The Dual Role of Mesenchymal Stem Cells in Cancer Pathophysiology: Pro-Tumorigenic Effects versus Therapeutic Potential
Abstract
:1. Introduction
2. Origin of Mesenchymal Stem Cells and Other Stromal Cells in the Tumor Microenvironment
| Cell Types | Markers | Comments | References |
|---|---|---|---|
| Endothelial cells | CD31 | PECAM-1, homotypic cell adhesion | [60] |
| CD93 | CD248 family member | [60,74] | |
| CLDN5 | Claudin 5 | [60] | |
| CDH5 | Cadherin 5 | [60] | |
| Pericytes (PCs) | PDGFRβ | Receptor for platelet-derived growth factor | [59] |
| NG2 | Encoded by Chondroitin sulfate proteoglycan 4 CSPG4 gene. | [59,60] | |
| Desmin | [59] | ||
| CD13 | Alanyl Aminopeptidase N | [59] | |
| CD248 | Endosialin (TEM-1), highly expressed in tumor tissues (PCs > pMSCs) | [59,61,75] | |
| KCNJ8 | Kir6.1, Potassium inwardly rectifying channel, associates with Abcc9 | [59,60,76] | |
| Vascular smooth muscle cells (vSMCs) | PDGFRβ | Levels in PCs > vSMCs | [77] |
| NG2 | [77] | ||
| Desmin | Muscle class III intermediate filament | [77] | |
| CD13 | |||
| RGS5 | Regulator of G protein signaling 5 GTPase activating protein | [59,64,78] | |
| CD146 | Melanoma cell adhesion molecule (MCAM) | [59,78] | |
| α-SMA | Alpha smooth muscle cell actin encoded by ACTA2. Level of expression in vSMCs >> PCs | [59] | |
| TAGLN | Transgelin, smooth muscle protein 22 alpha (SM22). | [59] | |
| Perivascular MSCs (also known as vascular fibroblasts) | VIM FSP FAP PDGFRβ | Vimentin, intermediate-filament ass. protein intermediate-filament ass. protein Fib activation protein alpha, serine protease | [79] |
| PDGFRα | [60] | ||
| CD13 | [79] | ||
| COL1A1 | pMSCs also express high levels of COL1A2, COL3A1, and COL4A1. Much less expressed by PCs’ | ||
| LAMA-1 | laminin subunit alpha 1, also expressed by epithelial cells | [60] | |
| LUM | Lumican | [60] | |
| DCN | Decorin | [60] | |
| MPZL2 | Myelin protein zero-like 2, adhesion | [60] | |
| SRPX2 | Sushi repeat-containing protein X-linked 2 | [60] | |
| FN | Fibronectin, also expressed by PCs/scar tissue | [80] | |
| Tumor-associated Macrophages (TAMs) | CD11b/CD18 | Complement receptor type 3 involved in phagocytosis of host cell debris | [81,82] |
| CD163 | Scavenger receptor, M2 anti-inflammatory | [83] | |
| C1q/C3 CD68 | Complement factors | [84] |
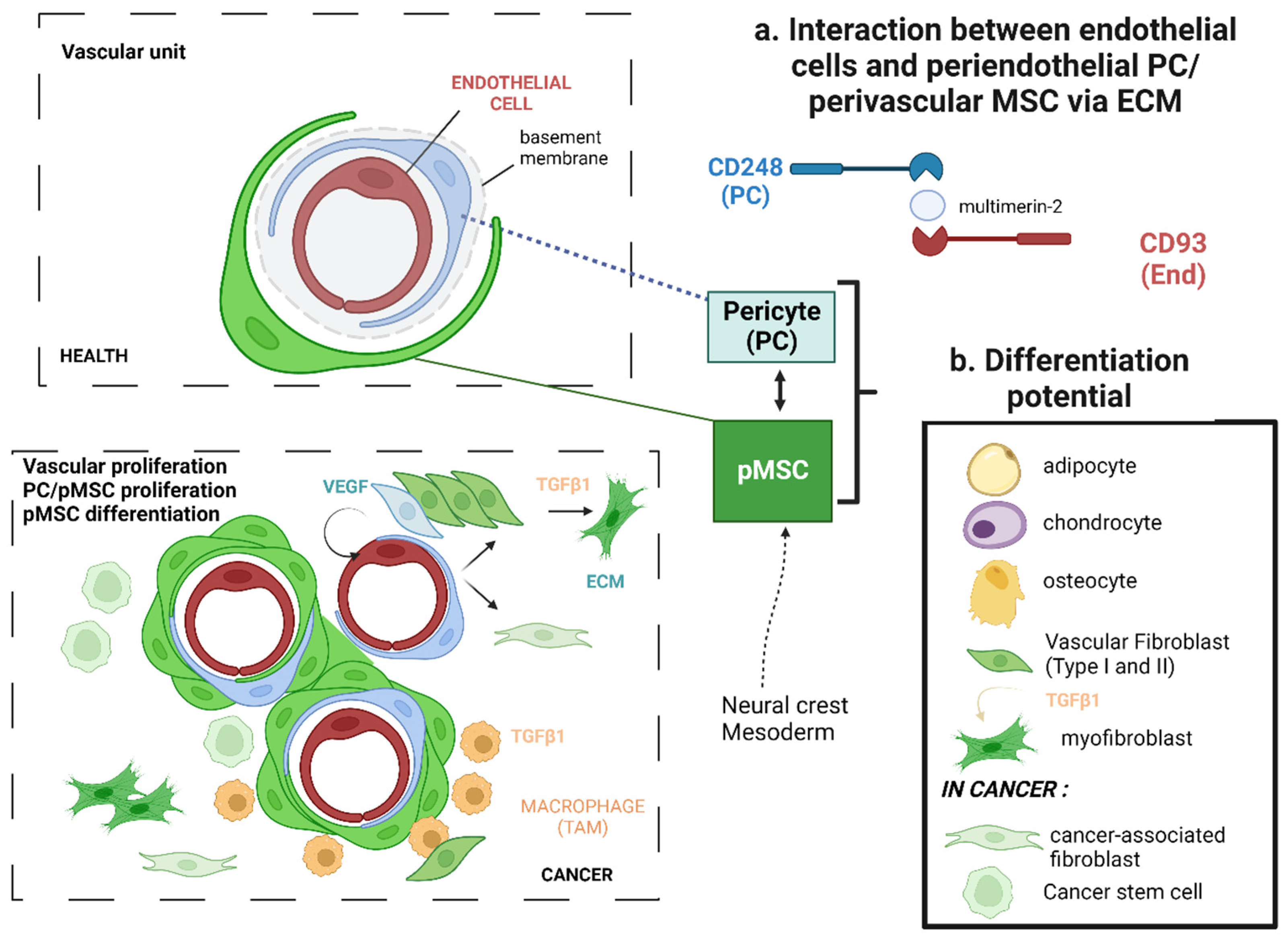
2.1. Recruitment of Mesenchymal Stem Cells
2.2. Mesenchymal Stem Cell Activation and Multi-Lineage Differentiation
2.3. Interplays between Mesenchymal Stem Cells and Components of the Tumor Microenvironment
2.3.1. Innate and Adaptative Immune Cells
2.3.2. Tumor Cells
2.3.3. Extracellular Matrix
3. Role of MSCs-1 and MSCs-2 Subtypes in Cancer Progression
3.1. The Epithelial-Mesenchymal Transition
3.2. Angiogenesis
3.3. Cell Survival
3.4. Metastasis
4. Mesenchymal Stem Cells as a Therapeutic Tool against Cancer Progression
4.1. Resistance to Treatments
4.1.1. Role of Mesenchymal Stem Cells in Tumor Resistance and Therapeutic Interest
4.1.2. Radioresistance and Mesenchymal Stem Cells
4.2. Mesenchymal Stem Cells as a Therapeutic Vector
4.2.1. Types of Vectoring Making Use of Mesenchymal Stem Cells
- Therapeutic macromolecules
- 2.
- Gene-directed enzyme prodrug therapy: suicide genes
- 3.
- Oncolytic virus
- 4.
- Metallic nanoparticles
4.2.2. Therapeutic Potential of Mesenchymal Stem Cells in the Case of Melanoma
4.2.3. Therapeutic Potential of Mesenchymal Stem Cells in the Case of Lung Cancer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal Stem Cells in Health and Disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Bianco, P.; Robey, P.G.; Simmons, P.J. Mesenchymal Stem Cells: Revisiting History, Concepts, and Assays. Cell Stem Cell 2008, 2, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Tolar, J.; Le Blanc, K.; Keating, A.; Blazar, B.R. Concise Review: Hitting the Right Spot with Mesenchymal Stromal Cells. Stem Cells 2010, 28, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Keating, A. Mesenchymal Stromal Cells: New Directions. Cell Stem Cell 2012, 10, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Lazennec, G.; Lam, P.Y. Recent Discoveries Concerning the Tumor—Mesenchymal Stem Cell Interactions. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2016, 1866, 290–299. [Google Scholar] [CrossRef]
- Ridge, S.M.; Sullivan, F.J.; Glynn, S.A. Mesenchymal Stem Cells: Key Players in Cancer Progression. Mol. Cancer 2017, 16, 31. [Google Scholar] [CrossRef]
- Papaccio, F.; Paino, F.; Regad, T.; Papaccio, G.; Desiderio, V.; Tirino, V. Concise Review: Cancer Cells, Cancer Stem Cells, and Mesenchymal Stem Cells: Influence in Cancer Development. Stem Cells Transl. Med. 2017, 6, 2115–2125. [Google Scholar] [CrossRef]
- Poggi, A.; Varesano, S.; Zocchi, M.R. How to Hit Mesenchymal Stromal Cells and Make the Tumor Microenvironment Immunostimulant Rather Than Immunosuppressive. Front. Immunol. 2018, 9, 262. [Google Scholar] [CrossRef]
- Leuning, D.G.; Beijer, N.R.M.; du Fossé, N.A.; Vermeulen, S.; Lievers, E.; van Kooten, C.; Rabelink, T.J.; de Boer, J. The Cytokine Secretion Profile of Mesenchymal Stromal Cells Is Determined by Surface Structure of the Microenvironment. Sci. Rep. 2018, 8, 7716. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A Framework for Advancing Our Understanding of Cancer-Associated Fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Piatetzky-Shapiro, I.; Petrakova, K.V. Osteogenesis in Transplants of Bone Marrow Cells. J. Embryol. Exp. Morphol. 1966, 16, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal Stem Cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Goshima, J.; Goldberg, V.M.; Caplan, A.I. The Osteogenic Potential of Culture-Expanded Rat Marrow Mesenchymal Cells Assayed In Vivo in Calcium Phosphate Ceramic Blocks. Clin. Orthop. Relat. Res. 1991, 262, 298–311. [Google Scholar] [CrossRef]
- Spees, J.L.; Olson, S.D.; Ylostalo, J.; Lynch, P.J.; Smith, J.; Perry, A.; Peister, A.; Wang, M.Y.; Prockop, D.J. Differentiation, Cell Fusion, and Nuclear Fusion during Ex Vivo Repair of Epithelium by Human Adult Stem Cells from Bone Marrow Stroma. Proc. Natl. Acad. Sci. USA 2003, 100, 2397–2402. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Teng, F.Y.H.; Tang, B.L. Coaxing Bone Marrow Stromal Mesenchymal Stem Cells towards Neuronal Differentiation: Progress and Uncertainties. Cell. Mol. Life Sci. 2006, 63, 1649–1657. [Google Scholar] [CrossRef]
- Tohill, M.; Terenghi, G. Stem-Cell Plasticity and Therapy for Injuries of the Peripheral Nervous System. Biotechnol. Appl. Biochem. 2004, 40, 17–24. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. Clarification of the Nomenclature for MSC: The International Society for Cellular Therapy Position Statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- da Silva Meirelles, L.; Caplan, A.I.; Nardi, N.B. In Search of the In Vivo Identity of Mesenchymal Stem Cells. Stem Cells 2008, 26, 2287–2299. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal Stem Cells Reside in Virtually All Post-Natal Organs and Tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise Review: Mesenchymal Stem Cells: Their Phenotype, Differentiation Capacity, Immunological Features, and Potential for Homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef]
- Lebeau, G.; Ah-Pine, F.; Daniel, M.; Bedoui, Y.; Vagner, D.; Frumence, E.; Gasque, P. Perivascular Mesenchymal Stem/Stromal Cells, an Immune Privileged Niche for Viruses? Int. J. Mol. Sci. 2022, 23, 8038. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; Ugarte, D.A.D.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Sabatini, F.; Petecchia, L.; Tavian, M.; de Villeroché, V.J.; Rossi, G.A.; Brouty-Boyé, D. Human Bronchial Fibroblasts Exhibit a Mesenchymal Stem Cell Phenotype and Multilineage Differentiating Potentialities. Lab. Investig. 2005, 85, 962–971. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent Mesenchymal Stem Cells from Adult Human Synovial Membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X.; Chen, E.; Li, L. Liver-Derived Human Mesenchymal Stem Cells: A Novel Therapeutic Source for Liver Diseases. Stem Cell Res. Ther. 2016, 7, 71. [Google Scholar] [CrossRef]
- Short, B.; Wagey, R. Isolation and Culture of Mesenchymal Stem Cells from Mouse Compact Bone. In Basic Cell Culture Protocols; Helgason, C.D., Miller, C.L., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; Volume 946, pp. 335–347. ISBN 978-1-62703-127-1. [Google Scholar]
- Lecourt, S.; Marolleau, J.-P.; Fromigué, O.; Vauchez, K.; Andriamanalijaona, R.; Ternaux, B.; Lacassagne, M.-N.; Robert, I.; Boumédiene, K.; Chéreau, F.; et al. Characterization of Distinct Mesenchymal-like Cell Populations from Human Skeletal Muscle In Situ and In Vitro. Exp. Cell Res. 2010, 316, 2513–2526. [Google Scholar] [CrossRef]
- Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Potential Therapeutic Applications of Muscle-Derived Mesenchymal Stem and Progenitor Cells. Expert Opin. Biol. Ther. 2010, 10, 505–517. [Google Scholar] [CrossRef]
- Trivanovic, D.; Kocic, J.; Mojsilovic, S.; Krstic, A.; Ilic, V.; Okic-Djordjevic, I.; Santibanez, J.; Jovcic, G.; Terzic, M.; Bugarski, D. Mesenchymal Stem Cells Isolated from Peripheral Blood and Umbilical Cord Wharton’s Jelly. Srp. Arh. Celok. Lek. 2013, 141, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Hassan, G.; Kasem, I.; Soukkarieh, C.; Aljamali, M. A Simple Method to Isolate and Expand Human Umbilical Cord Derived Mesenchymal Stem Cells: Using Explant Method and Umbilical Cord Blood Serum. Int. J. Stem Cells 2017, 10, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Beeravolu, N.; McKee, C.; Alamri, A.; Mikhael, S.; Brown, C.; Perez-Cruet, M.; Chaudhry, G.R. Isolation and Characterization of Mesenchymal Stromal Cells from Human Umbilical Cord and Fetal Placenta. J. Vis. Exp. 2017, 55224. [Google Scholar] [CrossRef]
- Vellasamy, S. Isolation and Characterisation of Mesenchymal Stem Cells Derived from Human Placenta Tissue. World J. Stem Cells 2012, 4, 53–61. [Google Scholar] [CrossRef]
- Pelekanos, R.A.; Sardesai, V.S.; Futrega, K.; Lott, W.B.; Kuhn, M.; Doran, M.R. Isolation and Expansion of Mesenchymal Stem/Stromal Cells Derived from Human Placenta Tissue. J. Vis. Exp. 2016, 54204. [Google Scholar] [CrossRef]
- Li, S.; Huang, K.-J.; Wu, J.-C.; Hu, M.S.; Sanyal, M.; Hu, M.; Longaker, M.T.; Lorenz, H.P. Peripheral Blood-Derived Mesenchymal Stem Cells: Candidate Cells Responsible for Healing Critical-Sized Calvarial Bone Defects. Stem Cells Transl. Med. 2015, 4, 359–368. [Google Scholar] [CrossRef]
- Williams, A.R.; Hare, J.M. Mesenchymal Stem Cells: Biology, Pathophysiology, Translational Findings, and Therapeutic Implications for Cardiac Disease. Circ. Res. 2011, 109, 923–940. [Google Scholar] [CrossRef]
- Lv, F.-J.; Tuan, R.S.; Cheung, K.M.C.; Leung, V.Y.L. Concise Review: The Surface Markers and Identity of Human Mesenchymal Stem Cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef]
- Fonseca, L.N.; Bolívar-Moná, S.; Agudelo, T.; Beltrán, L.D.; Camargo, D.; Correa, N.; Del Castillo, M.A.; Fernández de Castro, S.; Fula, V.; García, G.; et al. Cell Surface Markers for Mesenchymal Stem Cells Related to the Skeletal System: A Scoping Review. Heliyon 2023, 9, e13464. [Google Scholar] [CrossRef]
- Phinney, D.G.; Sensebé, L. Mesenchymal Stromal Cells: Misconceptions and Evolving Concepts. Cytotherapy 2013, 15, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Wood, B. Multicolor Immunophenotyping: Human Immune System Hematopoiesis. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2004; Volume 75, pp. 559–576. ISBN 978-0-12-564170-8. [Google Scholar]
- Watt, S.M.; Gilmore, D.J.; Davis, J.M.; Clark, M.R.; Waldmann, H. Cell-Surface Markers on Haemopoietic Precursors. Reagents for the Isolation and Analysis of Progenitor Cell Subpopulations. Mol. Cell. Probes 1987, 1, 297–326. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.P.; Alexander, W.S. Haematopoietic Stem Cells: Past, Present and Future. Cell Death Discov. 2017, 3, 17002. [Google Scholar] [CrossRef]
- Denu, R.A.; Nemcek, S.; Bloom, D.D.; Goodrich, A.D.; Kim, J.; Mosher, D.F.; Hematti, P. Fibroblasts and Mesenchymal Stromal/Stem Cells Are Phenotypically Indistinguishable. Acta Haematol. 2016, 136, 85–97. [Google Scholar] [CrossRef]
- Kuznetsov, S.A.; Krebsbach, P.H.; Satomura, K.; Kerr, J.; Riminucci, M.; Benayahu, D.; Robey, P.G. Single-Colony Derived Strains of Human Marrow Stromal Fibroblasts Form Bone after Transplantation In Vivo. J. Bone Miner. Res. 1997, 12, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Colter, D.C.; Sekiya, I.; Prockop, D.J. Identification of a Subpopulation of Rapidly Self-Renewing and Multipotential Adult Stem Cells in Colonies of Human Marrow Stromal Cells. Proc. Natl. Acad. Sci. USA 2001, 98, 7841–7845. [Google Scholar] [CrossRef]
- Galland, S.; Stamenkovic, I. Mesenchymal Stromal Cells in Cancer: A Review of Their Immunomodulatory Functions and Dual Effects on Tumor Progression. J. Pathol. 2020, 250, 555–572. [Google Scholar] [CrossRef]
- Salmon, H.; Remark, R.; Gnjatic, S.; Merad, M. Host Tissue Determinants of Tumour Immunity. Nat. Rev. Cancer 2019, 19, 215–227. [Google Scholar] [CrossRef]
- Petitprez, F.; Sun, C.-M.; Lacroix, L.; Sautès-Fridman, C.; de Reyniès, A.; Fridman, W.H. Quantitative Analyses of the Tumor Microenvironment Composition and Orientation in the Era of Precision Medicine. Front. Oncol. 2018, 8, 390. [Google Scholar] [CrossRef]
- Hass, R. Role of MSC in the Tumor Microenvironment. Cancers 2020, 12, 2107. [Google Scholar] [CrossRef]
- Xuan, X.; Tian, C.; Zhao, M.; Sun, Y.; Huang, C. Mesenchymal Stem Cells in Cancer Progression and Anticancer Therapeutic Resistance. Cancer Cell Int. 2021, 21, 595. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The Tumor Microenvironment at a Glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef] [PubMed]
- Takashima, Y.; Era, T.; Nakao, K.; Kondo, S.; Kasuga, M.; Smith, A.G.; Nishikawa, S.-I. Neuroepithelial Cells Supply an Initial Transient Wave of MSC Differentiation. Cell 2007, 129, 1377–1388. [Google Scholar] [CrossRef]
- Le Douarin, N.M.; Creuzet, S.; Couly, G.; Dupin, E. Neural Crest Cell Plasticity and Its Limits. Development 2004, 131, 4637–4650. [Google Scholar] [CrossRef] [PubMed]
- Majesky, M.W. Developmental Basis of Vascular Smooth Muscle Diversity. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1248–1258. [Google Scholar] [CrossRef]
- Hungerford, J.E.; Little, C.D. Developmental Biology of the Vascular Smooth Muscle Cell: Building a Multilayered Vessel Wall. J. Vasc. Res. 1999, 36, 2–27. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, Physiological, and Pathological Perspectives, Problems, and Promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef]
- Vanlandewijck, M.; He, L.; Mae, M.A.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Lavina, B.; Gouveia, L.; et al. A Molecular Atlas of Cell Types and Zonation in the Brain Vasculature. Nature 2018, 554, 475–480. [Google Scholar] [CrossRef]
- Brady, J.; Neal, J.; Sadakar, N.; Gasque, P. Human Endosialin (Tumor Endothelial Marker 1) Is Abundantly Expressed in Highly Malignant and Invasive Brain Tumors. J. Neuropathol. Exp. Neurol. 2004, 63, 1274–1283. [Google Scholar] [CrossRef]
- Khan, K.A.; Naylor, A.J.; Khan, A.; Noy, P.J.; Mambretti, M.; Lodhia, P.; Athwal, J.; Korzystka, A.; Buckley, C.D.; Willcox, B.E.; et al. Multimerin-2 Is a Ligand for Group 14 Family C-Type Lectins CLEC14A, CD93 and CD248 Spanning the Endothelial Pericyte Interface. Oncogene 2017, 36, 6097–6108. [Google Scholar] [CrossRef]
- Garcia, F.J.; Sun, N.; Lee, H.; Godlewski, B.; Mathys, H.; Galani, K.; Zhou, B.; Jiang, X.; Ng, A.P.; Mantero, J.; et al. Single-Cell Dissection of the Human Brain Vasculature. Nature 2022, 603, 893–899. [Google Scholar] [CrossRef]
- Travaglini, K.J.; Nabhan, A.N.; Penland, L.; Sinha, R.; Gillich, A.; Sit, R.; Chang, S.; Conley, S.D.; Mori, Y.; Seita, J.; et al. A Molecular Cell Atlas of the Human Lung from Single-Cell RNA Sequencing. Nature 2020, 587, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the Adult Human Heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Lendahl, U.; Muhl, L.; Betsholtz, C. Identification, Discrimination and Heterogeneity of Fibroblasts. Nat. Commun. 2022, 13, 3409. [Google Scholar] [CrossRef] [PubMed]
- Ah-Pine, F.; Khettab, M.; Bedoui, Y.; Slama, Y.; Daniel, M.; Doray, B.; Gasque, P. On the Origin and Development of Glioblastoma: Multifaceted Role of Perivascular Mesenchymal Stromal Cells. Acta Neuropathol. Commun. 2023, 11, 104. [Google Scholar] [CrossRef]
- Rajan, A.M.; Ma, R.C.; Kocha, K.M.; Zhang, D.J.; Huang, P. Dual Function of Perivascular Fibroblasts in Vascular Stabilization in Zebrafish. PLoS Genet. 2020, 16, e1008800. [Google Scholar] [CrossRef]
- Kalluri, R. The Biology and Function of Fibroblasts in Cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Sun, R.; Kong, X.; Qiu, X.; Huang, C.; Wong, P.-P. The Emerging Roles of Pericytes in Modulating Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 676342. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, J.; Li, L.; Liao, S.; He, J.; Zhou, S.; Zhou, Y. Pericytes in the Tumor Microenvironment. Cancer Lett. 2023, 556, 216074. [Google Scholar] [CrossRef]
- Bule, P.; Aguiar, S.I.; Aires-Da-Silva, F.; Dias, J.N.R. Chemokine-Directed Tumor Microenvironment Modulation in Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 9804. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, Y.; Behnan, J.; Ribeiro, M.M.; Kalantzi, C.; Zhang, M.-D.; Lou, D.; Häring, M.; Sharma, N.; Okawa, S.; et al. Neural Network Learning Defines Glioblastoma Features to Be of Neural Crest Perivascular or Radial Glia Lineages. Sci. Adv. 2022, 8, eabm6340. [Google Scholar] [CrossRef]
- Griffiths, M.R.; Botto, M.; Morgan, B.P.; Neal, J.W.; Gasque, P. CD93 Regulates Central Nervous System Inflammation in Two Mouse Models of Autoimmune Encephalomyelitis. Immunology 2018, 155, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A. CD248: A Therapeutic Target in Cancer and Fibrotic Diseases. Oncotarget 2019, 10, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.; Macosko, E.Z.; Wysoker, A.; Goldman, M.; Krienen, F.M.; de Rivera, H.; Bien, E.; Baum, M.A.; Bortolin, L.; Wang, S.; et al. Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain. Cell 2018, 174, 1015–1030. [Google Scholar] [CrossRef]
- Bergers, G.; Song, S. The Role of Pericytes in Blood-Vessel Formation and Maintenance. Neuro-Oncology 2005, 7, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Bergers, G.; Arnold, B.; Hämmerling, G.J.; Ganss, R. Regulator of G-Protein Signaling-5 Induction in Pericytes Coincides with Active Vessel Remodeling during Neovascularization. Blood 2005, 105, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Zeisberg, M. Fibroblasts in Cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Goritz, C.; Dias, D.O.; Tomilin, N.; Barbacid, M.; Shupliakov, O.; Frisen, J. A Pericyte Origin of Spinal Cord Scar Tissue. Science 2011, 333, 238–242. [Google Scholar] [CrossRef]
- Dorrier, C.E.; Aran, D.; Haenelt, E.A.; Sheehy, R.N.; Hoi, K.K.; Pintaric, L.; Chen, Y.; Lizama, C.O.; Cautivo, K.M.; Weiner, G.A.; et al. CNS Fibroblasts Form a Fibrotic Scar in Response to Immune Cell Infiltration. Nat. Neurosci. 2021, 24, 234–244. [Google Scholar] [CrossRef]
- Lapenna, A.; De Palma, M.; Lewis, C.E. Perivascular Macrophages in Health and Disease. Nat. Rev. Immunol. 2018, 18, 689–702. [Google Scholar] [CrossRef]
- Van Hove, H.; Martens, L.; Scheyltjens, I.; De Vlaminck, K.; Pombo Antunes, A.R.; De Prijck, S.; Vandamme, N.; De Schepper, S.; Van Isterdael, G.; Scott, C.L.; et al. A Single-Cell Atlas of Mouse Brain Macrophages Reveals Unique Transcriptional Identities Shaped by Ontogeny and Tissue Environment. Nat. Neurosci. 2019, 22, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Dorrier, C.E.; Jones, H.E.; Pintarić, L.; Siegenthaler, J.A.; Daneman, R. Emerging Roles for CNS Fibroblasts in Health, Injury and Disease. Nat. Rev. Neurosci. 2022, 23, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Nör, J. The Perivascular Niche and Self-Renewal of Stem Cells. Front. Physiol. 2015, 6, 367. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.; Albarenque, S.M.; Deedigan, L.; Yu, R.; Reidy, M.; Fulda, S.; Zwacka, R.M. Targeting of XIAP Combined with Systemic Mesenchymal Stem Cell-Mediated Delivery of STRAIL Ligand Inhibits Metastatic Growth of Pancreatic Carcinoma Cells. Stem Cells 2010, 28, 2109–2120. [Google Scholar] [CrossRef]
- Stoff-Khalili, M.A.; Rivera, A.A.; Mathis, J.M.; Banerjee, N.S.; Moon, A.S.; Hess, A.; Rocconi, R.P.; Numnum, T.M.; Everts, M.; Chow, L.T.; et al. Mesenchymal Stem Cells as a Vehicle for Targeted Delivery of CRAds to Lung Metastases of Breast Carcinoma. Breast Cancer Res. Treat. 2007, 105, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Mohammadi, S.; Moradian-Tehrani, R.; Noureddini, M.; Alani, B. Novel Application of Adipose-Derived Mesenchymal Stem Cells via Producing Antiangiogenic Factor TSP-1 in Lung Metastatic Melanoma Animal Model. Biologicals 2020, 68, 9–18. [Google Scholar] [CrossRef]
- Birbrair, A. (Ed.) Tumor Microenvironment: Non-Hematopoietic Cells; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; Volume 1234, ISBN 978-3-030-37183-8. [Google Scholar]
- Ren, G.; Zhao, X.; Wang, Y.; Zhang, X.; Chen, X.; Xu, C.; Yuan, Z.; Roberts, A.I.; Zhang, L.; Zheng, B.; et al. CCR2-Dependent Recruitment of Macrophages by Tumor-Educated Mesenchymal Stromal Cells Promotes Tumor Development and Is Mimicked by TNFα. Cell Stem Cell 2012, 11, 812–824. [Google Scholar] [CrossRef]
- Coffman, L.G.; Pearson, A.T.; Frisbie, L.G.; Freeman, Z.; Christie, E.; Bowtell, D.D.; Buckanovich, R.J. Ovarian Carcinoma-Associated Mesenchymal Stem Cells Arise from Tissue-Specific Normal Stroma. Stem Cells 2019, 37, 257–269. [Google Scholar] [CrossRef]
- Yang, L.; Lin, P.C. Mechanisms That Drive Inflammatory Tumor Microenvironment, Tumor Heterogeneity, and Metastatic Progression. Semin. Cancer Biol. 2017, 47, 185–195. [Google Scholar] [CrossRef]
- Dvorak, H.F. Tumors: Wounds That Do Not Heal—Redux. Cancer Immunol. Res. 2015, 3, 1–11. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal Stem Cells as Trophic Mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Rustad, K.C.; Gurtner, G.C. Mesenchymal Stem Cells Home to Sites of Injury and Inflammation. Adv. Wound Care 2012, 1, 147–152. [Google Scholar] [CrossRef] [PubMed]
- McLean, K.; Gong, Y.; Choi, Y.; Deng, N.; Yang, K.; Bai, S.; Cabrera, L.; Keller, E.; McCauley, L.; Cho, K.R.; et al. Human Ovarian Carcinoma–Associated Mesenchymal Stem Cells Regulate Cancer Stem Cells and Tumorigenesis via Altered BMP Production. J. Clin. Investig. 2011, 121, 3206–3219. [Google Scholar] [CrossRef] [PubMed]
- Khakoo, A.Y.; Pati, S.; Anderson, S.A.; Reid, W.; Elshal, M.F.; Rovira, I.I.; Nguyen, A.T.; Malide, D.; Combs, C.A.; Hall, G.; et al. Human Mesenchymal Stem Cells Exert Potent Antitumorigenic Effects in a Model of Kaposi’s Sarcoma. J. Exp. Med. 2006, 203, 1235–1247. [Google Scholar] [CrossRef]
- O’Malley, G.; Heijltjes, M.; Houston, A.M.; Rani, S.; Ritter, T.; Egan, L.J.; Ryan, A.E. Mesenchymal Stromal Cells (MSCs) and Colorectal Cancer: A Troublesome Twosome for the Anti-Tumour Immune Response? Oncotarget 2016, 7, 60752–60774. [Google Scholar] [CrossRef] [PubMed]
- Moniri, M.R.; Sun, X.-Y.; Rayat, J.; Dai, D.; Ao, Z.; He, Z.; Verchere, C.B.; Dai, L.-J.; Warnock, G.L. TRAIL-Engineered Pancreas-Derived Mesenchymal Stem Cells: Characterization and Cytotoxic Effects on Pancreatic Cancer Cells. Cancer Gene Ther. 2012, 19, 652–658. [Google Scholar] [CrossRef]
- Nakamizo, A.; Marini, F.; Amano, T.; Khan, A.; Studeny, M.; Gumin, J.; Chen, J.; Hentschel, S.; Vecil, G.; Dembinski, J.; et al. Human Bone Marrow–Derived Mesenchymal Stem Cells in the Treatment of Gliomas. Cancer Res. 2005, 65, 3307–3318. [Google Scholar] [CrossRef]
- Ren, C.; Kumar, S.; Chanda, D.; Kallman, L.; Chen, J.; Mountz, J.D.; Ponnazhagan, S. Cancer Gene Therapy Using Mesenchymal Stem Cells Expressing Interferon-β in a Mouse Prostate Cancer Lung Metastasis Model. Gene Ther. 2008, 15, 1446–1453. [Google Scholar] [CrossRef]
- Studeny, M.; Marini, F.C.; Dembinski, J.L.; Zompetta, C.; Cabreira-Hansen, M.; Bekele, B.N.; Champlin, R.E.; Andreeff, M. Mesenchymal Stem Cells: Potential Precursors for Tumor Stroma and Targeted-Delivery Vehicles for Anticancer Agents. JNCI J. Natl. Cancer Inst. 2004, 96, 1593–1603. [Google Scholar] [CrossRef]
- Quante, M.; Tu, S.P.; Tomita, H.; Gonda, T.; Wang, S.S.W.; Takashi, S.; Baik, G.H.; Shibata, W.; DiPrete, B.; Betz, K.S.; et al. Bone Marrow-Derived Myofibroblasts Contribute to the Mesenchymal Stem Cell Niche and Promote Tumor Growth. Cancer Cell 2011, 19, 257–272. [Google Scholar] [CrossRef]
- Ahn, J.o.; Lee, H.w.; Seo, K.w.; Kang, S.k.; Ra, J.c.; Youn, H.y. Anti-Tumor Effect of Adipose Tissue Derived-Mesenchymal Stem Cells Expressing Interferon-β and Treatment with Cisplatin in a Xenograft Mouse Model for Canine Melanoma. PLoS ONE 2013, 8, e74897. [Google Scholar] [CrossRef] [PubMed]
- Komarova, S.; Kawakami, Y.; Stoff-Khalili, M.A.; Curiel, D.T.; Pereboeva, L. Mesenchymal Progenitor Cells as Cellular Vehicles for Delivery of Oncolytic Adenoviruses. Mol. Cancer Ther. 2006, 5, 755–766. [Google Scholar] [CrossRef]
- Becker, A.D.; Riet, I.V. Homing and Migration of Mesenchymal Stromal Cells: How to Improve the Efficacy of Cell Therapy? World J. Stem Cells 2016, 8, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Ponte, A.L.; Marais, E.; Gallay, N.; Langonné, A.; Delorme, B.; Hérault, O.; Charbord, P.; Domenech, J. The In Vitro Migration Capacity of Human Bone Marrow Mesenchymal Stem Cells: Comparison of Chemokine and Growth Factor Chemotactic Activities. Stem Cells 2007, 25, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Madu, C.O.; Lu, A.; Lu, Y. HIF-1α Promotes A Hypoxia-Independent Cell Migration. Open Biol. J. 2010, 3, 8–14. [Google Scholar] [CrossRef]
- Schmidt, A.; Ladage, D.; Schinköthe, T.; Klausmann, U.; Ulrichs, C.; Klinz, F.; Brixius, K.; Arnhold, S.; Desai, B.; Mehlhorn, U.; et al. Basic Fibroblast Growth Factor Controls Migration in Human Mesenchymal Stem Cells. Stem Cells 2006, 24, 1750–1758. [Google Scholar] [CrossRef]
- Dwyer, R.M.; Potter-Beirne, S.M.; Harrington, K.A.; Lowery, A.J.; Hennessy, E.; Murphy, J.M.; Barry, F.P.; O’Brien, T.; Kerin, M.J. Monocyte Chemotactic Protein-1 Secreted by Primary Breast Tumors Stimulates Migration of Mesenchymal Stem Cells. Clin. Cancer Res. 2007, 13, 5020–5027. [Google Scholar] [CrossRef]
- Chen, M.-S.; Lin, C.-Y.; Chiu, Y.-H.; Chen, C.-P.; Tsai, P.-J.; Wang, H.-S. IL-1 β -Induced Matrix Metalloprotease-1 Promotes Mesenchymal Stem Cell Migration via PAR1 and G-Protein-Coupled Signaling Pathway. Stem Cells Int. 2018, 2018, 3524759. [Google Scholar] [CrossRef]
- Dubon, M.J.; Yu, J.; Choi, S.; Park, K. Transforming Growth Factor β Induces Bone Marrow Mesenchymal Stem Cell Migration via Noncanonical Signals and N-cadherin. J. Cell. Physiol. 2018, 233, 201–213. [Google Scholar] [CrossRef]
- Lourenco, S.; Teixeira, V.H.; Kalber, T.; Jose, R.J.; Floto, R.A.; Janes, S.M. Macrophage Migration Inhibitory Factor–CXCR4 Is the Dominant Chemotactic Axis in Human Mesenchymal Stem Cell Recruitment to Tumors. J. Immunol. 2015, 194, 3463–3474. [Google Scholar] [CrossRef]
- Abarbanell, A.M.; Coffey, A.C.; Fehrenbacher, J.W.; Beckman, D.J.; Herrmann, J.L.; Weil, B.; Meldrum, D.R. Proinflammatory Cytokine Effects on Mesenchymal Stem Cell Therapy for the Ischemic Heart. Ann. Thorac. Surg. 2009, 88, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, S.; Zhao, R.C. The Roles of Mesenchymal Stem Cells in Tumor Inflammatory Microenvironment. J. Hematol. Oncol. 2014, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.S.; Pelagalli, A.; Passaro, N.; Zannetti, A. Tumor-Educated Mesenchymal Stem Cells Promote pro-Metastatic Phenotype. Oncotarget 2017, 8, 73296–73311. [Google Scholar] [CrossRef]
- Ho, I.A.W.; Chan, K.Y.W.; Ng, W.-H.; Guo, C.M.; Hui, K.M.; Cheang, P.; Lam, P.Y.P. Matrix Metalloproteinase 1 Is Necessary for the Migration of Human Bone Marrow-Derived Mesenchymal Stem Cells toward Human Glioma. Stem Cells 2009, 27, 1366–1375. [Google Scholar] [CrossRef]
- Anton, K.; Banerjee, D.; Glod, J. Macrophage-Associated Mesenchymal Stem Cells Assume an Activated, Migratory, pro-Inflammatory Phenotype with Increased IL-6 and CXCL10 Secretion. PLoS ONE 2012, 7, e35036. [Google Scholar] [CrossRef] [PubMed]
- Barcellos-de-Souza, P.; Comito, G.; Pons-Segura, C.; Taddei, M.L.; Gori, V.; Becherucci, V.; Bambi, F.; Margheri, F.; Laurenzana, A.; Del Rosso, M.; et al. Mesenchymal Stem Cells Are Recruited and Activated into Carcinoma-Associated Fibroblasts by Prostate Cancer Microenvironment-Derived TGF-Β1. Stem Cells 2016, 34, 2536–2547. [Google Scholar] [CrossRef]
- Uchibori, R.; Tsukahara, T.; Mizuguchi, H.; Saga, Y.; Urabe, M.; Mizukami, H.; Kume, A.; Ozawa, K. NF-ΚB Activity Regulates Mesenchymal Stem Cell Accumulation at Tumor Sites. Cancer Res. 2013, 73, 364–372. [Google Scholar] [CrossRef]
- Papait, A.; Stefani, F.R.; Cargnoni, A.; Magatti, M.; Parolini, O.; Silini, A.R. The Multifaceted Roles of MSCs in the Tumor Microenvironment: Interactions with Immune Cells and Exploitation for Therapy. Front. Cell Dev. Biol. 2020, 8, 447. [Google Scholar] [CrossRef]
- Spaeth, E.L.; Dembinski, J.L.; Sasser, A.K.; Watson, K.; Klopp, A.; Hall, B.; Andreeff, M.; Marini, F. Mesenchymal Stem Cell Transition to Tumor-Associated Fibroblasts Contributes to Fibrovascular Network Expansion and Tumor Progression. PLoS ONE 2009, 4, e4992. [Google Scholar] [CrossRef]
- Mishra, P.J.; Mishra, P.J.; Humeniuk, R.; Medina, D.J.; Alexe, G.; Mesirov, J.P.; Ganesan, S.; Glod, J.W.; Banerjee, D. Carcinoma-Associated Fibroblast–Like Differentiation of Human Mesenchymal Stem Cells. Cancer Res. 2008, 68, 4331–4339. [Google Scholar] [CrossRef]
- Lee, K. Exosomes from Breast Cancer Cells Can Convert Adipose Tissue-Derived Mesenchymal Stem Cells into Myofibroblast-like Cells. Int. J. Oncol. 2011, 40, 130–138. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Oda, T.; Inagaki, Y.; Kushige, H.; Saito, Y.; Mori, N.; Takayama, Y.; Kumagai, Y.; Mitsuyama, T.; Kida, Y.S. Adipose-Derived Mesenchymal Stem Cells Differentiate into Heterogeneous Cancer-Associated Fibroblasts in a Stroma-Rich Xenograft Model. Sci. Rep. 2021, 11, 4690. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, B.; Li, Z.; Li, J.; Sun, S.; Sun, S. Cancer-Associated Adipocytes: Key Players in Breast Cancer Progression. J. Hematol. Oncol. 2019, 12, 95. [Google Scholar] [CrossRef]
- Fujisaki, K.; Fujimoto, H.; Sangai, T.; Nagashima, T.; Sakakibara, M.; Shiina, N.; Kuroda, M.; Aoyagi, Y.; Miyazaki, M. Cancer-Mediated Adipose Reversion Promotes Cancer Cell Migration via IL-6 and MCP-1. Breast Cancer Res. Treat. 2015, 150, 255–263. [Google Scholar] [CrossRef] [PubMed]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental Regulation of Tumour Angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Teng, I.-W.; Hou, P.-C.; Lee, K.-D.; Chu, P.-Y.; Yeh, K.-T.; Jin, V.X.; Tseng, M.-J.; Tsai, S.-J.; Chang, Y.-S.; Wu, C.-S.; et al. Targeted Methylation of Two Tumor Suppressor Genes Is Sufficient to Transform Mesenchymal Stem Cells into Cancer Stem/Initiating Cells. Cancer Res. 2011, 71, 4653–4663. [Google Scholar] [CrossRef]
- Melzer, C.; Yang, Y.; Hass, R. Interaction of MSC with Tumor Cells. Cell Commun. Signal. 2016, 14, 20. [Google Scholar] [CrossRef]
- Melzer, C.; von der Ohe, J.; Hass, R. Enhanced Metastatic Capacity of Breast Cancer Cells after Interaction and Hybrid Formation with Mesenchymal Stroma/Stem Cells (MSC). Cell Commun. Signal. 2018, 16, 2. [Google Scholar] [CrossRef]
- Yang, Y.; Otte, A.; Hass, R. Human Mesenchymal Stroma/Stem Cells Exchange Membrane Proteins and Alter Functionality during Interaction with Different Tumor Cell Lines. Stem Cells Dev. 2015, 24, 1205–1222. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The Immune Contexture in Human Tumours: Impact on Clinical Outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef]
- Qin, Z.; Richter, G.; Schüler, T.; Ibe, S.; Cao, X.; Blankenstein, T. B Cells Inhibit Induction of T Cell-Dependent Tumor Immunity. Nat. Med. 1998, 4, 627–630. [Google Scholar] [CrossRef]
- Kuroda, H.; Jamiyan, T.; Yamaguchi, R.; Kakumoto, A.; Abe, A.; Harada, O.; Enkhbat, B.; Masunaga, A. Prognostic Value of Tumor-Infiltrating B Lymphocytes and Plasma Cells in Triple-Negative Breast Cancer. Breast Cancer 2021, 28, 904–914. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Q.; Chen, X. The Immunomodulatory Effects of Mesenchymal Stem Cells on Regulatory B Cells. Front. Immunol. 2020, 11, 1843. [Google Scholar] [CrossRef] [PubMed]
- Krueger, T.E.; Thorek, D.L.J.; Meeker, A.K.; Isaacs, J.T.; Brennen, W.N. Tumor-Infiltrating Mesenchymal Stem Cells: Drivers of the Immunosuppressive Tumor Microenvironment in Prostate Cancer? Prostate 2019, 79, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Volarevic, A.; Djonov, V.G.; Jovicic, N.; Volarevic, V. Mesenchymal Stem Cell: A Friend or Foe in Anti-Tumor Immunity. Int. J. Mol. Sci. 2021, 22, 12429. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Tian, Z.; Lv, G.; Zhang, L.; Jiang, G.; Sun, K.; Wang, C.; Bu, X.; Li, R.; Shi, Y.; et al. Immunosuppressive Effect of Bone Marrow-Derived Mesenchymal Stem Cells in Inflammatory Microenvironment Favours the Growth of B16 Melanoma Cells. J. Cell. Mol. Med. 2011, 15, 2343–2352. [Google Scholar] [CrossRef]
- Guo, Y.; Chan, K.-H.; Lai, W.-H.; Siu, C.-W.; Kwan, S.-C.; Tse, H.-F.; Wing-Lok Ho, P.; Wing-Man Ho, J. Human Mesenchymal Stem Cells Upregulate CD1dCD5(+) Regulatory B Cells in Experimental Autoimmune Encephalomyelitis. Neuroimmunomodulation 2013, 20, 294–303. [Google Scholar] [CrossRef]
- Hermankova, B.; Zajicova, A.; Javorkova, E.; Chudickova, M.; Trosan, P.; Hajkova, M.; Krulova, M.; Holan, V. Suppression of IL-10 Production by Activated B Cells via a Cell Contact-Dependent Cyclooxygenase-2 Pathway Upregulated in IFN-γ-Treated Mesenchymal Stem Cells. Immunobiology 2016, 221, 129–136. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal Stem Cell-Mediated Immunosuppression Occurs via Concerted Action of Chemokines and Nitric Oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef]
- Frisbie, L.; Buckanovich, R.J.; Coffman, L. Carcinoma-Associated Mesenchymal Stem/Stromal Cells: Architects of the Pro-Tumorigenic Tumor Microenvironment. Stem Cells 2022, 40, 705–715. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, L.; Gong, C.; Shi, H.; Zeng, Y.; Wang, X.; Zhao, Y.; Wei, Y. Prognostic Significance of Tumor-Associated Macrophages in Solid Tumor: A Meta-Analysis of the Literature. PLoS ONE 2012, 7, e50946. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ke, S.Q.; Huang, Z.; Flavahan, W.; Fang, X.; Paul, J.; Wu, L.; Sloan, A.E.; McLendon, R.E.; Li, X.; et al. Periostin Secreted by Glioblastoma Stem Cells Recruits M2 Tumour-Associated Macrophages and Promotes Malignant Growth. Nat. Cell Biol. 2015, 17, 170–182. [Google Scholar] [CrossRef]
- Ah-Pine, F.; Malaterre-Septembre, A.; Bedoui, Y.; Khettab, M.; Neal, J.W.; Freppel, S.; Gasque, P. Complement Activation and Up-Regulated Expression of Anaphylatoxin C3a/C3aR in Glioblastoma: Deciphering the Links with TGF-β and VEGF. Cancers 2023, 15, 2647. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.; Efremova, M.; Riedel, A.; Mahata, B.; Pramanik, J.; Huuhtanen, J.; Kar, G.; Vento-Tormo, R.; Hagai, T.; Chen, X.; et al. Single-Cell RNA Sequencing Reveals a Dynamic Stromal Niche That Supports Tumor Growth. Cell Rep. 2020, 31, 107628. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.-H.; Feng, G.-W.; Wang, Z.-L.; Du, Y.; Shen, C.; Hui, H.; Peng, D.; Li, Z.-J.; Kong, D.-L.; Tian, J. Activation of Mesenchymal Stem Cells by Macrophages Promotes Tumor Progression through Immune Suppressive Effects. Oncotarget 2016, 7, 20934–20944. [Google Scholar] [CrossRef]
- Condeelis, J.; Pollard, J.W. Macrophages: Obligate Partners for Tumor Cell Migration, Invasion, and Metastasis. Cell 2006, 124, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, H.; Chiu, C.; Hanahan, D. Infiltrating Neutrophils Mediate the Initial Angiogenic Switch in a Mouse Model of Multistage Carcinogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 12493–12498. [Google Scholar] [CrossRef]
- Youn, J.-I.; Gabrilovich, D.I. The Biology of Myeloid-Derived Suppressor Cells: The Blessing and the Curse of Morphological and Functional Heterogeneity. Eur. J. Immunol. 2010, 40, 2969–2975. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, Y.; Dong, K.; Sun, Z.; Zhao, D.; Wang, W.; Yu, G.; Liu, W.; Xu, G.; Han, Z.; et al. Programming of the Development of Tumor-Promoting Neutrophils by Mesenchymal Stromal Cells. Cell. Physiol. Biochem. 2014, 33, 1802–1814. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, C.; Li, W.; Mao, Z.; Shi, Y.; Shi, H.; Ji, R.; Qian, H.; Xu, W.; Zhang, X. Tumor-Educated Neutrophils Activate Mesenchymal Stem Cells to Promote Gastric Cancer Growth and Metastasis. Front. Cell Dev. Biol. 2020, 8, 788. [Google Scholar] [CrossRef]
- Guo, S.; Huang, C.; Han, F.; Chen, B.; Ding, Y.; Zhao, Y.; Chen, Z.; Wen, S.; Wang, M.; Shen, B.; et al. Gastric Cancer Mesenchymal Stem Cells Inhibit NK Cell Function through MTOR Signalling to Promote Tumour Growth. Stem Cells Int. 2021, 2021, 9989790. [Google Scholar] [CrossRef] [PubMed]
- Pradier, A.; Passweg, J.; Villard, J.; Kindler, V. Human Bone Marrow Stromal Cells and Skin Fibroblasts Inhibit Natural Killer Cell Proliferation and Cytotoxic Activity. Cell Transpl. 2011, 20, 681–691. [Google Scholar] [CrossRef]
- Gazdic, M.; Simovic Markovic, B.; Jovicic, N.; Misirkic-Marjanovic, M.; Djonov, V.; Jakovljevic, V.; Arsenijevic, N.; Lukic, M.L.; Volarevic, V. Mesenchymal Stem Cells Promote Metastasis of Lung Cancer Cells by Downregulating Systemic Antitumor Immune Response. Stem Cells Int. 2017, 2017, 6294717. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.; Shamsasenjan, K.; Ahmadi, M.; Beheshti, S.A.; Saleh, M. Mesenchymal Stem Cells and Natural Killer Cells Interaction Mechanisms and Potential Clinical Applications. Stem Cell Res. Ther. 2022, 13, 97. [Google Scholar] [CrossRef]
- Swamydas, M.; Ricci, K.; Rego, S.L.; Dréau, D. Mesenchymal Stem Cell-Derived CCL-9 and CCL-5 Promote Mammary Tumor Cell Invasion and the Activation of Matrix Metalloproteinases. Cell Adhes. Migr. 2013, 7, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Mi, Z.; Bhattacharya, S.D.; Kim, V.M.; Guo, H.; Talbot, L.J.; Kuo, P.C. Osteopontin Promotes CCL5-Mesenchymal Stromal Cell-Mediated Breast Cancer Metastasis. Carcinogenesis 2011, 32, 477–487. [Google Scholar] [CrossRef]
- Gallo, M.; De Luca, A.; Lamura, L.; Normanno, N. Zoledronic Acid Blocks the Interaction between Mesenchymal Stem Cells and Breast Cancer Cells: Implications for Adjuvant Therapy of Breast Cancer. Ann. Oncol. 2012, 23, 597–604. [Google Scholar] [CrossRef]
- Naderi, E.H.; Skah, S.; Ugland, H.; Myklebost, O.; Sandnes, D.L.; Torgersen, M.L.; Josefsen, D.; Ruud, E.; Naderi, S.; Blomhoff, H.K. Bone Marrow Stroma-Derived PGE2 Protects BCP-ALL Cells from DNA Damage-Induced P53 Accumulation and Cell Death. Mol. Cancer 2015, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Loussouarn, C.; Pers, Y.-M.; Bony, C.; Jorgensen, C.; Noël, D. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Regulate the Mitochondrial Metabolism via Transfer of MiRNAs. Front. Immunol. 2021, 12, 623973. [Google Scholar] [CrossRef]
- Jothimani, G.; Pathak, S.; Dutta, S.; Duttaroy, A.K.; Banerjee, A. A Comprehensive Cancer-Associated MicroRNA Expression Profiling and Proteomic Analysis of Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes. Tissue Eng. Regen. Med. 2022, 19, 1013–1031. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Y.; Fan, Z.; Xu, J.; Jin, L.; Gao, Z.; Wu, Z.; Hu, L.; Wang, J.; Zhang, C.; et al. MiR-21 Modulates the Immunoregulatory Function of Bone Marrow Mesenchymal Stem Cells Through the PTEN/Akt/TGF-Β1 Pathway. Stem Cells 2015, 33, 3281–3290. [Google Scholar] [CrossRef]
- Kandouz, M.; Batist, G. Gap Junctions and Connexins as Therapeutic Targets in Cancer. Expert Opin. Ther. Targets 2010, 14, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Rafii, A.; Mirshahi, P.; Poupot, M.; Faussat, A.-M.; Simon, A.; Ducros, E.; Mery, E.; Couderc, B.; Lis, R.; Capdet, J.; et al. Oncologic Trogocytosis of an Original Stromal Cells Induces Chemoresistance of Ovarian Tumours. PLoS ONE 2008, 3, e3894. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Luo, M.; Wei, X. Mesenchymal Stem/Stromal Cells in Cancer Therapy. J. Hematol. Oncol. 2021, 14, 195. [Google Scholar] [CrossRef]
- Bartsch, J.E.; Staren, E.D.; Appert, H.E. Matrix Metalloproteinase Expression in Breast Cancer. J. Surg. Res. 2003, 110, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deng, Z.; Wang, Z.; Wang, D.; Zhang, L.; Su, Q.; Lai, Y.; Li, B.; Luo, Z.; Chen, X.; et al. Zipper-Interacting Protein Kinase Promotes Epithelial-Mesenchymal Transition, Invasion and Metastasis through AKT and NF-ΚB Signaling and Is Associated with Metastasis and Poor Prognosis in Gastric Cancer Patients. Oncotarget 2015, 6, 8323–8338. [Google Scholar] [CrossRef]
- Yang, H.-L.; Thiyagarajan, V.; Shen, P.-C.; Mathew, D.C.; Lin, K.-Y.; Liao, J.-W.; Hseu, Y.-C. Anti-EMT Properties of CoQ0 Attributed to PI3K/AKT/NFKB/MMP-9 Signaling Pathway through ROS-Mediated Apoptosis. J. Exp. Clin. Cancer Res. 2019, 38, 186. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Z.Z.; Aoshima, K.; Cai, W.L.; Sun, H.; Xu, T.; Zhang, Y.; An, Y.; Chen, J.F.; Chan, L.H.; et al. CECR2 Drives Breast Cancer Metastasis by Promoting NF-ΚB Signaling and Macrophage-Mediated Immune Suppression. Sci. Transl. Med. 2022, 14, eabf5473. [Google Scholar] [CrossRef]
- Atiya, H.; Frisbie, L.; Pressimone, C.; Coffman, L. Mesenchymal Stem Cells in the Tumor Microenvironment. In Tumor Microenvironment: Non-Hematopoietic Cells; Birbrair, A., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; pp. 31–42. ISBN 978-3-030-37184-5. [Google Scholar]
- Hayashi, Y.; Tsujii, M.; Kodama, T.; Akasaka, T.; Kondo, J.; Hikita, H.; Inoue, T.; Tsujii, Y.; Maekawa, A.; Yoshii, S.; et al. P53 Functional Deficiency in Human Colon Cancer Cells Promotes Fibroblast-Mediated Angiogenesis and Tumor Growth. Carcinogenesis 2016, 37, 972–984. [Google Scholar] [CrossRef]
- Monteran, L.; Erez, N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front. Immunol. 2019, 10, 1835. [Google Scholar] [CrossRef]
- Meulmeester, E.; Ten Dijke, P. The Dynamic Roles of TGF-β in Cancer. J. Pathol. 2011, 223, 205–218. [Google Scholar] [CrossRef]
- Yang, J.; Lu, Y.; Lin, Y.-Y.; Zheng, Z.-Y.; Fang, J.-H.; He, S.; Zhuang, S.-M. Vascular Mimicry Formation Is Promoted by Paracrine TGF-β and SDF1 of Cancer-Associated Fibroblasts and Inhibited by MiR-101 in Hepatocellular Carcinoma. Cancer Lett. 2016, 383, 18–27. [Google Scholar] [CrossRef]
- Zhou, B.; Zhuang, X.; Wang, Y.; Lin, Z.; Zhang, D.; Fan, S.; Li, J.; Chen, W. Tumor Necrosis Factor α Induces Myofibroblast Differentiation in Human Tongue Cancer and Promotes Invasiveness and Angiogenesis via Secretion of Stromal Cell-Derived Factor-1. Oral Oncol. 2015, 51, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Zigrino, P.; Kuhn, I.; Bäuerle, T.; Zamek, J.; Fox, J.W.; Neumann, S.; Licht, A.; Schorpp-Kistner, M.; Angel, P.; Mauch, C. Stromal Expression of MMP-13 Is Required for Melanoma Invasion and Metastasis. J. Investig. Dermatol. 2009, 129, 2686–2693. [Google Scholar] [CrossRef] [PubMed]
- Crawford, Y.; Kasman, I.; Yu, L.; Zhong, C.; Wu, X.; Modrusan, Z.; Kaminker, J.; Ferrara, N. PDGF-C Mediates the Angiogenic and Tumorigenic Properties of Fibroblasts Associated with Tumors Refractory to Anti-VEGF Treatment. Cancer Cell 2009, 15, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.M.; Jung, J.; Aziz, N.; Kissil, J.L.; Puré, E. Targeting Fibroblast Activation Protein Inhibits Tumor Stromagenesis and Growth in Mice. J. Clin. Investig. 2009, 119, 3613–3625. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of Tumor Microenvironment in Tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef]
- Ahn, S.Y. The Role of MSCs in the Tumor Microenvironment and Tumor Progression. Anticancer Res. 2020, 40, 3039–3047. [Google Scholar] [CrossRef]
- Zhu, J.; Liang, L.; Jiao, Y.; Liu, L.; on behalf of the U.S.-China Physical Sciences-Oncology Alliance. Enhanced Invasion of Metastatic Cancer Cells via Extracellular Matrix Interface. PLoS ONE 2015, 10, e0118058. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Knittel, J.G.; Yan, L.; Rueden, C.T.; White, J.G.; Keely, P.J. Collagen Density Promotes Mammary Tumor Initiation and Progression. BMC Med. 2008, 6, 11. [Google Scholar] [CrossRef]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular Matrix: A Dynamic Microenvironment for Stem Cell Niche. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- Konstantinov, A.S.; Kovaleva, O.V.; Samoilova, D.V.; Shelekhova, K.V. Role of Macrophages in Progression of Colorectal Cancer: A Contrast with the Traditional Paradigm. Int. J. Clin. Exp. Pathol. 2022, 15, 403–411. [Google Scholar] [PubMed]
- Gonzalez, M.E.; Martin, E.; Anwar, T.; Arellano-Garcia, C.; Medhora, N.; Lama, A.; Chen, Y.-C.; Tanager, K.S.; Yoon, E.; Kidwell, K.; et al. Mesenchymal Stem Cell Induced DDR2 Mediates Stromal-Breast Cancer Interactions and Metastasis Growth. Cell Rep. 2017, 18, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Zeltz, C.; Primac, I.; Erusappan, P.; Alam, J.; Noel, A.; Gullberg, D. Cancer-Associated Fibroblasts in Desmoplastic Tumors: Emerging Role of Integrins. Semin. Cancer Biol. 2020, 62, 166–181. [Google Scholar] [CrossRef]
- Eiro, N.; Fraile, M.; Fernández-Francos, S.; Sánchez, R.; Costa, L.A.; Vizoso, F.J. Importance of the Origin of Mesenchymal (Stem) Stromal Cells in Cancer Biology: “Alliance” or “War” in Intercellular Signals. Cell Biosci. 2021, 11, 109. [Google Scholar] [CrossRef]
- Liang, W.; Chen, X.; Zhang, S.; Fang, J.; Chen, M.; Xu, Y.; Chen, X. Mesenchymal Stem Cells as a Double-Edged Sword in Tumor Growth: Focusing on MSC-Derived Cytokines. Cell. Mol. Biol. Lett. 2021, 26, 3. [Google Scholar] [CrossRef]
- Rhee, K.-J.; Lee, J.; Eom, Y. Mesenchymal Stem Cell-Mediated Effects of Tumor Support or Suppression. Int. J. Mol. Sci. 2015, 16, 30015–30033. [Google Scholar] [CrossRef]
- Waterman, R.S.; Henkle, S.L.; Betancourt, A.M. Mesenchymal Stem Cell 1 (MSC1)-Based Therapy Attenuates Tumor Growth Whereas MSC2-Treatment Promotes Tumor Growth and Metastasis. PLoS ONE 2012, 7, e45590. [Google Scholar] [CrossRef]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A New Mesenchymal Stem Cell (MSC) Paradigm: Polarization into a Pro-Inflammatory MSC1 or an Immunosuppressive MSC2 Phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef]
- Ohlsson, L.B.; Varas, L.; Kjellman, C.; Edvardsen, K.; Lindvall, M. Mesenchymal Progenitor Cell-Mediated Inhibition of Tumor Growth In Vivo and In Vitro in Gelatin Matrix. Exp. Mol. Pathol. 2003, 75, 248–255. [Google Scholar] [CrossRef]
- Qiao, L.; Xu, Z.; Zhao, T.; Ye, L.; Zhang, X. Dkk-1 Secreted by Mesenchymal Stem Cells Inhibits Growth of Breast Cancer Cells via Depression of Wnt Signalling. Cancer Lett. 2008, 269, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yuan, Y.; Wang, X.; Wei, L.; Chen, Y.; Cong, C.; Li, S.; Long, D.; Tan, W.; Mao, Y.; et al. The Growth Inhibitory Effect of Mesenchymal Stem Cells on Tumor Cells In Vitro and In Vivo. Cancer Biol. Ther. 2008, 7, 245–251. [Google Scholar] [CrossRef]
- Gu, H.; Yan, C.; Wan, H.; Wu, L.; Liu, J.; Zhu, Z.; Gao, D. Mesenchymal Stem Cell-Derived Exosomes Block Malignant Behaviors of Hepatocellular Carcinoma Stem Cells through a LncRNA C5orf66-AS1/MicroRNA-127-3p/DUSP1/ERK Axis. Hum. Cell 2021, 34, 1812–1829. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Poggi, A.; Giuliani, M. Mesenchymal Stromal Cells Can Regulate the Immune Response in the Tumor Microenvironment. Vaccines 2016, 4, 41. [Google Scholar] [CrossRef]
- Li, G.-C.; Zhang, H.-W.; Zhao, Q.-C.; Sun, L.; Yang, J.-J.; Hong, L.; Feng, F.; Cai, L. Mesenchymal Stem Cells Promote Tumor Angiogenesis via the Action of Transforming Growth Factor Β1. Oncol. Lett. 2016, 11, 1089–1094. [Google Scholar] [CrossRef]
- Zhang, T.; Lee, Y.W.; Rui, Y.F.; Cheng, T.Y.; Jiang, X.H.; Li, G. Bone Marrow-Derived Mesenchymal Stem Cells Promote Growth and Angiogenesis of Breast and Prostate Tumors. Stem Cell Res. Ther. 2013, 4, 70. [Google Scholar] [CrossRef]
- Fregni, G.; Quinodoz, M.; Möller, E.; Vuille, J.; Galland, S.; Fusco, C.; Martin, P.; Letovanec, I.; Provero, P.; Rivolta, C.; et al. Reciprocal Modulation of Mesenchymal Stem Cells and Tumor Cells Promotes Lung Cancer Metastasis. eBioMedicine 2018, 29, 128–145. [Google Scholar] [CrossRef]
- Bergfeld, S.A.; Blavier, L.; DeClerck, Y.A. Bone Marrow–Derived Mesenchymal Stromal Cells Promote Survival and Drug Resistance in Tumor Cells. Mol. Cancer Ther. 2014, 13, 962–975. [Google Scholar] [CrossRef]
- Peinado, H.; Quintanilla, M.; Cano, A. Transforming Growth Factor β-1 Induces Snail Transcription Factor in Epithelial Cell Lines. J. Biol. Chem. 2003, 278, 21113–21123. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular Mechanisms of Epithelial–Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.Y.-J.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Tsai, J.H.; Yang, J. Epithelial–Mesenchymal Plasticity in Carcinoma Metastasis. Genes Dev. 2013, 27, 2192–2206. [Google Scholar] [CrossRef] [PubMed]
- Pawitan, J.A.; Bui, T.A.; Mubarok, W.; Antarianto, R.D.; Nurhayati, R.W.; Dilogo, I.H.; Oceandy, D. Enhancement of the Therapeutic Capacity of Mesenchymal Stem Cells by Genetic Modification: A Systematic Review. Front. Cell Dev. Biol. 2020, 8, 587776. [Google Scholar] [CrossRef] [PubMed]
- Beckermann, B.M.; Kallifatidis, G.; Groth, A.; Frommhold, D.; Apel, A.; Mattern, J.; Salnikov, A.V.; Moldenhauer, G.; Wagner, W.; Diehlmann, A.; et al. VEGF Expression by Mesenchymal Stem Cells Contributes to Angiogenesis in Pancreatic Carcinoma. Br. J. Cancer 2008, 99, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Du, W.J.; Chi, Y.; Yang, Z.X.; Li, Z.J.; Cui, J.J.; Song, B.Q.; Li, X.; Yang, S.G.; Han, Z.B.; Han, Z.C. Heterogeneity of Proangiogenic Features in Mesenchymal Stem Cells Derived from Bone Marrow, Adipose Tissue, Umbilical Cord, and Placenta. Stem Cell Res. Ther. 2016, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-H.; Chang, M.-C.; Tsai, K.-S.; Hung, M.-C.; Chen, H.-L.; Hung, S.-C. Mesenchymal Stem Cells Promote Growth and Angiogenesis of Tumors in Mice. Oncogene 2013, 32, 4343–4354. [Google Scholar] [CrossRef]
- Coffman, L.G.; Choi, Y.-J.; McLean, K.; Allen, B.L.; di Magliano, M.P.; Buckanovich, R.J. Human Carcinoma-Associated Mesenchymal Stem Cells Promote Ovarian Cancer Chemotherapy Resistance via a BMP4/HH Signaling Loop. Oncotarget 2016, 7, 6916–6932. [Google Scholar] [CrossRef]
- Jeon, E.S.; Heo, S.C.; Lee, I.H.; Choi, Y.J.; Park, J.H.; Choi, K.U.; Park, D.Y.; Suh, D.-S.; Yoon, M.-S.; Kim, J.H. Ovarian Cancer-Derived Lysophosphatidic Acid Stimulates Secretion of VEGF and Stromal Cell-Derived Factor-1α from Human Mesenchymal Stem Cells. Exp. Mol. Med. 2010, 42, 280–293. [Google Scholar] [CrossRef]
- Li, S.; Han, Y.; Lu, M.; Liu, Z.; Jin, J.; Guo, Q.; Wang, Y.; Liu, H. Mesenchymal Stem Cell-exosome-mediated Matrix Metalloproteinase 1 Participates in Oral Leukoplakia and Carcinogenesis by Inducing Angiogenesis. J. Oral Pathol. Med. 2022, 51, 638–648. [Google Scholar] [CrossRef]
- Hung, S.-C.; Pochampally, R.R.; Chen, S.-C.; Hsu, S.-C.; Prockop, D.J. Angiogenic Effects of Human Multipotent Stromal Cell Conditioned Medium Activate the PI3K-Akt Pathway in Hypoxic Endothelial Cells to Inhibit Apoptosis, Increase Survival, and Stimulate Angiogenesis. Stem Cells 2007, 25, 2363–2370. [Google Scholar] [CrossRef]
- Brogi, E.; Wu, T.; Namiki, A.; Isner, J.M. Indirect Angiogenic Cytokines Upregulate VEGF and BFGF Gene Expression in Vascular Smooth Muscle Cells, Whereas Hypoxia Upregulates VEGF Expression Only. Circulation 1994, 90, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.; Choy, M.; Alitalo, K.; Rafii, S. Vascular Endothelial Growth Factor (VEGF)–C Signaling through FLT-4 (VEGFR-3) Mediates Leukemic Cell Proliferation, Survival, and Resistance to Chemotherapy. Blood 2002, 99, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Konopleva, M.; Konoplev, S.; Hu, W.; Zaritskey, A.; Afanasiev, B.; Andreeff, M. Stromal Cells Prevent Apoptosis of AML Cells by Up-Regulation of Anti-Apoptotic Proteins. Leukemia 2002, 16, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-B.; Liu, Y.; Wang, G.-H.; Xu, X.; Cai, Y.; Wang, H.-Y.; Li, Y.-Q.; Meng, H.-F.; Dai, F.; Jin, J.-D. Mesenchymal Stem Cells Promote Colorectal Cancer Progression through AMPK/MTOR-Mediated NF-ΚB Activation. Sci. Rep. 2016, 6, 21420. [Google Scholar] [CrossRef]
- Burger, J.A.; Tsukada, N.; Burger, M.; Zvaifler, N.J.; Dell’Aquila, M.; Kipps, T.J. Blood-Derived Nurse-like Cells Protect Chronic Lymphocytic Leukemia B Cells from Spontaneous Apoptosis through Stromal Cell–Derived Factor-1. Blood 2000, 96, 2655–2663. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Weinberg, R.A. A Perspective on Cancer Cell Metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Yang, Y.; Bucan, V.; Baehre, H.; Von Der Ohe, J.; Otte, A.; Hass, R. Acquisition of New Tumor Cell Properties by MSC-Derived Exosomes. Int. J. Oncol. 2015, 47, 244–252. [Google Scholar] [CrossRef]
- Tu, B.; Zhu, J.; Liu, S.; Wang, L.; Fan, Q.; Hao, Y.; Fan, C.; Tang, T.-T. Mesenchymal Stem Cells Promote Osteosarcoma Cell Survival and Drug Resistance through Activation of STAT3. Oncotarget 2016, 7, 48296–48308. [Google Scholar] [CrossRef]
- Correa, D.; Somoza, R.A.; Lin, P.; Schiemann, W.P.; Caplan, A.I. Mesenchymal Stem Cells Regulate Melanoma Cancer Cells Extravasation to Bone and Liver at Their Perivascular Niche. Int. J. Cancer 2016, 138, 417–427. [Google Scholar] [CrossRef]
- Balch, C.M.; Houghton, A.N.; Sober, A.J.; Soong, S. Cutaneous Melanoma, 4th Edition. Dermatol. Surg. 2005, 31, 1715. [Google Scholar] [CrossRef]
- Kodet, O.; Kučera, J.; Strnadová, K.; Dvořánková, B.; Štork, J.; Lacina, L.; Smetana, K. Cutaneous Melanoma Dissemination Is Dependent on the Malignant Cell Properties and Factors of Intercellular Crosstalk in the Cancer Microenvironment (Review). Int. J. Oncol. 2020, 57, 619–630. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Gilkes, D.M.; Wong, C.C.L.; Kshitiz; Luo, W.; Zhang, H.; Wei, H.; Takano, N.; Schito, L.; Levchenko, A.; et al. Hypoxia-Inducible Factor–Dependent Breast Cancer–Mesenchymal Stem Cell Bidirectional Signaling Promotes Metastasis. J. Clin. Investig. 2013, 123, 189–205. [Google Scholar] [CrossRef]
- Kudo-Saito, C.; Fuwa, T.; Murakami, K.; Kawakami, Y. Targeting FSTL1 Prevents Tumor Bone Metastasis and Consequent Immune Dysfunction. Cancer Res. 2013, 73, 6185–6193. [Google Scholar] [CrossRef]
- McGuire, J.J.; Frieling, J.S.; Lo, C.H.; Li, T.; Muhammad, A.; Lawrence, H.R.; Lawrence, N.J.; Cook, L.M.; Lynch, C.C. Mesenchymal Stem Cell-Derived Interleukin-28 Drives the Selection of Apoptosis Resistant Bone Metastatic Prostate Cancer. Nat. Commun. 2021, 12, 723. [Google Scholar] [CrossRef]
- Rodini, C.O.; Gonçalves da Silva, P.B.; Assoni, A.F.; Carvalho, V.M.; Okamoto, O.K. Mesenchymal Stem Cells Enhance Tumorigenic Properties of Human Glioblastoma through Independent Cell-Cell Communication Mechanisms. Oncotarget 2018, 9, 24766–24777. [Google Scholar] [CrossRef]
- McAndrews, K.M.; McGrail, D.J.; Ravikumar, N.; Dawson, M.R. Mesenchymal Stem Cells Induce Directional Migration of Invasive Breast Cancer Cells through TGF-β. Sci. Rep. 2015, 5, 16941. [Google Scholar] [CrossRef]
- Lin, R.; Wang, S.; Zhao, R.C. Exosomes from Human Adipose-Derived Mesenchymal Stem Cells Promote Migration through Wnt Signaling Pathway in a Breast Cancer Cell Model. Mol. Cell. Biochem. 2013, 383, 13–20. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Q.; Huang, D.-B.; Sun, Q.-K.; Wu, S.-S.; Zhao, Y.-J.; Jia, W.; Hu, D.-S.; He, Y.-F. Tumor-Associated Mesenchymal Stem Cells Promote Hepatocellular Carcinoma Metastasis via a DNM3OS/KDM6B/TIAM1 Axis. Cancer Lett. 2021, 503, 19–31. [Google Scholar] [CrossRef]
- Martin, F.T.; Dwyer, R.M.; Kelly, J.; Khan, S.; Murphy, J.M.; Curran, C.; Miller, N.; Hennessy, E.; Dockery, P.; Barry, F.P.; et al. Potential Role of Mesenchymal Stem Cells (MSCs) in the Breast Tumour Microenvironment: Stimulation of Epithelial to Mesenchymal Transition (EMT). Breast Cancer Res. Treat. 2010, 124, 317–326. [Google Scholar] [CrossRef]
- Xue, Z.; Wu, X.; Chen, X.; Liu, Y.; Wang, X.; Wu, K.; Nie, Y.; Fan, D. Mesenchymal Stem Cells Promote Epithelial to Mesenchymal Transition and Metastasis in Gastric Cancer Though Paracrine Cues and Close Physical Contact: MSCs Promote EMT. J. Cell. Biochem. 2015, 116, 618–627. [Google Scholar] [CrossRef]
- Bates, R.C.; Mercurio, A.M. Tumor Necrosis Factor-Alpha Stimulates the Epithelial-to-Mesenchymal Transition of Human Colonic Organoids. Mol. Biol. Cell 2003, 14, 1790–1800. [Google Scholar] [CrossRef]
- De Wever, O.; Demetter, P.; Mareel, M.; Bracke, M. Stromal Myofibroblasts Are Drivers of Invasive Cancer Growth. Int. J. Cancer 2008, 123, 2229–2238. [Google Scholar] [CrossRef]
- Xu, M.-H.; Gao, X.; Luo, D.; Zhou, X.-D.; Xiong, W.; Liu, G.-X. EMT and Acquisition of Stem Cell-like Properties Are Involved in Spontaneous Formation of Tumorigenic Hybrids between Lung Cancer and Bone Marrow-Derived Mesenchymal Stem Cells. PLoS ONE 2014, 9, e87893. [Google Scholar] [CrossRef]
- Fan, X.-L.; Zhang, Y.; Li, X.; Fu, Q.-L. Mechanisms Underlying the Protective Effects of Mesenchymal Stem Cell-Based Therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef]
- Szewc, M.; Radzikowska-Bűchner, E.; Wdowiak, P.; Kozak, J.; Kuszta, P.; Niezabitowska, E.; Matysiak, J.; Kubiński, K.; Masłyk, M. MSCs as Tumor-Specific Vectors for the Delivery of Anticancer Agents—A Potential Therapeutic Strategy in Cancer Diseases: Perspectives for Quinazoline Derivatives. Int. J. Mol. Sci. 2022, 23, 2745. [Google Scholar] [CrossRef]
- Li, Z.; Fan, D.; Xiong, D. Mesenchymal Stem Cells as Delivery Vectors for Anti-Tumor Therapy. Stem Cell Investig. 2015, 2, 6. [Google Scholar] [CrossRef]
- Mirzaei, H.; Sahebkar, A.; Avan, A.; Jaafari, M.R.; Salehi, R.; Salehi, H.; Baharvand, H.; Rezaei, A.; Hadjati, J.; Pawelek, J.M.; et al. Application of Mesenchymal Stem Cells in Melanoma: A Potential Therapeutic Strategy for Delivery of Targeted Agents. Curr. Med. Chem. 2016, 23, 455–463. [Google Scholar] [CrossRef]
- Zhang, T.-Y.; Huang, B.; Yuan, Z.-Y.; Hu, Y.-L.; Tabata, Y.; Gao, J.-Q. Gene Recombinant Bone Marrow Mesenchymal Stem Cells as a Tumor-Targeted Suicide Gene Delivery Vehicle in Pulmonary Metastasis Therapy Using Non-Viral Transfection. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 257–267. [Google Scholar] [CrossRef]
- Katz, O.B.; Shaked, Y. Host Effects Contributing to Cancer Therapy Resistance. Drug Resist. Updates 2015, 19, 33–42. [Google Scholar] [CrossRef]
- Meads, M.B.; Gatenby, R.A.; Dalton, W.S. Environment-Mediated Drug Resistance: A Major Contributor to Minimal Residual Disease. Nat. Rev. Cancer 2009, 9, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Houthuijzen, J.M.; Daenen, L.G.M.; Roodhart, J.M.L.; Voest, E.E. The Role of Mesenchymal Stem Cells in Anti-Cancer Drug Resistance and Tumour Progression. Br. J. Cancer 2012, 106, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Borst, P. Cancer Drug Pan-Resistance: Pumps, Cancer Stem Cells, Quiescence, Epithelial to Mesenchymal Transition, Blocked Cell Death Pathways, Persisters or What? Open Biol. 2012, 2, 120066. [Google Scholar] [CrossRef] [PubMed]
- Rizzolio, S.; Giordano, S.; Corso, S. The Importance of Being CAFs (in Cancer Resistance to Targeted Therapies). J. Exp. Clin. Cancer Res. 2022, 41, 319. [Google Scholar] [CrossRef]
- Lis, R.; Touboul, C.; Mirshahi, P.; Ali, F.; Mathew, S.; Nolan, D.J.; Maleki, M.; Abdalla, S.A.; Raynaud, C.M.; Querleu, D.; et al. Tumor Associated Mesenchymal Stem Cells Protects Ovarian Cancer Cells from Hyperthermia through CXCL12. Int. J. Cancer 2011, 128, 715–725. [Google Scholar] [CrossRef]
- Dreuw, A.; Hermanns, H.M.; Heise, R.; Joussen, S.; Rodríguez, F.; Marquardt, Y.; Jugert, F.; Merk, H.F.; Heinrich, P.C.; Baron, J.M. Interleukin-6-Type Cytokines Upregulate Expression of Multidrug Resistance-Associated Proteins in NHEK and Dermal Fibroblasts. J. Investig. Dermatol. 2005, 124, 28–37. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Hemann, M.T. DNA Damage-Mediated Induction of a Chemoresistant Niche. Cell 2010, 143, 355–366. [Google Scholar] [CrossRef]
- Roodhart, J.M.L.; Daenen, L.G.M.; Stigter, E.C.A.; Prins, H.-J.; Gerrits, J.; Houthuijzen, J.M.; Gerritsen, M.G.; Schipper, H.S.; Backer, M.J.G.; van Amersfoort, M.; et al. Mesenchymal Stem Cells Induce Resistance to Chemotherapy through the Release of Platinum-Induced Fatty Acids. Cancer Cell 2011, 20, 370–383. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ Attenuates Tumour Response to PD-L1 Blockade by Contributing to Exclusion of T Cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and Drug Resistance: The Mechanistic Link and Clinical Implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.-J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Lam, E.W.-F.; Soeiro, I.; Tisato, V.; Bonnet, D.; Dazzi, F. Mesenchymal Stem Cells Inhibit Proliferation and Apoptosis of Tumor Cells: Impact on In Vivo Tumor Growth. Leukemia 2007, 21, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Melzer, C.; von der Ohe, J.; Lehnert, H.; Ungefroren, H.; Hass, R. Cancer Stem Cell Niche Models and Contribution by Mesenchymal Stroma/Stem Cells. Mol. Cancer 2017, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-J.; Reinhardt, F.; Herschman, H.R.; Weinberg, R.A. Cancer-Stimulated Mesenchymal Stem Cells Create a Carcinoma Stem Cell Niche via Prostaglandin E2 Signaling. Cancer Discov. 2012, 2, 840–855. [Google Scholar] [CrossRef] [PubMed]
- Skolekova, S.; Matuskova, M.; Bohac, M.; Toro, L.; Durinikova, E.; Tyciakova, S.; Demkova, L.; Gursky, J.; Kucerova, L. Cisplatin-Induced Mesenchymal Stromal Cells-Mediated Mechanism Contributing to Decreased Antitumor Effect in Breast Cancer Cells. Cell Commun. Signal. 2016, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Kabashima-Niibe, A.; Higuchi, H.; Takaishi, H.; Masugi, Y.; Matsuzaki, Y.; Mabuchi, Y.; Funakoshi, S.; Adachi, M.; Hamamoto, Y.; Kawachi, S.; et al. Mesenchymal Stem Cells Regulate Epithelial-Mesenchymal Transition and Tumor Progression of Pancreatic Cancer Cells. Cancer Sci. 2013, 104, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Peitzsch, C.; Kurth, I.; Kunz-Schughart, L.; Baumann, M.; Dubrovska, A. Discovery of the Cancer Stem Cell Related Determinants of Radioresistance. Radiother. Oncol. 2013, 108, 378–387. [Google Scholar] [CrossRef]
- Kreso, A.; Dick, J.E. Evolution of the Cancer Stem Cell Model. Cell Stem Cell 2014, 14, 275–291. [Google Scholar] [CrossRef]
- Meacham, C.E.; Morrison, S.J. Tumor Heterogeneity and Cancer Cell Plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef]
- Phillips, T.M.; McBride, W.H.; Pajonk, F. The Response of CD24−/Low /CD44 + Breast Cancer–Initiating Cells to Radiation. J. Natl. Cancer Inst. 2006, 98, 1777–1785. [Google Scholar] [CrossRef]
- Woodward, W.A.; Chen, M.S.; Behbod, F.; Alfaro, M.P.; Buchholz, T.A.; Rosen, J.M. WNT/β-Catenin Mediates Radiation Resistance of Mouse Mammary Progenitor Cells. Proc. Natl. Acad. Sci. USA 2007, 104, 618–623. [Google Scholar] [CrossRef]
- Wagner, V.P.; Martins, M.A.T.; Martins, M.D.; Warner, K.A.; Webber, L.P.; Squarize, C.H.; Nör, J.E.; Castilho, R.M. Overcoming Adaptive Resistance in Mucoepidermoid Carcinoma through Inhibition of the IKK-β/IκBα/NFκB Axis. Oncotarget 2016, 7, 73032–73044. [Google Scholar] [CrossRef]
- Bighetti-Trevisan, R.L.; Sousa, L.O.; Castilho, R.M.; Almeida, L.O. Cancer Stem Cells: Powerful Targets to Improve Current Anticancer Therapeutics. Stem Cells Int. 2019, 2019, 9618065. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jeong, E.K.; Ju, M.K.; Jeon, H.M.; Kim, M.Y.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Induction of Metastasis, Cancer Stem Cell Phenotype, and Oncogenic Metabolism in Cancer Cells by Ionizing Radiation. Mol. Cancer 2017, 16, 10. [Google Scholar] [CrossRef]
- Moncharmont, C.; Levy, A.; Guy, J.-B.; Falk, A.T.; Guilbert, M.; Trone, J.-C.; Alphonse, G.; Gilormini, M.; Ardail, D.; Toillon, R.-A.; et al. Radiation-Enhanced Cell Migration/Invasion Process: A Review. Crit. Rev. Oncol./Hematol. 2014, 92, 133–142. [Google Scholar] [CrossRef]
- Pei, J.; Park, I.-H.; Ryu, H.-H.; Li, S.-Y.; Li, C.-H.; Lim, S.-H.; Wen, M.; Jang, W.-Y.; Jung, S. Sublethal Dose of Irradiation Enhances Invasion of Malignant Glioma Cells through P53-MMP 2 Pathway in U87MG Mouse Brain Tumor Model. Radiat. Oncol. 2015, 10, 164. [Google Scholar] [CrossRef]
- Nowakowski, A.; Drela, K.; Rozycka, J.; Janowski, M.; Lukomska, B. Engineered Mesenchymal Stem Cells as an Anti-Cancer Trojan Horse. Stem Cells Dev. 2016, 25, 1513–1531. [Google Scholar] [CrossRef]
- Hagenhoff, A.; Bruns, C.J.; Zhao, Y.; von Lüttichau, I.; Niess, H.; Spitzweg, C.; Nelson, P.J. Harnessing Mesenchymal Stem Cell Homing as an Anticancer Therapy. Expert Opin. Biol. Ther. 2016, 16, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-H.; Zhou, Y.; Tabata, Y.; Gao, J.-Q. Mesenchymal Stem Cell-Based Drug Delivery Strategy: From Cells to Biomimetic. J. Control. Release 2019, 294, 102–113. [Google Scholar] [CrossRef]
- Studeny, M.; Marini, F.C.; Champlin, R.E.; Zompetta, C.; Fidler, I.J.; Andreeff, M. Bone Marrow-Derived Mesenchymal Stem Cells as Vehicles for Interferon-β Delivery into Tumors1. Cancer Res. 2002, 62, 3603–3608. [Google Scholar]
- Ding, Y.; Wang, C.; Sun, Z.; Wu, Y.; You, W.; Mao, Z.; Wang, W. Mesenchymal Stem Cells Engineered by Nonviral Vectors: A Powerful Tool in Cancer Gene Therapy. Pharmaceutics 2021, 13, 913. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cheng, M.; Yang, Z.; Zeng, C.-Y.; Chen, J.; Xie, Y.; Luo, S.-W.; Zhang, K.-H.; Zhou, S.-F.; Lu, N.-H. Mesenchymal Stem Cell-Based NK4 Gene Therapy in Nude Mice Bearing Gastric Cancer Xenografts. Drug Des. Dev. Ther. 2014, 8, 2449–2462. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Ding, Q.; Wu, Z.; Jiang, H.; Fang, Z. Therapeutic Potential of Human Mesenchymal Stem Cells Producing IL-12 in a Mouse Xenograft Model of Renal Cell Carcinoma. Cancer Lett. 2010, 290, 157–166. [Google Scholar] [CrossRef]
- Seo, S.H.; Kim, K.S.; Park, S.H.; Suh, Y.S.; Kim, S.J.; Jeun, S.-S.; Sung, Y.C. The Effects of Mesenchymal Stem Cells Injected via Different Routes on Modified IL-12-Mediated Antitumor Activity. Gene Ther. 2011, 18, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Mirlekar, B.; Pylayeva-Gupta, Y. IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers 2021, 13, 167. [Google Scholar] [CrossRef]
- Guiho, R.; Biteau, K.; Grisendi, G.; Taurelle, J.; Chatelais, M.; Gantier, M.; Heymann, D.; Dominici, M.; Redini, F. TRAIL Delivered by Mesenchymal Stromal/Stem Cells Counteracts Tumor Development in Orthotopic Ewing Sarcoma Models. Int. J. Cancer 2016, 139, 2802–2811. [Google Scholar] [CrossRef] [PubMed]
- Rossignoli, F.; Spano, C.; Grisendi, G.; Foppiani, E.M.; Golinelli, G.; Mastrolia, I.; Bestagno, M.; Candini, O.; Petrachi, T.; Recchia, A.; et al. MSC-Delivered Soluble TRAIL and Paclitaxel as Novel Combinatory Treatment for Pancreatic Adenocarcinoma. Theranostics 2019, 9, 436–448. [Google Scholar] [CrossRef]
- Tamura, R.; Miyoshi, H.; Yoshida, K.; Okano, H.; Toda, M. Recent Progress in the Research of Suicide Gene Therapy for Malignant Glioma. Neurosurg. Rev. 2021, 44, 29–49. [Google Scholar] [CrossRef]
- Zhang, T.-Y.; Huang, B.; Wu, H.-B.; Wu, J.-H.; Li, L.-M.; Li, Y.-X.; Hu, Y.-L.; Han, M.; Shen, Y.-Q.; Tabata, Y.; et al. Synergistic Effects of Co-Administration of Suicide Gene Expressing Mesenchymal Stem Cells and Prodrug-Encapsulated Liposome on Aggressive Lung Melanoma Metastases in Mice. J. Control. Release 2015, 209, 260–271. [Google Scholar] [CrossRef]
- Cavarretta, I.T.; Altanerova, V.; Matuskova, M.; Kucerova, L.; Culig, Z.; Altaner, C. Adipose Tissue–Derived Mesenchymal Stem Cells Expressing Prodrug-Converting Enzyme Inhibit Human Prostate Tumor Growth. Mol. Ther. 2010, 18, 223–231. [Google Scholar] [CrossRef]
- Kucerova, L.; Altanerova, V.; Matuskova, M.; Tyciakova, S.; Altaner, C. Adipose Tissue–Derived Human Mesenchymal Stem Cells Mediated Prodrug Cancer Gene Therapy. Cancer Res. 2007, 67, 6304–6313. [Google Scholar] [CrossRef]
- Segaliny, A.I.; Cheng, J.L.; Farhoodi, H.P.; Toledano, M.; Yu, C.C.; Tierra, B.; Hildebrand, L.; Liu, L.; Liao, M.J.; Cho, J.; et al. Combinatorial Targeting of Cancer Bone Metastasis Using MRNA Engineered Stem Cells. EBioMedicine 2019, 45, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Parato, K.A.; Senger, D.; Forsyth, P.A.J.; Bell, J.C. Recent Progress in the Battle between Oncolytic Viruses and Tumours. Nat. Rev. Cancer 2005, 5, 965–976. [Google Scholar] [CrossRef]
- Liang, M. Oncorine, the World First Oncolytic Virus Medicine and Its Update in China. Curr. Cancer Drug Targets 2018, 18, 171–176. [Google Scholar] [CrossRef]
- Mondal, M.; Guo, J.; He, P.; Zhou, D. Recent Advances of Oncolytic Virus in Cancer Therapy. Hum. Vaccines Immunother. 2020, 16, 2389–2402. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; He, X.; Sun, Q.; Chen, S.; Wan, K.; Xu, X.; Feng, X.; Li, P.; Chen, B.; Xiong, M. The Oncolytic Virus in Cancer Diagnosis and Treatment. Front. Oncol. 2020, 10, 1786. [Google Scholar] [CrossRef] [PubMed]
- Goradel, N.H.; Baker, A.T.; Arashkia, A.; Ebrahimi, N.; Ghorghanlu, S.; Negahdari, B. Oncolytic Virotherapy: Challenges and Solutions. Curr. Probl. Cancer 2021, 45, 100639. [Google Scholar] [CrossRef] [PubMed]
- Maroun, J.; Muñoz-Alía, M.; Ammayappan, A.; Schulze, A.; Peng, K.-W.; Russell, S. Designing and Building Oncolytic Viruses. Future Virol. 2017, 12, 193–213. [Google Scholar] [CrossRef]
- Russell, S.J.; Peng, K.-W.; Bell, J.C. Oncolytic Virotherapy. Nat. Biotechnol. 2012, 30, 658–670. [Google Scholar] [CrossRef]
- Keshavarz, M.; Mohammad Miri, S.; Behboudi, E.; Arjeini, Y.; Dianat-Moghadam, H.; Ghaemi, A. Oncolytic Virus Delivery Modulated Immune Responses toward Cancer Therapy: Challenges and Perspectives. Int. Immunopharmacol. 2022, 108, 108882. [Google Scholar] [CrossRef]
- Pidelaserra-Martí, G.; Engeland, C.E. Mechanisms of Measles Virus Oncolytic Immunotherapy. Cytokine Growth Factor Rev. 2020, 56, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Darestani, N.; Gilmanova, A.I.; Al-Gazally, M.E.; Zekiy, A.O.; Ansari, M.J.; Zabibah, R.S.; Jawad, M.A.; Al-Shalah, S.A.J.; Rizaev, J.A.; Alnassar, Y.S.; et al. Mesenchymal Stem Cell-Released Oncolytic Virus: An Innovative Strategy for Cancer Treatment. Cell Commun. Signal. 2023, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, H.; Liang, J.; Li, K.; Zhu, W.; Fu, L.; Wang, F.; Zheng, X.; Shi, H.; Wu, S.; et al. Identification and Characterization of Alphavirus M1 as a Selective Oncolytic Virus Targeting ZAP-Defective Human Cancers. Proc. Natl. Acad. Sci. USA 2014, 111, E4504–E4512. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic Viruses: A New Class of Immunotherapy Drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef]
- Toth, K.; Dhar, D.; Wold, W.S. Oncolytic (Replication-Competent) Adenoviruses as Anticancer Agents. Expert Opin. Biol. Ther. 2010, 10, 353–368. [Google Scholar] [CrossRef]
- Kwon, O.-J.; Kang, E.; Choi, J.-W.; Kim, S.W.; Yun, C.-O. Therapeutic Targeting of Chitosan–PEG–Folate-Complexed Oncolytic Adenovirus for Active and Systemic Cancer Gene Therapy. J. Control. Release 2013, 169, 257–265. [Google Scholar] [CrossRef]
- Alonso-Padilla, J.; Papp, T.; Kaján, G.L.; Benkő, M.; Havenga, M.; Lemckert, A.; Harrach, B.; Baker, A.H. Development of Novel Adenoviral Vectors to Overcome Challenges Observed with HAdV-5–Based Constructs. Mol. Ther. 2016, 24, 6–16. [Google Scholar] [CrossRef]
- Hendrickx, R.; Stichling, N.; Koelen, J.; Kuryk, L.; Lipiec, A.; Greber, U.F. Innate Immunity to Adenovirus. Hum. Gene Ther. 2014, 25, 265–284. [Google Scholar] [CrossRef]
- Taipale, K.; Liikanen, I.; Juhila, J.; Turkki, R.; Tähtinen, S.; Kankainen, M.; Vassilev, L.; Ristimäki, A.; Koski, A.; Kanerva, A.; et al. Chronic Activation of Innate Immunity Correlates with Poor Prognosis in Cancer Patients Treated with Oncolytic Adenovirus. Mol. Ther. 2016, 24, 175–183. [Google Scholar] [CrossRef]
- Msaouel, P.; Opyrchal, M.; Dispenzieri, A.; Peng, K.W.; Federspiel, M.J.; Russell, S.J.; Galanis, E. Clinical Trials with Oncolytic Measles Virus: Current Status and Future Prospects. Curr. Cancer Drug Targets 2018, 18, 177–187. [Google Scholar] [CrossRef]
- Kazimirsky, G.; Jiang, W.; Slavin, S.; Ziv-Av, A.; Brodie, C. Mesenchymal Stem Cells Enhance the Oncolytic Effect of Newcastle Disease Virus in Glioma Cells and Glioma Stem Cells via the Secretion of TRAIL. Stem Cell Res. Ther. 2016, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Mader, E.K.; Maeyama, Y.; Lin, Y.; Butler, G.W.; Russell, H.M.; Galanis, E.; Russell, S.J.; Dietz, A.B.; Peng, K.-W. Mesenchymal Stem Cell Carriers Protect Oncolytic Measles Viruses from Antibody Neutralization in an Orthotopic Ovarian Cancer Therapy Model. Clin. Cancer Res. 2009, 15, 7246–7255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, H.; Chen, C.; Liu, H.; He, Y.; Zhao, J.; Yang, P.; Mao, Q.; Xia, H. Systemic Administration of Mesenchymal Stem Cells Loaded with a Novel Oncolytic Adenovirus Carrying IL-24/Endostatin Enhances Glioma Therapy. Cancer Lett. 2021, 509, 26–38. [Google Scholar] [CrossRef]
- Ahmed, A.U.; Ulasov, I.V.; Mercer, R.W.; Lesniak, M.S. Maintaining and Loading Neural Stem Cells for Delivery of Oncolytic Adenovirus to Brain Tumors. In Oncolytic Viruses; Kirn, D.H., Liu, T.-C., Thorne, S.H., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 797, pp. 97–109. ISBN 978-1-61779-339-4. [Google Scholar]
- Tyler, M.A.; Ulasov, I.V.; Sonabend, A.M.; Nandi, S.; Han, Y.; Marler, S.; Roth, J.; Lesniak, M.S. Neural Stem Cells Target Intracranial Glioma to Deliver an Oncolytic Adenovirus In Vivo. Gene Ther. 2009, 16, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Jia, X.; Liu, X.; Hu, L.; Lin, K.; Xiao, T.; Qiao, Y.; Zhang, J.; Dan, J.; Wong, C.; et al. Identification of the Receptor of Oncolytic Virus M1 as a Therapeutic Predictor for Multiple Solid Tumors. Signal Transduct. Target. Ther. 2022, 7, 100. [Google Scholar] [CrossRef]
- Xiao, J.; Zeng, L.; Ding, S.; Chen, Y.; Zhang, X.; Bian, X.; Tian, G. Tumor-Tropic Adipose-Derived Mesenchymal Stromal Cell Mediated Bi2Se3 Nano-Radiosensitizers Delivery for Targeted Radiotherapy of Non-Small Cell Lung Cancer. Adv. Healthc. Mater. 2022, 11, 2200143. [Google Scholar] [CrossRef] [PubMed]
- Cihan, Y.B. Nanoparticle-Based Radiosensitizers in Radiotherapy Applications. Cancer Biother. Radiopharm. 2021, 36, 305–306. [Google Scholar] [CrossRef]
- Choi, J.; Kim, G.; Cho, S.B.; Im, H.-J. Radiosensitizing High-Z Metal Nanoparticles for Enhanced Radiotherapy of Glioblastoma multiforme. J. Nanobiotechnol. 2020, 18, 122. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Fu, S.; Wu, J. Gold Nanoparticles as Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2020, 15, 9407–9430. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, P.; Ma, J.; Li, D.; Yang, H.; Chen, W.; Jiang, Y. Enhancement of Radiosensitization by Silver Nanoparticles Functionalized with Polyethylene Glycol and Aptamer As1411 for Glioma Irradiation Therapy. Int. J. Nanomed. 2019, 14, 9483–9496. [Google Scholar] [CrossRef]
- Yao, Y.; Li, P.; He, J.; Wang, D.; Hu, J.; Yang, X. Albumin-Templated Bi2Se3-MnO2 Nanocomposites with Promoted Catalase-like Activity for Enhanced Radiotherapy of Cancer. ACS Appl. Mater. Interfaces 2021, 13, 28650–28661. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Gu, Z.; Yan, L.; Yong, Y.; Yi, X.; Zhang, X.; Liu, J.; Wu, R.; Ge, C.; Chen, C.; et al. Poly(Vinylpyrollidone)- and Selenocysteine-Modified Bi 2 Se 3 Nanoparticles Enhance Radiotherapy Efficacy in Tumors and Promote Radioprotection in Normal Tissues. Adv. Mater. 2017, 29, 1701268. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Kiessling, F.; Ashford, M.; Hennink, W.; Crommelin, D.; Storm, G. Cancer Nanomedicine: Is Targeting Our Target? Nat. Rev. Mater. 2016, 1, 16069. [Google Scholar] [CrossRef]
- Wolfram, J.; Ferrari, M. Clinical Cancer Nanomedicine. Nano Today 2019, 25, 85–98. [Google Scholar] [CrossRef]
- Huang, R.-Y.; Lin, Y.-H.; Lin, S.-Y.; Li, Y.-N.; Chiang, C.-S.; Chang, C.-W. Magnetic Ternary Nanohybrids for Nonviral Gene Delivery of Stem Cells and Applications on Cancer Therapy. Theranostics 2019, 9, 2411–2423. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Song, X.; Zhao, Z.; Barber, B.; Farr, A.M.; Ivanov, B.; Novich, M. Overall Survival in Patients with Metastatic Melanoma. Curr. Med. Res. Opin. 2015, 31, 987–991. [Google Scholar] [CrossRef]
- Pollack, L.A.; Li, J.; Berkowitz, Z.; Weir, H.K.; Wu, X.-C.; Ajani, U.A.; Ekwueme, D.U.; Li, C.; Pollack, B.P. Melanoma Survival in the United States, 1992 to 2005. J. Am. Acad. Dermatol. 2011, 65, S78.e1–S78.e10. [Google Scholar] [CrossRef] [PubMed]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef]
- Zhang, G.; Frederick, D.T.; Wu, L.; Wei, Z.; Krepler, C.; Srinivasan, S.; Chae, Y.C.; Xu, X.; Choi, H.; Dimwamwa, E.; et al. Targeting Mitochondrial Biogenesis to Overcome Drug Resistance to MAPK Inhibitors. J. Clin. Investig. 2016, 126, 1834–1856. [Google Scholar] [CrossRef]
- Tyciakova, S.; Matuskova, M.; Bohovic, R.; Polakova, K.; Toro, L.; Skolekova, S.; Kucerova, L. Genetically Engineered Mesenchymal Stromal Cells Producing TNFα Have Tumour Suppressing Effect on Human Melanoma Xenograft: AT-MSC/HTNFα in Treatment of A375 Xenografts. J. Gene Med. 2015, 17, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Kumar, S.; Chanda, D.; Chen, J.; Mountz, J.D.; Ponnazhagan, S. Therapeutic Potential of Mesenchymal Stem Cells Producing Interferon-α in a Mouse Melanoma Lung Metastasis Model. Stem Cells 2008, 26, 2332–2338. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.-W.; Lee, H.-W.; Oh, Y.-I.; Ahn, J.-O.; Koh, Y.-R.; Oh, S.-H.; Kang, S.-K.; Youn, H.-Y. Anti-Tumor Effects of Canine Adipose Tissue-Derived Mesenchymal Stromal Cell-Based Interferon-β Gene Therapy and Cisplatin in a Mouse Melanoma Model. Cytotherapy 2011, 13, 944–955. [Google Scholar] [CrossRef]
- Wong, V.L.Y.; Rieman, D.J.; Aronson, L.; Dalton, B.J.; Greig, R.; Anzano, M.A. Growth-Inhibitory Activity of Interferon-Beta against Human Colorectal Carcinoma Cell Lines. Int. J. Cancer 1989, 43, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Audsley, K.M.; Wagner, T.; Ta, C.; Newnes, H.V.; Buzzai, A.C.; Barnes, S.A.; Wylie, B.; Armitage, J.; Kaisho, T.; Bosco, A.; et al. IFNβ Is a Potent Adjuvant for Cancer Vaccination Strategies. Front. Immunol. 2021, 12, 735133. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Fu, M.L.; Weichselbaum, R.R.; Gajewski, T.F.; Guo, Y.; Fu, Y.-X. Targeting the Tumor Microenvironment with Interferon-β Bridges Innate and Adaptive Immune Responses. Cancer Cell 2014, 25, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-M.; Hsieh, Y.-Y.; Du, J.-L.; Yen, S.-C.; Hung, C.-F. Sequential Interferon β-Cisplatin Treatment Enhances the Surface Exposure of Calreticulin in Cancer Cells via an Interferon Regulatory Factor 1-Dependent Manner. Biomolecules 2020, 10, 643. [Google Scholar] [CrossRef]
- Ahn, J.-O.; Coh, Y.-R.; Lee, H.-W.; Shin, I.-S.; Kang, S.-K.; Youn, H.-Y. Human Adipose Tissue-Derived Mesenchymal Stem Cells Inhibit Melanoma Growth In Vitro and In Vivo. Anticancer Res. 2015, 35, 159–168. [Google Scholar]
- Petrov, V.N.; Isaeva, E.V.; Ulyanenko, S.E.; Beketov, E.E.; Yatsenko, E.M.; Sayapina, E.V.; Lepekhina, L.A.; Nasedkina, N.V.; Grivtsova, L.Y.; Kaprin, A.D. In Vivo Effects of Human Bone Marrow Mesenchymal Stromal Cells on the Development of Experimental B16 Melanoma in Mice. Bull. Exp. Biol. Med. 2020, 168, 561–565. [Google Scholar] [CrossRef]
- Çakır, U.; Hajdara, A.; Széky, B.; Mayer, B.; Kárpáti, S.; Mezey, É.; Silló, P.; Szakács, G.; Füredi, A.; Pós, Z.; et al. Mesenchymal-Stromal Cell-like Melanoma-Associated Fibroblasts Increase IL-10 Production by Macrophages in a Cyclooxygenase/Indoleamine 2,3-Dioxygenase-Dependent Manner. Cancers 2021, 13, 6173. [Google Scholar] [CrossRef]
- Kucerova, L.; Matuskova, M.; Pastorakova, A.; Tyciakova, S.; Jakubikova, J.; Bohovic, R.; Altanerova, V.; Altaner, C. Cytosine Deaminase Expressing Human Mesenchymal Stem Cells Mediated Tumour Regression in Melanoma Bearing Mice. J. Gene Med. 2008, 10, 1071–1082. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Sun, R.; Origuchi, M.; Kanehira, M.; Takahata, T.; Itoh, J.; Umezawa, A.; Kijima, H.; Fukuda, S.; Saijo, Y. Mesenchymal Stromal Cells Promote Tumor Growth through the Enhancement of Neovascularization. Mol. Med. 2011, 17, 579–587. [Google Scholar] [CrossRef]
- Gu, J.J.; Hoj, J.; Rouse, C.; Pendergast, A.M. Mesenchymal Stem Cells Promote Metastasis through Activation of an ABL-MMP9 Signaling Axis in Lung Cancer Cells. PLoS ONE 2020, 15, e0241423. [Google Scholar] [CrossRef]
- Kolluri, K.K.; Laurent, G.J.; Janes, S.M. Mesenchymal Stem Cells as Vectors for Lung Cancer Therapy. Respiration 2013, 85, 443–451. [Google Scholar] [CrossRef]
- Loebinger, M.R.; Kyrtatos, P.G.; Turmaine, M.; Price, A.N.; Pankhurst, Q.; Lythgoe, M.F.; Janes, S.M. Magnetic Resonance Imaging of Mesenchymal Stem Cells Homing to Pulmonary Metastases Using Biocompatible Magnetic Nanoparticles. Cancer Res. 2009, 69, 8862–8867. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Cargioli, T.G.; Machluf, M.; Yang, W.; Sun, Y.; Al-Hashem, R.; Kim, S.U.; Black, P.M.; Carroll, R.S. PEX-Producing Human Neural Stem Cells Inhibit Tumor Growth in a Mouse Glioma Model. Clin. Cancer Res. 2005, 11, 5965–5970. [Google Scholar] [CrossRef] [PubMed]
- Loebinger, M.R.; Eddaoudi, A.; Davies, D.; Janes, S.M. Mesenchymal Stem Cell Delivery of TRAIL Can Eliminate Metastatic Cancer. Cancer Res. 2009, 69, 4134–4142. [Google Scholar] [CrossRef]
- Mueller, L.P.; Luetzkendorf, J.; Widder, M.; Nerger, K.; Caysa, H.; Mueller, T. TRAIL-Transduced Multipotent Mesenchymal Stromal Cells (TRAIL-MSC) Overcome TRAIL Resistance in Selected CRC Cell Lines In Vitro and In Vivo. Cancer Gene Ther. 2011, 18, 229–239. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, X.; Qian, M.; Song, Y.; Wu, D.; Zhang, W. Knockdown of TGF-Β1 Expression in Human Umbilical Cord Mesenchymal Stem Cells Reverts Their Exosome-Mediated EMT Promoting Effect on Lung Cancer Cells. Cancer Lett. 2018, 428, 34–44. [Google Scholar] [CrossRef]
- Bechet, D.; Auger, F.; Couleaud, P.; Marty, E.; Ravasi, L.; Durieux, N.; Bonnet, C.; Plénat, F.; Frochot, C.; Mordon, S.; et al. Multifunctional Ultrasmall Nanoplatforms for Vascular-Targeted Interstitial Photodynamic Therapy of Brain Tumors Guided by Real-Time MRI. Nanomed.: Nanotechnol. Biol. Med. 2015, 11, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Li, X.; Liu, J.; Sun, Y.; Wang, Z.; Jiang, Y. Maximized Nanodrug-Loaded Mesenchymal Stem Cells by a Dual Drug-Loaded Mode for the Systemic Treatment of Metastatic Lung Cancer. Drug Deliv. 2017, 24, 1372–1383. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, T.; Särkioja, M.; Lehenkari, P.; Miettinen, S.; Ylikomi, T.; Suuronen, R.; Desmond, R.A.; Kanerva, A.; Hemminki, A. Human Mesenchymal Stem Cells Lack Tumor Tropism but Enhance the Antitumor Activity of Oncolytic Adenoviruses in Orthotopic Lung and Breast Tumors. Hum. Gene Ther. 2007, 18, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Hoyos, V.; Yagyu, S.; Tao, W.; Ramos, C.A.; Dotti, G.; Brenner, M.K.; Bouchier-Hayes, L. Bortezomib Sensitizes Non-Small Cell Lung Cancer to Mesenchymal Stromal Cell-Delivered Inducible Caspase-9-Mediated Cytotoxicity. Cancer Gene Ther. 2014, 21, 472–482. [Google Scholar] [CrossRef] [PubMed]
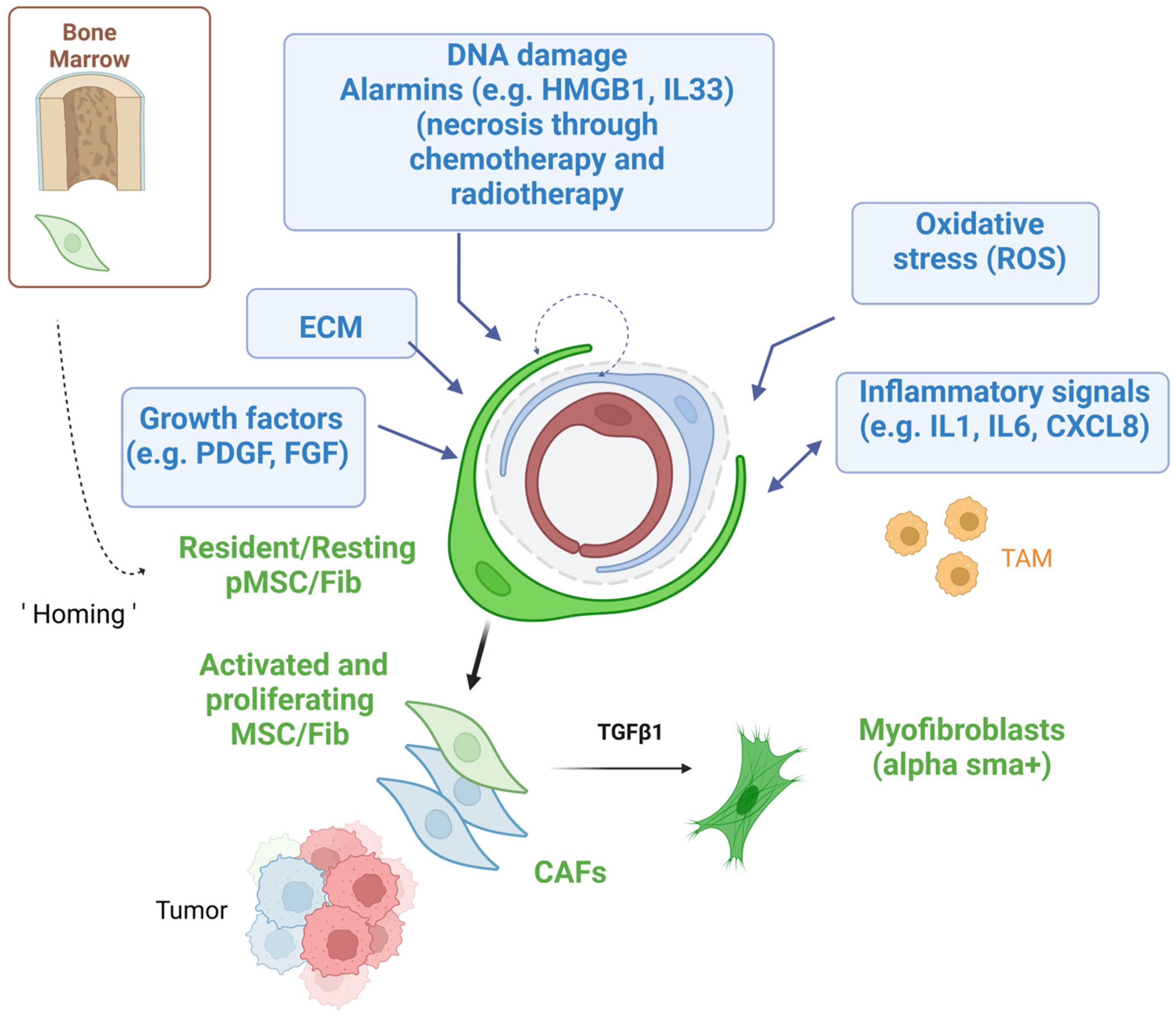
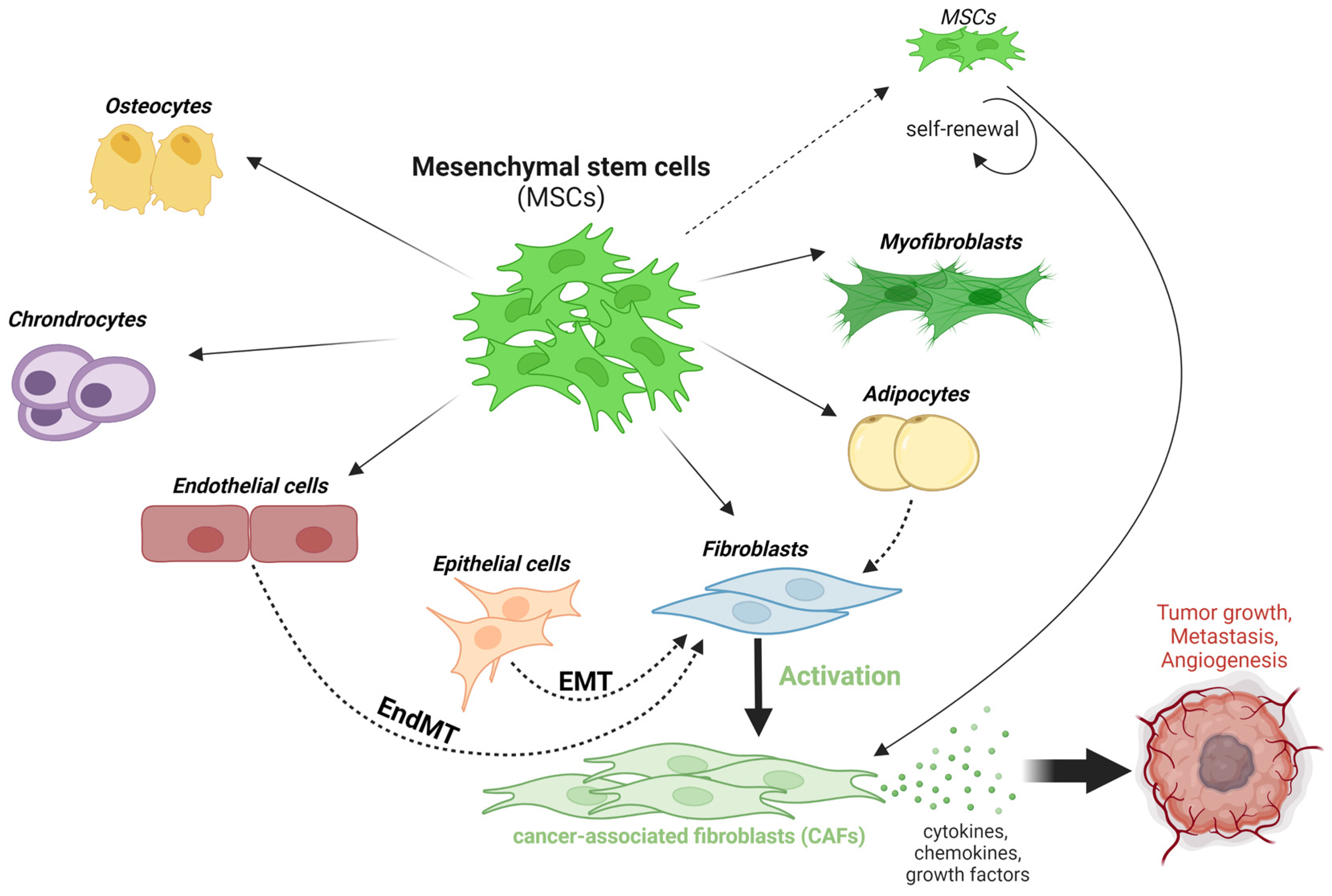
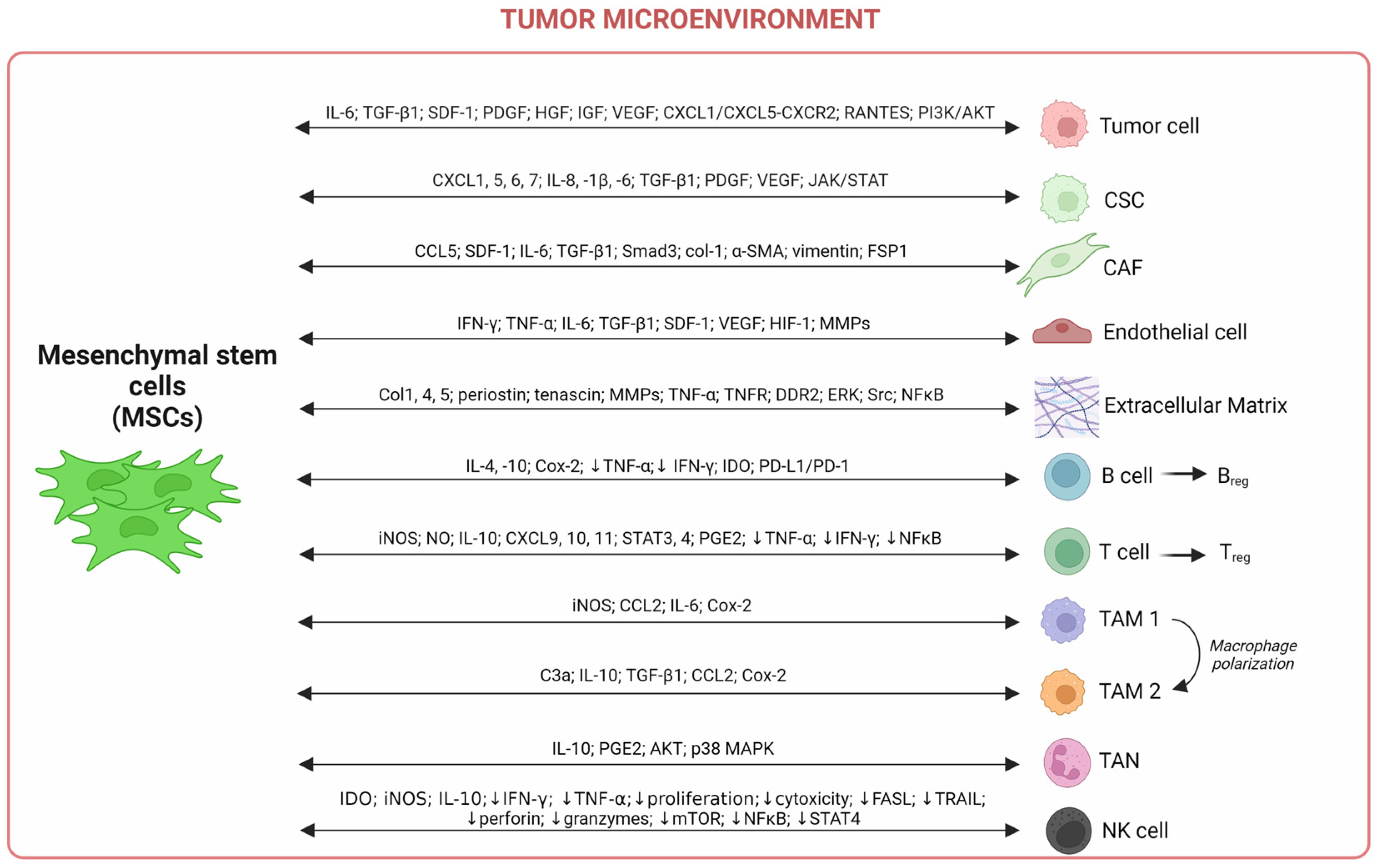
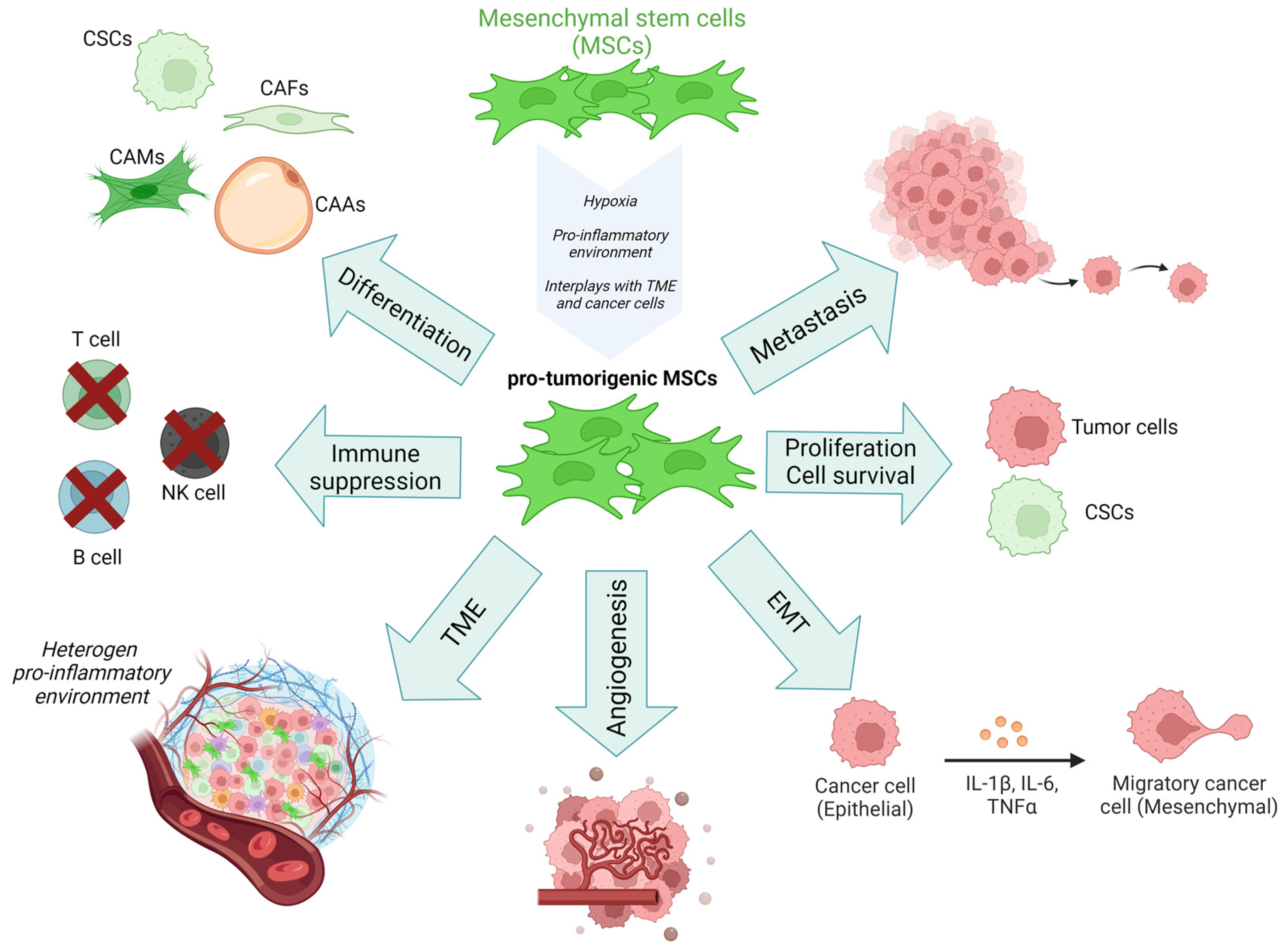
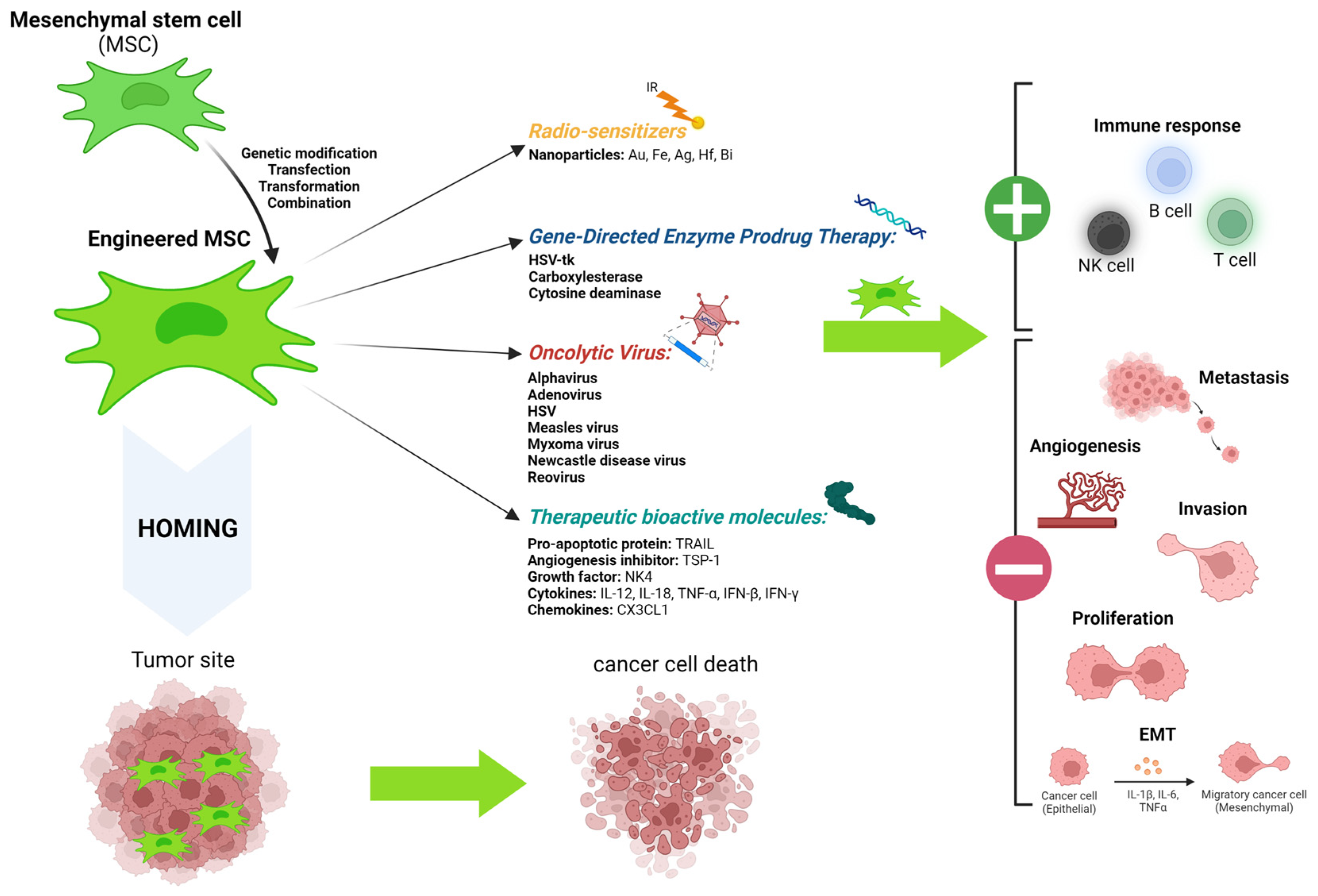
| Expressed Surface Markers | Negative Selection Surface Markers | Characteristics |
|---|---|---|
| CD105 | CD11b | Plastic adherence |
| CD90/THY-1 | CD14 | Multi-lineage differentiation |
| CD73 | CD19 | Plasticity |
| CD44 | CD34 | |
| CD166 | CD45 | |
| CD29 | CD79a | |
| STRO-1 | HLA-DR | |
| CD146/MUCIN | ||
| CD248/ENDOSIALIN | ||
| CD140β/PDGFRβ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slama, Y.; Ah-Pine, F.; Khettab, M.; Arcambal, A.; Begue, M.; Dutheil, F.; Gasque, P. The Dual Role of Mesenchymal Stem Cells in Cancer Pathophysiology: Pro-Tumorigenic Effects versus Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 13511. https://doi.org/10.3390/ijms241713511
Slama Y, Ah-Pine F, Khettab M, Arcambal A, Begue M, Dutheil F, Gasque P. The Dual Role of Mesenchymal Stem Cells in Cancer Pathophysiology: Pro-Tumorigenic Effects versus Therapeutic Potential. International Journal of Molecular Sciences. 2023; 24(17):13511. https://doi.org/10.3390/ijms241713511
Chicago/Turabian StyleSlama, Youssef, Franck Ah-Pine, Mohamed Khettab, Angelique Arcambal, Mickael Begue, Fabien Dutheil, and Philippe Gasque. 2023. "The Dual Role of Mesenchymal Stem Cells in Cancer Pathophysiology: Pro-Tumorigenic Effects versus Therapeutic Potential" International Journal of Molecular Sciences 24, no. 17: 13511. https://doi.org/10.3390/ijms241713511
APA StyleSlama, Y., Ah-Pine, F., Khettab, M., Arcambal, A., Begue, M., Dutheil, F., & Gasque, P. (2023). The Dual Role of Mesenchymal Stem Cells in Cancer Pathophysiology: Pro-Tumorigenic Effects versus Therapeutic Potential. International Journal of Molecular Sciences, 24(17), 13511. https://doi.org/10.3390/ijms241713511






