A Novel Rat Model of ADHD-like Hyperactivity/Impulsivity after Delayed Reward Has Selective Loss of Dopaminergic Neurons in the Right Ventral Tegmental Area
Abstract
1. Introduction
2. Results
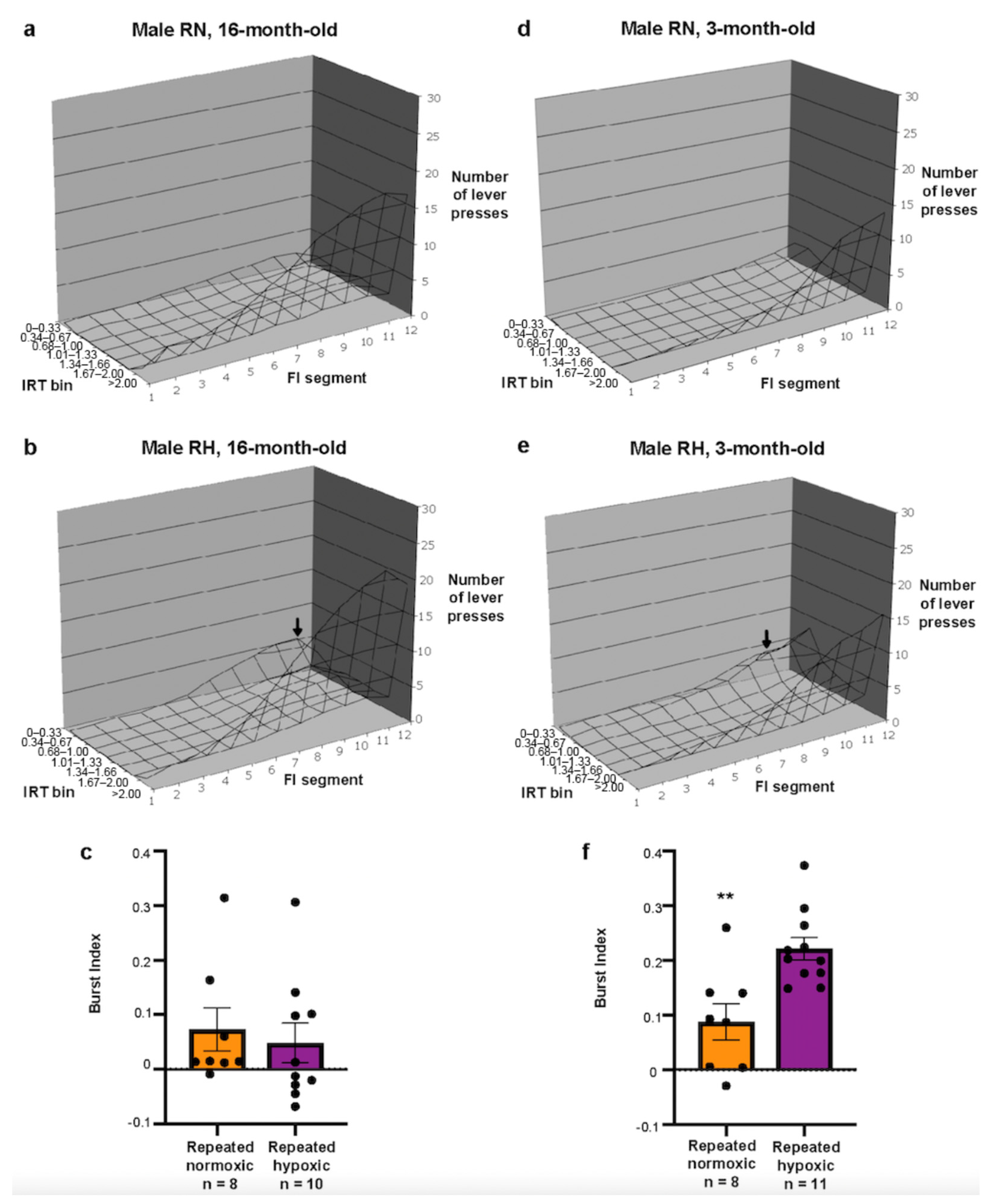
2.1. Repeated Hypoxia Did Not Induce ADHD-like Impulsivity at 16 Months of Age
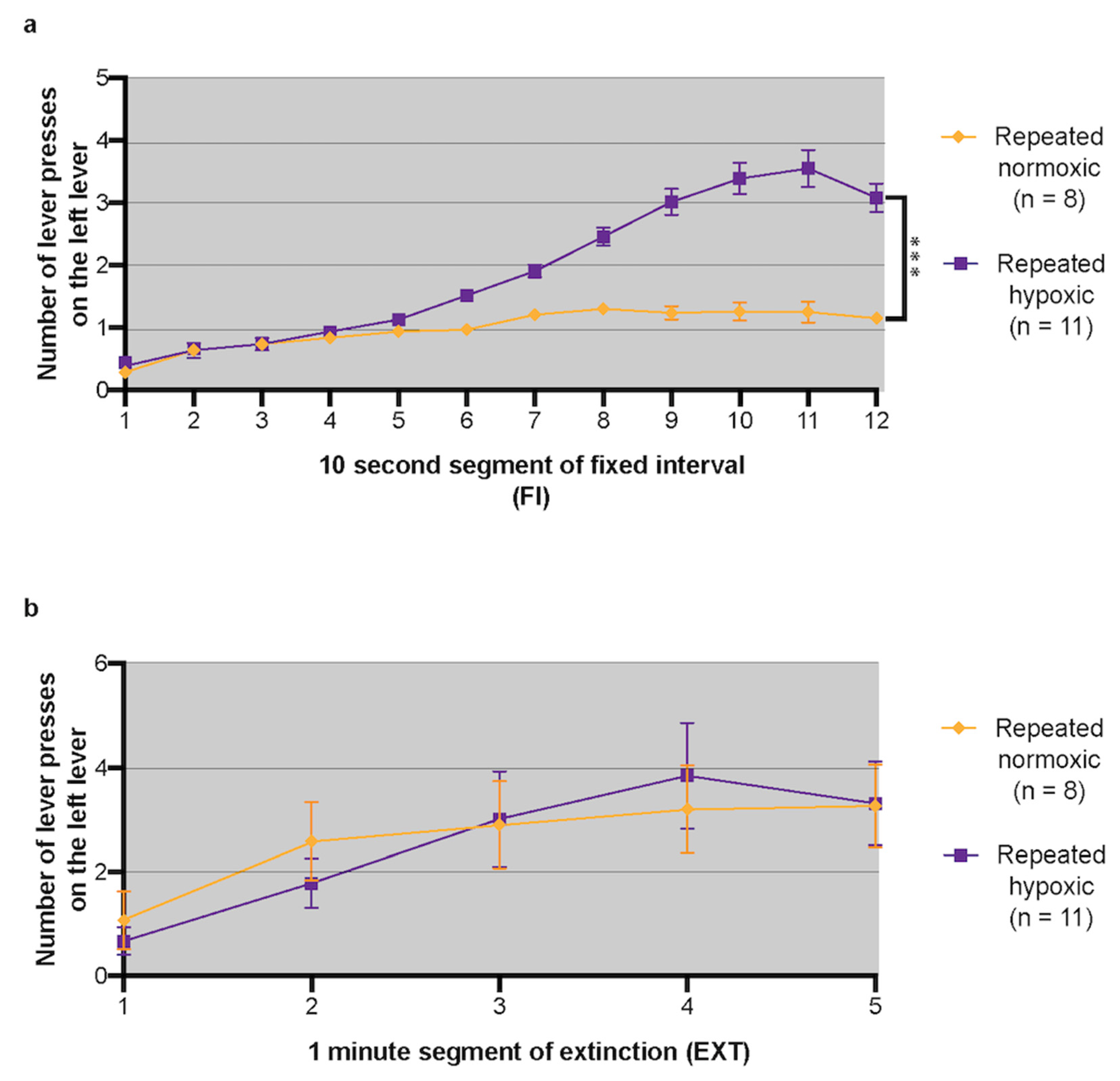
2.2. Repeated Hypoxia Induced ADHD-like Impulsivity at 3 Months of Age
2.3. Repeated Hypoxia Induced ADHD-like Hyperactivity at 3 Months of Age
2.4. Repeated Hypoxia Did Not Induce an ADHD-like Attention Deficit at 3 Months of Age
2.5. Repeated Hypoxia Rats Were Not Hyperactive in the Open Field Test
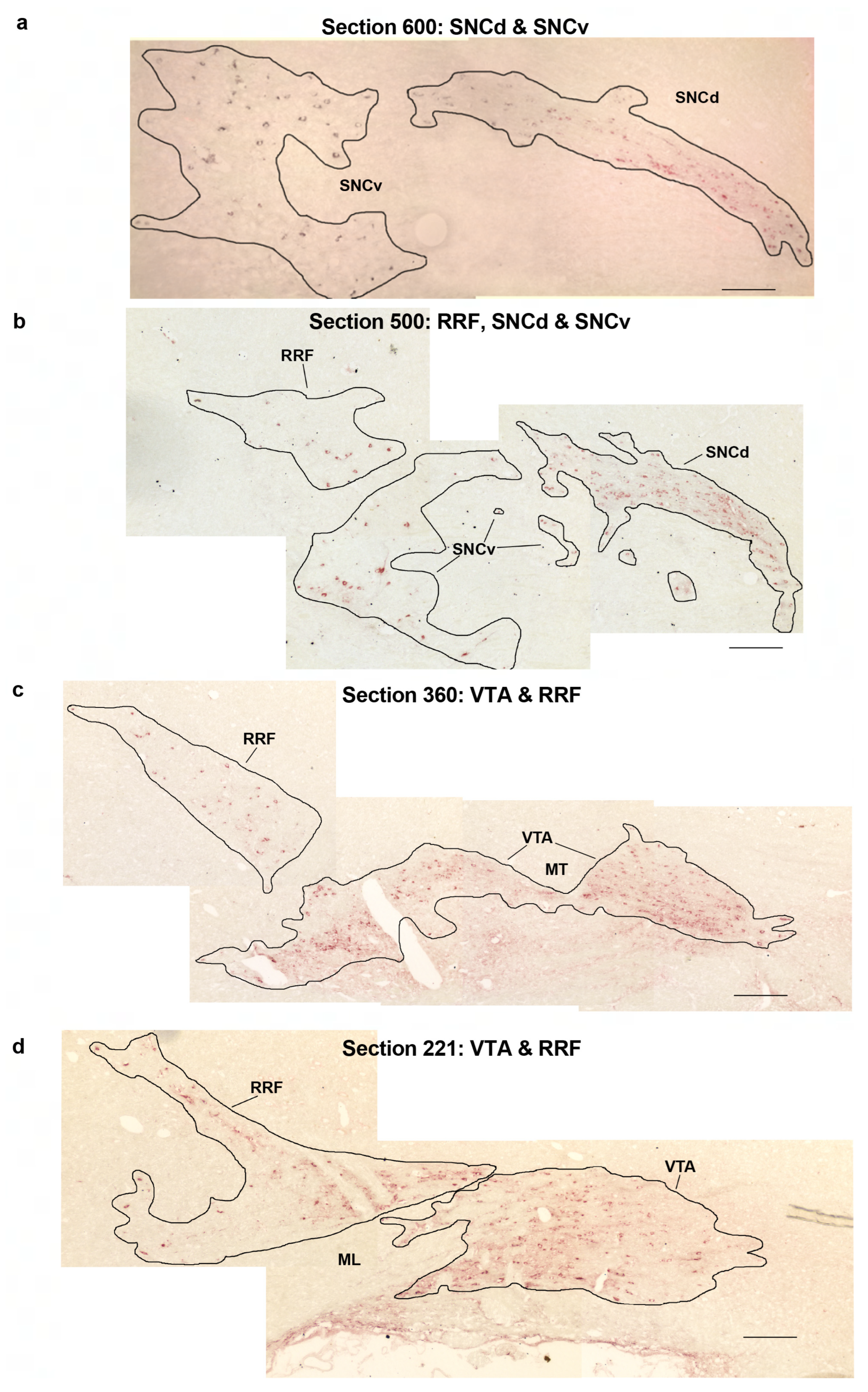
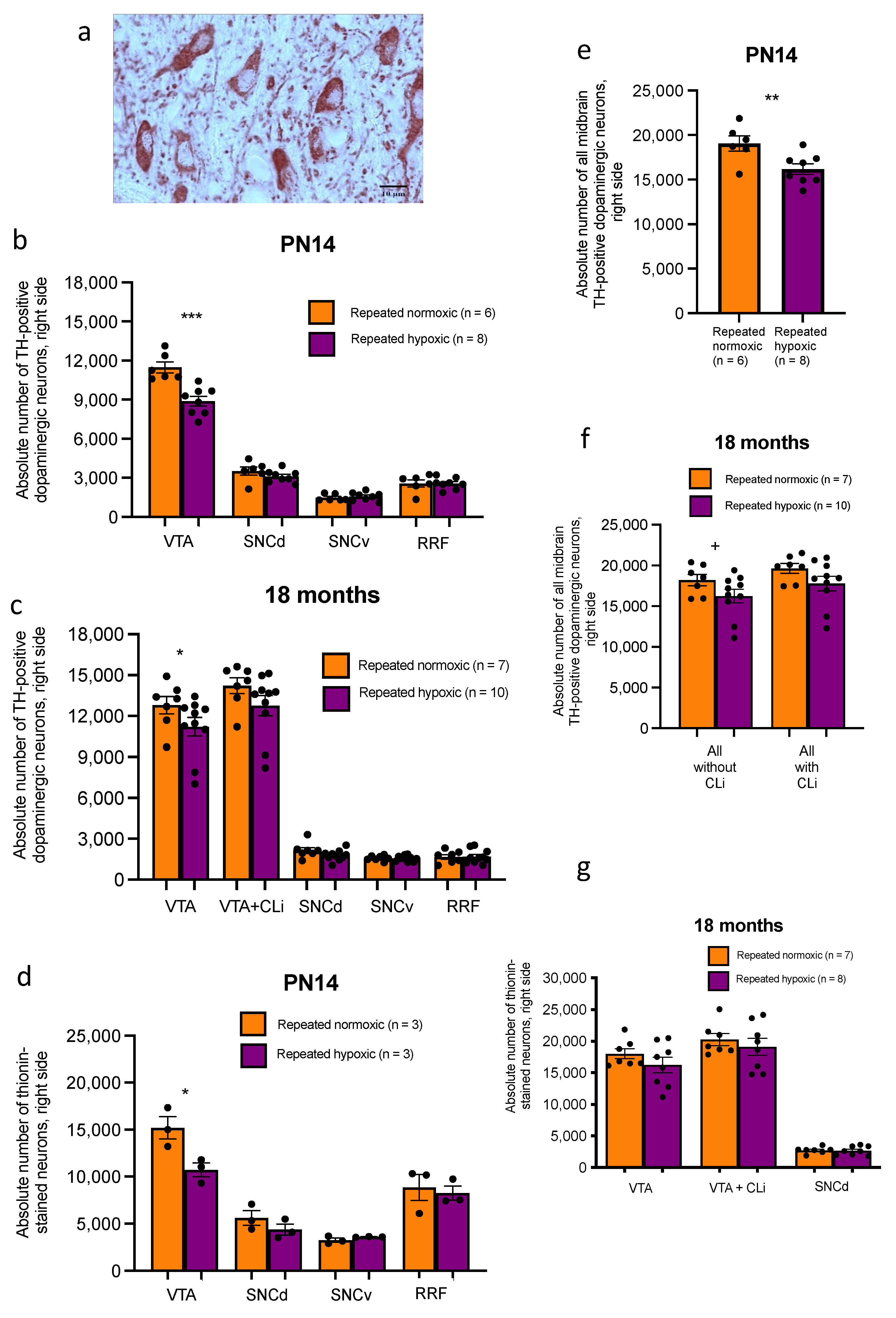
2.6. Repeated Hypoxia Rats at PN14 and at 18 Months of Age Have a Decreased Absolute Number of Dopaminergic Neurons in the Right VTA but Not the Right Substantia Nigra Compacta or Retrorubral Field
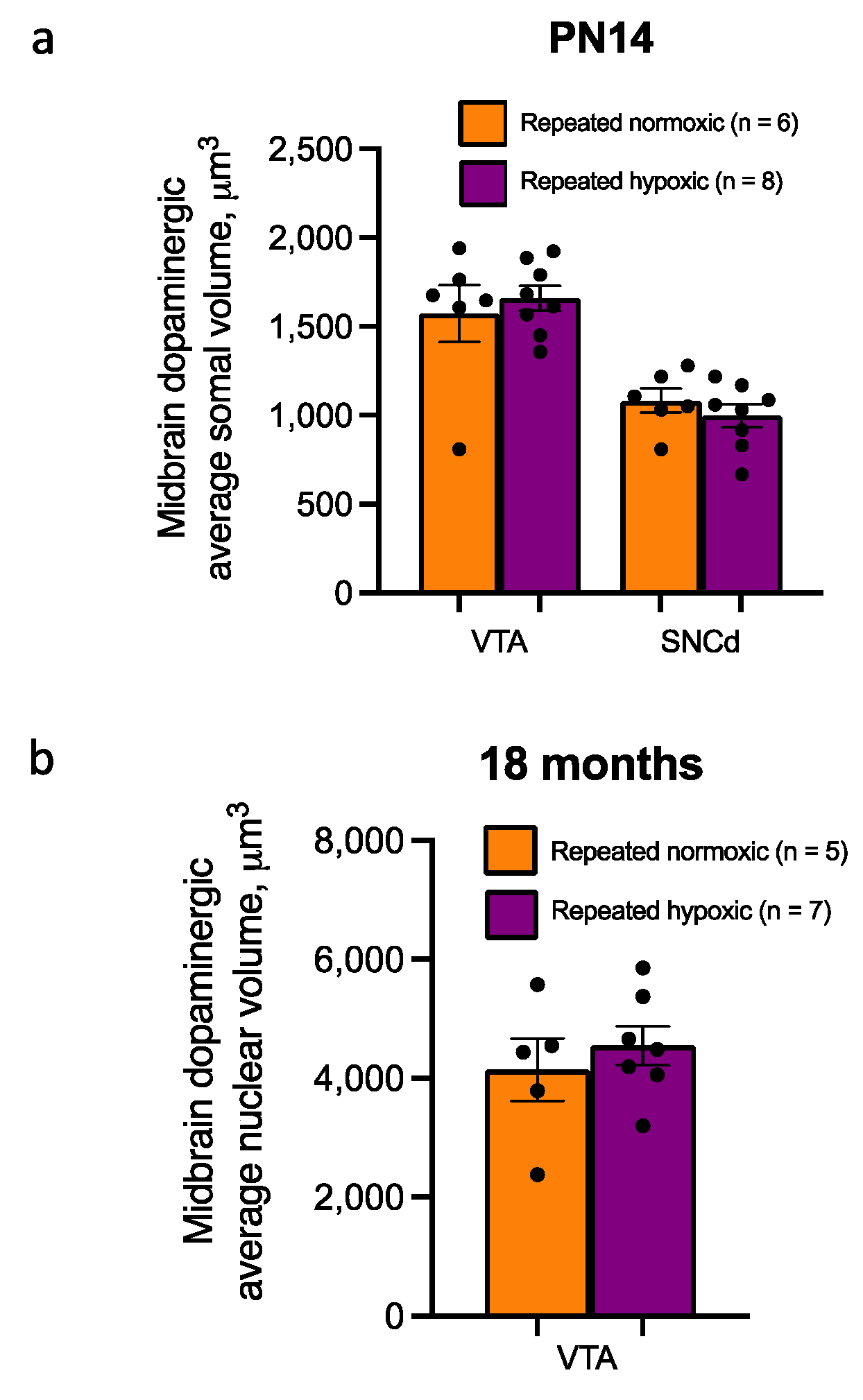
2.7. Repeated Hypoxia Had No Effect on Midbrain Dopaminergic Somal/Nuclear Volume
2.8. Reliable Stereological Data
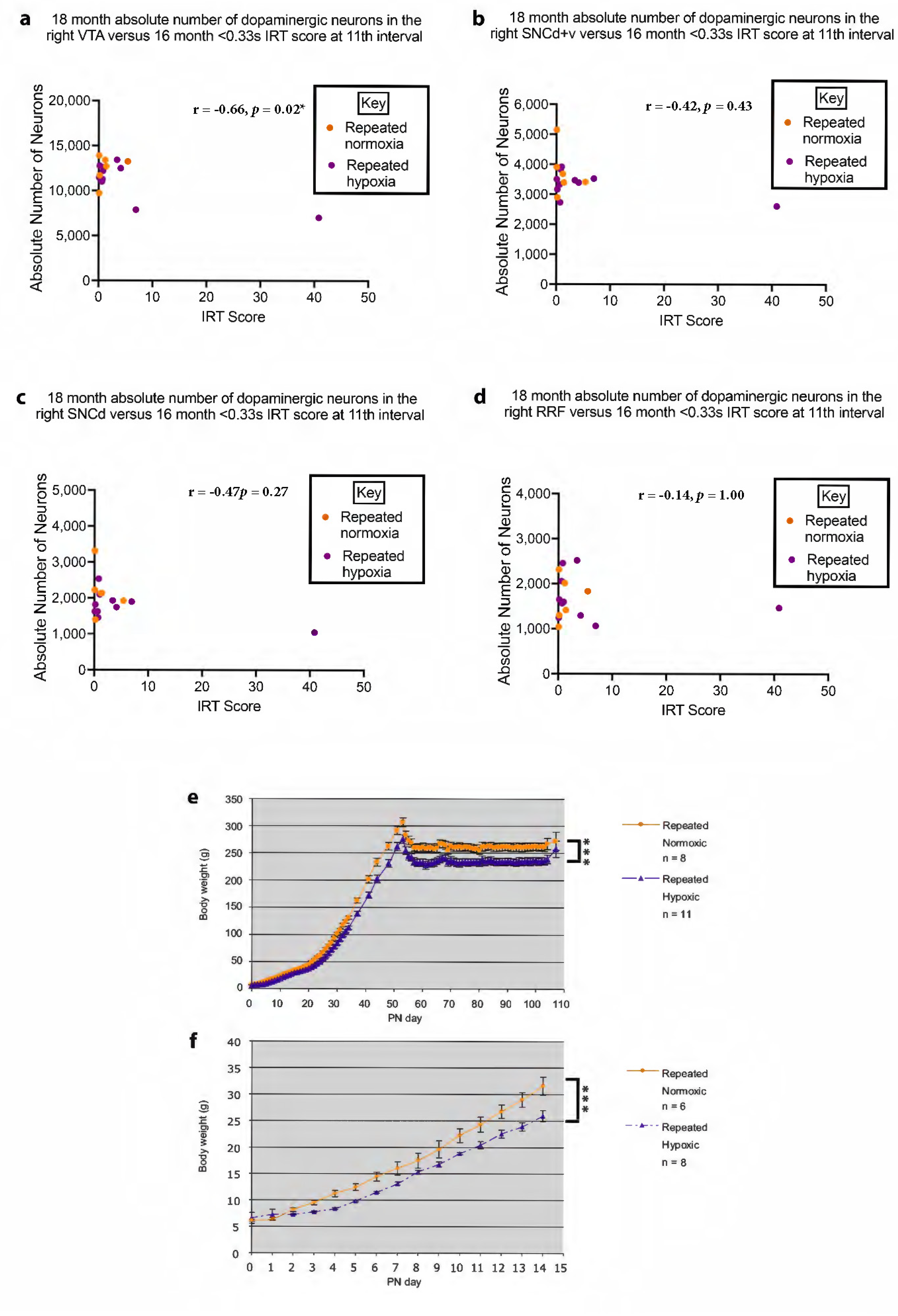
2.9. Changes in Midbrain Anatomy Correlate with Behaviour
2.10. Repeated Hypoxia Rats Have a Significant Reduction in Body Weight
3. Discussion
3.1. Major Findings
3.2. Ethical Considerations
3.3. Limitations and Future Directions
4. Materials and Methods
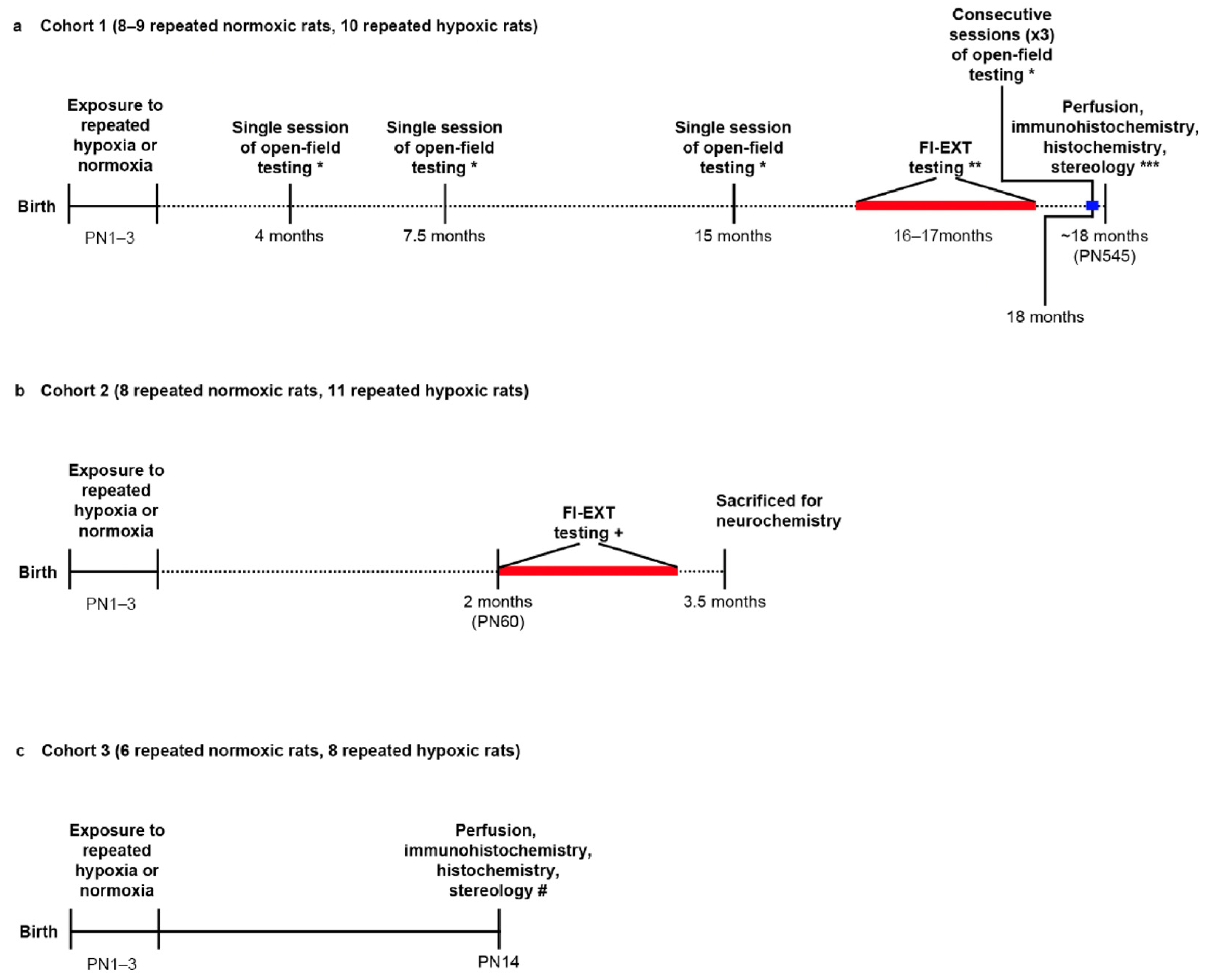
4.1. Repeated Hypoxia and Behaviour: Overview
4.2. Repeated Hypoxia or Repeated Normoxia
4.3. FI-EXT Testing of ADHD-like Impulsivity, Hyperactivity, and Inattention
4.4. Open Field Testing for Hyperactivity
4.5. Stereological Analyses of the Absolute Number and Average Somal or Nuclear Volume of Immunostained Dopaminergic Neurons in the Midbrain
4.6. Histochemical and Stereological Analyses of the Absolute Number of Midbrain Neurons
4.7. Stereological Measurement of the Average Somal Volume of Midbrain Dopaminergic Neurons
4.8. Coefficient of Error of the Stereological Estimates
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Posner, J.; Polanczyk, G.V.; Sonuga-Barke, E. Attention-deficit hyperactivity disorder. Lancet 2020, 395, 450–462. [Google Scholar] [CrossRef]
- Daley, D.; Jacobsen, R.H.; Lange, A.M.; Sørensen, A.; Walldorf, J. The economic burden of adult attention deficit hyperactivity disorder: A sibling comparison cost analysis. Eur. Psychiatry 2019, 61, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Page, T.F.; Altszuler, A.R.; Pelham, W.E., III; Kipp, H.; Gnagy, E.M.; Coxe, S.; Schatz, N.K.; Merrill, B.M.; Macphee, F.L.; et al. Family burden of raising a child with ADHD. J. Abnorm. Child. Psychol. 2019, 47, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.; Tomlinson, A.; Neill, J.C. Low attentive and high impulsive rats: A translational animal model of ADHD and disorders of attention and impulse control. Pharmacol. Ther. 2015, 158, 41–51. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-V, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR, 4th ed.; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Sagvolden, T.; Aase, H.; Zeiner, P.; Berger, D. Altered reinforcement mechanisms in attention-deficit/hyperactivity disorder. Behav. Brain Res. 1998, 94, 61–71. [Google Scholar] [CrossRef]
- Sagvolden, T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD). Neurosci. Biobehav. Rev. 2000, 24, 31–39. [Google Scholar] [CrossRef]
- Blondeau, C.; Dellu-Hagedorn, F. Dimensional Analysis of ADHD Subtypes in Rats. Biol. Psychiatry 2007, 61, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Sagvolden, T.; DasBanerjee, T.; Zhang-James, Y.; Middleton, F.; Faraone, S. Behavioral and genetic evidence for a novel animal model of Attention-Deficit/Hyperactivity Disorder Predominantly Inattentive Subtype. Behav. Brain Funct. 2008, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Holene, E.; Nafstad, I.; Skaare, J.U.; Sagvolden, T. Behavioral hyperactivity in rats following postnatal exposure to sub-toxic doses of polychlorinated biphenyl congeners 153 and 126. Behav. Brain. Res. 1998, 94, 213–224. [Google Scholar] [CrossRef]
- Paz, R.; Barsness, B.; Martenson, T.; Tanner, D.; Allan, A.M. Behavioral Teratogenicity Induced by Nonforced Maternal Nicotine Consumption. Neuropsychopharmacology 2006, 32, 693–699. [Google Scholar] [CrossRef]
- Tomlinson, A.; Grayson, B.; Marsh, S.; Hayward, A.; Marshall, K.M.; Neill, J.C. Putative therapeutic targets for symptom subtypes of adult ADHD: D4 receptor agonism and COMT inhibition improve attention and response inhibition in a novel translational animal model. Eur. Neuropsychopharmacol. 2015, 25, 454–467. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Wetsel, W.C.; Jones, S.R.; Levin, E.D.; Jaber, M.; Caron, M.G. Role of Serotonin in the Paradoxical Calming Effect of Psychostimulants on Hyperactivity. Science 1999, 283, 397–401. [Google Scholar] [CrossRef]
- Herpfer, I.; Hunt, S.P.; Stanford, S.C. A comparison of neurokinin 1 receptor knock-out (NK 1−/−) and wildtype mice: Exploratory behaviour and extracellular noradrenaline concentration in the cerebral cortex of anaesthetised subjects. Neuropharmacol. 2005, 48, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Majdak, P.; Ossyra, J.R.; Ossyra, J.M.; Cobert, A.J.; Hofmann, G.C.; Tse, S.; Panozzo, B.; Grogan, E.L.; Sorokina, A.; Rhodes, J.S. A new mouse model of ADHD for medication development. Sci. Rep. 2016, 6, 39472. [Google Scholar] [CrossRef] [PubMed]
- de la Peña, I.J.I.; Botanas, C.J.; de la Peña, J.B.; Custodio, R.J.; de la Peña, I.; Ryoo, Z.Y.; Kim, B.-N.; Ryu, J.H.; Kim, H.J.; Cheong, J.H. The Atxn7-overexpressing mice showed hyperactivity and impulsivity which were ameliorated by atomoxetine treatment: A possible animal model of the hyperactive-impulsive phenotype of ADHD. Prog. Neuro-Psychopharmacol. 2019, 88, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Regan, S.L.; Williams, M.T.; Vorhees, C.V. Review of rodent models of attention deficit hyperactivity disorder. Neurosci. Biobehav. Rev. 2021, 132, 621–637. [Google Scholar] [CrossRef]
- Kantak, K.M. Rodent models of attention-deficit hyperactivity disorder: An updated framework for model validation and therapeutic drug discovery. Pharmacol. Biochem. Behav. 2022, 216, 173378. [Google Scholar] [CrossRef]
- Oorschot, D.E.; Voss, L.; Covey, M.V.; Bilkey, D.K.; Saunders, S.E. ADHD-like hyperactivity, with no attention deficit, in adult rats after repeated hypoxia during the equivalent of extreme prematurity. J. Neurosci. Methods 2007, 166, 315–322. [Google Scholar] [CrossRef]
- Oorschot, D.E.; Voss, L.; Covey, M.V.; Goddard, L.; Huang, W.; Birchall, P.; Bilkey, D.K.; Kohe, S.E. Spectrum of Short- and Long-Term Brain Pathology and Long-Term Behavioral Deficits in Male Repeated Hypoxic Rats Closely Resembling Human Extreme Prematurity. J. Neurosci. 2013, 33, 11863–11877. [Google Scholar] [CrossRef]
- Wickens, J.R.; Hyland, B.I.; Tripp, G. Animal models to guide clinical drug development in ADHD: Lost in translation? Br. J. Pharmacol. 2011, 164, 1107–1128. [Google Scholar] [CrossRef]
- Sucksdorff, M.; Lehtonen, L.; Chudal, R.; Suominen, A.; Joelsson, P.; Gissler, M.; Sourander, A. Preterm Birth and Poor Fetal Growth as Risk Factors of Attention-Deficit/Hyperactivity Disorder. Pediatrics 2015, 136, e599–e608. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.P.; Bolat, G.U.; Bolat, H.; Matijasevich, A.; Santos, I.S.; Silveira, R.C.; Procianoy, R.S.; Rohde, L.A.; Moreira-Maia, C.R. Attention-Deficit/Hyperactivity Disorder and Very Preterm/Very Low Birth Weight: A Meta-analysis. Pediatrics 2017, 141, e20171645. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.; Girchenko, P.; Pulakka, A.; Heinonen, K.; Lähdepuro, A.; Lahti-Pulkkinen, M.; Hovi, P.; Tikanmäki, M.; Bartmann, P.; Lano, A.; et al. ADHD symptoms and diagnosis in adult preterms: Systematic review, IPD meta-analysis, and register-linkage study. Pediatr. Res. 2022, 93, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Vederhus, B.J.; Markestad, T.; Eide, G.E.; Graue, M.; Halvorsen, T. Health related quality of life after extremely preterm birth: A matched controlled cohort study. Health Qual. Life Outcomes 2010, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Scheres, A.; Milham, M.P.; Knutson, B.; Castellanos, F.X. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol. Psychiatry 2007, 61, 720–724. [Google Scholar] [CrossRef]
- Ströhle, A.; Stoy, M.; Wrase, J.; Schwarzer, S.; Schlagenhauf, F.; Huss, M.; Hein, J.; Nedderhut, A.; Neumann, B.; Gregor, A.; et al. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage 2008, 39, 966–972. [Google Scholar] [CrossRef]
- Heilman, K.M.; Voeller, K.K.; Nadeau, S.E. A Possible Pathophysiologic Substrate of Attention Deficit Hyperactivity Disorder. J. Child. Neurol. 1991, 6, S76–S81. [Google Scholar] [CrossRef]
- Carmona, S.; Proal, E.; Hoekzema, E.A.; Gispert, J.-D.; Picado, M.; Moreno, I.; Soliva, J.C.; Bielsa, A.; Rovira, M.; Hilferty, J.; et al. Ventro-Striatal Reductions Underpin Symptoms of Hyperactivity and Impulsivity in Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2009, 66, 972–977. [Google Scholar] [CrossRef]
- Andén, N.-E.; Dahlström, A.; Fuxe, K.; Larsson, K.; Olson, L.; Ungerstedt, U. Ascending monoamine neurons to the telencephalon and diencephalon. Acta Physiol. Scand. 1966, 67, 313–326. [Google Scholar] [CrossRef]
- Björklund, A.; Dunnett, S.B. Dopamine neuron systems in the brain: An update. Trends Neurosci. 2007, 30, 194–202. [Google Scholar] [CrossRef]
- Tanaka, M.; Spekker, E.; Szabo, A.; Polyak, H.; Vecsei, L. Modelling the neurodevelopmental pathogenesis in neuro-psychiatric disorders. Bioactive kynurenines and their analogues as neuroprotective agents—In celebration of 80th birthday of Professor Peter Riederer. Psychiatry Preclin. Pyschiatric Stud. 2022, 129, 627–642. [Google Scholar]
- Biederman, J.; Spencer, T.; Wilens, T. Evidence-based pharmacotherapy for attention-deficit hyperactivity disorder. Int. J. Neuropsychopharmacol. 1999, 7, 77–97. [Google Scholar] [CrossRef]
- Arnsten, A.F.; Pliszka, S.R. Catecholamine influences on prefrontal cortical function: Relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol. Biochem. Behav. 2011, 99, 211–216. [Google Scholar] [CrossRef]
- Cortese, S. The neurobiology and genetics of Attention-Deficit/Hyperactivity Disorder (ADHD): What every clinician should know. Eur. J. Paediatr. Neurol. 2012, 16, 422–433. [Google Scholar] [CrossRef]
- Mehta, T.R.; Monegro, A.; Nene, Y.; Fayyaz, M.; Bollu, P.C. Neurobiology of ADHD: A review. Curr. Dev. Disord. Rep. 2019, 6, 235–240. [Google Scholar] [CrossRef]
- Lewis, B.L.; O’Donnell, P. Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential ’up’ states in pyramidal neurons via D(1) dopamine receptors. Cereb. Cortex 2000, 10, 1168–1175. [Google Scholar] [CrossRef]
- Klanker, M.; Feenstra, M.; Denys, D. Dopaminergic control of cognitive flexibility in humans and animals. Front. Neurosci. 2013, 7, 201. [Google Scholar] [CrossRef]
- Leisman, G.; Melillo, R. Front and center: Maturational dysregulation of frontal lobe functional neuroanatomic connections in attention deficit hyperactivity disorder. Front. Neuroanat. 2022, 16, 936025. [Google Scholar] [CrossRef]
- Sagvolden, T.; Pettersen, M.B.; Larsen, M.C. Spontaneously hypertensive rats (SHR) as a putative animal model of childhood hyperkinesis: SHR behavior compared to four other rat strains. Physiol. Behav. 1993, 54, 1047–1055. [Google Scholar] [CrossRef]
- Sagvolden, T.; Sergeant, J.A. Attention deficit/hyperactivity disorder-from brain dysfunctions to behavior. Behav Brain Res 1998, 94, 1–10. [Google Scholar]
- Wickens, J.; Macfarlane, J.; Booker, C.; McNaughton, N. Dissociation of hypertension and fixed interval responding in two separate strains of genetically hypertensive rat. Behav. Brain Res. 2004, 152, 393–401. [Google Scholar] [CrossRef]
- Gundersen, H.J.G. Stereology of arbitrary particles: A review of unbiased number and size estimators and the presentation of some new ones, in memory of William, R. Thompson. J. Microsc. 1986, 143, 3–45. [Google Scholar] [CrossRef] [PubMed]
- Swaab, D.F.; Uylings, H.B.M. Density measures: Parameters to avoid. Neurobiol. Aging 1987, 8, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Oorschot, D. Are you using neuronal densities, synaptic densities or neurochemical densities as your definitive data? there is a better way to go. Prog. Neurobiol. 1994, 44, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Galvin, K.A.; Oorschot, D.E. Continuous low-dose treatment with brain-derived neurotrophic factor or neurotrophin-3 protects striatal medium spiny neurons from mild neonatal hypoxia/ischemia: A stereological study. Neuroscience 2003, 118, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Oorschot, D.E. Indirect measures of neuroprotection are parameters to avoid: Examples from research on neonatal rat hypoxia-ischemia. Image Anal. Stereol. 2011, 30, 1–9. [Google Scholar] [CrossRef]
- Napper, R.M.A. Total Number Is Important: Using the Disector Method in Design-Based Stereology to Understand the Structure of the Rodent Brain. Front. Neuroanat. 2018, 12, 16. [Google Scholar] [CrossRef]
- Braendgaard, H.; Evans, S.M.; Howard, C.V.; Gundersen, H.J.G. The total number of neurons in the human neocortex unbiasedly estimated using optical disectors. J. Microsc. 1990, 157, 285–304. [Google Scholar] [CrossRef]
- West, M.J.; Gundersen, H.J.G. Unbiased stereological estimation of the number of neurons in the human hippocampus. J. Comp. Neurol. 1990, 296, 1–22. [Google Scholar] [CrossRef]
- Oorschot, D.E. The total number of neurons in the neostriatal, pallidal, subthalamic and substantia nigral nuclei of the rat basal ganglia: A stereological study using the Cavalieri and optical disector methods. J. Comp. Neurol. 1996, 366, 580–599. [Google Scholar] [CrossRef]
- Gundersen, H.J.G.; Jensen, E.B.V.; Kieu, K.; Nielsen, J. The efficiency of systematic sampling in stereology–reconsidered. J. Microsc. 1999, 193, 199–211. [Google Scholar] [CrossRef]
- Slomianka, L.; West, M.J. Estimators of the precision of stereological estimates: An example based on the CA1 pyramidal cell layer of rats. Neuroscience 2005, 136, 757–767. [Google Scholar] [CrossRef]
- Korbo, L.; Andersen, B.B.; Ladefoged, O.; Moller, A. Total numbers of various cell types in rat cerebellar cortex estimated using an unbiased stereological method. Brain Res. 1993, 609, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, H.J.G.; Bagger, P.; Bendtsen, T.F.; Evans, S.M.; Korbo, L.; Marcussen, N.; Møller, A.; Nielsen, K.; Nyengaard, J.R.; Pakkenberg, B.; et al. The new stereological tools: Disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. Apmis 1988, 96, 857–881. [Google Scholar] [CrossRef]
- Faraone, S.V.; Banaschewski, T.; Coghill, D.; Zheng, Y.; Biederman, J.; Bellgrove, M.A.; Wang, Y. The World Federation of ADHD International Consensus Statement: 208 evidence-based conclusions about the disorder. Neurosci. Biobehav. Rev. 2021, 128, 789–818. [Google Scholar] [CrossRef] [PubMed]
- Barkley, R.A.; Ullman, D.G. A comparison of objective measures of activity and distractibility in hyperactive and non-hyperative children. J. Abnorm. Child. Psychol. 1976, 3, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, S.R.; Milar, C.; Wool, R.; Routh, D.K. Multiple measurement, transsituational diagnosis, and the concept of generaised overactivity. J. Pediatr. Psychol. 1980, 5, 365–375. [Google Scholar] [CrossRef]
- Bayless, D.W.; Perez, M.C.; Daniel, J.M. Comparison of the validity of the use of the spontaneously hypertensive rat as a model of attention deficit hyperactivity disorder in males and females. Behav. Brain Res. 2015, 286, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Brake, W.G.; Sullivan, R.M.; Gratton, A. Perinatal Distress Leads to Lateralized Medial Prefrontal Cortical Dopamine Hypofunction in Adult Rats. J. Neurosci. 2000, 20, 5538–5543. [Google Scholar] [CrossRef]
- Kaur, C.; Sivakumar, V.; Ling, E.A. Melatonin protects periventricular white matter from damage due to acute hypoxia. J. Pineal Res. 2010, 48, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Anantharam, P.; Hoffmann, A.; Meade, M.L.; Grobe, N.; Gearhart, J.M.; Whitley, E.M.; Mahama, B.; Rumbeiha, W.K. Broad spectrum proteomics analysis of the inferior colliculus following acute hydrogen sulphide exposure. Toxicol. Appl. Pharmacol. 2018, 355, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Paxinos, G.; Watson, C.; Halliday, G.M. The substantia nigra and ventral tegmental dopaminergic neurons from development to degeneration. J. Chem. Neuroanat. 2016, 76, 98–107. [Google Scholar] [CrossRef]
- Hack, M.B.; Schluchter, M.; Cartar, L.; Rahman, M.; Cuttler, L.; Borawski, E. Growth of Very Low Birth Weight Infants to Age 20 Years. Pediatrics 2003, 112, e30–e38. [Google Scholar] [CrossRef]
- Hall, C.S. Drive and emotionality: Factors associated with adjustment in the rat. Comp. Psych. 1934, 27, 89–108. [Google Scholar] [CrossRef]
- Pare, W.P. Relationship of various behaviours in the open-field test of emotionality. Psych. Rep. 1964, 14, 19–22. [Google Scholar] [CrossRef]
- Bronstein, P.M. Open-field behavior of the rat as a function of age: Cross-sectional and longitudinal investigations. J. Comp. Physiol. Psychol. 1972, 80, 335–341. [Google Scholar] [CrossRef]
- Russell, P.; Williams, D. Effects of repeated testing on rats’ locomotor activity in the open-field. Anim. Behav. 1973, 21, 109–111. [Google Scholar] [CrossRef]
- Ferguson, S.A.; Cada, A.M. A longitudinal study of short- and long-term activity levels in male and female spontaneously hypertensive, Wistar-Kyoto, and Sprague-Dawley rats. Behav. Neurosci. 2003, 117, 271–282. [Google Scholar] [CrossRef]
- Fowler, S.C.; Muma, N.A. Use of a force-sensing automated open field apparatus in a longitudinal study of multiple behavioral deficits in CAG140 Huntington’s disease model mice. Behav Brain Res 2015, 294, 7–16. [Google Scholar] [CrossRef]
- Scott, M.N.; Taylor, H.G.; Fristad, M.A.; Klein, N.; Espy, K.A.; Minich, N.B.; Hack, M.M. Behavior Disorders in Extremely Preterm/Extremely Low Birth Weight Children in Kindergarten. J. Dev. Behav. Pediatr. 2012, 33, 202–213. [Google Scholar] [CrossRef]
- Johnson, S.P.; Kochhar, P.M.; Hennessy, E.M.; Marlow, N.D.; Wolke, D.P.; Hollis, C.P. Antecedents of Attention-Deficit/Hyperactivity Disorder Symptoms in Children Born Extremely Preterm. J. Dev. Behav. Pediatr. 2016, 37, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Snelgrove, J.W.; Murphy, K.E. Preterm birth and social inequality: Assessing the effects of material and psychosocial disadvantage in a UK birth cohort. Acta Obstet. Gynecol. Scand. 2015, 94, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Hack, M.M.; Taylor, H.G.; Schluchter, M.; Andreias, L.; Drotar, D.; Klein, N. Behavioral Outcomes of Extremely Low Birth Weight Children at Age 8 Years. J. Dev. Behav. Pediatr. 2009, 30, 122–130. [Google Scholar] [CrossRef]
- Lindström, K.; Lindblad, F.; Hjern, A. Preterm Birth and Attention-Deficit/Hyperactivity Disorder in Schoolchildren. Pediatrics 2011, 127, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Tucha, L.; Tucha, O.; Laufkötter, R.; Walitza, S.; Klein, H.E.; Lange, K.W. Neuropsychological assessment of attention in adults with different subtypes of attention-deficit/hyperactivity disorder. J. Neural Transm. 2008, 115, 269–278. [Google Scholar] [CrossRef]
- van der Kooij, M.A.; Glennon, J.C. Animal models concerning the role of dopamine in attention-deficit hyperactivity disorder. Neurosci. Biobehav. Rev. 2007, 31, 597–618. [Google Scholar] [CrossRef]
- Sagvolden, T.; Hendley, E.D.; Knardahl, S. Behavior of hypertensive and hyperactive rat strains: Hyperactivity is not unitarily determined. Physiol. Behav. 1992, 52, 49–57. [Google Scholar] [CrossRef]
- Sagvolden, T.; Metzger, M.A.; Schiorbeck, H.K.; Rugland, A.L.; Spinnangr, I.; Sagvolden, G. The spontaneously hypertensive rat (SHR) as an animal model of childhood hyperactivity (ADHD): Changed reactivity to reinforcers and to pyschomotor stimulants. Behav. Neural Biol. 1992, 58, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Russell, V.A.; Sagvolden, T.; Johansen, E.B. Animal models of attention-deficit hyperactivity disorder. Behav. Brain Funct. 2005, 1, 9. [Google Scholar] [CrossRef]
- Damasceno, F.; Skinner, G.O.; Cordeiro, J.F.; Ferraz, M.R.; Almeida, O.M. Sleep deprivation affects sexual behavior and tyrosine hydroxylase (TH) levels in sexually experienced male rats. Physiol. Behav. 2008, 94, 405–411. [Google Scholar] [CrossRef]
- Sterio, D.C. The unbiased estimation of number and sizes of arbitrary particles using the disector. J. Microsc. 1984, 134, 127–136. [Google Scholar] [CrossRef]
- Basham, H.K.; Aghoghovwia, B.E.; Papaioannou, P.; Seo, S.; Oorschot, D.E. Delayed Double Treatment with Adult-Sourced Adipose-Derived Mesenchymal Stem Cells Increases Striatal Medium-Spiny Neuronal Number, Decreases Striatal Microglial Number, and Has No Subventricular Proliferative Effect, after Acute Neonatal Hypoxia-Ischemia in Male Rats. Int. J. Mol. Sci. 2021, 22, 7862. [Google Scholar] [CrossRef] [PubMed]
- German, D.C.; Manaye, Y.E. Midbrain dopaminergic neurons (nuclei A8, A9 and A10): Three-dimensional recon-struction in the rat. J. Comp. Neurol. 1993, 331, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: London, UK, 2007; ISBN 9780123919496. [Google Scholar]
- Gundersen, H.J.G.; Jensen, E.B. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 1987, 147, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, T.M. A review of recent advances in stereology for quantifying neural structure. J. Neurocytol. 1992, 21, 313–328. [Google Scholar] [CrossRef]
- Larsen, M.; Bjarkam, C.R.; Ostergaard, K.; West, M.J.; Sørensen, J.C. The anatomy of the porcine subthalamic nucleus evaluated with immunohistochemistry and design-based stereology. Anat. Embryol. 2004, 208, 239–247. [Google Scholar] [CrossRef]
- Dorph-Petersen, K.-A.; Pierri, J.N.; Sun, Z.; Sampson, A.R.; Lewis, D.A. Stereological analysis of the mediodorsal thalamic nucleus in schizophrenia: Volume, neuron number, and cell types. J. Comp. Neurol. 2004, 472, 449–462. [Google Scholar] [CrossRef]


| Measured Parameter | Number of Rats | Vref (mm3) of VTA | Nv (per mm3) of Dopaminergic Neurons in the VTA | N, Dopaminergic Neurons in the VTA |
|---|---|---|---|---|
| Experimental Condition | ||||
| VTA dopaminergic | ||||
| neurons, PN14 | ||||
| Repeated normoxia | 6 | |||
| Mean | 0.682 | 17,034 | 11,479 | |
| SD | 0.114 | 1689 | 1042 | |
| SEM | 0.051 | 755 | 466 | |
| Repeated hypoxia | 8 | |||
| Mean | 0.585 * | 15,269 ** | 8894 *** | |
| SD | 0.058 | 1855 | 1060 | |
| SEM | 0.022 | 701 | 400 | |
| VTA dopaminergic | ||||
| neurons, PN545 | ||||
| Repeated normoxia | 7 | |||
| Mean | 0.989 | 13,277 | 12,798 | |
| SD | 0.145 | 2970 | 1682 | |
| SEM | 0.059 | 1213 | 687 | |
| Repeated hypoxia | 10 | |||
| Mean | 1.007 † | 11,163 †† | 11,213 ††† | |
| SD | 0.148 | 1830 | 2125 | |
| SEM | 0.049 | 610 | 708 |
| Measured Parameter | CE Vref * | CE Nv ** | CE N *** |
|---|---|---|---|
| Experimental Condition † | |||
| Absolute number of VTA dopaminergic | |||
| neurons, PN14 | |||
| Repeated normoxia | 0.013 | 0.037 | 0.039 |
| Repeated hypoxia | 0.014 | 0.044 | 0.045 |
| Absolute number of SNCd dopaminergic | |||
| neurons, PN14 | |||
| Repeated normoxia | 0.018 | 0.069 | 0.071 |
| Repeated hypoxia | 0.018 | 0.071 | 0.074 |
| Absolute number of SNCv dopaminergic | |||
| neurons, PN14 | |||
| Repeated normoxia | 0.021 | 0.101 | 0.102 |
| Repeated hypoxia | 0.021 | 0.1 | 0.101 |
| Absolute number of RRF dopaminergic | |||
| neurons, PN14 | |||
| Repeated normoxia | 0.014 | 0.078 | 0.079 |
| Repeated hypoxia | 0.014 | 0.08 | 0.081 |
| Absolute number of VTA dopaminergic | |||
| neurons, PN545 | |||
| Repeated normoxia | 0.017 | 0.06 | 0.061 |
| Repeated hypoxia | 0.018 | 0.062 | 0.063 |
| Absolute number of VTA + CLi dopaminergic | |||
| neurons, PN545 | |||
| Repeated normoxia | 0.017 | 0.054 | 0.054 |
| Repeated hypoxia | 0.017 | 0.056 | 0.058 |
| Absolute number of SNCd dopaminergic | |||
| neurons, PN545 | |||
| Repeated normoxia | 0.052 | 0.087 | 0.09 |
| Repeated hypoxia | 0.05 | 0.081 | 0.082 |
| Absolute number of SNCv dopaminergic | |||
| neurons, PN545 | |||
| Repeated normoxia | 0.034 | 0.097 | 0.099 |
| Repeated hypoxia | 0.027 | 0.094 | 0.096 |
| Absolute number of RRF dopaminergic | |||
| neurons, PN545 | |||
| Repeated normoxia | 0.031 | 0.117 | 0.117 |
| Repeated hypoxia | 0.029 | 0.122 | 0.127 |
| Absolute number of VTA thionin- | |||
| stained neurons, PN14 | |||
| Repeated normoxia | 0.012 | 0.035 | 0.04 |
| Repeated hypoxia | 0.014 | 0.041 | 0.043 |
| Absolute number of SNCd thionin- | |||
| stained neurons, PN14 | |||
| Repeated normoxia | 0.018 | 0.056 | 0.059 |
| Repeated hypoxia | 0.017 | 0.06 | 0.063 |
| Absolute number of SNCv thionin- | |||
| stained neurons, PN14 | |||
| Repeated normoxia | 0.019 | 0.075 | 0.077 |
| Repeated hypoxia | 0.018 | 0.064 | 0.065 |
| Absolute number of RRF thionin- | |||
| stained neurons, PN14 | |||
| Repeated normoxia | 0.014 | 0.048 | 0.049 |
| Repeated hypoxia | 0.013 | 0.049 | 0.051 |
| Absolute number of VTA thionin- | |||
| stained neurons, PN545 | |||
| Repeated normoxia | 0.022 | 0.049 | 0.051 |
| Repeated hypoxia | 0.023 | 0.051 | 0.052 |
| Absolute number of VTA + CLi thionin- | |||
| stained neurons, PN545 | |||
| Repeated normoxia | 0.018 | 0.044 | 0.045 |
| Repeated hypoxia | 0.018 | 0.045 | 0.046 |
| Absolute number of SNCd thionin- | |||
| stained neurons, PN545 | |||
| Repeated normoxia | 0.059 | 0.072 | 0.076 |
| Repeated hypoxia | 0.056 | 0.065 | 0.067 |
| Measured Parameter | (Mean CE)2 | (CV)2 * | (Mean CE)2 < 1/2 (CV)2? |
|---|---|---|---|
| Experimental Condition Absolute number of VTA dopaminergic neurons, PN14 | |||
| Repeated normoxia, Vref | (0.013)2 | (0.168)2 | Yes |
| Repeated hypoxia, Vref | (0.014)2 | (0.098)2 | Yes |
| Repeated normoxia, Nv | (0.037)2 | (0.099)2 | Yes |
| Repeated hypoxia, Nv | (0.044)2 | (0.121)2 | Yes |
| Repeated normoxia, N | (0.039)2 | (0.091)2 | Yes |
| Repeated hypoxia, N | (0.045)2 | (0.119)2 | Yes |
| Absolute number of SNCd dopaminergic neurons, PN14 | |||
| Repeated normoxia, Vref | (0.018)2 | (0.139)2 | Yes |
| Repeated hypoxia, Vref | (0.018)2 | (0.106)2 | Yes |
| Repeated normoxia, Nv | (0.069)2 | (0.129)2 | Yes |
| Repeated hypoxia, Nv | (0.071)2 | (0.143)2 | Yes |
| Repeated normoxia, N | (0.071)2 | (0.220)2 | Yes |
| Repeated hypoxia, N | (0.074)2 | (0.148)2 | Yes |
| Absolute number of SNCv dopaminergic neurons, PN14 | |||
| Repeated normoxia, Vref | (0.021)2 | (0.092)2 | Yes |
| Repeated hypoxia, Vref | (0.021)2 | (0.153)2 | Yes |
| Repeated normoxia, Nv | (0.101)2 | (0.199)2 | Yes |
| Repeated hypoxia, Nv | (0.100)2 | (0.191)2 | Yes |
| Repeated normoxia, N | (0.102)2 | (0.169)2 | Yes |
| Repeated hypoxia, N | (0.101)2 | (0.204)2 | Yes |
| Absolute number of RRF dopaminergic neurons, PN14 | |||
| Repeated normoxia, Vref | (0.014)2 | (0.181)2 | Yes |
| Repeated hypoxia, Vref | (0.014)2 | (0.123)2 | Yes |
| Repeated normoxia, Nv | (0.078)2 | (0.151)2 | Yes |
| Repeated hypoxia, Nv | (0.080)2 | (0.144)2 | Yes |
| Repeated normoxia, N | (0.079)2 | (0.263)2 | Yes |
| Repeated hypoxia, N | (0.081)2 | (0.172)2 | Yes |
| Absolute number of VTA dopaminergic neurons, PN545 | |||
| Repeated normoxia, Vref | (0.017)2 | (0.146)2 | Yes |
| Repeated hypoxia, Vref | (0.018)2 | (0.147)2 | Yes |
| Repeated normoxia, Nv | (0.060)2 | (0.225)2 | Yes |
| Repeated hypoxia, Nv | (0.062)2 | (0.164)2 | Yes |
| Repeated normoxia, N | (0.061)2 | (0.131)2 | Yes |
| Repeated hypoxia, N | (0.063)2 | (0.190)2 | Yes |
| Absolute number of VTA + CLi dopaminergic neurons, PN545 | |||
| Repeated normoxia, Vref | (0.017)2 | (0.208)2 | Yes |
| Repeated hypoxia, Vref | (0.017)2 | (0.154)2 | Yes |
| Repeated normoxia, Nv | (0.054)2 | (0.201)2 | Yes |
| Repeated hypoxia, Nv | (0.056)2 | (0.174)2 | Yes |
| Repeated normoxia, N | (0.054)2 | (0.108)2 | Yes |
| Repeated hypoxia, N | (0.058)2 | (0.182)2 | Yes |
| Absolute number of SNCd dopaminergic neurons, PN545 | |||
| Repeated normoxia, Vref | (0.052)2 | (0.185)2 | Yes |
| Repeated hypoxia, Vref | (0.050)2 | (0.158)2 | Yes |
| Repeated normoxia, Nv | (0.087)2 | (0.139)2 | Yes |
| Repeated hypoxia, Nv | (0.081)2 | (0.234)2 | Yes |
| Repeated normoxia, N | (0.090)2 | (0.269)2 | Yes |
| Repeated hypoxia, N | (0.082)2 | (0.221)2 | Yes |
| Absolute number of SNCv dopaminergic neurons, PN545 | |||
| Repeated normoxia, Vref | (0.034)2 | (0.153)2 | Yes |
| Repeated hypoxia, Vref | (0.027)2 | (0.111)2 | Yes |
| Repeated normoxia, Nv | (0.097)2 | (0.222)2 | Yes |
| Repeated hypoxia, Nv | (0.094)2 | (0.197)2 | Yes |
| Repeated normoxia, N | (0.099)2 | (0.123)2 | No, 12.3% CV is low |
| Repeated hypoxia, N | (0.096)2 | (0.134)2 | No, 13.4% CV is low |
| Absolute number of RRF dopaminergic neurons, PN545 | |||
| Repeated normoxia, Vref | (0.031)2 | (0.205)2 | Yes |
| Repeated hypoxia, Vref | (0.029)2 | (0.288)2 | Yes |
| Repeated normoxia, Nv | (0.117)2 | (0.210)2 | Yes |
| Repeated hypoxia, Nv | (0.122)2 | (0.102)2 | No, 10.2% CV is low |
| Repeated normoxia, N | (0.117)2 | (0.265)2 | Yes |
| Repeated hypoxia, N | (0.127)2 | (0.295)2 | Yes |
| Somal volume of VTA dopaminergic neurons, PN14 | |||
| Repeated normoxia, ln(v) | (0.057)2 | (0.249)2 | Yes |
| Repeated hypoxia, ln(v) | (0.056)2 | (0.121)2 | Yes |
| Somal volume of SNCd dopaminergic neurons, PN14 | |||
| Repeated normoxia, ln(v) | (0.086)2 | (0.153)2 | Yes |
| Repeated hypoxia, ln(v) | (0.085)2 | (0.183)2 | Yes |
| Nuclear volume of VTA dopaminergic neurons, PN545 | |||
| Repeated normoxia, ln(v) | (0.097)2 | (0.284)2 | Yes |
| Repeated hypoxia, ln(v) | (0.117)2 | (0.192)2 | Yes |
| Measured Parameter | (Mean CE)2 | (CV)2 * | (Mean CE)2 < 1/2 (CV)2? |
|---|---|---|---|
| Experimental Condition Absolute number of VTA thionin-stained neurons, PN14 | |||
| Repeated normoxia, Vref | (0.012)2 | (0.146)2 | Yes |
| Repeated hypoxia, Vref | (0.014)2 | (0.059)2 | Yes |
| Repeated hormoxia, Nv | (0.035)2 | (0.037)2 | No, 3.7% CV is low *** |
| Repeated hypoxia, Nv | (0.041)2 | (0.080)2 | Yes |
| Repeated normoxia, N | (0.040)2 | (0.136)2 | Yes |
| Repeated hypoxia, N | (0.043)2 | (0.119)2 | Yes |
| Absolute number of SNCd thionin-stained neurons, PN14 | |||
| Repeated normoxia, Vref | (0.018)2 | (0.034)2 | Yes |
| Repeated hypoxia, Vref | (0.017)2 | (0.036)2 | Yes |
| Repeated normoxia, Nv | (0.056)2 | (0.082)2 | Yes |
| Repeated hypoxia, Nv | (0.060)2 | (0.196)2 | Yes |
| Repeated normoxia, N | (0.059)2 | (0.241)2 | Yes |
| Repeated hypoxia, N | (0.063)2 | (0.232)2 | Yes |
| Absolute number of SNCv thionin-stained neurons, PN14 | |||
| Repeated normoxia, Vref | (0.019)2 | (0.206)2 | Yes |
| Repeated hypoxia, Vref | (0.018)2 | (0.110)2 | Yes |
| Repeated normoxia, Nv | (0.075)2 | (0.002)2 | No, 0.2% CV is low |
| Repeated hypoxia, Nv | (0.064)2 | (0.117)2 | Yes |
| Repeated normoxia, N | (0.077)2 | (0.1222 | Yes |
| Repeated hypoxia, N | (0.065)2 | (0.012)2 | No, 1.2% CV is low |
| Absolute number of RRF thionin-stained neurons, PN14 | |||
| Repeated normoxia, Vref | (0.014)2 | (0.198)2 | Yes |
| Repeated hypoxia, Vref | (0.013)2 | (0.149)2 | Yes |
| Repeated normoxia, Nv | (0.048)2 | (0.236)2 | Yes |
| Repeated hypoxia, Nv | (0.049)2 | (0.053)2 | No, 5.3% CV is low |
| Repeated normoxia, N | (0.049)2 | (0.270)2 | Yes |
| Repeated hypoxia, N | (0.051)2 | (0.159)2 | Yes |
| Absolute number of VTA thionin-stained neurons, PN545 | |||
| Repeated normoxia, Vref | (0.022)2 | (0.079)2 | Yes |
| Repeated hypoxia, Vref | (0.023)2 | (0.141)2 | Yes |
| Repeated normoxia, Nv | (0.049)2 | (0.095)2 | Yes |
| Repeated hypoxia, Nv | (0.051)2 | (0.113)2 | Yes |
| Repeated normoxia, N | (0.051)2 | (0.118)2 | Yes |
| Repeated hypoxia, N | (0.052)2 | (0.214)2 | Yes |
| Absolute number of VTA + CLi thionin-stained neurons, PN545 | |||
| Repeated normoxia, Vref | (0.018)2 | (0.222)2 | Yes |
| Repeated hypoxia, Vref | (0.018)2 | (0.187)2 | Yes |
| Repeated normoxia, Nv | (0.044)2 | (0.112)2 | Yes |
| Repeated hypoxia, Nv | (0.045)2 | (0.124)2 | Yes |
| Repeated normoxia, N | (0.045)2 | (0.123)2 | Yes |
| Repeated hypoxia, N | (0.046)2 | (0.195)2 | Yes |
| Absolute number of SNCd thionin-stained neurons, PN545 | |||
| Repeated normoxia, Vref | (0.059)2 | (0.371)2 | Yes |
| Repeated hypoxia, Vref | (0.056)2 | (0.168)2 | Yes |
| Repeated normoxia, Nv | (0.072)2 | (0.474)2 | Yes |
| Repeated hypoxia, Nv | (0.065)2 | (0.218)2 | Yes |
| Repeated normoxia, N | (0.076)2 | (0.178)2 | Yes |
| Repeated hypoxia, N | (0.067)2 | (0.260)2 | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohe, S.E.; Gowing, E.K.; Seo, S.; Oorschot, D.E. A Novel Rat Model of ADHD-like Hyperactivity/Impulsivity after Delayed Reward Has Selective Loss of Dopaminergic Neurons in the Right Ventral Tegmental Area. Int. J. Mol. Sci. 2023, 24, 11252. https://doi.org/10.3390/ijms241411252
Kohe SE, Gowing EK, Seo S, Oorschot DE. A Novel Rat Model of ADHD-like Hyperactivity/Impulsivity after Delayed Reward Has Selective Loss of Dopaminergic Neurons in the Right Ventral Tegmental Area. International Journal of Molecular Sciences. 2023; 24(14):11252. https://doi.org/10.3390/ijms241411252
Chicago/Turabian StyleKohe, Sarah E., Emma K. Gowing, Steve Seo, and Dorothy E. Oorschot. 2023. "A Novel Rat Model of ADHD-like Hyperactivity/Impulsivity after Delayed Reward Has Selective Loss of Dopaminergic Neurons in the Right Ventral Tegmental Area" International Journal of Molecular Sciences 24, no. 14: 11252. https://doi.org/10.3390/ijms241411252
APA StyleKohe, S. E., Gowing, E. K., Seo, S., & Oorschot, D. E. (2023). A Novel Rat Model of ADHD-like Hyperactivity/Impulsivity after Delayed Reward Has Selective Loss of Dopaminergic Neurons in the Right Ventral Tegmental Area. International Journal of Molecular Sciences, 24(14), 11252. https://doi.org/10.3390/ijms241411252





