Regulation of V-ATPase by Jasmonic Acid: Possible Role of Persulfidation
Abstract
1. Introduction
2. Results
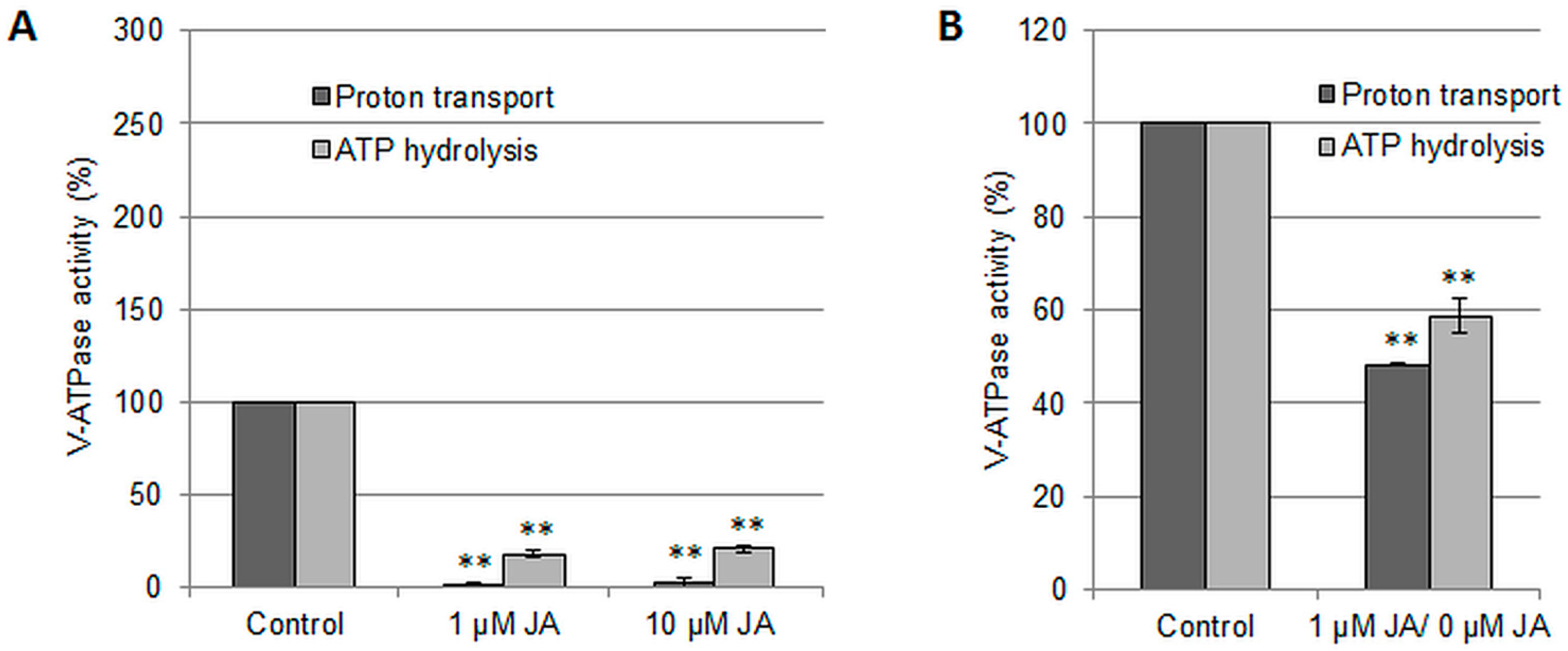
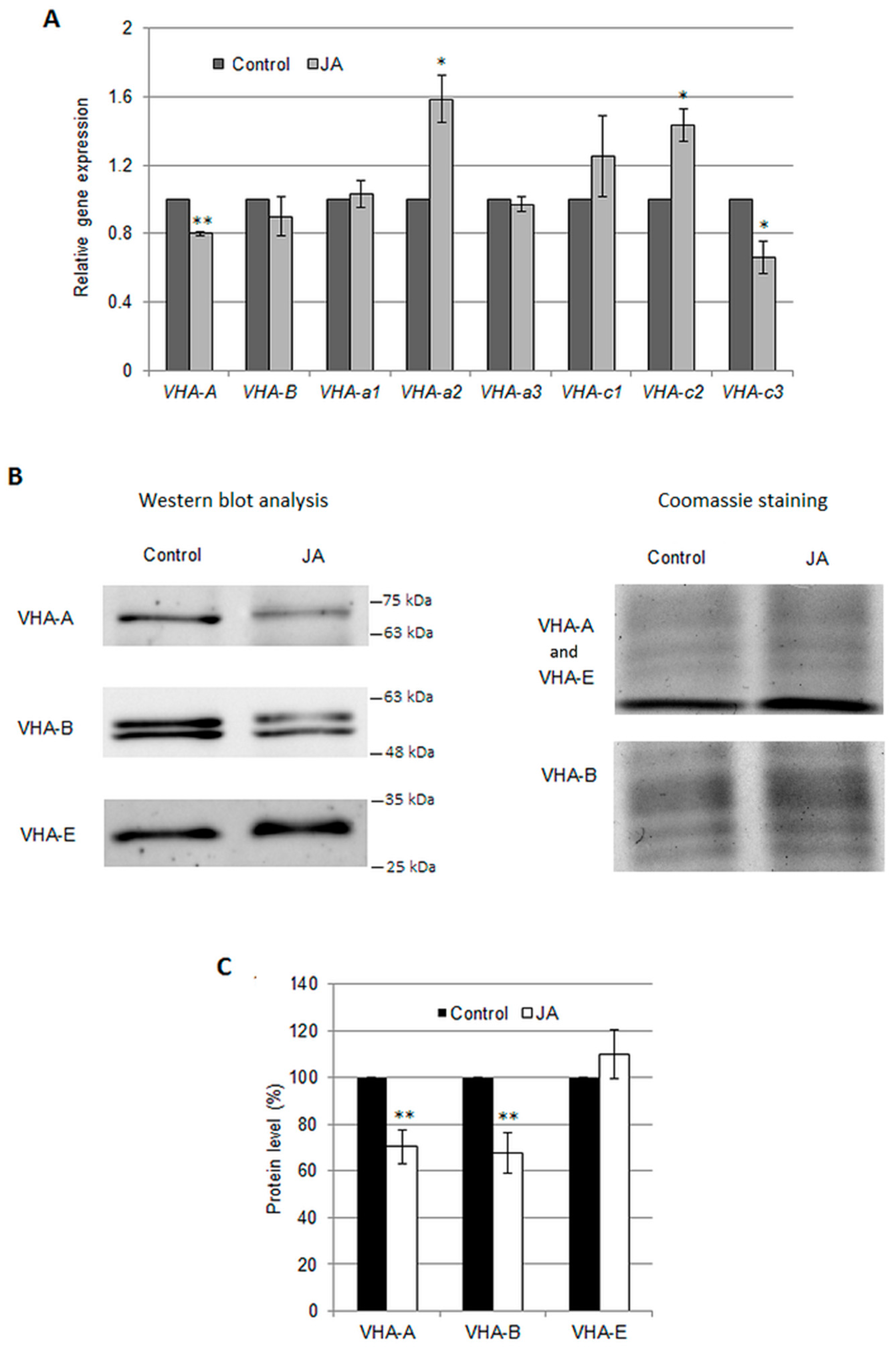
2.1. Regulation of the Vacuolar H+-ATPase by Jasmonic Acid
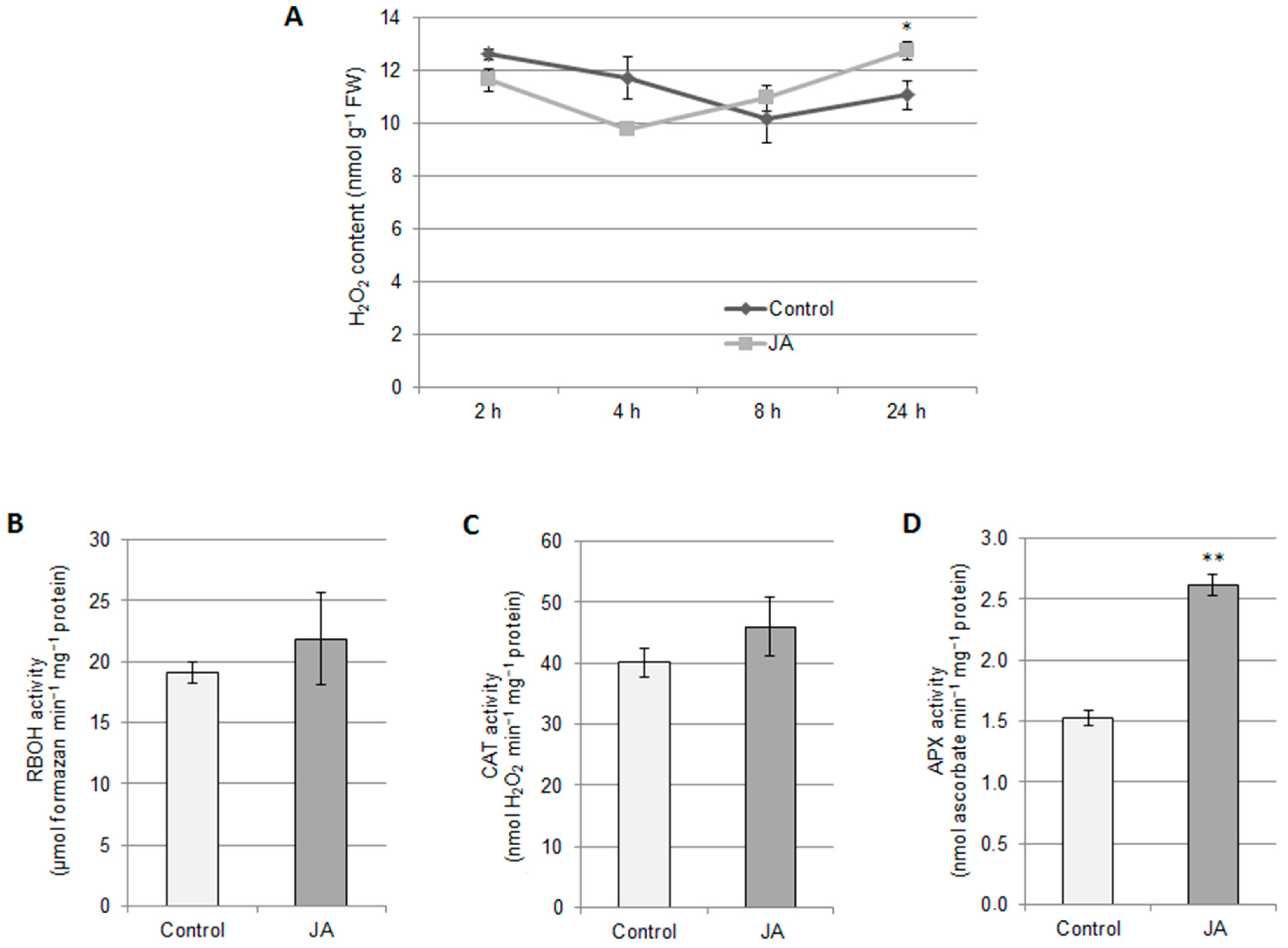
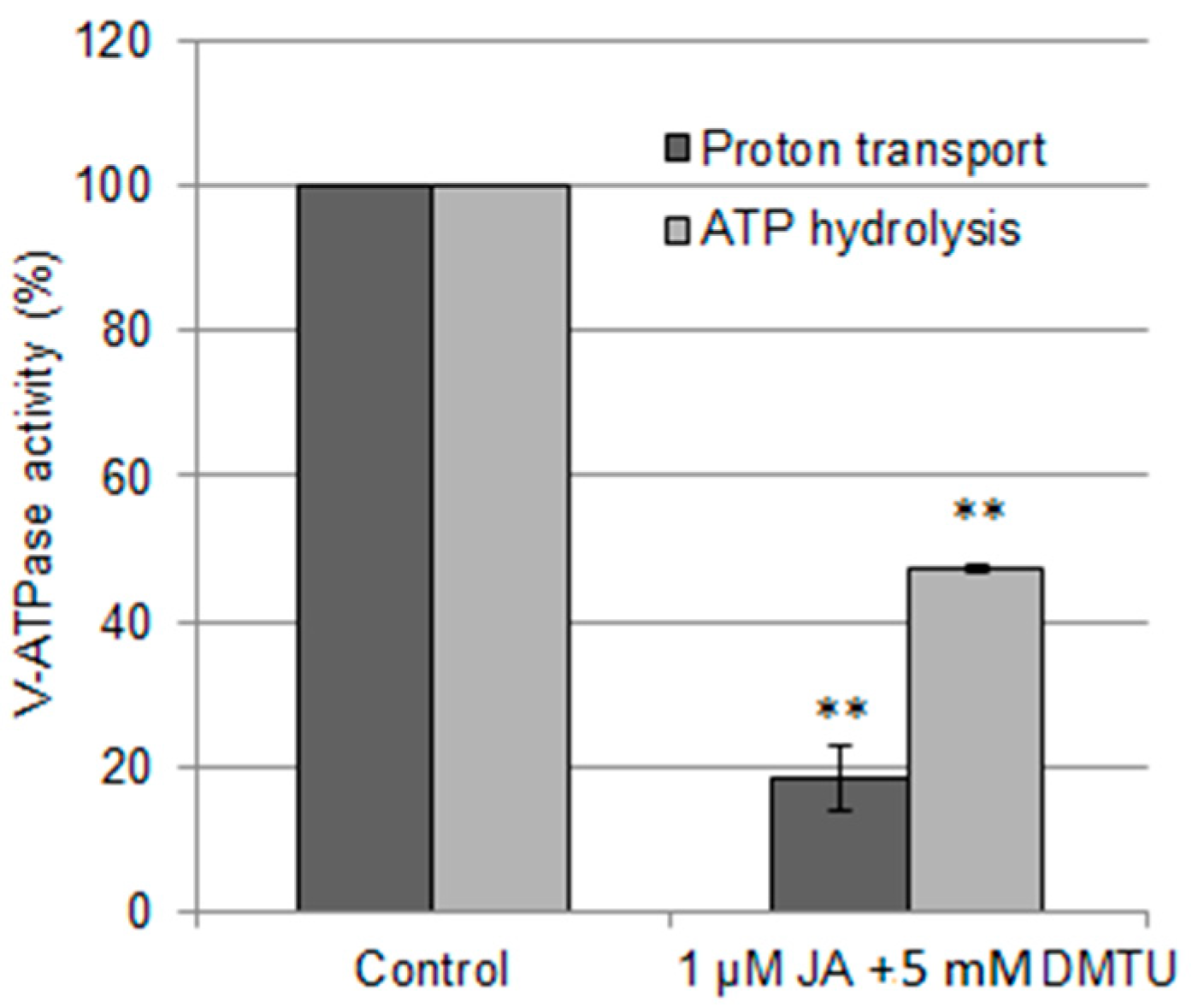
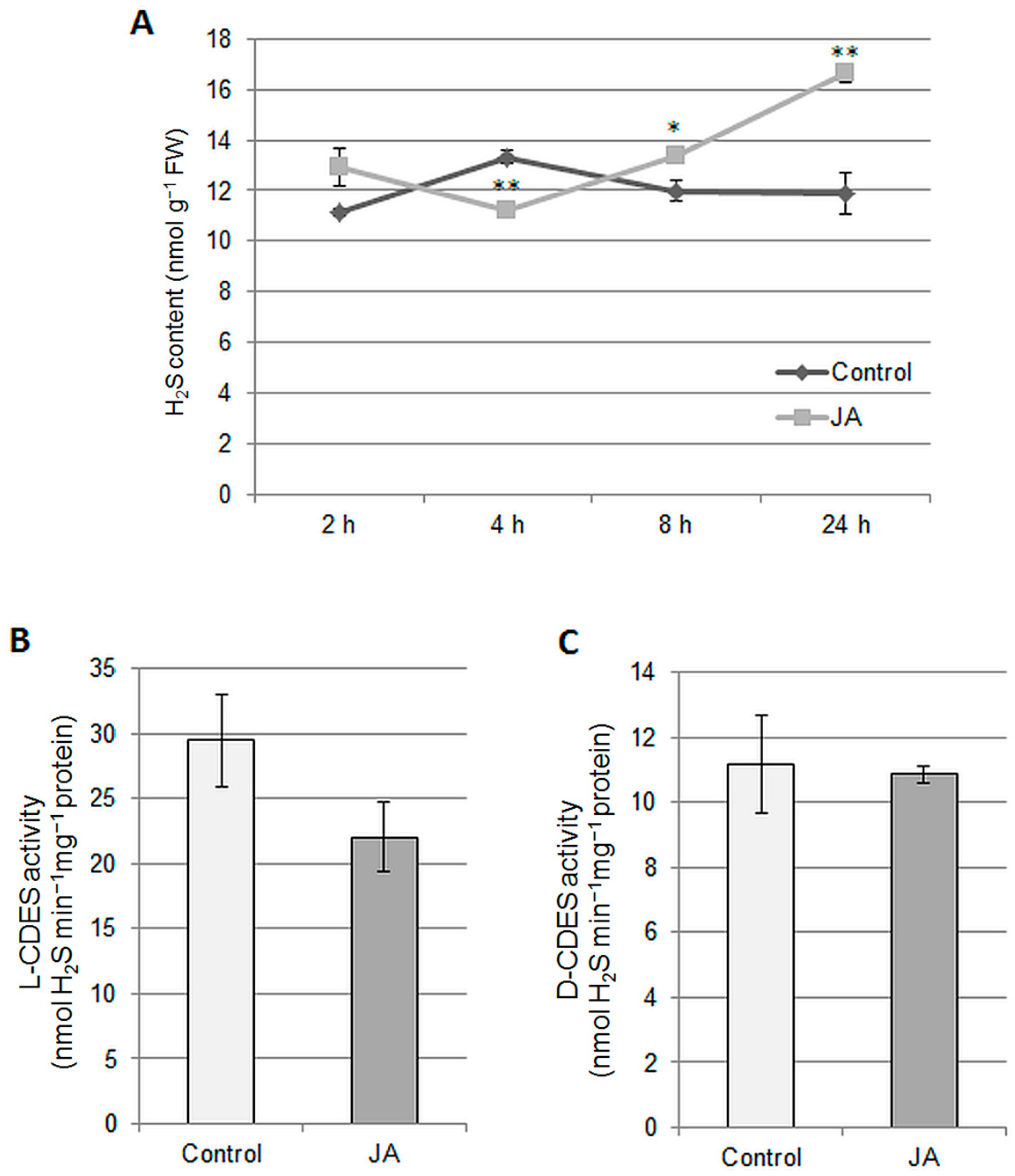
2.2. Involvement of H2S and H2O2 in the Regulation of Vacuolar H+-ATPase by Jasmonic Acid
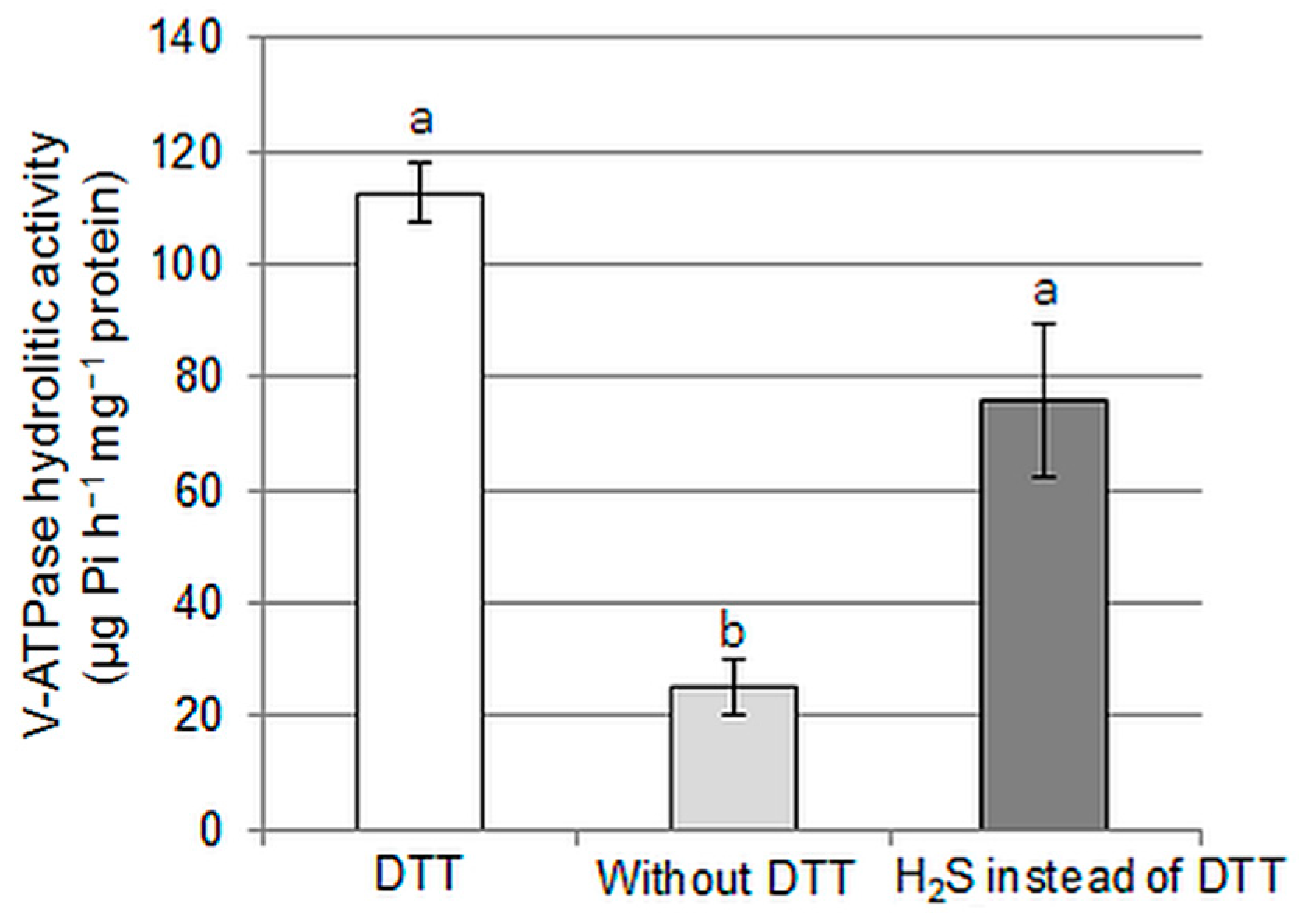
2.3. Direct Effect of H2S on the Vacuolar H+-ATPase Activity and Persulfidation of Its Subunits
3. Discussion
3.1. Jasmonic Acid Treatment Reduces V-ATPase Activity in Cucumber Roots
3.2. Jasmonic Acid Decreases the Protein Level of V-ATPase Subunits
3.3. H2O2 Participates in JA-Dependent V-ATPase Downregulation
3.4. H2S and Persulfidation Can Function as Elements of the JA Signaling Involved in V-ATPase Regulation
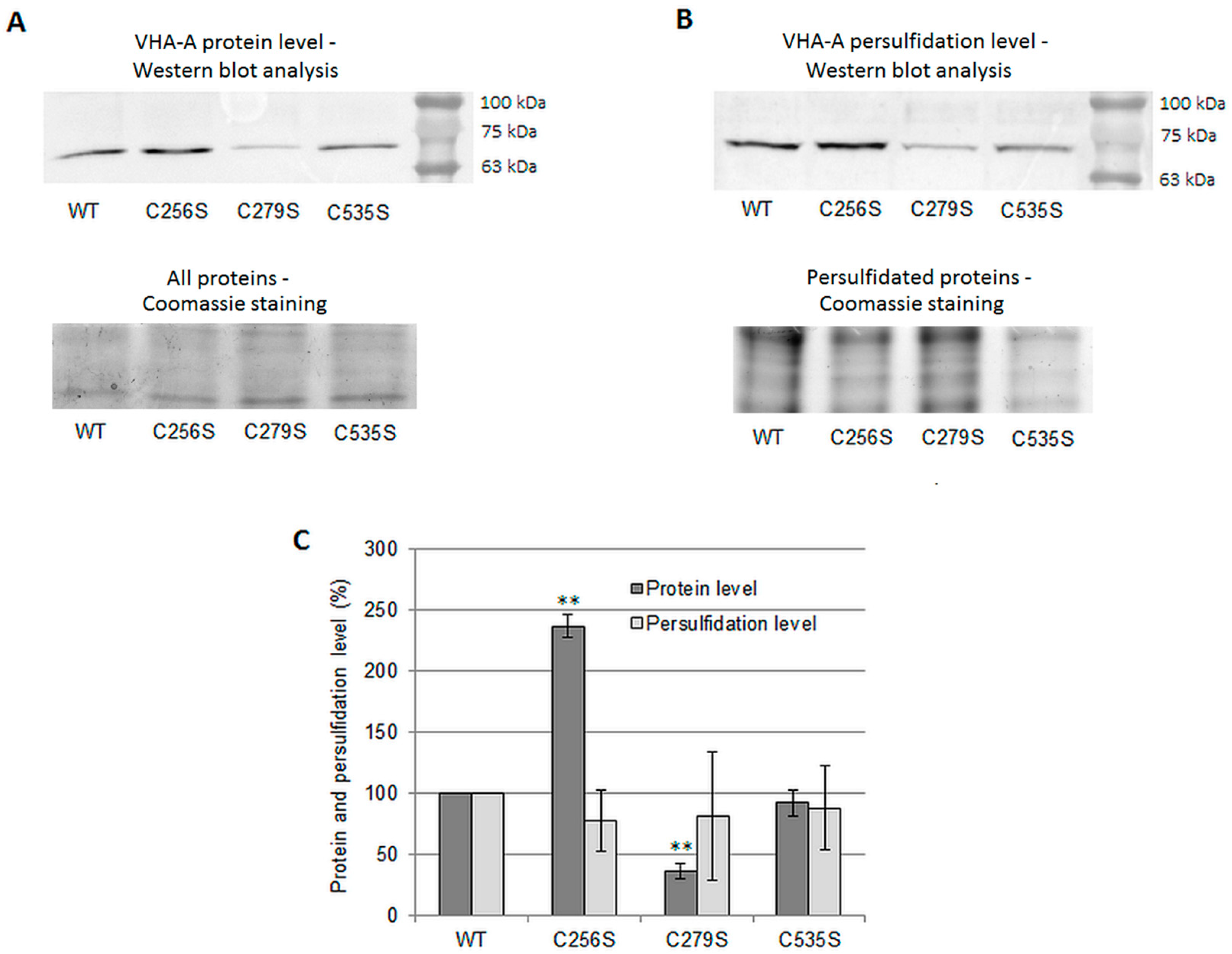
3.5. Persulfidation Level of VHA-A Does Not Change in Arabidopsis Mutants with a Substitution of Conserved Cys Residues within This Subunit
3.6. The Mechanisms of V-ATPase Downregulation by JA and Cd Are Different
4. Materials and Methods
4.1. The Plant Material, Growth Conditions and Treatments
4.2. Isolation of Membrane Fractions
4.3. Content of Signaling Molecules
4.4. Determination of Enzyme Activities
4.5. Gene Expression Analysis
4.6. Determination of Protein Level
4.7. Preparation of Protein Extract
4.8. Purification of Persulfidated Proteins Using the Tag-Switch Method
4.9. Western Blot and Coomassie Staining
4.10. Quantification of the Protein Level of V-ATPase Subunits and Their Level of Persulfidation
- -
- intensity of the bands of the pre-tag-switch trials in the immunoblots (protein level of the sample before the tag-switch procedure compared to the control or wild type)
- -
- Coomassie staining (protein amount in 9 µL of sample after the tag-switch procedure)
- -
- intensity of the signal of samples after the tag-switch procedure in the immunoblots (persulfidated proteins).
4.11. Databases and Bioinformatics Tools
4.12. Statistical Analysis
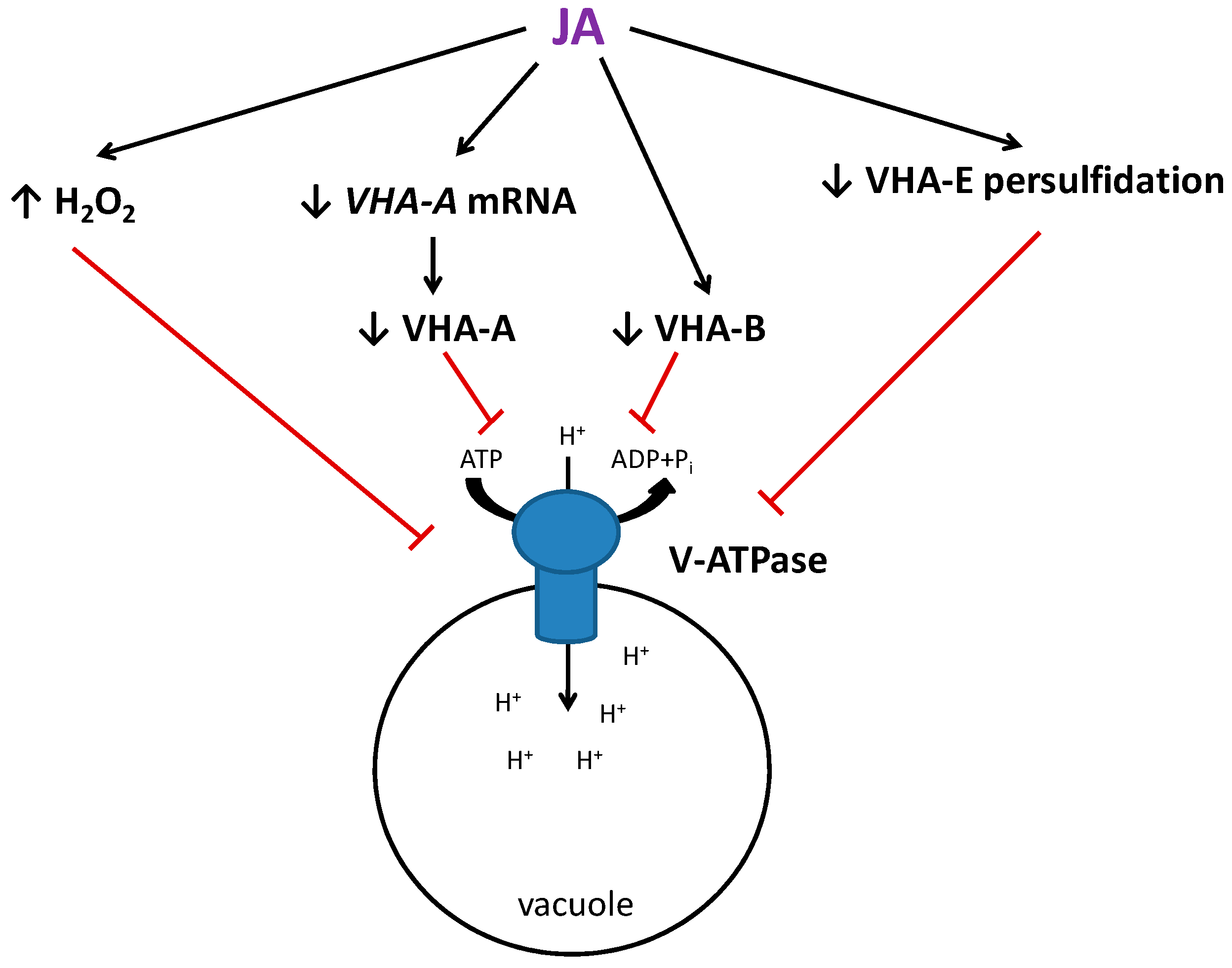
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lupanga, U.; Röhrich, R.; Askani, J.; Hilmer, S.; Kiefer, C.; Krebs, M.; Kanazawa, T.; Ueda, T.; Schumacher, K. The Arabidopsis V-ATPase is localized to the TGN/EE via a seed plant-specific motif. eLife 2020, 9, e60568. [Google Scholar] [CrossRef] [PubMed]
- Seidel, T. The Plant V-ATPase. Front. Plant Sci. 2022, 13, 931777. [Google Scholar] [CrossRef]
- Schumacher, K.; Krebs, M. The V-ATPase: Small cargo, large effects. Curr. Opin. Plant Biol. 2010, 13, 724–730. [Google Scholar] [CrossRef]
- Eaton, A.F.; Merkulova, M.; Brown, D. The H+-ATPase (V-ATPase): From proton pump to signaling complex in health and disease. Am. J. Physiol. Cell Physiol. 2021, 320, C392–C414. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, J.; Schubert, D.; Calvo-Weimar, O.; Stierhof, Y.-D.; Schmidt, R.; Schumacher, K. Essential role of the V-ATPase in male gametophyte development. Plant J. 2005, 41, 117–124. [Google Scholar] [CrossRef]
- Li, W.; Luo, L.; Gu, L.; Li, H.; Zhang, Q.; Ye, Y.; Li, L. Vacuolar H+-ATPase subunit VAB3 regulates cell growth and ion homeostasis in Arabidopsis. Plant J. 2022, 112, 664–676. [Google Scholar] [CrossRef]
- Zhang, H.; Niu, X.; Liu, J.; Xiao, F.; Cao, S.; Liu, Y. RNAi-directed downregulation of Vacuolar H+-ATPase subunit a results in enhanced stomatal aperture and density in Rice. PLoS ONE 2013, 8, e69046. [Google Scholar] [CrossRef] [PubMed]
- Gaxiola, R.A.; Palmgren, M.G.; Schumacher, K. Plant Proton pumps. FEBS Lett. 2007, 581, 2204–2214. [Google Scholar] [CrossRef]
- Feng, Y.; Forgac, M. Inhibition of vacuolar H+-ATPase by disulfide bond formation between cysteine 254 and cysteine 532 in subunit A. J. Biol. Chem. 1994, 269, 13224–13230. [Google Scholar] [CrossRef]
- Oluwatosin, Y.E.; Kane, P.M. Mutations in the CYS4 gene provide evidence for regulation of the yeast vacuolar H+-ATPase by oxidation and reduction in vivo. J. Biol. Chem. 1997, 272, 28149–28157. [Google Scholar] [CrossRef]
- Tavakoli, N.; Kluge, C.; Golldack, D.; Mimura, T.; Dietz, K.-J. Reversible redox control of plant vacuolar H+-ATPase activity is related to disulfide bridge formation in subunit E as well as subunit A. Plant J. 2001, 28, 51–59. [Google Scholar] [CrossRef]
- Kawamura, Y.; Arakawa, K.; Maeshima, M.; Yoshida, S. ATP analogue binding to the A subunit induces conformational changes in the E subunit that involves a disulfide bond formation in plant V-ATPase. Eur. J. Biochem. 2001, 268, 2801–2809. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Zhang, Q.; Wang, P.; Cao, B.; Jia, C.; Cheng, B.; Shi, Y.; Guo, W.-F.; Wang, Z.; Liu, Z.-X.; et al. QPTMplants: An integrative database of quantitative post-translational modifications in plants. Nucleic Acids Res. 2022, 50, D1491–D1499. [Google Scholar] [CrossRef]
- Puyaubert, J.; Fares, A.; Rézé, N.; Peltier, J.-B.; Baudouin, E. Identification of endogenously S-nitrosylated proteins in Arabidopsis plantlets: Effect of cold stress on cysteine nitrosylation level. Plant Sci. 2014, 215–216, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Huang, X.; Chen, L.; Sun, X.; Lu, C.; Zhang, L.; Wang, Y.; Zuo, J. Site-specific nitrosoproteomic identification of endogenously S-nitrosylated proteins in Arabidopsis. Plant Physiol. 2015, 167, 1731–1746. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, C.; Akter, S.; Eeckhout, D.; Persiau, G.; Wahni, K.; Bodra, N.; Van Molle, I.; De Smet, B.; Vertommen, D.; Gevaert, K.; et al. Sulfenome mining in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2014, 111, 11545–11550. [Google Scholar] [CrossRef] [PubMed]
- Aroca, A.; Benito, J.M.; Gotor, C.; Romero, L.C. Persulfidation proteome reveals the regulation of protein function by hydrogen sulfide in diverse biological processes in Arabidopsis. J. Exp. Bot. 2017, 68, 4915–4927. [Google Scholar] [CrossRef]
- Jurado-Flores, A.; Romero, L.C.; Gotor, C. Label-free quantitative proteomic analysis of nitrogen starvation in Arabidopsis root reveals new aspects of H2S signaling by protein persulfidation. Antioxidants 2021, 10, 508. [Google Scholar] [CrossRef]
- Corpas, F.J.; González-Gordo, S.; Cañas, A.; Palma, J.M. Nitric oxide and hydrogen sulfide in plants: Which comes first? J. Exp. Bot. 2019, 70, 4391–4404. [Google Scholar] [CrossRef]
- Aroca, A.; Zhang, J.; Xie, Y.; Romero, L.C.; Gotor, C. Hydrogen sulfide signaling in plant adaptations to adverse conditions: Molecular mechanisms. J. Exp. Bot. 2021, 72, 5893–5904. [Google Scholar] [CrossRef]
- Ohno, K.; Okuda, K.; Uehara, T. Endogenous S-sulfhydration of PTEN helps protect against modification by nitric oxide. Biochem. Biophys. Res. Commun. 2015, 456, 245–249. [Google Scholar] [CrossRef]
- Gotor, C.; García, I.; Aroca, Á.; Laureano-Marín, A.M.; Arenas-Alfonseca, L.; Jurado-Flores, A.; Moreno, I.; Romero, L.C. Signaling by hydrogen sulfide and cyanide through post-translational modification. J. Exp. Bot. 2019, 70, 4251–4265. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. an update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar]
- Świątek, A.; Lenjou, M.; Van Bockstaele, D.; Inzé, D.; Van Onckelen, H. Differential effect of jasmonic acid and abscisic acid on cell cycle progression in tobacco BY-2 cells. Plant Physiol. 2002, 128, 201–211. [Google Scholar] [CrossRef]
- Ghorbel, M.; Brini, F.; Sharma, A.; Landi, M. Role of jasmonic acid in plants: The molecular point of view. Plant Cell Rep. 2021, 40, 1471–1494. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Feussner, I. The oxylipin pathways: Biochemistry and function. Annu. Rev. Plant Biol. 2018, 69, 363–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of jasmonic acid in plant regulation and response to abiotic stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Lubyanova, A.R.; Bezrukova, M.V.; Shakirova, F.M. Involvement of nitric oxide in methyl jasmonate-mediated regulation of water metabolism in wheat plants under drought stress. Stresses 2022, 2, 477–492. [Google Scholar] [CrossRef]
- Shan, C.; Sun, H.; Zhou, Y.; Wang, W. Jasmonic acid-induced hydrogen sulfide activates MEK1/2 in regulating the redox state of ascorbate in Arabidopsis thaliana leaves. Plant Signal. Behav. 2019, 14, 1629265. [Google Scholar] [CrossRef]
- Kabała, K.; Zboińska, M.; Głowiak, D.; Reda, M.; Jakubowska, D.; Janicka, M. Interaction between the signaling molecules hydrogen sulfide and hydrogen peroxide and their role in vacuolar H+-ATPase regulation in cadmium-stressed cucumber roots. Physiol. Plant. 2019, 166, 688–704. [Google Scholar] [CrossRef]
- Kabała, K.; Janicka-Russak, M.; Reda, M.; Migocka, M. Transcriptional regulation of the V-ATPase subunit c and V-PPase isoforms in Cucumis sativus under heavy metal stress. Physiol. Plant. 2014, 150, 32–45. [Google Scholar] [CrossRef]
- Seidel, T.; Scholl, S.; Krebs, M.; Rienmüller, F.; Marten, I.; Hedrich, R.; Hanitzsch, M.; Janetzki, P.; Dietz, K.-J.; Schumacher, K. Regulation of the V-type ATPase by redox modulation. Biochem. J. 2012, 448, 243–251. [Google Scholar] [CrossRef]
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, J.; Hong-Hermesdorf, A.; Stierhof, Y.-D.; Schumacher, K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 2006, 18, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.; Beyhl, D.; Görlich, E.; Al-Rasheid, K.A.; Marten, I.; Stierhof, Y.-D.; Hedrich, R.; Schumacher, K. Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc. Natl. Acad. Sci. USA 2010, 107, 3251–3256. [Google Scholar] [CrossRef] [PubMed]
- Brüx, A.; Liu, T.Y.; Krebs, M.; Stierhof, Y.D.; Lohmann, J.U.; Miersch, O.; Wasternack, C.; Schumacher, K. Reduced V-ATPase activity in the trans-Golgi network causes oxylipin-dependent hypocotyl growth inhibition in Arabidopsis. Plant Cell 2008, 20, 1088–1100. [Google Scholar] [CrossRef]
- Liu, J.; Ji, Y.; Zhou, J.; Xing, D. Phosphatidylinositol 3-kinase promotes V-ATPase activation and vacuolar acidification and delays methyl jasmonate-induced leaf senescence. Plant Physiol. 2016, 170, 1714–1731. [Google Scholar] [CrossRef]
- Ratajczak, R.; Feussner, I.; Hause, B.; Böhm, A.; Parthier, B.; Wasternack, C. Alteration of V-type H+-ATPase during methyl jasmonate-induced senescence in barley (Hordeum vulgare L. cv. Salome). J. Plant Physiol. 1998, 152, 199–206. [Google Scholar] [CrossRef]
- Wasternack, C.; Strnad, M. Jasmonates: News on occurrence, biosynthesis, metabolism and action of an ancient group of signaling compounds. Int. J. Mol. Sci. 2018, 19, 2539. [Google Scholar] [CrossRef]
- Wojciechowska, N.; Michalak, K.M.; Bagniewska-Zadworna, A. Autophagy—An underestimated coordinator of construction and destruction during plant root ontogeny. Planta 2021, 254, 15. [Google Scholar] [CrossRef] [PubMed]
- Löw, R.; Rockel, B.; Kirsch, M.; Ratajczak, R.; Hortensteiner, S.; Martinoia, E.; Luttge, U.; Rausch, T. Early salt stress effects on the differential expression of vacuolar H+-ATPase genes in roots and leaves of Mesembryanthemum crystallinum. Plant Physiol. 1996, 110, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, R. Structure, function and regulation of the plant vacuolar H+-translocating ATPase. Biochim. Biophys. Acta (BBA) Biomembr. 2000, 1465, 17–36. [Google Scholar] [CrossRef]
- Zhang, M.; Fang, Y.; Liang, Z.; Huang, L. Enhanced expression of vacuolar H+-ATPase subunit E in the roots is associated with the adaptation of Broussonetia papyrifera to salt stress. PLoS ONE 2012, 7, e48183. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, K.; Vafeados, D.; McCarthy, M.; Sze, H.; Wilkins, T.; Chory, J. The Arabidopsis det3 mutant reveals a central role for the vacuolar H+-ATPase in plant growth and development. Genes Dev. 1999, 13, 3259–3270. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, Y.; Ge, Y.; Peng, J.; Dong, M.; Yang, G. Characterization of a vacuolar H+-ATPase G subunit gene from Juglans regia (JrVHAG1) involved in mannitol-induced osmotic stress tolerance. Plant Cell Rep. 2017, 36, 407–418. [Google Scholar] [CrossRef]
- Yang, X.; Gong, P.; Li, K.; Huang, F.; Cheng, F.; Pan, G. A single cytosine deletion in the ospls1 gene encoding vacuolar-type H+-atpase subunit A1 leads to premature leaf senescence and seed dormancy in Rice. J. Exp. Bot. 2016, 67, 2761–2776. [Google Scholar] [CrossRef]
- de Lourdes Oliveira Otoch, M.; Menezes Sobreira, A.C.; Farias de Aragão, M.E.; Orellano, E.G.; da Guia Silva Lima, M.; Fernandes de Melo, D. Salt modulation of vacuolar H+-ATPase and H+-pyrophosphatase activities in Vigna unguiculata. J. Plant Physiol. 2001, 158, 545–551. [Google Scholar] [CrossRef]
- Sarry, J.-E.; Kuhn, L.; Ducruix, C.; Lafaye, A.; Junot, C.; Hugouvieux, V.; Jourdain, A.; Bastien, O.; Fievet, J.B.; Vailhen, D.; et al. The early responses ofarabidopsis thaliana cells to cadmium exposure explored by protein and metabolite profiling analyses. Proteomics 2006, 6, 2180–2198. [Google Scholar] [CrossRef]
- Zhigang, A.; Löw, R.; Rausch, T.; Lüttge, U.; Ratajczak, R. The 32 kDa tonoplast polypeptide Di associated with the V0-type H+-ATPase of Mesembryanthemum crystallinum L. in the CAM state: A proteolytically processed subunit B? FEBS Lett. 1996, 389, 314–318. [Google Scholar] [CrossRef]
- Hernando, N.; David, P.; Tarsio, M.; Bartkiewicz, M.; Horne, W.C.; Kane, P.M.; Baron, R. The presence of the alternatively spliced A2 cassette in the vacuolar H+-atpase subunit a prevents assembly of the V1 Catalytic Domain. Eur. J. Biochem. 1999, 266, 293–301. [Google Scholar] [CrossRef][Green Version]
- Morris, C.A.; Owen, J.R.; Thomas, M.C.; El-Hiti, G.A.; Harwood, J.L.; Kille, P. Intracellular localization and induction of a dynamic RNA-editing event of macro-algal V-ATPase subunit A (VHA-A) in response to copper. Plant Cell Environ. 2013, 37, 189–203. [Google Scholar] [CrossRef]
- Magnotta, S.M.; Gogarten, J. Multisite polyadenylation and transcriptional response to stress of a vacuolar type H+-ATPase subunit A gene in Arabidopsis thaliana. BMC Plant Biol. 2002, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, J.; Liu, T.-Y.; Schumacher, K. Functional analysis of Arabidopsis V-ATPase subunit VHA-E isoforms. Eur. J. Cell Biol. 2010, 89, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, W.; Zhan, J.; Pan, Q. Systemic induction of H2O2 in pea seedlings pretreated by wounding and exogenous jasmonic acid. Sci. China Ser. C Life Sci. 2005, 48, 202–212. [Google Scholar] [CrossRef]
- Hung, K.T.; Hsu, Y.T.; Kao, C.H. Hydrogen peroxide is involved in methyl jasmonate-induced senescence of rice leaves. Physiol. Plant 2006, 127, 293–303. [Google Scholar] [CrossRef]
- Maruta, T.; Inoue, T.; Tamoi, M.; Yabuta, Y.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. Arabidopsis NADPH oxidases, AtrbohD and AtrbohF, are essential for jasmonic acid-induced expression of genes regulated by MYC2 transcription factor. Plant Sci. 2011, 180, 655–660. [Google Scholar] [CrossRef]
- Nafie, E.; Hathout, T.; Al Mokadem, A.S. Jasmonic acid elicits oxidative defense and detoxification systems in Cucumis melo L. cells. Braz. J. Plant Physiol. 2011, 23, 161–174. [Google Scholar] [CrossRef]
- Cuypers, A.; Hendrix, S.; Amaral dos Reis, R.; De Smet, S.; Deckers, J.; Gielen, H.; Jozefczak, M.; Loix, C.; Vercampt, H.; Vangronsveld, J.; et al. Hydrogen peroxide, signaling in disguise during metal phytotoxicity. Front. Plant Sci. 2016, 7, 470. [Google Scholar] [CrossRef]
- Harada, E.; Kusano, T.; Sano, H. Differential expression of genes encoding enzymes involved in sulfur assimilation pathways in response to wounding and jasmonate in Arabidopsis thaliana. J. Plant Physiol. 2000, 156, 272–276. [Google Scholar] [CrossRef]
- Jost, R.; Altschmied, L.; Bloem, E.; Bogs, J.; Gershenzon, J.; Hähnel, U.; Hänsch, R.; Hartmann, T.; Kopriva, S.; Kruse, C.; et al. Expression profiling of metabolic genes in response to methyl jasmonate reveals regulation of genes of primary and secondary sulfur-related pathways in Arabidopsis thaliana. Photosynth. Res. 2005, 86, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Zhang, Y.; Jin, Z.; Liu, Z.; Pei, Y. Role of hydrogen sulfide in the methyl jasmonate response to cadmium stress in foxtail millet. Front. Biosci. 2017, 22, 530–538. [Google Scholar]
- Deng, G.; Zhou, L.; Wang, Y.; Zhang, G.; Chen, X. Hydrogen sulfide acts downstream of jasmonic acid to inhibit stomatal development in Arabidopsis. Planta 2020, 251, 42. [Google Scholar] [CrossRef] [PubMed]
- Mikami, Y.; Shibuya, N.; Kimura, Y.; Nagahara, N.; Yamada, M.; Kimura, H. Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J. Biol. Chem. 2011, 286, 39379–39386. [Google Scholar] [CrossRef]
- Kabała, K.; Janicka-Russak, M.; Anklewicz, A. Mechanism of Cd and Cu action on the tonoplast proton pumps in cucumber roots. Physiol. Plant. 2013, 147, 207–217. [Google Scholar] [CrossRef]
- Wei, B.; Willems, P.; Huang, J.; Tian, C.; Yang, J.; Messens, J.; Van Breusegem, F. Identification of sulfenylated cysteines in Arabidopsis thaliana proteins using a disulfide-linked peptide reporter. Front. Plant Sci. 2020, 11, 777. [Google Scholar] [CrossRef]
- Bromberg, Y.; Rost, B. Correlating protein function and stability through the analysis of single amino acid substitutions. BMC Bioinform. 2009, 10, S8. [Google Scholar] [CrossRef]
- Maksymiec, W.; Wianowska, D.; Dawidowicz, A.L.; Radkiewicz, S.; Mardarowicz, M.; Krupa, Z. The level of jasmonic acid in Arabidopsis thaliana and Phaseolus coccineus plants under heavy metal stress. J. Plant Physiol. 2005, 162, 1338–1346. [Google Scholar] [CrossRef]
- Maksymiec, W.; Wójcik, M.; Krupa, Z. Variation in oxidative stress and photochemical activity in Arabidopsis thaliana leaves subjected to cadmium and excess copper in the presence or absence of jasmonate and Ascorbate. Chemosphere 2007, 66, 421–427. [Google Scholar] [CrossRef]
- Cabot, C.; Gallego, B.; Martos, S.; Barceló, J.; Poschenrieder, C. Signal cross talk in Arabidopsis exposed to cadmium, silicon, and botrytis cinerea. Planta 2013, 237, 337–349. [Google Scholar] [CrossRef]
- Keunen, E.; Remans, T.; Opdenakker, K.; Jozefczak, M.; Gielen, H.; Guisez, Y.; Vangronsveld, J.; Cuypers, A. A mutant of the Arabidopsis thaliana LIPOXYGENASE1 gene shows altered signalling and oxidative stress related responses after cadmium exposure. Plant Physiol. Biochem. 2013, 63, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Chen, J.; Li, X. Methyl jasmonate as modulator of Cd toxicity in Capsicum frutescens var. Fasciculatum seedlings. Ecotoxicol. Environ. Saf. 2013, 98, 203–209. [Google Scholar] [CrossRef]
- Lei, G.J.; Sun, L.; Sun, Y.; Zhu, X.F.; Li, G.X.; Zheng, S.J. Jasmonic acid alleviates cadmium toxicity in Arabidopsis via suppression of cadmium uptake and translocation. J. Integr. Plant Biol. 2020, 62, 218–227. [Google Scholar] [CrossRef]
- Mei, Y.; Chen, H.; Shen, W.; Shen, W.; Huang, L. Hydrogen peroxide is involved in hydrogen sulfide-induced lateral root formation in tomato seedlings. BMC Plant Biol. 2017, 17, 162. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gu, Q.; Yu, X.; Huang, L.; Xu, S.; Wang, R.; Shen, W.; Shen, W. Hydrogen peroxide acts downstream of melatonin to induce lateral root formation. Ann. Bot. 2018, 121, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Kabała, K.; Kłobus, G. Characterization of the tonoplast proton pumps in cucumis sativus L. Root Cells. Acta Physiol. Plant. 2001, 23, 55–63. [Google Scholar] [CrossRef]
- Larsson, C. Plasma membranes. In Modern Methods of Plant Analysis. Cell Components; Jackson, J.F., Linskens, H.F., Eds.; Springer: Berlin/Heidelberg, Germany, 1985; Volume 1, pp. 85–104. [Google Scholar]
- Kłobus, G. The role of plasma membrane-bound activities in nitrate transport into sealed plasma membrane vesicles from Cucumis sativus L. roots. In Structure and Function of Roots; Baluška, F., Ĉiamporová, M., Găsparíková, O., Barlow, P.W., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 133–140. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Li, Z.-G. Quantification of hydrogen sulfide concentration using methylene blue and 5,5′-dithiobis(2-nitrobenzoic acid) methods in plants. Methods Enzymol. 2015, 554, 101–110. [Google Scholar]
- Gallagher, S.R.; Leonard, R.T. Effect of Vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982, 70, 1335–1340. [Google Scholar] [CrossRef]
- Ames, B.N. Assay of inorganicphosphate, totalphosphate and phosphatases. Methods Enzymol. 1966, 8, 115–118. [Google Scholar]
- Sagi, M.; Fluhr, R. Superoxide production by plant homologues of the gp91phox NADPH oxidase. modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol. 2001, 126, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Jakubowska, D.; Janicka-Russak, M.; Kabała, K.; Migocka, M.; Reda, M. Modification of plasma membrane NADPH oxidase activity in cucumber seedling roots in response to cadmium stress. Plant Sci. 2015, 234, 50–59. [Google Scholar] [CrossRef]
- Siegel, L.M. A direct microdetermination for sulfide. Anal. Biochem. 1965, 11, 126–132. [Google Scholar] [CrossRef]
- Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A.; Ghassemi-Golezani, K. Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (‘Glycine max’ L. ) Plant Omics 2012, 5, 60. [Google Scholar]
- Migocka, M.; Papierniak, A. Identification of suitable reference genes for studying gene expression in cucumber plants subjected to abiotic stress and growth regulators. Mol. Breed. 2011, 28, 343–357. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitivemethod for the quantitation of microgramquantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Janicka, M.; Reda, M.; Mroczko, E.; Wdowikowska, A.; Kabała, K. Jasmonic acid effect on Cucumis sativus L. growth is related to inhibition of plasma membrane proton pump and the uptake and assimilation of nitrates. Int. J. Mol. Sci. 2023. submitted. [Google Scholar]
- Zboińska, M. Ścieżki sygnałowe uczestniczące w regulacji wakuolarnej H+-ATPazy w warunkach stresu kadmowego [Signaling pathways involved in the regulation of vacuolar H+-ATPase under cadmium stress]. Doctoral Thesis, University of Wrocław, Wrocław, Poland, 2021. [Google Scholar]
- Zboińska, M.; Janeczko, A.; Kabała, K. Involvement of NO in V-ATPase regulation in cucumber roots under control and cadmium stress conditions. Plants 2023, 12, 2884. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zboińska, M.; Romero, L.C.; Gotor, C.; Kabała, K. Regulation of V-ATPase by Jasmonic Acid: Possible Role of Persulfidation. Int. J. Mol. Sci. 2023, 24, 13896. https://doi.org/10.3390/ijms241813896
Zboińska M, Romero LC, Gotor C, Kabała K. Regulation of V-ATPase by Jasmonic Acid: Possible Role of Persulfidation. International Journal of Molecular Sciences. 2023; 24(18):13896. https://doi.org/10.3390/ijms241813896
Chicago/Turabian StyleZboińska, Magdalena, Luis C. Romero, Cecilia Gotor, and Katarzyna Kabała. 2023. "Regulation of V-ATPase by Jasmonic Acid: Possible Role of Persulfidation" International Journal of Molecular Sciences 24, no. 18: 13896. https://doi.org/10.3390/ijms241813896
APA StyleZboińska, M., Romero, L. C., Gotor, C., & Kabała, K. (2023). Regulation of V-ATPase by Jasmonic Acid: Possible Role of Persulfidation. International Journal of Molecular Sciences, 24(18), 13896. https://doi.org/10.3390/ijms241813896







