.; Aguirre, J.I. Bone structural, biomechanical, and histomorphometric characteristics of the hindlimb skeleton in the marsh rice rat (Oryzomys palustris). Anat. Rec. 2022, 305, 3133–3149. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashby, F.J.; Castillo, E.J.; Ludwig, Y.; Andraka, N.K.; Chen, C.; Jamieson, J.C.; Kabbej, N.; Sommerville, J.D.; Aguirre, J.I.; Heldermon, C.D. Femoral Structure and Biomechanical Characteristics in Sanfilippo Syndrome Type-B Mice. Int. J. Mol. Sci. 2023, 24, 13988. https://doi.org/10.3390/ijms241813988
Ashby FJ, Castillo EJ, Ludwig Y, Andraka NK, Chen C, Jamieson JC, Kabbej N, Sommerville JD, Aguirre JI, Heldermon CD. Femoral Structure and Biomechanical Characteristics in Sanfilippo Syndrome Type-B Mice. International Journal of Molecular Sciences. 2023; 24(18):13988. https://doi.org/10.3390/ijms241813988
Chicago/Turabian StyleAshby, Frederick James, Evelyn J. Castillo, Yan Ludwig, Natalia K. Andraka, Cong Chen, Julia C. Jamieson, Nadia Kabbej, John D. Sommerville, Jose I. Aguirre, and Coy D. Heldermon. 2023. "Femoral Structure and Biomechanical Characteristics in Sanfilippo Syndrome Type-B Mice" International Journal of Molecular Sciences 24, no. 18: 13988. https://doi.org/10.3390/ijms241813988
APA StyleAshby, F. J., Castillo, E. J., Ludwig, Y., Andraka, N. K., Chen, C., Jamieson, J. C., Kabbej, N., Sommerville, J. D., Aguirre, J. I., & Heldermon, C. D. (2023). Femoral Structure and Biomechanical Characteristics in Sanfilippo Syndrome Type-B Mice. International Journal of Molecular Sciences, 24(18), 13988. https://doi.org/10.3390/ijms241813988






