Abstract
Four diastereomers of 16-azidomethyl substituted 3-O-benzyl estradiol (1–4) and their two estrone analogs (16AABE and 16BABE) were tested for their antiproliferative properties against human gynecological cancer cell lines. The estrones were selected for additional experiments based on their outstanding cell growth-inhibiting activities. Both compounds increased hypodiploid populations of breast cancer cells, and 16AABE elicited cell cycle disturbance as evidenced by flow cytometry. The two analogs substantially increased the rate of tubulin polymerization in vitro. 16AABE and 16BABE inhibited breast cancer cells’ migration and invasive ability, as evidenced by wound healing and Boyden chamber assays. Since both estrone analogs exerted remarkable estrogenic activities, as documented by a luciferase reporter gene assay, they can be considered as promising drug candidates for hormone-independent malignancies.
1. Introduction
Cancer is prominent cause of death worldwide, with mortality rates comparable to those of stroke and coronary heart disease. According to the latest update from the International Agency for Research on Cancer (IARC) database, 19.3 million new cancer cases and almost 10 million cancer-related deaths occurred globally in 2020. Moreover, the global cancer burden is expected to reach 28.4 million cases by 2040, corresponding to a 47% rise in all cancer cases. Lung cancer is the leading cause of cancer death, responsible for 18% of tumor-related mortality, followed by colorectal (9.4%), liver (8.3%), stomach (7.7%), and female breast (6.9%) cancers. Concerning the incidence of different female tumors, breast carcinomas are the most common, accounting for 24.5% of all new cases. Altogether, 38.9% of new cancer cases in females involve gynecological malignancies [1]. The 2.26 million breast cancer cases diagnosed in 2020 show unequal geographical distribution, with the highest age-standardized incidence rate in Europe (69.7/100,000) and the lowest in South-East Asia (28.3/100,000). A statistically significant inverse correlation was observed between the mortality-to-incidence ratio (MIR) and the human development index (HDI), indicating poorer prognosis for patients living in less developed regions of the world [2]. All these epidemiological findings suggest that the prevention and treatment of female breast cancers are not yet resolved, despite the impressive therapeutic progress evidenced in past decades.
Substantial improvement of the global cancer burden is impossible without innovative therapeutic options, including original drugs. Studying compounds with steroidal skeleton as potential anticancer agents has a long history. Over the past few decades, several new steroids, such as cyproterone, finasteride, exemestane and fulvestrant, have been integrated into clinical practice. A feasible strategy for developing novel drug candidates involves the chemical modification of endogenous molecules to produce semi-synthetic analogs with diverse biological activities [3].
The initial application of steroid-based compounds in the field of anticancer therapy emerged from the utilization of diverse botanical extracts. Evidence suggests that steroid-like triterpenes, including betulinic acid, oleanolic acid, and related derivatives, exhibit potent proapoptotic and antimigratory effects against numerous human cancer cell lines [4,5,6,7,8,9,10]. Many estrane-based compounds modified in rings A or D have been investigated recently, demonstrating that triazolyl estranes exert promising anticancer actions [11,12]. Studies have also demonstrated that numerous core-modified estradiol analogs exhibit considerable antiproliferative activity against human cancer cell lines derived from gynecological malignancies [13]. The position, specific nature, size and polarity of the substituents newly introduced into the molecule have been shown to substantially impact the anticancer properties of the designed derivatives. The antiproliferative mechanism of certain core-modified estrones is based on their direct effects on the tubule-microtubule system, resulting in disturbed tubulin polymerization rates [11,14,15].
We have recently reported on certain 16,17-functionalized 3-methoxy or 3-benzyloxy estrone derivatives behaving as potent antiproliferative compounds [16,17]. The substitution pattern of ring D, and the nature of the protecting group at C-3-O was demonstrated to influence the cell growth-inhibitory potential of these compounds markedly. Overall, 3-benzyl ethers were found to be more potent [16]. The substituents’ nature and orientation affected the antitumoral behavior of these previously tested agents [17].
Based on these promising findings regarding the antiproliferative activities of 16,17-functionalized estrone 3-benzyl ethers, in the present study we aimed to assess the antiproliferative, antimetastatic and anticancer properties of these novel substituted steroidal compounds, including four 16-azidomethyl-17-hydroxy derivatives (1–4) and their 17-keto counterparts (16AABE and 16BABE, Figure 1).
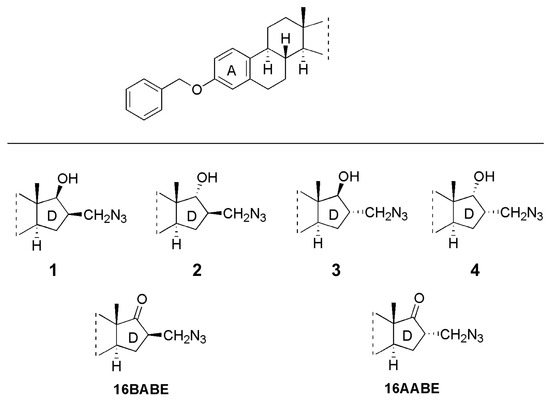
Figure 1.
Structures of the tested starting compounds (1–4) and the newly synthesized agents 16β-azidomethyl-3-O-benzyl estrone (16BABE) and 16α-azidomethyl-3-O-benzyl estrone (16AABE).
2. Results
2.1. Chemistry
Compounds 16AABE and 16BABE were synthesized from their 17-hydroxy precursors (1 and 3, Scheme 1). The starting compounds were subjected to oxidation using the Jones reagent. The reactions furnished the products 16AABE and 16BABE in high yields. The structures of 17-keto compounds were deduced from 1H and 13C NMR assessments.
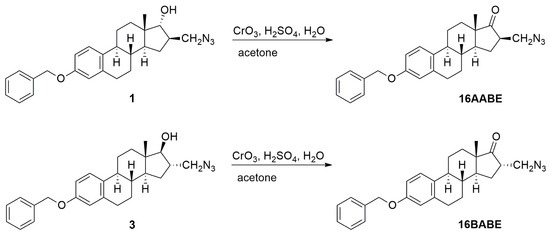
Scheme 1.
Syntheses of 16BABE and 16AABE.
2.2. Antiproliferative Assay
The antiproliferative capacities of the prepared compounds were determined by employing the MTT assay against a panel of human adherent cancer cell lines isolated from breast (MCF-7 and MDA-MB-231) or cervical (HeLa and SiHa) tumors. All compounds were tested at two concentrations (10 and 30 μM). When >50% of antiproliferative capacity was obtained at 10 μM, the assays were repeated with a broader concentration range (0.1–30 μM), and IC50 values were calculated (Figure 2, Supplementary Table S1, Supplementary Figure S1). Starting molecules 1–4 exerted negligible action at 10 μM, but substantial cell growth inhibition was observed at the higher concentration (30 μM). On the other hand, the 17-keto analogs (16AABE and 16BABE) elicited over 90% inhibition even at the lower concentration, and their calculated IC50 values were lower than that of the reference agent cisplatin. MTT assays were performed against the non-cancerous fibroblast cell line NIH/3T3 to obtain preliminary data on cancer selectivity of 16AABE and 16BABE. The fibroblast cells proved to be less sensitive, with calculated IC50 values >10 μM. The ratios of IC50 values obtained against cancer cells and fibroblasts were in the range of 0.2 and 0.5, indicating substantial cancer selectivity of these two compounds (Table 1).
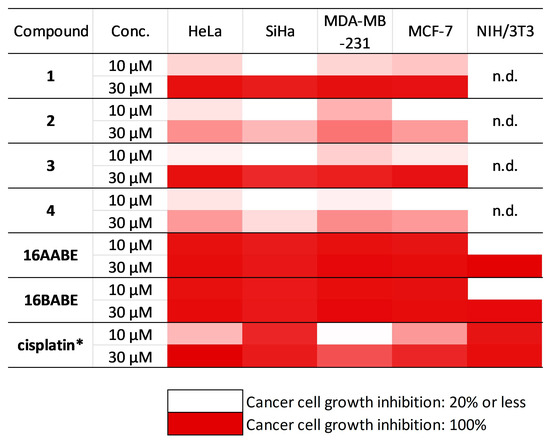
Figure 2.
Antiproliferative properties of the investigated molecules. Inhibition values <20% are considered negligible and are not given numerically. n.d.: not determined. *: data are from refererence [17]. Numeric results with calculated IC50 values are presented in Supplementary Table S1.

Table 1.
Tumor selectivity indices of 16AABE and 16BABE expressed as the ratio of IC50 values obtained against cancer cells and fibroblasts.
2.3. Propidium Iodide-Based Cell Cycle Analysis
16AABE and 16BABE were subjected to propidium iodide-based cell cycle analysis by flow cytometry to elucidate their mechanism of action. MDA-MB-231 cells were treated with various concentrations of the test agents for 24 h, and DNA content of the cells was determined. 16AABE induced a moderate but significant increase in the hypodiploid (subG1) cell population at 1 μM (Figure 3 and Figure 4). At 2 μM, which approximately equals the IC50 value for this agent, a more profound cell cycle disturbance was observed with a pronounced increase in the subG1 and G2/M populations at the expense of G1 and S phases. Conversely, 16BABE induced a minor but significant accumulation of subG1 cells at 8 μM, a concentration roughly equaling its IC50, indicating the proapoptotic activity of this compound (Figure 3).
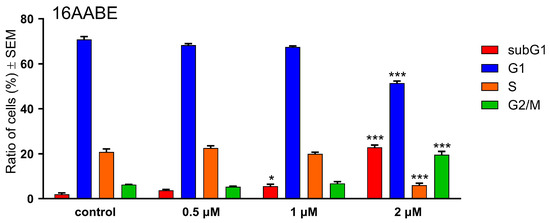
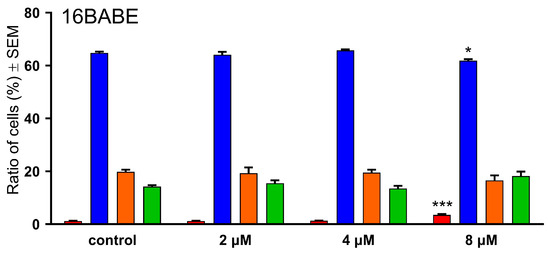
Figure 3.
Effects of 16AABE (upper panel) and 16BABE (lower panel) on cell cycle distribution of MDA-MB-231 cells treated with the indicated concentrations for 24 h. * and *** indicate significant differences at p < 0.05 and p < 0.001, respectively. Data are from three independent experiments performed in triplicate.
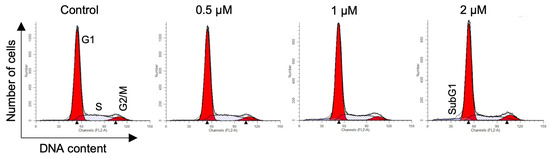
Figure 4.
Representative histograms for MDA-MB-231 cells treated with 16AABE. Histograms were generated using the ModFit LT 3.3.11 software.
2.4. Tubulin Polymerization Assay
The impact of 16AABE and 16BABE on microtubule polymerization was assessed using a cell-free system with a photometric kinetic determination. Concentrations of the test compounds were selected based on their IC50 values, as recommended by the kit’s manufacturer. Both compounds exhibited a stimulating effect on tubulin polymerization compared with control. Notably, the calculated maximum rates of tubulin polymerization (Vmax) were significantly higher than those observed for the control (Figure 5). Additionally, the Vmax values for the test compounds were higher than that for the reference agent paclitaxel (PAC, 10 μM), indicating their profound activity on tubulin polymerization.
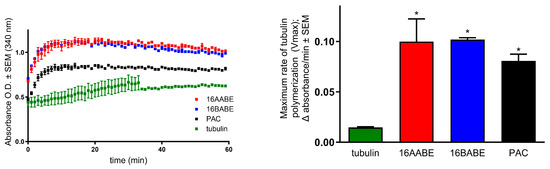
Figure 5.
Direct effects of 16AABE and 16BABE (500 μM for both) on tubulin polymerization. Left panel: recorded kinetic curves; paclitaxel (10 μM PAC) was included as a reference agent. Right panel: calculated maximum values for the rate of tubulin polymerization. * indicates significance at p < 0.05 compared with untreated control. Data are from two independent experiments performed in duplicate.
2.5. Wound Healing Assay
To investigate the antimigratory activity of the test compounds, we conducted a wound-healing assay using the MCF-7 breast cancer cell line. Using an in vitro model of wound closure, a wound was created by removing silicone inserts from a cell-covered chamber, followed by incubating the cells in a minimal serum-containing (2%) medium for 0, 24, and 48 h. Microscope image analysis was performed to measure the reduction in cell-free areas, serving as an indicator of wound closure. Our findings demonstrated a significant decrease in the migratory capacity of cancer cells (Figure 6 and Figure 7). Notably, both compounds exhibited remarkable antimigratory effects at subantiproliferative concentrations (1.5 μM), with 16BABE demonstrating a more pronounced action after 24 h of incubation.
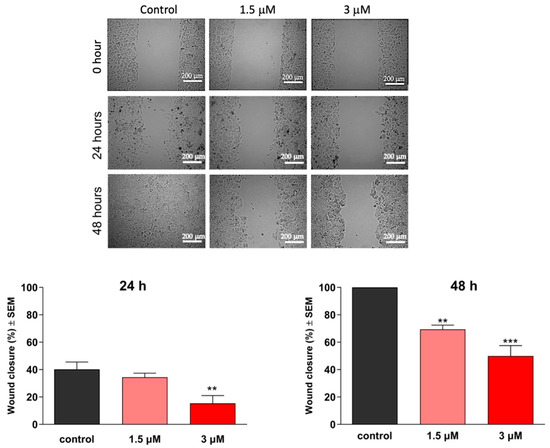
Figure 6.
Effects of 16AABE on the migration of MCF-7 cells. Upper panels: representative images taken at 24 or 48 h post-treatment with 16AABE. Lower panels: calculated wound closure values determined at 24 or 48 h post-treatment. ** and *** indicate significance at p < 0.01 and p < 0.001, respectively. Data are based on 4 independent experiments, all performed in triplicate.
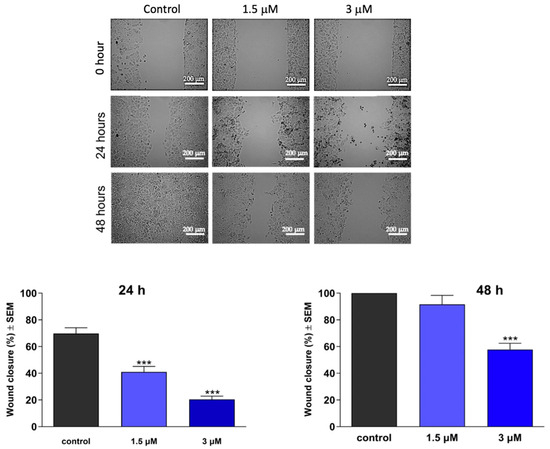
Figure 7.
Effects of 16BABE on the migration of MCF-7 cells. Upper panels: representative images taken at 24 or 48 h post-treatment with 16BABE. Lower panels: calculated wound closure values determined at 24 or 48 h post-treatment. *** indicates significance at p < 0.001. Data are based on 4 independent experiments, all performed in triplicate.
2.6. Boyden Chamber Assay
As the invasive capacity of cancer cells plays a pivotal role in metastatic behavior, it is crucial to assess the antimetastatic potential of any promising anticancer agents, in addition to characterizing their impact on cell migration. Boyden chambers with Matrigel Matrix-coated membranes (pore diameter: 8.0 μm) were employed to evaluate invasiveness, as they permit the passage of invasive cells while impeding the migration of non-invading cells. Remarkably, the test compounds hindered the invasion of MDA-MB-231 cells efficiently, even at low concentrations of 0.5 or 1 μM at 24 h post-treatment (Figure 8 and Figure 9). Moreover, both compounds exhibited a significant decrease in invading cells after 48 h of treatment, supporting their remarkable anti-invasive potential.
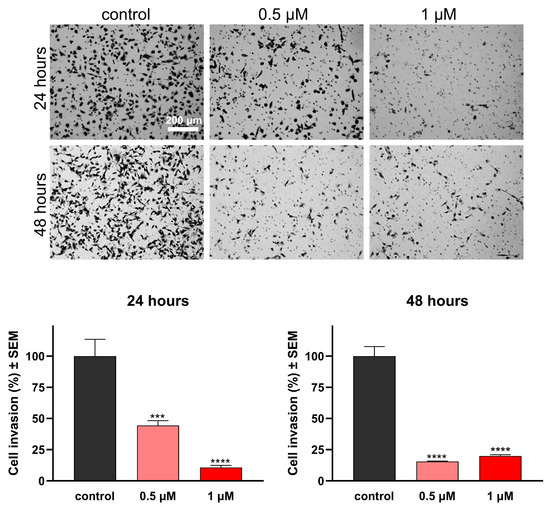
Figure 8.
Effects of 16AABE on the invasion capacity of MBA-MD-231 cells. Upper panels: representative images taken at 24 or 48 h post-treatment with 16AABE. Lower panels: 16AABE significantly reduced invasion of MDA-MB-231 cells at 24 h and 48 h post-treatment. Data are based on at least 4 independent experiments performed in duplicate. *** and **** indicate significance at p < 0.001 and p < 0.0001, respectively.
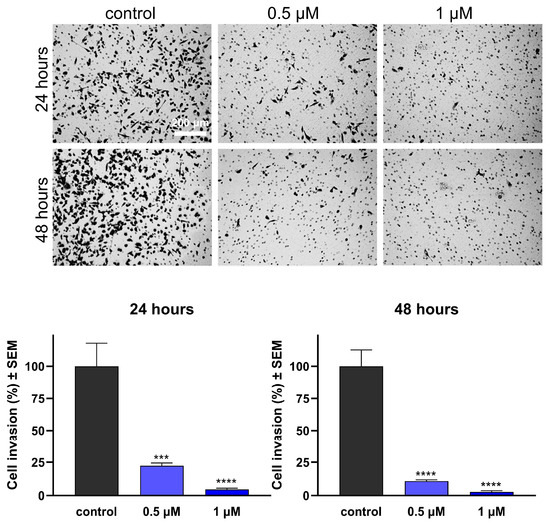
Figure 9.
Effects of 16BABE on the invasion capacity of MBA-MD-231 cells. Upper panels: representative images taken at 24 or 48 h post-treatment with 16BABE. Lower panels: 16BABE significantly reduced the invasion of MDA-MB-231 cells at 24 h and 48 h post-treatment. Data are based on at least 4 independent experiments performed in duplicate. *** and **** indicate significance at p < 0.001 and p < 0.0001, respectively.
2.7. Estrogenic Activities of the Test Compounds
Since 16AABE and 16BABE are structurally closely related to the natural estrogen 17β-estradiol, their hormonal activities are considered crucial elements of their pharmacological profile. A T47D breast cancer cell line transfected with an estrogen-responsive luciferase reporter gene was utilized to clarify the estrogenic activity of the test compounds (Figure 10). Both agents were found to exert estrogenic activity at concentrations several orders of magnitude higher than the reference agent 17β-estradiol. The calculated concentrations eliciting 50% of maximum estrogenic stimulation were approximately 5.5 nM and 178 nM, respectively. These results indicate that the tested estrone analogs possess considerable hormonal activity at their antiproliferative or antimetastatic concentrations.
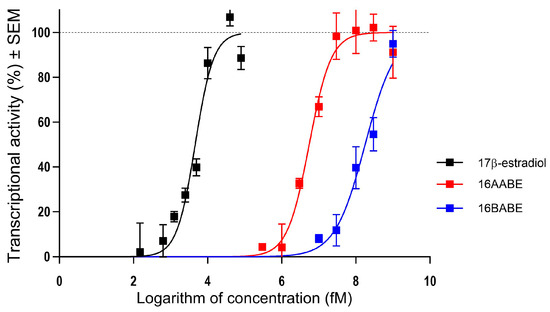
Figure 10.
Estrogenic effects of 16AABE and 16BABE expressed as the intensity of the estrogen-responsive luciferase in transfected T47D breast cancer cell line. Data are based on 3 independent experiments performed in triplicate.
3. Discussion
Breast cancer is the most frequent malignancy in females globally. Based on crucial molecular markers, including estrogen and progestin receptors and human epidermal growth factor receptor 2 (HER2), the disease entity is classified into major subtypes: hormone receptor (HR) positive, HER2-positive, and triple-negative breast cancers (TNBC). TNBC accounts for approximately 15–20% of all cases, and its prevalence seems to be higher in younger patients, below 40 years of age [18,19]. TNBC exhibits aggressive behavior compared with other subtypes, and has a poorer prognosis. Due to the lack of targeted pharmacological interventions, current treatment of TNBC is limited to traditional cytotoxic agents [20].
Although estrogens, including the natural hormone 17β-estradiol, are generally considered to promote cell growth, several estrane-based molecules have been identified as potent anticancer drug candidates [13].
The 16-substituted triazolyl estranes represent a class of compounds with a unique structural framework combining a triazole ring with an estrane scaffold. The design and synthesis of these compounds involve click chemistry and structure-activity relationship studies to optimize their pharmacological profiles. Continued research and optimization of 16-substituted triazolyl estranes hold promise for developing novel therapeutics across multiple disease areas [11].
Our current study has focused on investigating the antiproliferative properties of four 16-azidomethyl estradiol analogs (1–4) previously utilized as intermediaries in synthesizing 16-triazolyl estranes [17]. Our screens for antiproliferative activity were extended to cover two estrone congeners (16AABE and 16BABE), and compounds with a 17-keto function were found to be more active than the reference agent cisplatin. Moreover, the ratios of IC50 values obtained against cancer cells and NIH/3T3 fibroblasts were below 1 (within the range of 0.166 and 0.429), indicating reliable cancer selectivity. At the same time, 17-hydroxy analogs exhibited modest actions only. According to the calculated IC50 values, the stereochemical difference, i.e., the configuration of the 16-azidomethyl group, is not a crucial factor in the activity of these compounds. Based on these findings, the two estrone analogs were subjected to additional investigations to characterize their anticancer activities in detail.
Cell cycle analysis generally provides valuable insights into the mechanisms responsible for disturbing cell proliferation. Both selected compounds, 16AABE and 16BABE induced cell cycle disturbance in MDA-MB-231 TNBC cells. Treatment with 16AABE resulted in a concentration-dependent increase in the hypodiploid (subG1) population after 24 h of incubation. This action was detected at concentrations below the IC50 (1 and 2 μM), indicating the proapoptotic potency of this compound [21]. Additionally, profound accumulation of cells in the G2/M phase at the expense of the G1 and S populations was observed. On the other hand, 16BABE elicited a detectable change in cell cycle distribution at its IC50 only (8 μM), and this action was limited to a modest increase in the subG1 and a decrease in the G1 cell population.
Based on these cell cycle disturbances, investigations into the effects on tubulin polymerization seemed rational. Microtubules, these highly dynamic filamentous proteins within the cytoskeleton, are considered significant targets for anticancer interventions [22]. Both compounds were found to induce a considerable increase in tubulin polymerization rate at a concentration of 500 μM, indicating their ability to enhance microtubule assembly and stability. These effects were comparable or even superior to that of the positive control paclitaxel, highlighting that direct action on tubulin seems to be a crucial component of our test compounds’ pharmacological profile.
According to epidemiological data, approximately 90% of cancer-related deaths can be attributed to metastases [23]. This complex sequence of events encompasses several stages, including the local migration and invasion of tumor cells into neighboring tissues, penetration into the vascular system, survival, and exit from the circulatory system, followed by proliferation in distant organs, resulting in the establishment of new colonies [24]. Epidemiological evidence highlights the significant prevalence of invasive cervical cancer, ranking the fourth most frequent female malignancy after breast, colon, and lung cancers globally. Metastases of cervical carcinomas occur through either the hematogenous or lymphatic pathways. Patients with hematogenous metastases generally exhibit lower survival rates than those with lymphatic metastases [25,26,27,28]. These epidemiological characteristics illustrate the importance of developing effective antimetastatic compounds as potential drug candidates to hinder these tendencies.
Both 16AABE and 16BABE exhibited significant inhibitory effects on the migration of MDA-MB-231 cells. Both compounds demonstrated time- and concentration-dependent inhibition of cell migration as evidenced by the wound healing assay. Moreover, this antimigratory action was detected at a concentration of 1.5 μM, much lower than the IC50 values for cell growth inhibition in any cell lines tested. Based on these findings, the antimigratory properties of the test compounds may be explained by a separate pharmacological mechanism, rather than a consequence of cell growth inhibition.
A Boyden chamber assay was employed to evaluate the anti-invasive properties of our estrone analogs. After 24 h of treatment, both compounds demonstrated highly significant inhibition of breast cancer cell invasion at concentrations of 0.5 μM and 1 μM. Their actions became even more pronounced after 48 h of incubation. The exact characterization of the mechanism of their antimetastatic activities is beyond the scope of this study. However, in a previous study we investigated a set of 3-O-sulfamoyl-13α-estrone derivatives, and their pharmacological profile showed features similar to these currently tested compounds [29]. In that series, molecular docking studies were performed for three 13α-estrones to elucidate their binding properties to β-tubulin, and their binding affinity was found to correlate with their positive action on tubulin polymerization. Based on these findings, β-tubulin can be suggested as the probable site of action for 16AABE and 16BABE.
Since the role of microtubules is not limited to constructing the mitotic spindle, a tubulin disruptor may exert additional activities besides the expected antimitotic action. As tubulin dynamics are deeply involved in the mobility of cancer cells, pharmacological interventions affecting tubulin polymerization may influence metastatic potency, independently of the direct cytotoxicity of a given agent [30].
Finally, the estrane skeleton justified the characterization of the estrogenic activity of the tested analogs. Our findings indicate that 16AABE and 16BABE exhibit substantial hormonal activity at concentrations required for the antiproliferative and antimetastatic actions. Since a drug with estrogenic effect may promote the proliferation of estrogen sensitive cancer cells, this characteristic seems to be disadvantageous in most gynecological cancers. However, in a subclass of hormone-independent malignancies including triple-negative breast cancer, the hormonal agonist action may not limit the usability of such an agent. Therefore, our currently presented estrone analogs can be considered as innovative drug candidates for such hormone-neutral cancerous disorders.
4. Materials and Methods
4.1. Chemistry
Melting points (Mp) were determined with a Kofler hot-stage apparatus and were uncorrected. Elemental analyses were performed with a PerkinElmer CHN analyzer model 2400 (PerkinElmer, Waltham, MA, USA). Thin-layer chromatography involved silica gel 60 F254; layer thickness 0.2 mm (Merck, Budapest, Hungary); eluent (ss): 20% ethyl acetate/80% hexane; detection with I2 or UV (365 nm) after spraying with 5% phosphomolybdic acid in 50% aqueous phosphoric acid and heating at 100–120 °C for 10 min. Flash chromatography involved: silica gel 60, 40–63 μm (Merck). 1H NMR spectra were recorded in CDCl3 solution with a Bruker DRX-500 instrument (Bruker, Billerica, MA, USA) at 500 MHz, with Me4Si as the internal standard. 13C NMR spectra were recorded with the same instrument at 125 MHz under the same conditions (Supplementary Figures S2 and S3). Mass spectrometry: full scan mass spectra of the compounds were acquired in the range of 50 to 1000 m/z with a Finnigan TSQ-7000 triple quadrupole mass spectrometer (Finnigan-MAT, San Jose, CA, USA) equipped with a Finnigan electrospray ionization source. Analyses were performed in positive ion mode using flow injection mass spectrometry with a mobile phase of 50% aqueous acetonitrile containing 0.1% (v/v) formic acid. The flow rate was 0.3 mL/min. Five µL aliquot of the samples were loaded into the flow. The ESI capillary was adjusted to 4.5 kV and N2 was used as a nebulizer gas.
The general procedure for the synthesis of 16β-azidomethyl-3-benzyloxyestra-1,3,5(10)-trien-17-on (16BABE) and 16α-azidomethyl-3-benzyloxyestra-1,3,5(10)-trien-17-on (16AABE) was as follows.
Compound 1 or 3 (417 mg, 1.00 mmol) was dissolved in acetone (5 mL), then cooled in an ice-water bath, and Jones reagent (0.4 mL, 8 N) was added in five portions. The reaction mixture was allowed to stand at room temperature for 1 h, then it was diluted with water and extracted with ethyl acetate. The combined organic phases were washed with water until neutral and dried over sodium sulfate, and the crude product was subjected to column chromatography with dichloromethane/hexane = 8/2 as eluent.
Compound 16BABE was obtained as a white solid (382 mg, 92%). Mp 86–88 °C, Rf = 0.63. Anal. calcd. for C26H29N3O2: C, 75,15; H, 7.03. Found: C, 75, 27; H, 7.07. 1H NMR (500 MHz, CDCl3) δ ppm: 0.90 (m, 1H); 0.92 (s, 3H, 13-CH3); 1.27–1.63 (overlapping multiplets with hexanes solvent peaks, 16H); 1.98–2.08 (overlapping multiplets, 3H); 2.22–2.32 (overlapping multiplets, 2H); 2.40 (m, 1H); 2.90 (m, 2H, 6-H2); 3.62 (m, 2H, 16a-H2); 5.04 (s, 2H, OCH2); 6.74 (d, 1H, J = 2.5 Hz, 4-H); 6.79 (dd, 1H, J = 8.5 Hz, J = 2.6 Hz, 2-H); 7.20 (d, 1H, J = 8.6 Hz, 1-H); 7.32 (t, 1H, J = 7.7 Hz, 4′-H); 7.39 (t, 2H, J = 7.7 Hz, 3′- and 5′-H); 7.43 (d, 2H, J = 7.7 Hz, 2′- and 6′-H). 13C NMR (CDCl3) δ ppm: 13.4 (C-18); 25.8 (CH2); 26.3 (CH2); 26.7 (CH2); 29.6 (CH2); 31.9 (CH2); 37.7 (CH); 44.1 (CH); 48.3 (C-13); 48.9 (CH); 49.4 (CH); 51.6 (C-16a); 69.9 (OCH2); 112.4 (CH); 114.9 (CH); 126.2 (C-1); 127.4 (2C, 2× CH); 127.8 (CH); 128.5 (2C, 2× CH); 132.1 (C-10); 137.2 (C); 137.7 (C); 156.9 (C-3); 219.0 (C=O). MS m/z (%) 416 (100, [M+H]+).
Compound 16AABE was obtained as a white solid (374 mg, 90%). Mp 80–82 °C, Rf = 0.63. Anal. calcd. for C26H29N3O2: C, 75, 15; H, 7.03. Found: C, 75, 22; H, 7.09. 1H NMR (500 MHz, CDCl3) δ ppm: 0.88 (m, 1H); 0.97 (s, 3H, 13-CH3); 1.26–1.56 (overlapping multiplets with hexanes solvent peaks, 18H); 1.91–2.00 (overlapping multiplets, 4H); 2.27 (m, 1H); 2.39 (m, 1H); 2.75 (m, 1H); 2.90 (m, 2H, 6-H2); 3.51–3.62 (overlapping multiplets, 2H, 16a-H2); 5.05 (s, 2H, OCH2); 6.74 (d, 1H, J = 2.5 Hz, 4-H); 6.79 (dd, 1H, J = 8.5 Hz, J = 2.6 Hz, 2-H); 7.19 (d, 1H, J = 8.6 Hz, 1-H); 7.32 (t, 1H, J = 7.7 Hz, 4′-H); 7.38 (t, 2H, J = 7.7 Hz, 3′- and 5′-H); 7.43 (d, 2H, J = 7.7 Hz, 2′- and 6′-H). 13C NMR (CDCl3) δ ppm: 14.4 (C-18); 25.8 (CH2); 26.0 (CH2); 26.4 (CH2); 29.6 (CH2); 31.4 (CH2); 38.3 (CH); 43.9 (CH); 44.4 (C-13); 48.2 (CH); 48.6 (C-13); 51.8 (C-16a); 70.0 (OCH2); 112.4 (CH); 114.9 (CH); 126.3 (C-1); 127.4 (2C, 2× CH); 127.9 (CH); 128.5 (2C, 2× CH); 132.2 (C-10); 137.2 (C); 137.8 (C); 156.9 (C-3); 218.5 (C=O). MS m/z (%) 416 (100, [M+H]+).
4.2. Cell CUlture and CHemicals
The utilized cell lines (HeLa, MDA-MB-231, MCF-7, and NIH/3T3) were obtained from ECACC (European Collection of Cell Cultures, Salisbury, UK), except for SiHa cells which were obtained from ATCC (American Tissue Culture Collection, Manassas, VA, USA). All cell lines were cultured in Eagle’s Minimum Essential Medium (EMEM) at 37 °C in a humidified atmosphere with 5% carbon dioxide. The medium was supplemented with 10% fetal bovine serum (FBS), 1% non-essential amino acid solution, and 1% penicillin, streptomycin, and amphotericin B mixture. All cell culture mediums and supplements were obtained from Lonza Group Ltd. (Basel, Switzerland). Chemicals for the described in vitro experiments were purchased from Merck Ltd. (Budapest, Hungary) unless stated otherwise.
4.3. Determination of Antiproliferative Activity (MTT Assay)
The antiproliferative activity of the presented compounds were evaluated against a panel of human gynecological cancer cell lines. MCF-7 and MDA-MB-231 cell lines were derived from breast cancers, while HeLa and SiHa cell lines originated from cervical cancers of different pathological backgrounds. Non-cancerous human fibroblast cells (NIH/3T3) were used exclusively to assess cancer selectivity of the two azidomethyl compounds.
Cancer cells were seeded onto a 96-well microplate for the proliferation assay at a density of 5000 cells/well. After 24 h of incubation, 200 μL of new medium containing the test compounds at 10 or 30 µM concentrations was added.
Following incubation for 72 h at 37 °C in a humidified atmosphere containing 5% CO2, cell viability was assessed by adding 20 μL of 5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution. After 4 h of incubation, the yellow MTT solution was converted to violet crystals by mitochondrial reductases in viable cells. Subsequently, the medium was removed, and the formazan crystals were dissolved in 100 μL of DMSO with shaking at 37 °C for 60 min.
Absorbance of the reduced MTT solution was measured at 545 nm using a microplate reader, with untreated cells serving as the negative control [31]. In the case of active compounds (i.e., >50% cell growth inhibition at 10 μM), the assay was repeated with a series of dilutions, and sigmoidal dose–response curves were fitted to the obtained data. The IC50 values, representing the concentration at which cell proliferation was reduced by 50% compared with the untreated control, were calculated using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). Each in vitro experiment was conducted on two microplates with a minimum of five parallel wells. Stock solutions of the test substances (10 mM) were prepared in DMSO, with the highest DMSO concentration in the medium not exceeding 0.3%, which did not significantly affect cell proliferation. Cisplatin was used as a reference agent.
4.4. Propidium Iodide-Based Cell Cycle Analysis
Cell cycle analysis was conducted to investigate the mechanism of action of azidomethyl compounds in human breast cancer cell lines. Specifically, MDA-MB-231 cells were seeded onto 24-well plates at a density of 80,000 cells per well. The cells were treated with two concentrations of 16AABE (0.5 or 1 μM) and 16BABE (2 or 4 μM), respectively, for 24 h.
After treatment, the cells were washed with phosphate-buffered saline (PBS) and harvested using trypsin. The harvested cells were combined with the supernatants and PBS from the washing process. Subsequently, centrifugation at 1700 rpm for 5 min at room temperature was performed, followed by resuspending the cell pellets in a DNA staining solution. The DNA staining solution consisted of 10 μg/mL propidium iodide (PI), 0.1% Triton-X, 10 μg/mL RNase A, and 0.1% sodium citrate dissolved in PBS. The resuspended cells were then incubated in dark at room temperature for 30 min.
At least 20,000 events per sample were analyzed using a FACSCalibur (BD Biosciences, Franklin Lakes, NJ, USA) flow cytometer to assess the DNA content. Data obtained were analyzed using the ModFit LT 3.3.11 software (Verity Software House, Topsham, ME, USA). Untreated cells served as the control, and the hypodiploid (subG1) phase indicated the apoptotic cell population [21].
4.5. Tubulin Polymerization Assay
Following the manufacturer’s instructions, a tubulin polymerization assay kit (Cytoskeleton Inc., Denver, CO, USA) was employed to assess cell-independent direct effects of 16AABE and 16BABE on tubulin polymerization in vitro. Initially, 10 μL of a 500 μM solution of the desired compound was added to a UV-transparent microplate prewarmed to 37 °C. Positive control samples containing 10 μL of 10 μM paclitaxel, as well as untreated controls with general tubulin buffer (80 mM PIPES pH 6.9, 2 mM MgCl2, 0.5 mM EGTA) were also prepared. Next, 100 μL of a 3.0 mg/mL tubulin solution dissolved in polymerization buffer (80 mM PIPES pH 6.9, 2 mM MgCl2, 0.5 mM EGTA, 1 mM GTP, 10.2% glycerol) was added to each sample present in separate wells of a 96-well plate. The plate was immediately placed in an ultraviolet spectrophotometer (SPECTROstarNano, BMG Labtech, Ortenberg, Germany) prewarmed to 37 °C. A 60-min kinetic reaction was initiated, during which the absorbance was measured at 340 nm every minute to evaluate the effects of the test compounds. The tubulin polymerization curve was constructed by plotting the optical density against time. Maximum reaction rate (Vmax; Δabsorbance/min) was calculated based on the highest difference in absorbance observed over three consecutive time points on the kinetic curve.
4.6. Migration Assay
As previously described, MCF-7 cell suspension was prepared in a supplemented EMEM. The cells were then seeded onto 12-well plates using specialized silicone inserts (Ibidi GmbH, Grafelfing, Germany) at a concentration of 25,000 cells per well. The silicone inserts were gently removed after an overnight incubation, and the cells were washed with PBS. Subsequently, the cells were subjected to a wound healing assay by treating them with low concentrations of the test compounds (1.5 and 3 μM) prepared in EMEM medium with reduced serum content (2% FBS).
Antimigratory effect of the test compounds was assessed by measuring the size of cell-free areas. Images of the cell monolayer were captured at 0, 24, and 48 h using the QCapture Pro 6.0 software. Based on the captured images, the size of cell-free areas was determined using the ImageJ 1.53e software (National Institutes of Health, Bethesda, MD, USA).
4.7. Invasion Assay
To assess the impact of our test compounds on the invasion capacity of malignant MDA-MB-231 cells, we employed Boyden chambers equipped with a reconstituted membrane that mimics the basement membrane (BD Biosciences, Bedford, MA, USA). Treated cells were carefully pipetted onto the hydrated membranes in the upper chamber. In the lower chamber, EMEM supplemented with 10% FBS served as a chemoattractant. After a 24 h incubation period, the supernatants were removed, and non-invading cells on the upper side of the membrane were gently wiped using a cotton swab. The membrane was then rinsed twice with PBS and fixed with ice-cold 96% ethanol. Subsequently, invading cells were stained with 1% crystal violet dye solution for 30 min in the dark at room temperature. Multiple images (at least three per insert) were captured using a Nikon Eclipse TS100 microscope (Nikon Instruments Europe, Amstelveen, The Netherlands). Finally, invading cells were quantified and compared with untreated control samples.
4.8. Determination of Estrogenic Activity
T47D human breast adenocarcinoma cells expressing endogenous estrogen receptor (ERα), modified with an estrogen-responsive luciferase (Luc) reporter gene (T47D-KBluc, obtained from ATCC, Manassas, VA, USA) were used to assess the estrogenic activity of 16AABE and 16BABE [32]. Cells were maintained in phenol red-free MEM with 2 mM L-glutamine, 1 g/L glucose, 10% FBS and penicillin–streptomycin antibiotics. Before testing the compounds’ effect, cells were maintained in the medium described above, supplemented with 10% charcoal dextran-treated FBS for at least six days. Cells were seeded onto a 96-well white flat bottom plate (Greiner Bio-One, Mosonmagyaróvár, Hungary) at a density of 50,000 per well in 200 µL of the medium above, and were allowed to attach for 72 h. Then the indicated concentrations of the test compounds or the reference agent 17β-estradiol were added (less than 0.1% DMSO in the final concentration). Plates were incubated at 37 °C in a humidified 5% CO2 incubator before measuring luciferase activity. After 24 h of incubation, the dosing media was removed entirely, and 30 μL of One-Glo firefly luciferase reagent (Promega, Madison, WI, USA) per well was added to the plate, followed by incubation for 3 min at room temperature according to the manufacturer’s protocol, and then the luminescence signal was quantified (FLUOstar Optima, BMG Labtech, Ortenberg, Germany).
4.9. Statistical Analysis
Statistical data analysis was conducted using the GraphPad Prism 5 software (GraphPad, San Diego, CA, USA). One-way analysis of variance (ANOVA) was employed, followed by the Dunnett post-test, to assess the significance of the observed differences. Data are expressed as mean values ± standard error of the mean (SEM).
5. Conclusions
In conclusion, our findings provide compelling experimental evidence to support the relevance of 16-azidomethyl-estrone analogs as potential drug candidates with anticancer properties. The observed tumor-selective antiproliferative and antimetastatic effects, combined with their ability to induce cell cycle disturbances and exhibit tumor selectivity, highlight the promising prospects of these compounds as innovative anticancer agents. Furthermore, their potent antimigratory and anti-invasive properties are exerted below their growth-inhibitory concentrations. The tested compounds substantially increased the polymerization of tubulin, which may be the basis of their actions. Since 16AABE and 16BABE possess estrogenic activity, their further development seems rational for treating hormone-independent malignancies.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241813749/s1.
Author Contributions
I.Z. and E.M. conceived and designed the experiments; S.A.S.T., N.G., P.G., Á.K., Z.S. and A.K. performed the experiments; G.J.S. and R.M. analyzed experimental data; S.A.S.T. wrote the draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Hungarian Research Foundation (NKFI), grant numbers K143690, 142877 FK22, OTKA SNN 139323, and 2020-1.1.6-JÖVŐ−2021-00003. Projects no. TKP2021-EGA-32 and TKP2021-EGA-17 were implemented with support from the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-EGA funding scheme. The support provided by the Nemzet Fiatal Tehetségeiért Ösztöndíj (NTP-NFTÖ-21-B-0113) is also acknowledged. This work was supported by the ÚNKP-22-5-SZTE-535 New National Excellence Program of the Ministry for Innovation and Technology and by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences BO/00582/22/8, as well as by the KDP-2021 Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund for NG (C1764415 KDP/2021).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request.
Acknowledgments
The authors thank Dora Bokor, PharmD, for proofreading the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Azadnajafabad, S.; Saeedi Moghaddam, S.; Mohammadi, E.; Delazar, S.; Rashedi, S.; Baradaran, H.R.; Mansourian, M. Patterns of better breast cancer care in countries with higher human development index and healthcare expenditure: Insights from GLOBOCAN 2020. Front. Public Health 2023, 11, 1137286. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- AlQathama, A.; Shao, L.; Bader, A.; Khondkar, P.; Gibbons, S.; Prieto, J.M. Differential Antiproliferative and Anti-Migratory Activities of Ursolic Acid, 3-O-Acetylursolic Acid and Their Combination Treatments with Quercetin on Melanoma Cells. Biomolecules 2020, 10, 894. [Google Scholar] [CrossRef]
- Bednarczyk-Cwynar, B.; Ruszkowski, P.; Bobkiewicz-Kozlowska, T.; Zaprutko, L. Oleanolic Acid A-lactams Inhibit the Growth of HeLa, KB, MCF-7 and Hep-G2 Cancer Cell Lines at Micromolar Concentrations. Anticancer Agents Med. Chem. 2016, 16, 579–592. [Google Scholar] [CrossRef]
- Gheorgheosu, D.; Duicu, O.; Dehelean, C.; Soica, C.; Muntean, D. Betulinic acid as a potent and complex antitumor phytochemical: A minireview. Anticancer Agents Med. Chem. 2014, 14, 936–945. [Google Scholar] [CrossRef]
- Liu, J.; Wu, N.; Ma, L.N.; Zhong, J.T.; Liu, G.; Zheng, L.H.; Lin, X.K. p38 MAPK signaling mediates mitochondrial apoptosis in cancer cells induced by oleanolic acid. Asian Pac. J. Cancer Prev. 2014, 15, 4519–4525. [Google Scholar] [CrossRef]
- Sun, H.; Lv, C.; Yang, L.; Wang, Y.; Zhang, Q.; Yu, S.; Kong, H.; Wang, M.; Xie, J.; Zhang, C.; et al. Solanine induces mitochondria-mediated apoptosis in human pancreatic cancer cells. BioMed Res. Int. 2014, 2014, 805926. [Google Scholar] [CrossRef]
- Wu, J.; Yang, C.; Guo, C.; Li, X.; Yang, N.; Zhao, L.; Hang, H.; Liu, S.; Chu, P.; Sun, Z.; et al. SZC015, a synthetic oleanolic acid derivative, induces both apoptosis and autophagy in MCF-7 breast cancer cells. Chem. Biol. Interact. 2016, 244, 94–104. [Google Scholar] [CrossRef]
- Yang, S.J.; Liu, M.C.; Xiang, H.M.; Zhao, Q.; Xue, W.; Yang, S. Synthesis and in vitro antitumor evaluation of betulin acid ester derivatives as novel apoptosis inducers. Eur. J. Med. Chem. 2015, 102, 249–255. [Google Scholar] [CrossRef]
- Mernyák, E.; Kovács, I.; Minorics, R.; Sere, P.; Czégány, D.; Sinka, I.; Wölfling, J.; Schneider, G.; Újfaludi, Z.; Boros, I.; et al. Synthesis of trans-16-triazolyl-13α-methyl-17-estradiol diastereomers and the effects of structural modifications on their in vitro antiproliferative activities. J. Steroid Biochem. Mol. Biol. 2015, 150, 123–134. [Google Scholar] [CrossRef]
- Agarwal, D.S.; Sakhuja, R.; Beteck, R.M.; Legoabe, L.J. Steroid-triazole conjugates: A brief overview of synthesis and their application as anticancer agents. Steroids 2023, 197, 109258. [Google Scholar] [CrossRef]
- Gomes, A.R.; Pires, A.S.; Roleira, F.M.F.; Tavares-da-Silva, E.J. The structural diversity and biological activity of steroid oximes. Molecules 2023, 28, 1690. [Google Scholar] [CrossRef] [PubMed]
- Jurášek, M.; Černohorská, M.; Řehulka, J.; Spiwok, V.; Sulimenko, T.; Dráberová, E.; Darmostuk, M.; Gurská, S.; Frydrych, I.; Buriánová, R.; et al. Estradiol dimer inhibits tubulin polymerization and microtubule dynamics. J. Steroid Biochem. Mol. Biol. 2018, 183, 68–79. [Google Scholar] [CrossRef]
- Jójárt, R.; Senobar Tahaei, S.A.; Trungel-Nagy, P.; Kele, Z.; Minorics, R.; Paragi, G.; Zupkó, I.; Mernyák, E. Synthesis and evaluation of anticancer activities of 2- or 4-substituted 3-(N-benzyltriazolylmethyl)-13α-oestrone derivatives. J. Enzym. Inhib. Med. Chem. 2021, 36, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.; Mernyak, E.; Wolfling, J.; Sinka, I.; Zupko, I.; Schneider, G. Stereoselective synthesis of the four 16-hydroxymethyl-3-methoxy- and 16-hydroxymethyl-3-benzyloxy-13α-estra-1,3,5(10)-trien-17-ol isomers and their antiproliferative activities. Steroids 2018, 134, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.; Wölfling, J.; Mernyák, E.; Frank, É.; Benke, Z.; Senobar Tahaei, S.A.; Zupkó, I.; Mahó, S.; Schneider, G. Stereocontrolled synthesis of the four possible 3-methoxy and 3-benzyloxy-16-triazolyl-methyl-estra-17-ol hybrids and their antiproliferative activities. Steroids 2019, 152, 108500. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.D.; Khanna, S.; Ramchandani, S.; Kakoti, L.M.; Baruah, A.; Mamidala, V. Prevalence of Molecular Subtypes of Breast Carcinoma and Its Comparison between Two Different Age Groups: A Retrospective Study from a Tertiary Care Center of Northeast India. South Asian J. Cancer 2021, 10, 220–224. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef]
- Derakhshan, F.; Reis-Filho, J.S. Pathogenesis of Triple-Negative Breast Cancer. Annu. Rev. Pathol. 2022, 17, 181–204. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Reutelingsperger, C. Flow cytometry of apoptotic cell death. J. Immunol. Methods 2000, 243, 167–190. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Mehlen, P.; Puisieux, A. Metastasis: A question of life or death. Nat. Rev. Cancer 2006, 6, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Eger, A.; Mikulits, W. Models of epithelial–mesenchymal transition. Drug Discov. Today Dis. Model. 2005, 2, 57–63. [Google Scholar] [CrossRef]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef]
- Franco, E.L.; Schlecht, N.F.; Saslow, D. The epidemiology of cervical cancer. Cancer J. 2003, 9, 348–359. [Google Scholar] [CrossRef]
- Stolnicu, S.; Hoang, L.; Soslow, R.A. Recent advances in invasive adenocarcinoma of the cervix. Virchows Arch. 2019, 475, 537–549. [Google Scholar] [CrossRef]
- Vandeperre, A.; Van Limbergen, E.; Leunen, K.; Moerman, P.; Amant, F.; Vergote, I. Para-aortic lymph node metastases in locally advanced cervical cancer: Comparison between surgical staging and imaging. Gynecol. Oncol. 2015, 138, 299–303. [Google Scholar] [CrossRef]
- Ali, H.; Traj, P.; Szebeni, G.J.; Gémes, N.; Resch, V.; Paragi, G.; Mernyák, E.; Minorics, R.; Zupkó, I. Investigation of the antineoplastic effects of 2-(4-Chlorophenyl)-13α-estrone sulfamate against the HPV16-positive human invasive cervical carcinoma cell line SiHa. Int. J. Mol. Sci. 2023, 24, 6625. [Google Scholar] [CrossRef]
- Cermak, V.; Dostal, V.; Jelinek, M.; Libusova, L.; Kovar, J.; Rosel, D.; Brabek, J. Microtubule-targeting agents and their impact on cancer treatment. Eur. J. Cell Biol. 2020, 99, 151075. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Wilson, V.S.; Bobseine, K.; Gray, L.E., Jr. Development and characterization of a cell line that stably expresses an estrogen-responsive luciferase reporter for the detection of estrogen receptor agonist and antagonists. Toxicol. Sci. 2004, 81, 69–77. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).