High-Intensity Interval Training Ameliorates High-Fat Diet-Induced Metabolic Disorders via the Cyclic GMP-AMP Synthase-Stimulator of Interferon Gene Signaling Pathway
Abstract
:1. Introduction
2. Results
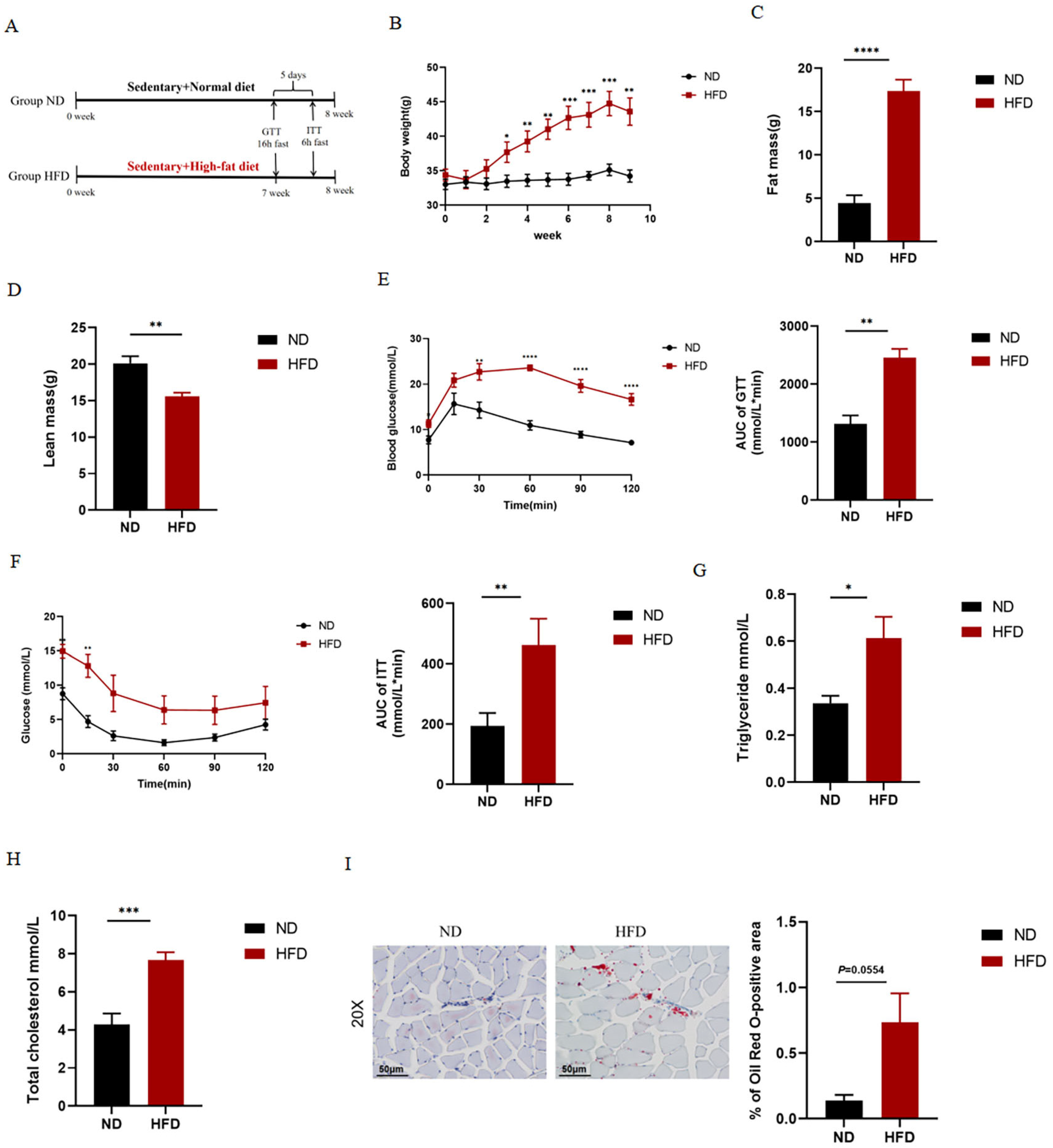
2.1. HFD Causes Obesity and Abnormal Glucolipid Metabolism in Mice
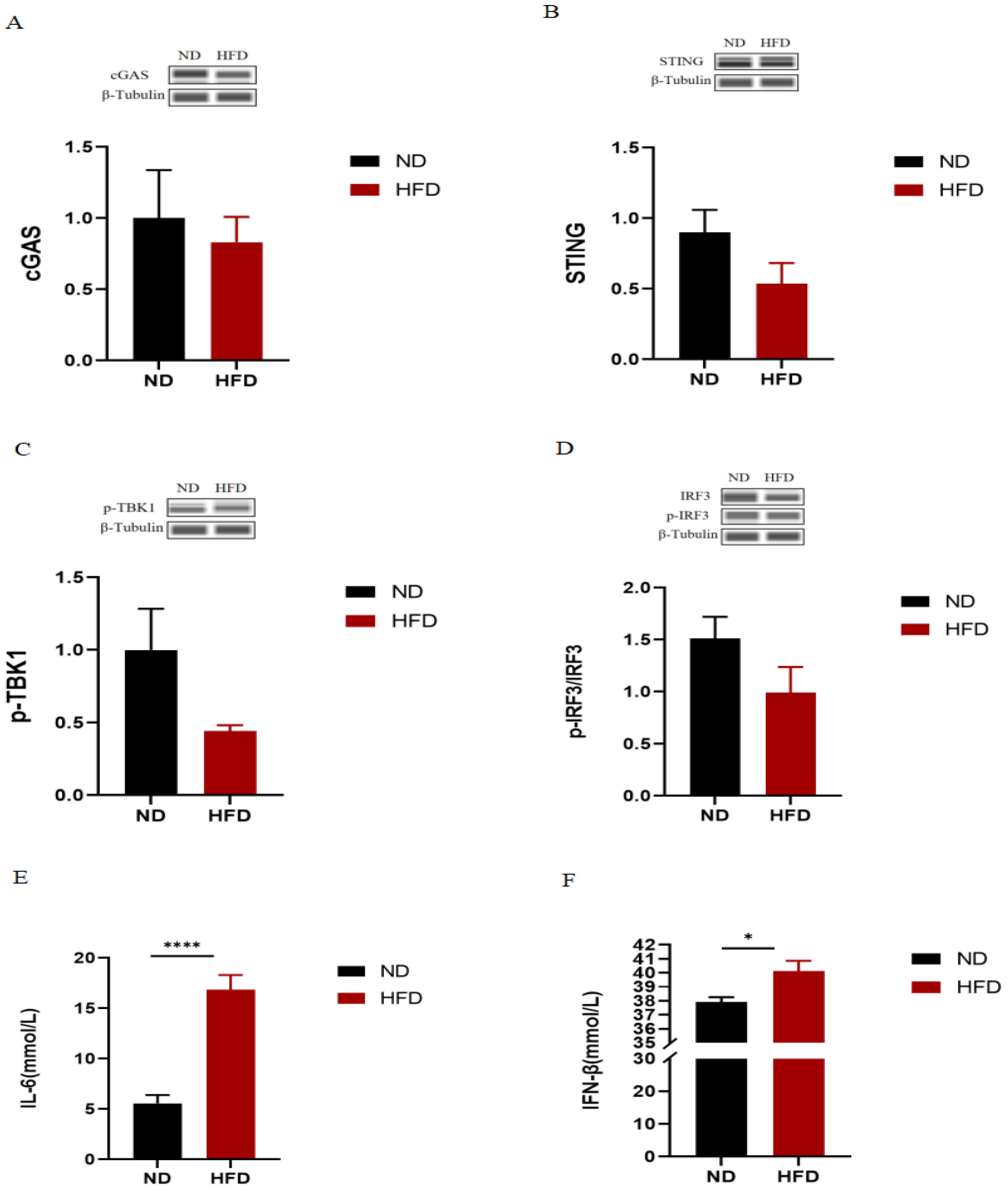
2.2. The cGAS-STING Signaling Pathway Is Not the Underlying Cause of Metabolic Disorders in HFD Mice
2.3. MICT and HIIT Ameliorate HFD-Induced Obesity and Abnormal Glucolipid Metabolism
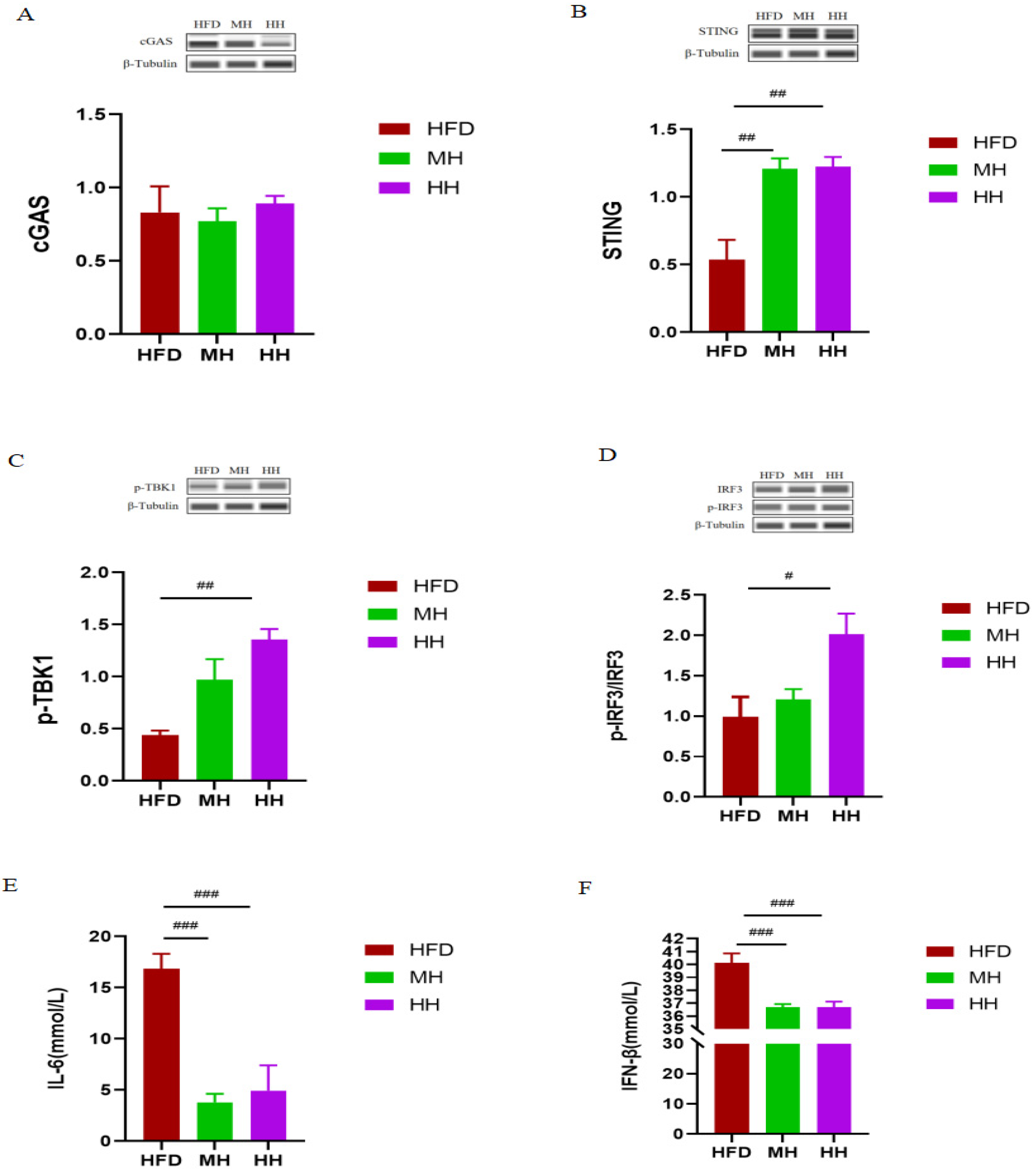
2.4. HIIT Ameliorates HFD-Induced Metabolic Disorders via the cGAS-STING Pathway
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Exercise Protocol
4.3. Blood and Tissue Samples Collection
4.4. Glucose Tolerance Test and Insulin Tolerance Test
4.5. Body Composition Analysis
4.6. Serum Total Cholesterol (TC) and Triglyceride (TG) Test
4.7. ELISAs of IL-6 and IFN-β
4.8. Oil Red O Staining
4.9. Wes Automated Western Blotting System
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Liu, F. The cGAS-cGAMP-STING Pathway: A Molecular Link Between Immunity and Metabolism. Diabetes 2019, 68, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Ablasser, A.; Goldeck, M.; Cavlar, T.; Deimling, T.; Witte, G.; Röhl, I.; Hopfner, K.P.; Ludwig, J.; Hornung, V. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 2013, 498, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Ascano, M.; Wu, Y.; Barchet, W.; Gaffney, B.L.; Zillinger, T.; Serganov, A.A.; Liu, Y.; Jones, R.A.; Hartmann, G.; et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 2013, 153, 1094–1107. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Barber, G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008, 455, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Du, F.; Xu, P.; Shu, C.; Sankaran, B.; Bell, S.L.; Liu, M.; Lei, Y.; Gao, X.; Fu, X.; et al. A conserved PLPLRT/SD motif of STING mediates the recruitment and activation of TBK1. Nature 2019, 569, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shang, G.; Gui, X.; Zhang, X.; Bai, X.C.; Chen, Z.J. Structural basis of STING binding with and phosphorylation by TBK1. Nature 2019, 567, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Chen, Z.J. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci. Signal 2012, 5, ra20. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef]
- Andzinski, L.; Spanier, J.; Kasnitz, N.; Kröger, A.; Jin, L.; Brinkmann, M.M.; Kalinke, U.; Weiss, S.; Jablonska, J.; Lienenklaus, S. Growing tumors induce a local STING dependent Type I IFN response in dendritic cells. Int. J. Cancer 2016, 139, 1350–1357. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Wu, X.; Ma, D.; Wu, J.; Wang, L.; Jiang, Y.; Fei, Y.; Zhu, C.; Tan, R.; et al. Nuclear cGAS suppresses DNA repair and promotes tumorigenesis. Nature 2018, 563, 131–136. [Google Scholar] [CrossRef]
- James, D.E.; Kraegen, E.W.; Chisholm, D.J. Effect of exercise training on whole-body insulin sensitivity and responsiveness. J. Appl. Physiol. 1984, 56, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Lanza, I.R.; Short, D.K.; Short, K.R.; Raghavakaimal, S.; Basu, R.; Joyner, M.J.; McConnell, J.P.; Nair, K.S. Endurance exercise as a countermeasure for aging. Diabetes 2008, 57, 2933–2942. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.; Nicol, C.W.; Bredin, S.S. Health benefits of physical activity: The evidence. CMAJ 2006, 174, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.M.; Peiffer, J.J.; Martins, R.N. Multiple effects of physical activity on molecular and cognitive signs of brain aging: Can exercise slow neurodegeneration and delay Alzheimer’s disease? Mol. Psychiatry 2013, 18, 864–874. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.S.; Dhaliwal, S.S.; Hills, A.P.; Pal, S. Effects of chronic exercise training on inflammatory markers in Australian overweight and obese individuals in a randomized controlled trial. Inflammation 2013, 36, 625–632. [Google Scholar] [CrossRef]
- da Luz, G.; Frederico, M.J.; da Silva, S.; Vitto, M.F.; Cesconetto, P.A.; de Pinho, R.A.; Pauli, J.R.; Silva, A.S.; Cintra, D.E.; Ropelle, E.R.; et al. Endurance exercise training ameliorates insulin resistance and reticulum stress in adipose and hepatic tissue in obese rats. Eur. J. Appl. Physiol. 2011, 111, 2015–2023. [Google Scholar] [CrossRef]
- Medeiros, C.; Frederico, M.J.; da Luz, G.; Pauli, J.R.; Silva, A.S.; Pinho, R.A.; Velloso, L.A.; Ropelle, E.R.; De Souza, C.T. Exercise training reduces insulin resistance and upregulates the mTOR/p70S6k pathway in cardiac muscle of diet-induced obesity rats. J. Cell Physiol. 2011, 226, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.G.; Carvalho, B.M.; Tobar, N.; Ropelle, E.R.; Pauli, J.R.; Bagarolli, R.A.; Guadagnini, D.; Carvalheira, J.B.; Saad, M.J. Physical exercise reduces circulating lipopolysaccharide and TLR4 activation and improves insulin signaling in tissues of DIO rats. Diabetes 2011, 60, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Sriwijitkamol, A.; Christ-Roberts, C.; Berria, R.; Eagan, P.; Pratipanawatr, T.; DeFronzo, R.A.; Mandarino, L.J.; Musi, N. Reduced skeletal muscle inhibitor of kappaB beta content is associated with insulin resistance in subjects with type 2 diabetes: Reversal by exercise training. Diabetes 2006, 55, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Zhe, G. Study on the Mechanism of Exercise Therapy on Urethane-Induced Lung Cancer in Mice—Based on Innate Immunit; East China Normal University: Shanghai, China, 2021. (In Chinese) [Google Scholar]
- MacInnis, M.J.; Gibala, M.J. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 2017, 595, 2915–2930. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, M.; Symonds, M.E. The impact of high-intensity interval training on inflammatory markers in metabolic disorders: A meta-analysis. Scand J. Med. Sci. Sports 2020, 30, 2020–2036. [Google Scholar] [CrossRef] [PubMed]
- Ross, L.M.; Porter, R.R.; Durstine, J.L. High-intensity interval training (HIIT) for patients with chronic diseases. J. Sport Health Sci. 2016, 5, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Rugbeer, N.; Constantinou, D.; Torres, G. Comparison of High-Intensity Training versus Moderate-Intensity Continuous Training on Cardiorespiratory Fitness and Body Fat Percentage in Persons with Overweight or Obesity: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Phys. Act. Health 2021, 18, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Mattioni Maturana, F.; Martus, P.; Zipfel, S.; Nieß, A.M. Effectiveness of HIIE versus MICT in Improving Cardiometabolic Risk Factors in Health and Disease: A Meta-analysis. Med. Sci. Sports Exerc. 2021, 53, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Fu, J.; Sun, S.; Zhao, G.; Cheng, W.; Dou, C.; Quan, M. Effects of HIIT and MICT on cardiovascular risk factors in adults with overweight and/or obesity: A meta-analysis. PLoS ONE 2019, 14, e0210644. [Google Scholar] [CrossRef] [PubMed]
- Vella, C.A.; Taylor, K.; Drummer, D. High-intensity interval and moderate-intensity continuous training elicit similar enjoyment and adherence levels in overweight and obese adults. Eur. J. Sport Sci. 2017, 17, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, J.; Wang, H.; Zhou, X.; Zeng, T.; Zhou, J.; Zhu, X. Metabolic Disease Animal Models induced by High-fat Diets. Lab. Anim. Comp. Med. 2021, 41, 70. [Google Scholar]
- Colpitts, B.H.; Smith, S.; Bouchard, D.R.; Boudreau, J.; Sénéchal, M. Are physical activity and sedentary behavior patterns contributing to diabetes and metabolic syndrome simultaneously? Transl. Sports Med. 2021, 4, 231–240. [Google Scholar] [CrossRef]
- Freyssin, C.; Verkindt, C.; Prieur, F.; Benaich, P.; Maunier, S.; Blanc, P. Cardiac rehabilitation in chronic heart failure: Effect of an 8-week, high-intensity interval training versus continuous training. Arch. Phys. Med. Rehabil. 2012, 93, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Karstoft, K.; Winding, K.; Knudsen, S.H.; Nielsen, J.S.; Thomsen, C.; Pedersen, B.K.; Solomon, T.P. The effects of free-living interval-walking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients: A randomized, controlled trial. Diabetes Care 2013, 36, 228–236. [Google Scholar] [CrossRef]
- Rognmo, Ø.; Hetland, E.; Helgerud, J.; Hoff, J.; Slørdahl, S.A. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur. J. Cardiovasc. Prev. Rehabil. 2004, 11, 216–222. [Google Scholar] [CrossRef]
- Wisløff, U.; Støylen, A.; Loennechen, J.P.; Bruvold, M.; Rognmo, Ø.; Haram, P.M.; Tjønna, A.E.; Helgerud, J.; Slørdahl, S.A.; Lee, S.J.; et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation 2007, 115, 3086–3094. [Google Scholar] [CrossRef]
- Wormgoor, S.G.; Dalleck, L.C.; Zinn, C.; Harris, N.K. Effects of High-Intensity Interval Training on People Living with Type 2 Diabetes: A Narrative Review. Can J. Diabetes 2017, 41, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Sylow, L.; Kleinert, M.; Richter, E.A.; Jensen, T.E. Exercise-stimulated glucose uptake—Regulation and implications for glycaemic control. Nat. Rev. Endocrinol. 2017, 13, 133–148. [Google Scholar] [CrossRef]
- Magalhães, J.P.; Júdice, P.B.; Ribeiro, R.; Andrade, R.; Raposo, J.; Dores, H.; Bicho, M.; Sardinha, L.B. Effectiveness of high-intensity interval training combined with resistance training versus continuous moderate-intensity training combined with resistance training in patients with type 2 diabetes: A one-year randomized controlled trial. Diabetes Obes. Metab. 2019, 21, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, H.; Li, C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods 2021, 10, 1854. [Google Scholar] [CrossRef]
- Kivimäki, M.; Bartolomucci, A.; Kawachi, I. The multiple roles of life stress in metabolic disorders. Nat. Rev. Endocrinol. 2023, 19, 10–27. [Google Scholar] [CrossRef]
- Lahera, V.; de Las Heras, N.; López-Farré, A.; Manucha, W.; Ferder, L. Role of Mitochondrial Dysfunction in Hypertension and Obesity. Curr. Hypertens Rep. 2017, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Bao, T. Correlation between Diabetic Cardiomyopathy and cGAS-STING Pathway in OVE26 Type 1 Diabetic Mice; Jilin University: Jilin, China, 2022. (In Chinese) [Google Scholar]
- Luo, X.; Li, H.; Ma, L.; Zhou, J.; Guo, X.; Woo, S.L.; Pei, Y.; Knight, L.R.; Deveau, M.; Chen, Y.; et al. Expression of STING Is Increased in Liver Tissues from Patients with NAFLD and Promotes Macrophage-Mediated Hepatic Inflammation and Fibrosis in Mice. Gastroenterology 2018, 155, 1971–1984.e4. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, Y.; An, W.; Song, J.; Zhang, Y.; Zhao, X. STING-mediated inflammation in Kupffer cells contributes to progression of nonalcoholic steatohepatitis. J. Clin. Investig. 2019, 129, 546–555. [Google Scholar] [CrossRef]
- Pham, P.T.; Fukuda, D.; Nishimoto, S.; Kim-Kaneyama, J.R.; Lei, X.F.; Takahashi, Y.; Sato, T.; Tanaka, K.; Suto, K.; Kawabata, Y.; et al. STING, a cytosolic DNA sensor, plays a critical role in atherogenesis: A link between innate immunity and chronic inflammation caused by lifestyle-related diseases. Eur. Heart J. 2021, 42, 4336–4348. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Barber, G.N. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-κB activation through TBK1. J. Virol. 2014, 88, 5328–5341. [Google Scholar] [CrossRef]
- Liu, D.; Wu, H.; Wang, C.; Li, Y.; Tian, H.; Siraj, S.; Sehgal, S.A.; Wang, X.; Wang, J.; Shang, Y.; et al. STING directly activates autophagy to tune the innate immune response. Cell Death Differ. 2019, 26, 1735–1749. [Google Scholar] [CrossRef]
- Vila, I.K.; Chamma, H.; Steer, A.; Saccas, M.; Taffoni, C.; Turtoi, E.; Reinert, L.S.; Hussain, S.; Marines, J.; Jin, L.; et al. STING orchestrates the crosstalk between polyunsaturated fatty acid metabolism and inflammatory responses. Cell Metab. 2022, 34, 125–139.e8. [Google Scholar] [CrossRef] [PubMed]
- Miles, M.P.; Naukam, R.J.; Hackney, A.C.; Clarkson, P.M. Blood leukocyte and glutamine fluctuations after eccentric exercise. Int. J. Sports Med. 1999, 20, 322–327. [Google Scholar] [CrossRef]
- Ablasser, A.; Chen, Z.J. cGAS in action: Expanding roles in immunity and inflammation. Science 2019, 363, eaat8657. [Google Scholar] [CrossRef] [PubMed]
- Marcuello, A.; González-Alonso, J.; Calbet, J.A.; Damsgaard, R.; López-Pérez, M.J.; Díez-Sánchez, C. Skeletal muscle mitochondrial DNA content in exercising humans. J. Appl. Physiol. 2005, 99, 1372–1377. [Google Scholar] [CrossRef]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Jiang, Q.; Jia, X.; Jiang, Z. ARMH3-mediated recruitment of PI4KB directs Golgi-to-endosome trafficking and activation of the antiviral effector STING. Immunity 2023, 56, 500–515.e6. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef]
- Schenk, S.; Saberi, M.; Olefsky, J.M. Insulin sensitivity: Modulation by nutrients and inflammation. J. Clin. Investig. 2008, 118, 2992–3002. [Google Scholar] [CrossRef] [PubMed]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Marcinko, K.; Sikkema, S.R.; Samaan, M.C.; Kemp, B.E.; Fullerton, M.D.; Steinberg, G.R. High intensity interval training improves liver and adipose tissue insulin sensitivity. Mol. Metab. 2015, 4, 903–915. [Google Scholar] [CrossRef] [PubMed]

| MICT | HIIT | ||||||
|---|---|---|---|---|---|---|---|
| Weeks | Exercise Speed (m/min) | Exercise Time (min) | Exercise Speed (m/min) | Exercise Time (min) | Rest Speed (m/min) | Rest Time (min) | Number of Repetitions |
| 1 | 14 | 60 | 22 | 2 | 8.8 | 2 | 12 |
| 2 | 14 | 60 | 23 | 2 | 9.2 | 2 | 12 |
| 3 | 14 | 60 | 24 | 2 | 9.6 | 2 | 12 |
| 4 | 14 | 60 | 25 | 2 | 10 | 2 | 12 |
| 5 | 14 | 60 | 26 | 2 | 10.4 | 2 | 12 |
| 6 | 14 | 60 | 27 | 2 | 10.8 | 2 | 12 |
| 7 | 14 | 60 | 28 | 2 | 11.2 | 2 | 12 |
| 8 | 14 | 60 | 29 | 2 | 11.6 | 2 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Li, X.; Yang, Y.; Zhang, Z.; Ding, S. High-Intensity Interval Training Ameliorates High-Fat Diet-Induced Metabolic Disorders via the Cyclic GMP-AMP Synthase-Stimulator of Interferon Gene Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 13840. https://doi.org/10.3390/ijms241813840
Hu Z, Li X, Yang Y, Zhang Z, Ding S. High-Intensity Interval Training Ameliorates High-Fat Diet-Induced Metabolic Disorders via the Cyclic GMP-AMP Synthase-Stimulator of Interferon Gene Signaling Pathway. International Journal of Molecular Sciences. 2023; 24(18):13840. https://doi.org/10.3390/ijms241813840
Chicago/Turabian StyleHu, Zhiwen, Xi Li, Yangjun Yang, Zhe Zhang, and Shuzhe Ding. 2023. "High-Intensity Interval Training Ameliorates High-Fat Diet-Induced Metabolic Disorders via the Cyclic GMP-AMP Synthase-Stimulator of Interferon Gene Signaling Pathway" International Journal of Molecular Sciences 24, no. 18: 13840. https://doi.org/10.3390/ijms241813840
APA StyleHu, Z., Li, X., Yang, Y., Zhang, Z., & Ding, S. (2023). High-Intensity Interval Training Ameliorates High-Fat Diet-Induced Metabolic Disorders via the Cyclic GMP-AMP Synthase-Stimulator of Interferon Gene Signaling Pathway. International Journal of Molecular Sciences, 24(18), 13840. https://doi.org/10.3390/ijms241813840





