Meiotic Cell Cycle Progression in Mouse Oocytes: Role of Cyclins
Abstract
1. Introduction
1.1. Overview of Cyclins
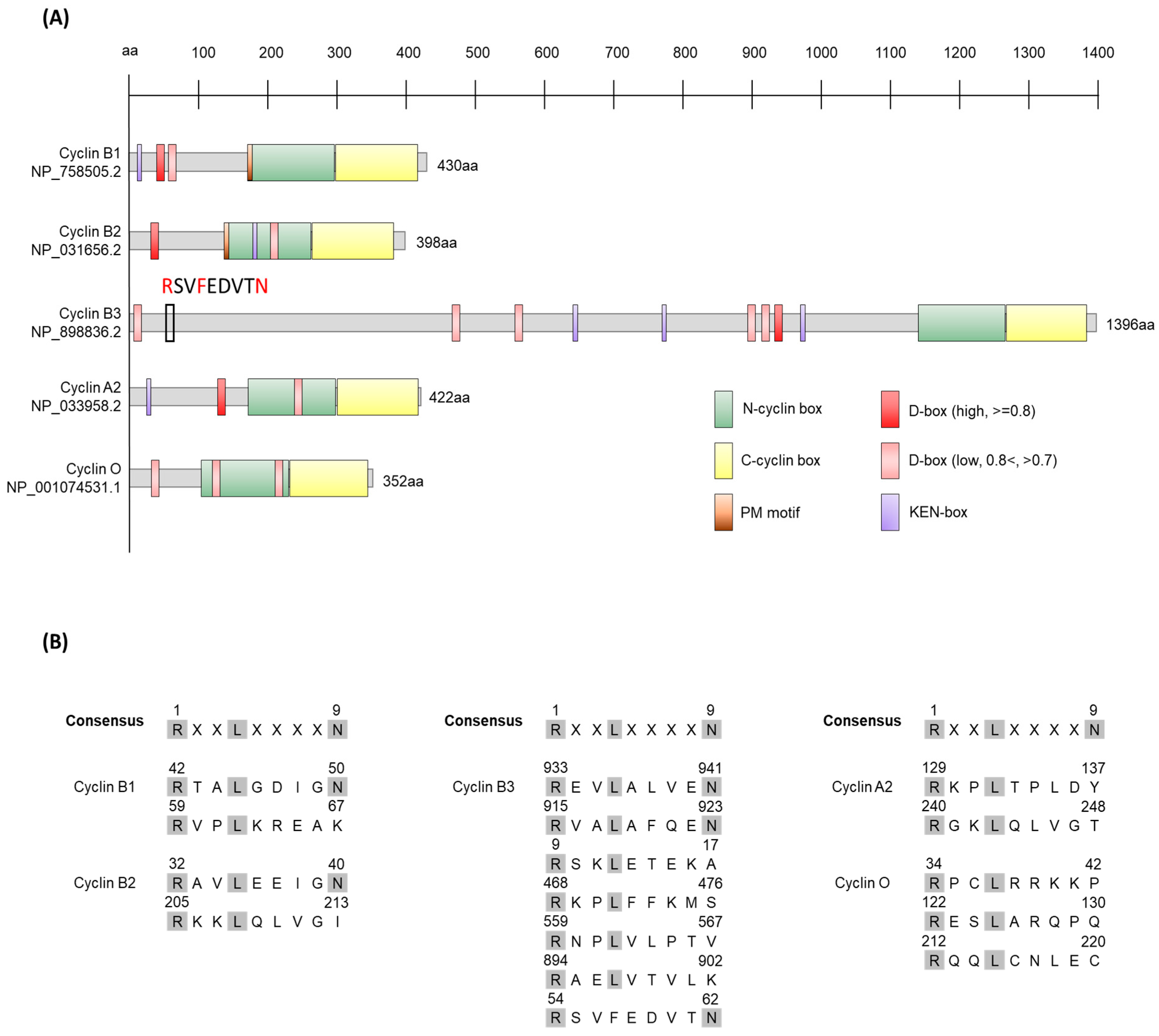
1.1.1. Cyclin B1
1.1.2. Cyclin B2
1.1.3. Cyclin B3
1.1.4. Cyclin A
1.1.5. Cyclin O
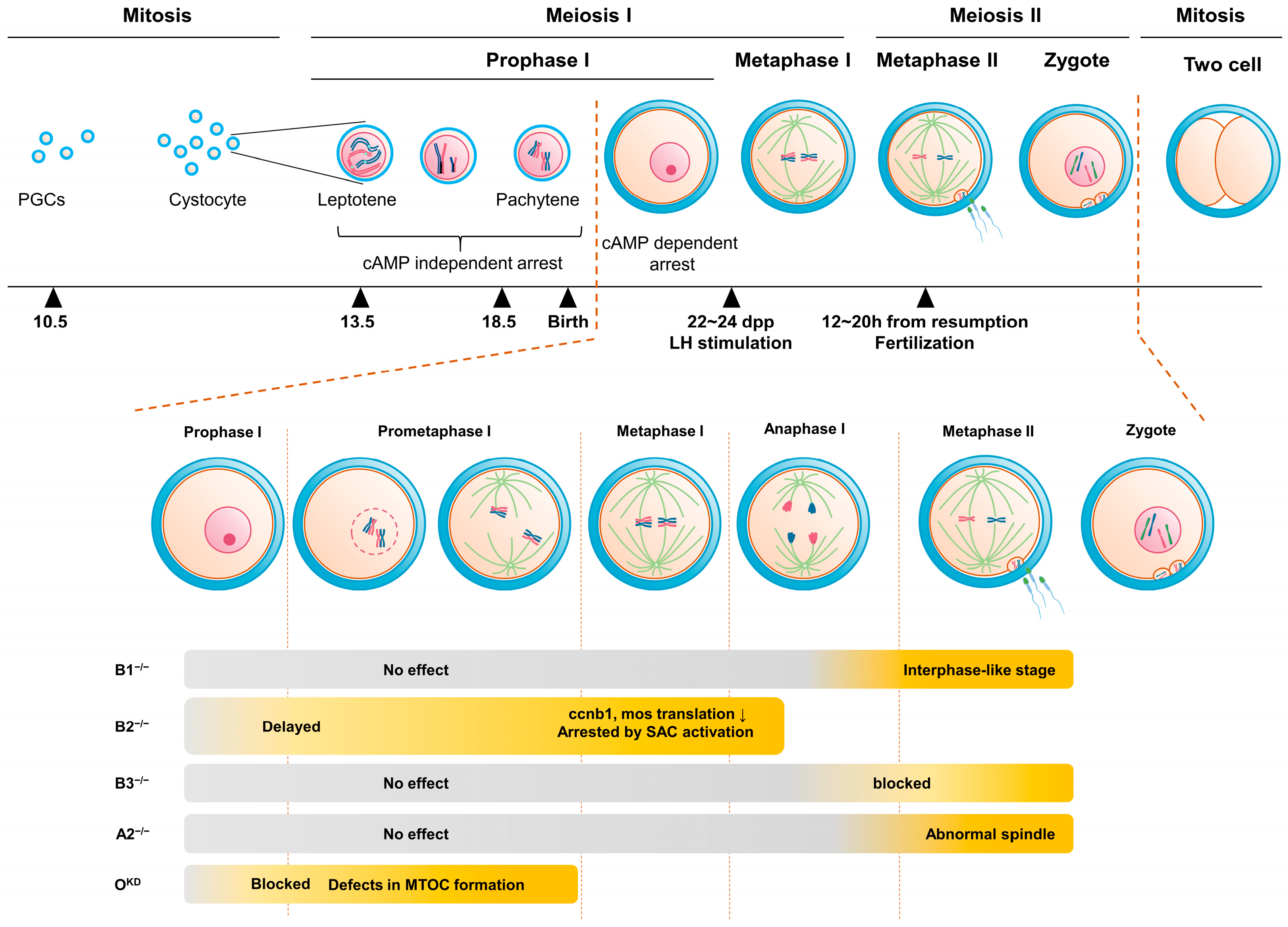
2. Germinal Vesicle (GV) Arrest
2.1. GV Arrest by Translational Regulation
2.2. GV Arrest by Degradation
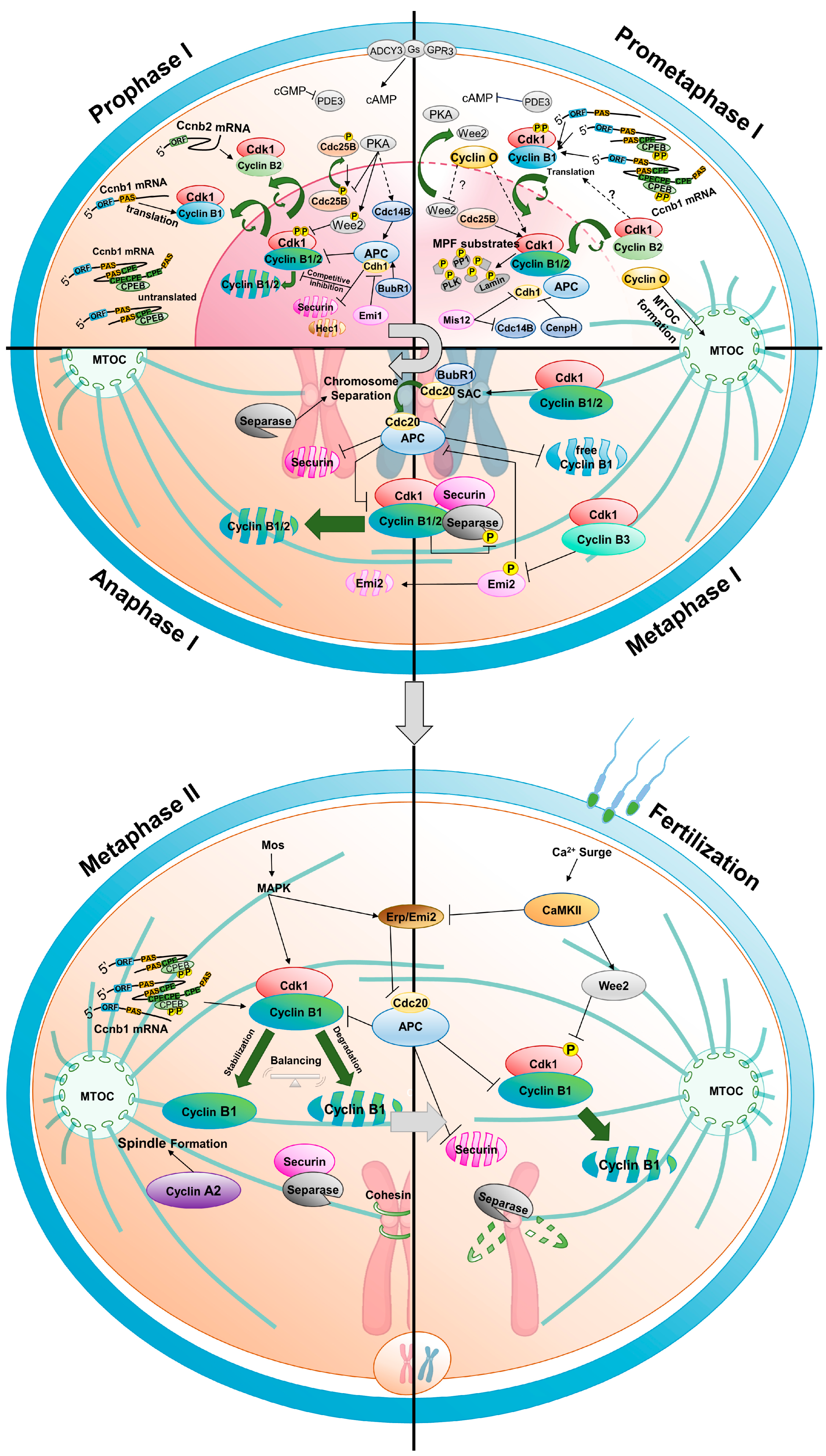
3. From Resumption of Oocyte Maturation to Metaphase I
3.1. Role of Cyclins from Resumption of Oocyte Maturation to Metaphase I
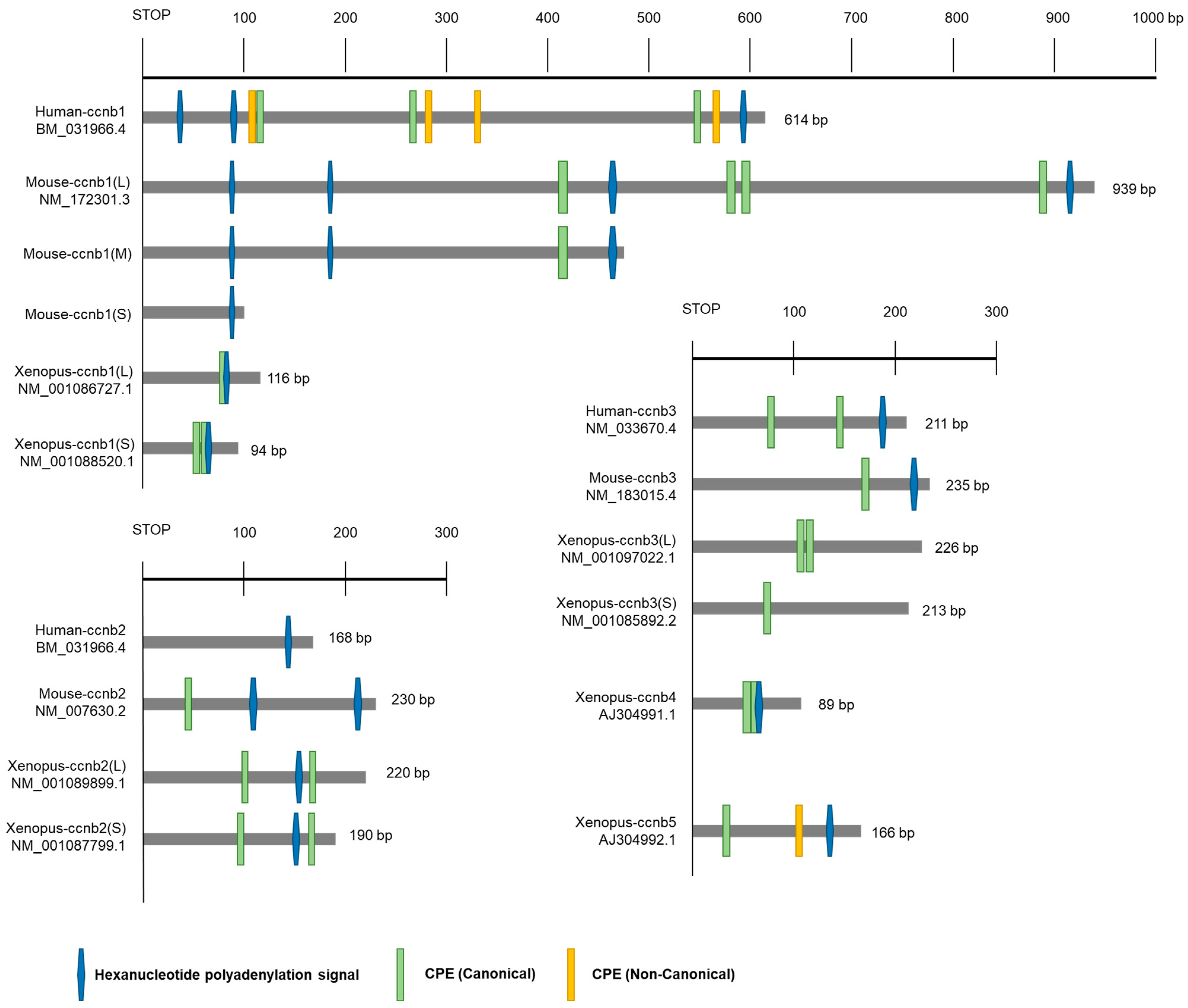
3.2. Translational Regulation of Cyclins
3.3. Regulation of Cyclin Degradation
4. Metaphase I-Anaphase I Transition
5. Anaphase I-Metaphase II Transition
6. Arrest at Metaphase II and Fertilization
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDC | Cell division cycle |
| MPF | Maturation-promoting factor |
| CDK | Cyclin-dependent kinase |
| CBD | Cyclin box domain |
| SAC | Spindle assembly checkpoint |
| APC/C | Anaphase-promoting complex/cyclosome |
| GV | Germinal vesicle |
| PKA | Protein kinase A |
| GVBD | Germinal vesicle breakdown |
| PDE3A | Phosphodiesterase 3A |
| CPSF | Cleavage and polyadenylation specificity factor |
| ePABP | Embryonic poly(A) binding protein |
| CPE | Cytoplasmic polyadenylation element |
| 3′ UTR | 3′ untranslated region |
| 5′ UTR | 5′ untranslated region |
| CaMKII, CaMK2A | Calmodulin-dependent protein kinase II |
References
- Masui, Y.; Markert, C.L. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J. Exp. Zool. 1971, 177, 129–145. [Google Scholar] [CrossRef]
- Evans, T.; Rosenthal, E.T.; Youngblom, J.; Distel, D.; Hunt, T. Cyclin: A protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell 1983, 33, 389–396. [Google Scholar] [CrossRef]
- Draetta, G.; Luca, F.; Westendorf, J.; Brizuela, L.; Ruderman, J.; Beach, D. Cdc2 protein kinase is complexed with both cyclin A and B: Evidence for proteolytic inactivation of MPF. Cell 1989, 56, 829–838. [Google Scholar] [CrossRef]
- Booher, R.N.; Alfa, C.E.; Hyams, J.S.; Beach, D.H. The fission yeast cdc2/cdc13/suc1 protein kinase: Regulation of catalytic activity and nuclear localization. Cell 1989, 58, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Nigg, E.A. Cyclin-dependent protein kinases: Key regulators of the eukaryotic cell cycle. Bioessays 1995, 17, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Chen, F.; Yang, X.; Xu, W.; Xie, J.; Yu, L. Phylogenetic analysis of CDK and cyclin proteins in premetazoan lineages. BMC Evol. Biol. 2014, 14, 10. [Google Scholar] [CrossRef]
- Kaldis, P.; Russo, A.A.; Chou, H.S.; Pavletich, N.P.; Solomon, M.J. Human and yeast cdk-activating kinases (CAKs) display distinct substrate specificities. Mol. Biol. Cell 1998, 9, 2545–2560. [Google Scholar] [CrossRef]
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef]
- Gunbin, K.V.; Suslov, V.V.; Turnaev, I.I.; Afonnikov, D.A.; Kolchanov, N.A. Molecular evolution of cyclin proteins in animals and fungi. BMC Evol. Biol. 2011, 11, 224. [Google Scholar] [CrossRef]
- Meyerson, M.; Harlow, E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol. Cell. Biol. 1994, 14, 2077–2086. [Google Scholar] [CrossRef]
- Sherr, C.J. D-type cyclins. Trends Biochem. Sci. 1995, 20, 187–190. [Google Scholar] [CrossRef]
- Obeyesekere, M.N.; Tucker, S.L.; Zimmerman, S.O. A model for regulation of the cell cycle incorporating cyclin A, cyclin B and their complexes. Cell Prolif. 1994, 27, 105–113. [Google Scholar] [CrossRef]
- Fung, T.K.; Poon, R.Y. A roller coaster ride with the mitotic cyclins. Semin. Cell Dev. Biol. 2005, 16, 335–342. [Google Scholar] [CrossRef]
- de Vant’ery, C.; Gavin, A.C.; Vassalli, J.D.; Schorderet-Slatkine, S. An accumulation of p34cdc2 at the end of mouse oocyte growth correlates with the acquisition of meiotic competence. Dev. Biol. 1996, 174, 335–344. [Google Scholar] [CrossRef]
- Capalbo, A.; Hoffmann, E.R.; Cimadomo, D.; Ubaldi, F.M.; Rienzi, L. Human female meiosis revised: New insights into the mechanisms of chromosome segregation and aneuploidies from advanced genomics and time-lapse imaging. Hum. Reprod. Update 2017, 23, 706–722. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Arur, S. Regulation of oocyte maturation: Role of conserved ERK signaling. Mol. Reprod. Dev. 2022, 89, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zhu, C.; Harper, J.W. A premature-termination mutation in the Mus musculus cyclin-dependent kinase 3 gene. Proc. Natl. Acad. Sci. USA 2001, 98, 1682–1686. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, T.; Hesabi, B.; Moons, D.S.; Pandolfi, P.P.; Hansel, K.S.; Koff, A.; Kiyokawa, H. Targeted disruption of CDK4 delays cell cycle entry with enhanced p27(Kip1) activity. Mol. Cell. Biol. 1999, 19, 7011–7019. [Google Scholar] [CrossRef]
- Malumbres, M.; Sotillo, R.; Santamaria, D.; Galan, J.; Cerezo, A.; Ortega, S.; Dubus, P.; Barbacid, M. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell 2004, 118, 493–504. [Google Scholar] [CrossRef]
- Adhikari, D.; Zheng, W.; Shen, Y.; Gorre, N.; Ning, Y.; Halet, G.; Kaldis, P.; Liu, K. Cdk1, but not Cdk2, is the sole Cdk that is essential and sufficient to drive resumption of meiosis in mouse oocytes. Hum. Mol. Genet. 2012, 21, 2476–2484. [Google Scholar] [CrossRef]
- Santamaría, D.; Barrière, C.; Cerqueira, A.; Hunt, S.; Tardy, C.; Newton, K.; Cáceres, J.F.; Dubus, P.; Malumbres, M.; Barbacid, M. Cdk1 is sufficient to drive the mammalian cell cycle. Nature 2007, 448, 811–815. [Google Scholar] [CrossRef]
- Diril, M.K.; Ratnacaram, C.K.; Padmakumar, V.C.; Du, T.; Wasser, M.; Coppola, V.; Tessarollo, L.; Kaldis, P. Cyclin-dependent kinase 1 (Cdk1) is essential for cell division and suppression of DNA re-replication but not for liver regeneration. Proc. Natl. Acad. Sci. USA 2012, 109, 3826–3831. [Google Scholar] [CrossRef] [PubMed]
- Nieduszynski, C.A.; Murray, J.; Carrington, M. Whole-genome analysis of animal A- and B-type cyclins. Genome Biol. 2002, 3, research0070.1. [Google Scholar] [CrossRef] [PubMed]
- Pines, J.; Hunter, T. Isolation of a human cyclin cDNA: Evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell 1989, 58, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.L.; Wolgemuth, D.J. Identification of a mouse B-type cyclin which exhibits developmentally regulated expression in the germ line. Mol. Reprod. Dev. 1992, 33, 259–269. [Google Scholar] [CrossRef]
- Han, S.J.; Martins, J.P.S.; Yang, Y.; Kang, M.K.; Daldello, E.M.; Conti, M. The Translation of Cyclin B1 and B2 is Differentially Regulated during Mouse Oocyte Reentry into the Meiotic Cell Cycle. Sci. Rep. 2017, 7, 14077. [Google Scholar] [CrossRef] [PubMed]
- Brandeis, M.; Rosewell, I.; Carrington, M.; Crompton, T.; Jacobs, M.A.; Kirk, J.; Gannon, J.; Hunt, T. Cyclin B2-null mice develop normally and are fertile whereas cyclin B1-null mice die in utero. Proc. Natl. Acad. Sci. USA 1998, 95, 4344–4349. [Google Scholar] [CrossRef]
- Fuchimoto, D.; Mizukoshi, A.; Schultz, R.M.; Sakai, S.; Aoki, F. Posttranscriptional regulation of cyclin A1 and cyclin A2 during mouse oocyte meiotic maturation and preimplantation development. Biol. Reprod. 2001, 65, 986–993. [Google Scholar] [CrossRef]
- Li, J.; Tang, J.X.; Cheng, J.M.; Hu, B.; Wang, Y.Q.; Aalia, B.; Li, X.Y.; Jin, C.; Wang, X.X.; Deng, S.L.; et al. Cyclin B2 can compensate for Cyclin B1 in oocyte meiosis I. J. Cell Biol. 2018, 217, 3901–3911. [Google Scholar] [CrossRef]
- Zhang, T.; Qi, S.T.; Huang, L.; Ma, X.S.; Ouyang, Y.C.; Hou, Y.; Shen, W.; Schatten, H.; Sun, Q.Y. Cyclin B3 controls anaphase onset independent of spindle assembly checkpoint in meiotic oocytes. Cell Cycle 2015, 14, 2648–2654. [Google Scholar] [CrossRef]
- Karasu, M.E.; Bouftas, N.; Keeney, S.; Wassmann, K. Cyclin B3 promotes anaphase I onset in oocyte meiosis. J. Cell Biol. 2019, 218, 1265–1281. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Zhang, L.; He, Z.; Feng, G.; Sun, H.; Wang, J.; Li, Z.; Liu, C.; Han, J.; et al. Cyclin B3 is required for metaphase to anaphase transition in oocyte meiosis I. J. Cell Biol. 2019, 218, 1553–1563. [Google Scholar] [CrossRef]
- Ma, J.Y.; Ou-Yang, Y.C.; Luo, Y.B.; Wang, Z.B.; Hou, Y.; Han, Z.M.; Liu, Z.; Schatten, H.; Sun, Q.Y. Cyclin O regulates germinal vesicle breakdown in mouse oocytes. Biol. Reprod. 2013, 88, 110. [Google Scholar] [CrossRef]
- Liu, D.; Matzuk, M.M.; Sung, W.K.; Guo, Q.; Wang, P.; Wolgemuth, D.J. Cyclin A1 is required for meiosis in the male mouse. Nat. Genet. 1998, 20, 377–380. [Google Scholar] [CrossRef]
- Wei, H.; Li, Y.; Zhao, C.; Jiang, X.; Chen, H.; Lang, M.F.; Sun, J. Cyclin A1 is expressed in mouse ovary. Int. J. Med. Sci. 2014, 11, 754–757. [Google Scholar] [CrossRef]
- Radonova, L.; Pauerova, T.; Jansova, D.; Danadova, J.; Skultety, M.; Kubelka, M.; Anger, M. Cyclin A1 in Oocytes Prevents Chromosome Segregation And Anaphase Entry. Sci. Rep. 2020, 10, 7455. [Google Scholar] [CrossRef] [PubMed]
- Touati, S.A.; Cladière, D.; Lister, L.M.; Leontiou, I.; Chambon, J.P.; Rattani, A.; Böttger, F.; Stemmann, O.; Nasmyth, K.; Herbert, M.; et al. Cyclin A2 is required for sister chromatid segregation, but not separase control, in mouse oocyte meiosis. Cell Rep. 2012, 2, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Bouftas, N.; Schneider, L.; Halder, M.; Demmig, R.; Baack, M.; Cladiere, D.; Walter, M.; Al Abdallah, H.; Kleinhempel, C.; Messaritaki, R.; et al. Cyclin B3 implements timely vertebrate oocyte arrest for fertilization. Dev. Cell 2022, 57, 2305–2320.e6. [Google Scholar] [CrossRef] [PubMed]
- Daldello, E.M.; Luong, X.G.; Yang, C.R.; Kuhn, J.; Conti, M. Cyclin B2 is required for progression through meiosis in mouse oocytes. Development 2019, 146, dev172734. [Google Scholar] [CrossRef]
- Funk, M.C.; Bera, A.N.; Menchen, T.; Kuales, G.; Thriene, K.; Lienkamp, S.S.; Dengjel, J.; Omran, H.; Frank, M.; Arnold, S.J. Cyclin O (Ccno) functions during deuterosome-mediated centriole amplification of multiciliated cells. EMBO J. 2015, 34, 1078–1089. [Google Scholar] [CrossRef]
- Mehmet, E.K.; Nora, B.; Scott, K.; Katja, W. Cyclin B3 promotes APC/C activation and anaphase I onset in oocyte meiosis. bioRxiv 2018. [Google Scholar] [CrossRef]
- Pines, J.; Hunter, T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J. Cell Biol. 1991, 115, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jackman, M.; Firth, M.; Pines, J. Human cyclins B1 and B2 are localized to strikingly different structures: B1 to microtubules, B2 primarily to the Golgi apparatus. EMBO J. 1995, 14, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.X.; Li, J.; Cheng, J.M.; Hu, B.; Sun, T.C.; Li, X.Y.; Batool, A.; Wang, Z.P.; Wang, X.X.; Deng, S.L.; et al. Requirement for CCNB1 in mouse spermatogenesis. Cell Death Dis. 2017, 8, e3142. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Newport, J.W. Evidence that the G1-S and G2-M transitions are controlled by different cdc2 proteins in higher eukaryotes. Cell 1991, 66, 731–742. [Google Scholar] [CrossRef]
- Lew, D.J.; Dulic, V.; Reed, S.I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell 1991, 66, 1197–1206. [Google Scholar] [CrossRef]
- Minshull, J.; Blow, J.J.; Hunt, T. Translation of cyclin mRNA is necessary for extracts of activated xenopus eggs to enter mitosis. Cell 1989, 56, 947–956. [Google Scholar] [CrossRef]
- Gautier, J.; Minshull, J.; Lohka, M.; Glotzer, M.; Hunt, T.; Maller, J.L. Cyclin is a component of maturation-promoting factor from Xenopus. Cell 1990, 60, 487–494. [Google Scholar] [CrossRef]
- Gallant, P.; Nigg, E.A. Identification of a novel vertebrate cyclin: Cyclin B3 shares properties with both A- and B-type cyclins. EMBO J. 1994, 13, 595–605. [Google Scholar] [CrossRef]
- Tschop, K.; Muller, G.A.; Grosche, J.; Engeland, K. Human cyclin B3. mRNA expression during the cell cycle and identification of three novel nonclassical nuclear localization signals. FEBS J. 2006, 273, 1681–1695. [Google Scholar] [CrossRef]
- Lozano, J.C.; Perret, E.; Schatt, P.; Arnould, C.; Peaucellier, G.; Picard, A. Molecular cloning, gene localization, and structure of human cyclin B3. Biochem. Biophys. Res. Commun. 2002, 291, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Kreutzer, M.A.; Richards, J.P.; De Silva-Udawatta, M.N.; Temenak, J.J.; Knoblich, J.A.; Lehner, C.F.; Bennett, K.L. Caenorhabditis elegans cyclin A- and B-type genes: A cyclin A multigene family, an ancestral cyclin B3 and differential germline expression. J. Cell Sci. 1995, 108 Pt 6, 2415–2424. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; O’Farrell, P.H. Cyclin B3 is a mitotic cyclin that promotes the metaphase-anaphase transition. Curr. Biol. 2015, 25, 811–816. [Google Scholar] [CrossRef]
- Bourouh, M.; Dhaliwal, R.; Rana, K.; Sinha, S.; Guo, Z.; Swan, A. Distinct and Overlapping Requirements for Cyclins A, B, and B3 in Drosophila Female Meiosis. G3 Genes Genomes Genet. 2016, 6, 3711–3724. [Google Scholar] [CrossRef]
- Michael, W.M. Cyclin CYB-3 controls both S-phase and mitosis and is asymmetrically distributed in the early C. elegans embryo. Development 2016, 143, 3119–3127. [Google Scholar] [CrossRef]
- Deyter, G.M.; Furuta, T.; Kurasawa, Y.; Schumacher, J.M. Caenorhabditis elegans cyclin B3 is required for multiple mitotic processes including alleviation of a spindle checkpoint-dependent block in anaphase chromosome segregation. PLoS Genet. 2010, 6, e1001218. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, H.W.; Knoblich, J.A.; Lehner, C.F. Drosophila Cyclin B3 is required for female fertility and is dispensable for mitosis like Cyclin B. Genes Dev. 1998, 12, 3741–3751. [Google Scholar] [CrossRef] [PubMed]
- Bouftas, N.; Wassmann, K. Cycling through mammalian meiosis: B-type cyclins in oocytes. Cell Cycle 2019, 18, 1537–1548. [Google Scholar] [CrossRef]
- Karasu, M.E.; Keeney, S. Cyclin B3 is dispensable for mouse spermatogenesis. Chromosoma 2019, 128, 473–487. [Google Scholar] [CrossRef]
- Howe, J.A.; Howell, M.; Hunt, T.; Newport, J.W. Identification of a developmental timer regulating the stability of embryonic cyclin A and a new somatic A-type cyclin at gastrulation. Genes Dev. 1995, 9, 1164–1176. [Google Scholar] [CrossRef]
- Sweeney, C.; Murphy, M.; Kubelka, M.; Ravnik, S.E.; Hawkins, C.F.; Wolgemuth, D.J.; Carrington, M. A distinct cyclin A is expressed in germ cells in the mouse. Development 1996, 122, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Morosetti, R.; Koeffler, H.P. Characterization of a second human cyclin A that is highly expressed in testis and in several leukemic cell lines. Cancer Res. 1997, 57, 913–920. [Google Scholar] [PubMed]
- Krämer, A.; Hochhaus, A.; Saussele, S.; Reichert, A.; Willer, A.; Hehlmann, R. Cyclin A1 is predominantly expressed in hematological malignancies with myeloid differentiation. Leukemia 1998, 12, 893–898. [Google Scholar] [CrossRef]
- Joshi, A.R.; Jobanputra, V.; Lele, K.M.; Wolgemuth, D.J. Distinct properties of cyclin-dependent kinase complexes containing cyclin A1 and cyclin A2. Biochem. Biophys. Res. Commun. 2009, 378, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Muller-Tidow, C.; Ji, P.; Diederichs, S.; Potratz, J.; Baumer, N.; Kohler, G.; Cauvet, T.; Choudary, C.; van der Meer, T.; Chan, W.Y.; et al. The cyclin A1-CDK2 complex regulates DNA double-strand break repair. Mol. Cell. Biol. 2004, 24, 8917–8928. [Google Scholar] [CrossRef] [PubMed]
- Vladar, E.K.; Stratton, M.B.; Saal, M.L.; Salazar-De Simone, G.; Wang, X.; Wolgemuth, D.; Stearns, T.; Axelrod, J.D. Cyclin-dependent kinase control of motile ciliogenesis. eLife 2018, 7, e36375. [Google Scholar] [CrossRef]
- Ravnik, S.E.; Wolgemuth, D.J. The developmentally restricted pattern of expression in the male germ line of a murine cyclin A, cyclin A2, suggests roles in both mitotic and meiotic cell cycles. Dev. Biol. 1996, 173, 69–78. [Google Scholar] [CrossRef][Green Version]
- Girard, F.; Strausfeld, U.; Fernandez, A.; Lamb, N.J. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell 1991, 67, 1169–1179. [Google Scholar] [CrossRef]
- Pagano, M.; Pepperkok, R.; Verde, F.; Ansorge, W.; Draetta, G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992, 11, 961–971. [Google Scholar] [CrossRef]
- Kabeche, L.; Compton, D.A. Cyclin A regulates kinetochore microtubules to promote faithful chromosome segregation. Nature 2013, 502, 110–113. [Google Scholar] [CrossRef]
- den Elzen, N.; Pines, J. Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J. Cell Biol. 2001, 153, 121–136. [Google Scholar] [CrossRef]
- Erlandsson, F.; Linnman, C.; Ekholm, S.; Bengtsson, E.; Zetterberg, A. A detailed analysis of cyclin A accumulation at the G(1)/S border in normal and transformed cells. Exp. Cell Res. 2000, 259, 86–95. [Google Scholar] [CrossRef]
- Wolthuis, R.; Clay-Farrace, L.; van Zon, W.; Yekezare, M.; Koop, L.; Ogink, J.; Medema, R.; Pines, J. Cdc20 and Cks direct the spindle checkpoint-independent destruction of cyclin A. Mol. Cell 2008, 30, 290–302. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Yuen, W.S.; Adhikari, D.; Flegg, J.A.; FitzHarris, G.; Conti, M.; Sicinski, P.; Nabti, I.; Marangos, P.; Carroll, J. Cyclin A2 modulates kinetochore-microtubule attachment in meiosis II. J. Cell Biol. 2017, 216, 3133–3143. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.; Stinnakre, M.G.; Senamaud-Beaufort, C.; Winston, N.J.; Sweeney, C.; Kubelka, M.; Carrington, M.; Bréchot, C.; Sobczak-Thépot, J. Delayed early embryonic lethality following disruption of the murine cyclin A2 gene. Nat. Genet. 1997, 15, 83–86. [Google Scholar] [CrossRef]
- Muller, S.J.; Caradonna, S. Cell cycle regulation of a human cyclin-like gene encoding uracil-DNA glycosylase. J. Biol. Chem. 1993, 268, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Hagen, L.; Kavli, B.; Sousa, M.M.; Torseth, K.; Liabakk, N.B.; Sundheim, O.; Pena-Diaz, J.; Otterlei, M.; Hørning, O.; Jensen, O.N.; et al. Cell cycle-specific UNG2 phosphorylations regulate protein turnover, activity and association with RPA. EMBO J. 2008, 27, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Roig, M.B.; Roset, R.; Ortet, L.; Balsiger, N.A.; Anfosso, A.; Cabellos, L.; Garrido, M.; Alameda, F.; Brady, H.J.; Gil-Gómez, G. Identification of a novel cyclin required for the intrinsic apoptosis pathway in lymphoid cells. Cell Death Differ. 2009, 16, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Firmani, L.D.; Uliasz, T.F.; Mehlmann, L.M. The switch from cAMP-independent to cAMP-dependent arrest of meiotic prophase is associated with coordinated GPR3 and CDK1 expression in mouse oocytes. Dev. Biol. 2018, 434, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Saitou, M.; Barton, S.C.; Surani, M.A. A molecular programme for the specification of germ cell fate in mice. Nature 2002, 418, 293–300. [Google Scholar] [CrossRef]
- Horner, K.; Livera, G.; Hinckley, M.; Trinh, K.; Storm, D.; Conti, M. Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev. Biol. 2003, 258, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Mehlmann, L.M. Oocyte-specific expression of Gpr3 is required for the maintenance of meiotic arrest in mouse oocytes. Dev. Biol. 2005, 288, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Bornslaeger, E.A.; Mattei, P.; Schultz, R.M. Involvement of cAMP-dependent protein kinase and protein phosphorylation in regulation of mouse oocyte maturation. Dev. Biol. 1986, 114, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Maller, J.L.; Krebs, E.G. Progesterone-stimulated meiotic cell division in Xenopus oocytes. Induction by regulatory subunit and inhibition by catalytic subunit of adenosine 3′:5’-monophosphate-dependent protein kinase. J. Biol. Chem. 1977, 252, 1712–1718. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Andersen, C.B.; Richard, F.; Mehats, C.; Chun, S.Y.; Horner, K.; Jin, C.; Tsafriri, A. Role of cyclic nucleotide signaling in oocyte maturation. Mol. Cell. Endocrinol. 2002, 187, 153–159. [Google Scholar] [CrossRef]
- Kalinowski, R.R.; Berlot, C.H.; Jones, T.L.; Ross, L.F.; Jaffe, L.A.; Mehlmann, L.M. Maintenance of meiotic prophase arrest in vertebrate oocytes by a Gs protein-mediated pathway. Dev. Biol. 2004, 267, 1–13. [Google Scholar] [CrossRef]
- Schultz, R.M.; Montgomery, R.R.; Ward-Bailey, P.F.; Eppig, J.J. Regulation of oocyte maturation in the mouse: Possible roles of intercellular communication, cAMP, and testosterone. Dev. Biol. 1983, 95, 294–304. [Google Scholar] [CrossRef]
- Han, S.J.; Chen, R.; Paronetto, M.P.; Conti, M. Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr. Biol. 2005, 15, 1670–1676. [Google Scholar] [CrossRef]
- Han, S.J.; Conti, M. New pathways from PKA to the Cdc2/cyclin B complex in oocytes: Wee1B as a potential PKA substrate. Cell Cycle 2006, 5, 227–231. [Google Scholar] [CrossRef]
- Oh, J.S.; Susor, A.; Conti, M. Protein tyrosine kinase Wee1B is essential for metaphase II exit in mouse oocytes. Science 2011, 332, 462–465. [Google Scholar] [CrossRef]
- Oh, J.S.; Han, S.J.; Conti, M. Wee1B, Myt1, and Cdc25 function in distinct compartments of the mouse oocyte to control meiotic resumption. J. Cell Biol. 2010, 188, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liu, C.; Hou, J.; Cui, C.; Wu, D.; Fan, H.; Sun, X.; Meng, J.; Yang, F.; Wang, E.; et al. Ser149 is another potential PKA phosphorylation target of Cdc25B in G2/M transition of fertilized mouse eggs. J. Biol. Chem. 2011, 286, 10356–10366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z.; Xu, X.Y.; Li, X.S.; Yu, M.; Yu, A.M.; Zong, Z.H.; Yu, B.Z. Protein kinase A modulates Cdc25B activity during meiotic resumption of mouse oocytes. Dev. Dyn. 2008, 237, 3777–3786. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, R.; Eppig, J.J. Transcriptional activity of the mouse oocyte genome: Companion granulosa cells modulate transcription and chromatin remodeling. Dev. Biol. 2001, 229, 224–236. [Google Scholar] [CrossRef]
- Zhang, Y.; Sheets, M.D. Analyses of zebrafish and Xenopus oocyte maturation reveal conserved and diverged features of translational regulation of maternal cyclin B1 mRNA. BMC Dev. Biol. 2009, 9, 7. [Google Scholar] [CrossRef]
- Kuge, H.; Inoue, A. Maturation of Xenopus laevis oocyte by progesterone requires poly(A) tail elongation of mRNA. Exp. Cell Res. 1992, 202, 52–58. [Google Scholar] [CrossRef]
- Chen, J.; Melton, C.; Suh, N.; Oh, J.S.; Horner, K.; Xie, F.; Sette, C.; Blelloch, R.; Conti, M. Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition. Genes Dev. 2011, 25, 755–766. [Google Scholar] [CrossRef]
- Fernández-Miranda, G.; Méndez, R. The CPEB-family of proteins, translational control in senescence and cancer. Ageing Res. Rev. 2012, 11, 460–472. [Google Scholar] [CrossRef]
- Ivshina, M.; Lasko, P.; Richter, J.D. Cytoplasmic polyadenylation element binding proteins in development, health, and disease. Annu. Rev. Cell Dev. Biol. 2014, 30, 393–415. [Google Scholar] [CrossRef]
- Richter, J.D.; Wasserman, W.J.; Smith, L.D. The mechanism for increased protein synthesis during Xenopus oocyte maturation. Dev. Biol. 1982, 89, 159–167. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Schultz, R.M.; Kopf, G.S. Acquisition of meiotic competence in mouse oocytes: Absolute amounts of p34(cdc2), cyclin B1, cdc25C, and wee1 in meiotically incompetent and competent oocytes. Biol. Reprod. 2000, 63, 1610–1616. [Google Scholar] [CrossRef]
- Arooz, T.; Yam, C.H.; Siu, W.Y.; Lau, A.; Li, K.K.; Poon, R.Y. On the concentrations of cyclins and cyclin-dependent kinases in extracts of cultured human cells. Biochemistry 2000, 39, 9494–9501. [Google Scholar] [CrossRef]
- Ledan, E.; Polanski, Z.; Terret, M.E.; Maro, B. Meiotic maturation of the mouse oocyte requires an equilibrium between cyclin B synthesis and degradation. Dev. Biol. 2001, 232, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Chang, H.Y.; Levasseur, M.; Jones, K.T. APCcdh1 activity in mouse oocytes prevents entry into the first meiotic division. Nat. Cell Biol. 2006, 8, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.E.; Weaver, J.; Jones, K.T. Spatial regulation of APCCdh1-induced cyclin B1 degradation maintains G2 arrest in mouse oocytes. Development 2010, 137, 1297–1304. [Google Scholar] [CrossRef]
- Reis, A.; Madgwick, S.; Chang, H.Y.; Nabti, I.; Levasseur, M.; Jones, K.T. Prometaphase APCcdh1 activity prevents non-disjunction in mammalian oocytes. Nat. Cell Biol. 2007, 9, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Schindler, K.; Schultz, R.M. CDC14B acts through FZR1 (CDH1) to prevent meiotic maturation of mouse oocytes. Biol. Reprod. 2009, 80, 795–803. [Google Scholar] [CrossRef]
- Homer, H.; Gui, L.; Carroll, J. A spindle assembly checkpoint protein functions in prophase I arrest and prometaphase progression. Science 2009, 326, 991–994. [Google Scholar] [CrossRef]
- Marangos, P.; Carroll, J. Securin regulates entry into M-phase by modulating the stability of cyclin B. Nat. Cell Biol. 2008, 10, 445–451. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, Y.; Li, L.; Wang, Z.B.; Shen, W.; Schatten, H.; Sun, Q.Y. CenpH regulates meiotic G2/M transition by modulating the APC/CCdh1-cyclin B1 pathway in oocytes. Development 2017, 144, 305–312. [Google Scholar] [CrossRef]
- Marangos, P.; Verschuren, E.W.; Chen, R.; Jackson, P.K.; Carroll, J. Prophase I arrest and progression to metaphase I in mouse oocytes are controlled by Emi1-dependent regulation of APC(Cdh1). J. Cell Biol. 2007, 176, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Homer, H. Hec1-dependent cyclin B2 stabilization regulates the G2-M transition and early prometaphase in mouse oocytes. Dev. Cell 2013, 25, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Norris, R.P.; Ratzan, W.J.; Freudzon, M.; Mehlmann, L.M.; Krall, J.; Movsesian, M.A.; Wang, H.; Ke, H.; Nikolaev, V.O.; Jaffe, L.A. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 2009, 136, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Norris, R.P.; Freudzon, M.; Mehlmann, L.M.; Cowan, A.E.; Simon, A.M.; Paul, D.L.; Lampe, P.D.; Jaffe, L.A. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: One of two paths to meiotic resumption. Development 2008, 135, 3229–3238. [Google Scholar] [CrossRef]
- Sela-Abramovich, S.; Edry, I.; Galiani, D.; Nevo, N.; Dekel, N. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology 2006, 147, 2280–2286. [Google Scholar] [CrossRef]
- Sela-Abramovich, S.; Chorev, E.; Galiani, D.; Dekel, N. Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology 2005, 146, 1236–1244. [Google Scholar] [CrossRef]
- Su, Y.Q.; Denegre, J.M.; Wigglesworth, K.; Pendola, F.L.; O’Brien, M.J.; Eppig, J.J. Oocyte-dependent activation of mitogen-activated protein kinase (ERK1/2) in cumulus cells is required for the maturation of the mouse oocyte-cumulus cell complex. Dev. Biol. 2003, 263, 126–138. [Google Scholar] [CrossRef]
- Su, Y.Q.; Wigglesworth, K.; Pendola, F.L.; O’Brien, M.J.; Eppig, J.J. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology 2002, 143, 2221–2232. [Google Scholar] [CrossRef]
- Conti, M. Signaling networks in somatic cells and oocytes activated during ovulation. Ann. Endocrinol. 2010, 71, 189–190. [Google Scholar] [CrossRef]
- Huo, L.J.; Yu, L.Z.; Liang, C.G.; Fan, H.Y.; Chen, D.Y.; Sun, Q.Y. Cell-cycle-dependent subcellular localization of cyclin B1, phosphorylated cyclin B1 and p34cdc2 during oocyte meiotic maturation and fertilization in mouse. Zygote 2005, 13, 45–53. [Google Scholar] [CrossRef]
- Marangos, P.; Carroll, J. The dynamics of cyclin B1 distribution during meiosis I in mouse oocytes. Reproduction 2004, 128, 153–162. [Google Scholar] [CrossRef][Green Version]
- Yoshitome, S.; Furuno, N.; Prigent, C.; Hashimoto, E. The subcellular localization of cyclin B2 is required for bipolar spindle formation during Xenopus oocyte maturation. Biochem. Biophys. Res. Commun. 2012, 422, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T. Entry into mitosis: A solution to the decades-long enigma of MPF. Chromosoma 2015, 124, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, S.; Robert, P.; Hached, K.; Sundermann, L.; Charrasse, S.; Labbé, J.C.; Castro, A.; Lorca, T. The master Greatwall kinase, a critical regulator of mitosis and meiosis. Int. J. Dev. Biol. 2016, 60, 245–254. [Google Scholar] [CrossRef]
- Mihajlovic, A.I.; FitzHarris, G. Segregating Chromosomes in the Mammalian Oocyte. Curr. Biol. 2018, 28, R895–R907. [Google Scholar] [CrossRef] [PubMed]
- Polanski, Z.; Ledan, E.; Brunet, S.; Louvet, S.; Verlhac, M.H.; Kubiak, J.Z.; Maro, B. Cyclin synthesis controls the progression of meiotic maturation in mouse oocytes. Development 1998, 125, 4989–4997. [Google Scholar] [CrossRef]
- Bellanger, S.; de Gramont, A.; Sobczak-Thepot, J. Cyclin B2 suppresses mitotic failure and DNA re-replication in human somatic cells knocked down for both cyclins B1 and B2. Oncogene 2007, 26, 7175–7184. [Google Scholar] [CrossRef]
- Levasseur, M.D.; Thomas, C.; Davies, O.R.; Higgins, J.M.G.; Madgwick, S. Aneuploidy in Oocytes Is Prevented by Sustained CDK1 Activity through Degron Masking in Cyclin B1. Dev. Cell 2019, 48, 672–684.e5. [Google Scholar] [CrossRef]
- Li, J.; Ouyang, Y.C.; Zhang, C.H.; Qian, W.P.; Sun, Q.Y. The cyclin B2/CDK1 complex inhibits separase activity in mouse oocyte meiosis I. Development 2019, 146, dev182519. [Google Scholar] [CrossRef]
- Li, J.; Dong, F.; Ouyang, Y.C.; Sun, Q.Y.; Qian, W.P. Overexpression of cyclin A1 promotes meiotic resumption but induces premature chromosome separation in mouse oocyte. J. Cell. Physiol. 2020, 235, 7136–7145. [Google Scholar] [CrossRef]
- Groisman, I.; Huang, Y.-S.; Mendez, R.; Cao, Q.; Theurkauf, W.; Richter, J.D. CPEB, Maskin, and Cyclin B1 mRNA at the Mitotic Apparatus: Implications for Local Translational Control of Cell Division. Cell 2000, 103, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.D. CPEB: A life in translation. Trends Biochem. Sci. 2007, 32, 279–285. [Google Scholar] [CrossRef] [PubMed]
- de Moor, C.H.; Meijer, H.; Lissenden, S. Mechanisms of translational control by the 3′ UTR in development and differentiation. Semin. Cell Dev. Biol. 2005, 16, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Y.; Hamilton, K.; Manley, J.L.; Shi, Y.; Walz, T.; Tong, L. Molecular basis for the recognition of the human AAUAAA polyadenylation signal. Proc. Natl. Acad. Sci. USA 2018, 115, E1419–E1428. [Google Scholar] [CrossRef]
- Colgan, D.F.; Manley, J.L. Mechanism and regulation of mRNA polyadenylation. Genes Dev. 1997, 11, 2755–2766. [Google Scholar] [CrossRef]
- Schönemann, L.; Kühn, U.; Martin, G.; Schäfer, P.; Gruber, A.R.; Keller, W.; Zavolan, M.; Wahle, E. Reconstitution of CPSF active in polyadenylation: Recognition of the polyadenylation signal by WDR33. Genes Dev. 2014, 28, 2381–2393. [Google Scholar] [CrossRef]
- Tay, J.; Hodgman, R.; Richter, J.D. The control of cyclin B1 mRNA translation during mouse oocyte maturation. Dev. Biol. 2000, 221, 1–9. [Google Scholar] [CrossRef]
- Hake, L.E.; Richter, J.D. CPEB is a specificity factor that mediates cytoplasmic polyadenylation during Xenopus oocyte maturation. Cell 1994, 79, 617–627. [Google Scholar] [CrossRef]
- Dejene, E.A.; Li, Y.; Showkatian, Z.; Ling, H.; Seto, E. Regulation of poly(a)-specific ribonuclease activity by reversible lysine acetylation. J. Biol. Chem. 2020, 295, 10255–10270. [Google Scholar] [CrossRef]
- Mendez, R.; Hake, L.E.; Andresson, T.; Littlepage, L.E.; Ruderman, J.V.; Richter, J.D. Phosphorylation of CPE binding factor by Eg2 regulates translation of c-mos mRNA. Nature 2000, 404, 302–307. [Google Scholar] [CrossRef]
- Radford, H.E.; Meijer, H.A.; de Moor, C.H. Translational control by cytoplasmic polyadenylation in Xenopus oocytes. Biochim. Biophys. Acta 2008, 1779, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Tarn, W.-Y.; Lai, M.-C. Translational control of cyclins. Cell Div. 2011, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Richter, J.D. Dissolution of the maskin-eIF4E complex by cytoplasmic polyadenylation and poly(A)-binding protein controls cyclin B1 mRNA translation and oocyte maturation. EMBO J. 2002, 21, 3852–3862. [Google Scholar] [CrossRef]
- Frolova, L.; Le Goff, X.; Zhouravleva, G.; Davydova, E.; Philippe, M.; Kisselev, L. Eukaryotic polypeptide chain release factor eRF3 is an eRF1- and ribosome-dependent guanosine triphosphatase. RNA 1996, 2, 334–341. [Google Scholar]
- Mendez, R.; Murthy, K.G.; Ryan, K.; Manley, J.L.; Richter, J.D. Phosphorylation of CPEB by Eg2 mediates the recruitment of CPSF into an active cytoplasmic polyadenylation complex. Mol. Cell 2000, 6, 1253–1259. [Google Scholar] [CrossRef]
- Piqué, M.; López, J.M.; Foissac, S.; Guigó, R.; Méndez, R. A Combinatorial Code for CPE-Mediated Translational Control. Cell 2008, 132, 434–448. [Google Scholar] [CrossRef]
- Hochegger, H.; Klotzbücher, A.; Kirk, J.; Howell, M.; le Guellec, K.; Fletcher, K.; Duncan, T.; Sohail, M.; Hunt, T. New B-type cyclin synthesis is required between meiosis I and II during Xenopus oocyte maturation. Development 2001, 128, 3795–3807. [Google Scholar] [CrossRef]
- Kotani, T.; Yasuda, K.; Ota, R.; Yamashita, M. Cyclin B1 mRNA translation is temporally controlled through formation and disassembly of RNA granules. J. Cell Biol. 2013, 202, 1041–1055. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Franciosi, F.; Daldello, E.M.; Luong, X.G.; Althoff, P.; Wang, X.; Conti, M. CPEB1-dependent disruption of the mRNA translation program in oocytes during maternal aging. Nat. Commun. 2023, 14, 416. [Google Scholar] [CrossRef]
- Fulka, J., Jr.; Motlík, J.; Fulka, J.; Jílek, F. Effect of cycloheximide on nuclear maturation of pig and mouse oocytes. J. Reprod. Fertil. 1986, 77, 281–285. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, C.R.; Han, S.J.; Daldello, E.M.; Cho, A.; Martins, J.P.S.; Xia, G.; Conti, M. Maternal mRNAs with distinct 3′ UTRs define the temporal pattern of Ccnb1 synthesis during mouse oocyte meiotic maturation. Genes Dev. 2017, 31, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, H.M.; Kang, M.K.; Sohn, D.H.; Han, S.J. 5’-UTR and ORF elements, as well as the 3′-UTR regulate the translation of Cyclin. Biochem. Biophys. Res. Commun. 2020, 527, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.Y.; Choe, M.H.; Kim, J.S.; Oh, J.S. Mis12 controls cyclin B1 stabilization via Cdc14B-mediated APC/C(Cdh1) regulation during meiotic G2/M transition in mouse oocytes. Development 2020, 147, dev185322. [Google Scholar] [CrossRef] [PubMed]
- Hellmuth, S.; Pöhlmann, C.; Brown, A.; Böttger, F.; Sprinzl, M.; Stemmann, O. Positive and negative regulation of vertebrate separase by Cdk1-cyclin B1 may explain why securin is dispensable. J. Biol. Chem. 2015, 290, 8002–8010. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Orr-Weaver, T.L. The molecular basis of sister-chromatid cohesion. Annu. Rev. Cell Dev. Biol. 2001, 17, 753–777. [Google Scholar] [CrossRef]
- Stemmann, O.; Zou, H.; Gerber, S.A.; Gygi, S.P.; Kirschner, M.W. Dual inhibition of sister chromatid separation at metaphase. Cell 2001, 107, 715–726. [Google Scholar] [CrossRef]
- Yu, J.; Raia, P.; Ghent, C.M.; Raisch, T.; Sadian, Y.; Cavadini, S.; Sabale, P.M.; Barford, D.; Raunser, S.; Morgan, D.O.; et al. Structural basis of human separase regulation by securin and CDK1-cyclin B1. Nature 2021, 596, 138–142. [Google Scholar] [CrossRef]
- Herbert, M.; Levasseur, M.; Homer, H.; Yallop, K.; Murdoch, A.; McDougall, A. Homologue disjunction in mouse oocytes requires proteolysis of securin and cyclin B1. Nat. Cell Biol. 2003, 5, 1023–1025. [Google Scholar] [CrossRef]
- McGuinness, B.E.; Anger, M.; Kouznetsova, A.; Gil-Bernabé, A.M.; Helmhart, W.; Kudo, N.R.; Wuensche, A.; Taylor, S.; Hoog, C.; Novak, B.; et al. Regulation of APC/C activity in oocytes by a Bub1-dependent spindle assembly checkpoint. Curr. Biol. 2009, 19, 369–380. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.Y.; Wang, F.; Sun, Q.Y.; Qian, W.P. The Cyclin B2/CDK1 Complex Conservatively Inhibits Separase Activity in Oocyte Meiosis II. Front. Cell Dev. Biol. 2021, 9, 648053. [Google Scholar] [CrossRef]
- Homer, H. The APC/C in female mammalian meiosis I. Reproduction 2013, 146, R61–R71. [Google Scholar] [CrossRef] [PubMed]
- Gorbsky, G.J. The spindle checkpoint and chromosome segregation in meiosis. FEBS J. 2015, 282, 2471–2487. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Kuo, W.T.; Chuang, Y.T.; Chen, C.Y.; Lin, C.C. Cyclin B1 destruction box-mediated protein instability: The enhanced sensitivity of fluorescent-protein-based reporter gene system. BioMed Res. Int. 2013, 2013, 732307. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Hamada, M.; Malureanu, L.; Jeganathan, K.B.; Zhou, W.; Morbeck, D.E.; van Deursen, J.M. Cdc20 is critical for meiosis I and fertility of female mice. PLoS Genet. 2010, 6, e1001147. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.R.; Jones, K.T. Regulation of the meiotic divisions of mammalian oocytes and eggs. Biochem. Soc. Trans. 2018, 46, 797–806. [Google Scholar] [CrossRef]
- Chen, L.; Ouyang, Y.C.; Gu, L.J.; Guo, J.N.; Han, Z.M.; Wang, Z.B.; Hou, Y.; Schatten, H.; Sun, Q.Y. Septin 9 controls CCNB1 stabilization via APC/C(CDC20) during meiotic metaphase I/anaphase I transition in mouse oocytes. Cell Prolif. 2023, 56, e13359. [Google Scholar] [CrossRef]
- Yamano, H.; Tsurumi, C.; Gannon, J.; Hunt, T. The role of the destruction box and its neighbouring lysine residues in cyclin B for anaphase ubiquitin-dependent proteolysis in fission yeast: Defining the D-box receptor. EMBO J. 1998, 17, 5670–5678. [Google Scholar] [CrossRef]
- Sigrist, S.; Jacobs, H.; Stratmann, R.; Lehner, C.F. Exit from mitosis is regulated by Drosophila fizzy and the sequential destruction of cyclins A, B and B3. EMBO J. 1995, 14, 4827–4838. [Google Scholar] [CrossRef]
- Nakajo, N.; Yoshitome, S.; Iwashita, J.; Iida, M.; Uto, K.; Ueno, S.; Okamoto, K.; Sagata, N. Absence of Wee1 ensures the meiotic cell cycle in Xenopus oocytes. Genes Dev. 2000, 14, 328–338. [Google Scholar] [CrossRef]
- Okamoto, K.; Nakajo, N.; Sagata, N. The existence of two distinct Wee1 isoforms in Xenopus: Implications for the developmental regulation of the cell cycle. EMBO J. 2002, 21, 2472–2484. [Google Scholar] [CrossRef]
- Nebreda, A.R.; Ferby, I. Regulation of the meiotic cell cycle in oocytes. Curr. Opin. Cell Biol. 2000, 12, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Verlhac, M.H.; Lefebvre, C.; Kubiak, J.Z.; Umbhauer, M.; Rassinier, P.; Colledge, W.; Maro, B. Mos activates MAP kinase in mouse oocytes through two opposite pathways. EMBO J. 2000, 19, 6065–6074. [Google Scholar] [CrossRef] [PubMed]
- Verlhac, M.H.; Kubiak, J.Z.; Clarke, H.J.; Maro, B. Microtubule and chromatin behavior follow MAP kinase activity but not MPF activity during meiosis in mouse oocytes. Development 1994, 120, 1017–1025. [Google Scholar] [CrossRef]
- Sagata, N.; Watanabe, N.; Vande Woude, G.F.; Ikawa, Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature 1989, 342, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, J.Z.; Weber, M.; de Pennart, H.; Winston, N.J.; Maro, B. The metaphase II arrest in mouse oocytes is controlled through microtubule-dependent destruction of cyclin B in the presence of CSF. EMBO J. 1993, 12, 3773–3778. [Google Scholar] [CrossRef]
- Tunquist, B.J.; Maller, J.L. Under arrest: Cytostatic factor (CSF)-mediated metaphase arrest in vertebrate eggs. Genes Dev. 2003, 17, 683–710. [Google Scholar] [CrossRef]
- Yamamoto, T.M.; Iwabuchi, M.; Ohsumi, K.; Kishimoto, T. APC/C-Cdc20-mediated degradation of cyclin B participates in CSF arrest in unfertilized Xenopus eggs. Dev. Biol. 2005, 279, 345–355. [Google Scholar] [CrossRef]
- Liu, J.; Grimison, B.; Lewellyn, A.L.; Maller, J.L. The anaphase-promoting complex/cyclosome inhibitor Emi2 is essential for meiotic but not mitotic cell cycles. J. Biol. Chem. 2006, 281, 34736–34741. [Google Scholar] [CrossRef]
- Tung, J.J.; Hansen, D.V.; Ban, K.H.; Loktev, A.V.; Summers, M.K.; Adler, J.R., 3rd; Jackson, P.K. A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc. Natl. Acad. Sci. USA 2005, 102, 4318–4323. [Google Scholar] [CrossRef]
- Zernicka-Goetz, M.; Ciemerych, M.A.; Kubiak, J.Z.; Tarkowski, A.K.; Maro, B. Cytostatic factor inactivation is induced by a calcium-dependent mechanism present until the second cell cycle in fertilized but not in parthenogenetically activated mouse eggs. J. Cell Sci. 1995, 108 Pt 2, 469–474. [Google Scholar] [CrossRef]
- Tunquist, B.J.; Schwab, M.S.; Chen, L.G.; Maller, J.L. The spindle checkpoint kinase bub1 and cyclin e/cdk2 both contribute to the establishment of meiotic metaphase arrest by cytostatic factor. Curr. Biol. 2002, 12, 1027–1033. [Google Scholar] [CrossRef]
- Geng, Y.; Yu, Q.; Sicinska, E.; Das, M.; Schneider, J.E.; Bhattacharya, S.; Rideout, W.M.; Bronson, R.T.; Gardner, H.; Sicinski, P. Cyclin E ablation in the mouse. Cell 2003, 114, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Knoblich, J.A.; Sauer, K.; Jones, L.; Richardson, H.; Saint, R.; Lehner, C.F. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell 1994, 77, 107–120. [Google Scholar] [CrossRef]
- Fay, D.S.; Han, M. Mutations in cye-1, a Caenorhabditis elegans cyclin E homolog, reveal coordination between cell-cycle control and vulval development. Development 2000, 127, 4049–4060. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.T. Mammalian egg activation: From Ca2+ spiking to cell cycle progression. Reproduction 2005, 130, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.C.; Verlhac, M.H. Second meiotic arrest and exit in frogs and mice. EMBO Rep. 2008, 9, 246–251. [Google Scholar] [CrossRef]
- Markoulaki, S.; Matson, S.; Ducibella, T. Fertilization stimulates long-lasting oscillations of CaMKII activity in mouse eggs. Dev. Biol. 2004, 272, 15–25. [Google Scholar] [CrossRef]
- Sanders, J.R.; Swann, K. Molecular triggers of egg activation at fertilization in mammals. Reproduction 2016, 152, R41–R50. [Google Scholar] [CrossRef]
- Yang, X.; Shu, L.; Cai, L.; Sun, X.; Cui, Y.; Liu, J. Homozygous missense mutation Arg207Cys in the WEE2 gene causes female infertility and fertilization failure. J. Assist. Reprod. Genet. 2019, 36, 965–971. [Google Scholar] [CrossRef]
- Hansen, D.V.; Tung, J.J.; Jackson, P.K. CaMKII and polo-like kinase 1 sequentially phosphorylate the cytostatic factor Emi2/XErp1 to trigger its destruction and meiotic exit. Proc. Natl. Acad. Sci. USA 2006, 103, 608–613. [Google Scholar] [CrossRef]
- Nixon, V.L.; Levasseur, M.; McDougall, A.; Jones, K.T. Ca(2+) oscillations promote APC/C-dependent cyclin B1 degradation during metaphase arrest and completion of meiosis in fertilizing mouse eggs. Curr. Biol. 2002, 12, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Clift, D.; Schuh, M. Restarting life: Fertilization and the transition from meiosis to mitosis. Nat. Rev. Mol. Cell Biol. 2013, 14, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Treen, N.; Heist, T.; Wang, W.; Levine, M. Depletion of Maternal Cyclin B3 Contributes to Zygotic Genome Activation in the Ciona Embryo. Curr. Biol. 2018, 28, 1150–1156.e4. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.M.; Kang, M.K.; Seong, S.Y.; Jo, J.H.; Kim, M.J.; Shin, E.K.; Lee, C.G.; Han, S.J. Meiotic Cell Cycle Progression in Mouse Oocytes: Role of Cyclins. Int. J. Mol. Sci. 2023, 24, 13659. https://doi.org/10.3390/ijms241713659
Kim HM, Kang MK, Seong SY, Jo JH, Kim MJ, Shin EK, Lee CG, Han SJ. Meiotic Cell Cycle Progression in Mouse Oocytes: Role of Cyclins. International Journal of Molecular Sciences. 2023; 24(17):13659. https://doi.org/10.3390/ijms241713659
Chicago/Turabian StyleKim, Hye Min, Min Kook Kang, Se Yoon Seong, Jun Hyeon Jo, Min Ju Kim, Eun Kyeong Shin, Chang Geun Lee, and Seung Jin Han. 2023. "Meiotic Cell Cycle Progression in Mouse Oocytes: Role of Cyclins" International Journal of Molecular Sciences 24, no. 17: 13659. https://doi.org/10.3390/ijms241713659
APA StyleKim, H. M., Kang, M. K., Seong, S. Y., Jo, J. H., Kim, M. J., Shin, E. K., Lee, C. G., & Han, S. J. (2023). Meiotic Cell Cycle Progression in Mouse Oocytes: Role of Cyclins. International Journal of Molecular Sciences, 24(17), 13659. https://doi.org/10.3390/ijms241713659





