Prostate Cancer Stem Cells: Biology and Treatment Implications
Abstract
:1. Introduction
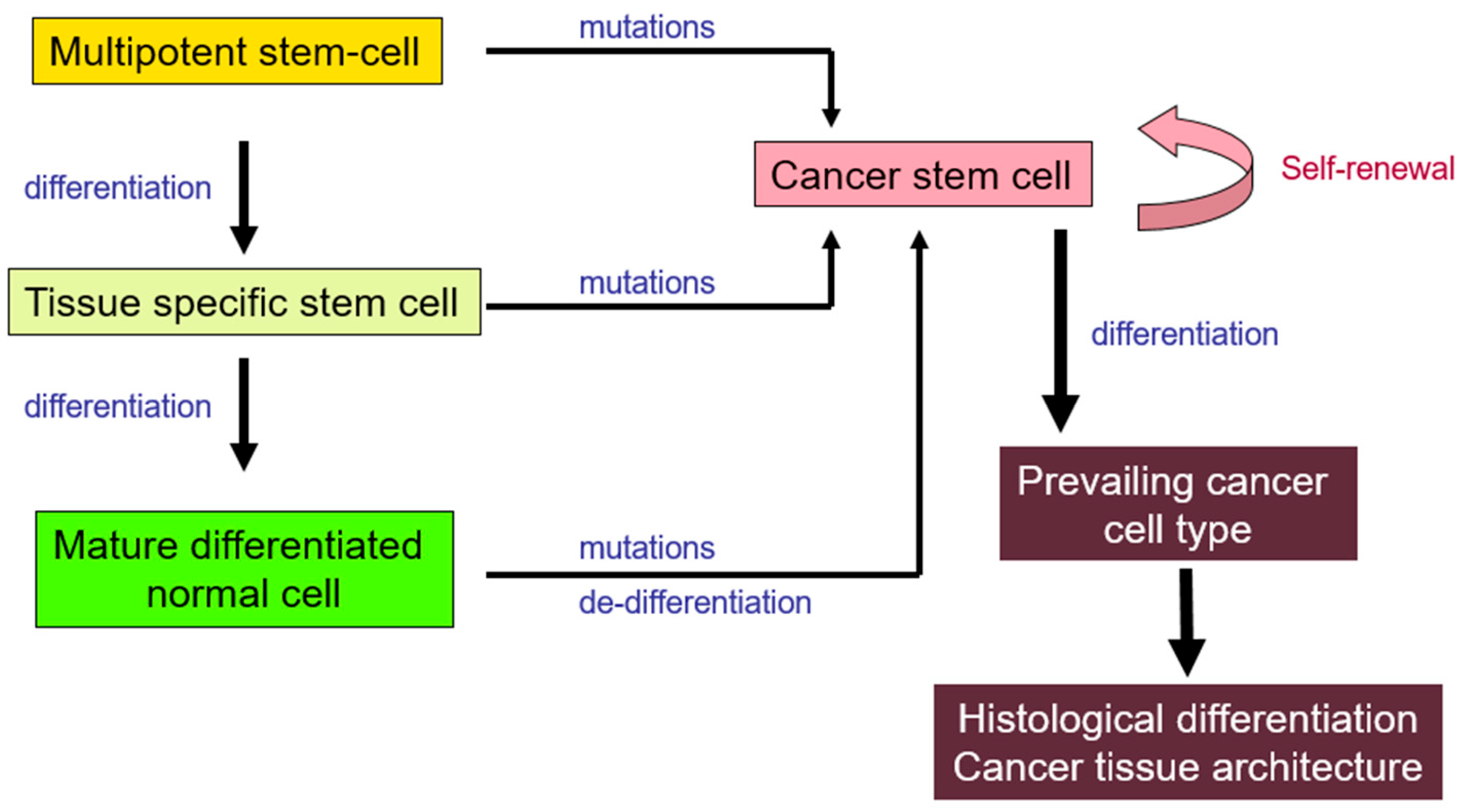
2. Cancer Stem Cells (CSCs)
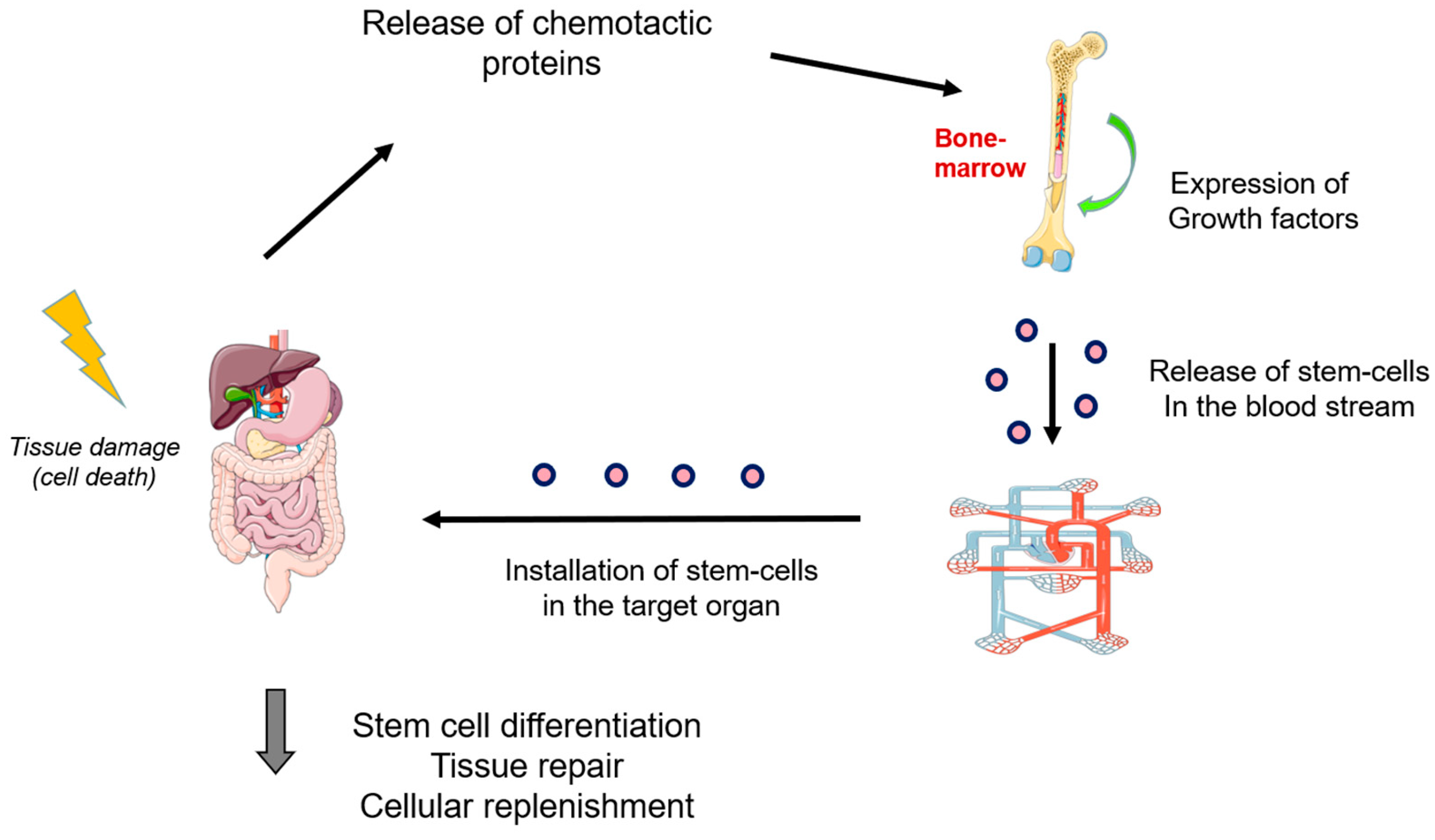
2.1. Origins
2.2. Tumor Microenvironment
3. Stem Cells in Prostate Cancer
3.1. Normal Prostate Gland
3.2. Prostate CSCs (PCSCs) Characterization
3.3. Active Biological Pathways in PCSCs
4. Prognostic and Therapeutic Implications
4.1. PCSCs and Resistance to Chemotherapy and Radiotherapy (RT)
4.2. PCSCs and Prognosis
4.3. Targeting PCSCs for Therapy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Armstrong, L.; Lako, M.; Buckley, N.; Lappin, T.R.; Murphy, M.J.; Nolta, J.A.; Pittenger, M.; Stojkovic, M. Editorial: Our top 10 developments in stem cell biology over the last 30 years. Stem Cells 2012, 30, 2–9. [Google Scholar] [CrossRef]
- Jensen, G.S.; Drapeau, C. The use of in situ bone marrow stem cells for the treatment of various degenerative diseases. Med. Hypotheses 2002, 59, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Maksym, R.B.; Tarnowski, M.; Grymula, K.; Tarnowska, J.; Wysoczynski, M.; Liu, R.; Czerny, B.; Ratajczak, J.; Kucia, M.; Ratajczak, M.Z. The role of stromal-derived factor-1–CXCR7 axis in development and cancer. Eur. J. Pharmacol. 2009, 625, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Tsolaki, E.; Yannaki, E. Stem cell-based regenerative opportunities for the liver: State of the art and beyond. World J. Gastroenterol. 2015, 21, 12334–12350. [Google Scholar] [CrossRef]
- Im, G.I. Endogenous Cartilage Repair by Recruitment of Stem Cells. Tissue Eng. Part B Rev. 2016, 22, 160–171. [Google Scholar] [CrossRef]
- Fisher, S.A.; Zhang, H.; Doree, C.; Mathur, A.; Martin-Rendon, E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst. Rev. 2015, 2015, CD006536. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading, C. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar]
- Sell, S. On the stem cell origin of cancer. Am. J. Pathol. 2010, 176, 2584–2594. [Google Scholar] [CrossRef]
- Walcher, L.; Kistenmacher, A.K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauss, A.; Blaudszun, A.R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer Stem Cells-Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y. Human bone marrow mesenchymal stromal/stem cells: Current clinical applications and potential for hematology. Int. J. Hematol. 2016, 103, 122–128. [Google Scholar] [CrossRef]
- Houghton, J.; Stoicov, C.; Nomura, S.; Rogers, A.B.; Carlson, J.; Li, H.; Cai, X.; Fox, J.G.; Goldenring, J.R.; Wang, T.C. Gastric cancer originating from bone marrow-derived cells. Science 2004, 306, 1568–1571. [Google Scholar] [CrossRef]
- Aractingi, S.; Kanitakis, J.; Euvrard, S.; Le Danff, C.; Peguillet, I.; Khosrotehrani, K.; Lantz, O.; Carosella, E.D. Skin carcinoma arising from donor cells in a kidney transplant recipient. Cancer Res. 2005, 65, 1755–1760. [Google Scholar] [CrossRef]
- Barozzi, P.; Luppi, M.; Facchetti, F.; Mecucci, C.; Alu, M.; Sarid, R.; Rasini, V.; Ravazzini, L.; Rossi, E.; Festa, S.; et al. Post-transplant Kaposi sarcoma originates from the seeding of donor-derived progenitors. Nat. Med. 2003, 9, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, Z.; Chen, Z.; Zhang, T.; Lu, Y. Multiple tumor types may originate from bone marrow-derived cells. Neoplasia 2006, 8, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Sell, S. Cellular origin of cancer: Dedifferentiation or stem cell maturation arrest? Environ. Health Perspect. 1993, 101 (Suppl. S5), 15–26. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Wernig, M.; Meissner, A.; Foreman, R.; Brambrink, T.; Ku, M.; Hochedlinger, K.; Bernstein, B.E.; Jaenisch, R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 2007, 448, 318–324. [Google Scholar] [CrossRef]
- Breton, A.; Sharma, R.; Diaz, A.C.; Parham, A.G.; Graham, A.; Neil, C.; Whitelaw, C.B.; Milne, E.; Donadeu, F.X. Derivation and characterization of induced pluripotent stem cells from equine fibroblasts. Stem Cells Dev. 2013, 22, 611–621. [Google Scholar] [CrossRef]
- Elaut, G.; Henkens, T.; Papeleu, P.; Snykers, S.; Vinken, M.; Vanhaecke, T.; Rogiers, V. Molecular mechanisms underlying the dedifferentiation process of isolated hepatocytes and their cultures. Curr. Drug Metab. 2006, 7, 629–660. [Google Scholar] [CrossRef]
- Uhrbom, L.; Dai, C.; Celestino, J.C.; Rosenblum, M.K.; Fuller, G.N.; Holland, E.C. Ink4a-Arf loss cooperates with KRas activation in astrocytes and neural progenitors to generate glioblastomas of various morphologies depending on activated Akt. Cancer Res. 2002, 62, 5551–5558. [Google Scholar]
- Katoh, M.; Katoh, M. WNT signaling pathway and stem cell signaling network. Clin. Cancer Res. 2007, 13, 4042–4045. [Google Scholar] [CrossRef] [PubMed]
- Katoh, Y.; Katoh, M. Hedgehog target genes: Mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr. Mol. Med. 2009, 9, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wu, H.; Xu, H.; Xiong, H.; Chu, Q.; Yu, S.; Wu, G.S.; Wu, K. Notch signaling: An emerging therapeutic target for cancer treatment. Cancer Lett. 2015, 369, 20–27. [Google Scholar] [CrossRef]
- Myant, K.; Sansom, O.J. Wnt/Myc interactions in intestinal cancer: Partners in crime. Exp. Cell Res. 2011, 317, 2725–2731. [Google Scholar] [CrossRef]
- Bayik, D.; Lathia, J.D. Cancer stem cell-immune cell crosstalk in tumour progression. Nat. Rev. Cancer 2021, 21, 526–536. [Google Scholar] [CrossRef]
- Plaks, V.; Kong, N.; Werb, Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef]
- Ye, J.; Wu, D.; Wu, P.; Chen, Z.; Huang, J. The cancer stem cell niche: Cross talk between cancer stem cells and their microenvironment. Tumour. Biol. 2014, 35, 3945–3951. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, Y.; Pedersen, E.A.; Havens, A.M.; Jung, Y.; Mishra, A.; Joseph, J.; Kim, J.K.; Patel, L.R.; Ying, C.; Ziegler, A.M.; et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J. Clin. Investig. 2011, 121, 1298–1312. [Google Scholar] [CrossRef]
- Williams, K.; Motiani, K.; Giridhar, P.V.; Kasper, S. CD44 integrates signaling in normal stem cell, cancer stem cell and (pre)metastatic niches. Exp. Biol. Med. 2013, 238, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Shiozawa, Y.; Taichman, R.S.; McCauley, L.K.; Pienta, K.; Keller, E. Prostate cancer and parasitism of the bone hematopoietic stem cell niche. Crit. Rev. Eukaryot. Gene Expr. 2012, 22, 131–148. [Google Scholar] [CrossRef]
- Keith, B.; Simon, M.C. Hypoxia-inducible factors, stem cells, and cancer. Cell 2007, 129, 465–472. [Google Scholar] [CrossRef]
- Gustafsson, M.V.; Zheng, X.; Pereira, T.; Gradin, K.; Jin, S.; Lundkvist, J.; Ruas, J.L.; Poellinger, L.; Lendahl, U.; Bondesson, M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev. Cell 2005, 9, 617–628. [Google Scholar] [CrossRef]
- Axelson, H.; Fredlund, E.; Ovenberger, M.; Landberg, G.; Pahlman, S. Hypoxia-induced dedifferentiation of tumor cells—A mechanism behind heterogeneity and aggressiveness of solid tumors. Semin. Cell Dev. Biol. 2005, 16, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Jogi, A.; Vallon-Christersson, J.; Holmquist, L.; Axelson, H.; Borg, A.; Pahlman, S. Human neuroblastoma cells exposed to hypoxia: Induction of genes associated with growth, survival, and aggressive behavior. Exp. Cell Res. 2004, 295, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, A.P.; Ramaekers, F.C.; Aalders, T.W.; Schaafsma, H.E.; Debruyne, F.M.; Schalken, J.A. Colocalization of basal and luminal cell-type cytokeratins in human prostate cancer. Cancer Res. 1992, 52, 6182–6187. [Google Scholar]
- Lee, D.K.; Liu, Y.; Liao, L.; Wang, F.; Xu, J. The prostate basal cell (BC) heterogeneity and the p63-positive BC differentiation spectrum in mice. Int. J. Biol. Sci. 2014, 10, 1007–1017. [Google Scholar] [CrossRef]
- Huang, Y.; Hamana, T.; Liu, J.; Wang, C.; An, L.; You, P.; Chang, J.Y.F.; Xu, J.; McKeehan, W.L.; Wang, F. Prostate Sphere-forming Stem Cells Are Derived from the P63-expressing Basal Compartment. J. Biol. Chem. 2015, 290, 17745–17752. [Google Scholar] [CrossRef]
- Richardson, G.D.; Robson, C.N.; Lang, S.H.; Neal, D.E.; Maitland, N.J.; Collins, A.T. CD133, a novel marker for human prostatic epithelial stem cells. J. Cell Sci. 2004, 117, 3539–3545. [Google Scholar] [CrossRef]
- Hudson, D.L.; O’Hare, M.; Watt, F.M.; Masters, J.R. Proliferative heterogeneity in the human prostate: Evidence for epithelial stem cells. Lab. Investig. 2000, 80, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.S.; Lawson, D.A.; Cheng, D.; Sun, W.; Garraway, I.P.; Witte, O.N. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc. Natl. Acad. Sci. USA 2008, 105, 20882–20887. [Google Scholar] [CrossRef]
- Garraway, I.P.; Sun, W.; Tran, C.P.; Perner, S.; Zhang, B.; Goldstein, A.S.; Hahm, S.A.; Haider, M.; Head, C.S.; Reiter, R.E.; et al. Human prostate sphere-forming cells represent a subset of basal epithelial cells capable of glandular regeneration in vivo. Prostate 2010, 70, 491–501. [Google Scholar] [CrossRef]
- Korsten, H.; Ziel-van der Made, A.; Ma, X.; van der Kwast, T.; Trapman, J. Accumulating progenitor cells in the luminal epithelial cell layer are candidate tumor initiating cells in a Pten knockout mouse prostate cancer model. PLoS ONE 2009, 4, e5662. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kruithof-de Julio, M.; Economides, K.D.; Walker, D.; Yu, H.; Halili, M.V.; Hu, Y.P.; Price, S.M.; Abate-Shen, C.; Shen, M.M. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 2009, 461, 495–500. [Google Scholar] [CrossRef]
- Collins, A.T.; Habib, F.K.; Maitland, N.J.; Neal, D.E. Identification and isolation of human prostate epithelial stem cells based on alpha(2)beta(1)-integrin expression. J. Cell Sci. 2001, 114, 3865–3872. [Google Scholar] [CrossRef]
- Harris, K.S.; Shi, L.; Foster, B.M.; Mobley, M.E.; Elliott, P.L.; Song, C.J.; Watabe, K.; Langefeld, C.D.; Kerr, B.A. CD117/c-kit defines a prostate CSC-like subpopulation driving progression and TKI resistance. Sci. Rep. 2021, 11, 1465. [Google Scholar] [CrossRef] [PubMed]
- Parimi, V.; Goyal, R.; Poropatich, K.; Yang, X.J. Neuroendocrine differentiation of prostate cancer: A review. Am. J. Clin. Exp. Urol. 2014, 2, 273–285. [Google Scholar]
- Ahlgren, G.; Pedersen, K.; Lundberg, S.; Aus, G.; Hugosson, J.; Abrahamsson, P.A. Regressive changes and neuroendocrine differentiation in prostate cancer after neoadjuvant hormonal treatment. Prostate 2000, 42, 274–279. [Google Scholar] [CrossRef]
- Pignon, J.C.; Grisanzio, C.; Geng, Y.; Song, J.; Shivdasani, R.A.; Signoretti, S. p63-expressing cells are the stem cells of developing prostate, bladder, and colorectal epithelia. Proc. Natl. Acad. Sci. USA 2013, 110, 8105–8110. [Google Scholar] [CrossRef] [PubMed]
- Butler, W.; Huang, J. Neuroendocrine cells of the prostate: Histology, biological functions, and molecular mechanisms. Precis. Clin. Med. 2021, 4, 25–34. [Google Scholar] [CrossRef]
- Leong, K.G.; Wang, B.E.; Johnson, L.; Gao, W.Q. Generation of a prostate from a single adult stem cell. Nature 2008, 456, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.; Yuan, J.; Wills, M.; Kasper, S. Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res. 2007, 67, 4807–4815. [Google Scholar] [CrossRef] [PubMed]
- Maitland, N.J.; Bryce, S.D.; Stower, M.J.; Collins, A.T. Prostate cancer stem cells: A target for new therapies. Cancer Stem Cells 2006, 2006, 155–179. [Google Scholar] [CrossRef]
- Collins, A.T.; Berry, P.A.; Hyde, C.; Stower, M.J.; Maitland, N.J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005, 65, 10946–10951. [Google Scholar] [CrossRef]
- Song, C.; Chen, H. Predictive significance of TMRPSS2-ERG fusion in prostate cancer: A meta-analysis. Cancer Cell Int. 2018, 18, 177. [Google Scholar] [CrossRef]
- Polson, E.S.; Lewis, J.L.; Celik, H.; Mann, V.M.; Stower, M.J.; Simms, M.S.; Rodrigues, G.; Collins, A.T.; Maitland, N.J. Monoallelic expression of TMPRSS2/ERG in prostate cancer stem cells. Nat. Commun. 2013, 4, 1623. [Google Scholar] [CrossRef]
- Khosh Kish, E.; Choudhry, M.; Gamallat, Y.; Buharideen, S.M.; Dhananjaya, D.; Bismar, T.A. The Expression of Proto-Oncogene ETS-Related Gene (ERG) Plays a Central Role in the Oncogenic Mechanism Involved in the Development and Progression of Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 4772. [Google Scholar] [CrossRef]
- Jamaspishvili, T.; Berman, D.M.; Ross, A.E.; Scher, H.I.; De Marzo, A.M.; Squire, J.A.; Lotan, T.L. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol. 2018, 15, 222–234. [Google Scholar] [CrossRef]
- Trotman, L.C.; Niki, M.; Dotan, Z.A.; Koutcher, J.A.; Di Cristofano, A.; Xiao, A.; Khoo, A.S.; Roy-Burman, P.; Greenberg, N.M.; Van Dyke, T.; et al. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003, 1, E59. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.; Wu, H. PTEN, stem cells, and cancer stem cells. J. Biol. Chem. 2009, 284, 11755–11759. [Google Scholar] [CrossRef] [PubMed]
- Boysen, G.; Barbieri, C.E.; Prandi, D.; Blattner, M.; Chae, S.S.; Dahija, A.; Nataraj, S.; Huang, D.; Marotz, C.; Xu, L.; et al. SPOP mutation leads to genomic instability in prostate cancer. eLife 2015, 4, e09207. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Zhu, Y.; Dai, X.; Dang, F.; Ren, J.; Ren, S.; Shulga, Y.V.; Beca, F.; Gan, W.; et al. SPOP Promotes Nanog Destruction to Suppress Stem Cell Traits and Prostate Cancer Progression. Dev. Cell 2019, 48, 329–344.e5. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N. The Molecular Taxonomy of Primary Prostate Cancer. Cell 2015, 163, 1011–1025. [Google Scholar] [CrossRef]
- Ghatak, D.; Das Ghosh, D.; Roychoudhury, S. Cancer Stemness: p53 at the Wheel. Front. Oncol. 2020, 10, 604124. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, G.J. Emerging roles of Myc in stem cell biology and novel tumor therapies. J. Exp. Clin. Cancer Res. 2018, 37, 173. [Google Scholar] [CrossRef]
- Grist, E.; Friedrich, S.; Brawley, C.; Mendes, L.; Parry, M.; Ali, A.; Haran, A.; Hoyle, A.; Gilson, C.; Lall, S.; et al. Accumulation of copy number alterations and clinical progression across advanced prostate cancer. Genome Med. 2022, 14, 102. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, P. High copy number variations, particular transcription factors, and low immunity contribute to the stemness of prostate cancer cells. J. Transl. Med. 2021, 19, 206. [Google Scholar] [CrossRef]
- Mazloumi, Z.; Farahzadi, R.; Rafat, A.; Dizaji Asl, K.; Karimipour, M.; Montazer, M.; Movassaghpour, A.A.; Dehnad, A.; Nozad Charoudeh, H. Effect of aberrant DNA methylation on cancer stem cell properties. Exp. Mol. Pathol. 2022, 125, 104757. [Google Scholar] [CrossRef]
- Majumdar, S.; Buckles, E.; Estrada, J.; Koochekpour, S. Aberrant DNA methylation and prostate cancer. Curr. Genom. 2011, 12, 486–505. [Google Scholar] [CrossRef] [PubMed]
- Castellon, E.A.; Valenzuela, R.; Lillo, J.; Castillo, V.; Contreras, H.R.; Gallegos, I.; Mercado, A.; Huidobro, C. Molecular signature of cancer stem cells isolated from prostate carcinoma and expression of stem markers in different Gleason grades and metastasis. Biol. Res. 2012, 45, 297–305. [Google Scholar] [CrossRef]
- Hurt, E.M.; Kawasaki, B.T.; Klarmann, G.J.; Thomas, S.B.; Farrar, W.L. CD44+ CD24(-) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br. J. Cancer 2008, 98, 756–765. [Google Scholar] [CrossRef]
- Sanchez, B.G.; Bort, A.; Vara-Ciruelos, D.; Diaz-Laviada, I. Androgen Deprivation Induces Reprogramming of Prostate Cancer Cells to Stem-Like Cells. Cells 2020, 9, 1441. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Su, Y.; Mei, Y.; Leng, Q.; Leng, B.; Liu, Z.; Stass, S.A.; Jiang, F. ALDH1A1 is a marker for malignant prostate stem cells and predictor of prostate cancer patients’ outcome. Lab. Investig. 2010, 90, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Shankar, E.; Kalayci, F.N.C.; Mukunda, A.; Alassfar, M.; Singh, V.; Chan, E.R.; MacLennan, G.T.; Gupta, S. Androgen Deprivation Induces Transcriptional Reprogramming in Prostate Cancer Cells to Develop Stem Cell-Like Characteristics. Int. J. Mol. Sci. 2020, 21, 9568. [Google Scholar] [CrossRef]
- Mathieu, J.; Zhang, Z.; Zhou, W.; Wang, A.J.; Heddleston, J.M.; Pinna, C.M.; Hubaud, A.; Stadler, B.; Choi, M.; Bar, M.; et al. HIF induces human embryonic stem cell markers in cancer cells. Cancer Res. 2011, 71, 4640–4652. [Google Scholar] [CrossRef]
- Kong, D.; Sethi, S.; Li, Y.; Chen, W.; Sakr, W.A.; Heath, E.; Sarkar, F.H. Androgen receptor splice variants contribute to prostate cancer aggressiveness through induction of EMT and expression of stem cell marker genes. Prostate 2015, 75, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lan, T. Molecular Origin, Expression Regulation, and Biological Function of Androgen Receptor Splicing Variant 7 in Prostate Cancer. Urol. Int. 2021, 105, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Mourkioti, I.; Polyzou, A.; Veroutis, D.; Theocharous, G.; Lagopati, N.; Gentile, E.; Stravokefalou, V.; Thanos, D.F.; Havaki, S.; Kletsas, D.; et al. A GATA2-CDC6 axis modulates androgen receptor blockade-induced senescence in prostate cancer. J. Exp. Clin. Cancer Res. 2023, 42, 187. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Kapse, P.; Siddique, S.; Kundu, M.; Choudhari, J.; Mohanty, V.; Malhotra, D.; Gosavi, S.W.; Gacche, R.N.; Kundu, G.C. Therapeutic implications of cancer stem cells in prostate cancer. Cancer Biol. Med. 2023, 20, 401–420. [Google Scholar] [CrossRef]
- Dubrovska, A.; Kim, S.; Salamone, R.J.; Walker, J.R.; Maira, S.M.; Garcia-Echeverria, C.; Schultz, P.G.; Reddy, V.A. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc. Natl. Acad. Sci. USA 2009, 106, 268–273. [Google Scholar] [CrossRef]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef]
- Gan, Y.; Shi, C.; Inge, L.; Hibner, M.; Balducci, J.; Huang, Y. Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene 2010, 29, 4947–4958. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol. Res. 2012, 66, 105–143. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.Y.; Choi, M.; Kim, B.H.; Cho, Y.M.; Moon, K.C.; Kang, G.H. BRAF and KRAS mutations in prostatic adenocarcinoma. Int. J. Cancer 2006, 119, 1858–1862. [Google Scholar] [CrossRef]
- Rybak, A.P.; Ingram, A.J.; Tang, D. Propagation of human prostate cancer stem-like cells occurs through EGFR-mediated ERK activation. PLoS ONE 2013, 8, e61716. [Google Scholar] [CrossRef] [PubMed]
- Prins, G.S.; Hu, W.Y.; Shi, G.B.; Hu, D.P.; Majumdar, S.; Li, G.; Huang, K.; Nelles, J.L.; Ho, S.M.; Walker, C.L.; et al. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology 2014, 155, 805–817. [Google Scholar] [CrossRef]
- Bakin, R.E.; Gioeli, D.; Sikes, R.A.; Bissonette, E.A.; Weber, M.J. Constitutive activation of the Ras/mitogen-activated protein kinase signaling pathway promotes androgen hypersensitivity in LNCaP prostate cancer cells. Cancer Res. 2003, 63, 1981–1989. [Google Scholar] [PubMed]
- Hong, S.K.; Kim, J.H.; Lin, M.F.; Park, J.I. The Raf/MEK/extracellular signal-regulated kinase 1/2 pathway can mediate growth inhibitory and differentiation signaling via androgen receptor downregulation in prostate cancer cells. Exp. Cell Res. 2011, 317, 2671–2682. [Google Scholar] [CrossRef] [PubMed]
- Dhir, R.; Ni, Z.; Lou, W.; DeMiguel, F.; Grandis, J.R.; Gao, A.C. Stat3 activation in prostatic carcinomas. Prostate 2002, 51, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Kroon, P.; Berry, P.A.; Stower, M.J.; Rodrigues, G.; Mann, V.M.; Simms, M.; Bhasin, D.; Chettiar, S.; Li, C.; Li, P.K.; et al. JAK-STAT blockade inhibits tumor initiation and clonogenic recovery of prostate cancer stem-like cells. Cancer Res. 2013, 73, 5288–5298. [Google Scholar] [CrossRef]
- Gonnissen, A.; Isebaert, S.; Haustermans, K. Hedgehog signaling in prostate cancer and its therapeutic implication. Int. J. Mol. Sci. 2013, 14, 13979–14007. [Google Scholar] [CrossRef]
- Wang, X.D.; Shou, J.; Wong, P.; French, D.M.; Gao, W.Q. Notch1-expressing cells are indispensable for prostatic branching morphogenesis during development and re-growth following castration and androgen replacement. J. Biol. Chem. 2004, 279, 24733–24744. [Google Scholar] [CrossRef]
- Cheng, J.W.; Duan, L.X.; Yu, Y.; Wang, P.; Feng, J.L.; Feng, G.Z.; Liu, Y. Bone marrow mesenchymal stem cells promote prostate cancer cell stemness via cell-cell contact to activate the Jagged1/Notch1 pathway. Cell Biosci. 2021, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Mourkioti, I.; Angelopoulou, A.; Belogiannis, K.; Lagopati, N.; Potamianos, S.; Kyrodimos, E.; Gorgoulis, V.; Papaspyropoulos, A. Interplay of Developmental Hippo-Notch Signaling Pathways with the DNA Damage Response in Prostate Cancer. Cells 2022, 11, 2449. [Google Scholar] [CrossRef]
- Chappell, W.H.; Abrams, S.L.; Lertpiriyapong, K.; Fitzgerald, T.L.; Martelli, A.M.; Cocco, L.; Rakus, D.; Gizak, A.; Terrian, D.; Steelman, L.S.; et al. Novel roles of androgen receptor, epidermal growth factor receptor, TP53, regulatory RNAs, NF-kappa-B, chromosomal translocations, neutrophil associated gelatinase, and matrix metalloproteinase-9 in prostate cancer and prostate cancer stem cells. Adv. Biol. Regul. 2016, 60, 64–87. [Google Scholar] [CrossRef]
- Jin, R.; Yi, Y.; Yull, F.E.; Blackwell, T.S.; Clark, P.E.; Koyama, T.; Smith, J.A., Jr.; Matusik, R.J. NF-kappaB gene signature predicts prostate cancer progression. Cancer Res. 2014, 74, 2763–2772. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, N.G.; Miller, A.; Titus, M.A.; Huss, W.J. The Efflux Transporter ABCG2 Maintains Prostate Stem Cells. Mol. Cancer Res. 2017, 15, 128–140. [Google Scholar] [CrossRef]
- Murillo-Garzon, V.; Kypta, R. WNT signalling in prostate cancer. Nat. Rev. Urol. 2017, 14, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Graham, P.H.; Power, C.A.; Hao, J.; Kearsley, J.H.; Li, Y. CD44 is a biomarker associated with human prostate cancer radiation sensitivity. Clin. Exp. Metastasis 2012, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, X.; Zheng, X.; Wang, X.; Li, S.; Zhang, L.; Yang, Z.; Xia, Z. Enrichment of prostate cancer stem-like cells from human prostate cancer cell lines by culture in serum-free medium and chemoradiotherapy. Int. J. Biol. Sci. 2013, 9, 472–479. [Google Scholar] [CrossRef]
- Guzel, E.; Karatas, O.F.; Duz, M.B.; Solak, M.; Ittmann, M.; Ozen, M. Differential expression of stem cell markers and ABCG2 in recurrent prostate cancer. Prostate 2014, 74, 1498–1505. [Google Scholar] [CrossRef]
- Kyjacova, L.; Hubackova, S.; Krejcikova, K.; Strauss, R.; Hanzlikova, H.; Dzijak, R.; Imrichova, T.; Simova, J.; Reinis, M.; Bartek, J.; et al. Radiotherapy-induced plasticity of prostate cancer mobilizes stem-like non-adherent, Erk signaling-dependent cells. Cell Death Differ. 2015, 22, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Frame, F.M.; Pellacani, D.; Collins, A.T.; Simms, M.S.; Mann, V.M.; Jones, G.D.; Meuth, M.; Bristow, R.G.; Maitland, N.J. HDAC inhibitor confers radiosensitivity to prostate stem-like cells. Br. J. Cancer 2013, 109, 3023–3033. [Google Scholar] [CrossRef]
- Lai, C.J.; Lin, C.Y.; Liao, W.Y.; Hour, T.C.; Wang, H.D.; Chuu, C.P. CD44 Promotes Migration and Invasion of Docetaxel-Resistant Prostate Cancer Cells Likely via Induction of Hippo-Yap Signaling. Cells 2019, 8, 295. [Google Scholar] [CrossRef]
- Dubrovska, A.; Elliott, J.; Salamone, R.J.; Kim, S.; Aimone, L.J.; Walker, J.R.; Watson, J.; Sauveur-Michel, M.; Garcia-Echeverria, C.; Cho, C.Y.; et al. Combination therapy targeting both tumor-initiating and differentiated cell populations in prostate carcinoma. Clin. Cancer Res. 2010, 16, 5692–5702. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Graham, P.H.; Hao, J.; Ni, J.; Bucci, J.; Cozzi, P.J.; Kearsley, J.H.; Li, Y. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 2013, 4, e875. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Cheung, B.B.; Beretov, J.; Duan, W.; Bucci, J.; Malouf, D.; Graham, P.; Li, Y. CD44 variant 6 is associated with prostate cancer growth and chemo-/radiotherapy response in vivo. Exp. Cell Res. 2020, 388, 111850. [Google Scholar] [CrossRef]
- Skvortsov, S.; Skvortsova, I.I.; Tang, D.G.; Dubrovska, A. Concise Review: Prostate Cancer Stem Cells: Current Understanding. Stem Cells 2018, 36, 1457–1474. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J. Wnt signaling pathway in non-small cell lung cancer. J. Natl. Cancer Inst. 2014, 106, djt356. [Google Scholar] [CrossRef] [PubMed]
- Takahashi-Yanaga, F.; Kahn, M. Targeting Wnt signaling: Can we safely eradicate cancer stem cells? Clin. Cancer Res. 2010, 16, 3153–3162. [Google Scholar] [CrossRef]
- Cristóbal, I.; Rojo, F.; Madoz-Gúrpide, J.; García-Foncillas, J. Cross Talk between Wnt/β-Catenin and CIP2A/Plk1 Signaling in Prostate Cancer: Promising Therapeutic Implications. Mol. Cell. Biol. 2016, 36, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.; Guo, Q.; Connelly, Z.; Cheng, S.; Yang, S.; Prieto-Dominguez, N.; Yu, X. Wnt/Beta-Catenin Signaling and Prostate Cancer Therapy Resistance. Adv. Exp. Med. Biol. 2019, 1210, 351–378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cheng, L.; Li, J.; Farah, E.; Atallah, N.M.; Pascuzzi, P.E.; Gupta, S.; Liu, X. Inhibition of the Wnt/beta-Catenin Pathway Overcomes Resistance to Enzalutamide in Castration-Resistant Prostate Cancer. Cancer Res. 2018, 78, 3147–3162. [Google Scholar] [CrossRef]
- Cojoc, M.; Peitzsch, C.; Kurth, I.; Trautmann, F.; Kunz-Schughart, L.A.; Telegeev, G.D.; Stakhovsky, E.A.; Walker, J.R.; Simin, K.; Lyle, S.; et al. Aldehyde Dehydrogenase Is Regulated by beta-Catenin/TCF and Promotes Radioresistance in Prostate Cancer Progenitor Cells. Cancer Res. 2015, 75, 1482–1494. [Google Scholar] [CrossRef]
- Noordzij, M.A.; van Steenbrugge, G.J.; Verkaik, N.S.; Schroder, F.H.; van der Kwast, T.H. The prognostic value of CD44 isoforms in prostate cancer patients treated by radical prostatectomy. Clin. Cancer Res. 1997, 3, 805–815. [Google Scholar]
- Aaltomaa, S.; Lipponen, P.; Viitanen, J.; Kankkunen, J.P.; Ala-Opas, M.; Kosma, V.M. Prognostic value of CD44 standard, variant isoforms 3 and 6 and -catenin expression in local prostate cancer treated by radical prostatectomy. Eur. Urol. 2000, 38, 555–562. [Google Scholar] [CrossRef]
- Korski, K.; Malicka-Durczak, A.; Breborowicz, J. Expression of stem cell marker CD44 in prostate cancer biopsies predicts cancer grade in radical prostatectomy specimens. Pol. J. Pathol. 2014, 65, 291–295. [Google Scholar] [CrossRef]
- Fawzy, M.S.; Mohamed, R.H.; Elfayoumi, A.R. Prostate stem cell antigen (PSCA) mRNA expression in peripheral blood in patients with benign prostatic hyperplasia and/or prostate cancer. Med. Oncol. 2015, 32, 74. [Google Scholar] [CrossRef] [PubMed]
- Zhigang, Z.; Wenlu, S. External beam radiotherapy (EBRT) suppressed prostate stem cell antigen (PSCA) mRNA expression in clinically localized prostate cancer. Prostate 2007, 67, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; He, M.; Wilson, T.; Liu, X.; Zhang, K.; Carmichael, C.; Torres, A.; Hernandez, S.; Lau, C.; Agarwal, N.; et al. Detection and phenotyping of circulating tumor cells in high-risk localized prostate cancer. Clin. Genitourin. Cancer 2015, 13, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, A.M.; Verhoef, E.I.; Roobol, M.J.; Schroder, F.H.; Wildhagen, M.F.; van der Kwast, T.H.; Jenster, G.; van Leenders, G.J. Validation of stem cell markers in clinical prostate cancer: Alpha6-integrin is predictive for non-aggressive disease. Prostate 2014, 74, 488–496. [Google Scholar] [CrossRef]
- Colombel, M.; Eaton, C.L.; Hamdy, F.; Ricci, E.; van der Pluijm, G.; Cecchini, M.; Mege-Lechevallier, F.; Clezardin, P.; Thalmann, G. Increased expression of putative cancer stem cell markers in primary prostate cancer is associated with progression of bone metastases. Prostate 2012, 72, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Obinata, D.; Takayama, K.; Urano, T.; Murata, T.; Kumagai, J.; Fujimura, T.; Ikeda, K.; Horie-Inoue, K.; Homma, Y.; Ouchi, Y.; et al. Oct1 regulates cell growth of LNCaP cells and is a prognostic factor for prostate cancer. Int. J. Cancer 2012, 130, 1021–1028. [Google Scholar] [CrossRef]
- Fujimura, T.; Takahashi, S.; Urano, T.; Takayama, K.; Sugihara, T.; Obinata, D.; Yamada, Y.; Kumagai, J.; Kume, H.; Ouchi, Y.; et al. Expression of androgen and estrogen signaling components and stem cell markers to predict cancer progression and cancer-specific survival in patients with metastatic prostate cancer. Clin. Cancer Res. 2014, 20, 4625–4635. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, M.; Li, X.; Weng, X.; Su, Z.; Zhang, M.; Tan, J.; Zeng, H.; Li, X.; Nie, L.; et al. SOX9 and HMGB3 co-operatively transactivate NANOG and promote prostate cancer progression. Prostate 2023, 83, 440–453. [Google Scholar] [CrossRef]
- Zadvornyi, T.V.; Lukianova, N.Y.; Borikun, T.V.; Vitruk, Y.V.; Stakhovsky, E.O.; Chekhun, V.F. NANOG as prognostic factor of prostate cancer course. Exp. Oncol. 2020, 42, 94–100. [Google Scholar] [CrossRef]
- Siddique, H.R.; Parray, A.; Zhong, W.; Karnes, R.J.; Bergstralh, E.J.; Koochekpour, S.; Rhim, J.S.; Konety, B.R.; Saleem, M. BMI1, stem cell factor acting as novel serum-biomarker for Caucasian and African-American prostate cancer. PLoS ONE 2013, 8, e52993. [Google Scholar] [CrossRef]
- Lv, L.; Yang, Z.; Ma, T.; Xuan, Y. Gli1, a potential cancer stem cell marker, is strongly associated with prognosis in prostate cancer. Int. J. Clin. Exp. Pathol. 2018, 11, 4957–4966. [Google Scholar] [PubMed]
- Allen, F.; Maillard, I. Therapeutic Targeting of Notch Signaling: From Cancer to Inflammatory Disorders. Front. Cell Dev. Biol. 2021, 9, 649205. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Tan, S.H.; Xavier, C.P.; Katta, S.; Huang, W.; Ravindranath, L.; Jamal, M.; Li, H.; Srivastava, M.; Srivatsan, E.S.; et al. Synergistic Activity with NOTCH Inhibition and Androgen Ablation in ERG-Positive Prostate Cancer Cells. Mol. Cancer Res. 2017, 15, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, Y.; Dong, B.; Qin, L.; Wang, C.; Zhou, P.; Wang, X.; Xu, H.; Xue, W.; Fang, Y.X.; et al. Pharmacological inhibition of the Notch pathway enhances the efficacy of androgen deprivation therapy for prostate cancer. Int. J. Cancer 2018, 143, 645–656. [Google Scholar] [CrossRef]
- Qiu, S.; Deng, L.; Bao, Y.; Jin, K.; Tu, X.; Li, J.; Liao, X.; Liu, Z.; Yang, L.; Wei, Q. Reversal of docetaxel resistance in prostate cancer by Notch signaling inhibition. Anticancer Drugs 2018, 29, 871–879. [Google Scholar] [CrossRef]
- Du, Z.; Li, L.; Sun, W.; Wang, X.; Zhang, Y.; Chen, Z.; Yuan, M.; Quan, Z.; Liu, N.; Hao, Y.; et al. HepaCAM inhibits the malignant behavior of castration-resistant prostate cancer cells by downregulating Notch signaling and PF-3084014 (a gamma-secretase inhibitor) partly reverses the resistance of refractory prostate cancer to docetaxel and enzalutamide in vitro. Int. J. Oncol. 2018, 53, 99–112. [Google Scholar] [CrossRef]
- Wang, L.; Zi, H.; Luo, Y.; Liu, T.; Zheng, H.; Xie, C.; Wang, X.; Huang, X. Inhibition of Notch pathway enhances the anti-tumor effect of docetaxel in prostate cancer stem-like cells. Stem Cell Res. Ther. 2020, 11, 258. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Messersmith, W.A.; Mikulski, S.M.; Papadopoulos, K.P.; Kwak, E.L.; Gibbon, D.G.; Patnaik, A.; Falchook, G.S.; Dasari, A.; Shapiro, G.I.; et al. Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J. Clin. Oncol. 2012, 30, 2348–2353. [Google Scholar] [CrossRef]
- Stein, M.N.; DiPaola, R.S.; Mayer, T.M.; Jeyamohan, C.; Metzger, D.; Anand, M.; Ivy, S.P.; Prostate Cancer Clinical Trials Consortium. A randomized phase II study of bicalutamide (BIC) followed by placebo or gamma secretase inhibitor RO4929097 (RO492) in men with rising PSA. J. Clin. Oncol. 2012, 30, 219. [Google Scholar] [CrossRef]
- Wu, M.H.; Wu, K.; Zhu, Y.B.; Li, D.C.; Yang, H.; Zeng, H. Baicalin Antagonizes Prostate Cancer Stemness via Inhibiting Notch1/NF-kappaB Signaling Pathway. Chin. J. Integr. Med. 2023; ahead of print. [Google Scholar] [CrossRef]
- Galperin, I.; Dempwolff, L.; Diederich, W.E.; Lauth, M. Inhibiting Hedgehog: An Update on Pharmacological Compounds and Targeting Strategies. J. Med. Chem. 2019, 62, 8392–8411. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.E.; Hughes, R.M.; Glavaris, S.; Ghabili, K.; He, P.; Anders, N.M.; Harb, R.; Tosoian, J.J.; Marchionni, L.; Schaeffer, E.M.; et al. Pharmacodynamic and pharmacokinetic neoadjuvant study of hedgehog pathway inhibitor Sonidegib (LDE-225) in men with high-risk localized prostate cancer undergoing prostatectomy. Oncotarget 2017, 8, 104182–104192. [Google Scholar] [CrossRef]
- Maughan, B.L.; Suzman, D.L.; Luber, B.; Wang, H.; Glavaris, S.; Hughes, R.; Sullivan, R.; Harb, R.; Boudadi, K.; Paller, C.; et al. Pharmacodynamic study of the oral hedgehog pathway inhibitor, vismodegib, in patients with metastatic castration-resistant prostate cancer. Cancer Chemother. Pharmacol. 2016, 78, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Gonnissen, A.; Isebaert, S.; McKee, C.M.; Dok, R.; Haustermans, K.; Muschel, R.J. The hedgehog inhibitor GANT61 sensitizes prostate cancer cells to ionizing radiation both in vitro and in vivo. Oncotarget 2016, 7, 84286–84298. [Google Scholar] [CrossRef] [PubMed]
- Gonnissen, A.; Isebaert, S.; McKee, C.M.; Muschel, R.J.; Haustermans, K. The Effect of Metformin and GANT61 Combinations on the Radiosensitivity of Prostate Cancer Cells. Int. J. Mol. Sci. 2017, 18, 399. [Google Scholar] [CrossRef]
- Sun, W.; Li, L.; Du, Z.; Quan, Z.; Yuan, M.; Cheng, H.; Gao, Y.; Luo, C.; Wu, X. Combination of phospholipase Cepsilon knockdown with GANT61 sensitizes castration-resistant prostate cancer cells to enzalutamide by suppressing the androgen receptor signaling pathway. Oncol. Rep. 2019, 41, 2689–2702. [Google Scholar] [CrossRef]
- Zhao, Y.; Alakhova, D.Y.; Kabanov, A.V. Can nanomedicines kill cancer stem cells? Adv. Drug Deliv. Rev. 2013, 65, 1763–1783. [Google Scholar] [CrossRef]
- Pudova, E.; Kobelyatskaya, A.; Katunina, I.; Snezhkina, A.; Nyushko, K.; Fedorova, M.; Pavlov, V.; Bulavkina, E.; Dalina, A.; Tkachev, S.; et al. Docetaxel Resistance in Castration-Resistant Prostate Cancer: Transcriptomic Determinants and the Effect of Inhibiting Wnt/beta-Catenin Signaling by XAV939. Int. J. Mol. Sci. 2022, 23, 12837. [Google Scholar] [CrossRef]
- Serttas, R.; Erdogan, S. Pretreatment of prostate cancer cells with salinomycin and Wnt inhibitor increases the efficacy of cabazitaxel by inducing apoptosis and decreasing cancer stem cells. Med. Oncol. 2023, 40, 194. [Google Scholar] [CrossRef]
- Navarro-Marchal, S.A.; Grinan-Lison, C.; Entrena, J.M.; Ruiz-Alcala, G.; Tristan-Manzano, M.; Martin, F.; Perez-Victoria, I.; Peula-Garcia, J.M.; Marchal, J.A. Anti-CD44-Conjugated Olive Oil Liquid Nanocapsules for Targeting Pancreatic Cancer Stem Cells. Biomacromolecules 2021, 22, 1374–1388. [Google Scholar] [CrossRef]
- Su, Z.; Liu, D.; Chen, L.; Zhang, J.; Ru, L.; Chen, Z.; Gao, Z.; Wang, X. CD44-Targeted Magnetic Nanoparticles Kill Head And Neck Squamous Cell Carcinoma Stem Cells In An Alternating Magnetic Field. Int. J. Nanomed. 2019, 14, 7549–7560. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Sun, J.; Liu, Y. Enhanced targeting of prostate cancer-initiating cells by salinomycin-encapsulated lipid-PLGA nanoparticles linked with CD44 antibodies. Oncol. Lett. 2019, 17, 4024–4033. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.A.; Lai, Y.R.; Wu, H.Y.; Lo, Y.J.; Chang, Y.F.; Hung, C.L.; Lin, C.J.; Lo, U.G.; Lin, H.; Hsieh, J.T.; et al. Bacterial Genotoxin-Coated Nanoparticles for Radiotherapy Sensitization in Prostate Cancer. Biomedicines 2021, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, C.; Wang, Y.; Lv, H.; Guo, Y.; Dai, H.; Wicha, M.S.; Chang, A.E.; Li, Q. Cytokine-induced killer (CIK) cells bound with anti-CD3/anti-CD133 bispecific antibodies target CD133(high) cancer stem cells in vitro and in vivo. Clin. Immunol. 2013, 149, 156–168. [Google Scholar] [CrossRef]
- Ning, S.T.; Lee, S.Y.; Wei, M.F.; Peng, C.L.; Lin, S.Y.; Tsai, M.H.; Lee, P.C.; Shih, Y.H.; Lin, C.Y.; Luo, T.Y.; et al. Targeting Colorectal Cancer Stem-Like Cells with Anti-CD133 Antibody-Conjugated SN-38 Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 17793–17804. [Google Scholar] [CrossRef]
- Tan, H.; Hou, N.; Liu, Y.; Liu, B.; Cao, W.; Zheng, D.; Li, W.; Liu, Y.; Xu, B.; Wang, Z.; et al. CD133 antibody targeted delivery of gold nanostars loading IR820 and docetaxel for multimodal imaging and near-infrared photodynamic/photothermal/chemotherapy against castration resistant prostate cancer. Nanomedicine 2020, 27, 102192. [Google Scholar] [CrossRef]
- Mitra Ghosh, T.; Mazumder, S.; Davis, J.; Yadav, J.; Akinpelu, A.; Alnaim, A.; Kumar, H.; Waliagha, R.; Church Bird, A.E.; Rais-Bahrami, S.; et al. Metronomic Administration of Topotecan Alone and in Combination with Docetaxel Inhibits Epithelial-mesenchymal Transition in Aggressive Variant Prostate Cancers. Cancer Res. Commun. 2023, 3, 1286–1311. [Google Scholar] [CrossRef]
- Zhu, X.; Prasad, S.; Gaedicke, S.; Hettich, M.; Firat, E.; Niedermann, G. Patient-derived glioblastoma stem cells are killed by CD133-specific CAR T cells but induce the T cell aging marker CD57. Oncotarget 2015, 6, 171–184. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Wu, Z.; Tong, C.; Dai, H.; Guo, Y.; Liu, Y.; Huang, J.; Lv, H.; Luo, C.; et al. CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. Oncoimmunology 2018, 7, e1440169. [Google Scholar] [CrossRef]
- Deng, Z.; Wu, Y.; Ma, W.; Zhang, S.; Zhang, Y.Q. Adoptive T-cell therapy of prostate cancer targeting the cancer stem cell antigen EpCAM. BMC Immunol. 2015, 16, 1. [Google Scholar] [CrossRef]
- Gogola, S.; Rejzer, M.; Bahmad, H.F.; Alloush, F.; Omarzai, Y.; Poppiti, R. Anti-Cancer Stem-Cell-Targeted Therapies in Prostate Cancer. Cancers 2023, 15, 1621. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koukourakis, I.M.; Platoni, K.; Kouloulias, V.; Arelaki, S.; Zygogianni, A. Prostate Cancer Stem Cells: Biology and Treatment Implications. Int. J. Mol. Sci. 2023, 24, 14890. https://doi.org/10.3390/ijms241914890
Koukourakis IM, Platoni K, Kouloulias V, Arelaki S, Zygogianni A. Prostate Cancer Stem Cells: Biology and Treatment Implications. International Journal of Molecular Sciences. 2023; 24(19):14890. https://doi.org/10.3390/ijms241914890
Chicago/Turabian StyleKoukourakis, Ioannis M., Kalliopi Platoni, Vassilis Kouloulias, Stella Arelaki, and Anna Zygogianni. 2023. "Prostate Cancer Stem Cells: Biology and Treatment Implications" International Journal of Molecular Sciences 24, no. 19: 14890. https://doi.org/10.3390/ijms241914890
APA StyleKoukourakis, I. M., Platoni, K., Kouloulias, V., Arelaki, S., & Zygogianni, A. (2023). Prostate Cancer Stem Cells: Biology and Treatment Implications. International Journal of Molecular Sciences, 24(19), 14890. https://doi.org/10.3390/ijms241914890







