Method Development for Determination of Doripenem in Human Plasma via Capillary Electrophoresis Coupled with Field-Enhanced Sample Stacking and Sweeping
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Process
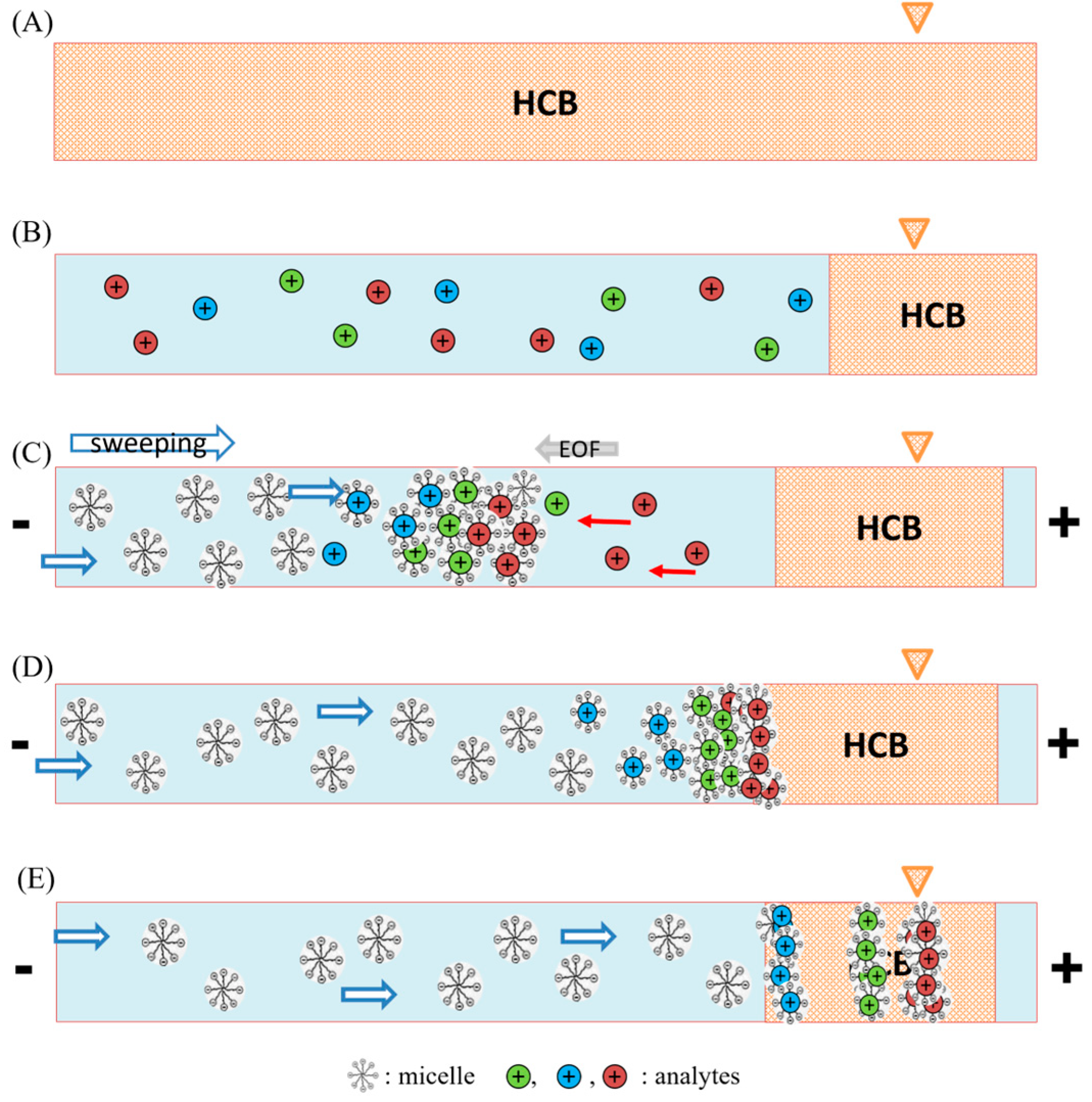
2.2. The Concept of FESS-Sweeping MEKC Model
2.3. Optimization of FESS-Sweeping MEKC Model
2.3.1. The pH Value of Background Solution
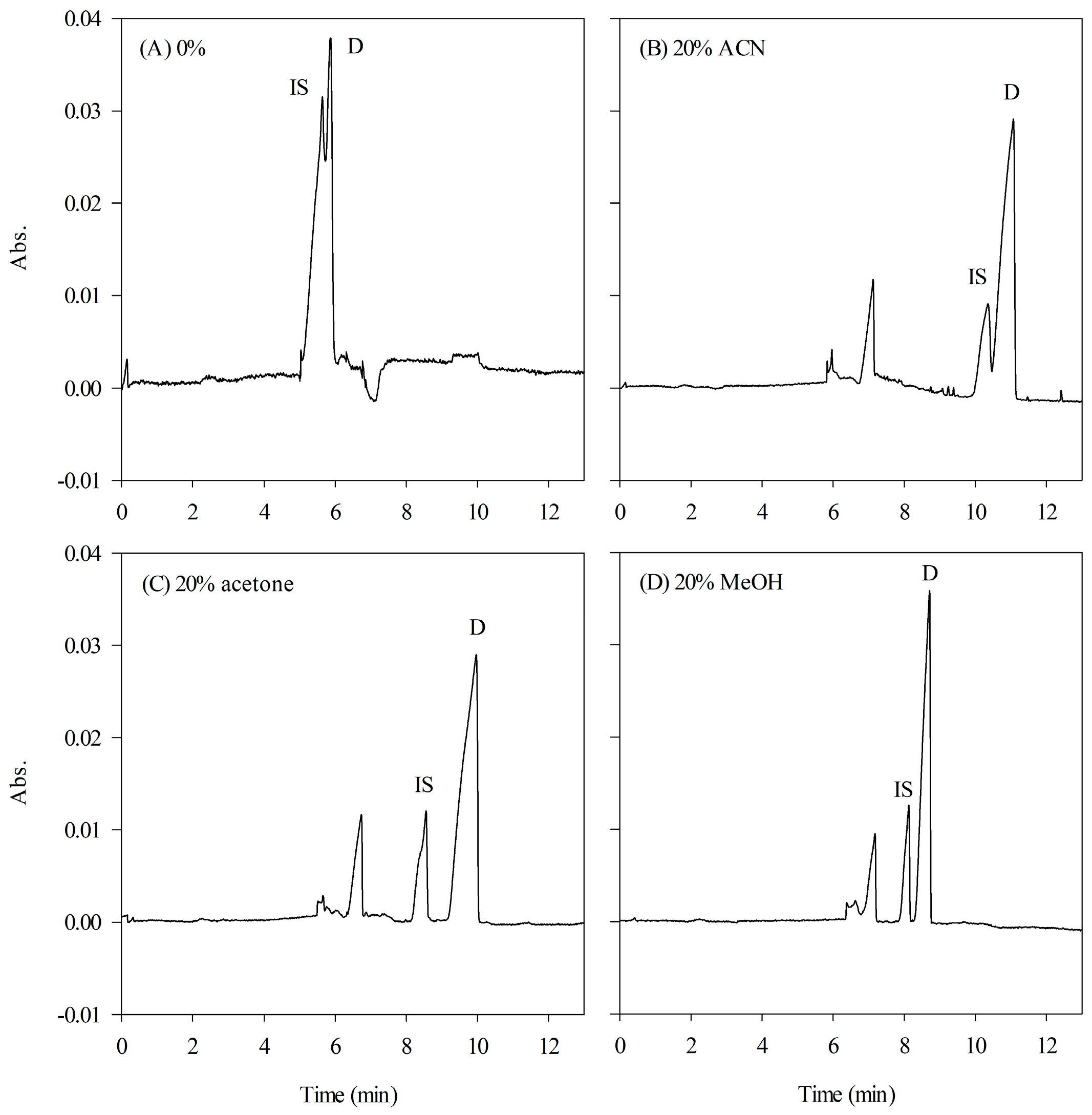
2.3.2. The Organic Modifier in HCB
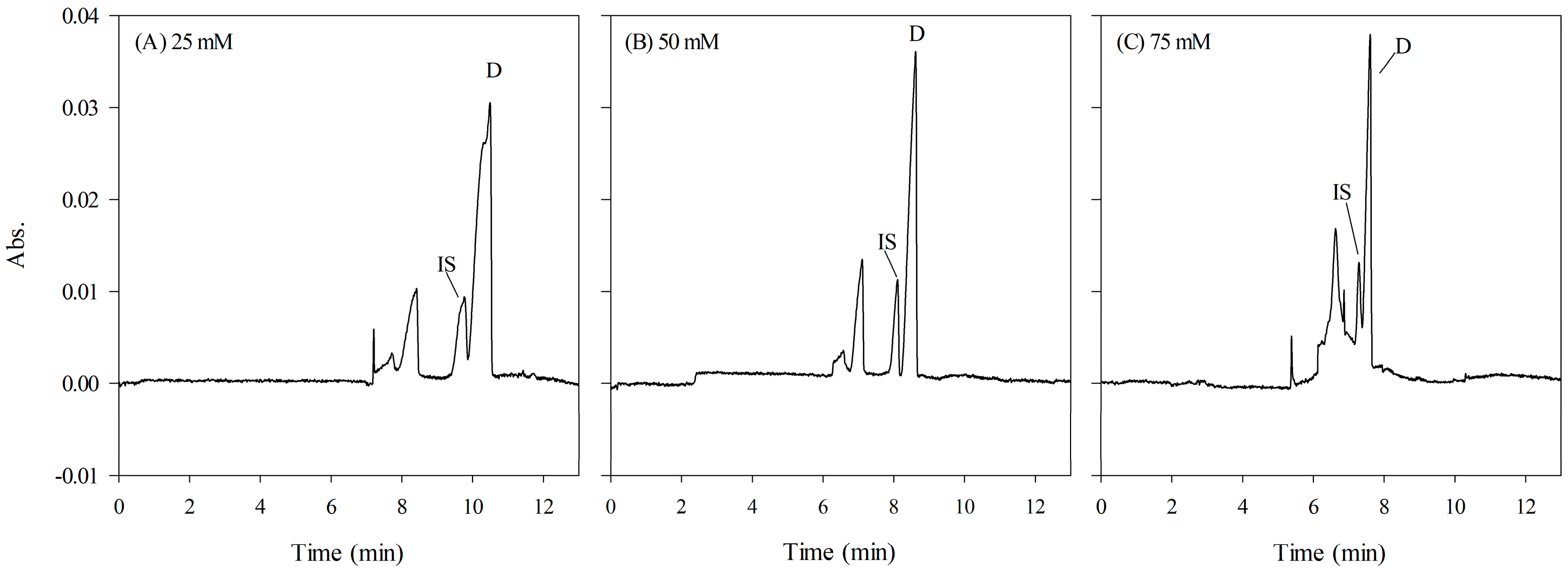
2.3.3. The Phosphate Concentration in HCB and Sample Matrix
2.3.4. Time for Sample Injection and the Concentration of SDS
2.4. Method Validation
2.4.1. Linearity
2.4.2. Accuracy, Precision, and Recovery
2.4.3. Selectivity and Specificity
2.4.4. Limit of Detection (LOD), Limit of Quantification (LOQ), and Sensitivity Enhancement
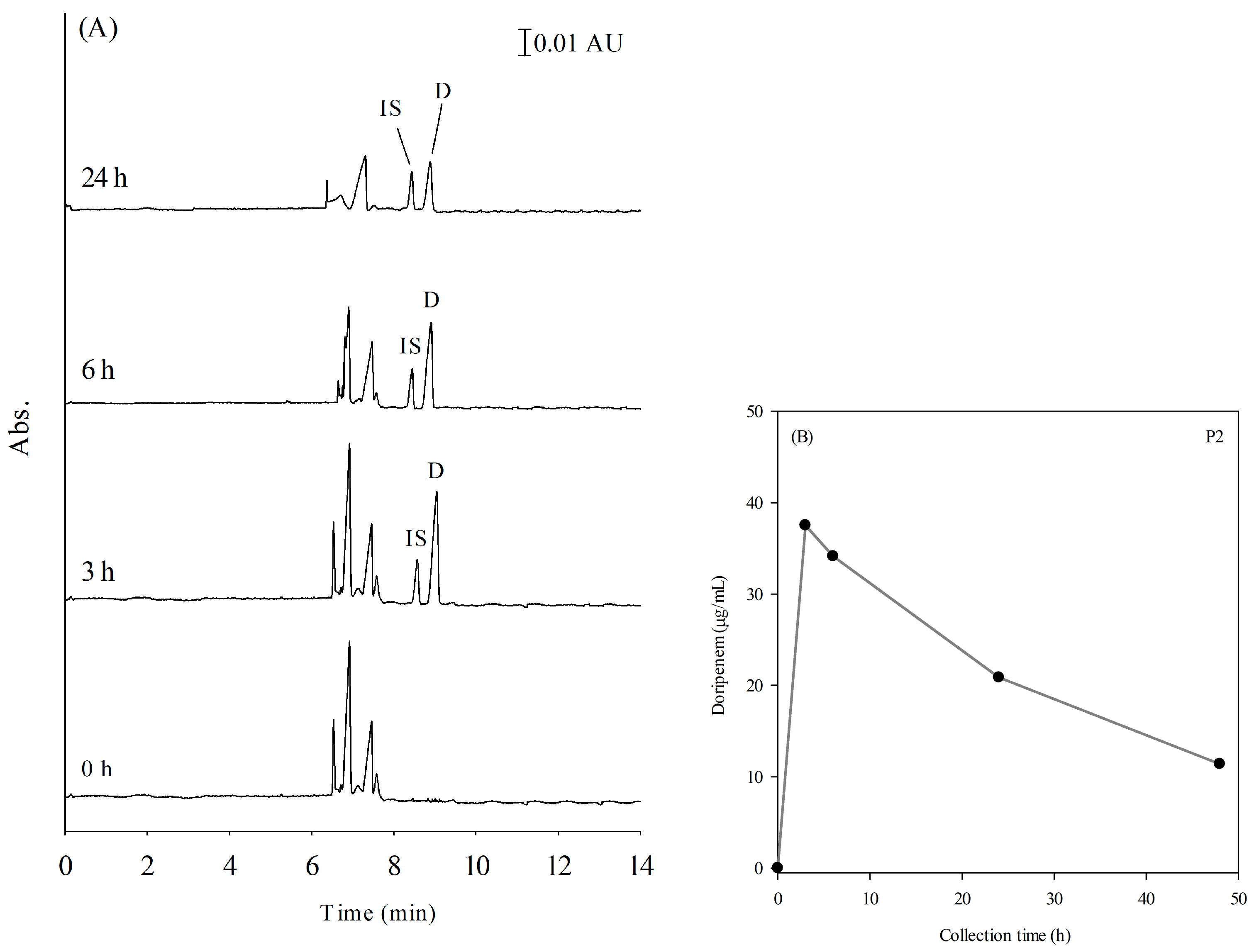
2.5. Application
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Pretreatment and Extraction of Plasma Samples
3.3. CE System
3.4. FESS-Sweeping MEKC Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2020. Available online: https://www.who.int/publications/i/item/9789240005587 (accessed on 30 June 2023).
- Core Elements of Hospital Antibiotic Stewardship Programs from the Centers for Disease Control and Prevention. Atlanta, GA. 2019. Available online: https://www.cdc.gov/antibiotic-use/core-elements/hospital.html (accessed on 30 June 2023).
- Arato, V.; Raso, M.M.; Gasperini, G.; Scorza, F.B.; Micoli, F. Prophylaxis and treatment against klebsiella pneumoniae: Current insights on this emerging anti-microbial resistant global threat. Int. J. Mol. Sci. 2021, 22, 4042. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Lee, J.H.; Lee, J.J.; Park, K.S.; Karim, A.M.; Lee, C.R.; Jeong, B.C.; Lee, S.H. Structural basis for carbapenem-hydrolyzing mechanisms of carbapenemases conferring antibiotic resistance. Int. J. Mol. Sci. 2015, 16, 9654–9692. [Google Scholar] [CrossRef] [PubMed]
- Shionogi & Co. Ltd. DORIBAX® (Doripenem for Injection); Shionogi & Co. Ltd.: Osaka, Japan, 2014. [Google Scholar]
- Patrier, J.; Timsit, J.F. Carbapenem use in critically ill patients. Curr. Opin. Infect. Dis. 2020, 33, 86–91. [Google Scholar] [CrossRef]
- Junior, R.M.; Pereira, G.O.; Tiguman, G.M.B.; Juodinis, V.D.; Telles, J.P.; de Souza, D.C.; Santos, S.R.C.J. Beta-lactams therapeutic monitoring in septic children-what target are we aiming for? a scoping review. Front. Pediatr. 2022, 10, 777854:1–777854:9. [Google Scholar] [CrossRef]
- CDC’s Antibiotic Resistance Reports. 2019. Available online: https://cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. (accessed on 30 June 2023).
- Mazzei, T. The pharmacokinetics and pharmacodynamics of the carbapenems: Focus on doripenem. J Chemother. 2010, 22, 219–225. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, M.I.; Brahim, I.; Hisham, N.; Aladdin, R.; Mohammed, H.; Bahaaeldin, A. Recent updates of carbapenem antibiotics. Eur. J. Med. Chem. 2017, 131, 185–195. [Google Scholar] [CrossRef]
- Monti, G.; Bradic, N.; Marzaroli, M.; Konkayev, A.; Fominskiy, E.; Kotani, Y.; Likhvantsev, V.V.; Momesso, E.; Nogtev, P.; Lobreglio, R.; et al. Continuous vs intermittent meropenem administration in critically ill patients with sepsis the mercy randomized clinical trial. Jama-J. Am. Med. Assoc. 2023, 330, 141–151. [Google Scholar] [CrossRef]
- Fratoni, A.J.; Nicolau, D.P.; Kuti, J.L. A guide to therapeutic drug monitoring of beta-lactam antibiotics. Pharmacotherapy 2021, 41, 220–233. [Google Scholar] [CrossRef]
- Lanckohr, C.; Boeing, C.; De Waele, J.J.; de Lange, D.W.; Schouten, J.; Prins, M.; Nijsten, M.; Povoa, P.; Morris, A.C.; Bracht, H. Antimicrobial stewardship, therapeutic drug monitoring and infection management in the ICU: Results from the international A-TEAMICU survey. Ann. Intensive Care 2021, 11, 131. [Google Scholar] [CrossRef]
- Mabilat, C.; Gros, M.F.; Nicolau, D.; Mouton, J.W.; Textoris, J.; Roberts, J.A.; Cotta, M.O.; van Belkum, A.; Caniaux, I. Diagnostic and medical needs for therapeutic drug monitoring of antibiotics. Eur. J. Clin. Microbiol. 2020, 39, 791–797. [Google Scholar] [CrossRef]
- Sutherland, C.; Nicolau, D.P. Development of an HPLC method for the determination of doripenem in human and mouse serum. J. Chromatogr. B 2007, 853, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Dailly, E.; Bouquié, R.; Deslandes, G.; Jolliet, P.; Le Floch, R. A liquid chromatography assay for a quantification of doripenem, ertapenem, imipenem, meropenem concentrations in human plasma: Application to a clinical pharmacokinetic study. J. Chromatogr. B 2011, 879, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, T.; Suzuki, A.; Niwa, T.; Ushikoshi, H.; Shirai, K.; Yoshida, S.; Ogura, S.; Itoh, Y. Simultaneous determination of eight β-lactam antibiotics in human serum by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2011, 879, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Colin, P.; De Bock, L.; T’Jollyn, H.; Boussery, K.; Van Bocxlaer, J. Development and validation of a fast and uniform approach to quantify β-lactam antibiotics in human plasma by solid phase extraction-liquid chromatography–electrospray-tandem mass spectrometry. Talanta 2013, 103, 285–293. [Google Scholar] [CrossRef]
- Kai, M.; Tanaka, R.; Suzuki, Y.; Goto, K.; Ohchi, Y.; Yasuda, N.; Tatsuta, R.; Kitano, T.; Itoh, H. Simultaneous quantification of plasma levels of 12 antimicrobial agents including carbapenem, anti-methicillin-resistant Staphylococcus aureus agent, quinolone and azole used in intensive care unit using UHPLC-MS/MS method. Clin. Biochem. 2021, 90, 40–49. [Google Scholar] [CrossRef]
- Grochocki, W.; Markuszewski, M.J.; Quirino, J.P. Three-step stacking of cationic analytes by field-enhanced sample injection, sweeping, and micelle to solvent stacking in capillary electrophoresis. J. Chromatogr. A 2015, 1424, 111–117. [Google Scholar] [CrossRef]
- Simpson, S.L., Jr.; Quirino, J.P.; Terabe, S. On-line sample preconcentration in capillary electrophoresis: Fundamentals and applications. J. Chromatogr. A 2008, 1184, 504–541. [Google Scholar] [CrossRef]
- Lin, H.-J.; Hsieh, K.-P.; Chiou, S.-S.; Kou, H.-S.; Wu, S.-M. Determination of deferasirox in human plasma by short-end injection and sweeping with a field-amplified sample stacking and micellar electrokinetic chromatography. J. Pharm. Biomed. Anal. 2016, 131, 497–502. [Google Scholar] [CrossRef]
- Liu, T.L.; Fang, L.S.; Liou, J.R.; Dai, J.S.; Chen, Y.L. Determination of quetiapine and its metabolites in plasma by field-enhanced sample stacking. J. Food Drug. Anal. 2021, 29, 709–716. [Google Scholar] [CrossRef]
- Wang, L.; MacDonald, D.; Huang, X.; Chen, D.D. Capture efficiency of dynamic pH junction focusing in capillary electrophoresis. Electrophoresis 2016, 37, 1143–1150. [Google Scholar] [CrossRef]
- Aranas, A.T.; Guidote, A.M.; Quirino, J.P. Sweeping and new on-line sample preconcentration techniques in capillary electrophoresis. Anal. Bioanal. Chem. 2009, 394, 175–185. [Google Scholar] [CrossRef]
- Hirokawa, T.; Okamoto, H.; Gaš, B. High-sensitive capillary zone electrophoresis analysis by electrokinetic injection with transient isotachophoretic preconcentration: Electrokinetic supercharging. Electrophoresis 2003, 24, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Paliosa, P.K.; Garcia, C.V.; Schapoval, E.E.S.; Mendez, A.S.L.; Steppe, M. Quantitative determination of the β-methyl carbapenem doripenem in powder for injection by a stability-indicating capillary zone electrophoresis method. Die Pharm. 2015, 70, 569–573. [Google Scholar] [CrossRef]
- Michalska, K.; Pajchel, G.; Tyski, S. Determination of doripenem and related substances in medicinal product using capillary electrophoresis. J. Sep. Sci. 2011, 34, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S.; Hamase, K.; Kinoshita, A.; Zaitsu, K. Simple and rapid analytical method for carbapenems using capillary zone electrophoresis. J. Chromatogr. B Biomed. 1999, 727, 219–225. [Google Scholar] [CrossRef]
- Pham, T.N.M.; Le, T.B.; Le, D.D.; Ha, T.H.; Nguyen, N.S.; Pham, T.D.; Hauser, P.C.; Nguyen, T.A.H.; Mai, T.D. Determination of carbapenem antibiotics using a purpose-made capillary electrophoresis instrument with contactless conductivity detection. J. Pharm. Biomed. Anal. 2020, 178, 112906:1–112906:8. [Google Scholar] [CrossRef] [PubMed]
- Michalska, K.; Cielecka-Piontek, J.; Pajchel, G.; Tyski, S. Determination of biapenem in a medicinal product by micellar electrokinetic chromatography with sweeping in an enhanced electric field. J. Chromatogr. A 2013, 1282, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Kitahashi, T.; Furuta, I. Determination of meropenem by capillary electrophoresis using direct injection of serum. J. Chromatogr. Sci. 2005, 43, 430–433. [Google Scholar] [CrossRef][Green Version]
- Michalska, K.; Pajchel, G.; Tyski, S. Different sample stacking strategies for the determination of ertapenem and its impurities by micellar electrokinetic chromatography in pharmaceutical formulation. J. Chromatogr. A 2009, 1216, 2934–2942. [Google Scholar] [CrossRef]
- Hansen, S.H.; Pedersen-Bjergaard, S. Bioanalysis of Pharmaceuticals: Sample Preparation, Separation Techniques, and Mass Spectrometry; Hansen, S.H., Pedersen-Bjergaard, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 73–122. [Google Scholar]
- Moldoveanu, S.; David, V. Chapter 7—Solid-Phase Extraction. In Modern Sample Preparation for Chromatography; Moldoveanu, S., David, V., Serban Moldoveanu, V.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 191–286. [Google Scholar]
- Hendriks, G.; Uges, D.; Franke, J. PH adjustment of human blood plasma prior to bioanalytical sample preparation. J. Pharm. Biomed. Anal. 2008, 47, 126–133. [Google Scholar] [CrossRef]
- ICH Harmonized Tripartite Guideline: Validation of Analytical Procedures. Text and Methodology, Q2(R1)-Step 4; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. 2005. Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 30 June 2023).
- Aboulatta, L.; Sugita, H.; Wakabayashi, H.; Noma, H.; Sasaki, T. Comparison of extended versus intermittent infusion of antipseudomonal beta-lactams for the treatment of critically ill patients with respiratory infections: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 98, 41–50. [Google Scholar] [CrossRef] [PubMed]


| (A) Doripenem | (B) Meropenem | ||
|---|---|---|---|
 | MW 1 = 438.52 pKa1 = 4.37; pKa2 = 9.39 | 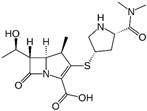 | MW 1 = 383.46 pKa1 = 4.37; pKa2 = 8.34 |
| Doripenem | Linearity (1–45 μg/mL) | Precision and Accuracy | |||
|---|---|---|---|---|---|
| Regression Equation | r2 | Spiked Conc. (μg/mL) | RSD (%) | RE (%) | |
| Intra-day (n = 3) | y = (0.106 ± 0.0059) x + (0.0337 ± 0.0259) | 0.9996 | 5.00 25.00 40.00 | 1.29 1.20 1.35 | −3.01 3.33 2.05 |
| Inter-day (n = 5) | y = (0.1087 ± 0.0037) x + (0.0394 ± 0.0394) | 0.9995 | 5.00 25.00 40.00 | 5.86 1.17 1.96 | −4.63 2.36 2.01 |
| Patient | Daily Dose | Dosage Interval | Collection Time Point (h) | Concentration Ranges (Premedication/Post Administration, μg/mL) | ||||
|---|---|---|---|---|---|---|---|---|
| Continuous administration | ||||||||
| P1 | 1.5 g | 1.5 g per day | 0 | 4.5 | 6 | 24 | 48 | 0/15.06–39.00 |
| P2 | 1.5 g | 1.5 g per day | 0 | 3 | 6 | 24 | 48 | 0/11.39–37.53 |
| Intermittent administration | ||||||||
| P3 | 1.5 g | 0.5 g per 8 h | 0 | 1 | 2 | 6 | 8 | 0/9.37–44.97 |
| P4 | 0.5 g | 0.25 g per 12 h | 0 | 0.75 | 2 | 4 | 8 | 0/9.64–44.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, H.-H.; Lin, Y.-C.; Hung, C.-C.; Hou, Y.-C.; Lin, Y.-H. Method Development for Determination of Doripenem in Human Plasma via Capillary Electrophoresis Coupled with Field-Enhanced Sample Stacking and Sweeping. Int. J. Mol. Sci. 2023, 24, 13751. https://doi.org/10.3390/ijms241813751
Liang H-H, Lin Y-C, Hung C-C, Hou Y-C, Lin Y-H. Method Development for Determination of Doripenem in Human Plasma via Capillary Electrophoresis Coupled with Field-Enhanced Sample Stacking and Sweeping. International Journal of Molecular Sciences. 2023; 24(18):13751. https://doi.org/10.3390/ijms241813751
Chicago/Turabian StyleLiang, Hsin-Hua, Yu-Chao Lin, Chin-Chuan Hung, Yu-Chi Hou, and Yi-Hui Lin. 2023. "Method Development for Determination of Doripenem in Human Plasma via Capillary Electrophoresis Coupled with Field-Enhanced Sample Stacking and Sweeping" International Journal of Molecular Sciences 24, no. 18: 13751. https://doi.org/10.3390/ijms241813751
APA StyleLiang, H.-H., Lin, Y.-C., Hung, C.-C., Hou, Y.-C., & Lin, Y.-H. (2023). Method Development for Determination of Doripenem in Human Plasma via Capillary Electrophoresis Coupled with Field-Enhanced Sample Stacking and Sweeping. International Journal of Molecular Sciences, 24(18), 13751. https://doi.org/10.3390/ijms241813751







