The Cation/Calcium Channel of Sperm (CatSper): A Common Role Played Despite Inter-Species Variation?
Abstract
1. Introduction
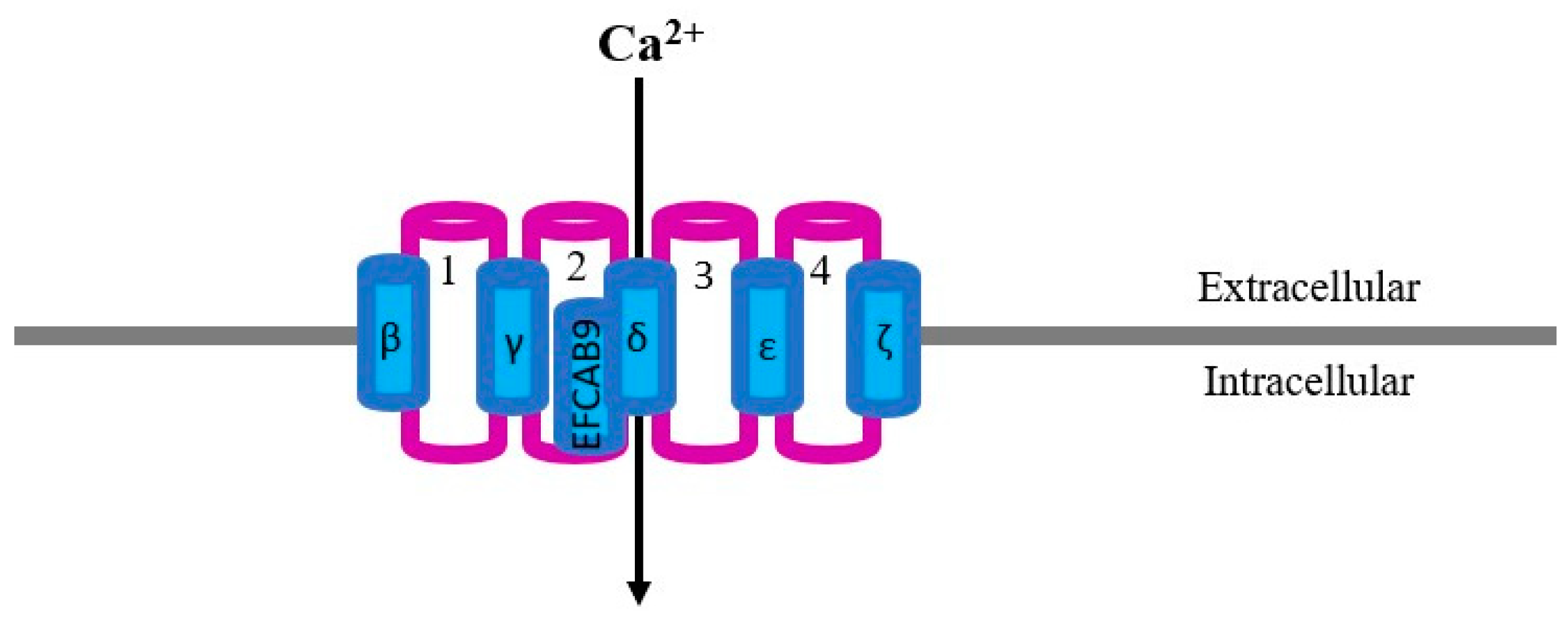
2. CatSper Structure and Localization
| Receptor | Species | Membrane Domain | Function | Method Used | Reference |
|---|---|---|---|---|---|
| CatSper | Mouse | Principal piece | Motility and hyperactivated motility | NB, WB, ICC | Ren et al., 2001 [16] |
| CatSper 1 | Human | Cytoplasmic droplets, midpiece, head | Motility and AR | WB and ICC | Tamburrino et al., 2014, 2015 [28,29] |
| CatSper 1 | Bovine | Principal piece | Hyperactivation | ICC | Johnson et al., 2017 [32] |
| CatSper 1 | Equine | Principal piece | Hyperactivation | PCR, ICC | Loux et al., 2013 [27] |
| CatSper 1 | Sea Urchin | Tail | - | ICC | Loyo-Celis et al., 2021 [34] |
| CatSper 1 | Pig | Acrosome, neck, tail and in cytoplasmic droplets | Motility | WB and ICC | Vicente-Carrillo et al., 2017 [31] |
| CatSper 1 | Ovine | Acrosome and post-acrosome | - | WB and ICC | Vicente-Carrillo et al., 2015 [30] |
| CatSper 2 | Mouse | Tail, principal piece | Motility and hyperactivated motility | WB, ICC | Quill et al., 2001, 2003 [18,38] |
| CatSper 2 | Human | Tail | Motility and male fertility | WB, ICC | Bhilawadikar et al., 2013; Smith et al., 2013 [42,43] |
| CatSper 2 | Pig | Post-acrosome, neck, tail and in cytoplasmic droplets | Motility | WB and ICC | Vicente-Carrillo et al., 2017 [30] |
| CatSper 2 | Ovine | Acrosome and neck | - | WB and ICC | Vicente-Carrillo et al., 2015 [30] |
| CatSper 3 and 4 | Mouse, Human | Acrosome, midpiece, cytoplasmic droplet | AR, hyperactivated motility during capacitation and male fertility | Prediction software, PCR, ICC (only mouse) | Lobley et al. 2003; Jin et al., 2005, 2007 [20,39,40] |
| CatSper 3 | Pig | Neck, tail and in cytoplasmic droplets | Motility | WB and ICC | Vicente-Carrillo et al., 2017 [31] |
| CatSper 3 | Ovine | Post-acrosome and principal piece | - | WB and ICC | Vicente-Carrillo et al., 2015 [30] |
| CatSper 3 | Sea Urchin | Principal piece | Chemotaxis and sperm motility | ICC | Seifert et al., 2015 [33] |
| CatSper 4 | Mouse | Principal piece | Hyperactivated motility and male fertility | ICC | Qi et al., 2007 [41] |
| CatSper 4 | Pig | Post-acrosome, neck, tail and in cytoplasmic droplets | Motility | WB and ICC | Vicente-Carrillo et al., 2017 [31] |
| CatSper 4 | Ovine | Post-acrosome | - | WB and ICC | Vicente-Carrillo et al., 2015 [30] |
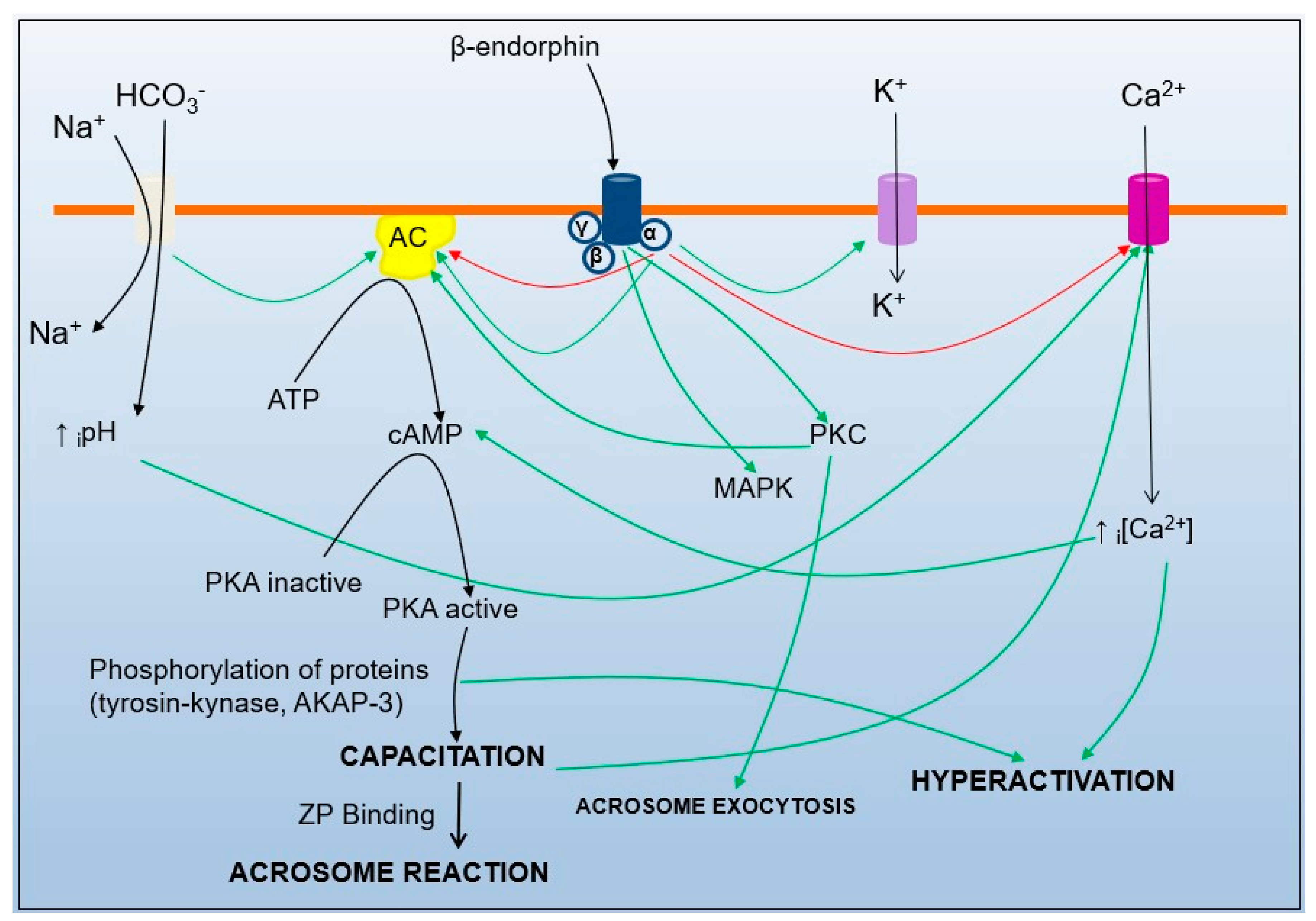
3. CatSper Activation
4. Role of CatSper in Fertilization
5. Mechanisms of Sperm Interaction with the Surroundings and Their Relationship to CatSper
6. CatSper, a Potential Target for Male Fertility Intervention?
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mann, T.; Lutwak-Mann, C. Male Reproductive Function and Semen, 1st ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1981; p. 507. [Google Scholar]
- Ho, K.; Wolff, C.A.; Suarez, S.S. CatSper-null mutant spermatozoa are unable to ascend beyond the oviductal reservoir. Reprod. Fertil. Dev. 2009, 21, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Vicente Carrillo, A. Sperm Membrane Channels, Receptors and Kinematics: Using Boar Spermatozoa for Drug Toxicity Screening. PhD Thesis, Linköping University, Linköping, Sweden, 2016. [Google Scholar]
- Gadella, B.M.; Tsai, P.S.; Boerke, A.; Brewis, I.A. Sperm head membrane reorganisation during capacitation. Int. J. Dev. Biol. 2008, 52, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.M. Tales from the tail: What do we really know about sperm motility? J. Androl. 2003, 24, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Publicover, S.J.; Giojalas, L.C.; Teves, M.E.; Mendes Machado de Oliveira, G.S.; Morales Garcia, A.A.; Barratt, C.L.R.; Harper, C.V. Ca2+ signalling in the control of motility and guidance in mammalian sperm. Front. Biosci. 2008, 13, 5623–5637. [Google Scholar] [CrossRef] [PubMed]
- Gadella, B.M.; Boerke, A. An update on post-ejaculatory remodeling of the sperm surface before mammalian fertilization. Theriogenology 2016, 85, 113–124. [Google Scholar] [CrossRef]
- Darszon, A.; López-Martínez, P.; Acevedo, J.J.; Hernández-Cruz, A.; Treviño, C.L. T-type Ca2+ channels in sperm function. Cell Calcium. 2006, 40, 241–252. [Google Scholar] [CrossRef]
- Lishko, P.V.; Botchkina, I.L.; Kirichok, Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011, 471, 387–391. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, H.; Ekstedt, E.; Einarsson, S. Acidification of the epididymal fluid in the boar. Int. J. Androl. 1990, 13, 238–243. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Roca, J.; Alvarez-Rodriguez, M.; Martinez-Serrano, C.A. How does the boar epididymis regulate the emission of fertile spermatozoa? Anim. Reprod. Sci. 2022, 246, 106829. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Martinez, E.A.; Calvete, J.J.; Peña-Vega, F.J.; Roca, J. Seminal Plasma: Relevant for fertility? Int. J. Mol. Sci. 2021, 22, 4368. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, H. Role of the oviduct in sperm capacitation. Theriogenology 2007, 68 (Suppl. S1), S138–S146. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, A.S.; Ballester, J.; Johannisson, A.; Hernandez, M.; Lundeheim, N.; Rodríguez-Martínez, H. In vitro capacitation of bull spermatozoa by oviductal fluid and its components. Zygote 2006, 3, 259–273. [Google Scholar] [CrossRef]
- Navarro, B.; Kirichok, Y.; Chung, J.J.; Clapham, D.E. Ion channels that control fertility in mammalian spermatozoa. Int. J. Dev. Biol. 2008, 52, 607–613. [Google Scholar] [CrossRef]
- Ren, D.; Navarro, B.; Perez, G.; Jackson, A.C.; Hsu, S.; Shi, Q.; Tilly, J.L.; Clapham, D.E. A sperm ion channel required for sperm motility and male fertility. Nature 2001, 413, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Xia, J. Calcium signalling through CatSper channels in mammalian fertilization. Physiology 2010, 25, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Quill, T.A.; Ren, D.; Clapham, D.E.; Garbers, D.L. A voltage-gated ion channel expressed specifically in spermatozoa. Proc. Natl. Acad. Sci. USA 2001, 98, 12527–12531. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Mannowetz, N.; Zhang, Y.; Everley, R.A.; Gygi, S.P.; Bewersdorf, J.; Lishko, P.V.; Chung, J.J. Dual sensing of physiologic pH and calcium by EFCAB9 regulates sperm motility. Cell 2019, 177, 1480–1494. [Google Scholar] [CrossRef]
- Lobley, A.; Pierron, V.; Reynolds, L.; Allen, L.; Michalovich, D. Identification of human and mouse CATSPER3 and CATSPER4 genes: Characterisation of a common interaction domain and evidence for expression in testis. Reprod. Biol. Endocrinol. 2003, 1, 53. [Google Scholar] [CrossRef]
- Liu, J.; Xia, J.; Cho, K.H.; Clapham, D.E.; Ren, D. CatSperβ, a novel transmembrane protein in the CatSper channel complex. J. Biol. Chem. 2007, 282, 18945–18952. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Cho, K.H.; Ren, D. A novel, single, transmembrane protein CATSPERG is associated with CATSPER1 channel protein. Biol. Reprod. 2009, 81, 539–544. [Google Scholar] [CrossRef]
- Chung, J.J.; Navarro, B.; Krapivinsky, G.; Krapivinsky, L.; Clapham, D.E. A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa. Nat. Commun. 2011, 2, 153. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.J.; Miki, K.; Kim, D.; Shim, S.H.; Shi, H.F.; Hwang, J.Y.; Cai, X.; Iseri, Y.; Zhuang, X.; Clapham, D.E. CatSperζ regulates the structural continuity of sperm Ca2+ signalling domains and is required for normal fertility. eLife 2017, 6, e23082. [Google Scholar] [CrossRef] [PubMed]
- Strünker, T.; Goodwin, N.; Brenker, C.; Kashikar, N.D.; Weyand, I.; Seifert, R.; Kaupp, U.B. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 2011, 471, 382–386. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Chung, J.J. CatSper Calcium Channels: 20 Years on. Physiology 2023, 38, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Loux, S.C.; Crawford, K.R.; Ing, N.H.; González-Fernández, L.; Macías-García, B.; Love, C.C.; Varner, D.D.; Velez, I.C.; Choi, Y.H.; Hinrichs, K. CatSper and the relationship of hyperactivated motility to intracellular calcium and pH kinetics in equine sperm. Biol. Reprod. 2013, 89, 123. [Google Scholar] [CrossRef] [PubMed]
- Tamburrino, L.; Marchiani, S.; Minetti, F.; Forti, G.; Muratori, M.; Baldi, E. The CatSper calcium channel in human sperm: Relation with motility and involvement in progesterone-induced acrosome reaction. Hum. Reprod. 2014, 29, 418–428. [Google Scholar] [CrossRef]
- Tamburrino, L.; Marchiani, S.; Vicini, E.; Muciaccia, B.; Cambi, M.; Pellegrini, S.; Forti, G.; Muratori, M.; Baldi, E. Quantification of CatSper1 expression in human spermatozoa and relation to functional parameters. Hum. Reprod. 2015, 30, 1532–1544. [Google Scholar] [CrossRef]
- Vicente-Carrillo, A.; Casao, A.; Pérez-Pé, R.; Cebrián-Pérez, J.A.; Muiño-Blanco, M.T.; Rodríguez-Martínez, H. Membrane receptor mapping in ejaculated ram spermatozoa. Reprod. Domest. Anim. 2015, 2105, 81–82. [Google Scholar]
- Vicente-Carrillo, A.; Álvarez-Rodríguez, M.; Rodríguez-Martínez, H. The CatSper channel modulates boar sperm motility during capacitation. Reprod. Biol. 2017, 17, 69–78. [Google Scholar] [CrossRef]
- Johnson, G.P.; English, A.M.; Cronin, S.; Hoey, D.A.; Meade, K.G.; Fair, S. Genomic identification, expression profiling, and functional characterization of CatSper channels in the bovine. Biol. Reprod. 2017, 97, 302–312. [Google Scholar] [CrossRef]
- Seifert, R.; Flick, M.; Bönigk, W.; Alvarez, L.; Trötschel, C.; Poetsch, A.; Müller, A.; Goodwin, N.; Pelzer, P.; Kashikar, N.D.; et al. The CatSper channel controls chemosensation in sea urchin sperm. EMBO J. 2015, 34, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Loyo-Celis, V.; Orta, G.; Beltrán, C.; Darszon, A. CatSper channels in sea urchin sperm. Cell Calcium. 2021, 99, 102466. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Ke, M.; Zhang, Y.; Yan, Z.; Wu, J. Structure of a mammalian sperm cation channel complex. Nature 2021, 595, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, H.; Wiesehoefer, C.; Shah, N.B.; Reetz, E.; Hwang, J.Y.; Huang, X.; Wang, T.E.; Lishko, P.V.; Davies, K.M.; et al. 3D structure and in situ arrangements of CatSper channel in the sperm flagellum. Nat. Commun. 2022, 13, 3439. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.J.; Shim, S.H.; Everley, R.A.; Gygi, S.P.; Zhuang, X.; Clapham, D.E. Structurally distinct Ca(2+) signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility. Cell 2014, 157, 808–822. [Google Scholar] [CrossRef]
- Quill, T.A.; Sugden, S.A.; Rossi, K.L.; Doolittle, L.K.; Hammer, R.E.; Garbers, D.L. Hyperactivated sperm motility driven by CatSper2 is required for fertilization. Proc. Natl. Acad. Sci. USA 2003, 100, 14869–14874. [Google Scholar] [CrossRef]
- Jin, J.; Jin, N.; Zheng, H.; Ro, S.; Tafolla, D.; Sanders, K.M.; Yan, W. CatSper3 and CatSper4 are essential for sperm hyperactivated motility and male fertility in the mouse. Biol. Reprod. 2007, 77, 37–44. [Google Scholar] [CrossRef]
- Jin, J.L.; O’Doherty, A.M.; Wang, S.; Zheng, H.; Sanders, K.M.; Yan, W. CatSper3 and CatSper4 encode two cation channel-like proteins exclusively expressed in the testis. Biol. Reprod. 2005, 73, 1235–1242. [Google Scholar] [CrossRef]
- Qi, H.; Moran, M.M.; Navarro, B.; Chong, J.A.; Krapivinsky, G.; Krapivinsky, L.; Kirichok, Y.; Ramsey, I.S.; Quill, T.A.; Clapham, D.E. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc. Natl. Acad. Sci. USA 2007, 104, 1219–1223. [Google Scholar] [CrossRef]
- Bhilawadikar, R.; Zaveri, K.; Mukadam, L.; Naik, S.; Kamble, K.; Modi, D.; Hinduja, I. Levels of Tektin 2 and CatSper 2 in normozoospermic and oligoasthenozoospermic men and its association with motility, fertilization rate, embryo quality and pregnancy rate. J. Assist. Reprod. Genet. 2013, 30, 513–523. [Google Scholar] [CrossRef][Green Version]
- Smith, J.F.; Syritsyna, O.; Fellous, M.; Serres, C.; Mannowetz, N.; Kirichok, Y.; Lishko, P.V. Disruption of the principal, progesterone-activated sperm Ca2+ channel in a CatSper2-deficient infertile patient. Proc. Natl. Acad. Sci. USA 2013, 110, 6823–6828. [Google Scholar] [CrossRef]
- Torrezan-Nitao, E.; Brown, S.; Lefievre, L.; Morris, J.; Correia, J.; Harper, C.; Publicover, S. SKF96365 modulates activity of CatSper channels in human sperm. Mol. Hum. Reprod. 2023, 29, gaad015. [Google Scholar] [CrossRef]
- Wang, T.; Young, S.; Krenz, H.; Tüttelmann, F.; Röpke, A.; Krallmann, C.; Kliesch, S.; Zeng, X.H.; Brenker, C.; Strünker, T. The Ca2+ channel CatSper is not activated by cAMP/PKA signaling but directly affected by chemicals used to probe the action of cAMP and PKA. J. Biol. Chem. 2020, 295, 13181–13193. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, H. State of the art in farm animal sperm evaluation. Reprod. Fertil. Dev. 2007, 19, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.A.; Sharif, M.; Wang, H.; Bovin, N.; Miller, D.J. Release of Porcine Sperm from Oviduct Cells Is Stimulated by Progesterone and Requires CatSper. Sci. Rep. 2019, 9, 19546. [Google Scholar] [CrossRef] [PubMed]
- Mirihagalle, S.; Hughes, J.R.; Miller, D.J. Progesterone-Induced Sperm Release from the Oviduct Sperm Reservoir. Cells 2022, 11, 1622. [Google Scholar] [CrossRef] [PubMed]
- Sánchez González, S.R.; Mata Martínez, E.; Torres Juárez, J.A.; Arias, R.J.; De Blas, G.A.; Sánchez Tusie, A.A. Cortisol modulates Ca2+ signalling and acrosome reaction in human sperm. Andrology 2023, 11, 134–142. [Google Scholar] [CrossRef]
- Forero-Forero, A.; López-Ramírez, S.; Felix, R.; Hernández-Sánchez, J.; Tesoro-Cruz, E.; Orozco-Suárez, S.; Murbartián, J.; Soria-Castro, E.; Olivares, A.; Bekker-Méndez, C.; et al. Down Regulation of CatSper1 Expression by Calmodulin Inhibitor (Calmidazolium): Possible Implications for Fertility. Int. J. Mol. Sci. 2022, 23, 8070. [Google Scholar] [CrossRef]
- Yeste, M.; Fernández-Novell, J.M.; Ramió-Lluch, L.; Estrada, E.; Rocha, L.G.; Cebrián-Pérez, J.A.; MuiñoBlanco, T.; Concha, I.I.; Ramírez, A.; Rodríguez-Gil, J.E. Intracellular calcium movements of boar spermatozoa during ‘in vitro’ capacitation and subsequent acrosome exocytosis follow a multiple storage place, extracellular calcium-dependent model. Andrology 2015, 3, 729–747. [Google Scholar] [CrossRef]
- Gualtieri, R.; Boni, R.; Tosti, E.; Zagami, M.; Talevi, R. Intracellular calcium and protein tyrosine phosphorylation during the release of bovine sperm adhering to the fallopian tube epithelium in vitro. Reproduction 2005, 129, 51–60. [Google Scholar] [CrossRef]
- Bruckman, N.G.; Nuñez, S.Y.; Puga Molina, L.D.C.; Buffone, M.G.; Darszon, A.; Cuasnicu, P.S.; Da Ros, V.G. Tyrosine phosphorylation signaling regulates Ca2+ entry by affecting intracellular pH during human sperm capacitation. J. Cell Physiol. 2019, 234, 5276–5288. [Google Scholar] [CrossRef] [PubMed]
- Cooray, A.; Chae, M.R.; Wijerathne, T.D.; Kim, D.G.; Kim, J.; Kim, C.Y.; Lee, S.W.; Lee, K.P. Hexane fraction of Prunus japonicahumbb. Seed extract enhances boar sperm motility via CatSper ion channel. Heliyon 2023, 9, e13616. [Google Scholar] [CrossRef] [PubMed]
- Italiya, J.M.; Patel, M.R.; Golaviya, A.V.; Patel, S.S.; Thakkar, B.K.; Jakhesara, S.J.; Joshi, C.G.; Koringa, P.G. RNA-sequencing attest increased sperm motility in bovine spermatozoa treated with ethanolic extract of Putranjiva roxburghii. 3 Biotech. 2023, 13, 33. [Google Scholar] [CrossRef]
- Baňas, Š.; Benko, F.; Ďuračka, M.; Lukáč, N.; Tvrdá, E. Epicatechin Prevents Cryocapacitation of Bovine Spermatozoa through Antioxidant Activity and Stabilization of Transmembrane Ion Channels. Int. J. Mol. Sci. 2023, 24, 2510. [Google Scholar] [CrossRef]
- Dolatkhah, M.A.; Khezri, S.; Shokoohi, M.; Alihemmati, A. The effect of Fumaria parviflora on the expression of sexual hormones along with their receptors in testicles of adult rats induced by varicocele. Andrologia 2022, 54, e14512. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Emam, M.M.; Ray, M.N.; Ozono, M.; Kogure, K. Heat stress disrupts spermatogenesis via modulation of sperm-specific calcium channels in rats. J. Therm. Biol. 2023, 112, 103465. [Google Scholar] [CrossRef]
- Huang, X.; Miyata, H.; Wang, H.; Mori, G.; Iida-Norita, R.; Ikawa, M.; Percudani, R.; Chung, J.J. A CUG-initiated CATSPERθ functions in the CatSper channel assembly and serves as a checkpoint for flagellar trafficking. bioRxiv 2023. [Google Scholar] [CrossRef]
- Lacalle, E.; Consuegra, C.; Martínez, C.A.; Hidalgo, M.; Dorado, J.; Martínez-Pastor, F.; Álvarez-Rodríguez, M.; Rodríguez-Martínez, H. Bicarbonate-Triggered In Vitro Capacitation of Boar Spermatozoa Conveys an Increased Relative Abundance of the Canonical Transient Receptor Potential Cation (TRPC) Channels 3, 4, 6 and 7 and of CatSper-γ Subunit mRNA Transcripts. Animals 2022, 12, 1012. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, L.; Han, H.; Xiong, B.; Zhong, R.; Jiang, Y.; Liu, L.; Sun, H.; Tan, J.; Cheng, X.; et al. Taxifolin increased semen quality of Duroc boars by improving gut microbes and blood metabolites. Front. Microbiol. 2022, 13, 1020628. [Google Scholar] [CrossRef]
- Castellano, L.E.; Treviño, C.L.; Rodríguez, D.; Serrano, C.J.; Pacheco, J.; Tsutsumi, V.; Felix, R.; Darszon, A. Transient receptor potential (TRPC) channels in human sperm: Expression, cellular localization and involvement in the regulation of flagellar motility. FEBS Lett. 2003, 541, 69–74. [Google Scholar] [CrossRef]
- Beech, D.J. Integration of transient receptor potential canonical channels with lipids. Acta. Physiol. 2012, 204, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Darszon, A.; Nishigaki, T.; Beltran, C.; Treviño, C.L. Calcium channels in the development, maturation, and function of spermatozoa. Physiol. Rev. 2011, 91, 1305–1355. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S.; Dai, X.B.; DeMott, R.P.; Redfern, K.; Mirando, M.A. Movement characteristics of boar sperm obtained from the oviduct or hyperactivated in vitro. J. Androl. 1992, 13, 75–80. [Google Scholar] [PubMed]
- Tienthai, P.; Johannisson, A.; Rodríguez-Martínez, H. Sperm capacitation in the porcine oviduct. Anim. Reprod. Sci. 2004, 80, 131–146. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, H. Aspects of the electrolytic composition of boar epididymal fluid with reference to sperm maturation and storage. Reprod. Domest. Anim. 1991, (Suppl. S1), 13–27. [Google Scholar]
- Ho, H.C.; Suarez, S.S. Characterization of the intracellular calcium store at the base of the sperm flagellum that regulates hyperactivated motility. Biol. Reprod. 2003, 68, 1590–1596. [Google Scholar] [CrossRef]
- Jaldety, Y.; Breitbart, H. ERK1/2 mediates sperm acrosome reaction through elevation of intracellular calcium concentration. Zygote 2015, 23, 652–661. [Google Scholar] [CrossRef]
- Austin, C.R. Observations on the penetration of the sperm in the mammalian egg. Aust. J. Sci. Res. B 1951, 4, 581–596. [Google Scholar] [CrossRef]
- Chang, M.C. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 1951, 168, 697–698. [Google Scholar] [CrossRef]
- Flesch, F.M.; Gadella, B.M. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim. Biophys. Acta. 2000, 1469, 197–235. [Google Scholar] [CrossRef]
- Gadella, B.M.; Harrison, R.A. The capacitating agent bicarbonate induces protein kinase A-dependent changes in phospholipid transbilayer behavior in the sperm plasma membrane. Development 2000, 127, 2407–2420. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Miller, N.G. cAMP-dependent protein kinase control of plasma membrane lipid architecture in boar sperm. Mol. Reprod. Dev. 2000, 55, 220–228. [Google Scholar] [CrossRef]
- Ickowicz, D.; Finkelstein, M.; Breitbart, H. Mechanism of sperm capacitation and the acrosome reaction: Role of protein kinases. Asian. J. Androl. 2012, 14, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R. The movement of golden hamster spermatozoa before and after capacitation. J. Reprod. Fertil. 1970, 23, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, R.E.; Carlson, A.E. Tiny Dancer: EFCAB9 Triggers Sperm Hyperactivation via CatSper. Trends Biochem. Sci. 2019, 44, 823–826. [Google Scholar] [CrossRef]
- Hildebrand, M.S.; Avenarius, M.R.; Fellous, M.; Zhang, Y.; Meyer, N.C.; Auer, J.; Serres, C.; Kahrizi, K.; Najmabadi, H.; Beckmann, J.S.; et al. Genetic male infertility and mutation of CATSPER ion channels. Eur. J. Hum. Genet. 2010, 18, 1178–1184. [Google Scholar] [CrossRef]
- Williams, H.L.; Mansell, S.; Alasmari, W.; Brown, S.G.; Wilson, S.M.; Sutton, K.A.; Miller, M.R.; Lishko, P.V.; Barratt, C.L.; Publicover, S.J.; et al. Specific loss of CatSper function is sufficient to compromise fertilizing capacity of human spermatozoa. Hum. Reprod. 2015, 30, 2737–2746. [Google Scholar] [CrossRef]
- Yang, F.; Gracia Gervasi, M.; Orta, G.; Tourzani, D.A.; De la Vega-Beltrán, J.L.; Ruthel, G.; Darszon, A.; Visconti, P.E.; Wang, P.J. C2CD6 regulates targeting and organization of the CatSper calcium channel complex in sperm flagella. Development 2022, 149, dev199988. [Google Scholar] [CrossRef]
- González-Arto, M.; Vicente-Carrillo, A.; Martínez-Pastor, F.; Fernández-Alegre, E.; Roca, J.; Miró, J.; Rigau, T.; Rodríguez-Gil, J.E.; Pérez-Pé, R.; Muiño-Blanco, T.; et al. Melatonin receptors MT1 and MT2 are expressed in spermatozoa from several seasonal and nonseasonal breeder species. Theriogenology 2016, 86, 1958–1968. [Google Scholar] [CrossRef]
- Zimmerman, K.J.; Crabo, B.G.; Moore, R.; Weisberg, S.; Deibel, F.C.; Graham, E.F. Movements of sodium and potassium into epididymal boar spermatozoa. Biol. Reprod. 1979, 21, 173–179. [Google Scholar] [CrossRef]
- Turner, R.M. Moving to the beat: A review of mammalian sperm motility regulation. Reprod. Fertil. Dev. 2006, 18, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Marquez, B.; Ignotz, G.; Suarez, S.S. Contributions of extracellular and intracellular Ca2+ to regulation of sperm motility: Release of intracellular stores can hyperactivate CatSper1 and CatSper2 null sperm. Dev. Biol. 2007, 303, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Meizel, S. The sperm, a neuron with a tail: “neurona” receptors in mammalian sperm. Biol. Rev. Camb. Philos. Soc. 2004, 79, 713–732. [Google Scholar] [CrossRef]
- Vicente-Carrillo, A.; Ekwall, H.; Álvarez-Rodríguez, M.; Rodríguez-Martínez, H. Membrane Stress During Thawing Elicits Redistribution of Aquaporin 7 But Not of Aquaporin 9 in Boar Spermatozoa. Reprod. Domest. Anim. 2016, 51, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.; Padilla, L.; Lucas, X.; Rodriguez-Martinez, H.; Barranco, I.; Roca, J. Seminal extracellular vesicles and their involvement in male (in)fertility: A systematic review. Int. J. Mol. Sci. 2023, 24, 4818. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, W.M.; Welch, G.R.; Johnson, L.A. Viability and membrane integrity of spermatozoa after dilution and flow cytometric sorting in the presence or absence of seminal plasma. Reprod. Fertil. Dev. 1996, 8, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, H.; Kvist, U.; Saravia, F.; Wallgren, M.; Johannisson, A.; Sanz, L.; Peña, F.J.; Martínez, E.A.; Roca, J.; Vázquez, J.M.; et al. The physiological roles of the boar ejaculate. Soc. Reprod. Fertil. Suppl. 2009, 66, 1–21. [Google Scholar] [CrossRef]
- Estienne, M.J.; Harper, A.F.; Day, J.L. Characteristics of sperm motility in boar semen diluted in different extenders and stored for seven days at 18 degrees C. Reprod. Biol. 2007, 7, 221–231. [Google Scholar]
- Knox, R.V. Artificial insemination in pigs today. Theriogenology 2016, 85, 83–93. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Z.; Gu, X.; Sheng, X.; Xiao, L.; Wang, X. Exosomes: New regulators of reproductive development. Mater Today Bio. 2023, 19, 100608. [Google Scholar] [CrossRef]
- Roca, J.; Rodriguez-Martinez, H.; Padilla, L.; Lucas, X.; Barranco, I. Extracellular vesicles in seminal fluid and its impact on male reproduction. An overview in pet and livestock species. Anim. Reprod. Sci. 2022, 246, 106853. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, H.; Roca, J. Extracellular vesicles in seminal plasma: A safe and relevant tool to improve fertility in livestock? Anim. Reprod. Sci. 2022, 244, 107051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, D.; Kang, H.; Zhou, W.; Chen, H.; Zeng, X. Seminal plasma exosomes evoke calcium signals via the CatSper channel to regulate human sperm function. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, M.; Song, D.; Huang, R.; Chen, C.; Chen, H.; Wang, Q.; Sun, X.; Song, J.; Zhang, J.; et al. Extracellular vesicles of seminal plasma and their derived peptides improve human sperm function via the CatSper-mediated calcium signals. Lancet 2023. prepint. [Google Scholar]
- Barranco, I.; Sanchez, C.; Bucci, C.; Alvarez-Barriento, A.; Rodriguez-Martinez, H.; Antonio Marcilla, A.; Roca, J. The proteome of large or small extracellular vesicles in pig seminal plasma differs, defining sources and biological functions. Mol. Cell. Proteomics. 2022, 22, 100514. [Google Scholar] [CrossRef]
- Padilla, L.; Barranco, I.; Martínez-Hernández, J.; Parra, A.; Parrilla, I.; Pastor, L.M.; Rodriguez-Martinez, H.; Lucas, X.; Roca, J. Extracellular vesicles would be involved in the release and delivery of seminal TGF-β isoforms in pigs. Front. Vet. Sci. 2023, 10, 1102049. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.K.; Rajesh, P.B. Role of tyrosine phosphorylation in sperm capacitation/acrosome reaction. Reprod. Biol. Endocrinol. 2004, 2, 75. [Google Scholar] [CrossRef][Green Version]
- Sun, X.H.; Zhu, Y.Y.; Wang, L.; Liu, H.L.; Ling, Y.; Li, Z.L.; Sun, L.B. The CatSper channel and its roles in male fertility: A systematic review. Reprod. Biol. Endocrinol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Kaminski, T.; Siawrys, G.; Bogacka, I.; Przala, J. The physiological role of beta-endorphin in porcine ovarian follicles. Reprod. Nutr. Dev. 2000, 40, 63–75. [Google Scholar] [CrossRef][Green Version]
- Subirán, N.; Casis, L.; Irazusta, J. Regulation of male fertility by the opioid system. Mol. Med. 2011, 17, 846–853. [Google Scholar] [CrossRef]
- Minoia, P.; Sciorci, R.L. Metabolic control through L calcium channel, PKC and opioid receptors modulation by an association of naloxone and calcium salts. Curr Drug Targets Immune. Endocr. Metabol. Disord. 2001, 1, 131–137. [Google Scholar]
- Law, P.Y.; Wong, Y.H.; Loh, H.H. Molecular mechanisms and regulation of opioid receptor signaling. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 389–430. [Google Scholar] [CrossRef] [PubMed]
- Feigenbaum, J.J.; Howard, S.G. Effects of naloxone on amphetamine induced striatal dopamine release in vivo: A microdialysis study. Life. Sci. 1997, 60, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Carrillo, A.; Álvarez-Rodríguez, M.; Rodríguez-Martínez, H. The mu (μ) and delta (δ) opioid receptors modulate boar sperm motility. Mol. Reprod. Dev. 2016, 83, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Long, J.E.; Lee, M.S.; Blithe, D.L. Male Contraceptive Development: Update on Novel Hormonal and Nonhormonal Methods. Clin. Chem. 2019, 65, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Mariani, N.A.P.; Silva, J.V.; Fardilha, M.; Silva, E.J.R. Advances in non-hormonal male contraception targeting sperm motility. Hum. Reprod. Update 2023, dmad008. [Google Scholar] [CrossRef] [PubMed]
- Luque, G.M.; Schiavi-Ehrenhaus, L.J.; Jabloñski, M.; Balestrini, P.A.; Novero, A.G.; Torres, N.I.; Osycka-Salut, C.E.; Darszon, A.; Krapf, D.; Buffone, M.G. High-throughput screening method for discovering CatSper inhibitors using membrane depolarization caused by external calcium chelation and fluorescent cell barcoding. Front. Cell. Dev. Biol. 2023, 11, 1010306. [Google Scholar] [CrossRef]
- Brown, S.G.; Publicover, S.J.; Barratt, C.L.R.; da Silva, S.J.M. Human sperm ion channel (dys)function: Implications for fertilization. Hum. Reprod. Update 2019, 25, 758–776. [Google Scholar] [CrossRef]
- Rennhack, A.; Schiffer, C.; Brenker, C.; Fridman, D.; Nitao, E.T.; Cheng, Y.M.; Tamburrino, L.; Balbach, M.; Stölting, G.; Berger, T.K.; et al. A novel cross-species inhibitor to study the function of CatSper Ca2+ channels in sperm. Br. J. Pharmacol. 2018, 175, 3144–3161. [Google Scholar] [CrossRef]
- Carlson, E.J.; Francis, R.; Liu, Y.; Li, P.; Lyon, M.; Santi, C.M.; Hook, D.J.; Hawkinson, J.E.; Georg, G.I. Discovery and Characterization of Multiple Classes of Human CatSper Blockers. Chem. Med. Chem. 2022, 17, e202000499. [Google Scholar] [CrossRef]
- Cai, X.; Clapham, D.E. Evolutionary genomics reveals lineage-specific gene loss and rapid evolution of a sperm-specific ion channel complex: CatSpers and CatSperbeta. PLoS ONE 2008, 3, e3569. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente-Carrillo, A.; Álvarez-Rodríguez, M.; Rodriguez-Martinez, H. The Cation/Calcium Channel of Sperm (CatSper): A Common Role Played Despite Inter-Species Variation? Int. J. Mol. Sci. 2023, 24, 13750. https://doi.org/10.3390/ijms241813750
Vicente-Carrillo A, Álvarez-Rodríguez M, Rodriguez-Martinez H. The Cation/Calcium Channel of Sperm (CatSper): A Common Role Played Despite Inter-Species Variation? International Journal of Molecular Sciences. 2023; 24(18):13750. https://doi.org/10.3390/ijms241813750
Chicago/Turabian StyleVicente-Carrillo, Alejandro, Manuel Álvarez-Rodríguez, and Heriberto Rodriguez-Martinez. 2023. "The Cation/Calcium Channel of Sperm (CatSper): A Common Role Played Despite Inter-Species Variation?" International Journal of Molecular Sciences 24, no. 18: 13750. https://doi.org/10.3390/ijms241813750
APA StyleVicente-Carrillo, A., Álvarez-Rodríguez, M., & Rodriguez-Martinez, H. (2023). The Cation/Calcium Channel of Sperm (CatSper): A Common Role Played Despite Inter-Species Variation? International Journal of Molecular Sciences, 24(18), 13750. https://doi.org/10.3390/ijms241813750









