DHA-Provoked Reduction in Adipogenesis and Glucose Uptake Could Be Mediated by Gps2 Upregulation in Immature 3T3-L1 Cells
Abstract
:1. Introduction
2. Results
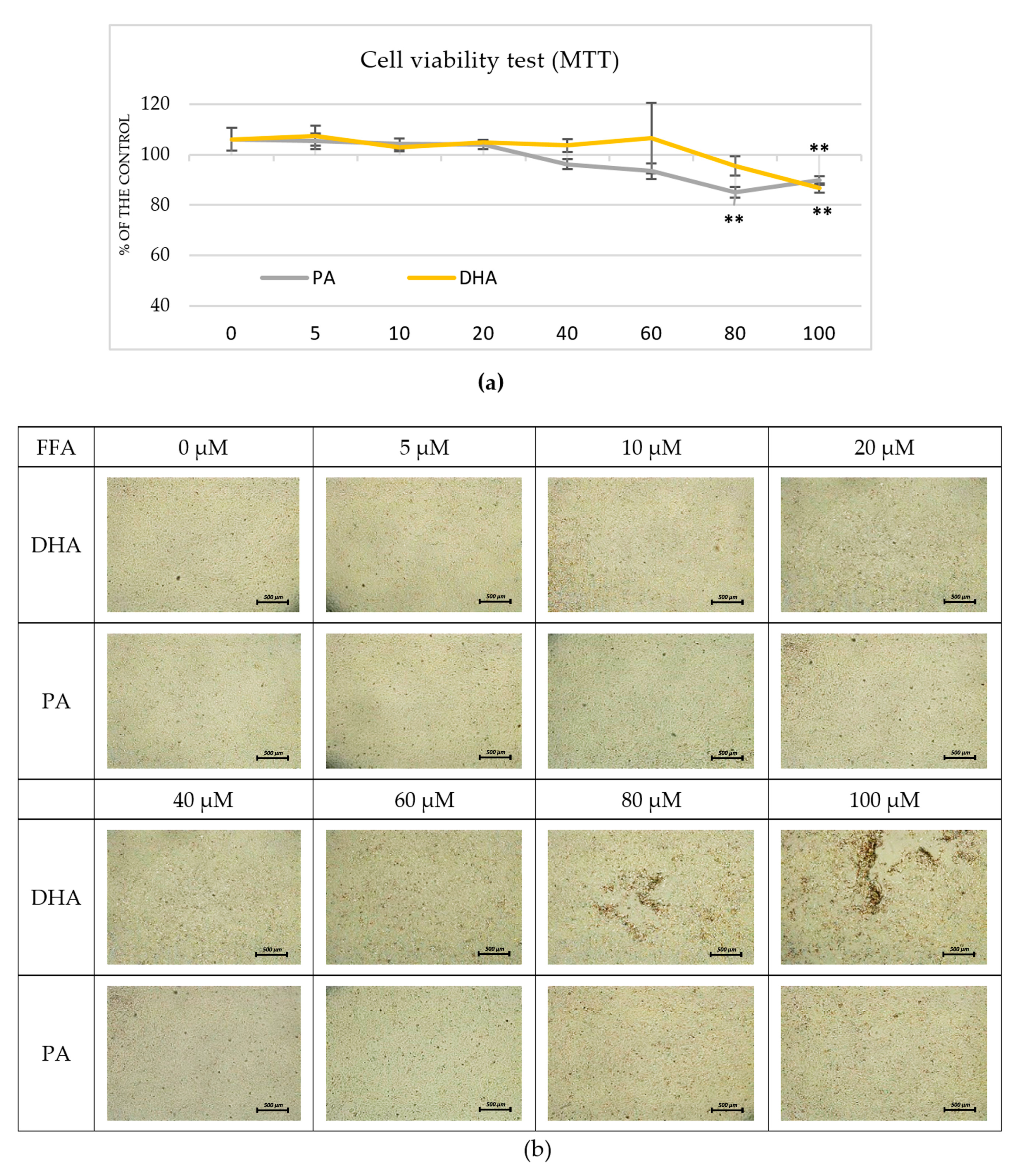
2.1. Pre-Experimental Procedure
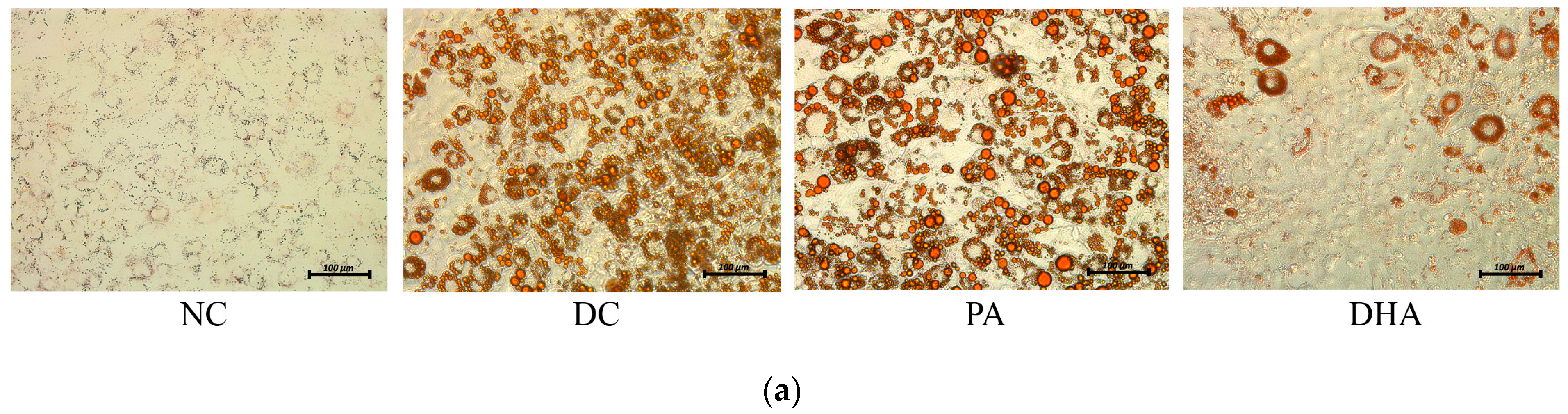
2.2. The Effect of DHA on Adipogenesis
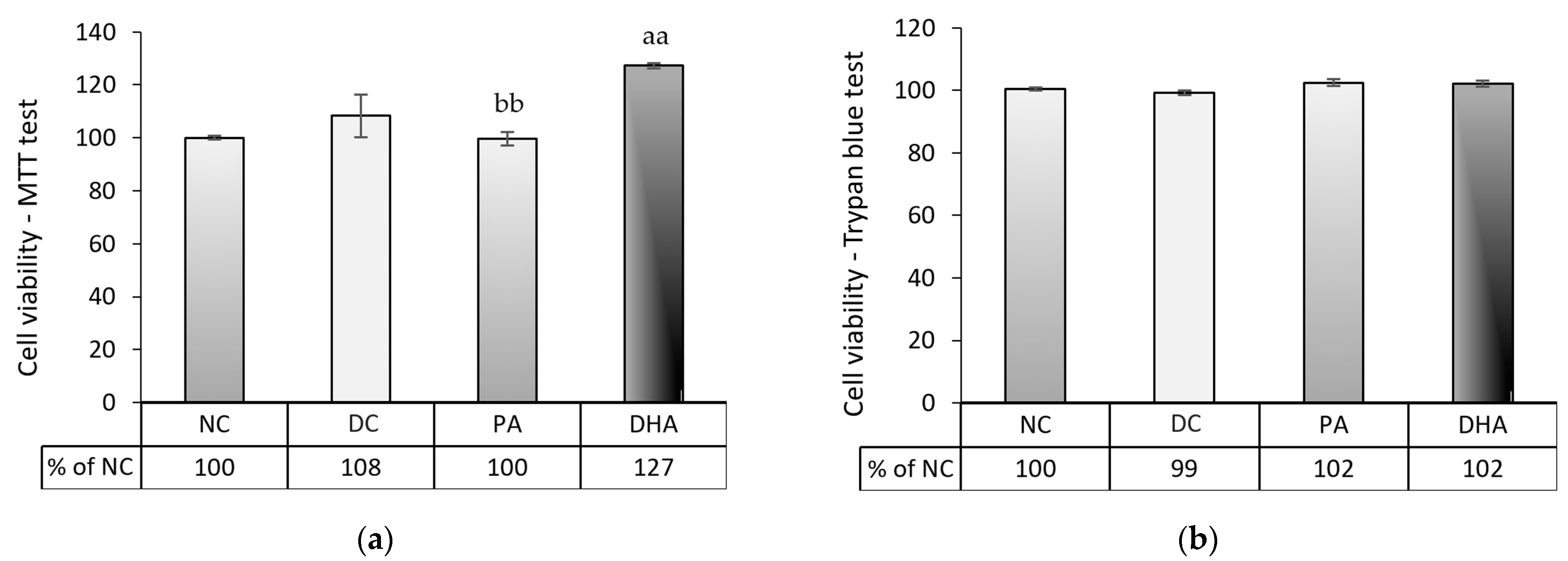
2.3. The Effect of DHA on Cell Number and Viability at the End of the Experiment
2.4. The Effect of DHA on Lipolysis
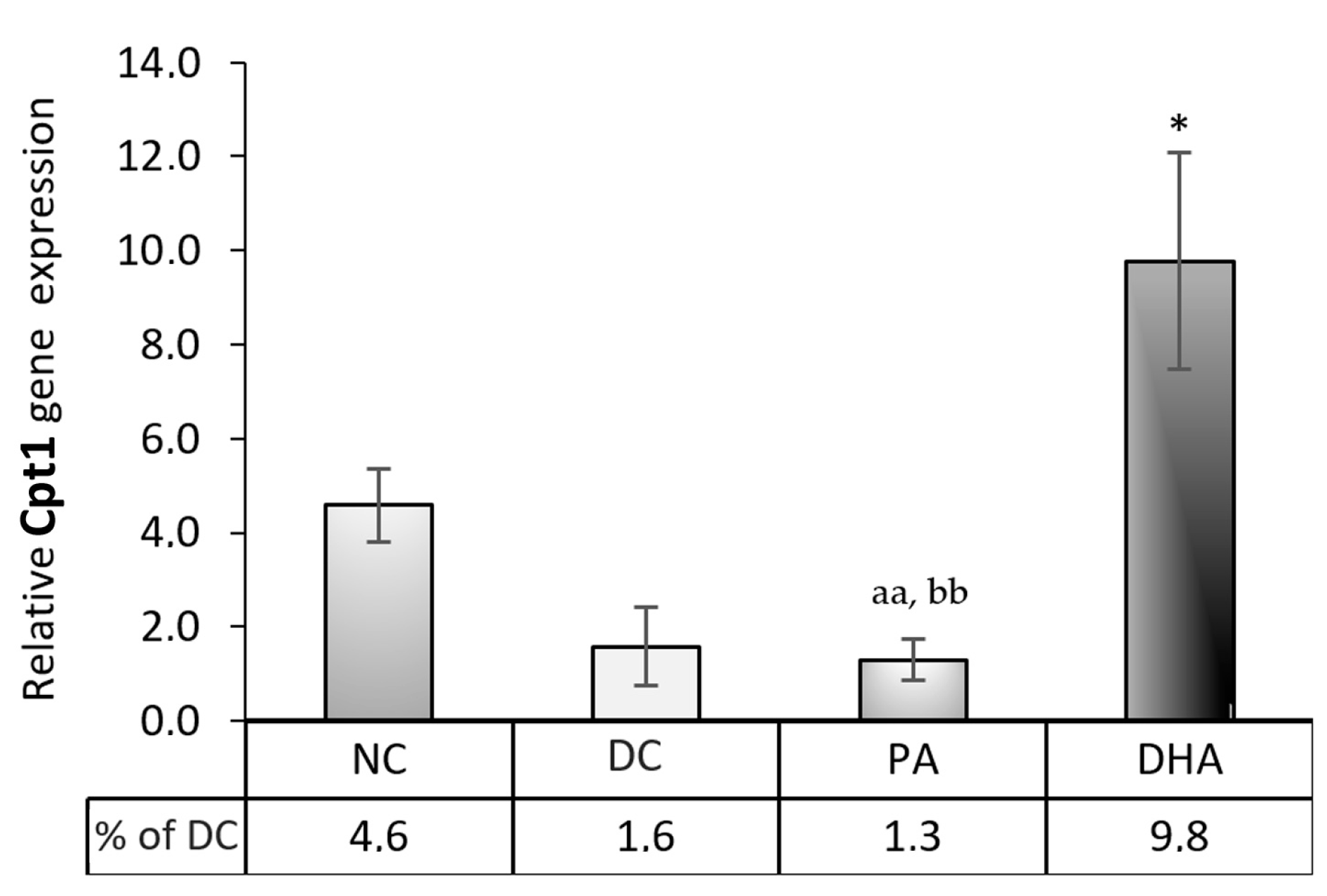
2.5. Carnitine Palmitoyltransferase 1 (Cpt1) Gene Expression
2.6. The Effect of DHA on Glucose Uptake
3. Discussion
4. Materials and Methods
4.1. Cell Culture Handling
4.2. Fatty Acid Dissolving Procedure
4.3. Pre-Experimental Procedure: Cell Viability Assays
4.4. Experimental Design
4.5. Assessment of Neutral Lipid Accumulation
4.6. Lipolysis Rate Evaluation
4.7. Glucose Consumption Estimation
4.8. Gene Expression Analyses
4.9. ELISA Adiponectin Quantification
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Chadt, A.; Al-Hasani, H. Glucose Transporters in Adipose Tissue, Liver, and Skeletal Muscle in Metabolic Health and Disease. Pflug. Arch. Eur. J. Physiol. 2020, 472, 1273–1298. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- D’Angelo, S.; Motti, M.L.; Meccariello, R. ω-3 and ω-6 Polyunsaturated Fatty Acids, Obesity and Cancer. Nutrients 2020, 12, 2751. [Google Scholar] [CrossRef]
- Martínez-Fernández, L.; Laiglesia, L.M.; Huerta, A.E.; Martínez, J.A.; Moreno-Aliaga, M.J. Omega-3 Fatty Acids and Adipose Tissue Function in Obesity and Metabolic Syndrome. Prostaglandins Other Lipid Mediat. 2015, 121, 24–41. [Google Scholar] [CrossRef]
- Magalhães, T.R.; Lourenço, A.L.; Gregório, H.; Queiroga, F.L. Therapeutic Effect of EPA/DHA Supplementation in Neoplastic and Non-Neoplastic Companion Animal Diseases: A Systematic Review. In Vivo 2021, 35, 1419–1436. [Google Scholar] [CrossRef]
- Labib, A.; Golpanian, R.S.; Aickara, D.; Smith, P.; Yosipovitch, G. The Effect of Fatty Acids, Vitamins, and Minerals on Pediatric Atopic Dermatitis: A Systematic Review. Pediatr. Dermatol. 2023, 40, 44–49. [Google Scholar] [CrossRef]
- Mirrahimi, B.; Moazemi, M.; Eslami, N.; Jamshidi, E.; Mir, M.; Mohebbi, R.; Esmaily, H. Evaluating the Effect of Eicosapentaenoic Acid in Children With Atopic Dermatitis: A Randomized Triple-Blind Clinical Trial. J. Pediatr. Pharmacol. Ther. 2023, 28, 29–35. [Google Scholar] [CrossRef]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Bukvić Mokos, Z. Omega-3 Versus Omega-6 Polyunsaturated Fatty Acids in the Prevention and Treatment of Inflammatory Skin Diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef]
- Sawada, Y.; Saito-Sasaki, N.; Nakamura, M. Omega 3 Fatty Acid and Skin Diseases. Front. Immunol. 2020, 11, 623052. [Google Scholar] [CrossRef]
- Wang, M.; Ma, L.-J.; Yang, Y.; Xiao, Z.; Wan, J.-B. N-3 Polyunsaturated Fatty Acids for the Management of Alcoholic Liver Disease: A Critical Review. Crit. Rev. Food Sci. Nutr. 2019, 59, S116–S129. [Google Scholar] [CrossRef]
- de Freitas Rodrigues, J.; Philippsen, H.K.; Dolabela, M.F.; Nagamachi, C.Y.; Pieczarka, J.C. The Potential of DHA as Cancer Therapy Strategies: A Narrative Review of In Vitro Cytotoxicity Trials. Nutrients 2023, 15, 2006. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Haque, R.; Khan, S.A. Chapter 30—Docosahexaenoic Acid (DHA): A Dietary Supplement With Promising Anticancer Potential. In The Molecular Nutrition of Fats; Patel, V.B., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 389–400. ISBN 978-0-12-811297-7. [Google Scholar]
- Jeong, S.; Kim, D.Y.; Kang, S.H.; Yun, H.K.; Kim, J.L.; Kim, B.R.; Park, S.H.; Na, Y.J.; Jo, M.J.; Jeong, Y.A.; et al. Docosahexaenoic Acid Enhances Oxaliplatin-Induced Autophagic Cell Death via the ER Stress/Sesn2 Pathway in Colorectal Cancer. Cancers 2019, 11, 982. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Górniak, B. Peroxisome Proliferator-Activated Receptors and Their Ligands: Nutritional and Clinical Implications—A Review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Kalupahana, N.S.; Goonapienuwala, B.L.; Moustaid-Moussa, N. Omega-3 Fatty Acids and Adipose Tissue: Inflammation and Browning. Annu. Rev. Nutr. 2020, 40, 25–49. [Google Scholar] [CrossRef]
- Pinel, A.; Rigaudière, J.-P.; Morio, B.; Capel, F. Adipose Tissue Dysfunctions in Response to an Obesogenic Diet Are Reduced in Mice after Transgenerational Supplementation with Omega 3 Fatty Acids. Metabolites 2021, 11, 838. [Google Scholar] [CrossRef]
- Harayama, T.; Shimizu, T. Roles of Polyunsaturated Fatty Acids, from Mediators to Membranes. J. Lipid Res. 2020, 61, 1150–1160. [Google Scholar] [CrossRef]
- Mersmann, H.J.; Ding, S.-T. Fatty Acids Modulate Porcine Adipocyte Differentiation and Transcripts for Transcription Factors and Adipocyte-Characteristic Proteins. J. Nutr. Biochem. 2001, 12, 101–108. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Liu, Y.; Chen, M.; Jiang, X.; Shen, T.; Li, Q.; Yang, Y.; Ling, W. N-3 Polyunsaturated Fatty Acids Increase Hepatic Fibroblast Growth Factor 21 Sensitivity via a PPAR-γ-β-Klotho Pathway. Mol. Nutr. Food Res. 2017, 61, 1601075. [Google Scholar] [CrossRef] [PubMed]
- Barquissau, V.; Ghandour, R.A.; Ailhaud, G.; Klingenspor, M.; Langin, D.; Amri, E.-Z.; Pisani, D.F. Control of Adipogenesis by Oxylipins, GPCRs and PPARs. Biochimie 2017, 136, 3–11. [Google Scholar] [CrossRef]
- Zhuang, P.; Lu, Y.; Shou, Q.; Mao, L.; He, L.; Wang, J.; Chen, J.; Zhang, Y.; Jiao, J. Differential Anti-Adipogenic Effects of Eicosapentaenoic and Docosahexaenoic Acids in Obesity. Mol. Nutr. Food Res. 2019, 63, 1801135. [Google Scholar] [CrossRef]
- Kim, H.-K.; Della-Fera, M.; Lin, J.; Baile, C.A. Docosahexaenoic Acid Inhibits Adipocyte Differentiation and Induces Apoptosis in 3T3-L1 Preadipocytes. J. Nutr. 2006, 136, 2965–2969. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-W.; Chien, Y.-S.; Chen, Y.-J.; Ajuwon, K.M.; Mersmann, H.M.; Ding, S.-T. Role of N-3 Polyunsaturated Fatty Acids in Ameliorating the Obesity-Induced Metabolic Syndrome in Animal Models and Humans. Int. J. Mol. Sci. 2016, 17, 1689. [Google Scholar] [CrossRef] [PubMed]
- Colitti, M.; Stefanon, B. Different Anti-Adipogenic Effects of Bio-Compounds on Primary Visceral Pre-Adipocytes and Adipocytes. EXCLI J. 2016, 15, 362–377. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-J.; Huang, C.J.; Xie, D. Anti-Obesity Effects of Conjugated Linoleic Acid, Docosahexaenoic Acid, and Eicosapentaenoic Acid. Mol. Nutr. Food Res. 2008, 52, 631–645. [Google Scholar] [CrossRef]
- Vackova, E.; Bosnakovski, D.; Bjørndal, B.; Yonkova, P.; Grigorova, N.; Ivanova, Z.; Penchev, G.; Simeonova, G.; Miteva, L.; Milanova, A.; et al. N-3 Polyunsaturated Fatty Acids Provoke a Specific Transcriptional Profile in Rabbit Adipose-Derived Stem Cells in Vitro. J. Anim. Physiol. Anim. Nutr. 2019, 103, 925–934. [Google Scholar] [CrossRef]
- Lipke, K.; Kubis-Kubiak, A.; Piwowar, A. Molecular Mechanism of Lipotoxicity as an Interesting Aspect in the Development of Pathological States—Current View of Knowledge. Cells 2022, 11, 844. [Google Scholar] [CrossRef]
- Glass, C.K.; Olefsky, J.M. Inflammation and Lipid Signaling in the Etiology of Insulin Resistance. Cell Metab. 2012, 15, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Cornall, L.M.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. GPR120 Agonism as a Countermeasure against Metabolic Diseases. Drug Discov. Today 2014, 19, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Paschoal, V.A.; Walenta, E.; Talukdar, S.; Pessentheiner, A.R.; Osborn, O.; Hah, N.; Chi, T.J.; Tye, G.L.; Armando, A.M.; Evans, R.M.; et al. Positive Reinforcing Mechanisms between GPR120 and PPARγ Modulate Insulin Sensitivity. Cell Metab. 2020, 31, 1173–1188.e5. [Google Scholar] [CrossRef] [PubMed]
- Drareni, K.; Ballaire, R.; Barilla, S.; Mathew, M.J.; Toubal, A.; Fan, R.; Liang, N.; Chollet, C.; Huang, Z.; Kondili, M.; et al. GPS2 Deficiency Triggers Maladaptive White Adipose Tissue Expansion in Obesity via HIF1A Activation. Cell Rep. 2018, 24, 2957–2971.e6. [Google Scholar] [CrossRef]
- English, J.; Orofino, J.; Cederquist, C.T.; Paul, I.; Li, H.; Auwerx, J.; Emili, A.; Belkina, A.; Cardamone, D.; Perissi, V. GPS2-Mediated Regulation of the Adipocyte Secretome Modulates Adipose Tissue Remodeling at the Onset of Diet-Induced Obesity. Mol. Metab. 2023, 69, 101682. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, Z.; Grigorova, N.; Vachkova, E.; Tacheva, T.; Penchev Georgiev, I. Docosahexaenoic and Oleic Acid Dissimilarly Modulate Lipid Metabolism of Immature and Already Mature Adipocytes in Vitro. Acta Vet. Eurasia 2023, 49, 19–25. [Google Scholar] [CrossRef]
- Sun, C.; Mao, S.; Chen, S.; Zhang, W.; Liu, C. PPARs-Orchestrated Metabolic Homeostasis in the Adipose Tissue. Int. J. Mol. Sci. 2021, 22, 8974. [Google Scholar] [CrossRef] [PubMed]
- Barber, E.; Sinclair, A.J.; Cameron-Smith, D. Comparative Actions of Omega-3 Fatty Acids on in-Vitro Lipid Droplet Formation. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.F.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Eicosapentaenoic and Docosapentaenoic Acids Lessen the Expression of PPARγ/Cidec Affecting Adipogenesis in Cultured 3T3-L1 Adipocytes. Acta Histochem. 2020, 122, 151504. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.; Ross, A.B.; Scheers, N.; Nookaew, I.; Gabrielsson, B.G.; Sandberg, A.-S. The Omega-3 Fatty Acids EPA and DHA, as a Part of a Murine High-Fat Diet, Reduced Lipid Accumulation in Brown and White Adipose Tissues. Int. J. Mol. Sci. 2019, 20, 5895. [Google Scholar] [CrossRef]
- Todorčević, M.; Kjær, M.A.; Djaković, N.; Vegusdal, A.; Torstensen, B.E.; Ruyter, B. N-3 HUFAs Affect Fat Deposition, Susceptibility to Oxidative Stress, and Apoptosis in Atlantic Salmon Visceral Adipose Tissue. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2009, 152, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.-S.; Xu, C.-W.; Xie, H.-Y.; Yao, A.-J.; Shen, Y.-Z.; Wan, J.-J.; Zhang, H.-Q.; Fu, J.-F.; Chen, Z.-M.; Zou, Z.-Q.; et al. DHA Induces Mitochondria-Mediated 3T3-L1 Adipocyte Apoptosis by down-Regulation of Akt and ERK. J. Funct. Foods 2016, 21, 517–524. [Google Scholar] [CrossRef]
- Oh, D.Y.; Walenta, E.; Akiyama, T.E.; Lagakos, W.S.; Lackey, D.; Pessentheiner, A.R.; Sasik, R.; Hah, N.; Chi, T.J.; Cox, J.M.; et al. A Gpr120 Selective Agonist Improves Insulin Resistance and Chronic Inflammation. Nat. Med. 2014, 20, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Yang, Y.; Zhou, Y.; Wei, H.; Peng, J. GPR120: A Critical Role in Adipogenesis, Inflammation, and Energy Metabolism in Adipose Tissue. Cell. Mol. Life Sci. 2017, 74, 2723–2733. [Google Scholar] [CrossRef]
- Tan, C.K.; Zhuang, Y.; Wahli, W. Synthetic and Natural Peroxisome Proliferator-Activated Receptor (PPAR) Agonists as Candidates for the Therapy of the Metabolic Syndrome. Expert Opin. Ther. Targets 2017, 21, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, C.; Hong, Y.-H.; Iga, T.; Hishikawa, D.; Suzuki, Y.; Song, S.-H.; Choi, K.-C.; Adachi, T.; Hirasawa, A.; Tsujimoto, G.; et al. The Regulation of Adipogenesis through GPR120. Biochem. Biophys. Res. Commun. 2007, 354, 591–597. [Google Scholar] [CrossRef]
- Miyauchi, S.; Hirasawa, A.; Iga, T.; Liu, N.; Itsubo, C.; Sadakane, K.; Hara, T.; Tsujimoto, G. Distribution and Regulation of Protein Expression of the Free Fatty Acid Receptor GPR120. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 379, 427–434. [Google Scholar] [CrossRef]
- Barilla, S. Role of GPS2 in the Regulation of Adipocyte Fate and Function: A Multi-Omics Approach; Institutionen för Biovetenskaper Och Näringslära/Department of Biosciences and Nutrition: Stockholm, Sweden, 2020; ISBN 978-91-7831-960-2. [Google Scholar]
- Cardamone, M.D.; Tanasa, B.; Cederquist, C.T.; Huang, J.; Mahdaviani, K.; Li, W.; Rosenfeld, M.G.; Liesa, M.; Perissi, V. Mitochondrial Retrograde Signaling in Mammals Is Mediated by the Transcriptional Cofactor GPS2 via Direct Mitochondria-to-Nucleus Translocation. Mol. Cell 2018, 69, 757–772.e7. [Google Scholar] [CrossRef]
- Barilla, S.; Liang, N.; Mileti, E.; Ballaire, R.; Lhomme, M.; Ponnaiah, M.; Lemoine, S.; Soprani, A.; Gautier, J.-F.; Amri, E.-Z.; et al. Loss of G Protein Pathway Suppressor 2 in Human Adipocytes Triggers Lipid Remodeling by Upregulating ATP Binding Cassette Subfamily G Member 1. Mol. Metab. 2020, 42, 101066. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Li, Y.; Gow, C.-H.; Wong, M.; Zha, J.; Yan, C.; Liu, H.; Wang, Y.; Burris, T.P.; Zhang, J. The Optimal Corepressor Function of Nuclear Receptor Corepressor (NCoR) for Peroxisome Proliferator-Activated Receptor γ Requires G Protein Pathway Suppressor 2. J. Biol. Chem. 2015, 290, 3666–3679. [Google Scholar] [CrossRef]
- Cederquist, C.T.; Lentucci, C.; Martinez-Calejman, C.; Hayashi, V.; Orofino, J.; Guertin, D.; Fried, S.K.; Lee, M.-J.; Cardamone, M.D.; Perissi, V. Systemic Insulin Sensitivity Is Regulated by GPS2 Inhibition of AKT Ubiquitination and Activation in Adipose Tissue. Mol. Metab. 2017, 6, 125–137. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Smith, E.; Gao, Y.; Kwan, J.; Blum, B.C.; Tilston-Lunel, A.M.; Turcinovic, I.; Varelas, X.; Cardamone, M.D.; Monti, S.; et al. Loss of G-Protein Pathway Suppressor 2 Promotes Tumor Growth Through Activation of AKT Signaling. Front. Cell Dev. Biol. 2020, 8, 608044. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K–AKT Network at the Interface of Oncogenic Signalling and Cancer Metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- Drareni, K.; Ballaire, R.; Alzaid, F.; Goncalves, A.; Chollet, C.; Barilla, S.; Nguewa, J.-L.; Dias, K.; Lemoine, S.; Riveline, J.-P.; et al. Adipocyte Reprogramming by the Transcriptional Coregulator GPS2 Impacts Beta Cell Insulin Secretion. Cell Rep. 2020, 32, 108141. [Google Scholar] [CrossRef]
- Grigorova, N.; Ivanova, Z.H.; Vachkova, E.; Tacheva, T.; Georgiev, I.P. Co-Administration of Oleic and Docosahexaenoic Acids Enhances Glucose Uptake Rather than Lipolysis in Mature 3T3-L1 Adipocytes Cell Culture. Bulg. J. Vet. Med. 2022, 25, 411–425. [Google Scholar] [CrossRef]
- Yang, Z.; Tu, Y.; Xia, H.; Jie, G.; Chen, X.; He, P. Suppression of Free-Radicals and Protection against H2O2-Induced Oxidative Damage in HPF-1 Cell by Oxidized Phenolic Compounds Present in Black Tea. Food Chem. 2007, 105, 1349–1356. [Google Scholar] [CrossRef]
- Park, Y.J.; Liang, J.F.; Ko, K.S.; Kim, S.W.; Yang, V.C. Low Molecular Weight Protamine as an Efficient and Nontoxic Gene Carrier: In Vitro Study. J. Gene Med. 2003, 5, 700–711. [Google Scholar] [CrossRef]
- Danesi, F.; Ferioli, F.; Caboni, M.F.; Boschetti, E.; Di Nunzio, M.; Verardo, V.; Valli, V.; Astolfi, A.; Pession, A.; Bordoni, A. Phytosterol Supplementation Reduces Metabolic Activity and Slows Cell Growth in Cultured Rat Cardiomyocytes. Br. J. Nutr. 2011, 106, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Chavez, J.A.; Summers, S.A. Characterizing the Effects of Saturated Fatty Acids on Insulin Signaling and Ceramide and Diacylglycerol Accumulation in 3T3-L1 Adipocytes and C2C12 Myotubes. Arch. Biochem. Biophys. 2003, 419, 101–109. [Google Scholar] [CrossRef]
- Sokolowska, E.; Blachnio-Zabielska, A. The Role of Ceramides in Insulin Resistance. Front. Endocrinol. 2019, 10, 577. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.T.; Fu, J.; Wang, Y.-K.; Desai, R.A.; Chen, C.S. Assaying Stem Cell Mechanobiology on Microfabricated Elastomeric Substrates with Geometrically Modulated Rigidity. Nat. Protoc. 2011, 6, 187–213. [Google Scholar] [CrossRef]
- Rivera Diaz, P.A.; Gómez Camargo, D.E.; Ondo-Méndez, A.; Gómez-Alegría, C.J. A Colorimetric Bioassay for Quantitation of Both Basal and Insulin-Induced Glucose Consumption in 3T3-L1 Adipose Cells. Heliyon 2020, 6, e03422. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, S.; Elashry, M.I.; Klymiuk, M.C.; Geburek, F. Investigation of Stemness and Multipotency of Equine Adipose-Derived Mesenchymal Stem Cells (ASCs) from Different Fat Sources in Comparison with Lipoma. Stem Cell Res. Ther. 2019, 10, 309. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. QBase Relative Quantification Framework and Software for Management and Automated Analysis of Real-Time Quantitative PCR Data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef] [PubMed]




| Abbreviation | Full Name | Forward Primer | Reverse Primer | Product Size (bp) |
|---|---|---|---|---|
| Pparg NM_001127330.2 | Peroxisome proliferator-activated receptor gamma, transcript variant 2 | AGGGCGATCTTGACAGGAAA | CGAAACTGGCACCCTTGAAA | 164 |
| Gpr120 NM_181748.2 | G protein-coupled receptor 120 | CCAACCGCATAGGAGAAATC | CAAGCTCAGCGTAAGCCTCT | 140 |
| Fasn NM_007988.3 | Fatty acid synthase | CTGAAGCCGAACACCTCTGT | GGGAATGTTACACCTTGCTCCT | 218 |
| Acaca NM_133360.3 | Acetyl-CoA carboxylase | TGCTCATGTTCCTTGCCCAA | TGCCACCACCATATTTGAGATT | 247 |
| Cpt1 NM_153679.2 | Carnitine palmitoyltransferase 1 | GTGTACTTCCAACTACGTCAGC | GCGATACAGGAGCAGGGTAT | 169 |
| Gps2 NM_001357906.2 | G protein pathway suppressor 2 | ACAAGTGCTTACGACCCGG | GGAAATGCTGATGGGGCTCT | 183 |
| Pik3r1 NM_001077495.2 | Phosphoinositide-3-kinase regulatory subunit 1 | ATACTTGATGTGGCTGACGC | GTCTCGCTTCCCTCGCAATA | 193 |
| Slc2a4 NM_009204.2 | Solute carrier family 2 member 4 | CGTTGGTCTCGGTGCTCTTA | AGCTCTGCCACAATGAACCA | 220 |
| Hprt NM_013556.2 | Hypoxanthine guanine phosphoribosyl transferase | ACAGGCCAGACTTTGTTGGA | ACTTGCGCTCATCTTAGGCT | 150 |
| Actb NM_007393.5 | β-actin | CCTCTATGCCAACACAGTGC | GTACTCCTGCTTGCTGATCC | 211 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigorova, N.; Ivanova, Z.; Vachkova, E.; Petrova, V.; Penev, T. DHA-Provoked Reduction in Adipogenesis and Glucose Uptake Could Be Mediated by Gps2 Upregulation in Immature 3T3-L1 Cells. Int. J. Mol. Sci. 2023, 24, 13325. https://doi.org/10.3390/ijms241713325
Grigorova N, Ivanova Z, Vachkova E, Petrova V, Penev T. DHA-Provoked Reduction in Adipogenesis and Glucose Uptake Could Be Mediated by Gps2 Upregulation in Immature 3T3-L1 Cells. International Journal of Molecular Sciences. 2023; 24(17):13325. https://doi.org/10.3390/ijms241713325
Chicago/Turabian StyleGrigorova, Natalia, Zhenya Ivanova, Ekaterina Vachkova, Valeria Petrova, and Toncho Penev. 2023. "DHA-Provoked Reduction in Adipogenesis and Glucose Uptake Could Be Mediated by Gps2 Upregulation in Immature 3T3-L1 Cells" International Journal of Molecular Sciences 24, no. 17: 13325. https://doi.org/10.3390/ijms241713325
APA StyleGrigorova, N., Ivanova, Z., Vachkova, E., Petrova, V., & Penev, T. (2023). DHA-Provoked Reduction in Adipogenesis and Glucose Uptake Could Be Mediated by Gps2 Upregulation in Immature 3T3-L1 Cells. International Journal of Molecular Sciences, 24(17), 13325. https://doi.org/10.3390/ijms241713325






