β-Catenin and SOX2 Interaction Regulate Visual Experience-Dependent Cell Homeostasis in the Developing Xenopus Thalamus
Abstract
1. Introduction
2. Results
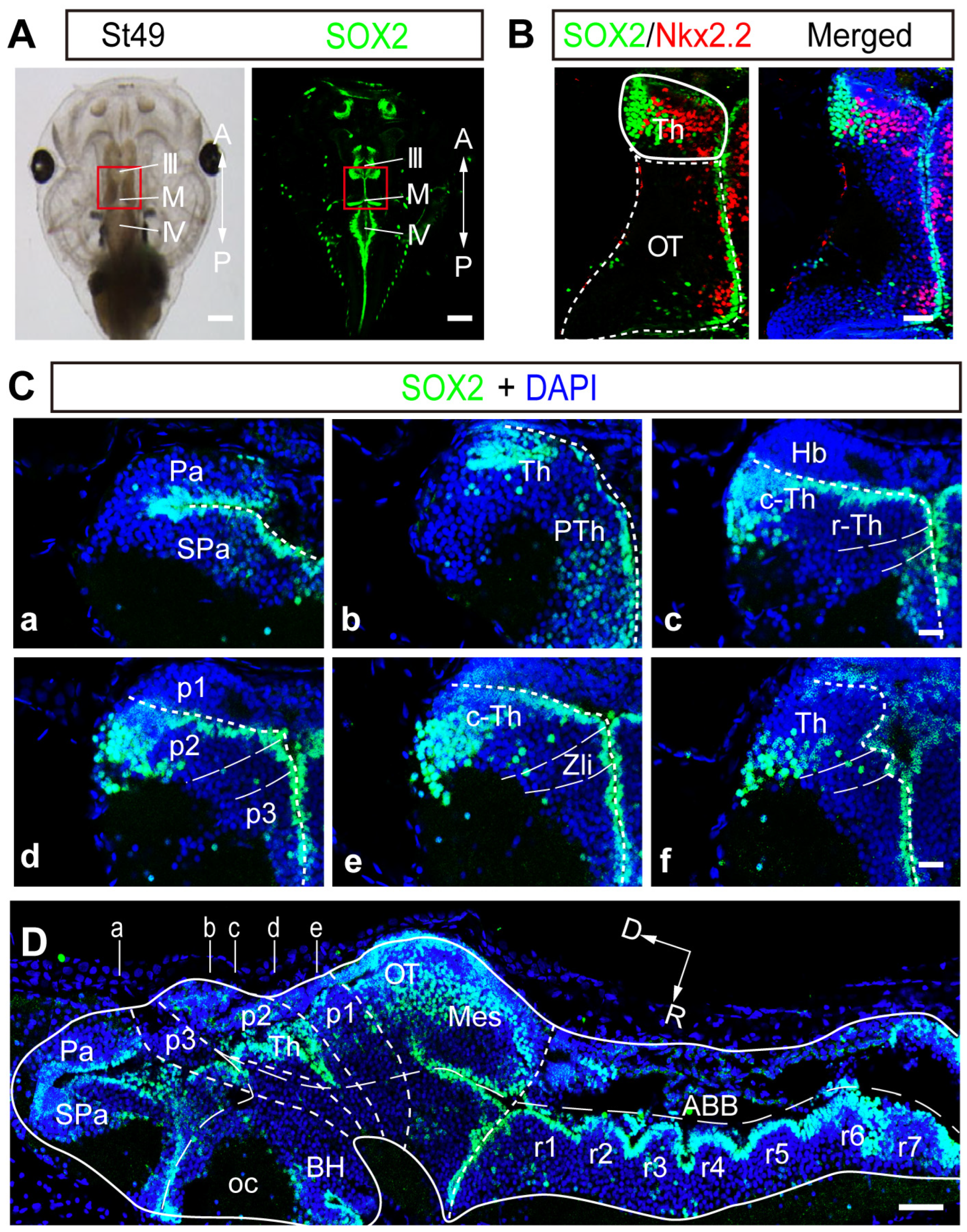
2.1. SOX2 Expression Is Highly Regionalized in the Developing Thalamus
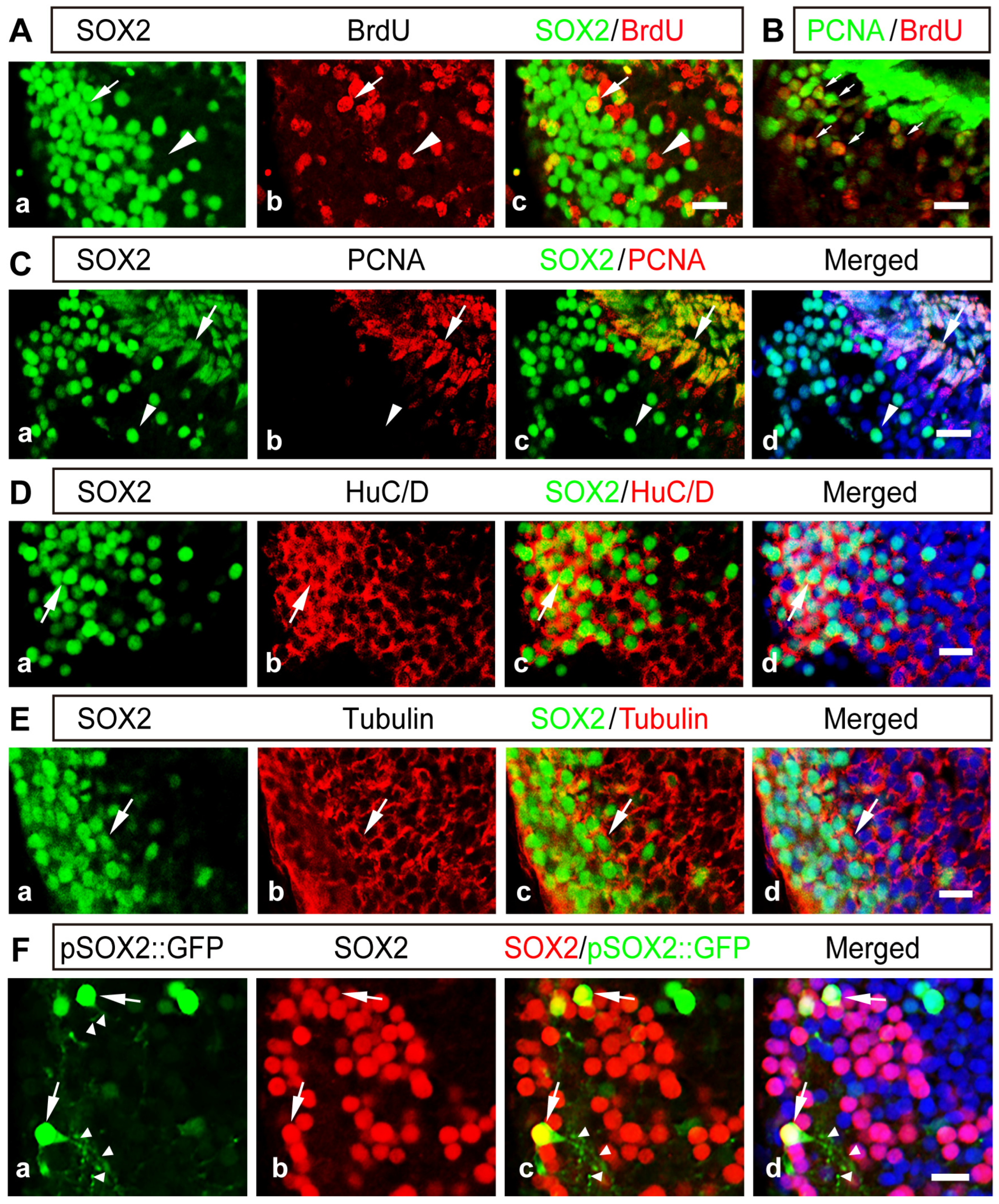
2.2. The Majority of SOX2+ Cells Are Differentiated Neurons in the Developing Thalamus
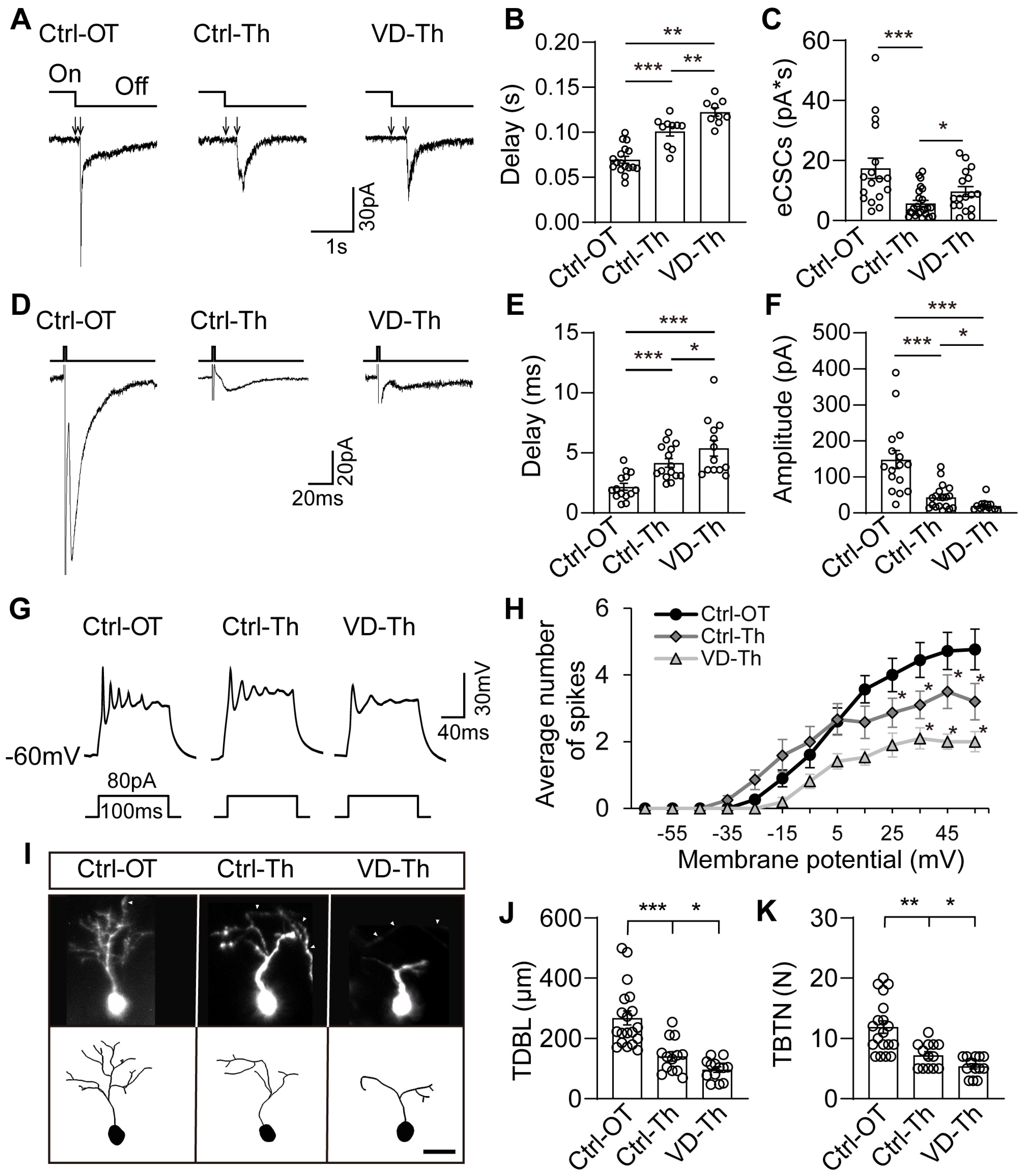
2.3. Thalamic Neurons Receive Retinal Signals and Respond to Visual Deprivation
2.4. Visual Deprivation Induces an Increase in Progenitor Cells and a Decrease in Differentiated Neurons
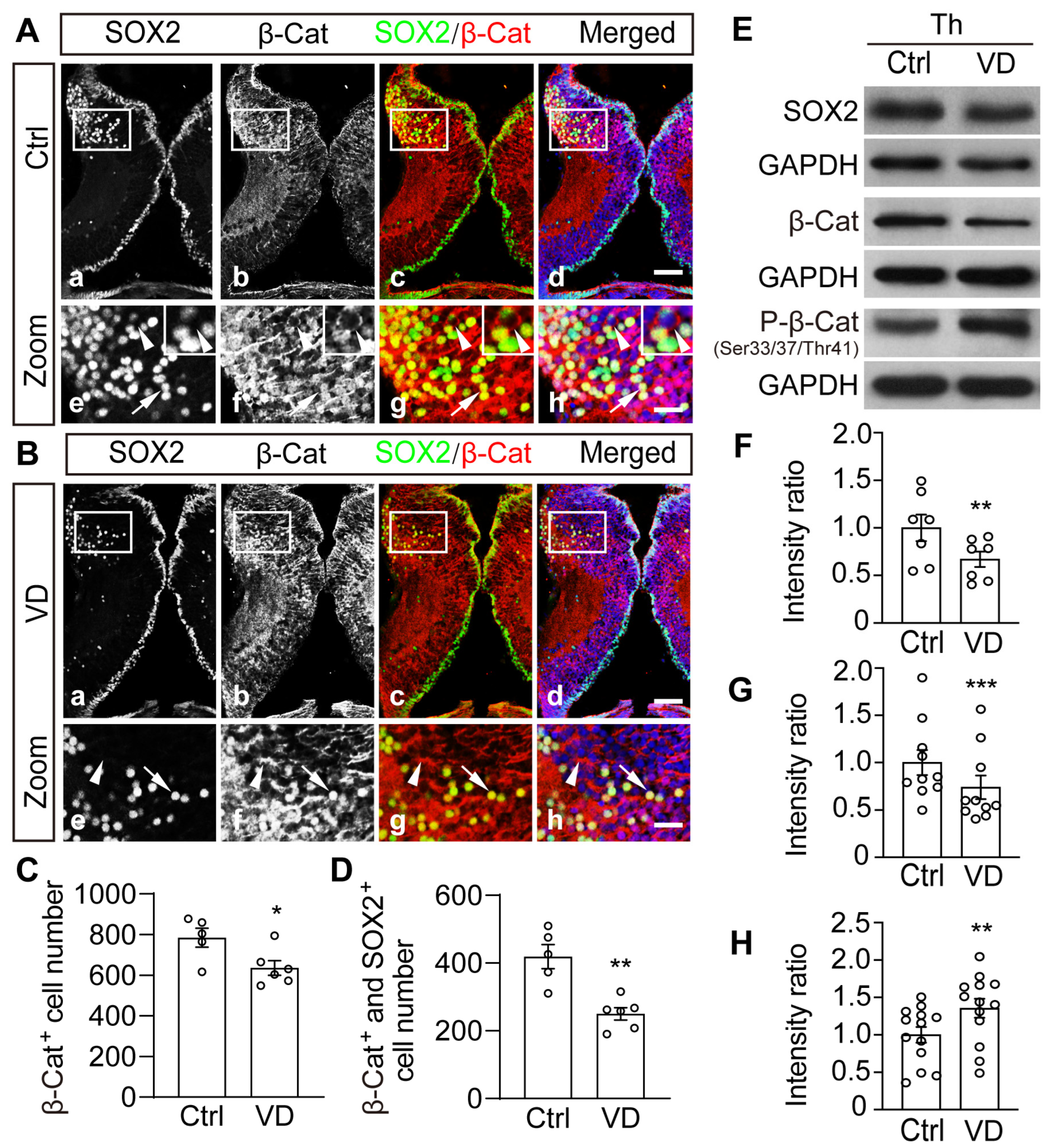
2.5. Visual Deprivation–Induced Homeostatic Regulation of Thalamic Cells Is Accompanied by Phosphorylation and Degradation of β-Catenin
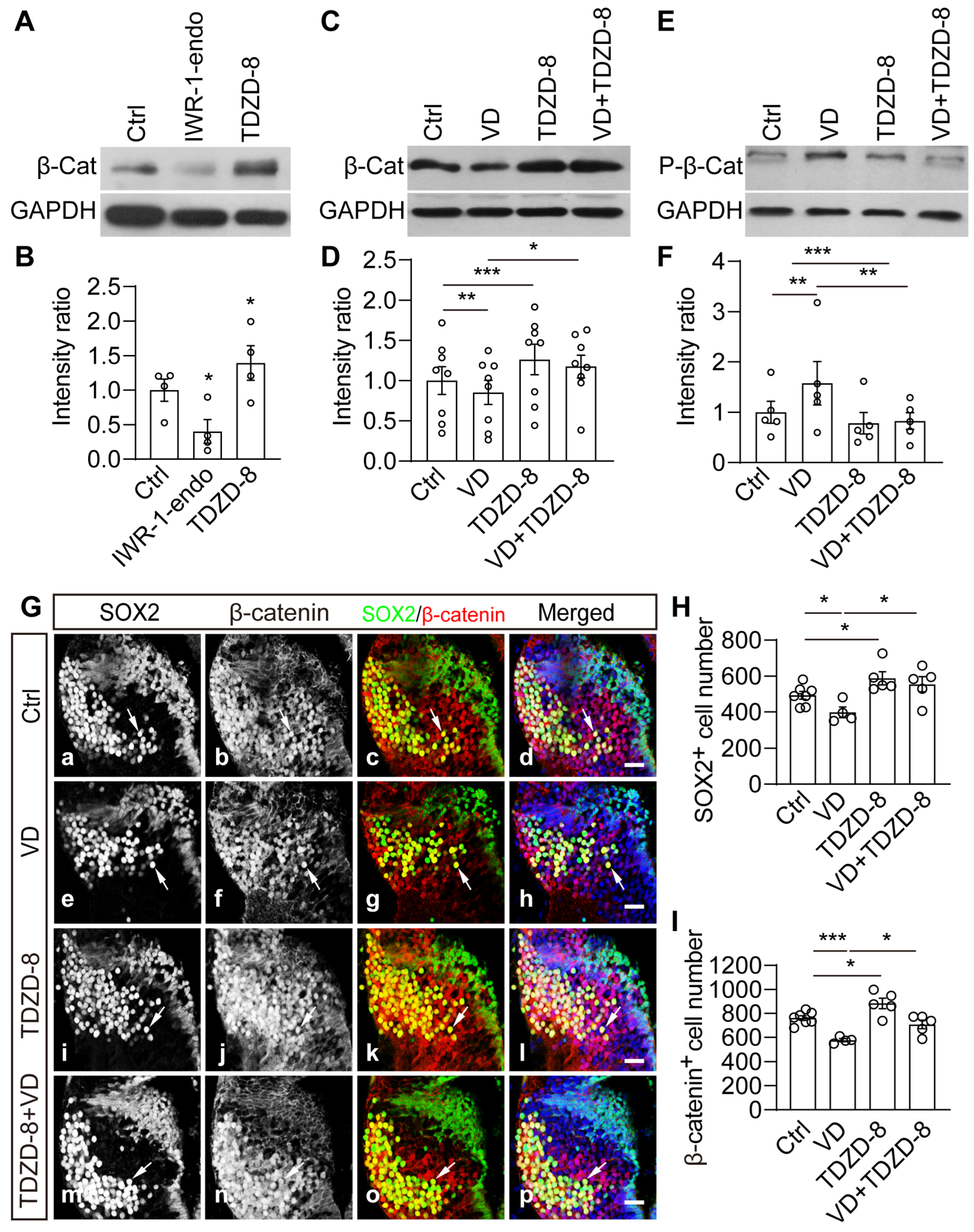
2.6. Wnt/β-Catenin Signaling Is Necessary and Sufficient to Mediate VD-Induced Thalamic Homeostasis
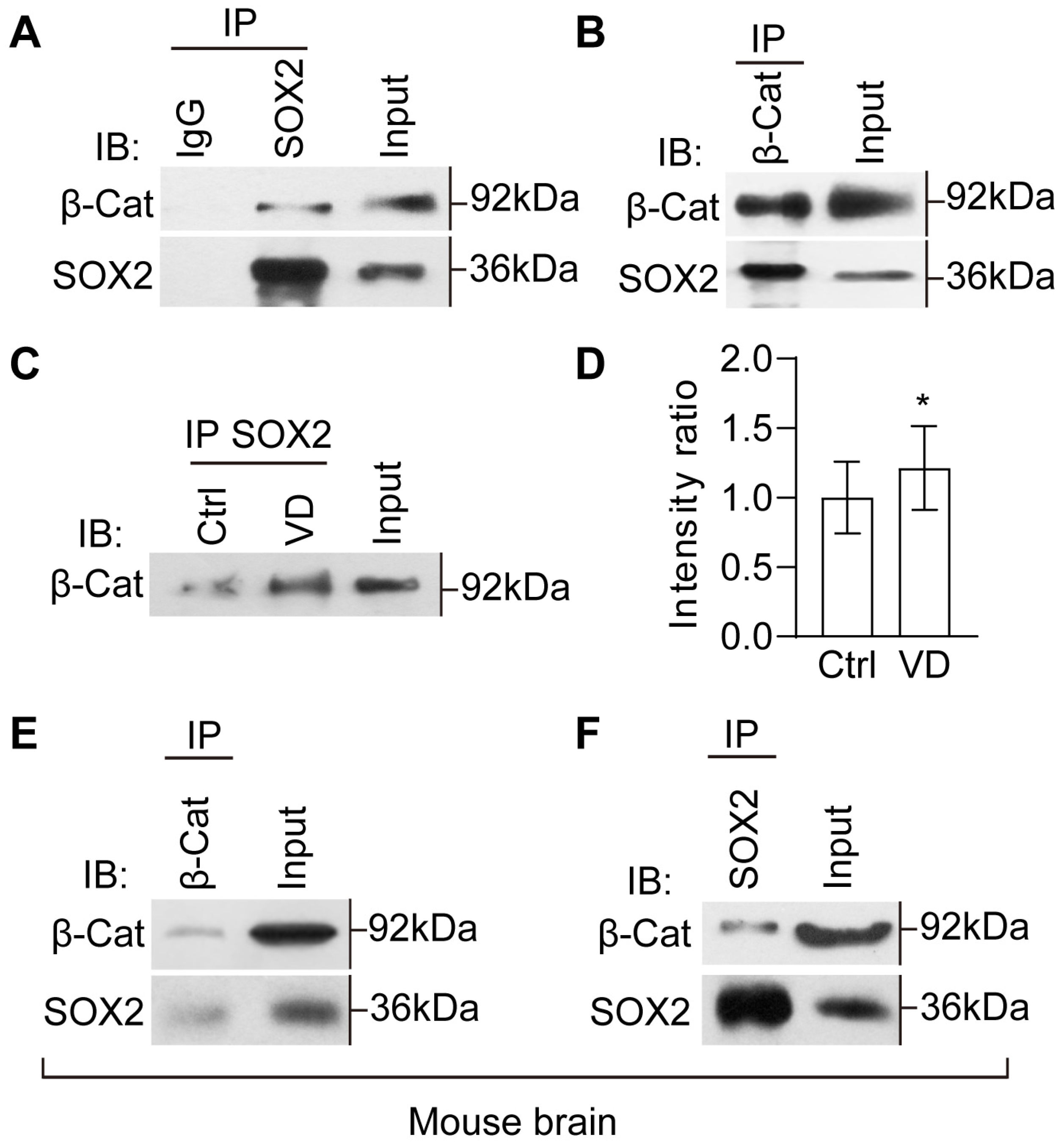
2.7. The Evolutionarily Conserved Crosstalk between SOX2 and β-Catenin
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Morpholinos and Transfection
4.3. BrdU Labeling
4.4. Immunohistochemistry and Image Analysis
4.5. Whole–Mount Immunofluorescence
4.6. Immunoblotting
4.7. Immunoprecipitation
4.8. Electrophysiology
4.9. Drugs and Treatment
4.10. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thuret, R.; Auger, H.; Papalopulu, N. Analysis of neural progenitors from embryogenesis to juvenile adult in Xenopus laevis reveals biphasic neurogenesis and continuous lengthening of the cell cycle. Biol. Open 2015, 4, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Scholpp, S.; Lumsden, A. Building a bridal chamber: Development of the thalamus. Trends Neurosci. 2010, 33, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ferre, A.; Martinez, S. Molecular regionalization of the diencephalon. Front. Neurosci. 2012, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Vue, T.Y.; Aaker, J.; Taniguchi, A.; Kazemzadeh, C.; Skidmore, J.M.; Martin, D.M.; Martin, J.F.; Treier, M.; Nakagawa, Y. Characterization of progenitor domains in the developing mouse thalamus. J. Comp. Neurol. 2007, 505, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Ferri, A.L.; Cavallaro, M.; Braida, D.; Di Cristofano, A.; Canta, A.; Vezzani, A.; Ottolenghi, S.; Pandolfi, P.P.; Sala, M.; DeBiasi, S.; et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development 2004, 131, 3805–3819. [Google Scholar] [CrossRef] [PubMed]
- Aizenman, C.D.; Munoz-Elias, G.; Cline, H.T. Visually driven modulation of glutamatergic synaptic transmission is mediated by the regulation of intracellular polyamines. Neuron 2002, 34, 623–634. [Google Scholar] [CrossRef]
- Tao, Y.; Ruan, H.; Guo, X.; Li, L.; Shen, W. HDAC1 regulates the proliferation of radial glial cells in the developing Xenopus tectum. PLoS ONE 2015, 10, e0120118. [Google Scholar] [CrossRef]
- Hall, Z.J.; Tropepe, V. Visual Experience Facilitates BDNF-Dependent Adaptive Recruitment of New Neurons in the Postembryonic Optic Tectum. J. Neurosci. 2018, 38, 2000–2014. [Google Scholar] [CrossRef]
- Shen, W.; Liu, H.H.; Schiapparelli, L.; McClatchy, D.; He, H.Y.; Yates, J.R., 3rd; Cline, H.T. Acute synthesis of CPEB is required for plasticity of visual avoidance behavior in Xenopus. Cell Rep. 2014, 6, 737–747. [Google Scholar] [CrossRef]
- Ruthazer, E.S.; Aizenman, C.D. Learning to see: Patterned visual activity and the development of visual function. Trends Neurosci. 2010, 33, 183–192. [Google Scholar] [CrossRef]
- Tremblay, M.; Fugere, V.; Tsui, J.; Schohl, A.; Tavakoli, A.; Travencolo, B.A.; Costa Lda, F.; Ruthazer, E.S. Regulation of radial glial motility by visual experience. J. Neurosci. 2009, 29, 14066–14076. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Gao, J.; Qi, X.; Tao, Y.; Guo, X.; Guo, Z.; Zheng, L.; Song, Y.; Liao, Y.; Shen, W. Visual experience dependent regulation of neuronal structure and function by histone deacetylase 1 in developing Xenopus tectum in vivo. Dev. Neurobiol. 2017, 77, 947–962. [Google Scholar] [CrossRef]
- Agathocleous, M.; Iordanova, I.; Willardsen, M.I.; Xue, X.Y.; Vetter, M.L.; Harris, W.A.; Moore, K.B. A directional Wnt/beta-catenin-Sox2-proneural pathway regulates the transition from proliferation to differentiation in the Xenopus retina. Development 2009, 136, 3289–3299. [Google Scholar] [CrossRef]
- Gao, J.; Ruan, H.; Qi, X.; Tao, Y.; Guo, X.; Shen, W. HDAC3 But not HDAC2 Mediates Visual Experience-Dependent Radial Glia Proliferation in the Developing Xenopus Tectum. Front. Cell Neurosci. 2016, 10, 221. [Google Scholar] [CrossRef]
- Morona, R.; Bandin, S.; Lopez, J.M.; Moreno, N.; Gonzalez, A. Amphibian thalamic nuclear organization during larval development and in the adult frog Xenopus laevis: Genoarchitecture and hodological analysis. J. Comp. Neurol. 2020, 528, 2361–2403. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.D.; Harafuji, N.; Archer, T.; Cunningham, D.D.; Casey, E.S. Xenopus Sox3 activates sox2 and geminin and indirectly represses Xvent2 expression to induce neural progenitor formation at the expense of non-neural ectodermal derivatives. Mech. Dev. 2009, 126, 42–55. [Google Scholar] [CrossRef]
- Mercurio, S.; Serra, L.; Pagin, M.; Nicolis, S.K. Deconstructing Sox2 Function in Brain Development and Disease. Cells 2022, 11, 1604. [Google Scholar] [CrossRef]
- Pevny, L.; Placzek, M. SOX genes and neural progenitor identity. Curr. Opin. Neurobiol. 2005, 15, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Kishi, M.; Mizuseki, K.; Sasai, N.; Yamazaki, H.; Shiota, K.; Nakanishi, S.; Sasai, Y. Requirement of Sox2-mediated signaling for differentiation of early Xenopus neuroectoderm. Development 2000, 127, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Graham, V.; Khudyakov, J.; Ellis, P.; Pevny, L. SOX2 functions to maintain neural progenitor identity. Neuron 2003, 39, 749–765. [Google Scholar] [CrossRef]
- Bylund, M.; Andersson, E.; Novitch, B.G.; Muhr, J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat. Neurosci. 2003, 6, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Favaro, R.; Valotta, M.; Ferri, A.L.; Latorre, E.; Mariani, J.; Giachino, C.; Lancini, C.; Tosetti, V.; Ottolenghi, S.; Taylor, V.; et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat. Neurosci. 2009, 12, 1248–1256. [Google Scholar] [CrossRef]
- Hoefflin, S.; Carter, D.A. Neuronal expression of SOX2 is enriched in specific hypothalamic cell groups. J. Chem. Neuroanat. 2014, 61–62, 153–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, A.H.; Bouchard-Cannon, P.; Hegazi, S.; Lowden, C.; Fung, S.W.; Chiang, C.K.; Ness, R.W.; Cheng, H.M. SOX2-Dependent Transcription in Clock Neurons Promotes the Robustness of the Central Circadian Pacemaker. Cell Rep. 2019, 26, 3191–3202.e8. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, S.; Serra, L.; Motta, A.; Gesuita, L.; Sanchez-Arrones, L.; Inverardi, F.; Foglio, B.; Barone, C.; Kaimakis, P.; Martynoga, B.; et al. Sox2 Acts in Thalamic Neurons to Control the Development of Retina-Thalamus-Cortex Connectivity. iScience 2019, 15, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Bandin, S.; Morona, R.; Gonzalez, A. Prepatterning and patterning of the thalamus along embryonic development of Xenopus laevis. Front. Neuroanat. 2015, 9, 107. [Google Scholar] [CrossRef]
- Gao, J.; Liao, Y.; Qiu, M.; Shen, W. Wnt/beta-Catenin Signaling in Neural Stem Cell Homeostasis and Neurological Diseases. Neuroscientist 2021, 27, 58–72. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Bem, J.; Brozko, N.; Chakraborty, C.; Lipiec, M.A.; Kozinski, K.; Nagalski, A.; Szewczyk, L.M.; Wisniewska, M.B. Wnt/beta-catenin signaling in brain development and mental disorders: Keeping TCF7L2 in mind. FEBS Lett. 2019, 593, 1654–1674. [Google Scholar] [CrossRef]
- Wodarz, A.; Nusse, R. Mechanisms of Wnt signaling in development. Annu. Rev. Cell. Dev. Biol. 1998, 14, 59–88. [Google Scholar] [CrossRef]
- Bengoa-Vergniory, N.; Kypta, R.M. Canonical and noncanonical Wnt signaling in neural stem/progenitor cells. Cell. Mol. Life Sci. 2015, 72, 4157–4172. [Google Scholar] [CrossRef] [PubMed]
- Dorsky, R.I.; Moon, R.T.; Raible, D.W. Control of neural crest cell fate by the Wnt signalling pathway. Nature 1998, 396, 370–373. [Google Scholar] [CrossRef]
- Braun, M.M.; Etheridge, A.; Bernard, A.; Robertson, C.P.; Roelink, H. Wnt signaling is required at distinct stages of development for the induction of the posterior forebrain. Development 2003, 130, 5579–5587. [Google Scholar] [CrossRef]
- Wang, X.; Kopinke, D.; Lin, J.; McPherson, A.D.; Duncan, R.N.; Otsuna, H.; Moro, E.; Hoshijima, K.; Grunwald, D.J.; Argenton, F.; et al. Wnt signaling regulates postembryonic hypothalamic progenitor differentiation. Dev. Cell 2012, 23, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Chen, J.; Zhu, X.; Sun, J.; Wu, X.; Shen, W.; Zhang, W.; Tao, Q.; Meng, A. Maternal Huluwa dictates the embryonic body axis through beta-catenin in vertebrates. Science 2018, 362, eaat1045. [Google Scholar] [CrossRef]
- Bluske, K.K.; Vue, T.Y.; Kawakami, Y.; Taketo, M.M.; Yoshikawa, K.; Johnson, J.E.; Nakagawa, Y. Beta-Catenin signaling specifies progenitor cell identity in parallel with Shh signaling in the developing mammalian thalamus. Development 2012, 139, 2692–2702. [Google Scholar] [CrossRef] [PubMed]
- Acosta, H.; Lopez, S.L.; Revinski, D.R.; Carrasco, A.E. Notch destabilises maternal beta-catenin and restricts dorsal-anterior development in Xenopus. Development 2011, 138, 2567–2579. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Lea, R.; Vleminckx, K.; Amaya, E. Fezf2 promotes neuronal differentiation through localised activation of Wnt/beta-catenin signalling during forebrain development. Development 2014, 141, 4794–4805. [Google Scholar] [CrossRef]
- Yost, C.; Torres, M.; Miller, J.R.; Huang, E.; Kimelman, D.; Moon, R.T. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes. Dev. 1996, 10, 1443–1454. [Google Scholar] [CrossRef]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell. Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef]
- Zhou, C.J.; Pinson, K.I.; Pleasure, S.J. Severe defects in dorsal thalamic development in low-density lipoprotein receptor-related protein-6 mutants. J. Neurosci. 2004, 24, 7632–7639. [Google Scholar] [CrossRef] [PubMed]
- Bluske, K.K.; Kawakami, Y.; Koyano-Nakagawa, N.; Nakagawa, Y. Differential activity of Wnt/beta-catenin signaling in the embryonic mouse thalamus. Dev. Dyn. 2009, 238, 3297–3309. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Y.; Smallwood, P.M.; Nathans, J. An essential role for Frizzled5 in neuronal survival in the parafascicular nucleus of the thalamus. J. Neurosci. 2008, 28, 5641–5653. [Google Scholar] [CrossRef]
- Wang, Y.; Thekdi, N.; Smallwood, P.M.; Macke, J.P.; Nathans, J. Frizzled-3 is required for the development of major fiber tracts in the rostral CNS. J. Neurosci. 2002, 22, 8563–8573. [Google Scholar] [CrossRef] [PubMed]
- Toledo, E.M.; Colombres, M.; Inestrosa, N.C. Wnt signaling in neuroprotection and stem cell differentiation. Prog. Neurobiol. 2008, 86, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Van Raay, T.J.; Moore, K.B.; Iordanova, I.; Steele, M.; Jamrich, M.; Harris, W.A.; Vetter, M.L. Frizzled 5 signaling governs the neural potential of progenitors in the developing Xenopus retina. Neuron 2005, 46, 23–36. [Google Scholar] [CrossRef]
- Zorn, A.M.; Barish, G.D.; Williams, B.O.; Lavender, P.; Klymkowsky, M.W.; Varmus, H.E. Regulation of Wnt signaling by Sox proteins: XSox17 alpha/beta and XSox3 physically interact with beta-catenin. Mol. Cell 1999, 4, 487–498. [Google Scholar] [CrossRef]
- Bernard, P.; Harley, V.R. Acquisition of SOX transcription factor specificity through protein-protein interaction, modulation of Wnt signalling and post-translational modification. Int. J. Biochem. Cell Biol. 2010, 42, 400–410. [Google Scholar] [CrossRef]
- Mueller, T. What is the Thalamus in Zebrafish? Front. Neurosci. 2012, 6, 64. [Google Scholar] [CrossRef]
- Hooks, B.M.; Chen, C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron 2006, 52, 281–291. [Google Scholar] [CrossRef]
- Penn, A.A.; Riquelme, P.A.; Feller, M.B.; Shatz, C.J. Competition in retinogeniculate patterning driven by spontaneous activity. Science 1998, 279, 2108–2112. [Google Scholar] [CrossRef] [PubMed]
- Huh, C.Y.L.; Abdelaal, K.; Salinas, K.J.; Gu, D.; Zeitoun, J.; Figueroa Velez, D.X.; Peach, J.P.; Fowlkes, C.C.; Gandhi, S.P. Long-term Monocular Deprivation during Juvenile Critical Period Disrupts Binocular Integration in Mouse Visual Thalamus. J. Neurosci. 2020, 40, 585–604. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kloc, M.; Gu, Y.; Ge, S.; Maffei, A. Layer-specific experience-dependent rewiring of thalamocortical circuits. J. Neurosci. 2013, 33, 4181–4191. [Google Scholar] [CrossRef] [PubMed]
- Catalano, S.M.; Shatz, C.J. Activity-dependent cortical target selection by thalamic axons. Science 1998, 281, 559–562. [Google Scholar] [CrossRef]
- Bestman, J.E.; Lee-Osbourne, J.; Cline, H.T. In vivo time-lapse imaging of cell proliferation and differentiation in the optic tectum of Xenopus laevis tadpoles. J. Comp. Neurol. 2012, 520, 401–433. [Google Scholar] [CrossRef]
- Sharma, P.; Cline, H.T. Visual activity regulates neural progenitor cells in developing xenopus CNS through musashi1. Neuron 2010, 68, 442–455. [Google Scholar] [CrossRef]
- Bruno, J.R.; Udoh, U.G.; Landen, J.G.; Osborn, P.O.; Asher, C.J.; Hunt, J.E.; Pratt, K.G. A circadian-dependent preference for light displayed by Xenopus tadpoles is modulated by serotonin. iScience 2022, 25, 105375. [Google Scholar] [CrossRef]
- Amador-Arjona, A.; Cimadamore, F.; Huang, C.T.; Wright, R.; Lewis, S.; Gage, F.H.; Terskikh, A.V. SOX2 primes the epigenetic landscape in neural precursors enabling proper gene activation during hippocampal neurogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, E1936–E1945. [Google Scholar] [CrossRef]
- Sarkar, A.; Hochedlinger, K. The sox family of transcription factors: Versatile regulators of stem and progenitor cell fate. Cell Stem Cell 2013, 12, 15–30. [Google Scholar] [CrossRef]
- Wang, L.; Bluske, K.K.; Dickel, L.K.; Nakagawa, Y. Basal progenitor cells in the embryonic mouse thalamus—Their molecular characterization and the role of neurogenins and Pax6. Neural Dev. 2011, 6, 35. [Google Scholar] [CrossRef]
- Shiraishi, A.; Muguruma, K.; Sasai, Y. Generation of thalamic neurons from mouse embryonic stem cells. Development 2017, 144, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Blackshaw, S.; Scholpp, S.; Placzek, M.; Ingraham, H.; Simerly, R.; Shimogori, T. Molecular pathways controlling development of thalamus and hypothalamus: From neural specification to circuit formation. J. Neurosci. 2010, 30, 14925–14930. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Z.H.; Scott, E.P.; Mu, W.; Guo, X.; Borgenheimer, E.; Freeman, M.; Ming, G.L.; Wu, Q.F.; Song, H.; Nakagawa, Y. In vivo clonal analysis reveals spatiotemporal regulation of thalamic nucleogenesis. PLoS Biol. 2018, 16, e2005211. [Google Scholar] [CrossRef]
- Dehay, C.; Savatier, P.; Cortay, V.; Kennedy, H. Cell-cycle kinetics of neocortical precursors are influenced by embryonic thalamic axons. J. Neurosci. 2001, 21, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Guido, W. Development, form, and function of the mouse visual thalamus. J. Neurophysiol. 2018, 120, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.K.; Krishnan, S.; Lin, Q.; Kibat, C.; Jesuthasan, S. Characterization of a thalamic nucleus mediating habenula responses to changes in ambient illumination. BMC Biol. 2017, 15, 104. [Google Scholar] [CrossRef]
- Schneider, S.; Steinbeisser, H.; Warga, R.M.; Hausen, P. Beta-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech. Dev. 1996, 57, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Larabell, C.A.; Torres, M.; Rowning, B.A.; Yost, C.; Miller, J.R.; Wu, M.; Kimelman, D.; Moon, R.T. Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in beta-catenin that are modulated by the Wnt signaling pathway. J. Cell Biol. 1997, 136, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Rowning, B.A.; Wells, J.; Wu, M.; Gerhart, J.C.; Moon, R.T.; Larabell, C.A. Microtubule-mediated transport of organelles and localization of beta-catenin to the future dorsal side of Xenopus eggs. Proc. Natl. Acad. Sci. USA 1997, 94, 1224–1229. [Google Scholar] [CrossRef]
- Brannon, M.; Gomperts, M.; Sumoy, L.; Moon, R.T.; Kimelman, D. A beta-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes. Dev. 1997, 11, 2359–2370. [Google Scholar] [CrossRef]
- Funayama, N.; Fagotto, F.; McCrea, P.; Gumbiner, B.M. Embryonic axis induction by the armadillo repeat domain of beta-catenin: Evidence for intracellular signaling. J. Cell Biol. 1995, 128, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Misztal, K.; Wisniewska, M.B.; Ambrozkiewicz, M.; Nagalski, A.; Kuznicki, J. WNT protein-independent constitutive nuclear localization of beta-catenin protein and its low degradation rate in thalamic neurons. J. Biol. Chem. 2011, 286, 31781–31788. [Google Scholar] [CrossRef] [PubMed]
- Pratt, T.; Davey, J.W.; Nowakowski, T.J.; Raasumaa, C.; Rawlik, K.; McBride, D.; Clinton, M.; Mason, J.O.; Price, D.J. The expression and activity of beta-catenin in the thalamus and its projections to the cerebral cortex in the mouse embryo. BMC Neurosci. 2012, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Heasman, J.; Crawford, A.; Goldstone, K.; Garner-Hamrick, P.; Gumbiner, B.; McCrea, P.; Kintner, C.; Noro, C.Y.; Wylie, C. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell 1994, 79, 791–803. [Google Scholar] [CrossRef]
- Elul, T.M.; Kimes, N.E.; Kohwi, M.; Reichardt, L.F. N- and C-terminal domains of beta-catenin, respectively, are required to initiate and shape axon arbors of retinal ganglion cells in vivo. J. Neurosci. 2003, 23, 6567–6575. [Google Scholar] [CrossRef]
- Otero, J.J.; Fu, W.; Kan, L.; Cuadra, A.E.; Kessler, J.A. Beta-catenin signaling is required for neural differentiation of embryonic stem cells. Development 2004, 131, 3545–3557. [Google Scholar] [CrossRef]
- Selvaraj, P.; Xiao, L.; Lee, C.; Murthy, S.R.; Cawley, N.X.; Lane, M.; Merchenthaler, I.; Ahn, S.; Loh, Y.P. Neurotrophic Factor-alpha1: A Key Wnt-beta-Catenin Dependent Anti-Proliferation Factor and ERK-Sox9 Activated Inducer of Embryonic Neural Stem Cell Differentiation to Astrocytes in Neurodevelopment. Stem Cells 2017, 35, 557–571. [Google Scholar] [CrossRef]
- Tang, M.; Villaescusa, J.C.; Luo, S.X.; Guitarte, C.; Lei, S.; Miyamoto, Y.; Taketo, M.M.; Arenas, E.; Huang, E.J. Interactions of Wnt/beta-catenin signaling and sonic hedgehog regulate the neurogenesis of ventral midbrain dopamine neurons. J. Neurosci. 2010, 30, 9280–9291. [Google Scholar] [CrossRef]
- Shitasako, S.; Ito, Y.; Ito, R.; Ueda, Y.; Shimizu, Y.; Ohshima, T. Wnt and Shh signals regulate neural stem cell proliferation and differentiation in the optic tectum of adult zebrafish. Dev. Neurobiol. 2017, 77, 1206–1220. [Google Scholar] [CrossRef]
- Chew, J.L.; Loh, Y.H.; Zhang, W.; Chen, X.; Tam, W.L.; Yeap, L.S.; Li, P.; Ang, Y.S.; Lim, B.; Robson, P.; et al. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol. Cell. Biol. 2005, 25, 6031–6046. [Google Scholar] [CrossRef]
- Masui, S.; Nakatake, Y.; Toyooka, Y.; Shimosato, D.; Yagi, R.; Takahashi, K.; Okochi, H.; Okuda, A.; Matoba, R.; Sharov, A.A.; et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat. Cell Biol. 2007, 9, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Sinner, D.; Rankin, S.; Lee, M.; Zorn, A.M. Sox17 and beta-catenin cooperate to regulate the transcription of endodermal genes. Development 2004, 131, 3069–3080. [Google Scholar] [CrossRef]
- Mansukhani, A.; Ambrosetti, D.; Holmes, G.; Cornivelli, L.; Basilico, C. Sox2 induction by FGF and FGFR2 activating mutations inhibits Wnt signaling and osteoblast differentiation. J. Cell Biol. 2005, 168, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Valenta, T.; Hausmann, G.; Basler, K. The many faces and functions of beta-catenin. EMBO J. 2012, 31, 2714–2736. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, M.; van de Wetering, M.; Oosterwegel, M.; Peterson-Maduro, J.; Godsave, S.; Korinek, V.; Roose, J.; Destree, O.; Clevers, H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell 1996, 86, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.S.; Saj, A.; Gocha, T.; Murphy, M.; Gonsalves, F.C.; Zhang, X.; Hayward, P.; Akgol Oksuz, B.; Shen, S.S.; Madar, A.; et al. Inhibition of beta-catenin-TCF1 interaction delays differentiation of mouse embryonic stem cells. J. Cell Biol. 2015, 211, 39–51. [Google Scholar] [CrossRef]
- Olson, L.E.; Tollkuhn, J.; Scafoglio, C.; Krones, A.; Zhang, J.; Ohgi, K.A.; Wu, W.; Taketo, M.M.; Kemler, R.; Grosschedl, R.; et al. Homeodomain-mediated beta-catenin-dependent switching events dictate cell-lineage determination. Cell 2006, 125, 593–605. [Google Scholar] [CrossRef]
- Wisniewska, M.B.; Nagalski, A.; Dabrowski, M.; Misztal, K.; Kuznicki, J. Novel beta-catenin target genes identified in thalamic neurons encode modulators of neuronal excitability. BMC Genom. 2012, 13, 635. [Google Scholar] [CrossRef]
- Nieuwkoop, P.; Faber, J. Normal Table of Xenopus laevis (Daudin); Garland Publishing Inc.: New York, NY, USA, 1994; p. 252. [Google Scholar]
- Mei, R.; Qiu, W.; Yang, Y.; Xu, S.; Rao, Y.; Li, Q.; Luo, Y.; Huang, H.; Yang, A.; Tao, H.; et al. Evidence That DDR1 Promotes Oligodendrocyte Differentiation during Development and Myelin Repair after Injury. Int. J. Mol. Sci. 2023, 24, 10318. [Google Scholar] [CrossRef]
- Gao, J.; Luo, Y.; Lu, Y.; Wu, X.; Chen, P.; Zhang, X.; Han, L.; Qiu, M.; Shen, W. Epigenetic regulation of GABAergic differentiation in the developing brain. Front. Cell Neurosci. 2022, 16, 988732. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, W.; Cline, H.T. Electrophysiological Recording for Study of Xenopus Retinotectal Circuitry. Cold Spring Harb. Protoc. 2021, 2021, 6880. [Google Scholar] [CrossRef] [PubMed]


| Antigen, Host Species | Immunogen | Source | Catalog No. | RRID | Dilution |
|---|---|---|---|---|---|
| β-catenin, mouse | C-terminus of human β-catenin | CST (Danvers, MA, USA) | 2677 | AB_1030943 | 1:200 (IF) |
| 1:50 (IP) | |||||
| 1:1000 (WB) | |||||
| β-catenin, rabbit | Residues surrounding Pro714 of human β-catenin protein | CST | 8480 | AB_11127855 | 1:200 (IF) |
| 1:50 (IP) | |||||
| 1:2000 (WB) | |||||
| BLBP, mouse | Amino acids 1–132 of human BLBP | Abcam (Cambridge, UK) | ab131137 | AB_11157091 | 1:100 (IF) |
| BrdU, mouse | BrdU conjugated to KLH | Sigma (St. Louis, MO, USA) | B2531 | AB_476793 | 1:100 (IF) |
| GAPDH, rabbit | C-terminus of human GAPDH | Millipore (Burlington, MA, USA) | ABS16 | AB_11211543 | 1:10,000 (WB) |
| HuC/D, mouse | Recombinant human HuC/HuD | Thermo Fisher (Waltham, MA, USA) | A-21271 | AB_221448 | 1:50 (IF) |
| 1:1000 (WB) | |||||
| Nkx2.2, mouse | Chicken Nkx2.2 | DSHB (Iowa City, IA, USA) | 74.5A5 | AB_531794 | 1:50 (IF) |
| PCNA, rabbit | Recombinant human PCNA | Abcam | ab18197 | AB_444313 | 1:200 (IF) |
| Phospho-β-catenin | Residues surrounding Ser33, Ser37 and Thr41 of human β-catenin | CST | 9561 | AB_331729 | 1:1000 (WB) |
| SOX2, mouse | Residues surrounding Gly179 of human SOX2 protein | CST | 4900 | AB_10560516 | 1:200 (IF) |
| 1:1000 (WB) | |||||
| 1:50 (IP) | |||||
| SOX2, rabbit | Recombinant human SOX2 | Abcam | ab97959 | AB_2341193 | 1:200 (IF) |
| 1:2000 (WB) | |||||
| 1:50 (IP) | |||||
| SOX9, rabbit | Recombinant human SOX9 | Abcam | ab185230 | AB_2715497 | 1:200 (IF) |
| Tubulin, mouse | C-terminal of mouse α-tubulin | Beyotime (Shanghai, China) | AT819 | 1:200 (IF) | |
| Vimentin, rabbit | Recombinant human vimentin | Abcam | ab16700 | AB_443435 | 1:200 (IF) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Lu, Y.; Luo, Y.; Duan, X.; Chen, P.; Zhang, X.; Wu, X.; Qiu, M.; Shen, W. β-Catenin and SOX2 Interaction Regulate Visual Experience-Dependent Cell Homeostasis in the Developing Xenopus Thalamus. Int. J. Mol. Sci. 2023, 24, 13593. https://doi.org/10.3390/ijms241713593
Gao J, Lu Y, Luo Y, Duan X, Chen P, Zhang X, Wu X, Qiu M, Shen W. β-Catenin and SOX2 Interaction Regulate Visual Experience-Dependent Cell Homeostasis in the Developing Xenopus Thalamus. International Journal of Molecular Sciences. 2023; 24(17):13593. https://doi.org/10.3390/ijms241713593
Chicago/Turabian StyleGao, Juanmei, Yufang Lu, Yuhao Luo, Xinyi Duan, Peiyao Chen, Xinyu Zhang, Xiaohua Wu, Mengsheng Qiu, and Wanhua Shen. 2023. "β-Catenin and SOX2 Interaction Regulate Visual Experience-Dependent Cell Homeostasis in the Developing Xenopus Thalamus" International Journal of Molecular Sciences 24, no. 17: 13593. https://doi.org/10.3390/ijms241713593
APA StyleGao, J., Lu, Y., Luo, Y., Duan, X., Chen, P., Zhang, X., Wu, X., Qiu, M., & Shen, W. (2023). β-Catenin and SOX2 Interaction Regulate Visual Experience-Dependent Cell Homeostasis in the Developing Xenopus Thalamus. International Journal of Molecular Sciences, 24(17), 13593. https://doi.org/10.3390/ijms241713593





