Unraveling the Angiogenic Puzzle: Pre-Treatment sVEGFR1 and sVEGFR2 Levels as Promising Prognostic Indicators in Early-Stage Breast Cancer Patients
Abstract
1. Introduction
2. Results
2.1. Patient-Specific Data
2.2. Assessment of Angiogenic Parameters Regarding the Type of Treatment
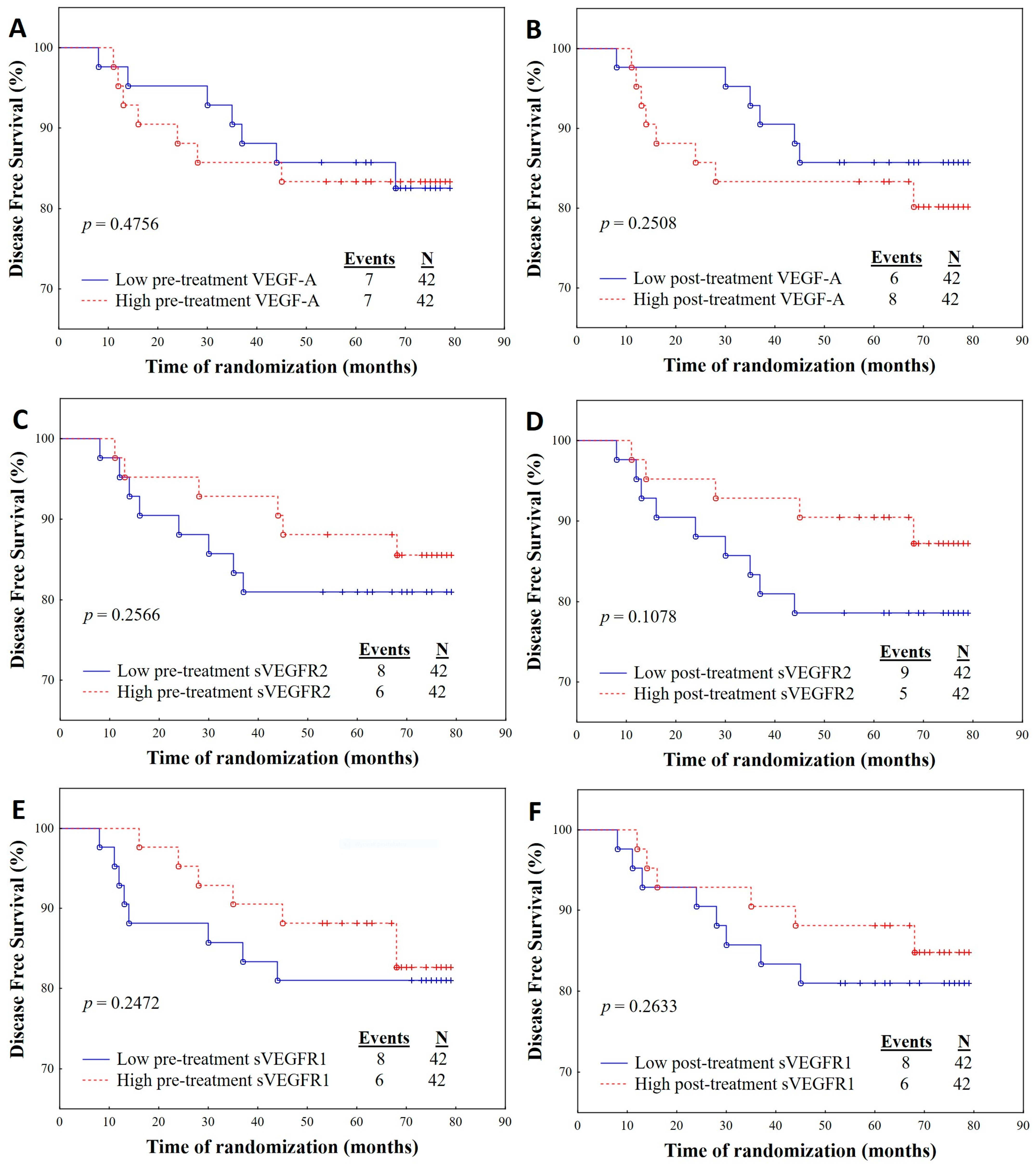
2.3. Survival Analysis Regarding Angiogenic Parameters
2.4. Association of OS and DFS with Angiogenic Parameters
2.5. Association of Angiogenic Parameters with DFS in Linear Regression Models
3. Discussion
Limitations and Strengths of the Study
4. Materials and Methods
4.1. Study Design
4.2. Adjuvant Treatment
4.3. Patient Outcomes
4.4. Methods
4.4.1. Blood Sampling and Angiogenic Parameters Evaluation
4.4.2. Immunohistochemistry
4.5. Statistical Analysis
4.6. Ethics Approval
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Tsang, J.Y.S.; Tse, G.M. Molecular Classification of Breast Cancer. Adv. Anat. Pathol. 2019, 27, 27–35. [Google Scholar] [CrossRef]
- Chen, W.; Hoffmann, A.D.; Liu, H.; Liu, X. Organotropism: New insights into molecular mechanisms of breast cancer metastasis. NPJ Precis. Oncol. 2018, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Farahmand, L. Breast cancer: Biology, biomarkers, and treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, H.; Song, X.; Yang, Q. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin. Cancer Biol. 2019, 60, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Electronic address: Clinicalguidelines@esmo.org (2019). Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef]
- Schneider, B.P.; Miller, K.D. Angiogenesis of breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, N.M.; Jaradat, S.K.; Al-Shami, K.M.; Alkhalifa, A.E. Targeting Angiogenesis in Breast Cancer: Current Evidence and Future Perspectives of Novel Anti-Angiogenic Approaches. Front. Pharmacol. 2022, 13, 838133. [Google Scholar] [CrossRef]
- Badodekar, N.; Sharma, A.; Patil, V.; Telang, G.; Sharma, R.; Patil, S.; Vyas, N.; Somasundaram, I. Angiogenesis induction in breast cancer: A paracrine paradigm. Cell Biochem. Funct. 2021, 39, 860–873. [Google Scholar] [CrossRef]
- Barron, G.A.; Goua, M.; Wahle, K.W.; Bermano, G. Circulating levels of angiogenesis-related growth factors in breast cancer: A study to profile proteins responsible for tubule formation. Oncol. Rep. 2017, 38, 1886–1894. [Google Scholar] [CrossRef]
- Longatto Filho, A.; Lopes, J.M.; Schmitt, F.C. Angiogenesis and breast cancer. J. Oncol. 2010, 2010, 576384. [Google Scholar] [CrossRef] [PubMed]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. Rev. Roum. De Morphol. Et Embryol. 2018, 59, 455–467. [Google Scholar]
- Vasudev, N.S.; Reynolds, A.R. Anti-angiogenic therapy for cancer: Current progress, unresolved questions and future directions. Angiogenesis 2014, 17, 471–494. [Google Scholar] [CrossRef]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Madu, C.O.; Wang, S.; Madu, C.O.; Lu, Y. Angiogenesis in Breast Cancer Progression, Diagnosis, and Treatment. J. Cancer 2020, 11, 4474–4494. [Google Scholar] [CrossRef] [PubMed]
- Wiszniak, S.; Schwarz, Q. Exploring the Intracrine Functions of VEGF-A. Biomolecules 2021, 11, 128. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69 (Suppl. 3), 4–10. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Ema, M. Roles of VEGF-A signalling in development, regeneration, and tumors. J. Biochem. 2014, 156, 1–10. [Google Scholar] [CrossRef]
- Thielemann, A.; Baszczuk, A.; Kopczyński, Z.; Kopczyński, P.; Grodecka-Gazdecka, S. Clinical usefulness of assessing VEGF and soluble receptors sVEGFR-1 and sVEGFR-2 in women with breast cancer. Ann. Agric. Environ. Med. AAEM 2013, 20, 293–297. [Google Scholar]
- Goedegebuure, R.; de Klerk, L.K.; Bass, A.J.; Derks, S.; Thijssen, V. Combining Radiotherapy with Anti-angiogenic Therapy and Immunotherapy; A Therapeutic Triad for Cancer? Front. Immunol. 2019, 9, 3107. [Google Scholar] [CrossRef]
- Grabham, P.; Sharma, P. The effects of radiation on angiogenesis. Vasc. Cell 2013, 5, 19. [Google Scholar] [CrossRef]
- Karsten, M.M.; Beck, M.H.; Rademacher, A.; Knabl, J.; Blohmer, J.U.; Jückstock, J.; Radosa, J.C.; Jank, P.; Rack, B.; Janni, W. VEGF-A165b levels are reduced in breast cancer patients at primary diagnosis but increase after completion of cancer treatment. Sci. Rep. 2020, 10, 3635. [Google Scholar] [CrossRef]
- Banerjee, S.; Pancholi, S.; A’hern, R.; Ghazoui, Z.; Smith, I.E.; Dowsett, M.; Martin, L.A. The effects of neoadjuvant anastrozole and tamoxifen on circulating vascular endothelial growth factor and soluble vascular endothelial growth factor receptor 1 in breast cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 2656–2663. [Google Scholar] [CrossRef][Green Version]
- Shibuya, M. Vascular endothelial growth factor and its receptor system: Physiological functions in angiogenesis and pathological roles in various diseases. J. Biochem. 2013, 153, 13–19. [Google Scholar] [CrossRef]
- Simons, M. An inside view: VEGF receptor trafficking and signaling. Physiology 2012, 27, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Claesson-Welsh, L. VEGF receptor signal transduction—A brief update. Vasc. Pharmacol. 2016, 86, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct. Funct. 2001, 26, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Toi, M.; Bando, H.; Ogawa, T.; Muta, M.; Hornig, C.; Weich, H.A. Significance of vascular endothelial growth factor (VEGF)/soluble VEGF receptor-1 relationship in breast cancer. Int. J. Cancer 2002, 98, 14–18. [Google Scholar] [CrossRef] [PubMed]
- El Tarhouny, S.; Seefeld, M.; Fan, A.X.; Hahn, S.; Holzgreve, W.; Zhong, X.Y. Comparison of serum VEGF and its soluble receptor sVEGFR1 with serum cell-free DNA in patients with breast tumor. Cytokine 2008, 44, 65–69. [Google Scholar] [CrossRef]
- Jain, R.K. Normalizing tumor microenvironment to treat cancer: Bench to bedside to biomarkers. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Pavlakovic, H.; Becker, J.; Albuquerque, R.; Wilting, J.; Ambati, J. Soluble VEGFR-2: An antilymphangiogenic variant of VEGF receptors. Ann. N. Y. Acad. Sci. 2010, 1207 (Suppl. 1), E7–E15. [Google Scholar] [CrossRef]
- Ebos, J.M.; Lee, C.R.; Bogdanovic, E.; Alami, J.; Van Slyke, P.; Francia, G.; Xu, P.; Mutsaers, A.J.; Dumont, D.J.; Kerbel, R.S. Vascular endothelial growth factor-mediated decrease in plasma soluble vascular endothelial growth factor receptor-2 levels as a surrogate biomarker for tumor growth. Cancer Res. 2008, 68, 521–529. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.; Hicks, D.G. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch. Pathol. Lab. Med. 2014, 138, 241–256. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | Number of Patients (%) |
|---|---|
| Age [years] | |
| <50 | 23 (27) |
| ≥50 | 61 (73) |
| Menopausal status | |
| Premenopausal | 26 (31) |
| Postmenopausal | 58 (69) |
| BMI [kg/m2] | |
| 18.5–24.9 | 42 (50) |
| 25–29.9 | 28 (33) |
| ≥30 | 14 (17) |
| Parity | |
| Nulliparous | 8 (10) |
| Parous | 76 (90) |
| Cigarette smoking | |
| Smoker | 20 (24) |
| Non-smoker | 64 (76) |
| Stage | |
| IA | 42 (50) |
| IB | 0 (0) |
| IIA | 37 (44) |
| IIB | 5 (6) |
| Tumor diameter | |
| T1a | 5 (6) |
| T1b | 8 (10) |
| T1c | 44 (52) |
| T2 | 27 (32) |
| Nodal involvement | |
| Negative | 64 (76) |
| Positive | 20 (24) |
| Intrinsic type | |
| Luminal A | 51 (61) |
| Luminal B HER2(-) | 16 (19) |
| Luminal B HER2(+) | 5 (6) |
| Non-luminal HER2(+) | 3 (3) |
| Triple-negative | 9 (11) |
| Histological type | |
| Invasive ductal | 73 (87) |
| Invasive lobular | 11 (13) |
| Grading | |
| 1 | 4 (5) |
| 2 | 64 (76) |
| 3 | 16 (19) |
| Disease recurrence | |
| Yes | 4 |
| No | 80 |
| DFS (months) | |
| Median (IQR) | 70.5 (62–77) |
| Died during follow-up | |
| Yes | 10 |
| No | 74 |
| OS (months) | |
| Median (IQR) | 72.5 (63–77) |
| Feature/ Number of Patients (%) | Pre-Treatment VEGF-A Concentration (pg/mL) | Post-Treatment VEGF-A Concentration (pg/mL) | |
|---|---|---|---|
| Median (Q1–Q3) | Median (Q1–Q3) | p-Values | |
| BCS + Radiotherapy n = 70 (83%) | 65.85 (44.51–116.35) | 106.90 (58.14–161.70) | 0.0028 |
| Mastectomy n = 14 (17%) | 53.17 (38.41–102.82) | 55.02 (34.78–110.20) | 0.7893 |
| Chemotherapy | |||
| Anthracycline n = 30 (36%) | 52.005 (38.41–76.83) | 83.33 (37.31–145.20) | 0.2012 |
| Non-anthracycline n = 8 (9%) | 62.69 (43.43–108.60) | 80.58 (61.44–115.76) | 0.2888 |
| No n= 46 (55%) | 82.63 (39.67–126.40) | 114.05 (56.43–161.70) | 0.1048 |
| Endocrine therapy | |||
| Tamoxifen n = 41 (49%) | 56.32 (39.67–111.09) | 98.43 (49.77–144.40) | 0.2115 |
| Inhibitor aromatase n = 19 (23%) | 66.83 (32.47–118.34) | 105.5 (56.43–163.10) | 0.0665 |
| Tamoxifen and inhibitor aromatase n = 7 (8%) | 74.12 (38.41–126.93) | 52.61 (28.98–161.70) | 1.0000 |
| Other type n = 3 (4%) | 64.87 (37.45–81.45) | 68.14 (52.25–96.96) | 0.2482 |
| No n = 14 (17%) | 80.84 (49.70–115.39) | 130.15 (81.75–156.70) | 0.4227 |
| Feature/ Number of Patients (%) | Pre-Treatment sVEGFR1 Concentration (pg/mL) | Post-Treatment sVEGFR1 Concentration (pg/mL) | |
|---|---|---|---|
| Median (Q1–Q3) | Median (Q1–Q3) | p-Values | |
| BCS + Radiotherapy n = 70 (83%) | 31.40 (22.27–87.12) | 343.20 (218.80–392.10) | 0.0001 |
| Mastectomy n = 14 (17%) | 27.09(19.60–76.27) | 282.95 (255.60–362.90) | 0.0005 |
| Chemotherapy | |||
| Anthracycline n = 30 (36%) | 29.58 (22.27–76.27) | 292.25 (217.90–363.90) | 0.0001 |
| Non-anthracycline n = 8 (9%) | 51.98 (18.89–84.39) | 264.40 (222.15–329.20) | 0.0133 |
| No n = 46 (55%) | 32.09 (19.68–97.24) | 354.00 (255.60–407.30) | 0.0001 |
| Endocrine therapy | |||
| Tamoxifen n = 41 (49%) | 30.00 (19.20–87.12) | 345.32 (249.20–407.30) | 0.0001 |
| Inhibitor aromatase n = 19 (23%) | 41.99 (19.60–97.24) | 343.20 (194.10–381.80) | 0.0001 |
| Tamoxifen and inhibitor aromatase n = 7 (8%) | 22.46 (15.25–37.81) | 295.50 (262.10–448.10) | 0.0233 |
| Other type n = 3 (4%) | 82.14 (79.80–131.29) | 262.10 (133.70–404.40) | 0.2482 |
| No n = 14 (17%) | 29.58 (22.27–83.31) | 320.70 (233.50–386.50) | 0.0005 |
| Feature/ Number of Patients (%) | Pre-Treatment sVEGFR2 Concentration (pg/mL) | Post-Treatment sVEGFR2 Concentration (pg/mL) | |
|---|---|---|---|
| Median (Q1–Q3) | Median (Q1–Q3) | p-Values | |
| BCS + Radiotherapy n = 70 (83%) | 9275.74 (7418.77–11,955.8) | 7397.15 (6800.0–8660.0) | 0.0001 |
| Mastectomy n = 14 (17%) | 10,140.80 (8206.39–12,913.9) | 7165.78 (6773.85–8770.0) | 0.0033 |
| Chemotherapy | |||
| Anthracycline n = 30 (36%) | 8751.85 (6680.40–11,092.93) | 7327.40 (6316.18–8605.25) | 0.0176 |
| Non-anthracycline n = 8 (9%) | 8773.58 (8287.31–11,612.07) | 7113.08 (6817.50–8909.08) | 0.0771 |
| No n = 46 (55%) | 9990.63 (7839.68–12,417.90) | 7422.15 (6800.00–8759.71) | 0.0002 |
| Endocrine therapy | |||
| Tamoxifen n = 41 (49%) | 9868.01 (7740.15–12,417.90) | 7458.00 (6825.00–8716.72) | 0.0018 |
| Inhibitor aromatase n = 19 (23%) | 9581.91 (7582.57–12,426.22) | 7400.00 (7061.47–8465.00) | 0.0059 |
| Tamoxifen and inhibitor aromatase n = 7 (8%) | 9863.15 (8967.11–10,989.35) | 8977.75 (6778.25–9392.83) | 0.4497 |
| Other type n = 3 (4%) | 8216.21 (7839.68–9805.00) | 6825.00 (6355.00–7310.00) | 0.2482 |
| No n = 14 (17%) | 8770.23 (6559.79–12,113.50) | 7089.92 (6109.41–8405.00) | 0.1814 |
| OS | DFS | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p-Values | HR (95% CI) | p-Values |
| VEGF-A pre-treatment Low High | 1.4607 (0.4121–5.1774) | 0.5572 | 0.9006 (0.5625–1.4422) | 0.6632 |
| VEGF-A post-treatment Low High | 0.9960 (0.2883–3.4415) | 0.9950 | 0.9412 (0.5878–1.5072) | 0.8009 |
| sVEGFR1 pre-treatment Low High | 2.3581 (0.6065–9.1689) | 0.2157 | 0.3104 (0.1898–0.5075) | 0.0001 |
| sVEGFR1 post-treatment Low High | 1.6293 0.4597–5.7753) | 0.4496 | 1.1525 (0.7201–1.8444) | 0.5541 |
| sVEGFR2 pre-treatment Low High | 1.6702 (0.4667–5.9767) | 0.4304 | 2.9558 (1.8071–4.8344) | 0.0001 |
| sVEGFR2 post-treatment Low High | 4.4138 (0.9362–20.8098) | 0.0606 | 0.9894 (0.6186–1.5825) | 0.9646 |
| OS | DFS | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p-Values | HR (95% CI) | p-Values |
| VEGF-A pre-treatment Low High | 2.0519 (0.5063–8.3163) | 0.3141 | 0.6273 (0.3656–1.0764) | 0.0905 |
| VEGF-A post-treatment Low High | 0.5997 (0.1345–2.6723) | 0.5024 | 0.7312 (0.3999–1.3370) | 0.3093 |
| sVEGFR1 pre-treatment Low High | 2.9007 (0.6533–12.8791) | 0.1614 | 0.2170 (0.1204–0.3912) | 0.0001 |
| sVEGFR1 post-treatment Low High | 3.0377 (0.7299–12.6419) | 0.1267 | 1.1074 (0.6279–1.9531) | 0.7243 |
| sVEGFR2 pre-treatment Low High | 2.1030 (0.5232–8.4531) | 0.2949 | 4.2506 (2.3292–7.7571) | 0.0001 |
| sVEGFR2 post-treatment Low High | 4.1921 (0.8591–20.4557) | 0.0764 | 1.0415 (0.6114–1.7743) | 0.8810 |
| Model 1 | Model 2 | Model 3 | Model 4 | ||
|---|---|---|---|---|---|
| VEGF-A pre-treatment | Beta p-value | 0.1898 0.1576 | 0.2047 0.1288 | 0.2183 0.0995 | 0.2958 0.0308 |
| VEGF-A post-treatment | Beta p-value | −0.0247 0.8580 | 0.0071 0.9590 | 0.0157 0.9090 | −0.0506 0.7116 |
| sVEGFR1 pre-treatment | Beta p-value | −0.1823 0.1696 | −0.2218 0.1003 | −0.2578 0.0499 | −0.1425 0.2836 |
| sVEGFR1 post-treatment | Beta p-value | −0.0541 0.6385 | −0.0815 0.4901 | −0.1149 0.3274 | −0.0312 0.7950 |
| sVEGFR2 pre-treatment | Beta p-value | −0.1335 0.3537 | −0.1819 0.2181 | −0.1600 0.2651 | −0.0816 0.5839 |
| sVEGFR2 post-treatment | Beta p-value | −0.0345 0.7741 | −0.0252 0.8357 | −0.0264 0.8257 | 0.0393 0.7471 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarychta, E.; Bielawski, K.; Wrzeszcz, K.; Rhone, P.; Ruszkowska-Ciastek, B. Unraveling the Angiogenic Puzzle: Pre-Treatment sVEGFR1 and sVEGFR2 Levels as Promising Prognostic Indicators in Early-Stage Breast Cancer Patients. Int. J. Mol. Sci. 2023, 24, 13508. https://doi.org/10.3390/ijms241713508
Zarychta E, Bielawski K, Wrzeszcz K, Rhone P, Ruszkowska-Ciastek B. Unraveling the Angiogenic Puzzle: Pre-Treatment sVEGFR1 and sVEGFR2 Levels as Promising Prognostic Indicators in Early-Stage Breast Cancer Patients. International Journal of Molecular Sciences. 2023; 24(17):13508. https://doi.org/10.3390/ijms241713508
Chicago/Turabian StyleZarychta, Elżbieta, Kornel Bielawski, Katarzyna Wrzeszcz, Piotr Rhone, and Barbara Ruszkowska-Ciastek. 2023. "Unraveling the Angiogenic Puzzle: Pre-Treatment sVEGFR1 and sVEGFR2 Levels as Promising Prognostic Indicators in Early-Stage Breast Cancer Patients" International Journal of Molecular Sciences 24, no. 17: 13508. https://doi.org/10.3390/ijms241713508
APA StyleZarychta, E., Bielawski, K., Wrzeszcz, K., Rhone, P., & Ruszkowska-Ciastek, B. (2023). Unraveling the Angiogenic Puzzle: Pre-Treatment sVEGFR1 and sVEGFR2 Levels as Promising Prognostic Indicators in Early-Stage Breast Cancer Patients. International Journal of Molecular Sciences, 24(17), 13508. https://doi.org/10.3390/ijms241713508








