Ion fluxes Involved in the Adaptation of the Estuarine Diatom Coscinodiscus centralis Ehrenberg to Salinity Stress
Abstract
1. Introduction
2. Results
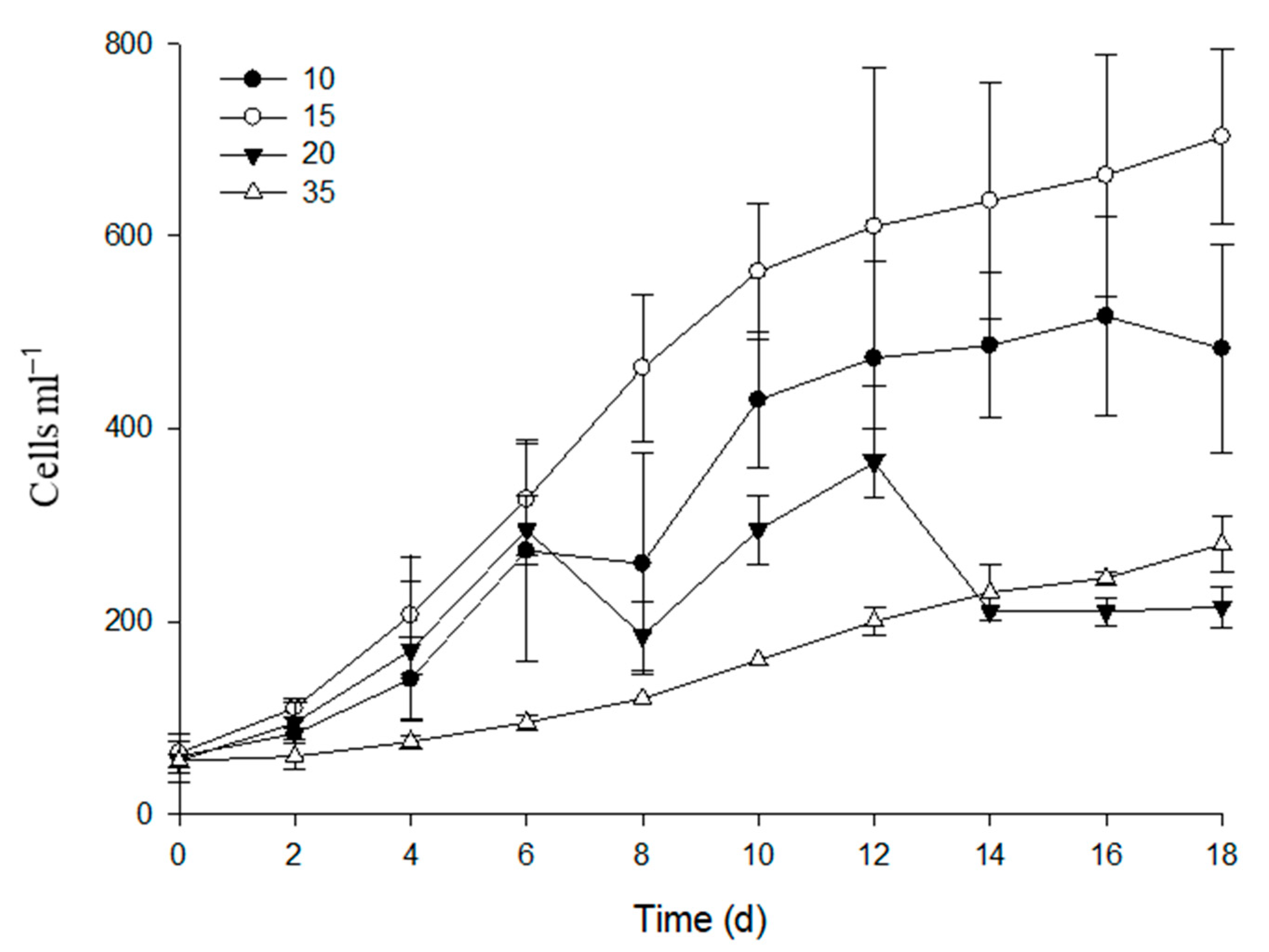
2.1. Salinity Optima for Growth in Cultures
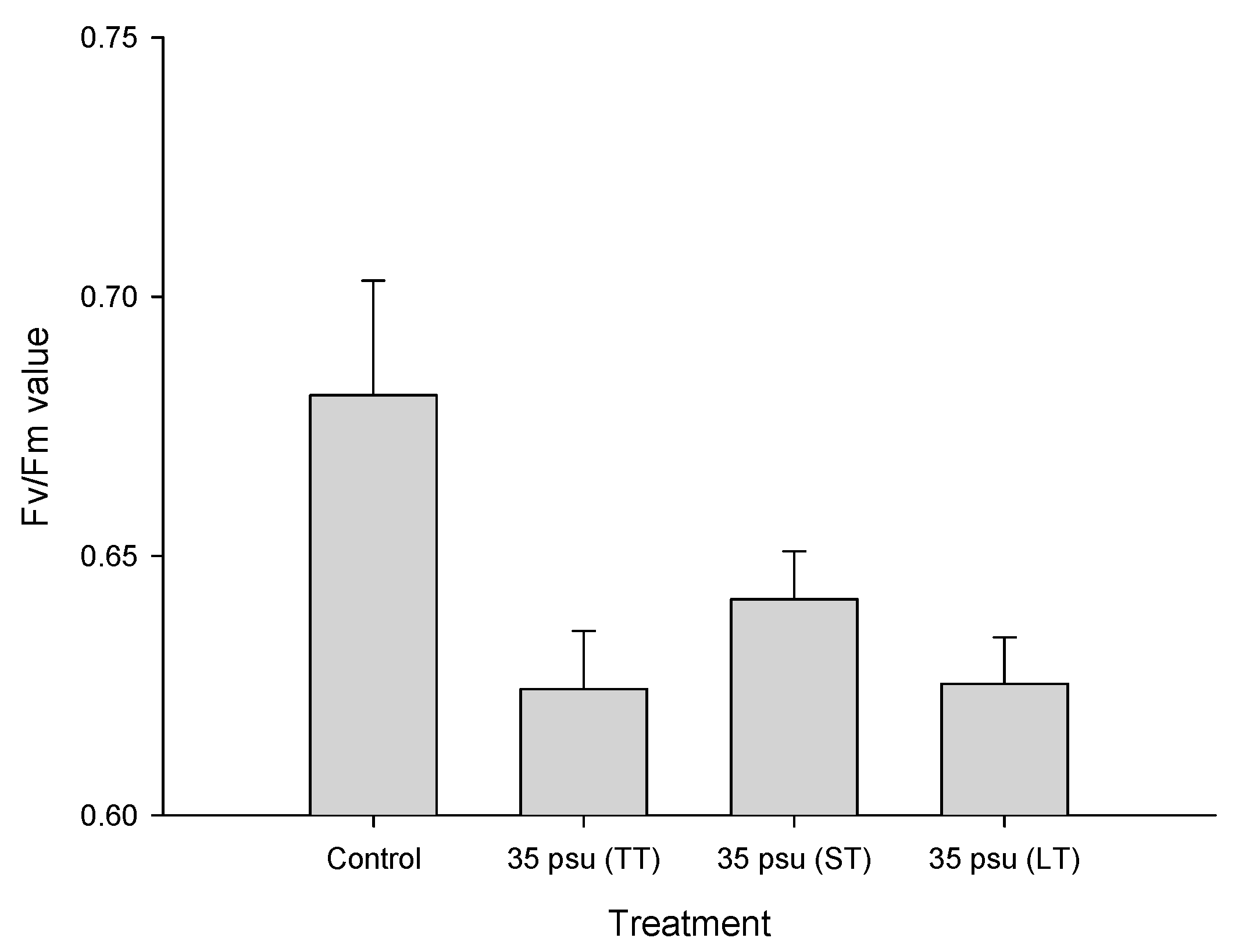
2.2. Response of Fv/Fm to Salinity Stress
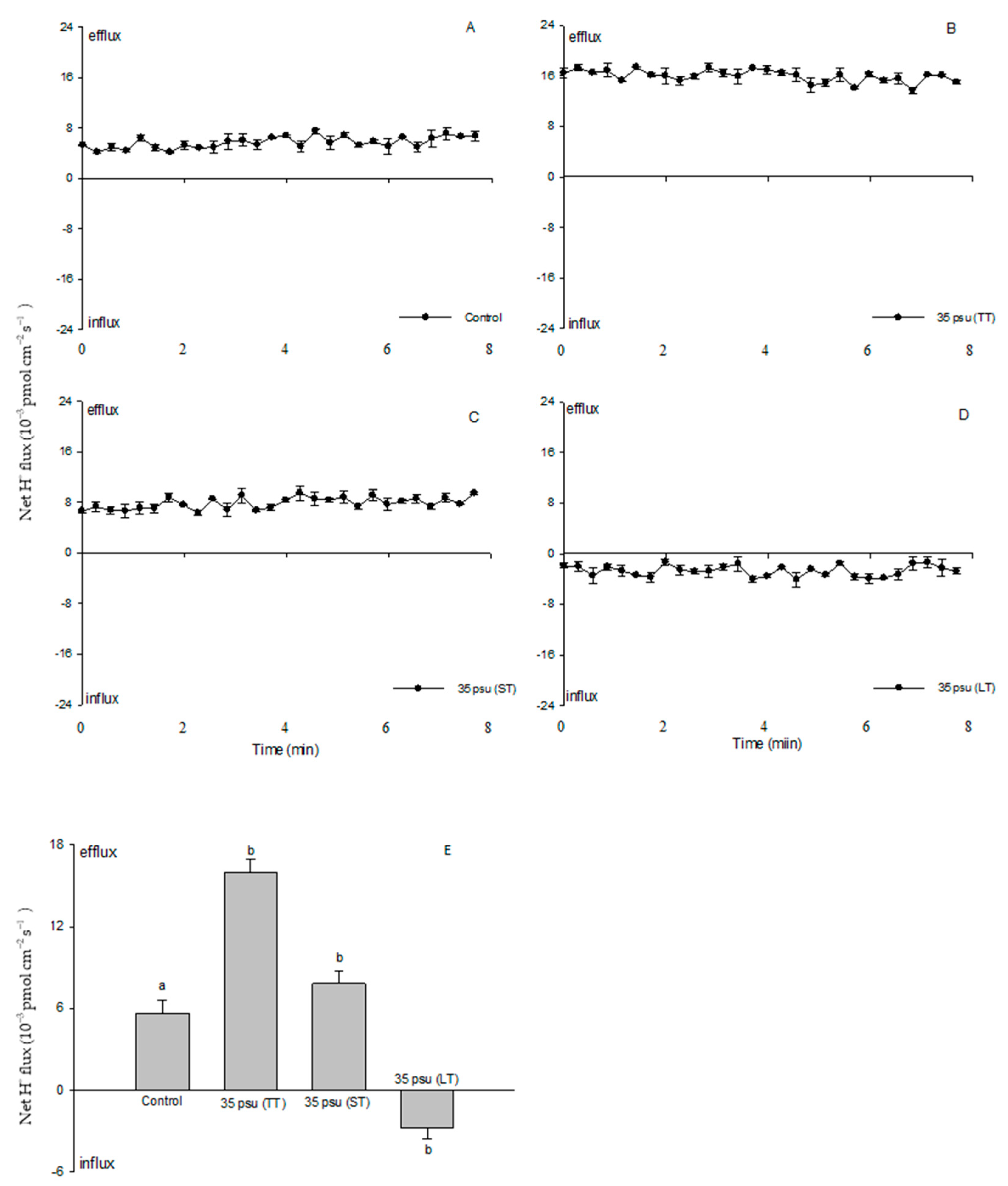
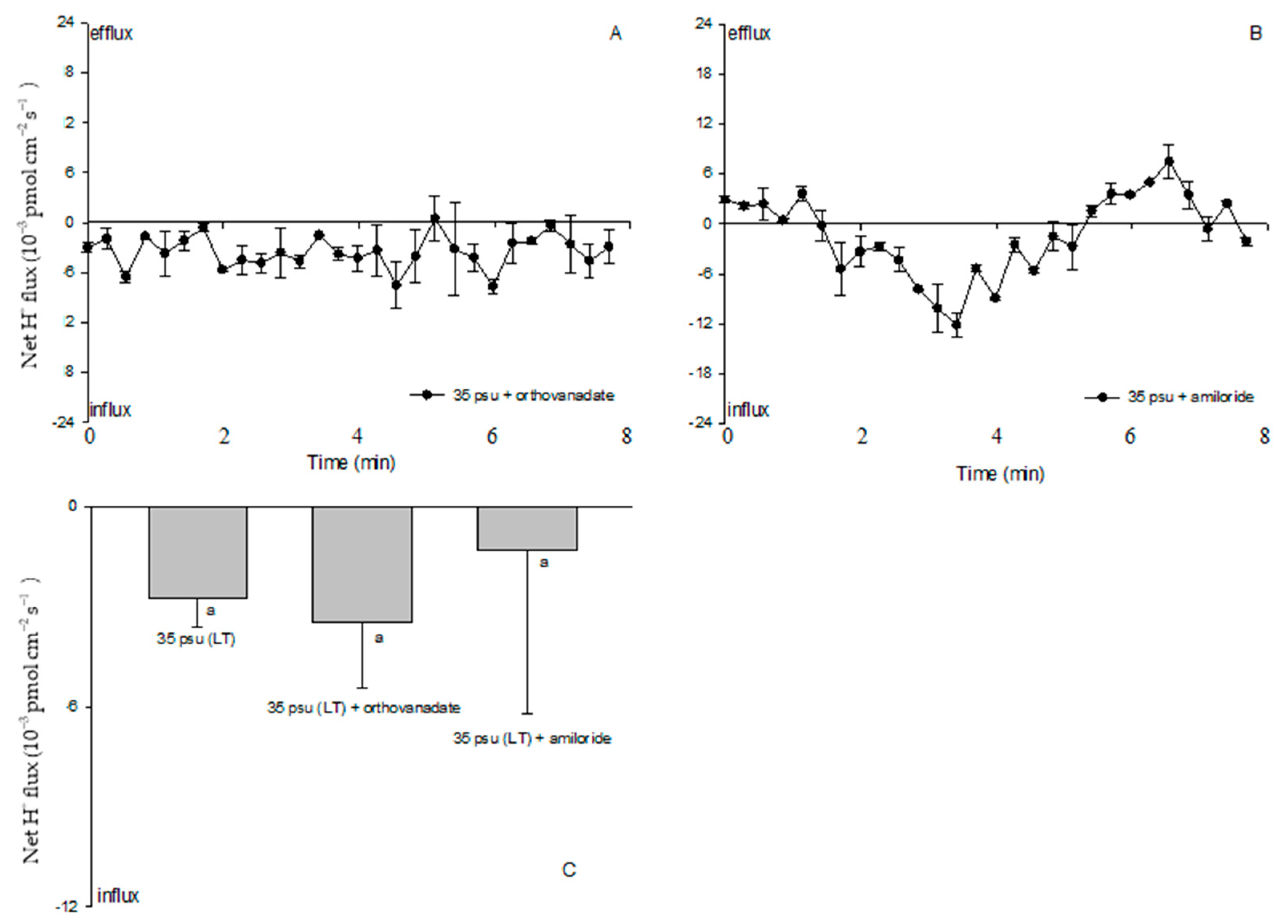
2.3. Ion Fluxes in Response to Salt Stress in the Estuarine Diatom C. centralis: H+ Fluxes
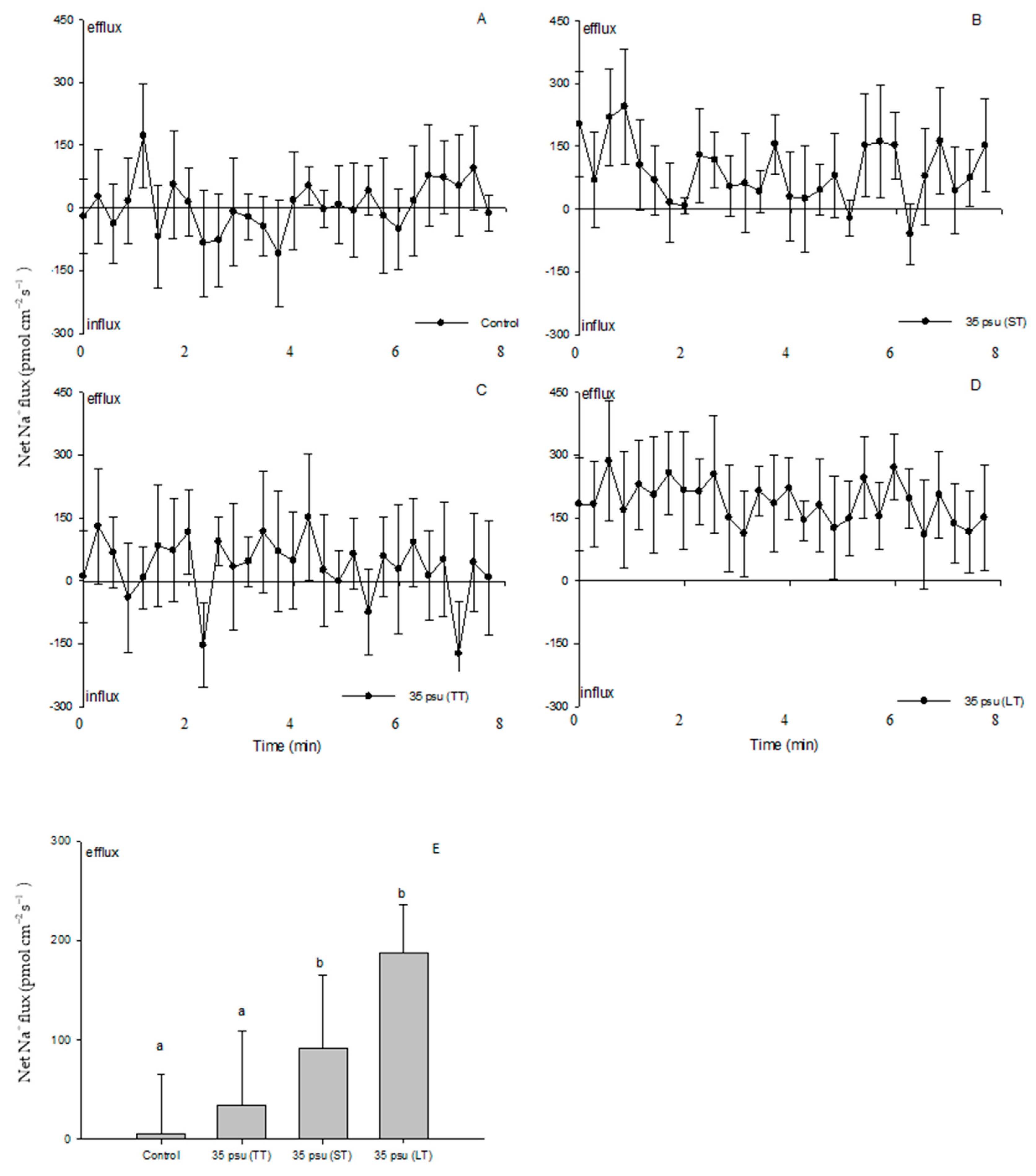
2.4. Na+ Fluxes
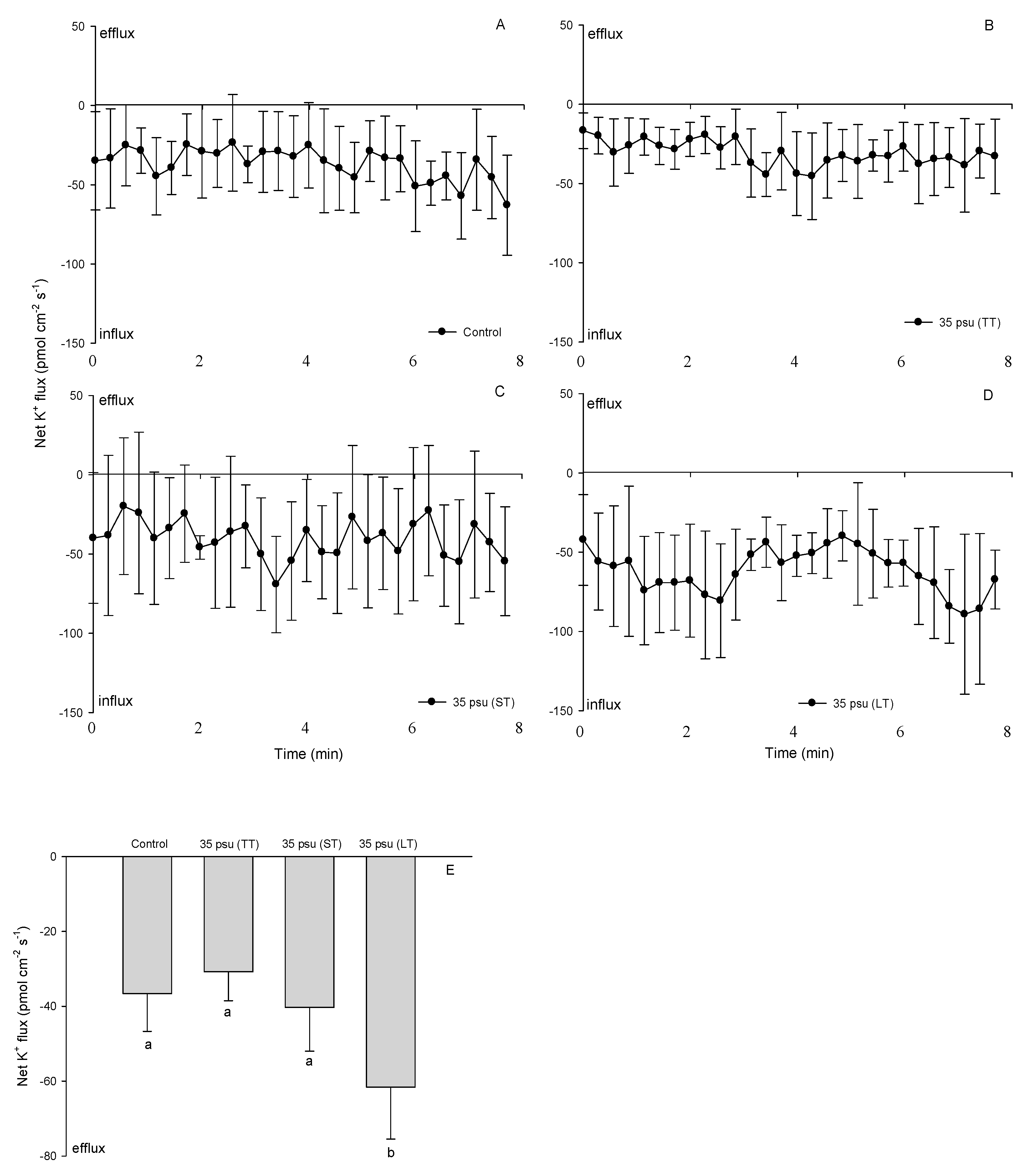
2.5. K+ Fluxes
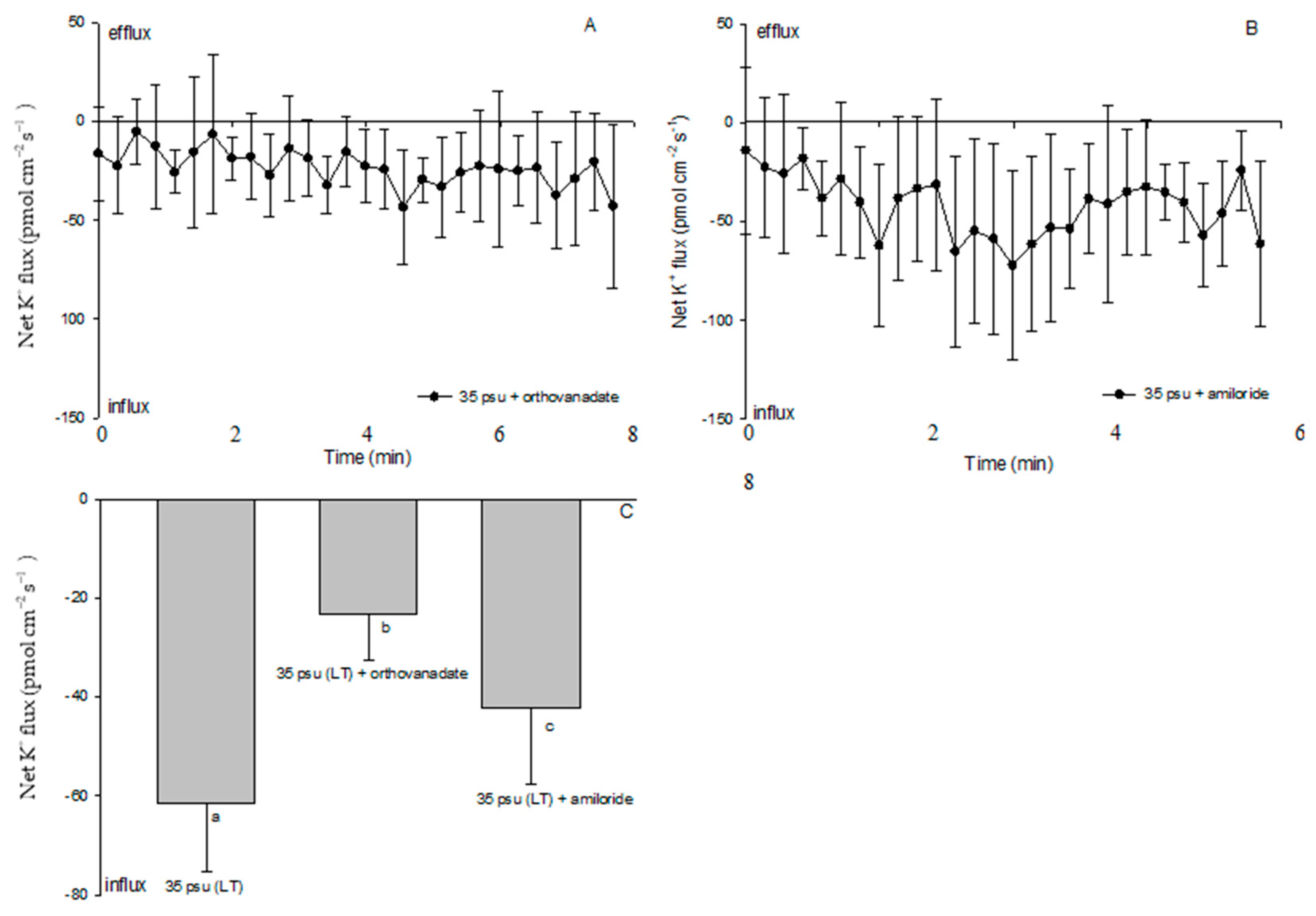
2.6. Effects of PM Transport Inhibitors on H+, Na+, and K+ Fluxes
3. Discussion
3.1. Growth under Salinity Stress
3.2. Na+/H+ Antiport across the PM under Salinity Stress
3.3. K+ Fluxes under Salinity Stress
4. Conclusions
5. Materials and Methods
5.1. Culture Conditions
5.2. Determination of Optimum Salinity
5.3. Treatments
5.4. Measurement of Maximal Quantum Yield of Photosystem II (Fv/Fm) under Different Salinity Stresses
5.5. Measurements of Net H+, Na+, and K+ Fluxes via a Scanning Ion-Selective Electrode Technique (SIET)
5.6. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Kirst, G.O. Salinity tolerance of eukaryotic marine algae. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 21–53. [Google Scholar] [CrossRef]
- Vos, P.C.; de Wolf, H. Diatoms as a tool for reconstructing sedimentary environments in coastal wetlands, methodolocial aspects. Hydrobiologia 1993, 269, 285–296. [Google Scholar] [CrossRef]
- Muylaert, K.; Sabbe, K.; Vyverman, W. Spatial and temporal dynamics of phytoplankton communities in a freshwater tidal estuary (Schelde, Belgium). Estuar. Coast. Shelf Sci. 2000, 50, 673–687. [Google Scholar] [CrossRef]
- Lionard, M.; Muylaert, K.; Van Gansbeke, D.; Vyverman, W. Influence of changes in salinity and light internsity on growth of phytoplankton communities form the Schelde river and estuary (Belgium/The Netherlands). Hydrobiologia 2005, 540, 105–115. [Google Scholar] [CrossRef]
- Brand, L.E. The salinity tolerance of forty-six marine phytoplankton isolates. Estuar. Coast. Shelf Sci. 1984, 18, 543–556. [Google Scholar] [CrossRef]
- Trobajo, R.; Rovira, L.; Mann, D.G.; Cox, E.J. Effects of salinity on growth and on valve morphology of five estuarine diatoms. Phycol. Res. 2011, 59, 83–90. [Google Scholar] [CrossRef]
- Balzano, S.; Sarno, D.; Kooistra, W.H.C.E. Effects of salinity on the growth rate and morphology of ten Skeletonema strains. J. Plankton Res. 2011, 33, 937–945. [Google Scholar] [CrossRef]
- Underwood, G.J.C. Seasonal and spatial variation in epipelic diatom assemblages in the Severn estuary. Diatom Res. 1994, 9, 451–472. [Google Scholar] [CrossRef]
- Snoeijs, P. Distribution of epiphytic diatom species compostion, diversity and biomass on different macroalgal hosts along seasonal and salinity gradients in the Baltic Sea. Diatom Res. 1994, 9, 189–211. [Google Scholar] [CrossRef]
- Underwood, G.J.C.; Philips, J.; Saunders, K. Distribution of estuarine benthic diatom species along salinity and nutrient gradients. Eur. J. Phycol. 1998, 33, 173–183. [Google Scholar] [CrossRef]
- Hellebust, J.A. Effect of salinity on photosynthesis and mannitol synthesis in the green flagellate Platymonas suecica. Can. J. Bot. 1976, 54, 1735–1741. [Google Scholar] [CrossRef]
- Kinraide, T.B. Interactions among Ca2+, Na+ and K+ in salinity toxicity: Quantitative resolution of multiple toxic and ameliorative effects. J. Exp. Bot. 1999, 50, 1495–1505. [Google Scholar] [CrossRef]
- Serrano, R.; Mulet, J.M.; Rios, G.; Marquez, J.A.; de Larrinoa, I.F.; Leube, M.P.; Mendizabal, I.; Pascualahuir, A.; Proft, M.; Ros, R.; et al. A glimpse of the mechanisms of ion homeostasis during salt stress. J. Exp. Bot. 1999, 50, 1023–1036. [Google Scholar] [CrossRef]
- Bental, M.; Degani, H.; Avron, M. 23Na-NMR studies of the intracellular sodium ion concentration in the halotolerant alga Dunaliella salina. Plant Physiol. 1988, 87, 813–817. [Google Scholar]
- Shabala, L.; Cuin, T.A.; Newman, I.A.; Shabala, S. Salinity-induced ion flux patterns from the excised roots of Arabidopsis sos mutants. Planta 2005, 222, 1041–1050. [Google Scholar]
- Katz, A.; Bental, M.; Degani, H.; Avron, M. In vivo pH regulation by a Na+/H+ antiporter in the halotolerant alga Dunaliella salina. Plant Physiol. 1991, 96, 110–115. [Google Scholar] [CrossRef][Green Version]
- Stamatakis, K.; Ladas, N.P.; Alygizakizoba, A.; Papageorgiou, G.C. Sodium chloride-induced volume changes of freshwater cyanobacterium Synechococcus sp. PCC 7942 cells can be probed by chlorophyll a fluorescence. Arch. Biochem. Biophys. 1999, 370, 240–249. [Google Scholar] [CrossRef]
- Taylor, A.R.; Brownlee, C.; Wheeler, G. Proton channels in algae: Reasons to be excited. Trends Plant Sci. 2012, 17, 675–684. [Google Scholar]
- DeCoursey, T.E. Voltage-gated proton channels find their dream job managing the respiratory burst in phagocytes. Physiology 2010, 25, 27–40. [Google Scholar]
- Borowitzka, L.; Brown, A.D. The salt relations of marine and halophylic species of the unicellular green alga Dunaliella. Arch. Microbiol. 1974, 96, 37–55. [Google Scholar] [CrossRef]
- Balnokin, Y.V.; Medveder, I.V.; Bodna, I.V. Potassium transport systems in cells of the halophylic alga Dunaliella. Sov. Plant Physiol. 1984, 30, 718–726. [Google Scholar]
- Fuji, S.; Nishimoto, N.; Notoya, A.; Hellebust, J.A. Growth and osmoregulation of Chaetoceros muelleri in relation to salinity. Plant Cell Physiol. 1995, 36, 759–764. [Google Scholar]
- Pick, U.; Karni, L.; Avron, M. Determination of ion content and ion fluxes in the halotolerant alga Dunaliella salina. Plant Physiol. 1986, 81, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Newman, I.; Zhou, M.; Mendham, N.; Zhang, G.; Shabala, S. Screening plants for salt tolerance by measuring K+ flux: A case study for barley. Plant Cell Environ. 2005, 28, 1230–1246. [Google Scholar]
- Chen, Z.; Zhou, M.; Zewman, I.A.; Zhang, G.; Shabala, S. Potassium and sodium relations in salinized barley tissues as a basis of differential salt tolerance. Funct. Plant Biol. 2007, 34, 150–162. [Google Scholar] [CrossRef]
- Wegmann, K. Osmoregulation in eukaryotic algae. FEMS Microbiol. Rev. 1986, 39, 37–43. [Google Scholar]
- Clavero, E.; Hernandez-Marine, M.; Grimalt, J.O.; Garcia-Pichel, F. Salinity tolerance of diatoms from thalassic Hypersalinie environments. J. Phycol. 2000, 36, 1021–1034. [Google Scholar]
- Leterme, S.C.; Prime, E.; Mitchell, J.; Brown, M.H.; Ellis, A.V. Diatom adaptability to environmental change: A case study of two Cocconeis specis from high salinity areas. Diatom Res. 2013, 28, 29–35. [Google Scholar]
- Chen, S.; Li, S.W. Relationships between the movement of chloroplasts and cytoskeletons in diatoms. Bot. Mar. 1991, 34, 501–511. [Google Scholar]
- Lew, R.R. Ion and oxygen fluxes in the unicellular alga Eremosphaera viridis. Plant Cell Physiol. 2010, 51, 1889–1899. [Google Scholar]
- Makita, N.; Shihira-Ishikawa, I. Chloroplast assemblage by mechanical stimulation and its intercellular transmission in diatom cells. Protoplasma 1997, 197, 86–95. [Google Scholar]
- Taylor, A.R.; Chrachri, A.; Wheeler, G.; Goddard, H.; Brownlee, C.A. Voltage-Gated H+ Channel Underlying pH Homeostasis in Calcifying Coccolithophores. PLoS Biol. 2011, 9, 1–14. [Google Scholar]
- Morth, J.P.; Pedersen, B.P.; Buch-Pedersen, M.J.; Andersen, J.P.; Vilsen, B.; Palmgren, M.G.; Nissen, P. A structural overview of the plasma membrane Na+, K+-ATPase and H+-ATPase ion pumps. Nat. Rev. Mol. Cell Biol. 2011, 12, 60–70. [Google Scholar] [PubMed]
- Boyd, C.N.; Gradmann, D. Electrophysiology of the marine diatom Coscinodiscus wailesii III. Uptake of nitrate and ammonium. J. Exp. Bot. 1999, 50, 461–467. [Google Scholar]
- Hildebrand, M. Cloning and functional characterization of ammonium transporters from the marine diatom Cylindrotheca fusiformis (Bacillariophyceae). J. Phycol. 2005, 41, 105–113. [Google Scholar]
- Gimmler, H.; Schirling, R. Cation permeability of the plasmalemma of the halotolerant alga Dunaliella parva. II. Cation content and glycerol concentration of the cells as dependent upon external NaCl concentration. Z. Pflanzenphysiol. 1978, 87, 435–444. [Google Scholar]
- Ginzburg, M. Measurements of ion concentrations in Dunaliella parva subjected to hypertonic shocks. J. Exp. Bot. 1981, 32, 333–340. [Google Scholar]
- Ehrenfeld, J.; Cousin, J.L. Ionic regulation of unicellular the green alga Dunaliella tertiolecta: Response to hypertonic shock. J. Membr. Biol. 1984, 77, 45–55. [Google Scholar] [CrossRef]
- Maathuis, F.J.M.; Amtmann, A. K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios. Annu. Bot. 1999, 84, 123–133. [Google Scholar]
- Kempf, B.; Bremer, E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 1998, 170, 319–330. [Google Scholar]
- Jin, D.X.; Cheng, Z.D.; Lin, J.M.; Liu, S. The Marine Benthic Diatoms in China; China Ocean Press: Beijing, China, 1985; Volume 1, 313p. [Google Scholar]
- Berges, J.A.; Franklin, D.J.; Harrison, P.J. Evolution of an artificial seawater medium: Improvements in enriched seawater, artificial water over the last two decades. J. Phycol. 2001, 37, 1138–1145. [Google Scholar] [CrossRef]
- Lin, A.P.; Wang, G.C.; Zhou, W.Q. Simultaneous measurements of H+ and O2 fluxes in Zostera marina and its physiological implications. Physiol. Plant. 2012, 148, 582–589. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Hu, X.; Gao, Y.; Liang, J.; Sun, L. Ion fluxes Involved in the Adaptation of the Estuarine Diatom Coscinodiscus centralis Ehrenberg to Salinity Stress. Int. J. Mol. Sci. 2023, 24, 13683. https://doi.org/10.3390/ijms241813683
Chen C, Hu X, Gao Y, Liang J, Sun L. Ion fluxes Involved in the Adaptation of the Estuarine Diatom Coscinodiscus centralis Ehrenberg to Salinity Stress. International Journal of Molecular Sciences. 2023; 24(18):13683. https://doi.org/10.3390/ijms241813683
Chicago/Turabian StyleChen, Changping, Xiao Hu, Yahui Gao, Junrong Liang, and Lin Sun. 2023. "Ion fluxes Involved in the Adaptation of the Estuarine Diatom Coscinodiscus centralis Ehrenberg to Salinity Stress" International Journal of Molecular Sciences 24, no. 18: 13683. https://doi.org/10.3390/ijms241813683
APA StyleChen, C., Hu, X., Gao, Y., Liang, J., & Sun, L. (2023). Ion fluxes Involved in the Adaptation of the Estuarine Diatom Coscinodiscus centralis Ehrenberg to Salinity Stress. International Journal of Molecular Sciences, 24(18), 13683. https://doi.org/10.3390/ijms241813683





