New Insights into Photobiomodulation of the Vaginal Microbiome—A Critical Review
Abstract
:1. Introduction
2. The Human Microbiome and Its Diseases
2.1. Vaginal Microbiome (VMB)
2.2. The Impact of Vaginal Dysbiosis on Health
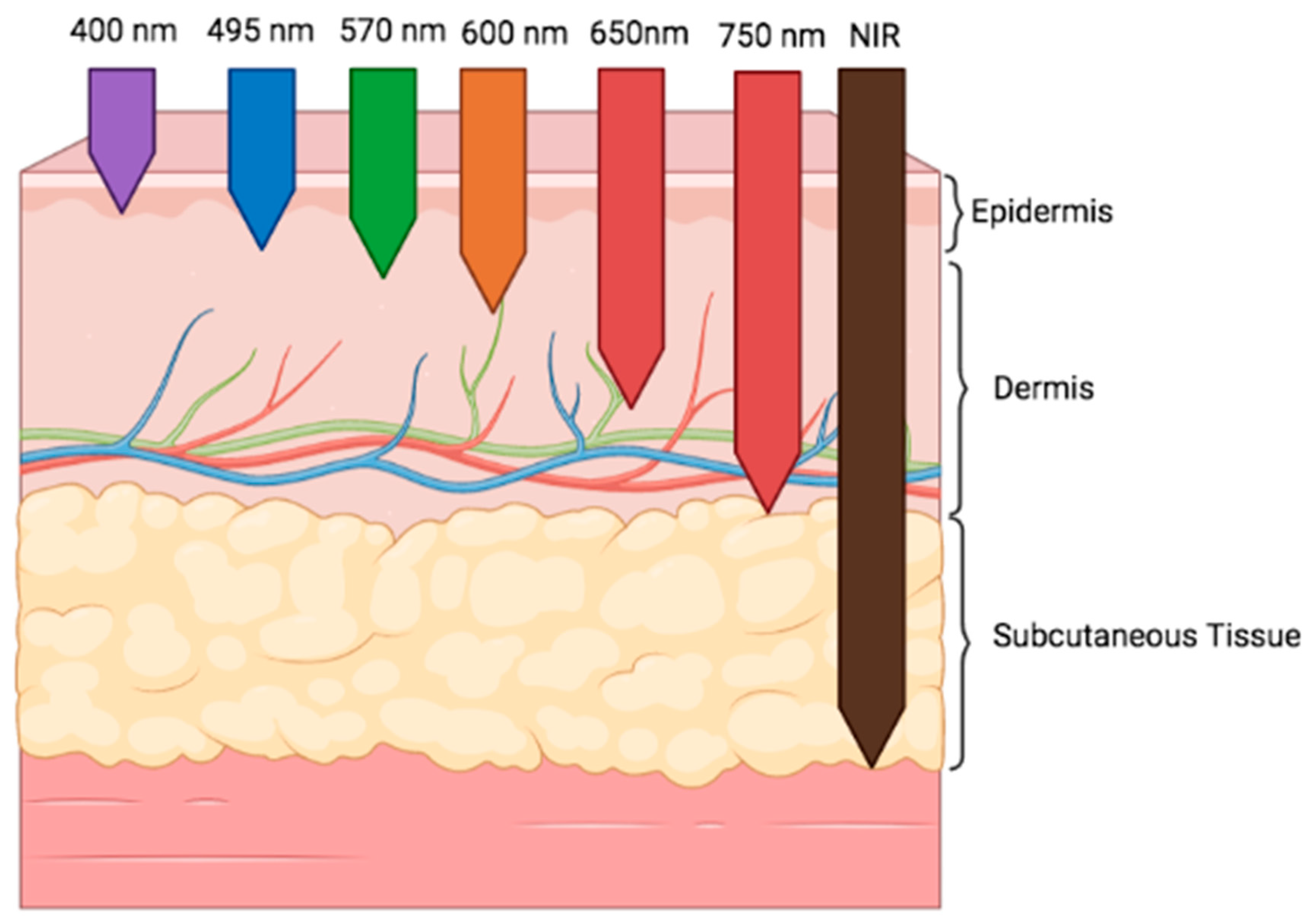
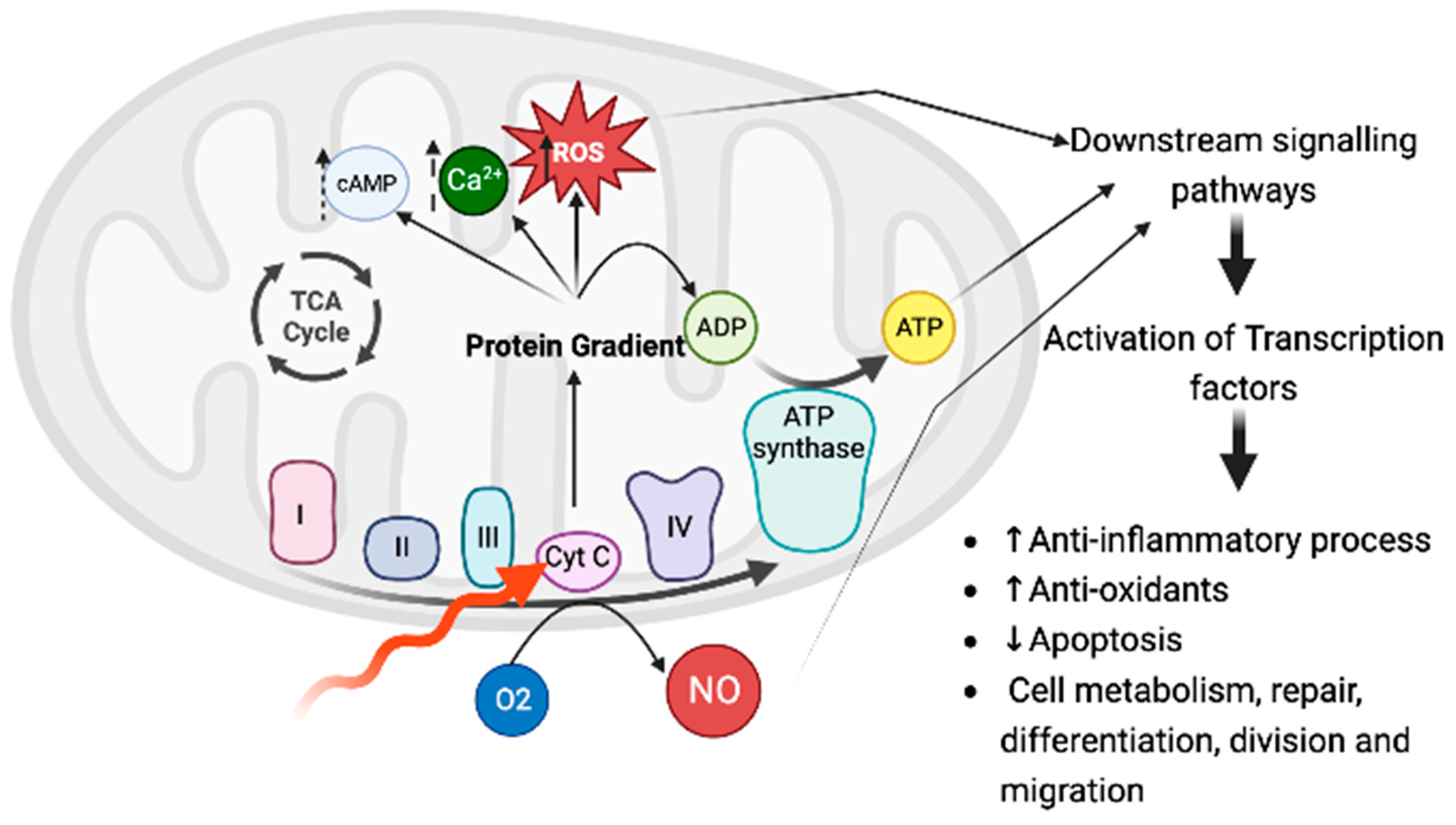
3. Photobiomodulation: Definition and Mechanism of Action
3.1. Photobiomodulation of the Microbiome
3.2. Potential Applications for the Vaginal Microbiome (VMB)
| Vaginal Microbiome Component | PBM Mechanism | Reference |
|---|---|---|
| NO, the main component of the vaginal immune response to bacterial vaginosis. Lactobacillus sp. could also produce NO from exogenous arginine. Disruptions of NO balance could lead to dysbiosis. | PBM augments NO bioavailability. NO stimulates the production of anti-inflammatory cytokines that prevent the invasion of pathogenic bacteria. | [94,95,96] |
| Gut–vagina axis | PBM induces changes in the human microbiome by increasing Akkermansia muciniphila, Bifidobacterium sp., and Faecalibacterium sp. (correlated with healthy microbiome) and decreasing the Firmicutes/Bacteroides ratio. | [26,32] |
| Chlamydia sp. | Water-filtered infrared A (λ 780 to 1400 nm), can significantly reduce infectivity of extracellular infectious elementary bodies in HeLa cell lines. | [97] |
| Candida sp. | 415 nm blue light had anti-fungal effects. | [99,100] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef]
- Aggarwal, N.; Kitano, S.; Puah, G.R.Y.; Kittelmann, S.; Hwang, I.Y.; Chang, M.W. Microbiome and Human Health: Current Under-standing, Engineering, and Enabling Technologies. Chem. Rev. 2023, 123, 31–72. [Google Scholar] [CrossRef]
- Bicknell, B.; Liebert, A.; Johnstone, D.; Kiat, H. Photobiomodulation of the microbiome: Implications for metabolic and inflammatory diseases. Lasers Med. Sci. 2019, 34, 317–327. [Google Scholar] [CrossRef]
- El Tekle, G.; Garrett, W.S. Bacteria in Cancer Initiation, Promotion and Progression. Nat. Rev. Cancer 2023, 23, 600–618. Available online: https://www.nature.com/articles/s41568-023-00594-2 (accessed on 20 July 2023). [CrossRef]
- Elkafas, H.; Walls, M.; Al-Hendy, A.; Ismail, N. Gut and genital tract microbiomes: Dysbiosis and link to gynecological disorders. Front. Cell. Infect. Microbiol. 2022, 12, 1059825. [Google Scholar] [CrossRef]
- Breton, J.; Galmiche, M.; Déchelotte, P. Dysbiotic Gut Bacteria in Obesity: An Overview of the Metabolic Mechanisms and Therapeutic Perspectives of Next-Generation Probiotics. Microorganisms 2022, 10, 452. [Google Scholar] [CrossRef]
- Qiu, P.; Ishimoto, T.; Fu, L.; Zhang, J.; Zhang, Z.; Liu, Y. The Gut Microbiota in Inflammatory Bowel Disease. Front. Cell. Infect. Microbiol. 2022, 12, 733992. [Google Scholar] [CrossRef]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The microbiome and irritable bowel syndrome—A review on the pathophysiology, current research and future therapy. Front. Microbiol. 2019, 10, 1136. [Google Scholar] [CrossRef]
- Wang, P.X.; Deng, X.R.; Zhang, C.H.; Yuan, H.J. Gut microbiota and metabolic syndrome. Chin. Med. J. 2020, 133, 808–816. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, B.; Yu, D.; Zhu, C. Gut Microbiota: An Important Player in Type 2 Diabetes Mellitus. Front. Cell. Infect. Microbiol. 2022, 12, 834485. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, F.; Or-Rashid, M.H.; Al Mamun, A.; Rahaman, M.S.; Islam, M.M.; Meem, A.F.K.; Sutradhar, P.R.; Mitra, S.; Mimi, A.A.; et al. The Gut Microbiota (Microbiome) in cardiovascular disease and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 903570. [Google Scholar] [CrossRef] [PubMed]
- Taniya, M.A.; Chung, H.-J.; Al Mamun, A.; Alam, S.; Aziz, A.; Emon, N.U.; Islam, M.; Hong, S.-T.S.; Podder, B.R.; Mimi, A.A.; et al. Role of Gut Microbiome in Autism Spectrum Disorder and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 915701. [Google Scholar] [CrossRef] [PubMed]
- Varesi, A.; Pierella, E.; Romeo, M.; Piccini, G.B.; Alfano, C.; Bjørklund, G.; Oppong, A.; Ricevuti, G.; Esposito, C.; Chirumbolo, S.; et al. The Potential Role of Gut Microbiota in Alzheimer’s Disease: From Diagnosis to Treatment. Nutrients 2022, 14, 668. [Google Scholar] [CrossRef]
- Hey, G.; Nair, N.; Klann, E.; Gurrala, A.; Safarpour, D.; Mai, V.; Ramirez-Zamora, A.; Vedam-Mai, V. Therapies for Parkinson’s disease and the gut microbiome: Evidence for bidirectional connection. Front. Aging Neurosci. 2023, 15, 1151850. [Google Scholar] [CrossRef]
- Verstraelen, H.; Vieira-Baptista, P.; De Seta, F.; Ventolini, G.; Lonnee-Hoffmann, R.; Lev-Sagie, A. The Vaginal Microbiome: I. Research Development, Lexicon, Defining “Normal” and the Dynamics throughout Women’s Lives. J. Low. Genit. Tract Dis. 2022, 26, 73–78. [Google Scholar] [CrossRef]
- Adapen, C.; Réot, L.; Menu, E. Role of the human vaginal microbiota in the regulation of inflammation and sexually transmitted infection acquisition: Contribution of the non-human primate model to a better understanding? Front. Reprod. Health 2022, 4, 992176. [Google Scholar] [CrossRef]
- Kosti, I.; Lyalina, S.; Pollard, K.S.; Butte, A.J.; Sirota, M. Meta-Analysis of Vaginal Microbiome Data Provides New Insights into Preterm Birth. Front. Microbiol. 2020, 11, 476. [Google Scholar] [CrossRef]
- Lev-Sagie, A.; De Seta, F.; Verstraelen, H.; Ventolini, G.; Lonnee-Hoffmann, R.; Vieira-Baptista, P. The Vaginal Microbiome: II. Vaginal Dysbiotic Conditions. J. Low. Genit. Tract Dis. 2022, 26, 79–84. [Google Scholar] [CrossRef]
- Vieira-Baptista, P.; De Seta, F.; Verstraelen, H.; Ventolini, G.M.; Lonnee-Hoffmann, R.; Lev-Sagie, A. The Vaginal Microbiome: V. Therapeutic Modalities of Vaginal Microbiome Engineering and Research Challenges. J. Low. Genit. Tract Dis. 2022, 26, 99–104. [Google Scholar] [CrossRef]
- Almeida, C.; Oliveira, R.; Baylina, P.; Fernandes, R.; Teixeira, F.G.; Barata, P. Current Trends and Challenges of Fecal Microbiota Transplantation—An Easy Method That Works for All? Biomedicines 2022, 10, 2742. [Google Scholar] [CrossRef]
- Wrønding, T.; Vomstein, K.; Bosma, E.F.; Mortensen, B.; Westh, H.; Heintz, J.E.; Mollerup, S.; Petersen, A.M.; Ensign, L.M.; DeLong, K.; et al. Antibiotic-free vaginal microbiota transplant with donor engraftment, dysbiosis resolution and live birth after recurrent pregnancy loss: A proof of concept case study. EClinicalMedicine 2023, 61, 102070. [Google Scholar] [CrossRef] [PubMed]
- Ayyar, V.S.; Sukumaran, S. Circadian rhythms: Influence on physiology, pharmacology, and therapeutic interventions. J. Pharmacokinet. Pharmacodyn. 2021, 48, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Liebert, A.; Bicknell, B.; Johnstone, D.M.; Gordon, L.C.; Kiat, H.; Hamblin, M.R. “photobiomics”: Can Light, Including Photobiomodulation, Alter the Microbiome? Photobiomodul. Photomed. Laser Surg. 2019, 37, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Ailioaie, L.M.; Litscher, G. Probiotics, Photobiomodulation, and Disease Management: Controversies and Challenges. Int. J. Mol. Sci. 2021, 22, 4942. [Google Scholar] [CrossRef]
- Lederberg, J.; Mccray, A.T. Commentary ’Ome Sweet ’Omics-A Genealogical Treasury of Words. Available online: www.-ics.com (accessed on 10 July 2023).
- Proctor, L. Priorities for the next 10 years of human microbiome research. Nature 2019, 569, 623–625. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Baquero, F.; Nombela, C. The Microbiome as a Human Organ. Clin. Microbiol. Infect. 2012, 18, 2–4. Available online: https://www.sciencedirect.com/science/article/pii/S1198743X14609587 (accessed on 1 July 2023). [CrossRef]
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. The gut-brain axis and the microbiome: Mechanisms and clinical implications. Clin. Gastroenterol. Hepatol. 2019, 17, 322–332. Available online: https://www.sciencedirect.com/science/article/pii/S1542356518310814 (accessed on 10 July 2023). [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Yeruva, T.; Lee, C.H. Regulation of Vaginal Microbiome by Nitric Oxide. Curr. Pharm. Biotechnol. 2019, 20, 17–31. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. Female Gut and Genital Tract Microbiota-Induced Crosstalk and Differential Effects of Short-Chain Fatty Acids on Immune Sequelae. Front. Immunol. 2020, 11, 2184. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen–gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef]
- Mirzaei, R.; Kavyani, B.; Nabizadeh, E.; Kadkhoda, H.; Ozma, M.A.; Abdi, M. Microbiota metabolites in the female reproductive system: Focused on the short-chain fatty acids. Heliyon 2023, 9, e14562. [Google Scholar] [CrossRef] [PubMed]
- Chancharoenthana, W.; Kamolratanakul, S.; Schultz, M.J.; Leelahavanichkul, A. The leaky gut and the gut microbiome in sepsis–targets in research and treatment. Clin. Sci. 2023, 137, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Voltolini, C.; Battersby, S.; Etherington, S.L.; Petraglia, F.; Norman, J.E.; Jabbour, H.N. A Novel Antiinflammatory Role for the Short-Chain Fatty Acids in Human Labor. Endocrinology 2012, 153, 395–403. [Google Scholar] [CrossRef]
- Holdcroft, A.M.; Ireland, D.J.; Payne, M.S. The Vaginal Microbiome in Health and Disease—What Role Do Common Intimate Hygiene Practices Play? Microorganisms 2023, 11, 298. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef]
- France, M.; Alizadeh, M.; Brown, S.; Ma, B.; Ravel, J. Towards a deeper understanding of the vaginal microbiota. Nat. Microbiol. 2022, 7, 367–378. [Google Scholar] [CrossRef]
- Gupta, K.; Stapleton, A.E.; Hooton, T.M.; Roberts, P.L.; Fennell, C.L.; Stamm, W.E. Inverse Association of H2O2-Producing Lactobacilli and Vaginal Escherichia coli Colonization in Women with Recurrent Urinary Tract Infections. Available online: https://academic.oup.com/jid/article/178/2/446/904810 (accessed on 10 July 2023).
- Kirjavainen, P.V.; Pautler, S.; Baroja, M.L.; Anukam, K.; Crowley, K.; Carter, K.; Reid, G. Abnormal Immunological Profile and Vaginal Microbiota in Women Prone to Urinary Tract Infections. Clin. Vaccine Immunol. 2009, 16, 29–36. [Google Scholar] [CrossRef]
- Haggerty, C.L.; Ness, R.B.; Totten, P.A.; Farooq, F.; Tang, G.; Ko, D.; Hou, X.; Fiedler, T.L.; Srinivasan, S.; Astete, S.G.; et al. Presence and Concentrations of Select Bacterial Vaginosis-Associated Bacteria Are Associated with Increased Risk of Pelvic Inflammatory Disease. Sex Transm Dis. 2020, 47, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, C.L.; Totten, P.A.; Tang, G.; Astete, S.G.; Ferris, M.J.; Norori, J.; Bass, D.C.; Martin, D.H.; Taylor, B.D.; Ness, R.B. Identification of novel microbes associated with pelvic inflammatory disease and infertility. Sex. Transm. Infect. 2016, 92, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Forney, L.J.; Ravel, J. Vaginal Microbiome: Rethinking Health and Disease. Annu. Rev. Microbiol. 2012, 66, 371–389. [Google Scholar] [CrossRef]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Galuppi, M.; Lamont, R.F.; Chaemsaithong, P.; Miranda, J.; et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, D.A.; Chandiramani, M.; Lee, Y.S.; Kindinger, L.; Smith, A.; Angelopoulos, N.; Lehne, B.; Arulkumaran, S.; Brown, R.; Teoh, T.G.; et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci. Rep. 2015, 5, 8988. [Google Scholar] [CrossRef]
- Nunn, K.L.; Witkin, S.S.; Schneider, G.M.; Boester, A.; Nasioudis, D.; Minis, E.; Gliniewicz, K.; Forney, L.J. Changes in the Vaginal Microbiome during the Pregnancy to Postpartum Transition. Reprod. Sci. 2021, 28, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Grewal, K.; Lee, Y.S.; Smith, A.; Brosens, J.J.; Bourne, T.; Al-Memar, M.; Kundu, S.; MacIntyre, D.A.; Bennett, P.R. Chromosomally normal miscarriage is associated with vaginal dysbiosis and local inflammation. BMC Med. 2022, 20, 38. [Google Scholar] [CrossRef]
- Fox, C.; Eichelberger, K. Maternal microbiome and pregnancy outcomes. Fertil. Steril. 2015, 104, 1358–1363. [Google Scholar] [CrossRef]
- Fransson, E.; Gudnadottir, U.; Hugerth, L.W.; Itzel, E.W.; Hamsten, M.; Boulund, F.; Pennhag, A.; Du, J.; Schuppe-Koistinen, I.; Brusselaers, N.; et al. Cohort profile: The Swedish Maternal Microbiome project (SweMaMi)-assessing the dynamic associations between the microbiome and maternal and neonatal adverse events. BMJ Open 2022, 12, e065825. [Google Scholar] [CrossRef]
- Dunlop, A.L.; Mulle, J.G.; Ferranti, E.P.; Edwards, S.; Dunn, A.B.; Corwin, E.J. Maternal Microbiome and Pregnancy Outcomes That Impact Infant Health: A Review. Adv. Neonatal Care 2015, 15, 377–385. [Google Scholar] [CrossRef]
- Haahr, T.; Jensen, J.; Thomsen, L.; Duus, L.; Rygaard, K.; Humaidan, P. Abnormal vaginal microbiota may be associated with poor reproductive outcomes: A prospective study in IVF patients. Hum. Reprod. 2016, 31, 795–803. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Hauth, J.C.; Andrews, W.W. Intrauterine Infection and Preterm Delivery. N. Engl. J. Med. 2000, 342, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Leitich, H.; Bodner-Adler, B.; Brunbauer, M.; Kaider, A.; Egarter, C.; Husslein, P. Bacterial vaginosis as a risk factor for preterm delivery: A meta-analysis. Am. J. Obstet. Gynecol. 2003, 189, 139–147. [Google Scholar] [CrossRef]
- Guerra, B.; Ghi, T.; Quarta, S.; Morselli-Labate, A.M.; Lazzarotto, T.; Pilu, G.; Rizzo, N. Pregnancy outcome after early detection of bacterial vaginosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 128, 40–45. [Google Scholar] [CrossRef] [PubMed]
- De Seta, F.; Sartore, A.; Piccoli, M.; Maso, G.; Zicari, S.; Panerari, F.; Guaschino, S. Bacterial vaginosis and preterm delivery: An open question. J. Reprod. Med. 2005, 50, 313–318. Available online: http://europepmc.org/abstract/MED/15971479 (accessed on 10 July 2023). [PubMed]
- Purwar, M.; Ughade, S.; Bhagat, B.; Agarwal, V.; Kulkarni, H. Bacterial Vaginosis in Early Pregnancy and Adverse Pregnancy Outcome. J. Obstet. Gynaecol. Res. 2001, 27, 175–181. [Google Scholar] [CrossRef]
- Gudnadottir, U.; Debelius, J.W.; Du, J.; Hugerth, L.W.; Danielsson, H.; Schuppe-Koistinen, I.; Fransson, E.; Brusselaers, N. The vaginal microbiome and the risk of preterm birth: A systematic review and network meta-analysis. Sci. Rep. 2022, 12, 7926. [Google Scholar] [CrossRef]
- Gáspár, L. Professor Endre Mester, the Father of Photobiomodulation. J. Laser Dent. 2009, 17, 146–148. [Google Scholar]
- De Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef]
- Anders, J.J.; Lanzafame, R.J.; Arany, P.R. Low-Level Light/Laser Therapy Versus Photobiomodulation Therapy. Photomed. Laser Surg. 2015, 33, 183–184. [Google Scholar] [CrossRef]
- da Silva, T.G.; Ribeiro, R.S.; Mencalha, A.L.; de Souza Fonseca, A. Photobiomodulation at molecular, cellular, and systemic levels. Lasers Med. Sci. 2023, 38, 136. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.M.C.T.; Sergio, L.P.d.S.; Fonseca, A.d.S.d. Photobiomodulation via multiple-wavelength radiations. Lasers Med. Sci. 2020, 35, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, L.C.; Berman, M.; Halper, J.; Chazot, P. Rationale for 1068 nm Photobiomodulation Therapy (PBMT) as a Novel, Non-Invasive Treatment for COVID-19 and Other Coronaviruses: Roles of NO and Hsp70. Int. J. Mol. Sci. 2022, 23, 5221. [Google Scholar] [CrossRef] [PubMed]
- Cios, A.; Ciepielak, M.; Szymański, Ł.; Lewicka, A.; Cierniak, S.; Stankiewicz, W.; Mendrycka, M.; Lewicki, S. Effect of Different Wavelengths of Laser Irradiation on the Skin Cells. Int. J. Mol. Sci. 2021, 22, 2437. [Google Scholar] [CrossRef] [PubMed]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Amaroli, A.; Pasquale, C.; Zekiy, A.; Utyuzh, A.; Benedicenti, S.; Signore, A.; Ravera, S. Photobiomodulation and Oxidative Stress: 980 nm Diode Laser Light Regulates Mitochondrial Activity and Reactive Oxygen Species Production. Oxidative Med. Cell. Longev. 2021, 2021, 6626286. [Google Scholar] [CrossRef]
- Ravera, S.; Colombo, E.; Pasquale, C.; Benedicenti, S.; Solimei, L.; Signore, A.; Amaroli, A. Mitochondrial Bioenergetic, Photo-biomodulation and Trigeminal Branches Nerve Damage, What’s the Connection? A Review. Int. J. Mol. Sci. 2021, 22, 4347. [Google Scholar] [CrossRef]
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers: Vs. light emitting diodes? Photochem. Photobiol. Sci. 2018, 17, 1003–1017. [Google Scholar] [CrossRef]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation—Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef]
- Sommer, A.P. Mitochondrial cytochrome c oxidase is not the primary acceptor for near infrared light—It is mitochondrial bound water: The principles of low-level light therapy. Ann. Transl. Med. 2019, 7 (Suppl. S1), S13. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.Y.; Wang, Y.; Lyu, P.; Hamblin, M.R. Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: Role of intracellular calcium and light-gated ion channels. Sci. Rep. 2016, 6, 33719. [Google Scholar] [CrossRef]
- Liebert, A.; Capon, W.; Pang, V.; Vila, D.; Bicknell, B.; McLachlan, C.; Kiat, H. Photophysical Mechanisms of Photobiomodulation Therapy as Precision Medicine. Biomedicines 2023, 11, 237. [Google Scholar] [CrossRef]
- Cheng, K.; Martin, L.F.; Slepian, M.J.; Patwardhan, A.M.; Ibrahim, M.M. Mechanisms and Pathways of Pain Photobiomodulation: A Narrative Review. J. Pain 2021, 22, 763–777. [Google Scholar] [CrossRef]
- Fernández-Guarino, M.; Bacci, S.; González, L.A.P.; Bermejo-Martínez, M.; Cecilia-Matilla, A.; Hernández-Bule, M.L. The Role of Physical Therapies in Wound Healing and Assisted Scarring. Int. J. Mol. Sci. 2023, 24, 7487. [Google Scholar] [CrossRef] [PubMed]
- Behroozian, T.; Bonomo, P.; Patel, P.; Kanee, L.; Finkelstein, S.; Hurk, C.v.D.; Chow, E.; Wolf, J.R.; Banerjee, S.; Becherini, C.; et al. Multinational Association of Supportive Care in Cancer (MASCC) clinical practice guidelines for the prevention and management of acute radiation dermatitis: International Delphi consensus-based recommendations. Lancet Oncol. 2023, 24, e172–e185. [Google Scholar] [CrossRef] [PubMed]
- Zipper, R.; Lamvu, G. Vaginal laser therapy for gynecologic conditions: Re-examining the controversy and where do we go from here. J. Comp. Eff. Res. 2022, 11, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Djavid, G.E.; Bigdeli, B.; Goliaei, B.; Nikoofar, A.; Hamblin, M.R. Photobiomodulation leads to enhanced radiosensitivity through induction of apoptosis and autophagy in human cervical cancer cells. J. Biophotonics 2017, 10, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Liebert, A. Emerging Applications of Photobiomodulation Therapy: The Interaction Between Metabolomics and the Microbiome. Photomed. Laser Surg. 2018, 36, 515–517. [Google Scholar] [CrossRef]
- Montazeri, K.; Farhadi, M.; Fekrazad, R.; Chaibakhsh, S.; Mahmoudian, S. Photobiomodulation therapy in mood disorders: A sys-tematic review. Lasers Med. Sci. 2022, 37, 3343–3351. [Google Scholar] [CrossRef]
- Da Silva, D.; Crous, A.; Abrahamse, H. Photobiomodulation: An Effective Approach to Enhance Proliferation and Differentiation of Adipose-Derived Stem Cells into Osteoblasts. Stem Cells Int. 2021, 2021, 8843179. [Google Scholar] [CrossRef] [PubMed]
- Ganipineni, V.D.P.; Gutlapalli, S.D.; Kumar, I.A.S.K.; Monica, P.; Vagdevi, M.; Sowrab, T.S.; Potru, M. Exploring the Potential of Energy-Based Therapeutics (Photobiomodulation/Low-Level Laser Light Therapy) in Cardiovascular Disorders: A Review and Perspective. Cureus 2023, 15, e37880. [Google Scholar] [CrossRef] [PubMed]
- Oron, U.; Tuby, H.; Maltz, L.; Sagi-Assif, O.; Abu-Hamed, R.; Yaakobi, T.; Doenyas-Barak, K.; Efrati, S. Autologous Bone-Marrow Stem Cells Stimulation Reverses Post-Ischemic-Reperfusion Kidney Injury in Rats. Am. J. Nephrol. 2014, 40, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Farfara, D.; Tuby, H.; Trudler, D.; Doron-Mandel, E.; Maltz, L.; Vassar, R.J.; Frenkel, D.; Oron, U. Low-Level Laser Therapy Ameliorates Disease Progression in a Mouse Model of Alzheimer’s Disease. J. Mol. Neurosci. 2015, 55, 430–436. [Google Scholar] [CrossRef]
- Johnstone, D.M.; Gordon, L.C. Remote photobiomodulation: An emerging strategy for neuroprotection. Neural Regen. Res. 2019, 14, 2086–2087. [Google Scholar] [CrossRef]
- Bicknell, B.; Laakso, E.-L.; Liebert, A.; Kiat, H. Modifying the Microbiome as a Potential Mechanism of Photo-biomodulation: A Case Report. Photobiomodul. Photomed. Laser Surg. 2022, 40, 88–97. [Google Scholar] [CrossRef]
- Karu, T. Photobiology of Low-power Laser Effects. Health Phys. 1989, 56, 691–704. [Google Scholar] [CrossRef]
- Zanotta, N.; Ottaviani, G.; Campisciano, G.; Poropat, A.; Bovenzi, M.; Rupel, K.; Gobbo, M.; Comar, M.; Di Lenarda, R.; Biasotto, M.; et al. Photobiomodulation modulates inflammation and oral microbiome: A pilot study. Biomarkers 2020, 25, 677–684. [Google Scholar] [CrossRef]
- Ravera, S.; Bertola, N.; Pasquale, C.; Bruno, S.; Benedicenti, S.; Ferrando, S.; Zekiy, A.; Arany, P.; Amaroli, A. 808-nm Photo-biomodulation Affects the Viability of a Head and Neck Squamous Carcinoma Cellular Model, Acting on Energy Metabolism and Oxidative Stress Production. Biomedicines 2021, 9, 1717. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Zekiy, A.; Benedicenti, S.; Pasquale, C. A Narrative Review on Oral and Periodontal Bacteria Micro-biota Photobiomodulation, through Visible and Near-Infrared Light: From the Origins to Modern Therapies. Int. J. Mol. Sci. 2022, 23, 1372. [Google Scholar] [CrossRef]
- Balle, C.; Esra, R.; Havyarimana, E.; Jaumdally, S.Z.; Lennard, K.; Konstantinus, I.N.; Barnabas, S.L.; Happel, A.-U.; Gill, K.; Pidwell, T.; et al. Relationship between the Oral and Vaginal Microbiota of South African Adolescents with High Prevalence of Bacterial Vaginosis. Microorganisms 2020, 8, 1004. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J. Leukoc. Biol. 2010, 88, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Factories 2020, 19, 203. [Google Scholar] [CrossRef] [PubMed]
- Mannick, J.B.; Stamler, J.S.; Teng, E.; Simpson, N.; Lawrence, J.; Jordan, J.; Finberg, R.W. Nitric Oxide Modulates HIV-1 Replication. J. Acquir. Immune Defic. Syndr. 1999, 22, 1–9. [Google Scholar] [CrossRef]
- Marti, H.; Koschwanez, M.; Pesch, T.; Blenn, C.; Borel, N. Water-Filtered Infrared Irradiation in Combination with Visible Light Inhibits Acute Chlamydial Infection. PLoS ONE 2014, 9, e102239. [Google Scholar] [CrossRef]
- Foxman, B.; Muraglia, R.; Dietz, J.P.; Sobel, J.D.; Wagner, J. Prevalence of Recurrent Vulvovaginal Candidiasis in 5 European Countries and the United States: Results From an Internet Panel Survey. J. Low. Genit. Tract Dis. 2013, 17, 340–345. [Google Scholar] [CrossRef]
- Pekmezovic, M.; Hovhannisyan, H.; Gresnigt, M.S.; Iracane, E.; Oliveira-Pacheco, J.; Siscar-Lewin, S.; Seemann, E.; Qualmann, B.; Kalkreuter, T.; Müller, S.; et al. Candida pathogens induce protective mitochondria-associated type I interferon signalling and a damage-driven response in vaginal epithelial cells. Nat. Microbiol. 2021, 6, 643–657. [Google Scholar] [CrossRef]
- Wang, T.; Dong, J.; Yin, H.; Zhang, G. Blue light therapy to treat candida vaginitis with comparisons of three wavelengths: An in vitro study. Lasers Med. Sci. 2020, 35, 1329–1339. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, F.P.; Carvalhos, C.A.; Figueiredo-Dias, M. New Insights into Photobiomodulation of the Vaginal Microbiome—A Critical Review. Int. J. Mol. Sci. 2023, 24, 13507. https://doi.org/10.3390/ijms241713507
Santos FP, Carvalhos CA, Figueiredo-Dias M. New Insights into Photobiomodulation of the Vaginal Microbiome—A Critical Review. International Journal of Molecular Sciences. 2023; 24(17):13507. https://doi.org/10.3390/ijms241713507
Chicago/Turabian StyleSantos, Fernanda P., Carlota A. Carvalhos, and Margarida Figueiredo-Dias. 2023. "New Insights into Photobiomodulation of the Vaginal Microbiome—A Critical Review" International Journal of Molecular Sciences 24, no. 17: 13507. https://doi.org/10.3390/ijms241713507
APA StyleSantos, F. P., Carvalhos, C. A., & Figueiredo-Dias, M. (2023). New Insights into Photobiomodulation of the Vaginal Microbiome—A Critical Review. International Journal of Molecular Sciences, 24(17), 13507. https://doi.org/10.3390/ijms241713507







