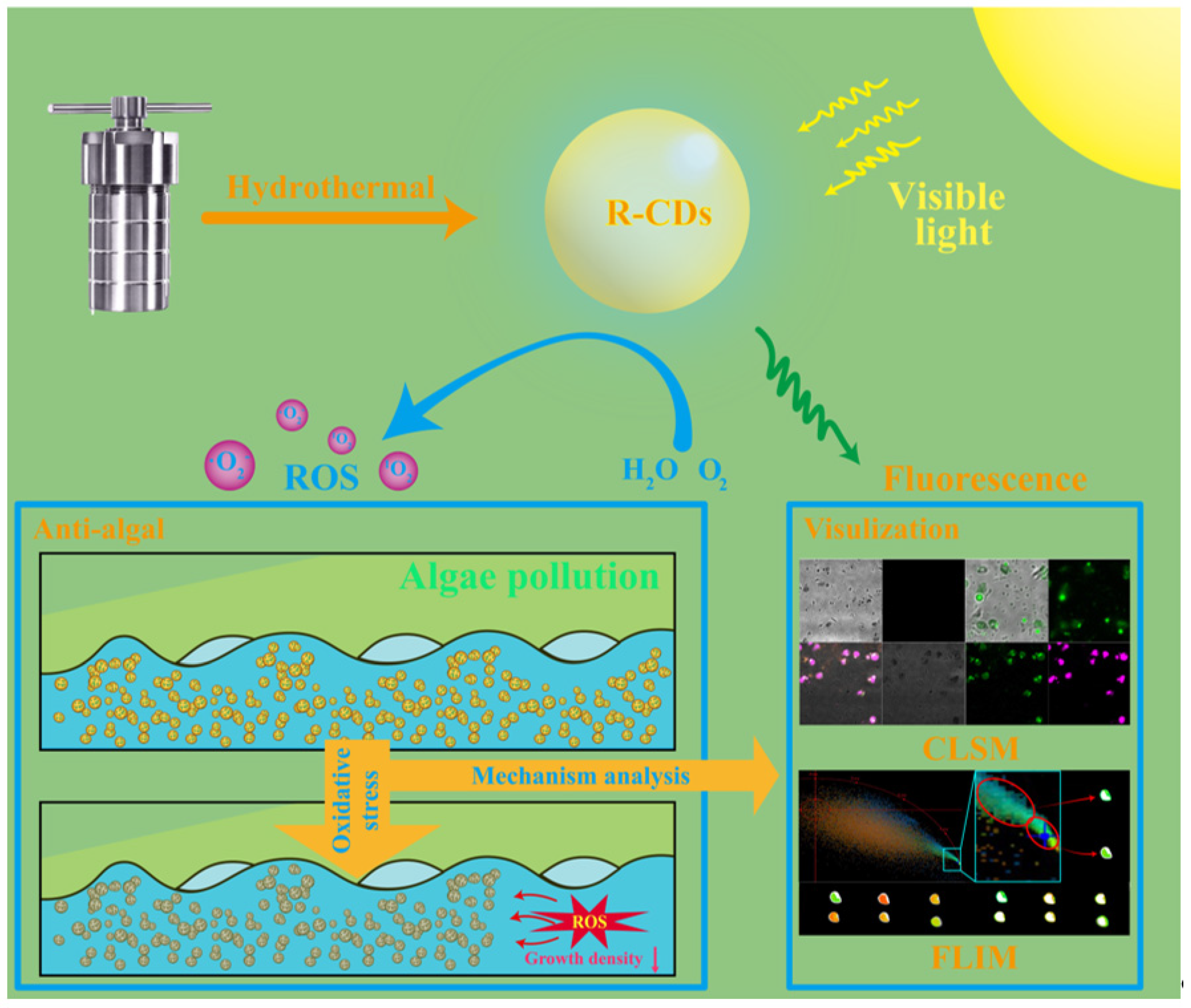
Visible-Light-Activated Carbon Dot Photocatalyst for ROS-Mediated Inhibition of Algae Growth
Abstract
:1. Introduction
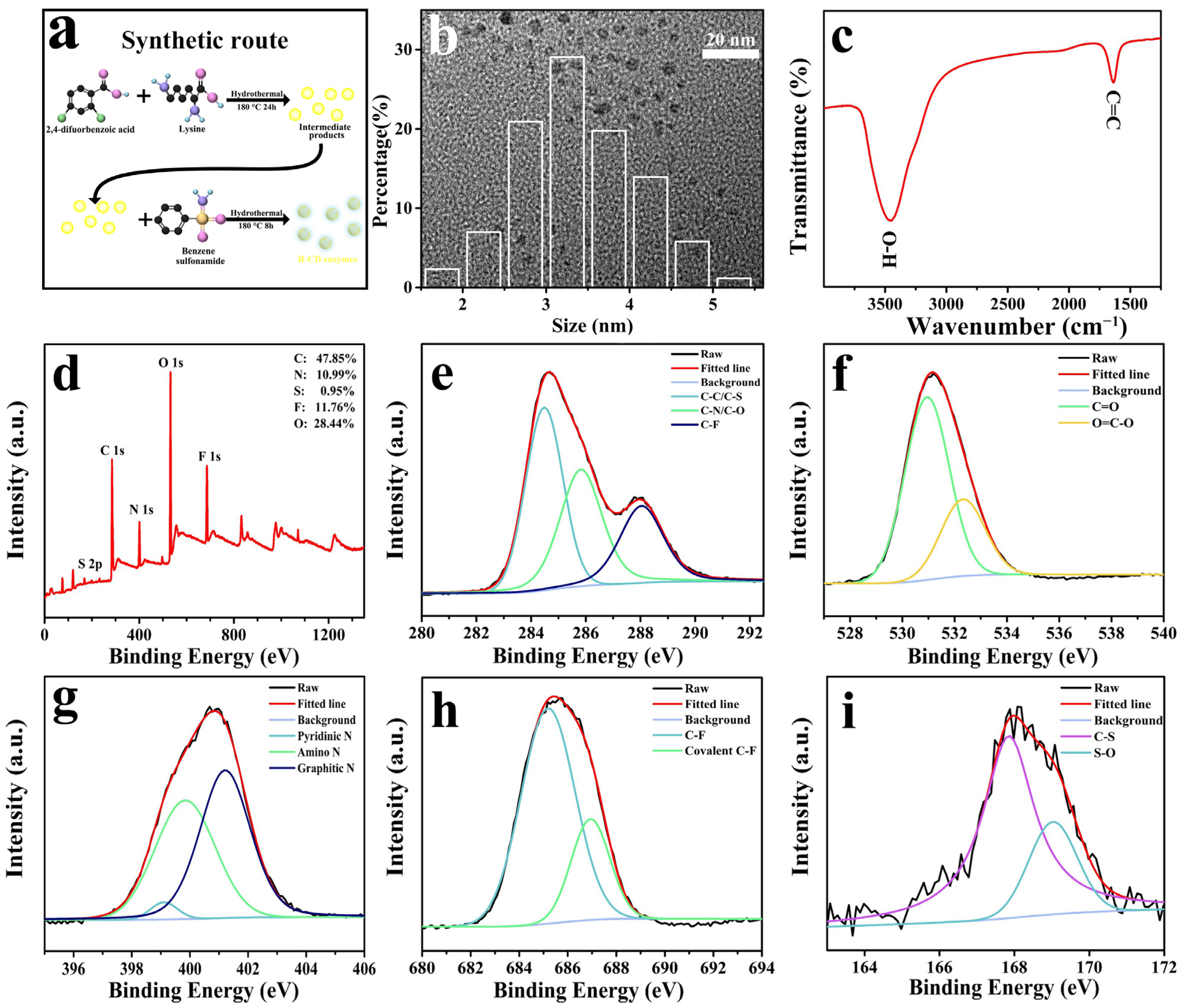
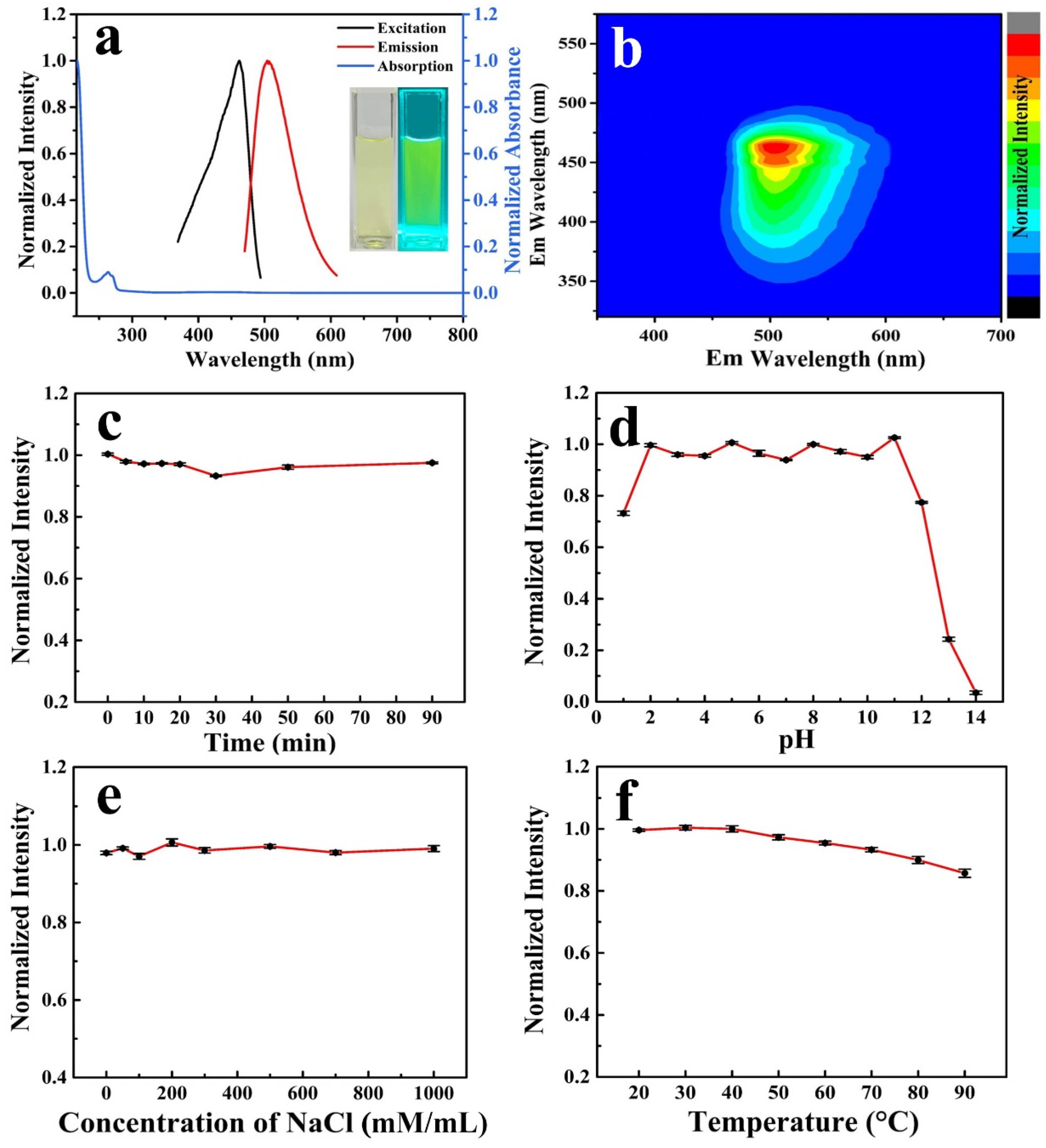
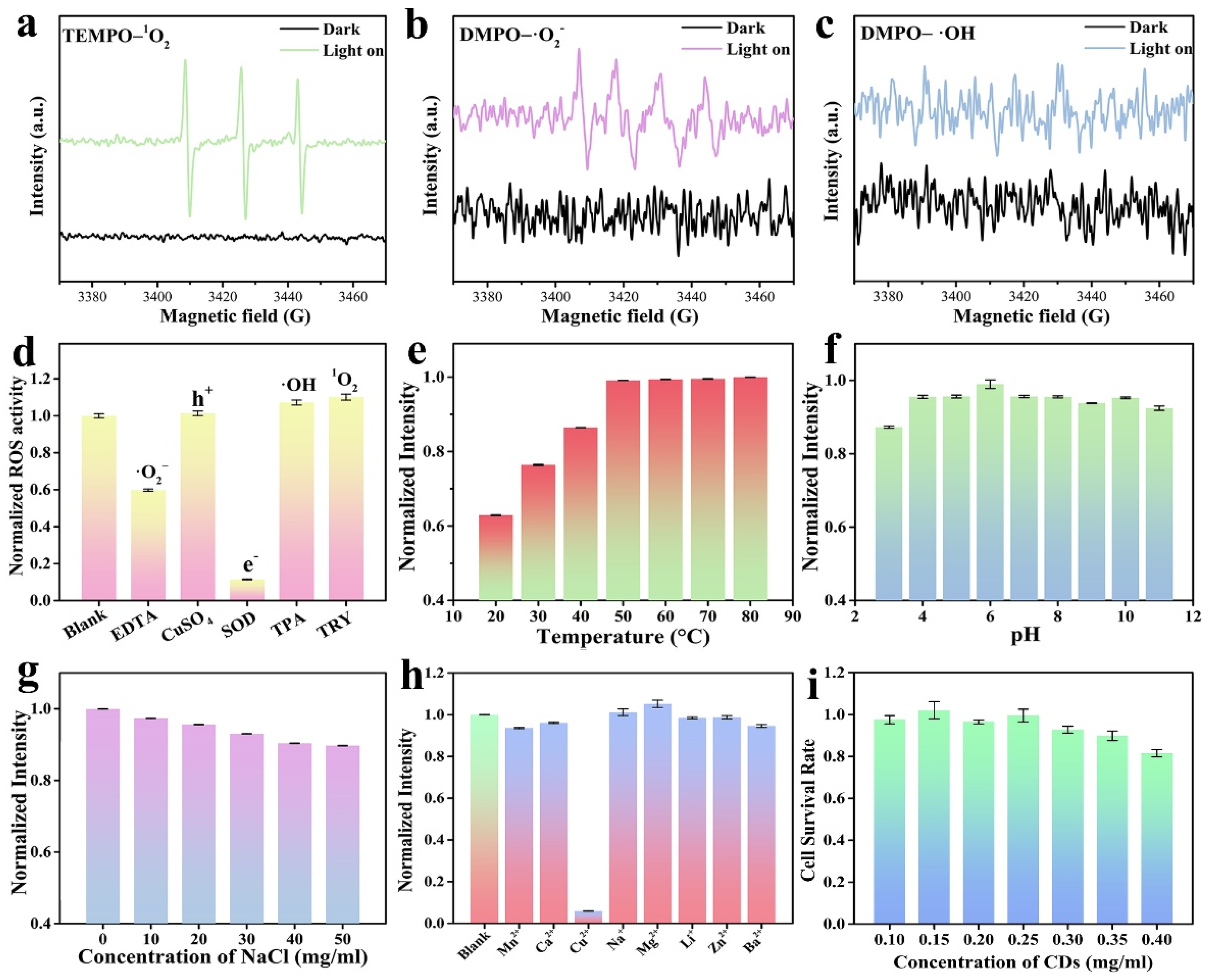
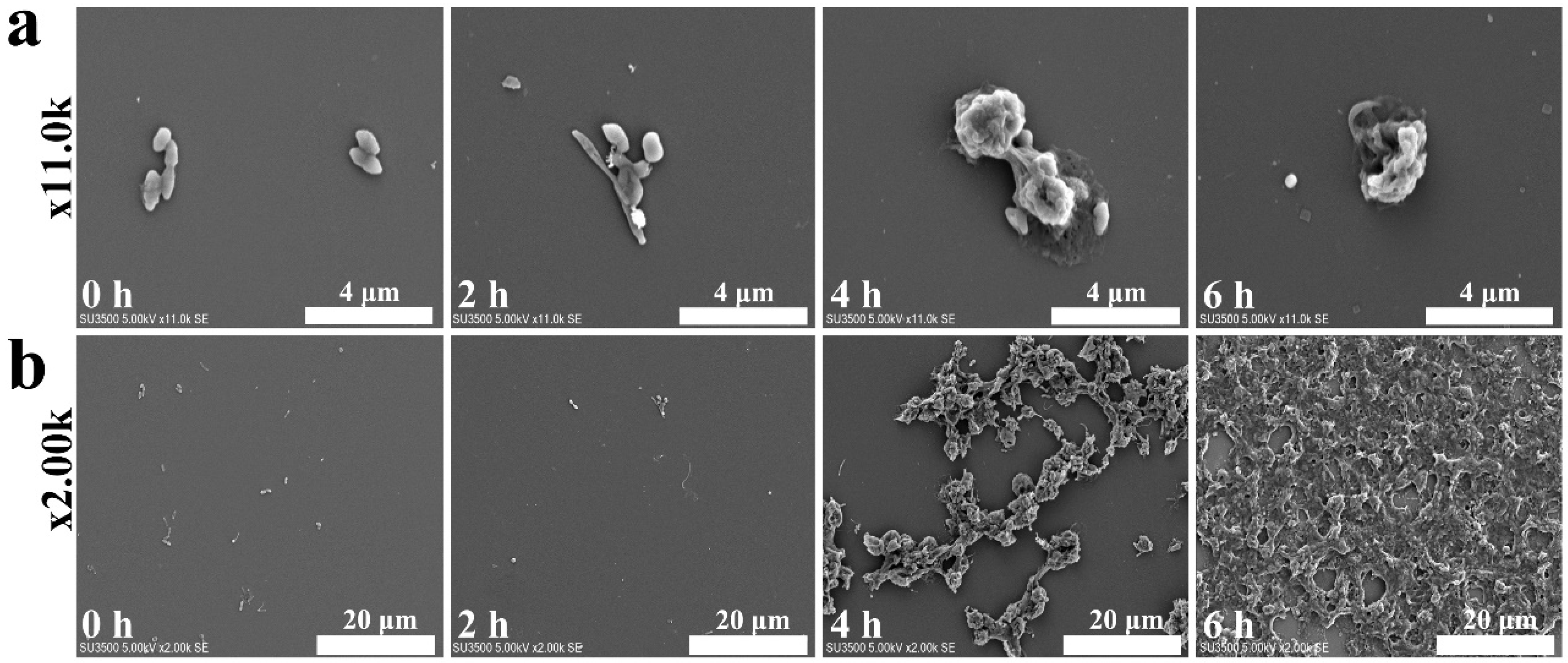
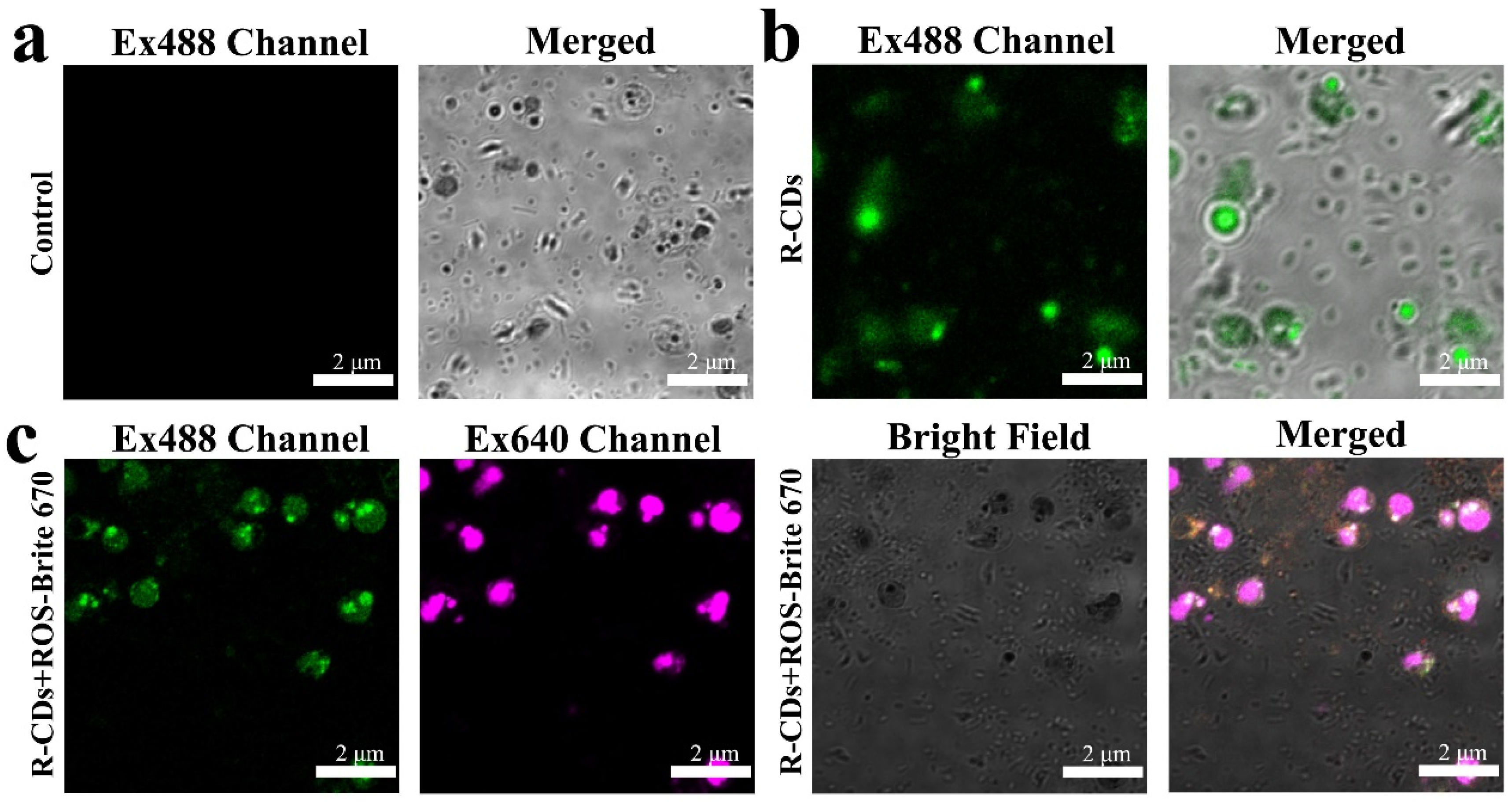
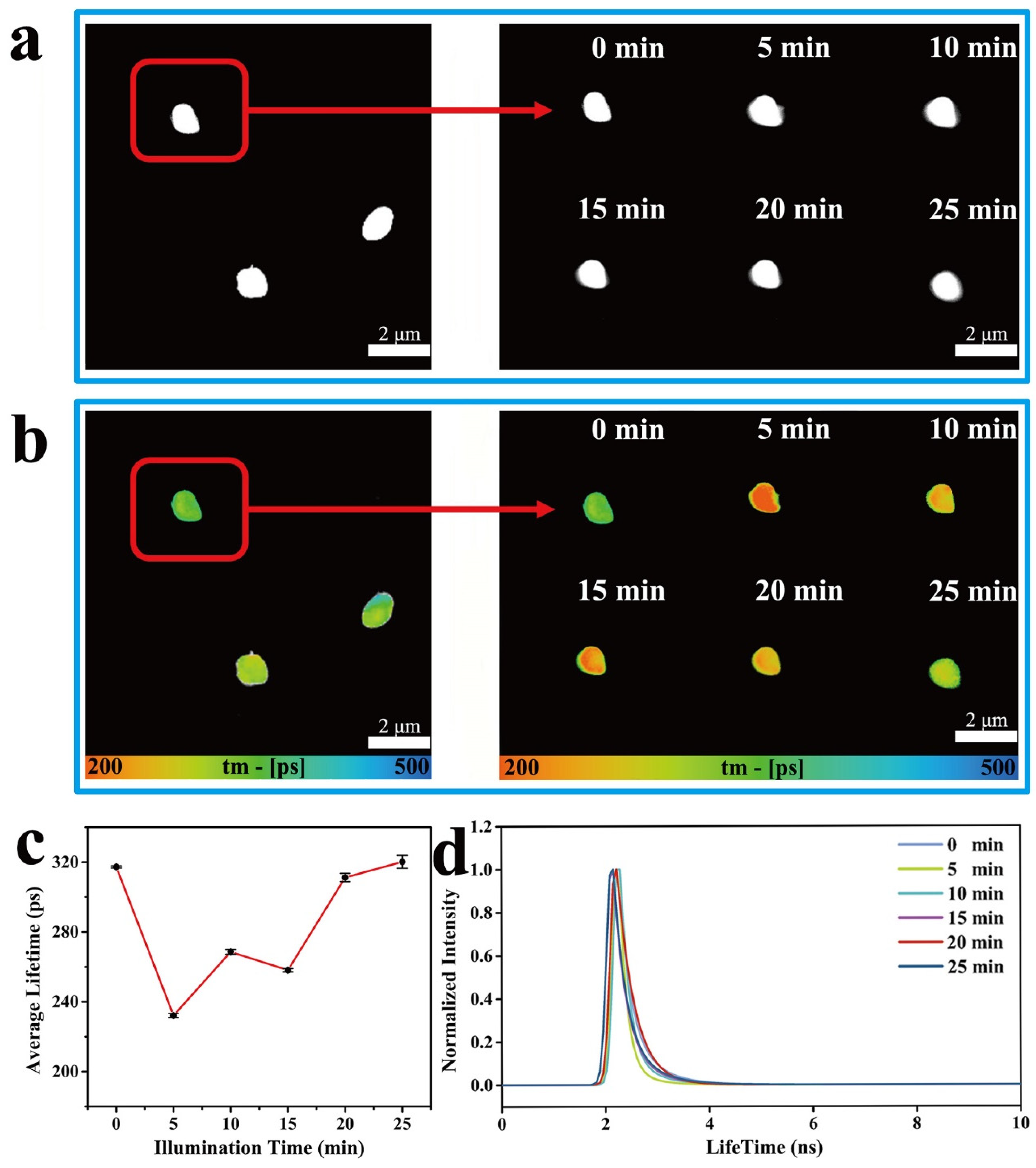
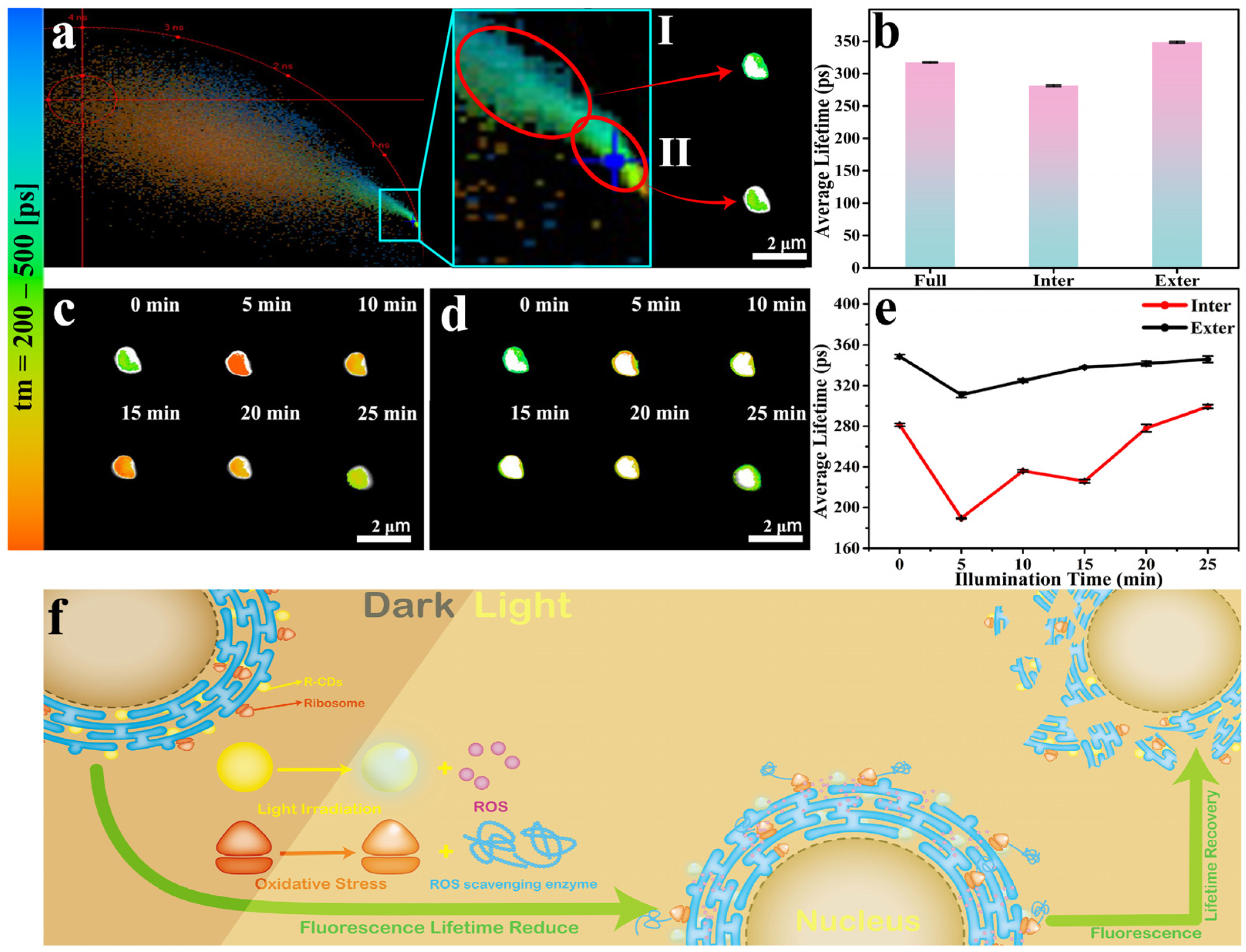
2. Results and Discussion
3. Methods and Materials
3.1. Instruments and Reagents
3.2. Preparation of R-CDs
3.3. PGS Scherffel Culture
3.4. Determination of the ROS Content
3.4.1. Determination of the ROS Content
3.4.2. Stability Experiment
3.5. Anti-Algae Experiment
3.6. Imaging Experience
3.6.1. TEM Imaging
3.6.2. SEM Sample Preparation of Algae
3.6.3. CLSM and FLIM Sample Preparation of Algae
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burkholder, J.M.; Noga, E.J.; Hobbs, C.H.; Glasgow, H.B. New ‘phantom’ dinoflagellate is the causative agent of major estuarine fish kills. Nature 1992, 358, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Hallegraeff, G.; Enevoldsen, H.; Zingone, A. Global harmful algal bloom status reporting. Harmful Algae 2021, 102, 101992. [Google Scholar] [CrossRef]
- Acuña-Alonso, C.; Álvarez, X.; Valero, E.; Pacheco, F.A.L. Modelling of threats that affect Cyano-HABs in an eutrophicated reservoir: First phase towards water security and environmental governance in watersheds. Sci. Total Environ. 2021, 809, 152155. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef]
- Kumar, K.P.; Kumar, S.P.; Nair, G.A. Risk assessment of the amnesic shellfish poison, domoic acid, on animals and humans. J. Environ. Biol. 2009, 30, 319–325. [Google Scholar]
- Paerl, H.W.; Otten, T.G. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Carmichael, W.W. Health effects of toxin-producing cyanobacteria: “The CyanoHABs”. Hum. Ecol. Risk Assess. 2001, 7, 1393–1407. [Google Scholar] [CrossRef]
- Sha, J.; Xiong, H.; Li, C.; Lu, Z.; Zhang, J.; Zhong, H.; Zhang, W.; Yan, B. Harmful algal blooms and their eco-environmental indication. Chemosphere 2021, 274, 129912. [Google Scholar] [CrossRef] [PubMed]
- Balaji-Prasath, B.; Wang, Y.; Su, Y.P.; Hamilton, D.P.; Lin, H.; Zheng, L.; Zhang, Y. Methods to control harmful algal blooms: A review. Environ. Chem. Lett. 2022, 20, 3133–3152. [Google Scholar] [CrossRef]
- Wang, J.; Beusen, A.H.W.; Liu, X.; Bouwman, A.F. Aquaculture Production is a Large, Spatially Concentrated Source of Nutrients in Chinese Freshwater and Coastal Seas. Environ. Sci. Technol. 2020, 54, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Pan, G. A Universal Method for Flocculating Harmful Algal Blooms in Marine and Fresh Waters Using Modified Sand. Environ. Sci. Technol. 2013, 47, 4555–4562. [Google Scholar] [CrossRef]
- Rosa, M.; Ward, J.E.; Holohan, B.A.; Shumway, S.E.; Wikfors, G.H. Physicochemical surface properties of microalgae and their combined effects on particle selection by suspension-feeding bivalve molluscs. J. Exp. Mar. Biol. Ecol. 2017, 486, 59–68. [Google Scholar] [CrossRef]
- Li, M.; Chen, D.; Liu, Y.; Chuang, C.Y.; Kong, F.; Harrison, P.J.; Zhu, X.; Jiang, Y. Exposure of engineered nanoparticles to Alexandrium tamarense (Dinophyceae): Healthy impacts of nanoparticles via toxin-producing dinoflagellate. Sci. Total Environ. 2018, 610–611, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Lee, H.-J.; Kim, M.S.; Park, N.-B.; Lee, C. Control of the red tide dinoflagellate Cochlodinium polykrikoides by ozone in seawater. Water Res. 2017, 109, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, L.; Aguilera, A.; Echenique, R.; Andrinolo, D.; Giannuzzi, L. Application of Hydrogen Peroxide to the Control of Eutrophic Lake Systems in Laboratory Assays. Toxins 2014, 6, 2657–2675. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Schideman, L.; Yu, G.; Zhang, Y. A synergistic combination of algal wastewater treatment and hydrothermal biofuel production maximized by nutrient and carbon recycling. Energy Environ. Sci. 2013, 6, 3765–3779. [Google Scholar] [CrossRef]
- Grattan, L.M.; Holobaugh, S.; Morris, J.G., Jr. Harmful algal blooms and public health. Harmful Algae 2016, 57, 2–8. [Google Scholar] [CrossRef]
- Beretta-Blanco, A.; Carrasco-Letelier, L. Relevant factors in the eutrophication of the Uruguay River and the Río Negro. Sci. Total Environ. 2020, 761, 143299. [Google Scholar] [CrossRef]
- Xiao, X.; Li, C.; Huang, H.; Lee, Y.P. Inhibition effect of natural flavonoids on red tide alga Phaeocystis globosa and its quantitative structure-activity relationship. Environ. Sci. Pollut. Res. 2019, 26, 23763–23776. [Google Scholar] [CrossRef]
- Coloma, S.E.; Dienstbier, A.; Bamford, D.H.; Sivonen, K.; Roine, E.; Hiltunen, T. Newly isolated Nodularia phage influences cyanobacterial community dynamics. Environ. Microbiol. 2017, 19, 273–286. [Google Scholar] [CrossRef]
- Humbert, J.-F.; Quiblier, C. The Suitability of Chemical Products and Other Short-Term Remedial Methods for the Control of Cyanobacterial Blooms in Freshwater Ecosystems. Front. Environ. Sci. 2019, 7, 176. [Google Scholar] [CrossRef]
- Lin, C.; Fugetsu, B.; Su, Y.; Watari, F. Studies on toxicity of multi-walled carbon nanotubes on Arabidopsis T87 suspension cells. J. Hazard. Mater. 2009, 170, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.A.K.; Reddy, P.V.L.; Kwon, E.; Kim, K.-H.; Akter, T.; Kalagara, S. Recent advances in photocatalytic treatment of pollutants in aqueous media. Environ. Int. 2016, 91, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.V.L.; Kavitha, B.; Reddy, P.A.K.; Kim, K.-H. TiO2-based photocatalytic disinfection of microbes in aqueous media: A review. Environ. Res. 2017, 154, 296–303. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, Z.; Yan, Z.; Zhou, G.; Zhang, W.; Wang, Y.; Li, X. Defense pathways of Chlamydomonas reinhardtii under silver nanoparticle stress: Extracellular biosorption, internalization and antioxidant genes. Chemosphere 2021, 291 Pt 1, 132764. [Google Scholar] [CrossRef]
- Fan, G.; Du, B.; Zhou, J.; Yu, W.; Chen, Z.; Yang, S. Stable Ag2O/g-C3N4 p-n heterojunction photocatalysts for efficient inactivation of harmful algae under visible light. Appl. Catal. B Environ. 2020, 265, 118610. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.; Lao, Y.; Lv, X.; Tao, Y.; Huang, B.; Wang, J.; Zhou, J.; Cai, Z. TiO2 nanoparticles in the marine environment: Physical effects responsible for the toxicity on algae Phaeodactylum tricornutum. Sci. Total Environ. 2016, 565, 818–826. [Google Scholar] [CrossRef]
- Hu, L.; Chen, J.; Wei, Y.; Wang, M.; Xu, Y.; Wang, C.; Gao, P.; Liu, Y.; Liu, C.; Song, Y.; et al. Photocatalytic degradation effect and mechanism of Karenia mikimotoi by non-noble metal modified TiO2 loading onto copper metal organic framework (SNP-TiO2@Cu-MOF) under visible light. J. Hazard. Mater. 2023, 442, 130059. [Google Scholar] [CrossRef]
- Miller, R.J.; Muller, E.B.; Cole, B.; Martin, T.; Nisbet, R.; Bielmyer-Fraser, G.K.; Jarvis, T.A.; Keller, A.A.; Cherr, G.; Lenihan, H.S. Photosynthetic efficiency predicts toxic effects of metal nanomaterials in phytoplankton. Aquat. Toxicol. 2017, 183, 85–93. [Google Scholar] [CrossRef]
- Aruoja, V.; Pokhrel, S.; Sihtmäe, M.; Mortimer, M.; Mädler, L.; Kahru, A. Toxicity of 12 metal-based nanoparticles to algae, bacteria and protozoa. Environ. Sci. Nano 2015, 2, 630–644. [Google Scholar] [CrossRef]
- Yang, Z.; Hou, J.; Wu, M.; Miao, L.; Wu, J.; Li, Y. A novel co-graft tannin-based flocculant for the mitigation of harmful algal blooms (HABs): The effect of charge density and molecular weight. Sci. Total Environ. 2021, 806 Pt 1, 150518. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.; Dumitrescu, E.; Andreescu, D.; Andreescu, S. Nanoporous Sorbents for the Removal and Recovery of Phosphorus from Eutrophic Waters: Sustainability Challenges and Solutions. ACS Sustain. Chem. Eng. 2018, 6, 12542–12561. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Jin, J.; Li, L.; Zhang, L.; Luan, Z.; Xin, S.; Song, K. Progress in the Application of Carbon Dots-Based Nanozymes. Front. Chem. 2021, 9, 748044. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, X.; Zhu, T.; Deng, M.; Ikechukwu, I.P.; Huang, W.; Yin, G.; Bai, Y.; Qu, D.; Huang, X.; et al. All-inorganic CsPbBr3 perovskite quantum dots as a photoluminescent probe for ultrasensitive Cu2+ detection. J. Mater. Chem. C 2018, 6, 4793–4799. [Google Scholar] [CrossRef]
- Du, M.; Huo, B.; Liu, J.; Li, M.; Shen, A.; Bai, X.; Lai, Y.; Fang, L.; Yang, Y. A turn-on fluorescent probe based on Si-rhodamine for sensitive and selective detection of phosgene in solution and in the gas phase. J. Mater. Chem. C 2018, 6, 10472–10479. [Google Scholar] [CrossRef]
- Wang, G.; Wang, S.; Yan, C.; Bai, G.; Liu, Y. DNA-functionalized gold nanoparticle-based fluorescence polarization for the sensitive detection of silver ions. Colloids Surf. B Biointerfaces 2018, 167, 150–155. [Google Scholar] [CrossRef]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Guo, Z.; Kim, G.-H.; Shin, I.; Yoon, J. A cyanine-based fluorescent sensor for detecting endogenous zinc ions in live cells and organisms. Biomaterials 2012, 33, 7818–7827. [Google Scholar] [CrossRef]
- Jovanović, S.; Marković, Z.; Budimir, M.; Prekodravac, J.; Zmejkoski, D.; Kepić, D.; Bonasera, A.; Marković, B.T. Lights and Dots toward Therapy—Carbon-Based Quantum Dots as New Agents for Photodynamic Therapy. Pharmaceutics 2023, 15, 1170. [Google Scholar] [CrossRef]
- Baranwal, A.; Srivastava, A.; Kumar, P.; Bajpai, V.K.; Maurya, P.K.; Chandra, P. Prospects of Nanostructure Materials and Their Composites as Antimicrobial Agents. Front. Microbiol. 2018, 9, 422. [Google Scholar] [CrossRef]
- Sakdaronnarong, C.; Sangjan, A.; Boonsith, S.; Kim, D.C.; Shin, H.S. Recent Developments in Synthesis and Photocatalytic Applications of Carbon Dots. Catalysts 2020, 10, 320. [Google Scholar] [CrossRef]
- Marković, Z.M.; Kováčová, M.; Jeremić, S.R.; Nagy, Š.; Milivojević, D.D.; Kubat, P.; Kleinová, A.; Budimir, M.D.; Mojsin, M.M.; Stevanović, M.J.; et al. Highly Efficient Antibacterial Polymer Composites Based on Hydrophobic Riboflavin Carbon Polymerized Dots. Nanomaterials 2022, 12, 4070. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, V.; Banerjee, S.; Roy, P.; Bhatt, A.K. Fluorescent xylitol carbon dots: A potent antimicrobial agent and drug carrier. Biotechnol. Appl. Biochem. 2021, 69, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sheng, L.; Yang, X.; Sun, J.; Ye, Y.; Geng, S.; Ning, D.; Zheng, J.; Fan, M.; Zhang, Y.; et al. Natural biomass-derived carbon dots as potent antimicrobial agents against multidrug-resistant bacteria and their biofilms. Sustain. Mater. Technol. 2023, 36, e00584. [Google Scholar] [CrossRef]
- Dong, X.; Liang, W.; Meziani, M.J.; Sun, Y.-P.; Yang, L. Carbon Dots as Potent Antimicrobial Agents. Theranostics 2020, 10, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kumar, G.; Agrawal, V. Green synthesis of silver nanoparticles using Holarrhena antidysenterica (L.) Wall. bark extract and their larvicidal activity against dengue and filariasis vectors. Parasitol. Res. 2017, 117, 377–389. [Google Scholar] [CrossRef]
- Liu, J.; Lu, J.-F.; Kan, J.; Tang, Y.-Q.; Jin, C.-H. Preparation, characterization and antioxidant activity of phenolic acids grafted carboxymethyl chitosan. Int. J. Biol. Macromol. 2013, 62, 85–93. [Google Scholar] [CrossRef]
- Guo, F.; Li, M.; Ren, H.; Huang, X.; Shu, K.; Shi, W.; Lu, C. Facile bottom-up preparation of Cl-doped porous g-C3N4 nanosheets for enhanced photocatalytic degradation of tetracycline under visible light. Sep. Purif. Technol. 2019, 228, 115770. [Google Scholar] [CrossRef]
- Li, H.; Ye, S.; Guo, J.; Wang, H.; Yan, W.; Song, J.; Qu, J. Biocompatible carbon dots with low-saturation-intensity and high-photobleaching-resistance for STED nanoscopy imaging of the nucleolus and tunneling nanotubes in living cells. Nano Res. 2019, 12, 3075–3084. [Google Scholar] [CrossRef]
- Dufour, T.; Minnebo, J.; Rich, S.A.; Neyts, E.C.; Bogaerts, A.; Reniers, F. Understanding polyethylene surface functionalization by an atmospheric He/O2plasma through combined experiments and simulations. J. Phys. D Appl. Phys. 2014, 47, 224007. [Google Scholar] [CrossRef]
- Weaver, J.F.; Hoflund, G.B. Surface Characterization Study of the Thermal Decomposition of AgO. J. Phys. Chem. 1994, 98, 8519–8524. [Google Scholar] [CrossRef]
- Zeng, Y.; Xu, Z.; Guo, J.; Yu, X.; Zhao, P.; Song, J.; Qu, J.; Chen, Y.; Li, H. Bifunctional Nitrogen and Fluorine Co-Doped Carbon Dots for Selective Detection of Copper and Sulfide Ions in Real Water Samples. Molecules 2022, 27, 5149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, C.; Sun, L.; Zhang, J.; Yang, X.; Ma, H. Defects coordination triggers red-shifted photoluminescence in carbon dots and their application in ratiometric Cr(VI) sensing. Microchem. J. 2021, 169, 106552. [Google Scholar] [CrossRef]
- Elistratova, J.; Mukhametshina, A.; Kholin, K.; Nizameev, I.; Mikhailov, M.; Sokolov, M.; Khairullin, R.; Miftakhova, R.; Shammas, G.; Kadirov, M.; et al. Interfacial uploading of luminescent hexamolybdenum cluster units onto amino-decorated silica nanoparticles as new design of nanomaterial for cellular imaging and photodynamic therapy. J. Colloid Interface Sci. 2019, 538, 387–396. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, Y.; Wamer, W.G.; Yin, J.-J. Electron spin resonance spectroscopy for the study of nanomaterial-mediated generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 49–63. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef]
- Král'Ová, K.; Sersen, F.; Blahová, M. Effects of Cu(II) complexes on photosynthesis in spinach chloroplasts. Aqua(aryloxyacetato)copper(II) complexes. Gen. Physiol. Biophys. 1994, 13, 483–491. [Google Scholar]
- Villeneuve, L.; Alberti, L.; Steghens, J.-P.; Lancelin, J.-M.; Mestas, J.-L. Assay of hydroxyl radicals generated by focused ultrasound. Ultrason. Sonochem. 2009, 16, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Watanabe, Y. Tryptophan protects hepatocytes against reactive oxygen species-dependent cell death via multiple pathways including Nrf2-dependent gene induction. Amino Acids 2016, 48, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Zhang, M.L.; Pu, L.; Gai, P.P.; Li, F. Nitrogen-Enriched Conjugated Polymer Enabled Metal-Free Carbon Nanozymes with Efficient Oxidase-Like Activity. Small 2022, 18, 2104993. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, C.; Tan, L.; Wang, J. Toxicity of Co nanoparticles on three species of marine microalgae. Environ. Pollut. 2018, 236, 454–461. [Google Scholar] [CrossRef]
- Gao, N.; Jing, J.; Zhao, H.; Liu, Y.; Yang, C.; Gao, M.; Chen, B.; Zhang, R.; Zhang, X. Defective Ag–In–S/ZnS quantum dots: An oxygen-derived free radical scavenger for mitigating macrophage inflammation. J. Mater. Chem. B 2021, 9, 8971–8979. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Xu, Z.; Li, H.; Chen, Y.; Guo, J. Visible-Light-Activated Carbon Dot Photocatalyst for ROS-Mediated Inhibition of Algae Growth. Int. J. Mol. Sci. 2023, 24, 13509. https://doi.org/10.3390/ijms241713509
Song J, Xu Z, Li H, Chen Y, Guo J. Visible-Light-Activated Carbon Dot Photocatalyst for ROS-Mediated Inhibition of Algae Growth. International Journal of Molecular Sciences. 2023; 24(17):13509. https://doi.org/10.3390/ijms241713509
Chicago/Turabian StyleSong, Jun, Zhibin Xu, Hao Li, Yu Chen, and Jiaqing Guo. 2023. "Visible-Light-Activated Carbon Dot Photocatalyst for ROS-Mediated Inhibition of Algae Growth" International Journal of Molecular Sciences 24, no. 17: 13509. https://doi.org/10.3390/ijms241713509
APA StyleSong, J., Xu, Z., Li, H., Chen, Y., & Guo, J. (2023). Visible-Light-Activated Carbon Dot Photocatalyst for ROS-Mediated Inhibition of Algae Growth. International Journal of Molecular Sciences, 24(17), 13509. https://doi.org/10.3390/ijms241713509




