Fluconazole-Induced Protein Changes in Osteogenic and Immune Metabolic Pathways of Dental Pulp Mesenchymal Stem Cells of Osteopetrosis Patients
Abstract
1. Introduction
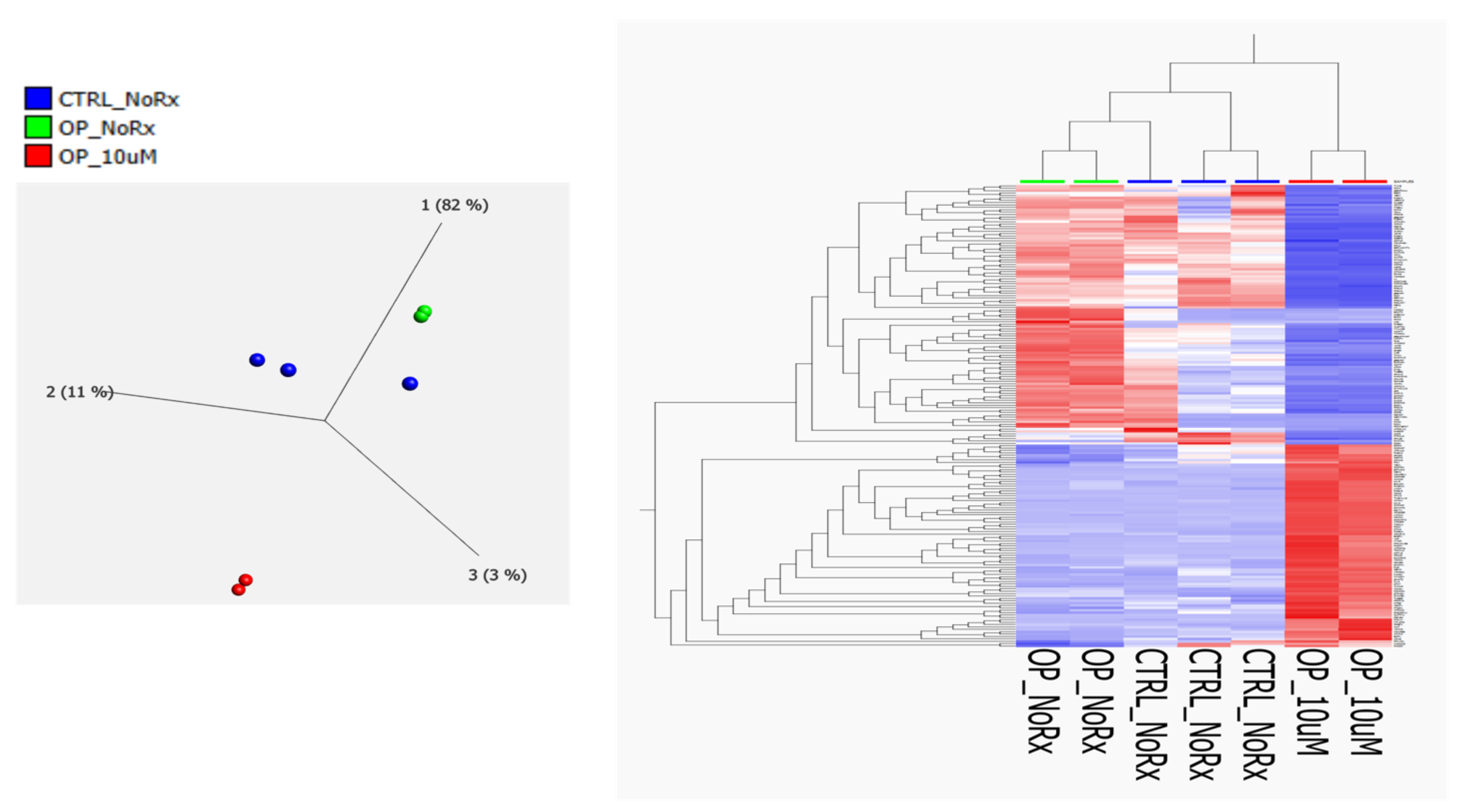
2. Results
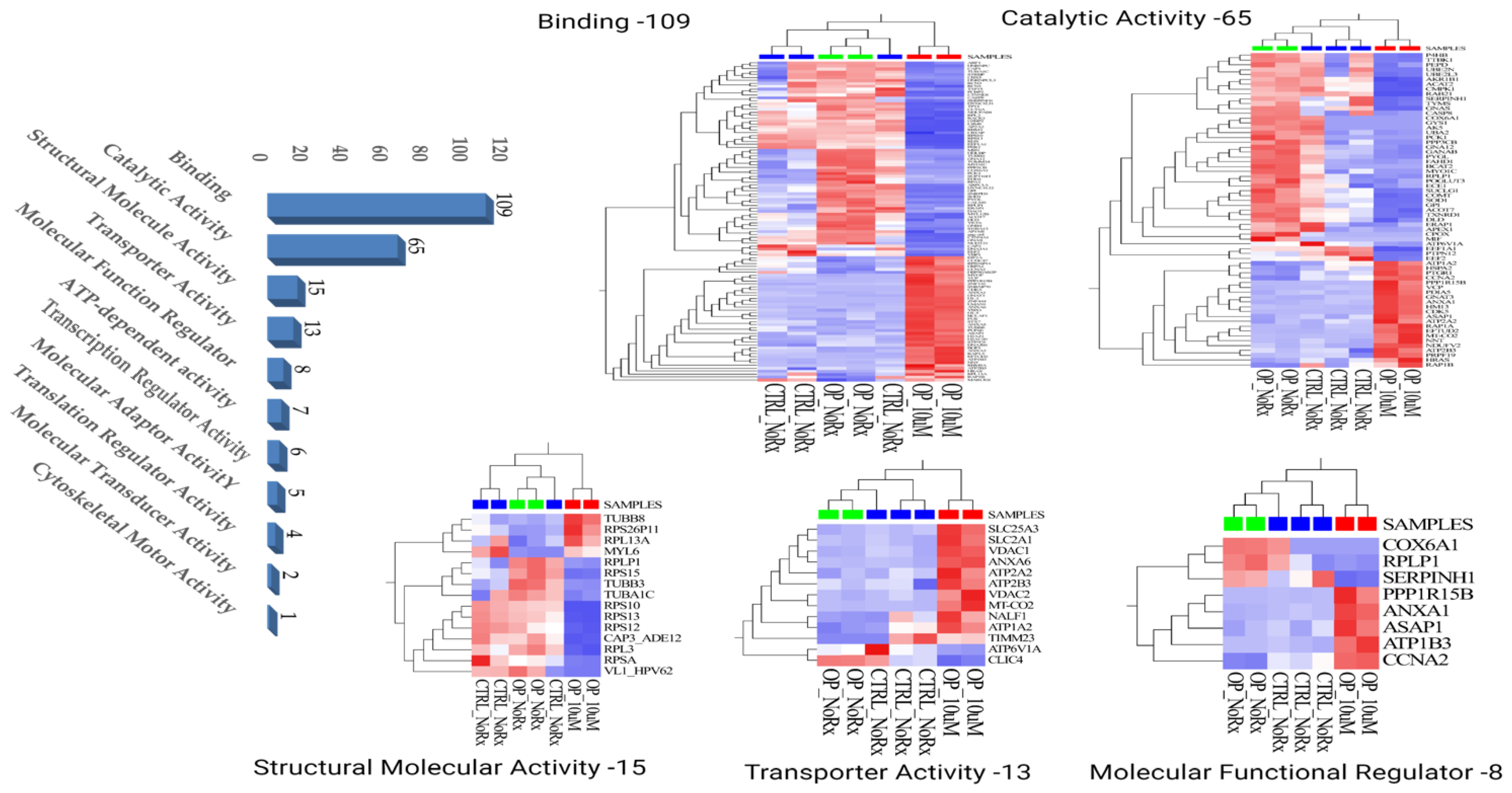
2.1. Molecular Functional Classification of the Treatment Induced Protein Changes
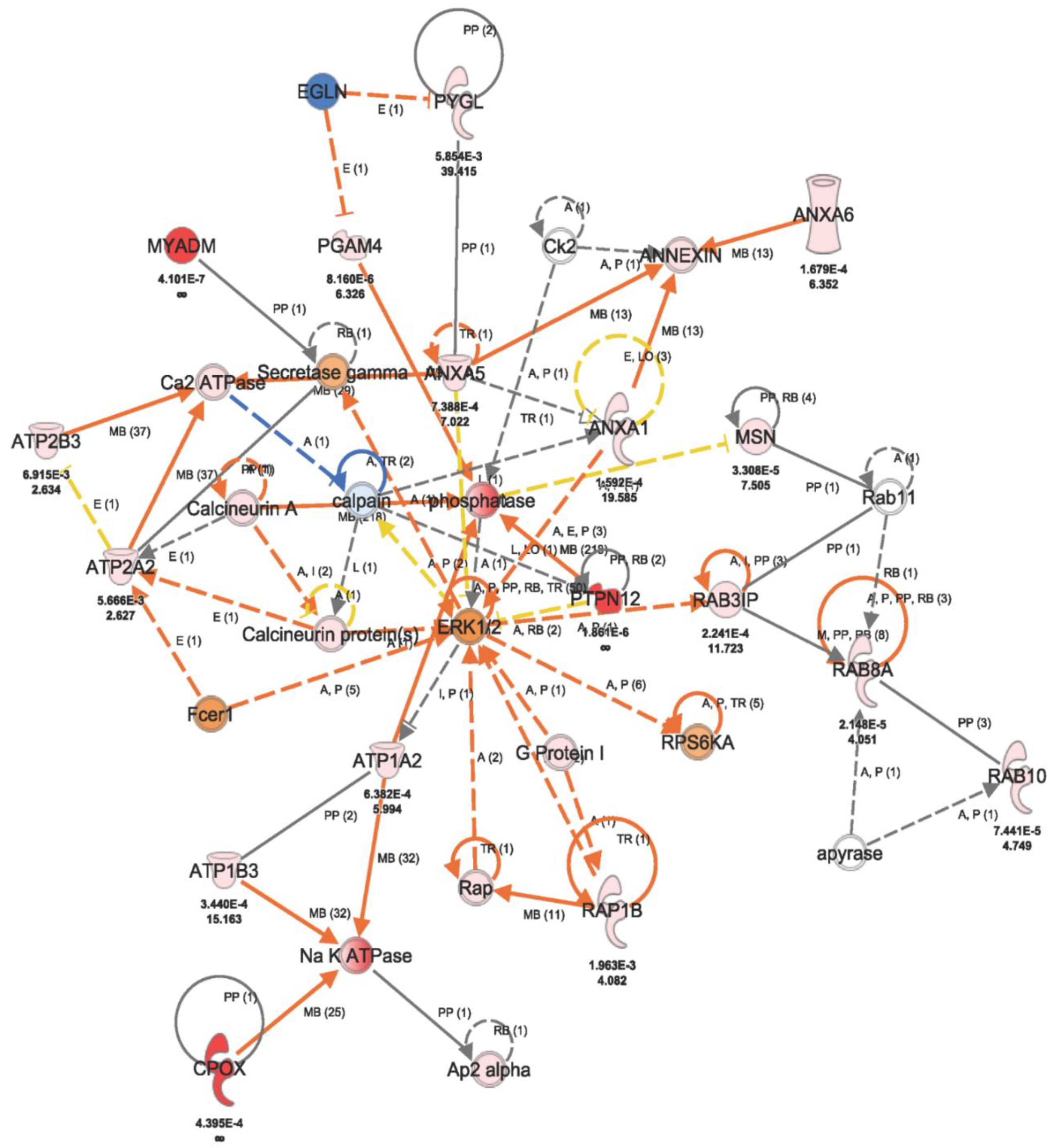
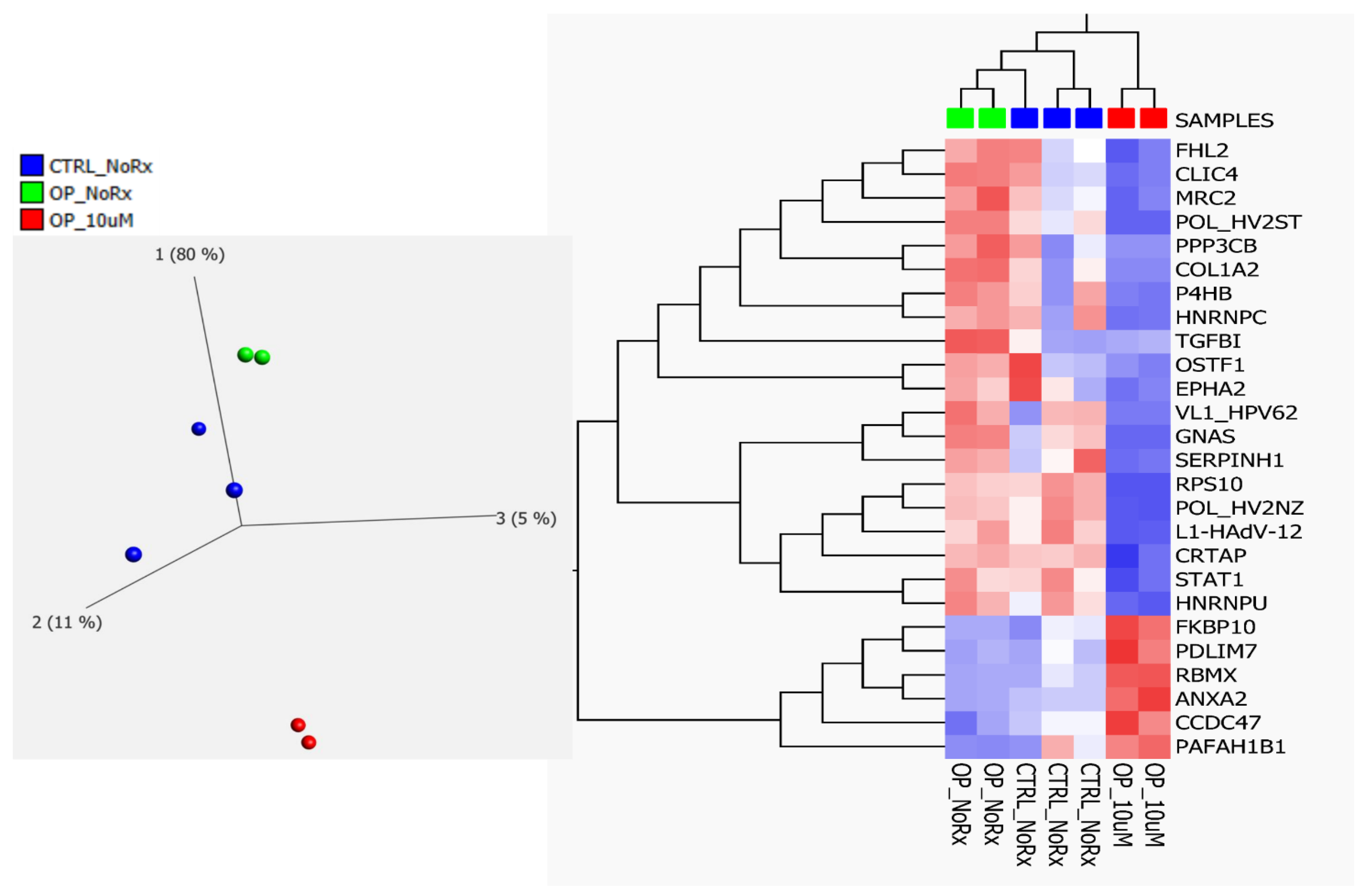
2.2. Fluconazole-Induced Protein Changes Involving Osteogenesis
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. Cell Isolation, Culture and Fluconazole Treatment of Dental Pulp Mesenchymal Cells
4.3. Whole-Cell Lysate of Mesenchymal Stromal Cells
4.4. In-Solution Digestion of Complex Protein Mixture
4.5. Protein Identification by Label-Free Liquid Chromatography Mass Spectrometry (LC/MSE)
4.6. Data Analysis and Informatics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SHED | Stem cells from human exfoliated deciduous teeth |
| MSCs | Mesenchymal stem cells |
| LC–MS/MS | Liquid chromatography–tandem mass spectrometry |
| CA-II | Carbonic Anhydrase II |
| CAA | Carbonic Anhydrase Activator |
| HC | Healthy control |
| OP | Osteopetrosis patients |
| DP | Dental pulp |
| MSSCs | Mesenchymal stem/stromal cells |
References
- Ohlsson, A.; Cumming, W.A.; Paul, A.; Sly, W.S. Carbonic anhydrase II deficiency syndrome: Recessive osteopetrosis with renal tubular acidosis and cerebral calcification. Pediatrics 1986, 77, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Stark, G.; Sakati, N. Marble brain disease: Recessive osteopetrosis, renal tubular acidosis and cerebral calcification in three Saudi Arabian families. Dev. Med. Child Neurol. 1980, 22, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.G. Control of bone resorption by hematopoietic tissue. The induction and reversal of congenital osteopetrosis in mice through use of bone marrow and splenic transplants. J. Exp. Med. 1975, 142, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, N.; Takahashi, N.; Akatsu, T.; Tanaka, H.; Sasaki, T.; Nishihara, T.; Koga, T.; Martin, T.J.; Suda, T. Origin of osteoclasts: Mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc. Natl. Acad. Sci. USA 1990, 87, 7260–7264. [Google Scholar] [CrossRef] [PubMed]
- Madel, M.B.; Ibanez, L.; Wakkach, A.; de Vries, T.J.; Teti, A.; Apparailly, F.; Blin-Wakkach, C. Immune Function and Diversity of Osteoclasts in Normal and Pathological Conditions. Front. Immunol. 2019, 10, 1408. [Google Scholar] [CrossRef] [PubMed]
- Cappariello, A.; Maurizi, A.; Veeriah, V.; Teti, A. The Great Beauty of the osteoclast. Arch. Biochem. Biophys. 2014, 558, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Penna, S.; Capo, V.; Palagano, E.; Sobacchi, C.; Villa, A. One Disease, Many Genes: Implications for the Treatment of Osteopetroses. Front. Endocrinol. 2019, 10, 85. [Google Scholar] [CrossRef]
- Alkhayal, Z.; Shinwari, Z.; Gaafar, A.; Alaiya, A. Proteomic Profiling of the First Human Dental Pulp Mesenchymal Stem/Stromal Cells from Carbonic Anhydrase II Deficiency Osteopetrosis Patients. Int. J. Mol. Sci. 2020, 22, 380. [Google Scholar] [CrossRef]
- Wu, C.C.; Econs, M.J.; DiMeglio, L.A.; Insogna, K.L.; Levine, M.A.; Orchard, P.J.; Miller, W.P.; Petryk, A.; Rush, E.T.; Shoback, D.M.; et al. Diagnosis and Management of Osteopetrosis: Consensus Guidelines From the Osteopetrosis Working Group. J. Clin. Endocrinol. Metab. 2017, 102, 3111–3123. [Google Scholar] [CrossRef]
- Stark, Z.; Savarirayan, R. Osteopetrosis. Orphanet J. Rare Dis. 2009, 4, 5. [Google Scholar] [CrossRef]
- Coudert, A.E.; de Vernejoul, M.C.; Muraca, M.; Del Fattore, A. Osteopetrosis and its relevance for the discovery of new functions associated with the skeleton. Int. J. Endocrinol. 2015, 2015, 372156. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Guerrini, M.M.; Cassani, B.; Pangrazio, A.; Sobacchi, C. Infantile malignant, autosomal recessive osteopetrosis: The rich and the poor. Calcif. Tissue Int. 2009, 84, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Zhang, B.; Zhou, J.; Li, T.; Liu, Z.; Li, Y.; Yang, M. Molecular insights into the human CLC-7/Ostm1 transporter. Sci. Adv. 2020, 6, eabb4747. [Google Scholar] [CrossRef] [PubMed]
- Landa, J.; Margolis, N.; Di Cesare, P. Orthopaedic management of the patient with osteopetrosis. J. Am. Acad. Orthop. Surg. 2007, 15, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Askmyr, M.K.; Fasth, A.; Richter, J. Towards a better understanding and new therapeutics of osteopetrosis. Br. J. Haematol. 2008, 140, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Kato, H.; Nonaka, K.; Nakanishi, H. Stem cells from human exfoliated deciduous teeth attenuate mechanical allodynia in mice through distinct from the siglec-9/MCP-1-mediated tissue-repairing mechanism. Sci. Rep. 2021, 11, 20053. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Nagai, R.; Kooh, S.W.; Balfe, J.W.; Fenton, T.; Halperin, M.L. Renal tubular acidosis and osteopetrosis with carbonic anhydrase II deficiency: Pathogenesis of impaired acidification. Pediatr. Nephrol. 1997, 11, 633–636. [Google Scholar] [CrossRef]
- Akocak, S.; Lolak, N.; Bua, S.; Nocentini, A.; Supuran, C.T. Activation of human α-carbonic anhydrase isoforms I, II, IV and VII with bis-histamine schiff bases and bis-spinaceamine substituted derivatives. J. Enzym. Inhib. Med. Chem. 2019, 34, 1193–1198. [Google Scholar] [CrossRef]
- Akocak, S.; Supuran, C.T. Activation of α-, β-, γ- δ-, ζ- and η- class of carbonic anhydrases with amines and amino acids: A review. J. Enzym. Inhib. Med. Chem. 2019, 34, 1652–1659. [Google Scholar] [CrossRef]
- Lolak, N.; Akocak, S.; Bua, S.; Koca, M.; Supuran, C.T. Design and synthesis of novel 1,3-diaryltriazene-substituted sulfonamides as potent and selective carbonic anhydrase II inhibitors. Bioorgan. Chem. 2018, 77, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Lolak, N.; Akocak, S.; Bua, S.; Supuran, C.T. Design, synthesis and biological evaluation of novel ureido benzenesulfonamides incorporating 1,3,5-triazine moieties as potent carbonic anhydrase IX inhibitors. Bioorgan. Chem. 2019, 82, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Lai, J.; Xu, W.; Zhu, W.; Chen, G. Protein tyrosine phosphatase non-receptor type 12 suppresses tumor progression in osteosarcoma cells. J. Orthop. Sci. 2023, 28, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.M.; Schwartz, G.J.; Alper, S.L. Urinary Acidification. In Fetal and Neonatal Physiology, 4th ed.; Polin, R.A., Abman, S.H., Rowitch, D.H., Benitz, W.E., Fox, W.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Blandina, P.; Supuran, C.T. Carbonic anhydrase activators and their potential in the pharmaceutical field. In Carbonic Anhydrases; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar] [CrossRef]
- Coban, T.A.; Beydemir, Ş.; Gücin, İ.; Ekinci, D.; Innocenti, A.; Vullo, D.; Supuran, C.T. Sildenafil is a strong activator of mammalian carbonic anhydrase isoforms I–XIV. Bioorgan. Med. Chem. 2009, 17, 5791–5795. [Google Scholar] [CrossRef] [PubMed]
- Akocak, S.; Lolak, N.; Bua, S.; Nocentini, A.; Karakoc, G.; Supuran, C.T. α-Carbonic anhydrases are strongly activated by spinaceamine derivatives. Bioorgan. Med. Chem. 2019, 27, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, A.; Ikeda, H.; Tsukamoto, H.; Kihira, K.; Ishioka, M.; Hirose, J.; Hata, T.; Fujioka, H.; Ono, Y. Timolol activates the enzyme activities of human carbonic anhydrase I and II. Biol. Pharm. Bull. 2010, 33, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Almstedt, K.; Rafstedt, T.; Supuran, C.T.; Carlsson, U.; Hammarstrom, P. Small-molecule suppression of misfolding of mutated human carbonic anhydrase II linked to marble brain disease. Biochemistry 2009, 48, 5358–5364. [Google Scholar] [CrossRef]
- Datta, R.; Shah, G.N.; Rubbelke, T.S.; Waheed, A.; Rauchman, M.; Goodman, A.G.; Katze, M.G.; Sly, W.S. Progressive renal injury from transgenic expression of human carbonic anhydrase IV folding mutants is enhanced by deficiency of p58IPK. Proc. Natl. Acad. Sci. USA 2010, 107, 6448–6452. [Google Scholar] [CrossRef]
- Feldshtein, M.; Elkrinawi, S.; Yerushalmi, B.; Marcus, B.; Vullo, D.; Romi, H.; Ofir, R.; Landau, D.; Sivan, S.; Supuran, C.T.; et al. Hyperchlorhidrosis caused by homozygous mutation in CA12, encoding carbonic anhydrase XII. Am. J. Hum. Genet. 2010, 87, 713–720. [Google Scholar] [CrossRef][Green Version]
- Van Karnebeek, C.D.; Sly, W.S.; Ross, C.J.; Salvarinova, R.; Yaplito-Lee, J.; Santra, S.; Shyr, C.; Horvath, G.A.; Eydoux, P.; Lehman, A.M.; et al. Mitochondrial carbonic anhydrase VA deficiency resulting from CA5A alterations presents with hyperammonemia in early childhood. Am. J. Hum. Genet. 2014, 94, 453–461. [Google Scholar] [CrossRef]
- Van Wesenbeeck, L.; Odgren, P.R.; Coxon, F.P.; Frattini, A.; Moens, P.; Perdu, B.; MacKay, C.A.; Van Hul, E.; Timmermans, J.P.; Vanhoenacker, F.; et al. Involvement of PLEKHM1 in osteoclastic vesicular transport and osteopetrosis in incisors absent rats and humans. J. Clin. Investig. 2007, 117, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, J.M.; Ohlemiller, K.K.; Shah, G.N.; Ulmasov, B.; Becker, T.A.; Waheed, A.; Hennig, A.K.; Lukasiewicz, P.D.; Sly, W.S. Carbonic anhydrase XIV deficiency produces a functional defect in the retinal light response. Proc. Natl. Acad. Sci. USA 2007, 104, 8514–8519. [Google Scholar] [CrossRef] [PubMed]
- Muller, W.E.; Schroder, H.C.; Schlossmacher, U.; Grebenjuk, V.A.; Ushijima, H.; Wang, X. Induction of carbonic anhydrase in SaOS-2 cells, exposed to bicarbonate and consequences for calcium phosphate crystal formation. Biomaterials 2013, 34, 8671–8680. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Schroder, H.C.; Muller, W.E. Biocalcite, a multifunctional inorganic polymer: Building block for calcareous sponge spicules and bioseed for the synthesis of calcium phosphate-based bone. Beilstein J. Nanotechnol. 2014, 5, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.S.; Szivek, J.A.; Lai, L.-W.; Lien, Y.-H.H. Phenotypic characteristics of bone in carbonic anhydrase II-deficient mice. Calcif. Tissue Int. 2008, 82, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zheng, Y.; Yang, Q.; Wang, L.; Pan, J.; Xia, Y.; Yan, X.; Han, J. Carbonic anhydrase I (CA1) is involved in the process of bone formation and is susceptible to ankylosing spondylitis. Arthritis Res. Ther. 2012, 14, R176. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Guitron, L.E.; Ceron-Torres, T.; Sobacchi, C.; Ochoa-Ruiz, E.; Villegas-Huesca, S. Autosomal recessive osteopetrosis type I: Description of pathogenic variant of TCIRG1 gene. Bol. Med. Hosp. Infant. Mex. 2018, 75, 255–259. [Google Scholar] [CrossRef]
- Athanasiadou, E.; Vlachou, C.; Theocharidou, A.; Tilaveridis, I.; Vargiami, E.; Antoniadis, K.; Arapostathis, K. When a pedodontic examination leads to the diagnosis of osteopetrosis: A case report. Spec. Care Dent. 2020, 40, 113–120. [Google Scholar] [CrossRef]
- Debruyne, D.; Ryckelynck, J.P. Clinical pharmacokinetics of fluconazole. Clin. Pharmacokinet. 1993, 24, 10–27. [Google Scholar] [CrossRef]
- Rose, A.S.; Bradley, A.R.; Valasatava, Y.; Duarte, J.M.; Prlić, A.; Rose, P.W. NGL viewer: Web-based molecular graphics for large complexes. Bioinformatics 2018, 34, 3755–3758. [Google Scholar] [CrossRef]
- Kim, J.M.; Yang, Y.S.; Hong, J.; Chaugule, S.; Chun, H.; van der Meulen, M.C.H.; Xu, R.; Greenblatt, M.B.; Shim, J.H. Biphasic regulation of osteoblast development via the ERK MAPK–mTOR pathway. Elife 2022, 11, e78069. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xu, Y.; Gan, Y.; Song, L.; Zhang, C.; Wang, L.; Zhou, Q. Role of the ERK1/2 Signaling Pathway in Osteogenesis of Rat Tendon-Derived Stem Cells in Normoxic and Hypoxic Cultures. Int. J. Med. Sci. 2016, 13, 629–637. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rousselle, A.V.; Heymann, D. Osteoclastic acidification pathways during bone resorption. Bone 2002, 30, 533–540. [Google Scholar] [CrossRef]
- Schlesinger, P.H.; Mattsson, J.P.; Blair, H.C. Osteoclastic acid transport: Mechanism and implications for physiological and pharmacological regulation. Miner. Electrolyte Metab. 1994, 20, 31–39. [Google Scholar] [PubMed]
- Schneider, M.R.; Sibilia, M.; Erben, R.G. The EGFR network in bone biology and pathology. Trends Endocrinol. Metab. TEM 2009, 20, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Alkhayal, Z.; Shinwari, Z.; Gaafar, A.; Alaiya, A. Carbonic Anhydrase II Activators in Osteopetrosis Treatment: A Review. Curr. Issues Mol. Biol. 2023, 45, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Even-Or, E.; NaserEddin, A.; Dinur Schejter, Y.; Shadur, B.; Zaidman, I.; Stepensky, P. Haploidentical stem cell transplantation with post-transplant cyclophosphamide for osteopetrosis and other nonmalignant diseases. Bone Marrow Transplant. 2021, 56, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Handler, D.C.; Pascovici, D.; Mirzaei, M.; Gupta, V.; Salekdeh, G.H.; Haynes, P.A. The Art of Validating Quantitative Proteomics Data. Proteomics 2018, 18, e1800222. [Google Scholar] [CrossRef]
- Franzen, B.; Hirano, T.; Okuzawa, K.; Uryu, K.; Alaiya, A.A.; Linder, S.; Auer, G. Sample preparation of human tumors prior to two-dimensional electrophoresis of proteins. Electrophoresis 1995, 16, 1087–1089. [Google Scholar] [CrossRef]
- Franzen, B.; Linder, S.; Okuzawa, K.; Kato, H.; Auer, G. Nonenzymatic extraction of cells from clinical tumor material for analysis of gene expression by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis 1993, 14, 1045–1053. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Alaiya, A.A.; Aljurf, M.; Shinwari, Z.; Almohareb, F.; Malhan, H.; Alzahrani, H.; Owaidah, T.; Fox, J.; Alsharif, F.; Mohamed, S.Y.; et al. Protein signatures as potential surrogate biomarkers for stratification and prediction of treatment response in chronic myeloid leukemia patients. Int. J. Oncol. 2016, 49, 913–933. [Google Scholar] [CrossRef]
- AlZahrani, S.; Shinwari, Z.; Gaafar, A.; Alaiya, A.; Al-Kahtani, A. Anti-Inflammatory Effect of Specialized Proresolving Lipid Mediators on Mesenchymal Stem Cells: An In Vitro Study. Cells 2022, 12, 122. [Google Scholar] [CrossRef]
- Alaiya, A.; Alshukairi, A.; Shinwari, Z.; Al-Fares, M.; Alotaibi, J.; AlOmaim, W.; Alsharif, I.; Bakheet, R.; Alharbi, L.; Allam, R.; et al. Alterations in the Plasma Proteome Induced by SARS-CoV-2 and MERS-CoV Reveal Biomarkers for Disease Outcomes for COVID-19 Patients. J. Inflamm. Res. 2021, 14, 4313–4328. [Google Scholar] [CrossRef]
- Dasouki, M.; Alaiya, A.; ElAmin, T.; Shinwari, Z.; Monies, D.; Abouelhoda, M.; Jabaan, A.; Almourfi, F.; Rahbeeni, Z.; Alsohaibani, F.; et al. Comprehensive multi-omics analysis of G6PC3 deficiency-related congenital neutropenia with inflammatory bowel disease. iScience 2021, 24, 102214. [Google Scholar] [CrossRef] [PubMed]
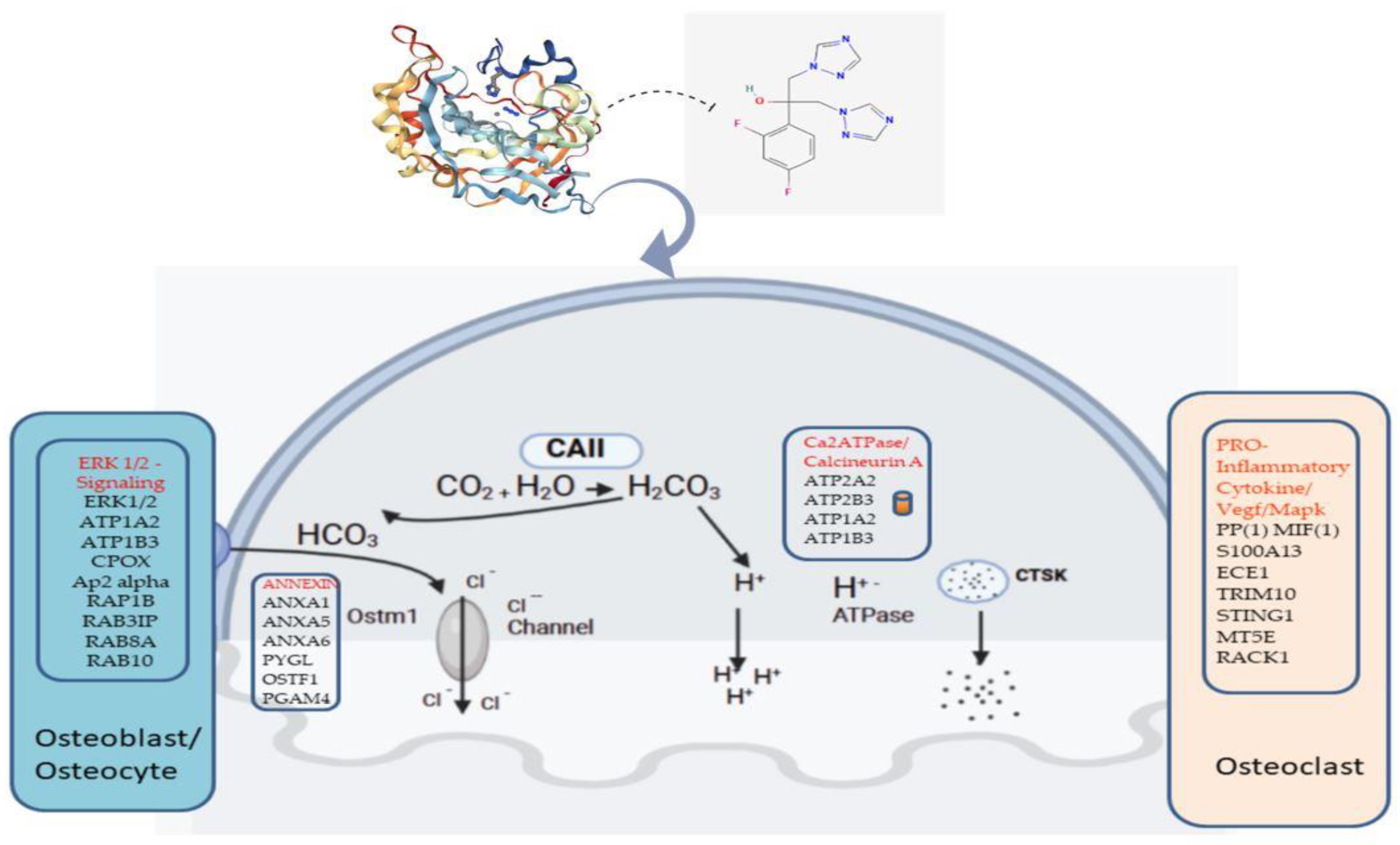
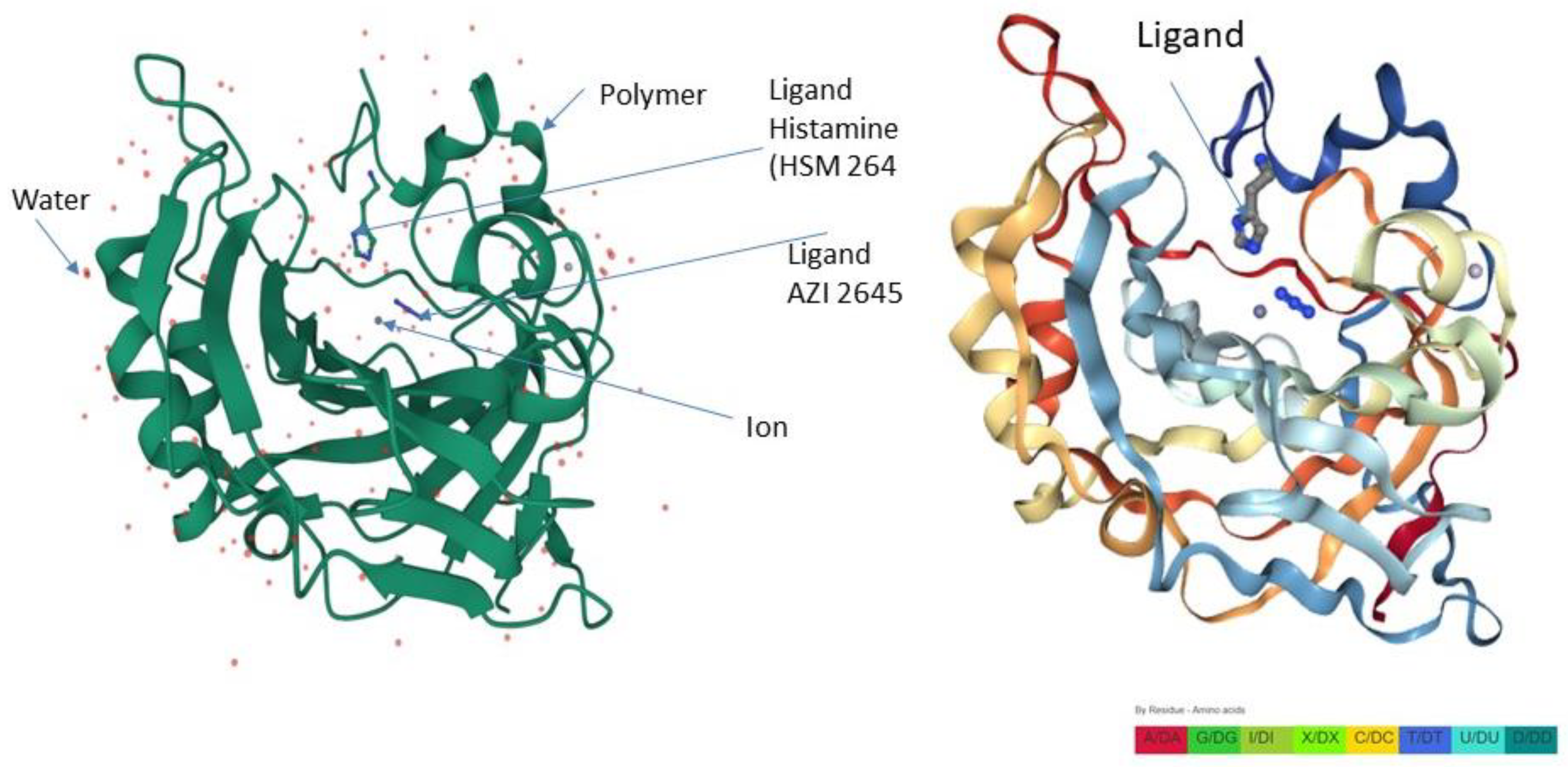
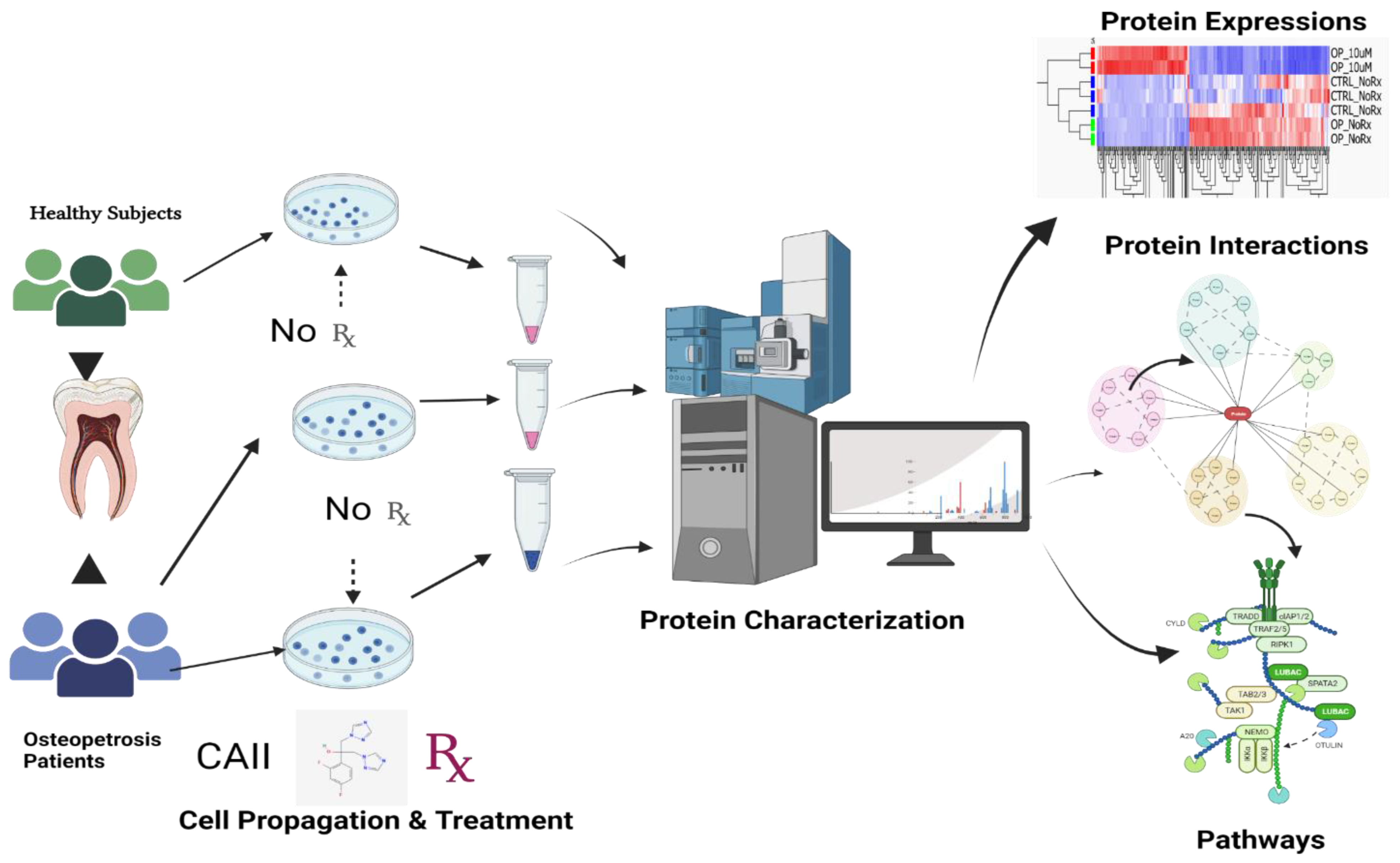
| Subjects | Age (Years) | Gender | Pathogenetics | Skeletal Radiology | Clinical Findings |
|---|---|---|---|---|---|
| OP#1 | 11 | M | c.232 + 1G > A, IVS2 + 1G > Aof the CA2 gene, (AR, RTA) | High frequency of fractures, dense bones | Dental abnormalities, developmental delay, optic atrophy/esotropia, ADHD |
| OP#2 | 10 | F | c.232 + 1G > A, IVS2 + 1G > Aof the CA2 gene (AR, RTA) | Dense skull bones | Short stature, esotropia and optic atrophy |
| OP#3 | 16 | F | CAII deficiency Renal Profile Low CO2, (RTA) | Diffuse increased density of skeletal bones, sclerosis more prominent in skull | ADHD, developmental delay learning disabilities, scoliosis |
| OP#4 | 15 | M | CAII deficiency Renal profile Low CO2, (RTA) | Brachiocephaly, increased bone density, Erlenmeyer flask deformity | Failure to thrive, fractures, intracranial calcification |
| OP#5 | 9 | M | c.232 + 1G > A;IVS2 + 1G > A (RTA), AD, CAII | Generalized increase in bone density shape normal | Calcification, fractures |
| HC#1 | 11 | F | Healthy | Not indicated | No Abnormalities |
| HC#2 | 9 | F | Healthy | Not indicated | No Abnormalities |
| HC#3 | 10 | M | Healthy | Not indicated | No Abnormalities |
| HC#4 | 16 | M | Healthy | Not indicated | No Abnormalities |
| HC#5 | 9 | M | Healthy | Not indicated | No Abnormalities |
| HC#6 | 13 | F | Healthy | Not indicated | No Abnormalities |
| Accession | GN | Anova (p) | Max Fold Change | Highest Mean Condition | Lowest Mean Condition | Role in Bone Related Disease Association |
|---|---|---|---|---|---|---|
| O75718 | CRTAP | 0.0193 | 3.30 | OP-NoRx | OP-Rx | Ostegenesis Imperfecta |
| P05962 | POL_HV2NZ | 0.0001 | 81.79 | OP-NoRx | OP-Rx | Not known |
| P07237 | P4HB | 0.0002 | 3.32 | OP-NoRx | OP-Rx | Ostegenesis Imperfecta |
| P07910 | HNRNPC | 0.0008 | 3.48 | OP-NoRx | OP-Rx | Osteoblast |
| P08123 | COL1A2 | 0.0305 | 170.43 | OP-NoRx | OP-Rx | Ostegenesis Imperfecta |
| P16298 | PPP3CB | 0.0010 | 42.88 | OP-NoRx | OP-Rx | Osteoclast |
| P20876 | POL_HV2ST | 0.0000 | 22.26 | OP-NoRx | OP-Rx | Not known |
| P29317 | EPHA2 | 0.0444 | 7.03 | OP-NoRx | OP-Rx | Osteoblast-Clast |
| P36712 | L1-HAdV-12 | 0.0004 | 5.12 | OP-NoRx | OP-Rx | Not known |
| P42224 | STAT1 | 0.0461 | 2.29 | OP-NoRx | OP-Rx | Osteoclast |
| P46783 | RPS10 | 0.0041 | 1850.80 | OP-NoRx | OP-Rx | Osteoblast-Clast |
| P50454 | SERPINH1 | 0.0000 | 5.35 | OP-NoRx | OP-Rx | Oste Imperfecta |
| P50823 | VL1_HPV62 | 0.0000 | ∞ | OP-NoRx | OP-Rx | Not known |
| Q5JWF2 | GNAS | 0.0015 | 42.22 | OP-NoRx | OP-Rx | Osteoblast-Clast |
| Q9UBG0 | MRC2 | 0.0151 | 8.64 | OP-NoRx | OP-Rx | Osteoblast |
| Q9Y696 | CLIC4 | 0.0007 | 9.14 | OP-NoRx | OP-Rx | Osteoblast |
| Q00839 | HNRNPU | 0.0117 | 2.46 | OP-NoRx | OP-Rx | Osteoblast |
| Q14192 | FHL2 | 0.0834 | 8.20 | OP-NoRx | OP-Rx | Osteoblast |
| Q92882 | OSTF1 | 0.0109 | 4.56 | OP-NoRx | OP-Rx | Osteoblast |
| Q15582 | TGFBI | 0.0009 | 4.02 | OP-NoRx | OP-Rx | Osteoblast |
| P07355 | ANXA2 | 0.0000 | 6.68 | OP-Rx | OP-NoRx | Osteoblast |
| Q9NR12 | PDLIM7 | 0.0004 | 4.86 | OP-Rx | OP-NoRx | Osteoblast |
| Q96A33 | CCDC47 | 0.0014 | 3.43 | OP-Rx | OP-NoRx | Osteoblast |
| Q96AY3 | FKBP10 | 0.0030 | 5.92 | OP-Rx | OP-NoRx | Oste Imperfata |
| P38159 | RBMX | 0.0020 | 979.35 | OP-Rx | OP-NoRx | Osteoblast |
| P43034 | PAFAH1B1 | 0.0085 | 39.22 | OP-Rx | OP-NoRx | Osteoclast |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhayal, Z.; Shinwari, Z.; Gaafar, A.; Alaiya, A. Fluconazole-Induced Protein Changes in Osteogenic and Immune Metabolic Pathways of Dental Pulp Mesenchymal Stem Cells of Osteopetrosis Patients. Int. J. Mol. Sci. 2023, 24, 13841. https://doi.org/10.3390/ijms241813841
Alkhayal Z, Shinwari Z, Gaafar A, Alaiya A. Fluconazole-Induced Protein Changes in Osteogenic and Immune Metabolic Pathways of Dental Pulp Mesenchymal Stem Cells of Osteopetrosis Patients. International Journal of Molecular Sciences. 2023; 24(18):13841. https://doi.org/10.3390/ijms241813841
Chicago/Turabian StyleAlkhayal, Zikra, Zakia Shinwari, Ameera Gaafar, and Ayodele Alaiya. 2023. "Fluconazole-Induced Protein Changes in Osteogenic and Immune Metabolic Pathways of Dental Pulp Mesenchymal Stem Cells of Osteopetrosis Patients" International Journal of Molecular Sciences 24, no. 18: 13841. https://doi.org/10.3390/ijms241813841
APA StyleAlkhayal, Z., Shinwari, Z., Gaafar, A., & Alaiya, A. (2023). Fluconazole-Induced Protein Changes in Osteogenic and Immune Metabolic Pathways of Dental Pulp Mesenchymal Stem Cells of Osteopetrosis Patients. International Journal of Molecular Sciences, 24(18), 13841. https://doi.org/10.3390/ijms241813841





