Synthesis of α-Diimine Complex Enabling Rapidly Covalent Attachment to Silica Supports and Application of Homo-/Heterogeneous Catalysts in Ethylene Polymerization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of α-Diimine Ligand and Complex CatA
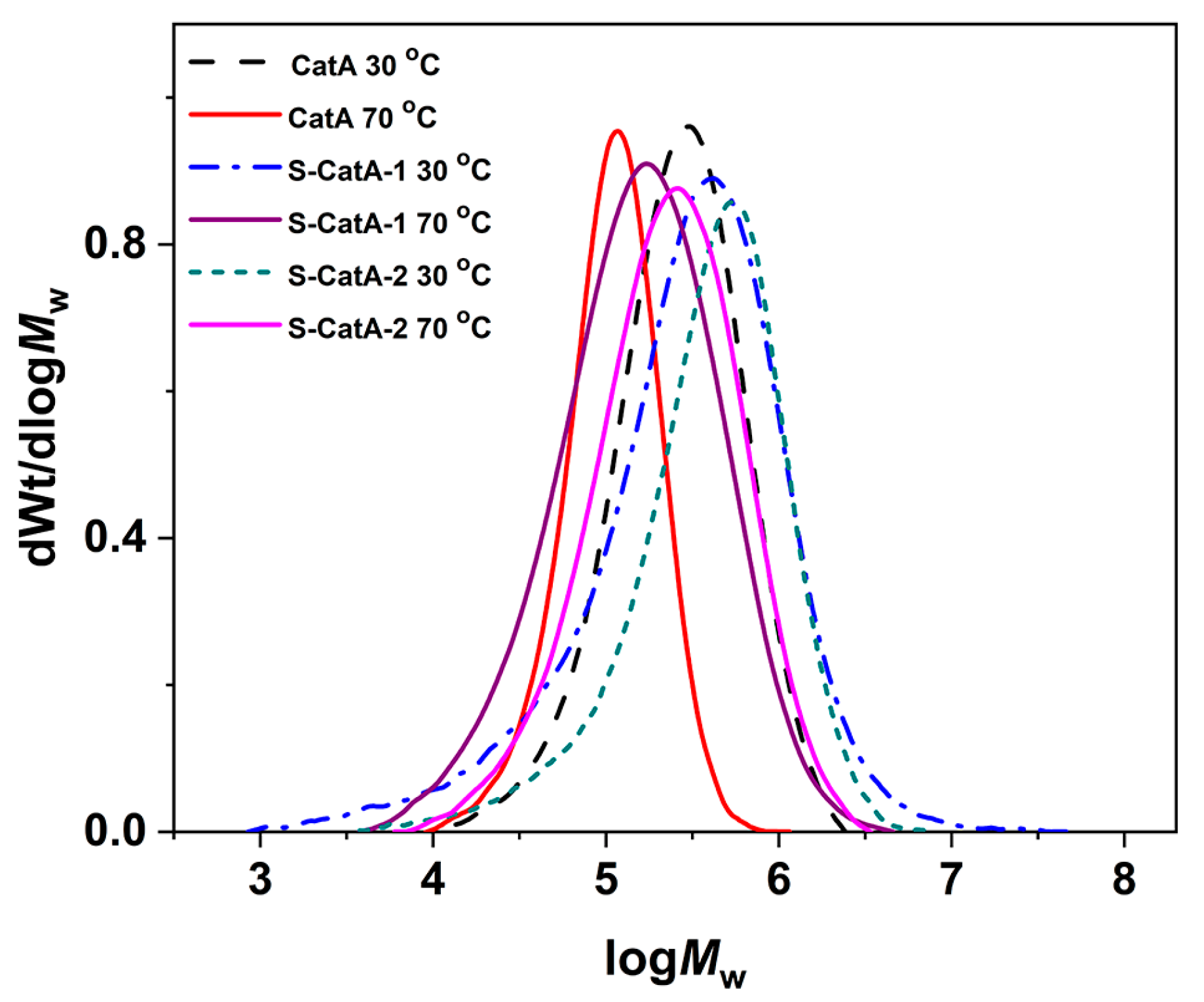
2.2. Ethylene Polymerization with α-Diimine Nickel (II) Complex CatA
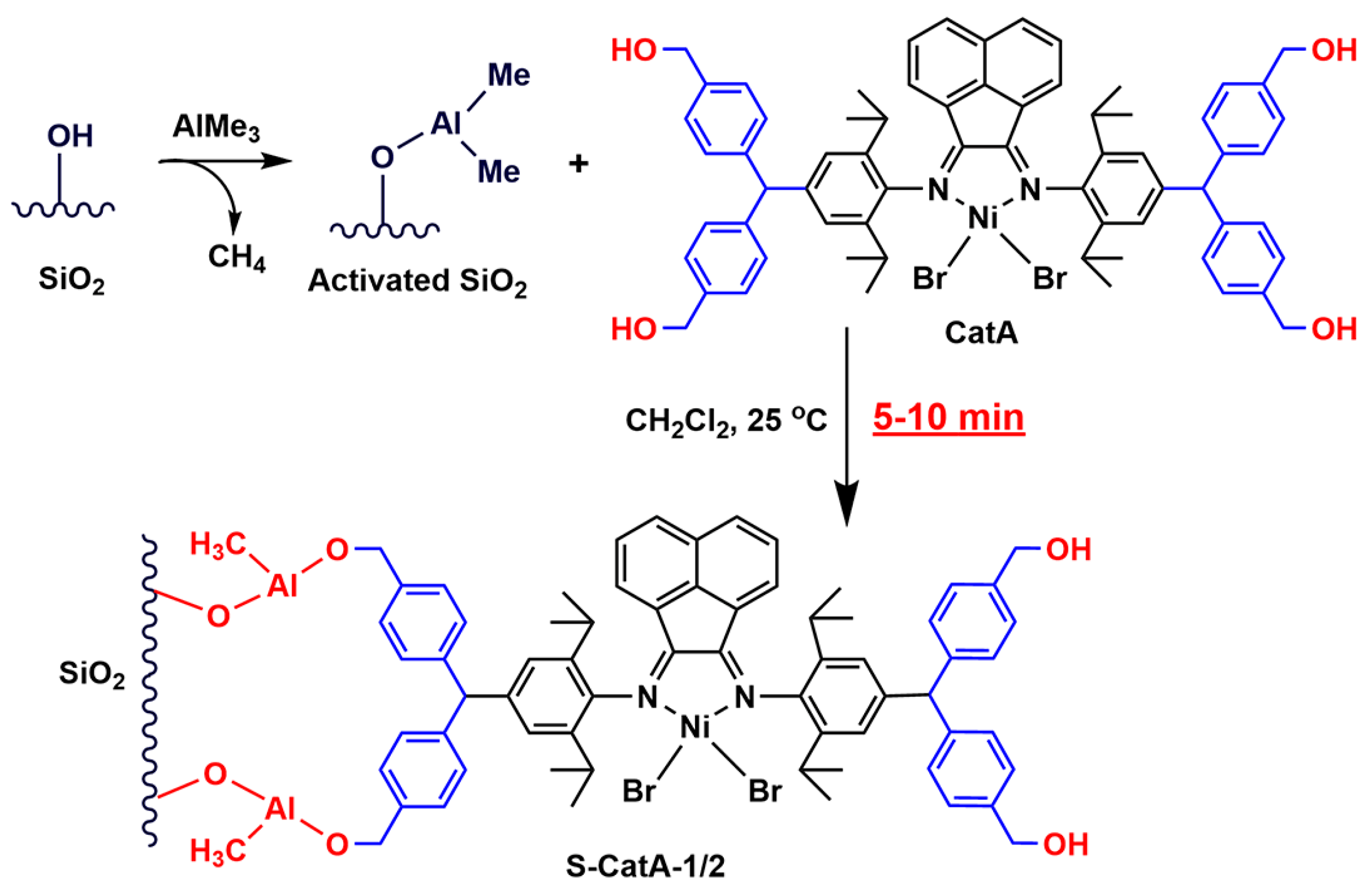
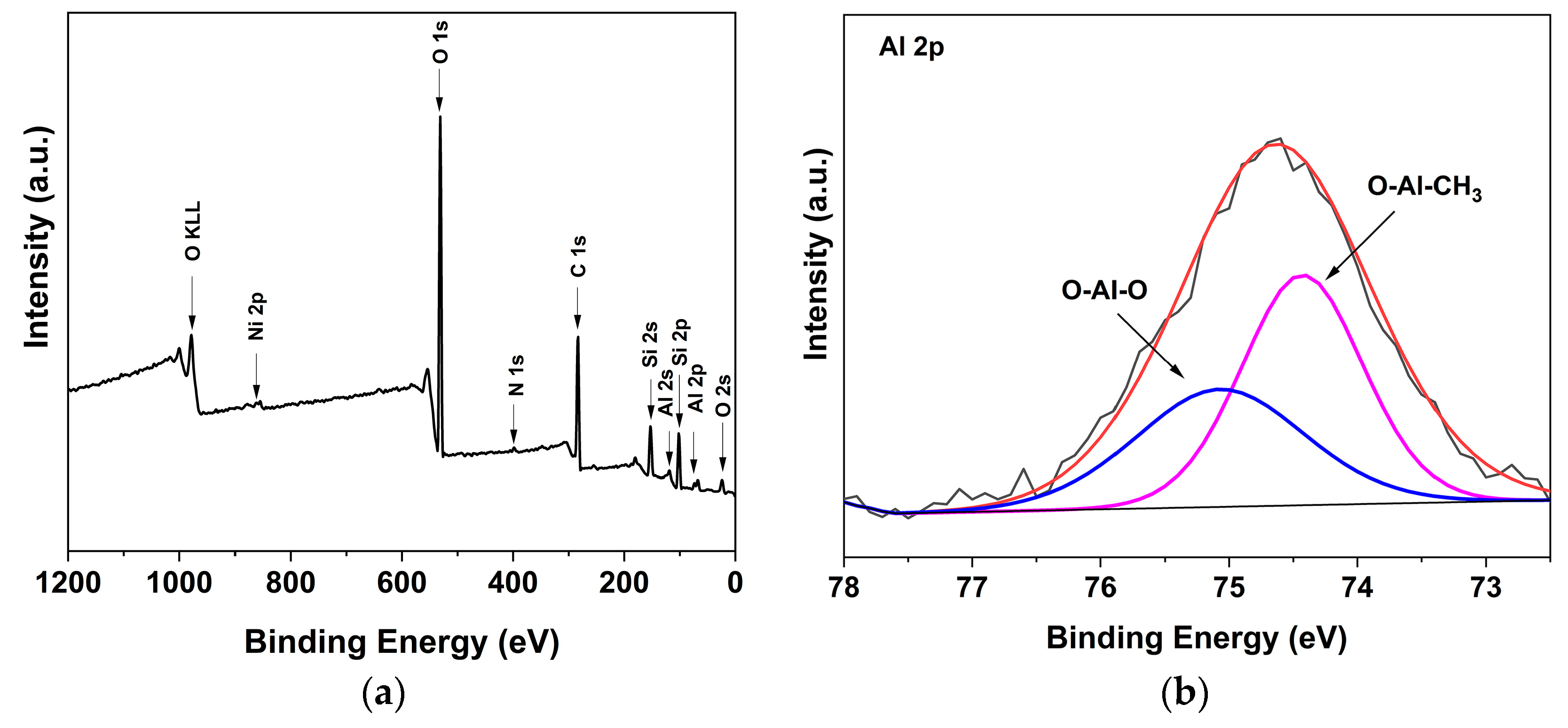
2.3. Preparation of Supported α-Diimine Nickel(II) Catalysts (S-CatA-1, S-CatA-2)
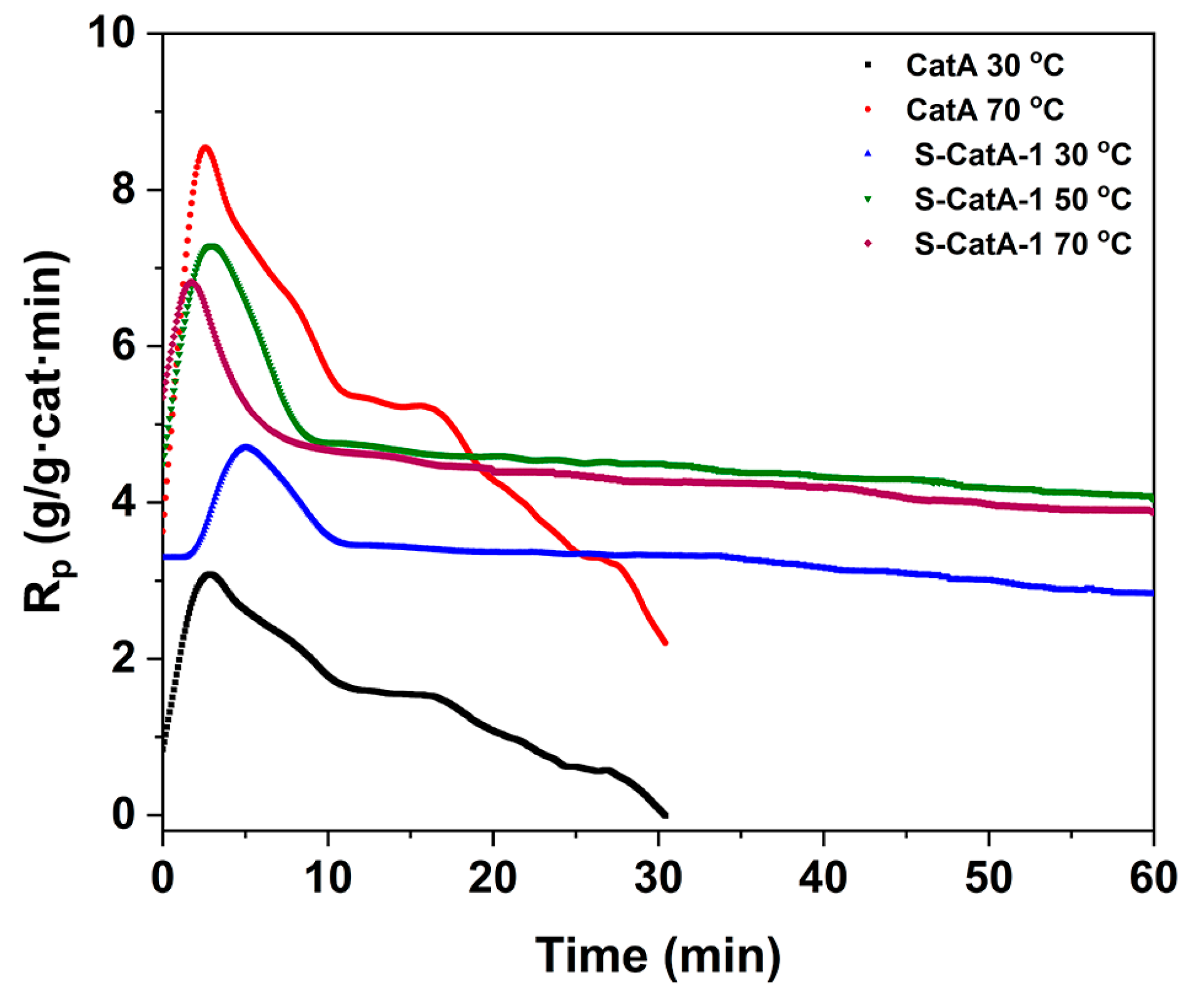
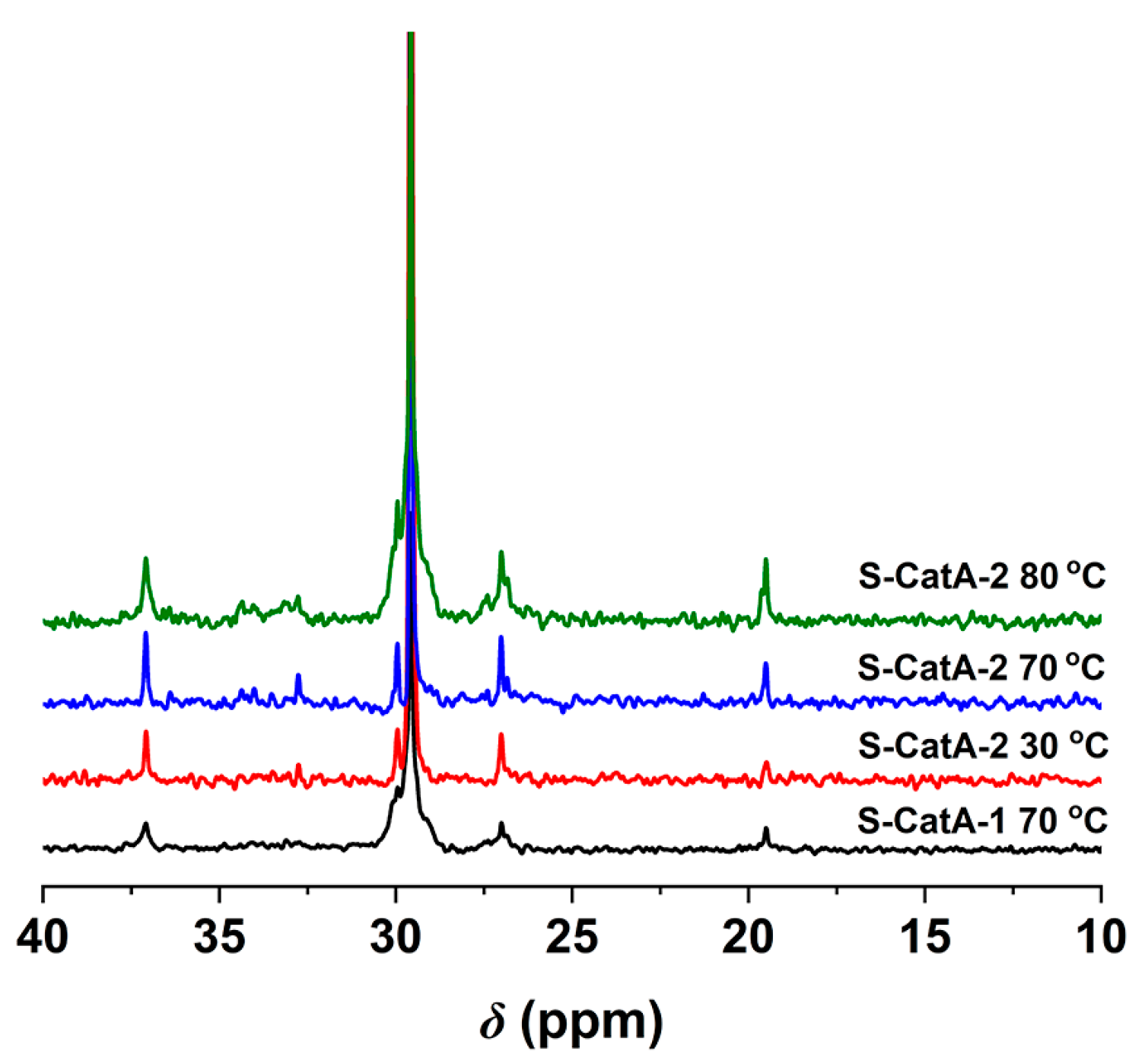
2.4. Ethylene Polymerization with Supported Catalysts (S-CatA-1, S-CatA-2)
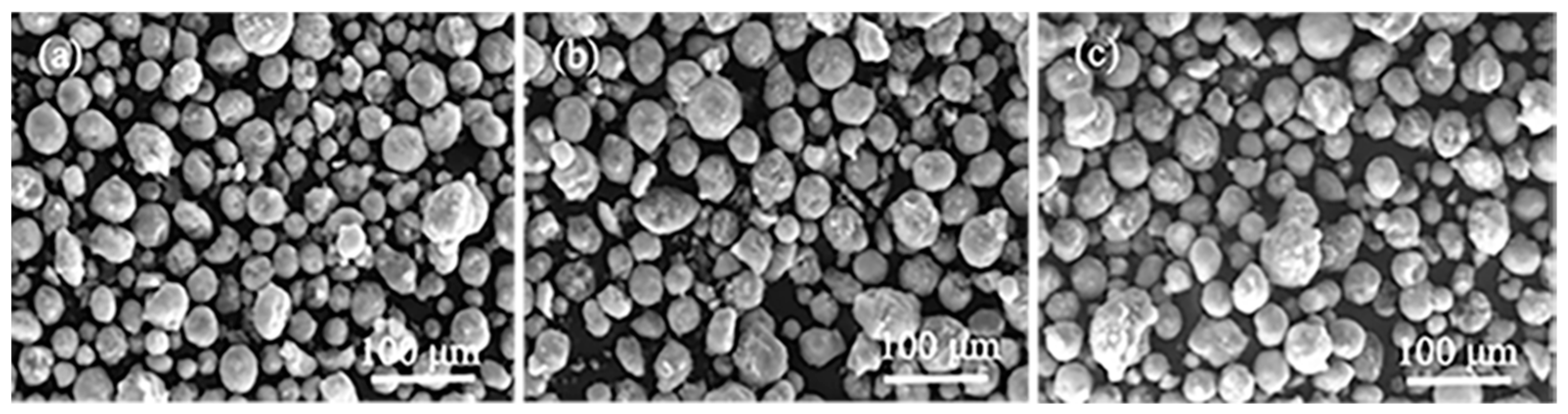
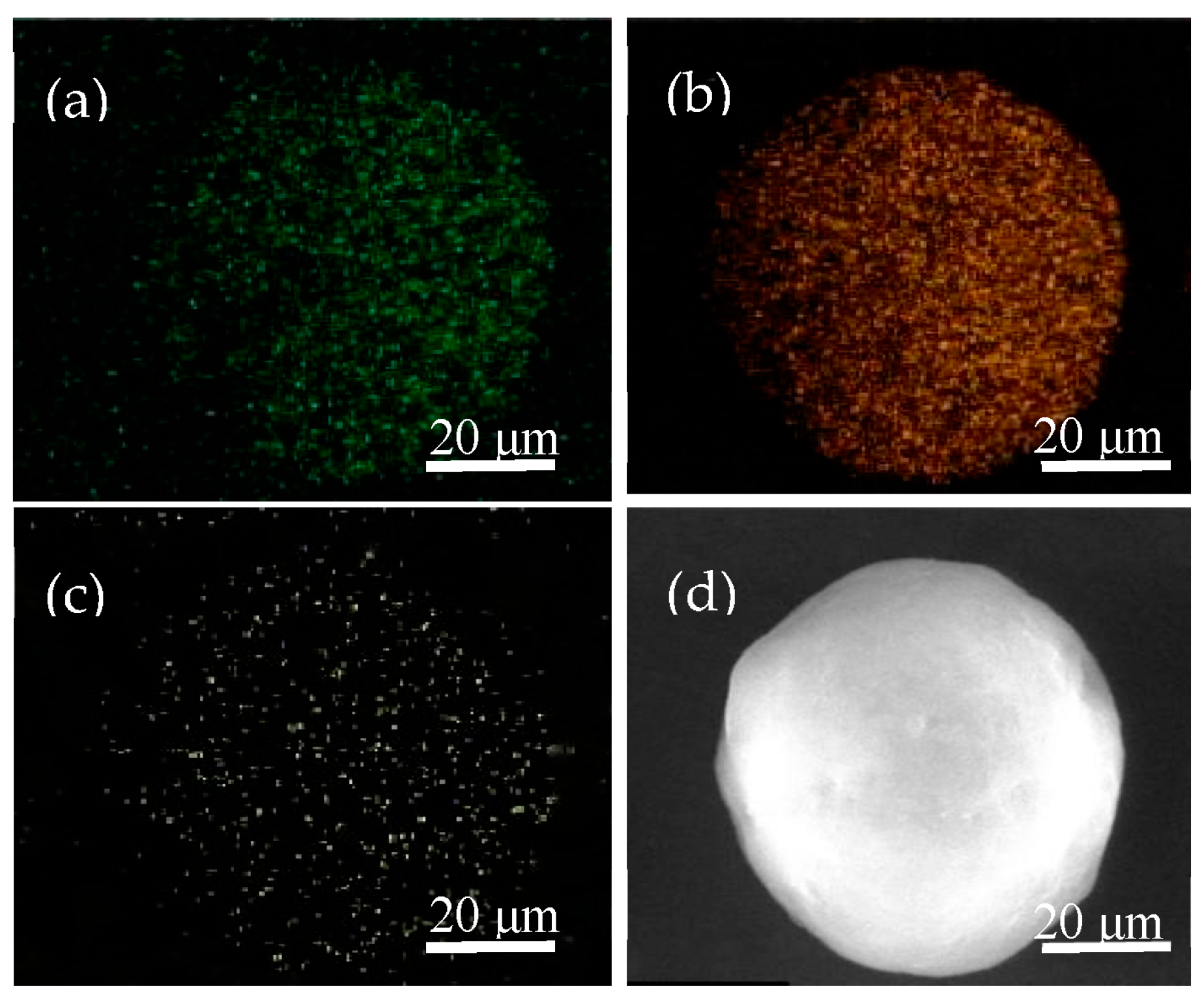
2.5. Morphology of Polymers Obtained over Homogeneous and Heterogeneous Catalytic Systems
3. Materials and Methods
3.1. General Methods and Materials
3.2. Characterizations
3.3. Preparation of α-Diimine Nickel (II) Complex
3.4. Synthesis of Supported Catalysts S-CatA-1 and S-CatA-2
3.5. Ethylene Polymerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Dholakiya, B.Z.; Jangir, R. Role of organometallic complexes in olefin polymerization: A review report. J. Organomet. Chem. 2021, 953, 122066. [Google Scholar] [CrossRef]
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd(II)- and Ni(II)-based catalysts for polymerization of ethylene and α-olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Small, B.L.; Brookhart, M.; Bennett, A.M.A. Highly Active Iron and Cobalt Catalysts for the Polymerization of Ethylene. J. Am. Chem. Soc. 1998, 120, 4049–4050. [Google Scholar] [CrossRef]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-Metal Catalysts for Ethylene Homo- and Copolymerization. Chem. Rev. 2000, 100, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Q.; Solanad, G.A.; Sun, W.-H. Recent advances in Ni-mediated ethylene chain growth: Nimine-donor ligand effects on catalytic activity, thermal stability and oligo-/polymer structure. Coord. Chem. Rev. 2017, 350, 68–83. [Google Scholar] [CrossRef]
- AlObaidi, F.; Ye, Z.; Zhu, S. Ethylene polymerization with homogeneous nickel–diimine catalysts: Effects of catalyst structure and polymerization conditions on catalyst activity and polymer properties. Polymer 2004, 45, 6823–6829. [Google Scholar] [CrossRef]
- Meinhard, D.; Wegner, M.; Kipiani, G.; Hearley, A.; Reuter, P.; Fischer, S.; Marti, A.O.; Rieger, B. New Nickel(II) Diimine Complexes and the Control of Polyethylene Microstructure by Catalyst Design. J. Am. Chem. Soc. 2007, 129, 9182–9191. [Google Scholar] [CrossRef]
- Bahuleyan, B.K.; Son, G.W.; Park, D.-W.; Ha, C.-S.; Kim, I. Ethylene polymerization by sterically and electronically modulated Ni(II) α-diimine complexes. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 1066–1082. [Google Scholar] [CrossRef]
- Rhinehart, J.L.; Brown, L.A.; Long, B.K. A robust Ni(II) α-diimine catalyst for high temperature ethylene polymerization. J. Am. Chem. Soc. 2013, 135, 16316–16319. [Google Scholar] [CrossRef]
- Jia, D.D.; Zhang, W.J.; Liu, W.L.; Wang, L.; Redshaw, C.; Sun, W.H. Unsymmetrical α-diiminonickel bromide complexes: Synthesis, characterization and their catalytic behavior toward ethylene. Catal. Sci. Technol. 2013, 3, 2737–2745. [Google Scholar] [CrossRef]
- Mu, H.L.; Pan, L.; Song, D.P.; Li, Y.S. Neutral nickel catalysts for olefin homo- and copolymerization: Relationships between catalyst structures and catalytic properties. Chem. Rev. 2015, 115, 12091–12137. [Google Scholar] [CrossRef] [PubMed]
- Rhinehart, J.L.; Mitchell, N.E.; Long, B.K. Enhancing α-Diimine Catalysts for High-Temperature Ethylene Polymerization. ACS Catal. 2014, 4, 2501–2504. [Google Scholar] [CrossRef]
- Dai, S.Y.; Sui, X.L.; Chen, C.L. Highly Robust Palladium(II) α-Diimine Catalysts for Slow-Chain Walking Polymerization of Ethylene and Copolymerization with Methyl Acrylate. Angew. Chem. Int. Ed. 2015, 54, 9948–9953. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.M.; Long, B.K. Recent developments in redox-active olefin polymerization catalysts. Coord. Chem. Rev. 2018, 372, 141–152. [Google Scholar] [CrossRef]
- Pei, L.X.; Liu, F.S.; Liao, H.; Gao, J.; Zhong, L.; Gao, H.Y.; Wu, Q. Synthesis of polyethylenes with controlled branching with α-diimine nickel catalysts and revisiting formation of long-chain branching. ACS Catal. 2018, 8, 1104–1113. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Zhang, R.; Ma, Y.P.; Solan, G.A.; Liang, T.L.; Sun, W.H. Branched polyethylenes attainable using thermally enhanced bis(imino)acenaphthene-nickel catalysts: Exploring the effects of temperature and pressure. Appl. Catal. A Gen. 2019, 573, 73–86. [Google Scholar] [CrossRef]
- Wang, F.Z.; Chen, C.L. A continuing legend: The Brookhart-type α-diimine nickel and palladium catalysts. Polym. Chem. 2019, 10, 2354–2369. [Google Scholar] [CrossRef]
- Padilla-Vélez, O.; O’Connor, K.S.; LaPointe, A.M.; MacMillan, S.N.; Coates, G.W. Switchable living nickel(II) α-diimine catalyst for ethylene polymerization. Chem. Commun. 2019, 55, 7607–7610. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Y.X.; Jian, Z.B. Tunable branching and living character in ethylene polymerization using “polyethylene glycol sandwich” α-diimine nickel catalysts. Polym. Chem. 2021, 12, 1236. [Google Scholar] [CrossRef]
- Liu, H.J.; Tian, W.L.; Wang, X.Y.; Lei, T.; Li, P.; Xu, G.Y.; Li, C.; Zhang, S.J.; Wang, F.Z. Controllable preparation of branched polyolefins with various microstructural units via chain walking ethylene and pentene polymerizations. Chin. J. Polym. Sci. 2023, 41, 905–914. [Google Scholar] [CrossRef]
- Chapleski, R.C.; Kern, J.L.; Anderson, W.C.; Long, B.K.; Roy, S.A. Mechanistic Study of Microstructure Modulation in Olefin Polymerizations using a Redox-Active Ni(II) α-Diimine Catalyst. Catal. Sci. Technol. 2020, 10, 2029–2039. [Google Scholar] [CrossRef]
- Zheng, H.D.; Zhong, L.; Du, C.; Du, W.B.; Cheung, C.S.; Ruan, J.J.; Gao, H.Y. Combining hydrogen bonding interactions with steric and electronic modifications for thermally robust α-diimine palladium catalysts toward ethylene (co)polymerization. Catal. Sci. Technol. 2021, 11, 124–135. [Google Scholar] [CrossRef]
- Schrekker, H.S.; Kotov, V.; Preishuber-Pflugl, P.; White, P.; Brookhart, M. Efficient Slurry-Phase Homopolymerization of Ethylene to Branched Polyethylenes Using α-Diimine Nickel(II) Catalysts Covalently Linked to Silica Supports. Macromolecules 2006, 39, 6341–6354. [Google Scholar] [CrossRef]
- Li, Y.G.; Pan, L.; Zheng, Z.J.; Li, Y.S. Polymerization of ethylene to branched polyethylene with silica and Merrifield resin supported nickel(II) catalysts with α-diimine ligands. J. Mol. Catal. A Chem. 2008, 287, 57–64. [Google Scholar] [CrossRef]
- Choi, Y.; Soares, J.B. Ethylene slurry polymerization using nickel diimine catalysts covalently-attached onto MgCl2-based supports. Polymer 2010, 51, 2271–2276. [Google Scholar] [CrossRef]
- Huang, C.; Zakharov, V.A.; Semikolenova, N.V.; Matsko, M.A.; Mahmood, Q.; Talsi, E.P.; Sun, W.H. Comparisons between homogeneous and immobilized 1-(2,6-dibenzhydryl-4-nitrophenylimino)-2-mesityliminoacenaphthylnickel bromide as a precatalyst in ethylene polymerization. J. Catal. 2019, 372, 103–108. [Google Scholar] [CrossRef]
- Favero, C.; Closs, M.B.; Galland, G.B.; Stieler, R.; Rossetto, E.; Bernardo-Gusmão, K. A binary nickel diimine-MCM-41 supported catalyst and its application in ethylene polymerization. J. Catal. 2019, 377, 63–71. [Google Scholar] [CrossRef]
- Zong, K.N.; Hou, Y.H.; Zhao, X.B.; Sun, Y.L.; Liu, B.Y.; Yang, M. Slurry homopolymerization of ethylene using thermostable α-diimine nickel catalysts covalently linked to silica supports via substituents on acenaphthequinone-backbone. Polymers 2022, 14, 3684. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, C.; Zhao, H.P.; Cai, Z.G.; Chen, C.L. Hydrogen-bonding-induced heterogenization of nickel and palladium catalysts for copolymerization of ethylene with polar monomers. Angew. Chem. Int. Ed. 2021, 60, 17446–17451. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q.; Yao, P.; Pang, W.M.; Zou, C.; Chen, M. SiO2-supported Ni(II) and Fe(II) catalysts bearing sodium-sulfonate group for olefin polymerization. Chin. J. Chem. 2022, 40, 2773–2779. [Google Scholar] [CrossRef]
- Zou, C.; Si, G.F.; Chen, C.L. A general strategy for heterogenizing olefin polymerization catalysts and the synthesis of polyolefins and composites. Nat. Commun. 2022, 13, 1954. [Google Scholar] [CrossRef]
- Ma, Z.S.; Xu, M.L.; Zhu, N.N.; Tan, C.; Chen, C.L. Heterogeneous α-diimine nickel catalysts with improved catalytic performance in ethylene polymerization. Chin. J. Chem. 2023, 41, 1155–1162. [Google Scholar] [CrossRef]
- Bahuleyan, B.K.; Oh, J.M.; Chandran, D.; Ha, J.Y.; Hur, A.Y.; Park, D.-W.; Ha, C.S.; Suh, H.; Kim, I. Highly Efficient Supported Diimine Ni(II) and Iminopyridyl Fe(II) Catalysts for Ethylene Polymerizations. Top. Catal. 2010, 53, 500–509. [Google Scholar] [CrossRef]
- Zhang, R.F.; Hou, Y.H.; Wei, X.L.; Zhao, D.D.; Cui, M.M.; Zhai, F.F.; Li, X.L.; Liu, B.Y.; Yang, M. Thermostable α-Diimine Nickel Complexes with Substituents on Acenaphthequinone-backbone for Ethylene Polymerization. Chin. J. Polym. Sci. 2020, 6, 134–136. [Google Scholar] [CrossRef]
- Zhao, D.D.; Hou, Y.H.; Zong, K.N.; Cui, M.M.; Liu, B.Y.; Yang, M. Linear/branched block polyethylene produced by α-diimine nickel(II) catalyst and bis(phenoxy-imine) zirconium binary catalyst system in the presence of diethyl zinc. Chin. J. Polym. Sci. 2021, 39, 1581–1589. [Google Scholar] [CrossRef]
- Gates, D.P.; Svejda, S.A.; Oñate, E. Synthesis of branched polyethylene using (α-diimine) nickel(II) catalysts: Influence of temperature, ethylene pressure, and ligand structure on polymer properties. Macromolecules 2000, 33, 2320–2334. [Google Scholar] [CrossRef]
- Galland, G.B.; de Souza, R.F.; Mauler, R.S.; Nunes, F.F. 13C NMR Determination of the Composition of Linear Low-Density Polyethylene Obtained with [η3-Methallyl-nickel-diimine]PF6 Complex. Macromolecules 1999, 32, 1620–1625. [Google Scholar] [CrossRef]
- Leatherman, M.D.; Svejda, S.A.; Johnson, L.K. Mechanistic studies of nickel (II) alkyl agostic cations and alkyl ethylene complexes: Investigations of chain propagation and isomerization in (α-diimine) Ni (II)-catalyzed ethylene polymerization. J. Am. Chem. Soc. 2003, 125, 3068–3081. [Google Scholar] [CrossRef]
- He, X.; Guo, Y.; Chen, X.; Wu, B.; Zou, J.; Wen, Y.; Chen, D. Synthesis of MWNTs/SiO2 Supported Nickel and Palladium Complexes and their Application as Catalysts for Cyclic Olefins Polymerization. J. Organomet. Chem. 2021, 949, 121953. [Google Scholar] [CrossRef]
- Clas, S.-D.; Heyding, R.D.; McFaddin, D.C.; Russell, K.E.; Scammell-Bullock, M.V.; Kelusky, E.C.; St-Cyr, D. Crystallinities of copolymers of ethylene and 1-alkenes. J. Polym. Sci. Pol. Phys. 1988, 26, 1271–1286. [Google Scholar] [CrossRef]
- ASTM D1895-69; Standard Test Methods for Apparent Density, Bulk Factor, and Pourability of Plastic Materials. American Society of Testing Materials: West Conshohocken, PA, USA, 2017.





| Entry | T °C | P MPa | t min | Yield g | Act. c | Tm d °C | χc e (%) | Mw e kg/mol | PDI e |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | 0.5 | 30 | 0.89 | 1.77 | 120 | 16 | 332 | 2.9 |
| 2 | 50 | 0.5 | 30 | 3.84 | 7.68 | 107 | 6 | n.d. | n.d. |
| 3 | 70 | 0.5 | 30 | 3.30 | 6.60 | 94 | 2 | 136 | 2.8 |
| 4 | 70 | 1.0 | 30 | 4.73 | 9.46 | 110 | 5 | 439 | 2.3 |
| 5 b | 30 | 0.5 | 30 | 1.02 | 2.04 | 119 | 38 | 141 | 2.3 |
| 6 b | 50 | 0.5 | 30 | 2.73 | 5.46 | 105 | 5 | n.d. | n.d. |
| 7 b | 70 | 0.5 | 30 | 1.98 | 3.96 | 74 | 1 | 114 | 2.4 |
| 8 | 30 | 0.5 | 60 | 2.60 | 2.60 | 118 | 28 | 359 | 2.4 |
| 9 | 50 | 0.5 | 60 | 6.51 | 6.51 | n.d. | n.d. | n.d. | n.d. |
| 10 | 70 | 0.5 | 60 | 3.90 | 3.90 | 95 | 2 | 152 | 2.1 |
| 11 f | 70 | 0.5 | 30 | 1.42 | 2.84 | n.d. | n.d. | n.d. | n.d. |
| Entry | T °C | P MPa | Branches (1000 C) | Percentage over Total Branching (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Methyl | Ethyl | Propyl | Butyl | Amyl | LCB b | ||||
| 1 | 30 | 0.5 | 35 | 80.8 | 9.1 | 0.9 | 0 | 0 | 9.2 |
| 3 | 70 | 0.5 | 90 | 63.5 | 11.3 | 9.0 | 0 | 0.8 | 15.4 |
| 4 | 70 | 1.0 | 82 | 70.9 | 9.8 | 1.0 | 4.7 | 0.6 | 13.0 |
| 5 c | 30 | 0.5 | 41 | 83.4 | 4.9 | 1.7 | 2.2 | 0.7 | 7.1 |
| 7 c | 70 | 0.5 | 108 | 78.9 | 4.6 | 1.5 | 3.0 | 1.5 | 10.5 |
| Entry | Catalyst | Ni b wt% | T °C | Act. c | Act. d | Tm e °C | χc e (%) | Mw f kg/mol | PDI f |
|---|---|---|---|---|---|---|---|---|---|
| 1 | S-CatA-1 | 0.94 | 30 | 2.12 | 353 | 130 | 39 | 503 | 4.6 |
| 2 | S-CatA-1 | 0.94 | 50 | 4.02 | 641 | 125 | 23 | n.d. | n.d. |
| 3 | S-CatA-1 | 0.94 | 70 | 3.86 | 615 | 120 | 13 | 190 | 3.0 |
| 4 g | S-CatA-1 | 0.94 | 70 | 4.28 | 681 | 125 | 19 | 205 | 4.3 |
| 5 | S-CatA-2 | 1.41 | 30 | 3.72 | 892 | 131 | 41 | 679 | 3.3 |
| 6 | S-CatA-2 | 1.41 | 50 | 6.92 | 1656 | 128 | 24 | n.d. | n.d. |
| 7 | S-CatA-2 | 1.41 | 70 | 4.07 | 982 | 121 | 14 | 244 | 2.7 |
| 8 | S-CatA-2 | 1.41 | 80 | 1.38 | 265 | 120 | 9 | n.d. | n.d. |
| 9 g | S-CatA-2 | 1.41 | 70 | 4.51 | 1079 | 123 | 16 | 449 | 4.6 |
| Entry | Catalyst | T °C | P MPa | Branches (1000C) | Percentage over Total Branching (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Methyl | Ethyl | Propyl | Butyl | Amyl | LCB b | |||||
| 3 | S-CatA-1 | 70 | 0.5 | 63 | 68.6 | 9.2 | 6.2 | 1.1 | 1.3 | 13.6 |
| 4 | S-CatA-1 | 70 | 1.0 | 55 | 69.0 | 8.1 | 2.4 | 3.2 | 1.4 | 15.9 |
| 5 | S-CatA-2 | 30 | 0.5 | 34 | 68.9 | 13.8 | 3.1 | 2.0 | 2.3 | 9.9 |
| 7 | S-CatA-2 | 70 | 0.5 | 52 | 61.1 | 10.7 | 4.2 | 5.8 | 4.5 | 13.7 |
| 8 | S-CatA-2 | 80 | 0.5 | 67 | 57.8 | 11.4 | 3.7 | 3.8 | 3.1 | 20.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Hou, Y.; Ye, L.; Zong, K.; An, Q.; Liu, B.; Yang, M. Synthesis of α-Diimine Complex Enabling Rapidly Covalent Attachment to Silica Supports and Application of Homo-/Heterogeneous Catalysts in Ethylene Polymerization. Int. J. Mol. Sci. 2023, 24, 13645. https://doi.org/10.3390/ijms241713645
Zhao X, Hou Y, Ye L, Zong K, An Q, Liu B, Yang M. Synthesis of α-Diimine Complex Enabling Rapidly Covalent Attachment to Silica Supports and Application of Homo-/Heterogeneous Catalysts in Ethylene Polymerization. International Journal of Molecular Sciences. 2023; 24(17):13645. https://doi.org/10.3390/ijms241713645
Chicago/Turabian StyleZhao, Xiaobei, Yanhui Hou, Linlin Ye, Kening Zong, Qingming An, Binyuan Liu, and Min Yang. 2023. "Synthesis of α-Diimine Complex Enabling Rapidly Covalent Attachment to Silica Supports and Application of Homo-/Heterogeneous Catalysts in Ethylene Polymerization" International Journal of Molecular Sciences 24, no. 17: 13645. https://doi.org/10.3390/ijms241713645
APA StyleZhao, X., Hou, Y., Ye, L., Zong, K., An, Q., Liu, B., & Yang, M. (2023). Synthesis of α-Diimine Complex Enabling Rapidly Covalent Attachment to Silica Supports and Application of Homo-/Heterogeneous Catalysts in Ethylene Polymerization. International Journal of Molecular Sciences, 24(17), 13645. https://doi.org/10.3390/ijms241713645






