SLO3: A Conserved Regulator of Sperm Membrane Potential
Abstract
1. Introduction
2. Potassium Channels in Sperm
3. Structure and Gating of the SLO3 Pore-Forming Subunits

4. Structure and Function of the SLO3 β and γ Subunits
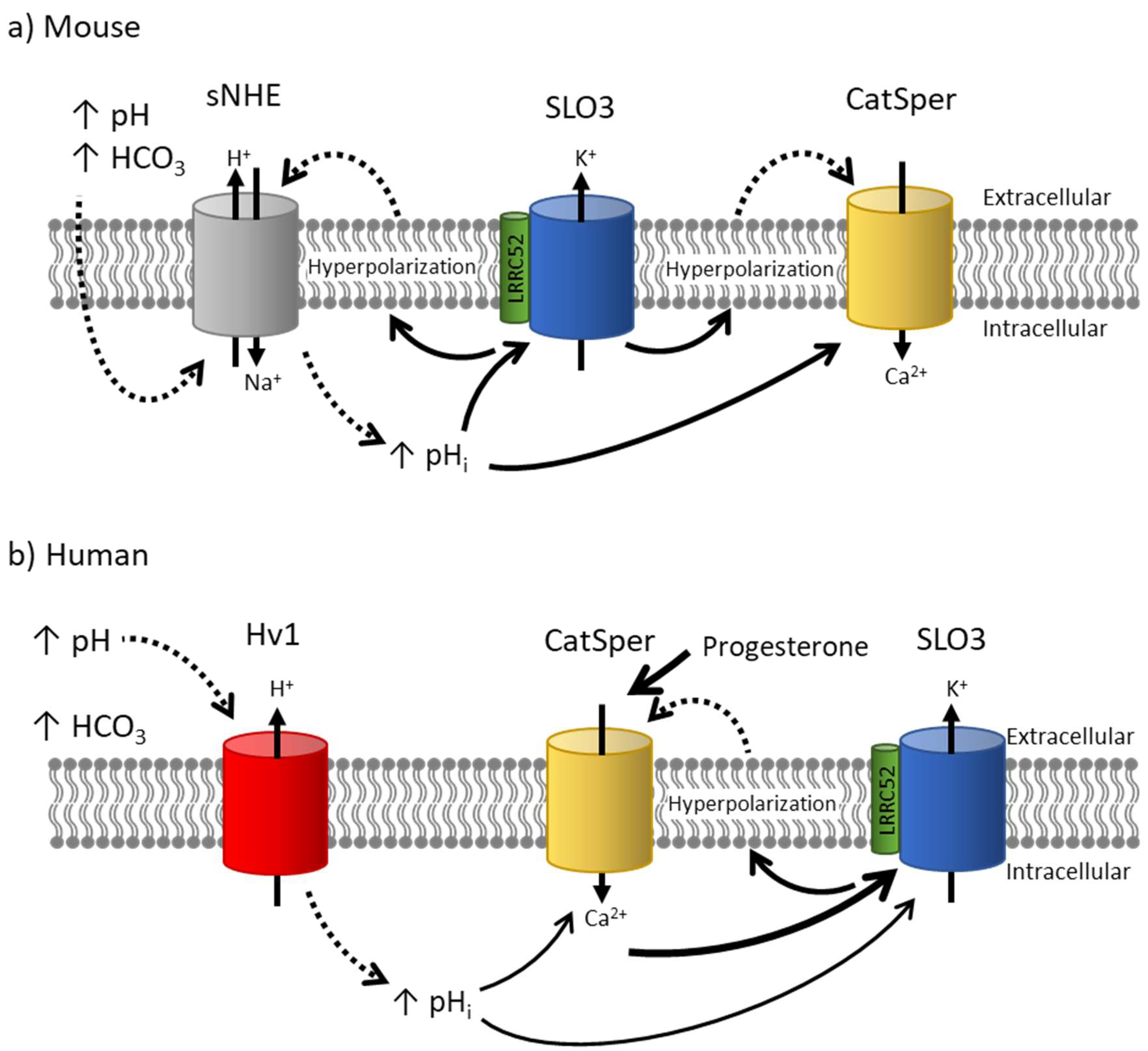
5. Challenges in Determining Whether SLO3 Is Responsible for Human Sperm Hyperpolarization
5.1. Challenge 1: Differences in pH and Ca2+ Regulation
5.2. Challenge 2: Differences in Voltage Sensitivity
5.3. Challenge 3: Differences in Functional Relationship with CatSper
5.4. Challenge 4: Differences in Pharmacology
6. Newly Discovered Variants and Inhibitors Confirm That SLO3 Is Responsible for Human Sperm Hyperpolarization
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, M.C. Fertilizing Capacity of Spermatozoa Deposited into the Fallopian Tubes. Nature 1951, 168, 697–698. [Google Scholar] [CrossRef]
- Austin, C. Observations on the Penetration of the Sperm into the Mammalian Egg. Aust. J. Biol. Sci. 1951, 4, 581. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Moore, G.D.; Bailey, J.L.; Leclerc, P.; Connors, S.A.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of Mouse Spermatozoa. II. Protein Tyrosine Phosphorylation and Capacitation Are Regulated by a CAMP-Dependent Pathway. Development 1995, 121, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Oberdorf, J.A.; Florman, H.M. PH Regulation in Mouse Sperm: Identification of Na(+)-, Cl(-)-, and HCO3(-)-Dependent and Arylaminobenzoate-Dependent Regulatory Mechanisms and Characterization of Their Roles in Sperm Capacitation. Dev. Biol. 1996, 173, 510–520. [Google Scholar] [CrossRef]
- Vredenburgh-Wilberg, W.L.; Parrish, J.J. Intracellular PH of Bovine Sperm Increases during Capacitation. Mol. Reprod. Dev. 1995, 40, 490–502. [Google Scholar] [CrossRef]
- Breitbart, H. Signaling Pathways in Sperm Capacitation and Acrosome Reaction. Cell. Mol. Biol. 2003, 49, 321–327. [Google Scholar]
- Ferreira, J.J.; Lybaert, P.; Puga-Molina, L.C.; Santi, C.M. Conserved Mechanism of Bicarbonate-Induced Sensitization of CatSper Channels in Human and Mouse Sperm. Front. Cell Dev. Biol. 2021, 9, 2614. [Google Scholar] [CrossRef]
- Chávez, J.C.; de la Vega-Beltrán, J.L.; Escoffier, J.; Visconti, P.E.; Treviño, C.L.; Darszon, A.; Salkoff, L.; Santi, C.M. Ion Permeabilities in Mouse Sperm Reveal an External Trigger for SLO3-Dependent Hyperpolarization. PLoS ONE 2013, 8, e60578. [Google Scholar] [CrossRef] [PubMed]
- DasGupta, S.; Mills, C.L.; Fraser, L.R. Ca2+-Related Changes in the Capacitation State of Human Spermatozoa Assessed by a Chlortetracycline Fluorescence Assay. J. Reprod. Fertil. 1993, 99, 135–143. [Google Scholar] [CrossRef]
- Baldi, E.; Casano, R.; Falsetti, C.; Krausz, C.; Maggi, M.; Forti, G. Intracellular Calcium Accumulation and Responsiveness to Progesterone in Capacitating Human Spermatozoa. J. Androl. 1991, 12, 323–330. [Google Scholar]
- Chávez, J.C.; Ferreira, J.J.; Butler, A.; De La Vega Beltrán, J.L.; Treviño, C.L.; Darszon, A.; Salkoff, L.; Santi, C.M. SLO3 K+ Channels Control Calcium Entry through CATSPER Channels in Sperm. J. Biol. Chem. 2014, 289, 32266–32275. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.J.; Cassina, A.; Irigoyen, P.; Ford, M.; Pietroroia, S.; Peramsetty, N.; Radi, R.; Santi, C.M.; Sapiro, R. Increased Mitochondrial Activity upon CatSper Channel Activation Is Required for Mouse Sperm Capacitation. Redox Biol. 2021, 48, 102176. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Clark, E.N.; Florman, H.M. Sperm Membrane Potential: Hyperpolarization during Capacitation Regulates Zona Pellucida-Dependent Acrosomal Secretion. Dev. Biol. 1995, 171, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Santi, C.M.; Orta, G.; Salkoff, L.; Visconti, P.E.; Darszon, A.; Treviño, C.L. K+ and Cl− Channels and Transporters in Sperm Function. Curr. Top. Dev. Biol. 2013, 102, 385–421. [Google Scholar] [CrossRef] [PubMed]
- Arnoult, C.; Kazam, I.G.; Visconti, P.E.; Kopf, G.S.; Villaz, M.; Florman, H.M. Control of the Low Voltage-Activated Calcium Channel of Mouse Sperm by Egg ZP3 and by Membrane Hyperpolarization during Capacitation. Proc. Natl. Acad. Sci. USA 1999, 96, 6757–6762. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Garay, C.; de la Vega-Beltrán, J.L.; Delgado, R.; Labarca, P.; Felix, R.; Darszon, A. Inwardly Rectifying K+ Channels in Spermatogenic Cells: Functional Expression and Implication in Sperm Capacitation. Dev. Biol. 2001, 234, 261–274. [Google Scholar] [CrossRef]
- Gunderson, S.J.; Puga Molina, L.C.; Spies, N.; Balestrini, P.A.; Buffone, M.G.; Jungheim, E.S.; Riley, J.; Santi, C.M. Machine-Learning Algorithm Incorporating Capacitated Sperm Intracellular PH Predicts Conventional in Vitro Fertilization Success in Normospermic Patients. Fertil. Steril. 2021, 115, 930–939. [Google Scholar] [CrossRef]
- Mortimer, S.T.; Swan, M.A.; Mortimer, D. Effect of Seminal Plasma on Capacitation and Hyperactivation in Human Spermatozoa. Human. Reprod. 1998, 13, 2139–2146. [Google Scholar] [CrossRef]
- Ishijima, S. Dynamics of Flagellar Force Generated by a Hyperactivated Spermatozoon. Reproduction 2011, 142, 409–415. [Google Scholar] [CrossRef]
- Demott, R.P.; Suarez, S.S. Hyperactivated Sperm Progress in the Mouse Oviduct. Biol. Reprod. 1992, 46, 779–785. [Google Scholar] [CrossRef]
- Suarez, S.S.; Dai, X.B.; DeMott, R.P.; Redfern, K.; Mirando, M.A. Movement Characteristics of Boar Sperm Obtained from the Oviduct or Hyperactivated in vitro. J. Androl. 1992, 13, 75–80. [Google Scholar]
- Yanagimachi, R. Fertility of Mammalian Spermatozoa: Its Development and Relativity. Zygote 1994, 2, 371–372. [Google Scholar] [CrossRef]
- Buffone, M.G.; Rodriguez-Miranda, E.; Storey, B.T.; Gerton, G.L. Acrosomal Exocytosis of Mouse Sperm Progresses in a Consistent Direction in Response to Zona Pellucida. J. Cell Physiol. 2009, 220, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, H.; Rubinstein, S.; Lax, Y. Regulatory Mechanisms in Acrosomal Exocytosis. Rev. Reprod. 1997, 2, 165–174. [Google Scholar] [CrossRef]
- Inoue, N.; Ikawa, M.; Okabe, M. The Mechanism of Sperm–Egg Interaction and the Involvement of IZUMO1 in Fusion. Asian J. Androl. 2011, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- López-González, I.; Torres-Rodríguez, P.; Sánchez-Carranza, O.; Solís-López, A.; Santi, C.M.; Darszon, A.; Treviño, C.L. Membrane Hyperpolarization during Human Sperm Capacitation. Mol. Hum. Reprod. 2014, 20, 619–629. [Google Scholar] [CrossRef]
- De La Vega-Beltran, J.L.; Sánchez-Cárdenas, C.; Krapf, D.; Hernandez-González, E.O.; Wertheimer, E.; Treviño, C.L.; Visconti, P.E.; Darszon, A. Mouse Sperm Membrane Potential Hyperpolarization Is Necessary and Sufficient to Prepare Sperm for the Acrosome Reaction. J. Biol. Chem. 2012, 287, 44384–44393. [Google Scholar] [CrossRef] [PubMed]
- González-Martínez, M.; Darszon, A. A Fast Transient Hyperpolarization Occurs during the Sea Urchin Sperm Acrosome Reaction Induced by Egg Jelly. FEBS Lett. 1987, 218, 247–250. [Google Scholar] [CrossRef]
- Linares-Hernández, L.; Guzmán-Grenfell, A.M.; Hicks-Gomez, J.J.; González-Martínez, M.T. Voltage-Dependent Calcium Influx in Human Sperm Assessed by Simultaneous Optical Detection of Intracellular Calcium and Membrane Potential. Biochim. Biophys. Acta Biomembr. 1998, 1372, 1–12. [Google Scholar] [CrossRef]
- Patrat, C.; Serres, C.; Jouannet, P. Progesterone Induces Hyperpolarization after a Transient Depolarization Phase in Human Spermatozoa. Biol. Reprod. 2002, 66, 1775–1780. [Google Scholar] [CrossRef]
- Brown, S.G.; Publicover, S.J.; Mansell, S.A.; Lishko, P.V.; Williams, H.L.; Ramalingam, M.; Wilson, S.M.; Barratt, C.L.R.; Sutton, K.A.; Da Silva, S.M. Depolarization of Sperm Membrane Potential Is a Common Feature of Men with Subfertility and Is Associated with Low Fertilization Rate at IVF. Human. Reprod. 2016, 31, 1147–1157. [Google Scholar] [CrossRef]
- Calzada, L.; Tellez, J. Defective Function of Membrane Potential (Ψ) on Sperm of Infertile Men. Arch. Androl. 1997, 38, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Brukman, N.G.; Nuñez, S.Y.; Puga Molina, L.d.C.; Buffone, M.G.; Darszon, A.; Cuasnicu, P.S.; Da Ros, V.G. Tyrosine Phosphorylation Signaling Regulates Ca2+ Entry by Affecting Intracellular PH during Human Sperm Capacitation. J. Cell Physiol. 2019, 234, 5276–5288. [Google Scholar] [CrossRef] [PubMed]
- Molina, L.C.P.; Gunderson, S.; Riley, J.; Lybaert, P.; Borrego-Alvarez, A.; Jungheim, E.S.; Santi, C.M. Membrane Potential Determined by Flow Cytometry Predicts Fertilizing Ability of Human Sperm. Front. Cell Dev. Biol. 2020, 7, 387. [Google Scholar] [CrossRef]
- Baro Graf, C.; Ritagliati, C.; Torres-Monserrat, V.; Stival, C.; Carizza, C.; Buffone, M.G.; Krapf, D. Membrane Potential Assessment by Fluorimetry as a Predictor Tool of Human Sperm Fertilizing Capacity. Front. Cell Dev. Biol. 2020, 7, 383. [Google Scholar] [CrossRef]
- Schreiber, M.; Wei, A.; Yuan, A.; Gaut, J.; Saito, M.; Salkoff, L. Slo3, a Novel PH-Sensitive K+ Channel from Mammalian Spermatocytes. J. Biol. Chem. 1998, 273, 3509–3516. [Google Scholar] [CrossRef]
- Navarro, B.; Kirichok, Y.; Clapham, D.E. KSper, a PH-Sensitive K+ Current That Controls Sperm Membrane Potential. Proc. Natl. Acad. Sci. USA 2007, 104, 7688–7692. [Google Scholar] [CrossRef]
- Santi, C.M.; Martínez-López, P.; de la Vega-Beltrán, J.L.; Butler, A.; Alisio, A.; Darszon, A.; Salkoff, L. The SLO3 Sperm-Specific Potassium Channel Plays a Vital Role in Male Fertility. FEBS Lett. 2010, 584, 1041–1046. [Google Scholar] [CrossRef]
- Zeng, X.H.; Yang, C.; Kim, S.T.; Lingle, C.J.; Xia, X.M. Deletion of the Slo3 Gene Abolishes Alkalization-Activated K+ Current in Mouse Spermatozoa. Proc. Natl. Acad. Sci. USA 2011, 108, 5879–5884. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.H.; Navarro, B.; Xia, X.M.; Clapham, D.E.; Lingle, C.J. Simultaneous Knockout of Slo3 and CatSper1 Abolishes All Alkalization- and Voltage-Activated Current in Mouse Spermatozoa. J. Gen. Physiol. 2013, 142, 305–313. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 18 July 2022).
- Macqueen, D.J.; Johnston, I.A. A Well-Constrained Estimate for the Timing of the Salmonid Whole Genome Duplication Reveals Major Decoupling from Species Diversification. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132881. [Google Scholar] [CrossRef]
- Braasch, I.; Gehrke, A.R.; Smith, J.J.; Kawasaki, K.; Manousaki, T.; Pasquier, J.; Amores, A.; Desvignes, T.; Batzel, P.; Catchen, J.; et al. The Spotted Gar Genome Illuminates Vertebrate Evolution and Facilitates Human-Teleost Comparisons. Nat. Genet. 2016, 48, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Vicens, A.; Andrade-López, K.; Cortez, D.; Gutiérrez, R.M.; Treviño, C.L. Premammalian Origin of the Sperm-Specific Slo3 Channel. FEBS Open Bio 2017, 7, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Ferreira, J.J.; Dzikunu, V.; Butler, A.; Lybaert, P.; Yuan, P.; Magleby, K.L.; Salkoff, L.; Santi, C.M. A Genetic Variant of the Sperm-Specific SLO3 K+ Channel Has Altered PH and Ca2+ Sensitivities. J. Biol. Chem. 2017, 292, 8978–8987. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A Multiple Sequence Alignment Editor and Analysis Workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Troshin, P.V.; Procter, J.B.; Sherstnev, A.; Barton, D.L.; Madeira, F.; Barton, G.J. JABAWS 2.2 Distributed Web Services for Bioinformatics: Protein Disorder, Conservation and RNA Secondary Structure. Bioinformatics 2018, 34, 1939–1940. [Google Scholar] [CrossRef] [PubMed]
- Troshin, P.V.; Procter, J.B.; Barton, G.J. Java Bioinformatics Analysis Web Services for Multiple Sequence Alignment-JABAWS:MSA. Bioinformatics 2011, 27, 2001–2002. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Joiner, W.J.; Wu, M.; Yang, Y.; Sigworth, F.J.; Kaczmarek, L.K. Slick (Slo2.1), a Rapidly-Gating Sodium-Activated Potassium Channel Inhibited by ATP. J. Neurosci. 2003, 23, 11681–11691. [Google Scholar] [CrossRef]
- Yuan, A.; Santi, C.M.; Wei, A.; Wang, Z.W.; Pollak, K.; Nonet, M.; Kaczmarek, L.; Crowder, C.M.; Salkoff, L. The Sodium-Activated Potassium Channel Is Encoded by a Member of the Slo Gene Family. Neuron 2003, 37, 765–773. [Google Scholar] [CrossRef]
- Jiang, Y.; Pico, A.; Cadene, M.; Chait, B.T.; MacKinnon, R. Structure of the RCK Domain from the E. Coli K+ Channel and Demonstration of Its Presence in the Human BK Channel. Neuron 2001, 29, 593–601. [Google Scholar] [CrossRef]
- Peng, Y.; Leonetti, M.D.; Pico, A.R.; Hsiung, Y.; MacKinnon, R. Structure of the Human BK Channel Ca2+-Activation Apparatus at 3.0 Å Resolution. Science 2010, 329, 182–186. [Google Scholar] [CrossRef]
- Marty, A. Ca-Dependent K Channels with Large Unitary Conductance in Chromaffin Cell Membranes. Nature 1981, 291, 497–500. [Google Scholar] [CrossRef]
- Pallotta, B.S.; Magleby, K.L.; Barrett, J.N. Single Channel Recordings of Ca2+-Activated K+ Currents in Rat Muscle Cell Culture. Nature 1981, 293, 471–474. [Google Scholar] [CrossRef]
- Latorre, R.; Vergara, C.; Hidalgo, C. Reconstitution in Planar Lipid Bilayers of a Ca2+-Dependent K+ Channel from Transverse Tubule Membranes Isolated from Rabbit Skeletal Muscle. Proc. Natl. Acad. Sci. USA 1982, 79, 805–809. [Google Scholar] [CrossRef]
- Schreiber, M.; Yuan, A.; Salkoff, L. Transplantable Sites Confer Calcium Sensitivity to BK Channels. Nat. Neurosci. 1999, 2, 416–421. [Google Scholar] [CrossRef]
- Xia, X.M.; Zeng, X.; Lingle, C.J. Multiple Regulatory Sites in Large-Conductance Calcium-Activated Potassium Channels. Nature 2002, 418, 880–884. [Google Scholar] [CrossRef]
- Geng, Y.; Deng, Z.; Zhang, G.; Budelli, G.; Butler, A.; Yuan, P.; Cui, J.; Salkoff, L.; Magleby, K.L. Coupling of Ca2+ and Voltage Activation in BK Channels through the AB Helix/Voltage Sensor Interface. Proc. Natl. Acad. Sci. USA 2020, 117, 14512–14521. [Google Scholar] [CrossRef]
- Schreiber, M.; Salkoff, L. A Novel Calcium-Sensing Domain in the BK Channel. Biophys. J. 1997, 73, 1355–1363. [Google Scholar] [CrossRef]
- Santi, C.M.; Ferreira, G.; Yang, B.; Gazula, V.-R.; Butler, A.; Wei, A.; Kaczmarek, L.K.; Salkoff, L. Opposite Regulation of Slick and Slack K Channels by Neuromodulators. J. Neurosci. 2006, 26, 5059–5068. [Google Scholar] [CrossRef]
- Li, P.; Halabi, C.M.; Stewart, R.; Butler, A.; Brown, B.; Xia, X.; Santi, C.; England, S.; Ferreira, J.; Mecham, R.P.; et al. Sodium-Activated Potassium Channels Moderate Excitability in Vascular Smooth Muscle. J. Physiol. 2019, 597, 5093–5108. [Google Scholar] [CrossRef]
- Ferreira, J.J.; Butler, A.; Stewart, R.; Gonzalez-Cota, A.L.; Lybaert, P.; Amazu, C.; Reinl, E.L.; Wakle-Prabagaran, M.; Salkoff, L.; England, S.K.; et al. Oxytocin Can Regulate Myometrial Smooth Muscle Excitability by Inhibiting the Na+-Activated K+ Channel, Slo2.1. J. Physiol. 2019, 597, 137–149. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Gan, L.; Kaczmarek, L.K. Localization of the Slack Potassium Channel in the Rat Central Nervous System. J. Comp. Neurol. 2002, 454, 241–254. [Google Scholar] [CrossRef]
- Hou, S.; Xu, R.; Heinemann, S.H.; Hoshi, T. Reciprocal Regulation of the Ca2+ and H+ Sensitivity in the SLO1 BK Channel Conferred by the RCK1 Domain. Nat. Struct. Mol. Biol. 2008, 15, 403–410. [Google Scholar] [CrossRef]
- Brenker, C.; Zhou, Y.; Muller, A.; Echeverry, F.A.; Trotschel, C.; Poetsch, A.; Xia, X.M.; Bonigk, W.; Lingle, C.J.; Kaupp, U.B.; et al. The Ca2+-Activated K+ Current of Human Sperm Is Mediated by Slo3. Elife 2014, 2014, e01438. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, X.; Lingle, C.J. Slo3 K+ Channels: Voltage and PH Dependence of Macroscopic Currents. J. Gen. Physiol. 2006, 128, 317–336. [Google Scholar] [CrossRef]
- Leonetti, M.D.; Yuan, P.; Hsiung, Y.; MacKinnon, R. Functional and Structural Analysis of the Human SLO3 PH- and Voltage-Gated K+ Channel. Proc. Natl. Acad. Sci. USA 2012, 109, 19274–19279. [Google Scholar] [CrossRef]
- Hite, R.K.; Tao, X.; MacKinnon, R. Structural Basis for Gating the High-Conductance Ca2+-Activated K+ Channel. Nature 2017, 541, 52–57. [Google Scholar] [CrossRef]
- Tao, X.; Hite, R.K.; MacKinnon, R. Cryo-EM Structure of the Open High-Conductance Ca2+-Activated K+ Channel. Nature 2017, 541, 46–51. [Google Scholar] [CrossRef]
- Tao, X.; Mackinnon, R. Molecular Structures of the Human Slo1 K+ Channel in Complex with B4. eLife 2019, 8, e51409. [Google Scholar] [CrossRef]
- Liu, G.; Niu, X.; Wu, R.S.; Chudasama, N.; Yao, Y.; Jin, X.; Weinberg, R.; Zakharov, S.I.; Motoike, H.; Marx, S.O.; et al. Location of Modulatory β Subunits in BK Potassium Channels. J. Gen. Physiol. 2010, 135, 449–459. [Google Scholar] [CrossRef]
- Chen, G.; Li, Q.; Yan, J. The Leucine-Rich Repeat Domains of BK Channel Auxiliary γ Subunits Regulate Their Expression, Trafficking, and Channel-Modulation Functions. J. Biol. Chem. 2022, 298, 101664. [Google Scholar] [CrossRef]
- Gonzalez-Perez, V.; Xia, X.-M.; Lingle, C.J. Functional Regulation of BK Potassium Channels by Γ1 Auxiliary Subunits. Proc. Natl. Acad. Sci. USA 2014, 111, 4868–4873. [Google Scholar] [CrossRef]
- Behrens, R.; Nolting, A.; Reimann, F.; Schwarz, M.; Waldscḧtz, R.; Pongs, O. HKCNMB3 and HKCNMB4, Cloning and Characterization of Two Members of the Large-Conductance Calcium-Activated Potassium Channel β Subunit Family. FEBS Lett. 2000, 474, 99–106. [Google Scholar] [CrossRef]
- Brenner, R.; Jegla, T.J.; Wickenden, A.; Liu, Y.; Aldrich, R.W. Cloning and Functional Characterization of Novel Large Conductance Calcium-Activated Potassium Channel β Subunits, HKCNMB3 and HKCNMB4. J. Biol. Chem. 2000, 275, 6453–6461. [Google Scholar] [CrossRef]
- Uebele, V.N.; Lagrutta, A.; Wade, T.; Figueroa, D.J.; Liu, Y.; McKenna, E.; Austin, C.P.; Bennett, P.B.; Swanson, R. Cloning and Functional Expression of Two Families of β-Subunits of the Large Conductance Calcium-Activated K+ Channel. J. Biol. Chem. 2000, 275, 23211–23218. [Google Scholar] [CrossRef]
- Yan, J.; Aldrich, R.W. LRRC26 Auxiliary Protein Allows BK Channel Activation at Resting Voltage without Calcium. Nature 2010, 466, 513–516. [Google Scholar] [CrossRef]
- Yan, J.; Aldrich, R.W. BK Potassium Channel Modulation by Leucine-Rich Repeat-Containing Proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 7917–7922. [Google Scholar] [CrossRef]
- Dolan, J.; Walshe, K.; Alsbury, S.; Hokamp, K.; O’Keeffe, S.; Okafuji, T.; Miller, S.F.C.; Tear, G.; Mitchell, K.J. The Extracellular Leucine-Rich Repeat Superfamily; a Comparative Survey and Analysis of Evolutionary Relationships and Expression Patterns. BMC Genom. 2007, 8, 1–24. [Google Scholar] [CrossRef]
- Yang, C.; Zeng, X.-H.; Zhou, Y.; Xia, X.-M.; Lingle, C.J. LRRC52 (Leucine-Rich-Repeat-Containing Protein 52), a Testis-Specific Auxiliary Subunit of the Alkalization-Activated Slo3 Channel. Proc. Natl. Acad. Sci. USA 2011, 108, 19419–19424. [Google Scholar] [CrossRef]
- Wang, G.M.; Zhong, Z.G.; Du, X.R.; Zhang, F.F.; Guo, Q.; Liu, Y.; Tang, Q.Y.; Zhang, Z. Cloning and Characterization of the Rat Slo3 (KCa5.1) Channel: From Biophysics to Pharmacology. Br. J. Pharmacol. 2020, 177, 3552–3567. [Google Scholar] [CrossRef]
- Zeng, X.-H.; Yang, C.; Xia, X.-M.; Liu, M.; Lingle, C.J. SLO3 Auxiliary Subunit LRRC52 Controls Gating of Sperm KSPER Currents and Is Critical for Normal Fertility. Proc. Natl. Acad. Sci. USA 2015, 112, 2599–2604. [Google Scholar] [CrossRef]
- Sánchez-Carranza, O.; Torres-Rodríguez, P.; Darszon, A.; Treviño, C.L.; López-González, I. Pharmacology of HSlo3 Channels and Their Contribution in the Capacitation-Associated Hyperpolarization of Human Sperm. Biochem. Biophys. Res. Commun. 2015, 466, 554–559. [Google Scholar] [CrossRef]
- Jiang, Z.; Wallner, M.; Meera, P.; Toro, L. Human and Rodent MaxiK Channel β-Subunit Genes: Cloning and Characterization. Genomics 1999, 55, 57–67. [Google Scholar] [CrossRef]
- Yang, C.-T.; Zeng, X.-H.; Xia, X.-M.; Lingle, C.J. Interactions between β Subunits of the KCNMB Family and Slo3: Β4 Selectively Modulates Slo3 Expression and Function. PLoS ONE 2009, 4, e6135. [Google Scholar] [CrossRef]
- Qian, X.; Nimigean, C.M.; Niu, X.; Moss, B.L.; Magleby, K.L. Slo1 Tail Domains, but Not the Ca2+ Bowl, Are Required for the Β1 Subunit to Increase the Apparent Ca2+ Sensitivity of BK Channels. J. Gen. Physiol. 2002, 120, 829–843. [Google Scholar] [CrossRef]
- Mannowetz, N.; Naidoo, N.M.; Choo, S.A.S.; Smith, J.F.; Lishko, P.V. Slo1 Is the Principal Potassium Channel of Human Spermatozoa. eLife 2013, 2013, e01009. [Google Scholar] [CrossRef]
- Santi, C.M.; Butler, A.; Kuhn, J.; Wei, A.; Salkoff, L. Bovine and Mouse SLO3 K+ Channels. J. Biol. Chem. 2009, 284, 21589–21598. [Google Scholar] [CrossRef]
- Wang, T.; Young, S.; Krenz, H.; Tüttelmann, F.; Röpke, A.; Krallmann, C.; Kliesch, S.; Zeng, X.H.; Brenker, C.; Strünker, T. The Ca2+ Channel CatSper Is Not Activated by CAMP/PKA Signaling but Directly Affected by Chemicals Used to Probe the Action of CAMP and PKA. J. Biol. Chem. 2020, 295, 13181. [Google Scholar] [CrossRef]
- Lyon, M.; Li, P.; Ferreira, J.J.; Lazarenko, R.M.; Kharade, S.V.; Kramer, M.; McClenahan, S.J.; Days, E.; Bauer, J.A.; Spitznagel, B.D.; et al. A Selective Inhibitor of the Sperm-Specific Potassium Channel SLO3 Impairs Human Sperm Function. Proc. Natl. Acad. Sci. USA 2023, 120, e2212338120. [Google Scholar] [CrossRef]
- Ren, D.; Navarro, B.; Perez, G.; Jackson, A.C.; Hsu, S.; Shi, Q.; Tilly, J.L.; Clapham, D.E. A Sperm Ion Channel Required for Sperm Motility and Male Fertility. Nature 2001, 413, 603–609. [Google Scholar] [CrossRef]
- Kirichok, Y.; Navarro, B.; Clapham, D.E. Whole-Cell Patch-Clamp Measurements of Spermatozoa Reveal an Alkaline-Activated Ca2+ Channel. Nature 2006, 439, 737–740. [Google Scholar] [CrossRef]
- Carlson, A.E.; Westenbroek, R.E.; Quill, T.; Ren, D.; Clapham, D.E.; Hille, B.; Garbers, D.L.; Babcock, D.F. CatSper1 Required for Evoked Ca2+ Entry and Control of Flagellar Function in Sperm. Proc. Natl. Acad. Sci. USA 2003, 100, 14864–14868. [Google Scholar] [CrossRef]
- Lv, M.; Liu, C.; Ma, C.; Yu, H.; Shao, Z.; Gao, Y.; Liu, Y.; Wu, H.; Tang, D.; Tan, Q.; et al. Homozygous Mutation in SLO3 Leads to Severe Asthenoteratozoospermia Due to Acrosome Hypoplasia and Mitochondrial Sheath Malformations. Reprod. Biol. Endocrinol. 2022, 20, 1–15. [Google Scholar] [CrossRef]
- Liu, R.; Yan, Z.; Fan, Y.; Qu, R.; Chen, B.; Li, B.; Wu, L.; Wu, H.; Mu, J.; Zhao, L.; et al. Bi-Allelic Variants in KCNU1 Cause Impaired Acrosome Reactions and Male Infertility. Hum. Reprod. 2022, 37, 1394–1405. [Google Scholar] [CrossRef]
- Chávez, J.C.; Darszon, A.; Treviño, C.L.; Nishigaki, T. Quantitative Intracellular PH Determinations in Single Live Mammalian Spermatozoa Using the Ratiometric Dye SNARF-5F. Front. Cell Dev. Biol. 2020, 7, 366. [Google Scholar] [CrossRef]
- Kaupp, U.B.; Strünker, T. Signaling in Sperm: More Different than Similar. Trends Cell Biol. 2017, 27, 101–109. [Google Scholar] [CrossRef]
- Miller, M.R.; Mansell, S.A.; Meyers, S.A.; Lishko, P.V. Flagellar Ion Channels of Sperm: Similarities and Differences between Species. Cell Calcium 2015, 58, 105–113. [Google Scholar] [CrossRef]
- Balestrini, P.A.; Sanchez-Cardenas, C.; Luque, G.M.; Baro Graf, C.; Sierra, J.M.; Hernández-Cruz, A.; Visconti, P.E.; Krapf, D.; Darszon, A.; Buffone, M.G. Membrane Hyperpolarization Abolishes Calcium Oscillations That Prevent Induced Acrosomal Exocytosis in Human Sperm. FASEB J. 2021, 35, e21478. [Google Scholar] [CrossRef]
- Torrezan-Nitao, E.; Brown, S.G.; Mata-Martínez, E.; Trevi No, C.L.; Barratt, C.; Publicover, S. [Ca2+]i Oscillations in Human Sperm Are Triggered in the Flagellum by Membrane Potential Sensitive Activity of CatSper. Hum. Reprod. 2020, 36, 293–304. [Google Scholar] [CrossRef]
- Lishko, P.V.; Botchkina, I.L.; Fedorenko, A.; Kirichok, Y. Acid Extrusion from Human Spermatozoa Is Mediated by Flagellar Voltage-Gated Proton Channel. Cell 2010, 140, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, Y.; Wu, H.; Zhang, H.; Zhang, H.; Mao, J.; Liu, D.; Zhao, L.; Lin, H.C.; Tang, W.; et al. Sodium-Hydrogen-Exchanger Expression in Human Sperm and Its Relationship with Semen Parameters. J. Assist. Reprod. Genet. 2017, 34, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Kirichok, Y. The Role of Hv1 and CatSper Channels in Sperm Activation. J. Physiol. 2010, 588, 4667–4672. [Google Scholar] [CrossRef] [PubMed]
- Wijerathne, T.D.; Kim, J.; Yang, D.; Lee, K.P. Intracellular Calcium-Dependent Regulation of the Sperm-Specific Calcium-Activated Potassium Channel, HSlo3, by the BKCa Activator LDD175. Korean J. Physiol. Pharmacol. 2017, 21, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Y.; Zhang, Z.; Xia, O.M.; Lingle, C.J. Block of Mouse Slo1 and Slo3 K+ Channels by CTX, IbTX, TEA, 4-AP and Quinidine. Channels 2010, 4, 22–41. [Google Scholar] [CrossRef]
- Mansell, S.A.; Publicover, S.J.; Barratt, C.L.R.; Wilson, S.M. Patch Clamp Studies of Human Sperm under Physiological Ionic Conditions Reveal Three Functionally and Pharmacologically Distinct Cation Channels. Mol. Hum. Reprod. 2014, 20, 392–408. [Google Scholar] [CrossRef]
- Brenker, C.; Goodwin, N.; Weyand, I.; Kashikar, N.D.; Naruse, M.; Krähling, M.; Müller, A.; Kaupp, U.B.; Strünker, T. The CatSper Channel: A Polymodal Chemosensor in Human Sperm. EMBO J. 2012, 31, 1654–1665. [Google Scholar] [CrossRef]
- Lishko, P.V.; Botchkina, I.L.; Kirichok, Y. Progesterone Activates the Principal Ca2+ Channel of Human Sperm. Nature 2011, 471, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Wrighton, D.C.; Muench, S.P.; Lippiat, J.D. Mechanism of Inhibition of Mouse Slo3 (KCa 5.1) Potassium Channels by Quinine, Quinidine and Barium. Br. J. Pharmacol. 2015, 172, 4355–4363. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, P.; Santi, C.M.; Treviño, C.L.; Ocampo-Gutiérrez, A.Y.; Acevedo, J.J.; Alisio, A.; Salkoff, L.B.; Darszon, A. Mouse Sperm K+ Currents Stimulated by PH and CAMP Possibly Coded by Slo3 Channels. Biochem. Biophys. Res. Commun. 2009, 381, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Cavarocchi, E.; Whitfield, M.; Saez, F.; Touré, A. Sperm Ion Transporters and Channels in Human Asthenozoospermia: Genetic Etiology, Lessons from Animal Models, and Clinical Perspectives. Int. J. Mol. Sci. 2022, 23, 3926. [Google Scholar] [CrossRef] [PubMed]

| Compound | Assay | Concentration | Effect | Study |
|---|---|---|---|---|
| 4-AP | hKSper | 2 mM | – | [108] |
| Bupivacaine | hKSper | 3 mM | ↓ | [108] |
| Charybdotoxin | hKSper, IKSper | 1 µM | ↓ | [89] |
| Clofilium | hKSper | 50 µM–5 mM | ↓ | [67,108] |
| Iberiotoxin | hKSper, IKSper | 100 nM | – | [67,89] |
| hKSper | 100 nM | ↓ | [89] | |
| Lidocaine | hKSper | 3 mM | ↓ | [108] |
| Paxilline | hKSper, IKSper | 100 nM | ↓ | [89] |
| Progesterone | hKSper, IKSper | 0.5–30 µM | ↓ | [89,108] |
| Quinidine | hKSper | 300–500 µM | ↓ | [67,108] |
| TEA | hKSper | 10 mM | – | [67] |
| VU0546110 | hKSper | 10 µM | ↓ | [92] |
| Compound | Assay | Concentration | Effect | Study |
|---|---|---|---|---|
| 4-AP | hSLO3 | 25 mM | – | [106] |
| rSLO3 | 100 mM | – | [83] | |
| Ba2+ | hSLO3 | 1 mM | ↓ | [85] |
| mSLO3 | 2 mM | ↓ | [111,112] | |
| Charybdotoxin | hSLO3 | 100 nM | ↓ | [85] |
| Clofilium | hSLO3 | 50 µM | – | [85] |
| mSLO3 | 50 µM | ↓ | [111] | |
| Iberiotoxin | hSLO3 | 100 nM | – | [85,106] |
| hSLO3 | 0.1–300 nM | ↓ | [92] | |
| Ketamine | rSLO3 | 25–500 µM | ↓ | [83] |
| LDD175 | hSLO3 | 30 µM | ↑ | [106] |
| NS1619 | hSLO3 | 50 µM | ↓ | [106] |
| Paxilline | hSLO3 | 1–30 µM | ↓ | [92] |
| Penitrem A | hSLO3 | 100 nM | ↓ | [85] |
| Progesterone | hSLO3 | 30 µM | ↓ | [67,85] |
| Propofol | rSLO3 | 100–700 µM | ↓ | [83] |
| Quinidine | hSLO3 | 0.1–100 µM | ↓ | [85,92] |
| rSLO3 | 10–500 µM | ↓ | [83] | |
| mSLO3 | 500 µM | ↓ | [111] | |
| Slotoxin | hSLO3 | 100 nM | – | [85] |
| TEA | hSLO3 | 20 mM | ↓ | [106] |
| mSLO3 | 60 mM | ↓ | [112] | |
| mSLO3 | 20 mM | – | [111] | |
| VU0546110 | hSLO3 | 0.3–30 µM | ↓ | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyon, M.D.; Ferreira, J.J.; Li, P.; Bhagwat, S.; Butler, A.; Anderson, K.; Polo, M.; Santi, C.M. SLO3: A Conserved Regulator of Sperm Membrane Potential. Int. J. Mol. Sci. 2023, 24, 11205. https://doi.org/10.3390/ijms241311205
Lyon MD, Ferreira JJ, Li P, Bhagwat S, Butler A, Anderson K, Polo M, Santi CM. SLO3: A Conserved Regulator of Sperm Membrane Potential. International Journal of Molecular Sciences. 2023; 24(13):11205. https://doi.org/10.3390/ijms241311205
Chicago/Turabian StyleLyon, Maximilian D., Juan J. Ferreira, Ping Li, Shweta Bhagwat, Alice Butler, Kelsey Anderson, Maria Polo, and Celia M. Santi. 2023. "SLO3: A Conserved Regulator of Sperm Membrane Potential" International Journal of Molecular Sciences 24, no. 13: 11205. https://doi.org/10.3390/ijms241311205
APA StyleLyon, M. D., Ferreira, J. J., Li, P., Bhagwat, S., Butler, A., Anderson, K., Polo, M., & Santi, C. M. (2023). SLO3: A Conserved Regulator of Sperm Membrane Potential. International Journal of Molecular Sciences, 24(13), 11205. https://doi.org/10.3390/ijms241311205






