MiRNAs in Lung Adenocarcinoma: Role, Diagnosis, Prognosis, and Therapy
Abstract
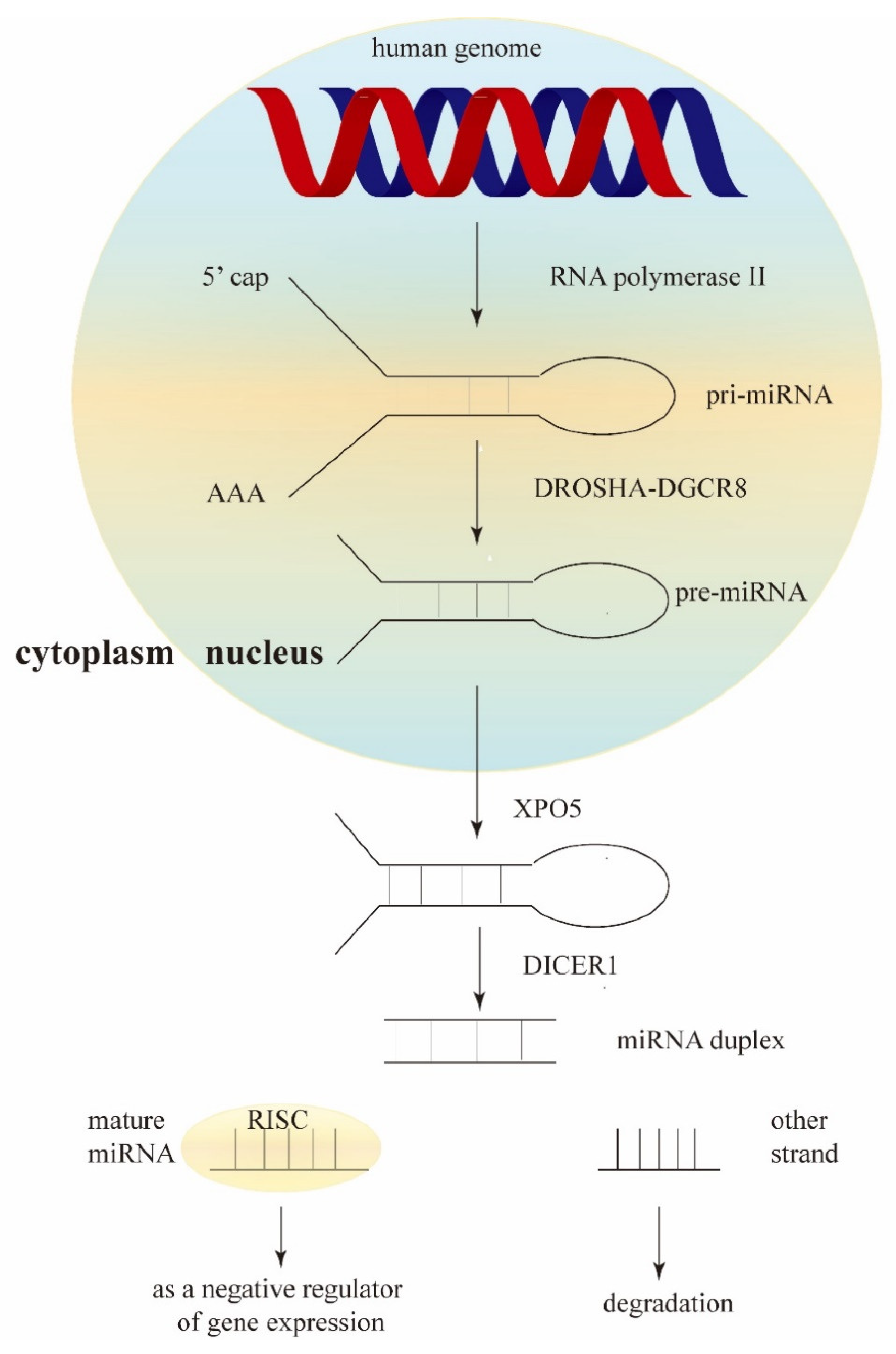
:1. Introduction
2. Tumor Suppressor and Oncogenic miRNAs in LUAD
2.1. Tumor Suppressor miRNAs in LUAD
2.2. Oncogenic miRNAs in LUAD
3. MiRNA Sponges: Competitive Endogenous RNAs (ceRNAs)
4. MiRNAs as Diagnostic Biomarkers in LUAD
4.1. Tissue Sample
4.2. Extracellular Fluid
4.2.1. Blood
4.2.2. Phlegm
4.2.3. Pleural Fluid
4.2.4. Exosome
5. MiRNAs as Prognostic Biomarkers in LUAD
6. MiRNAs in LUAD Therapy
6.1. MiRNAs as Potential Drugs for LUAD Treatment
6.2. MiRNA and Chemo-Resistance
6.3. MiRNA and Radiation Therapy
6.4. MiRNA and EGFR
7. MiRNA and Tumor Immunity in LUAD
8. Conclusions and Future Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bade, B.C.; Cruz, D.C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Vasudevan, S.; Tong, Y.; Steitz, J.A. Switching from repression to activation: MicroRNAs can up-regulate translation. Science 2007, 318, 1931–1934. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.I.; Yan, K.P.; Amuthan, G.; Chendrimada, T.; Doratotaj, B.; Cooch, N.; Shiekhattar, R. The Microprocessor complex mediates the genesis of microRNAs. Nature 2004, 432, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Denli, A.M.; Tops, B.B.; Plasterk, R.H.; Ketting, R.F.; Hannon, G.J. Processing of primary microRNAs by the Microprocessor complex. Nature 2004, 432, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ahn, C.; Han, J.; Choi, H.; Kim, J.; Yim, J.; Lee, J.; Provost, P.; Radmark, O.; Kim, S.; et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003, 425, 415–419. [Google Scholar] [CrossRef]
- Lund, E.; Guttinger, S.; Calado, A.; Dahlberg, J.E.; Kutay, U. Nuclear export of microRNA precursors. Science 2004, 303, 95–98. [Google Scholar] [CrossRef]
- Bohnsack, M.T.; Czaplinski, K.; Gorlich, D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA 2004, 10, 185–191. [Google Scholar] [CrossRef]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef]
- Esquela-Kerscher, A.; Slack, F.J. Oncomirs—microRNAs with a role in cancer. Nat. Rev. Cancer 2006, 6, 259–269. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging concepts of miRNA therapeutics: From cells to clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, J.; Wang, J.; Lin, X.; Ding, F. Role of miRNA in Lung Cancer-Potential Biomarkers and Therapies. Curr. Pharm. Des. 2018, 23, 5997–6010. [Google Scholar] [CrossRef] [PubMed]
- Shaker, F.; Nikravesh, A.; Arezumand, R.; Aghaee-Bakhtiari, S.H. Web-based tools for miRNA studies analysis. Comput. Biol. Med. 2020, 127, 104060. [Google Scholar] [CrossRef]
- Lee, Y.S.; Dutta, A. MicroRNAs in cancer. Annu. Rev. Pathol. 2009, 4, 199–227. [Google Scholar] [CrossRef] [PubMed]
- Takamizawa, J.; Konishi, H.; Yanagisawa, K.; Tomida, S.; Osada, H.; Endoh, H.; Harano, T.; Yatabe, Y.; Nagino, M.; Nimura, Y.; et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004, 64, 3753–3756. [Google Scholar] [CrossRef]
- Inamura, K.; Togashi, Y.; Nomura, K.; Ninomiya, H.; Hiramatsu, M.; Satoh, Y.; Okumura, S.; Nakagawa, K.; Ishikawa, Y. let-7 microRNA expression is reduced in bronchioloalveolar carcinoma, a non-invasive carcinoma, and is not correlated with prognosis. Lung Cancer 2007, 58, 392–396. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, J.X.; Hao, R.M.; Zhang, Q.; Guo, J.Q.; Li, Y.J.; Xie, N.; Liu, L.Y.; Wang, P.Y.; Zhang, C.; et al. Induction of microRNA-let-7a inhibits lung adenocarcinoma cell growth by regulating cyclin D1. Oncol. Rep. 2018, 40, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, R.; Lu, M.; Cheng, C.; Feng, Z.; Zhao, R.; Liang, J.; Han, J.; Jiang, J.; Xu-Welliver, M.; et al. Let-7b-3p inhibits tumor growth and metastasis by targeting the BRF2-mediated MAPK/ERK pathway in human lung adenocarcinoma. Transl. Lung Cancer Res. 2021, 10, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.Y.; Sun, Y.X.; Zhang, S.; Pang, M.; Zhang, H.H.; Gao, S.Y.; Zhang, C.; Lv, C.J.; Xie, S.Y. Let-7c inhibits A549 cell proliferation through oncogenic TRIB2 related factors. FEBS Lett. 2013, 587, 2675–2681. [Google Scholar] [CrossRef]
- Nelson, H.H.; Christensen, B.C.; Plaza, S.L.; Wiencke, J.K.; Marsit, C.J.; Kelsey, K.T. KRAS mutation, KRAS-LCS6 polymorphism, and non-small cell lung cancer. Lung Cancer 2010, 69, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, E.J.; Lee, S.; Tan, X.; Liu, X.; Park, S.; Kang, K.; Yoon, J.S.; Ko, Y.H.; Kurie, J.M.; et al. MiR-34a and miR-34b/c have distinct effects on the suppression of lung adenocarcinomas. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- Daugaard, I.; Knudsen, A.; Kjeldsen, T.E.; Hager, H.; Hansen, L.L. The association between miR-34 dysregulation and distant metastases formation in lung adenocarcinoma. Exp. Mol. Pathol. 2017, 102, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Shiba-Ishii, A.; Kim, Y.; Dai, T.; Husni, R.E.; Hong, J.; Kano, J.; Sakashita, S.; Iijima, T.; Noguchi, M. miR-3941: A novel microRNA that controls IGBP1 expression and is associated with malignant progression of lung adenocarcinoma. Cancer Sci. 2017, 108, 536–542. [Google Scholar] [CrossRef]
- Okada, N.; Lin, C.P.; Ribeiro, M.C.; Biton, A.; Lai, G.; He, X.; Bu, P.; Vogel, H.; Jablons, D.M.; Keller, A.C.; et al. A positive feedback between p53 and miR-34 miRNAs mediates tumor suppression. Genes. Dev. 2014, 28, 438–450. [Google Scholar] [CrossRef]
- Gibbons, D.L.; Lin, W.; Creighton, C.J.; Rizvi, Z.H.; Gregory, P.A.; Goodall, G.J.; Thilaganathan, N.; Du, L.; Zhang, Y.; Pertsemlidis, A.; et al. Contextual extracellular cues promote tumor cell EMT and metastasis by regulating miR-200 family expression. Genes. Dev. 2009, 23, 2140–2151. [Google Scholar] [CrossRef]
- Schliekelman, M.J.; Gibbons, D.L.; Faca, V.M.; Creighton, C.J.; Rizvi, Z.H.; Zhang, Q.; Wong, C.H.; Wang, H.; Ungewiss, C.; Ahn, Y.H.; et al. Targets of the tumor suppressor miR-200 in regulation of the epithelial-mesenchymal transition in cancer. Cancer Res. 2011, 71, 7670–7682. [Google Scholar] [CrossRef]
- Roy, B.C.; Kohno, T.; Iwakawa, R.; Moriguchi, T.; Kiyono, T.; Morishita, K.; Sanchez-Cespedes, M.; Akiyama, T.; Yokota, J. Involvement of LKB1 in epithelial-mesenchymal transition (EMT) of human lung cancer cells. Lung Cancer 2010, 70, 136–145. [Google Scholar] [CrossRef]
- Kim, J.S.; Kurie, J.M.; Ahn, Y.H. BMP4 depletion by miR-200 inhibits tumorigenesis and metastasis of lung adenocarcinoma cells. Mol. Cancer 2015, 14, 173. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yi, X.; Goswami, S.; Ahn, Y.H.; Roybal, J.D.; Yang, Y.; Diao, L.; Peng, D.; Peng, D.; Fradette, J.J.; et al. Growth and metastasis of lung adenocarcinoma is potentiated by BMP4-mediated immunosuppression. Oncoimmunology 2016, 5, e1234570. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Chuang, C.H.; Prosser, H.M.; Fuziwara, C.S.; Chan, C.; Sahasrabudhe, N.; Kuhn, M.; Wu, Y.; Chen, J.; Biton, A.; et al. miR-200 deficiency promotes lung cancer metastasis by activating Notch signaling in cancer-associated fibroblasts. Genes. Dev. 2021, 35, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ahn, Y.H.; Gibbons, D.L.; Zang, Y.; Lin, W.; Thilaganathan, N.; Alvarez, C.A.; Moreira, D.C.; Creighton, C.J.; Gregory, P.A.; et al. The Notch ligand Jagged2 promotes lung adenocarcinoma metastasis through a miR-200-dependent pathway in mice. J. Clin. Investig. 2011, 121, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Luo, P.; Song, Q.; Fei, X. DNMT1/miR-200a/GOLM1 signaling pathway regulates lung adenocarcinoma cells proliferation. Biomed. Pharmacother. 2018, 99, 839–847. [Google Scholar] [CrossRef]
- Guo, L.; Wang, J.; Yang, P.; Lu, Q.; Zhang, T.; Yang, Y. MicroRNA-200 promotes lung cancer cell growth through FOG2-independent AKT activation. IUBMB Life 2015, 67, 720–725. [Google Scholar] [CrossRef]
- Cho, W.C.; Chow, A.S.; Au, J.S. Restoration of tumour suppressor hsa-miR-145 inhibits cancer cell growth in lung adenocarcinoma patients with epidermal growth factor receptor mutation. Eur. J. Cancer 2009, 45, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C.; Chow, A.S.; Au, J.S. MiR-145 inhibits cell proliferation of human lung adenocarcinoma by targeting EGFR and NUDT1. RNA Biol. 2011, 8, 125–131. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Yan, Y.F.; Liu, Y.M.; Li, Y.J.; Zhang, H.H.; Pang, M.; Hu, J.X.; Zhao, W.; Xie, N.; Zhou, L.; et al. Smad3-related miRNAs regulated oncogenic TRIB2 promoter activity to effectively suppress lung adenocarcinoma growth. Cell Death Dis. 2016, 7, e2528. [Google Scholar] [CrossRef]
- Watt, K.; Newsted, D.; Voorand, E.; Gooding, R.J.; Majewski, A.; Truesdell, P.; Yao, B.; Tuschl, T.; Renwick, N.; Craig, A.W. MicroRNA-206 suppresses TGF-beta signalling to limit tumor growth and metastasis in lung adenocarcinoma. Cell. Signal. 2018, 50, 25–36. [Google Scholar] [CrossRef]
- Liu, L.; Bi, N.; Wu, L.; Ding, X.; Men, Y.; Zhou, W.; Li, L.; Zhang, W.; Shi, S.; Song, Y.; et al. MicroRNA-29c functions as a tumor suppressor by targeting VEGFA in lung adenocarcinoma. Mol. Cancer 2017, 16, 50. [Google Scholar] [CrossRef]
- Greenawalt, E.J.; Edmonds, M.D.; Jain, N.; Adams, C.M.; Mitra, R.; Eischen, C.M. Targeting of SGK1 by miR-576-3p Inhibits Lung Adenocarcinoma Migration and Invasion. Mol. Cancer Res. 2019, 17, 289–298. [Google Scholar] [CrossRef]
- Li, X.; Fu, Q.; Li, H.; Zhu, L.; Chen, W.; Ruan, T.; Xu, W.; Yu, X. MicroRNA-520c-3p functions as a novel tumor suppressor in lung adenocarcinoma. FEBS J. 2019, 286, 2737–2752. [Google Scholar] [CrossRef] [PubMed]
- Misono, S.; Seki, N.; Mizuno, K.; Yamada, Y.; Uchida, A.; Sanada, H.; Moriya, S.; Kikkawa, N.; Kumamoto, T.; Suetsugu, T.; et al. Molecular Pathogenesis of Gene Regulation by the miR-150 Duplex: MiR-150-3p Regulates TNS4 in Lung Adenocarcinoma. Cancers 2019, 11, 601. [Google Scholar] [CrossRef]
- Sanada, H.; Seki, N.; Mizuno, K.; Misono, S.; Uchida, A.; Yamada, Y.; Moriya, S.; Kikkawa, N.; Machida, K.; Kumamoto, T.; et al. Involvement of Dual Strands of miR-143 (miR-143-5p and miR-143-3p) and Their Target Oncogenes in the Molecular Pathogenesis of Lung Adenocarcinoma. Int. J. Mol. Sci. 2019, 20, 4482. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhu, J.; Du, W.; Ning, W.; Zhang, Y.; Zeng, Y.; Liu, Z.; Huang, J.A. AKT2 drives cancer progression and is negatively modulated by miR-124 in human lung adenocarcinoma. Respir. Res. 2020, 21, 227. [Google Scholar] [CrossRef]
- Cui, S.; Lou, S.; Guo, W.; Jian, S.; Wu, Y.; Liu, X.; Lan, X.; Jia, X. Prediction of MiR-21-5p in Promoting the Development of Lung Adenocarcinoma via PDZD2 Regulation. Med. Sci. Monit. 2020, 26, e923366. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhou, Y.; Chen, W.; Yang, Y.; Ye, J.; Ou, H.; Wu, H. miR-21-5p promotes lung adenocarcinoma cell proliferation, migration and invasion via targeting WWC2. Cancer Biomark 2020, 28, 549–559. [Google Scholar] [CrossRef]
- An, Y.; Zhang, Q.; Li, X.; Wang, Z.; Li, Y.; Tang, X. Upregulated microRNA miR-21 promotes the progression of lung adenocarcinoma through inhibition of KIBRA and the Hippo signaling pathway. Biomed. Pharmacother. 2018, 108, 1845–1855. [Google Scholar] [CrossRef]
- Zhong, J.; Ren, X.; Chen, Z.; Zhang, H.; Zhou, L.; Yuan, J.; Li, P.; Chen, X.; Liu, W.; Wu, D.; et al. miR-21-5p promotes lung adenocarcinoma progression partially through targeting SET/TAF-Ialpha. Life Sci. 2019, 231, 116539. [Google Scholar] [CrossRef]
- Kunita, A.; Morita, S.; Irisa, T.U.; Goto, A.; Niki, T.; Takai, D.; Nakajima, J.; Fukayama, M. MicroRNA-21 in cancer-associated fibroblasts supports lung adenocarcinoma progression. Sci. Rep. 2018, 8, 8838. [Google Scholar] [CrossRef]
- Seike, M.; Goto, A.; Okano, T.; Bowman, E.D.; Schetter, A.J.; Horikawa, I.; Mathe, E.A.; Jen, J.; Yang, P.; Sugimura, H.; et al. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc. Natl. Acad. Sci. USA 2009, 106, 12085–12090. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, S.; Zheng, M.; Chen, Z.; Wang, G.; Ma, J.; Zhang, B.; Huang, W.; Sun, X.; Wang, C. miR-31-5p modulates cell progression in lung adenocarcinoma through TNS1/p53 axis. Strahlenther. Onkol. 2022, 198, 304–314. [Google Scholar] [CrossRef]
- Edmonds, M.D.; Boyd, K.L.; Moyo, T.; Mitra, R.; Duszynski, R.; Arrate, M.P.; Chen, X.; Zhao, Z.; Blackwell, T.S.; Andl, T.; et al. MicroRNA-31 initiates lung tumorigenesis and promotes mutant KRAS-driven lung cancer. J. Clin. Investig. 2016, 126, 349–364. [Google Scholar]
- Wang, J.; Zhou, T.; Sun, Z.; Ye, T.; Zhou, S.; Li, J.; Liu, Y.; Kong, L.; Tang, J.; Liu, D.; et al. Zeb1 Regulates the Symmetric Division of Mouse Lewis Lung Carcinoma Stem Cells through Numb mediated by miR-31. Int. J. Biol. Sci. 2018, 14, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.S.; Jeon, H.S.; Sun, Z.; Aubry, M.C.; Tang, H.; Park, C.H.; Rakhshan, F.; Schultz, D.A.; Kolbert, C.P.; Lupu, R.; et al. Increased miR-708 expression in NSCLC and its association with poor survival in lung adenocarcinoma from never smokers. Clin. Cancer Res. 2012, 18, 3658–3667. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Hou, D.; Liang, H.; Gong, F.; Wang, Y.; Yan, X.; Jiang, X.; Wang, C.; Zhang, J.; Zen, K.; et al. miR-150 promotes the proliferation and migration of lung cancer cells by targeting SRC kinase signalling inhibitor 1. Eur. J. Cancer 2014, 50, 1013–1024. [Google Scholar]
- Song, Q.; Xu, Y.; Yang, C.; Chen, Z.; Jia, C.; Chen, J.; Zhang, Y.; Lai, P.; Fan, X.; Zhou, X.; et al. miR-483-5p promotes invasion and metastasis of lung adenocarcinoma by targeting RhoGDI1 and ALCAM. Cancer Res. 2014, 74, 3031–3042. [Google Scholar] [PubMed]
- Sun, Y.; Zhao, J.; Yin, X.; Yuan, X.; Guo, J.; Bi, J. miR-297 acts as an oncogene by targeting GPC5 in lung adenocarcinoma. Cell Prolif. 2016, 49, 636–643. [Google Scholar] [CrossRef]
- Sanchez, N.C.; Medrano-Jimenez, E.; Aguilar-Leon, D.; Perez-Martinez, L.; Pedraza-Alva, G. Tumor Necrosis Factor-Induced miR-146a Upregulation Promotes Human Lung Adenocarcinoma Metastasis by Targeting Merlin. DNA Cell Biol. 2020, 39, 484–497. [Google Scholar] [CrossRef]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef]
- Thomson, D.W.; Dinger, M.E. Endogenous microRNA sponges: Evidence and controversy. Nat. Rev. Genet. 2016, 17, 272–283. [Google Scholar] [PubMed]
- Li, L.; Chang, H.Y. Physiological roles of long noncoding RNAs: Insight from knockout mice. Trends Cell Biol. 2014, 24, 594–602. [Google Scholar] [PubMed]
- Ma, T.; Hu, Y.; Guo, Y.; Yan, B. Tumor-Promoting Activity of Long Noncoding RNA LINC00466 in Lung Adenocarcinoma via miR-144-Regulated HOXA10 Axis. Am. J. Pathol. 2019, 189, 2154–2170. [Google Scholar] [PubMed]
- Wang, L.; Zhang, X.; Liu, Y.; Xu, S. Long noncoding RNA FBXL19-AS1 induces tumor growth and metastasis by sponging miR-203a-3p in lung adenocarcinoma. J. Cell Physiol. 2020, 235, 3612–3625. [Google Scholar] [PubMed]
- Liu, J.; Feng, Y.; Zeng, X.; He, M.; Gong, Y.; Liu, Y. LncRNA VPS9D1-AS1 Promotes Malignant Progression of Lung Adenocarcinoma by Targeting miRNA-30a-5p/KIF11 Axis. Front. Genet. 2021, 12, 807628. [Google Scholar]
- Pei, Y.F.; He, Y.; Hu, L.Z.; Zhou, B.; Xu, H.Y.; Liu, X.Q. The Crosstalk between lncRNA-SNHG7/miRNA-181/cbx7 Modulates Malignant Character in Lung Adenocarcinoma. Am. J. Pathol. 2020, 190, 1343–1354. [Google Scholar]
- Ge, Z.; Liu, H.; Ji, T.; Liu, Q.; Zhang, L.; Zhu, P.; Li, L.; Zhu, L. Long non-coding RNA 00960 promoted the aggressiveness of lung adenocarcinoma via the miR-124a/SphK1 axis. Bioengineered 2022, 13, 1276–1287. [Google Scholar] [CrossRef]
- Du, J.; Zhang, G.; Qiu, H.; Yu, H.; Yuan, W. The novel circular RNA circ-CAMK2A enhances lung adenocarcinoma metastasis by regulating the miR-615-5p/fibronectin 1 pathway. Cell Mol. Biol. Lett. 2019, 24, 72. [Google Scholar]
- Xu, Y.; Yu, J.; Huang, Z.; Fu, B.; Tao, Y.; Qi, X.; Mou, Y.; Hu, Y.; Wang, Y.; Cao, Y.; et al. Circular RNA hsa_circ_0000326 acts as a miR-338-3p sponge to facilitate lung adenocarcinoma progression. J. Exp. Clin. Cancer Res. 2020, 39, 57. [Google Scholar]
- Sun, Z. Circular RNA hsa_circ_0001588 promotes the malignant progression of lung adenocarcinoma by modulating miR-524-3p/NACC1 signaling. Life Sci. 2020, 259, 118157. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Qiu, T.; Han, B.; Wang, Y.; Sun, X.; Qin, Y.; Liu, A.; Ge, N.; Jiao, W. Circular RNA circCSNK1G3 induces HOXA10 signaling and promotes the growth and metastasis of lung adenocarcinoma cells through hsa-miR-143-3p sponging. Cell Oncol. 2021, 44, 297–310. [Google Scholar]
- Shen, H.Y.; Shi, L.X.; Wang, L.; Fang, L.P.; Xu, W.; Xu, J.Q.; Fan, B.Q.; Fan, W.F. Hsa_circ_0001361 facilitates the progress of lung adenocarcinoma cells via targeting miR-525-5p/VMA21 axis. J. Transl. Med. 2021, 19, 389. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Guo, T.; Chen, W.; Lin, Z.; Ye, M.; Pan, X. CircMMD_007 promotes oncogenic effects in the progression of lung adenocarcinoma through microRNA-197-3p/protein tyrosine phosphatase non-receptor type 9 axis. Bioengineered 2022, 13, 4991–5004. [Google Scholar] [CrossRef]
- Nooreldeen, R.; Bach, H. Current and Future Development in Lung Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef]
- Landi, M.T.; Zhao, Y.; Rotunno, M.; Koshiol, J.; Liu, H.; Bergen, A.W.; Rubagotti, M.; Goldstein, A.M.; Linnoila, I.; Marincola, F.M.; et al. MicroRNA expression differentiates histology and predicts survival of lung cancer. Clin. Cancer Res. 2010, 16, 430–441. [Google Scholar]
- Hamamoto, J.; Soejima, K.; Yoda, S.; Naoki, K.; Nakayama, S.; Satomi, R.; Terai, H.; Ikemura, S.; Sato, T.; Yasuda, H.; et al. Identification of microRNAs differentially expressed between lung squamous cell carcinoma and lung adenocarcinoma. Mol. Med. Rep. 2013, 8, 456–462. [Google Scholar] [CrossRef]
- Patnaik, S.; Mallick, R.; Kannisto, E.; Sharma, R.; Bshara, W.; Yendamuri, S.; Dhillon, S.S. MiR-205 and MiR-375 microRNA assays to distinguish squamous cell carcinoma from adenocarcinoma in lung cancer biopsies. J. Thorac. Oncol. 2015, 10, 446–453. [Google Scholar] [CrossRef]
- Huang, W.; Hu, J.; Yang, D.W.; Fan, X.T.; Jin, Y.; Hou, Y.Y.; Wang, J.P.; Yuan, Y.F.; Tan, Y.S.; Zhu, X.Z.; et al. Two microRNA panels to discriminate three subtypes of lung carcinoma in bronchial brushing specimens. Am. J. Respir. Crit. Care Med. 2012, 186, 1160–1167. [Google Scholar] [CrossRef]
- Powrozek, T.; Mlak, R.; Dziedzic, M.; Malecka-Massalska, T.; Sagan, D. Analysis of primary-miRNA-3662 and its mature form may improve detection of the lung adenocarcinoma. J. Cancer Res. Clin. Oncol. 2017, 143, 1941–1946. [Google Scholar] [CrossRef]
- He, Y.; Yang, Y.; Kuang, P.; Ren, S.; Rozeboom, L.; Rivard, C.J.; Li, X.; Zhou, C.; Hirsch, F.R. Seven-microRNA panel for lung adenocarcinoma early diagnosis in patients presenting with ground-glass nodules. Onco Targets Ther. 2017, 10, 5915–5926. [Google Scholar]
- Wang, H.; Peng, R.; Wang, J.; Qin, Z.; Xue, L. Circulating microRNAs as potential cancer biomarkers: The advantage and disadvantage. Clin. Epigenetics 2018, 10, 59. [Google Scholar]
- Patnaik, S.K.; Yendamuri, S.; Kannisto, E.; Kucharczuk, J.C.; Singhal, S.; Vachani, A. MicroRNA expression profiles of whole blood in lung adenocarcinoma. PLoS ONE 2012, 7, e46045. [Google Scholar]
- Rani, S.; Gately, K.; Crown, J.; O’Byrne, K.; O’Driscoll, L. Global analysis of serum microRNAs as potential biomarkers for lung adenocarcinoma. Cancer Biol. Ther. 2013, 14, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Tai, M.C.; Yanagisawa, K.; Nakatochi, M.; Hotta, N.; Hosono, Y.; Kawaguchi, K.; Naito, M.; Taniguchi, H.; Wakai, K.; Yokoi, K.; et al. Blood-borne miRNA profile-based diagnostic classifier for lung adenocarcinoma. Sci. Rep. 2016, 6, 31389. [Google Scholar] [CrossRef]
- Gao, S.; Guo, W.; Liu, T.; Liang, N.; Ma, Q.; Gao, Y.; Tan, F.; Xue, Q.; He, J. Plasma extracellular vesicle microRNA profiling and the identification of a diagnostic signature for stage I lung adenocarcinoma. Cancer Sci. 2022, 113, 648–659. [Google Scholar] [PubMed]
- Gao, F.; Chang, J.; Wang, H.; Zhang, G. Potential diagnostic value of miR-155 in serum from lung adenocarcinoma patients. Oncol. Rep. 2014, 31, 351–357. [Google Scholar] [PubMed]
- Powrozek, T.; Krawczyk, P.; Kowalski, D.M.; Winiarczyk, K.; Olszyna-Serementa, M.; Milanowski, J. Plasma circulating microRNA-944 and microRNA-3662 as potential histologic type-specific early lung cancer biomarkers. Transl. Res. 2015, 166, 315–323. [Google Scholar]
- Yu, L.; Todd, N.W.; Xing, L.; Xie, Y.; Zhang, H.; Liu, Z.; Fang, H.; Zhang, J.; Katz, R.L.; Jiang, F. Early detection of lung adenocarcinoma in sputum by a panel of microRNA markers. Int. J. Cancer 2010, 127, 2870–2878. [Google Scholar] [PubMed]
- Shin, Y.M.; Yun, J.; Lee, O.J.; Han, H.S.; Lim, S.N.; An, J.Y.; Lee, K.H.; Lee, K.M.; Choe, K.H. Diagnostic Value of Circulating Extracellular miR-134, miR-185, and miR-22 Levels in Lung Adenocarcinoma-Associated Malignant Pleural Effusion. Cancer Res. Treat. 2014, 46, 178–185. [Google Scholar] [PubMed]
- Han, H.S.; Yun, J.; Lim, S.N.; Han, J.H.; Lee, K.H.; Kim, S.T.; Kang, M.H.; Son, S.M.; Lee, Y.M.; Choi, S.Y.; et al. Downregulation of cell-free miR-198 as a diagnostic biomarker for lung adenocarcinoma-associated malignant pleural effusion. Int. J. Cancer 2013, 133, 645–652. [Google Scholar] [PubMed]
- Zhang, Y.; Liu, F.; Yuan, Y.; Jin, C.; Chang, C.; Zhu, Y.; Zhang, X.; Tian, C.; He, F.; Wang, J. Inflammasome-Derived Exosomes Activate NF-kappaB Signaling in Macrophages. J. Proteome Res. 2017, 16, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Chen, Y.; Chen, H.; Fei, S.; Chen, D.; Cai, X.; Liu, L.; Lin, B.; Su, H.; Zhao, L.; et al. Evaluation of Tumor-Derived Exosomal miRNA as Potential Diagnostic Biomarkers for Early-Stage Non-Small Cell Lung Cancer Using Next-Generation Sequencing. Clin. Cancer Res. 2017, 23, 5311–5319. [Google Scholar]
- Han, Z.; Li, Y.; Zhang, J.; Guo, C.; Li, Q.; Zhang, X.; Lan, Y.; Gu, W.; Xing, Z.; Liang, L.; et al. Tumor-derived circulating exosomal miR-342-5p and miR-574-5p as promising diagnostic biomarkers for early-stage Lung Adenocarcinoma. Int. J. Med. Sci. 2020, 17, 1428–1438. [Google Scholar] [CrossRef]
- Yanaihara, N.; Caplen, N.; Bowman, E.; Seike, M.; Kumamoto, K.; Yi, M.; Stephens, R.M.; Okamoto, A.; Yokota, J.; Tanaka, T.; et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 2006, 9, 189–198. [Google Scholar]
- Saito, M.; Schetter, A.J.; Mollerup, S.; Kohno, T.; Skaug, V.; Bowman, E.D.; Mathe, E.A.; Takenoshita, S.; Yokota, J.; Haugen, A.; et al. The association of microRNA expression with prognosis and progression in early-stage, non-small cell lung adenocarcinoma: A retrospective analysis of three cohorts. Clin. Cancer Res. 2011, 17, 1875–1882. [Google Scholar]
- Rothschild, S.I.; Tschan, M.P.; Federzoni, E.A.; Jaggi, R.; Fey, M.F.; Gugger, M.; Gautschi, O. MicroRNA-29b is involved in the Src-ID1 signaling pathway and is dysregulated in human lung adenocarcinoma. Oncogene 2012, 31, 4221–4232. [Google Scholar] [PubMed]
- Rothschild, S.I.; Tschan, M.P.; Jaggi, R.; Fey, M.F.; Gugger, M.; Gautschi, O. MicroRNA-381 represses ID1 and is deregulated in lung adenocarcinoma. J. Thorac. Oncol. 2012, 7, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Nadal, E.; Chen, G.; Gallegos, M.; Lin, L.; Ferrer-Torres, D.; Truini, A.; Wang, Z.; Lin, J.; Reddy, R.M.; Llatjos, R.; et al. Epigenetic inactivation of microRNA-34b/c predicts poor disease-free survival in early-stage lung adenocarcinoma. Clin. Cancer Res. 2013, 19, 6842–6852. [Google Scholar]
- Zhang, J.; Wu, J. The Potential Roles of Exosomal miR-214 in Bone Metastasis of Lung Adenocarcinoma. Front. Oncol. 2020, 10, 611054. [Google Scholar]
- Zhao, S.; Yu, J.; Wang, L. Machine Learning Based Prediction of Brain Metastasis of Patients with IIIA-N2 Lung Adenocarcinoma by a Three-miRNA Signature. Transl. Oncol. 2018, 11, 157–167. [Google Scholar]
- Sun, G.; Ding, X.; Bi, N.; Wu, L.; Wang, J.; Zhang, W.; Dong, X.; Lv, N.; Song, Y.; Zhan, Q.; et al. MiR-423-5p in brain metastasis: Potential role in diagnostics and molecular biology. Cell Death Dis. 2018, 9, 936. [Google Scholar]
- Meng, W.; Ye, Z.; Cui, R.; Perry, J.; Dedousi-Huebner, V.; Huebner, A.; Wang, Y.; Li, B.; Volinia, S.; Nakanishi, H.; et al. MicroRNA-31 predicts the presence of lymph node metastases and survival in patients with lung adenocarcinoma. Clin. Cancer Res. 2013, 19, 5423–5433. [Google Scholar] [PubMed]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [PubMed]
- Esquela-Kerscher, A.; Trang, P.; Wiggins, J.F.; Patrawala, L.; Cheng, A.; Ford, L.; Weidhaas, J.B.; Brown, D.; Bader, A.G.; Slack, F.J. The let-7 microRNA reduces tumor growth in mouse models of lung cancer. Cell Cycle 2008, 7, 759–764. [Google Scholar] [CrossRef]
- Esposito, C.L.; Cerchia, L.; Catuogno, S.; De Vita, G.; Dassie, J.P.; Santamaria, G.; Swiderski, P.; Condorelli, G.; Giangrande, P.H.; de Franciscis, V. Multifunctional aptamer-miRNA conjugates for targeted cancer therapy. Mol. Ther. 2014, 22, 1151–1163. [Google Scholar]
- Chen, W.; Liu, Y.; Liang, X.; Huang, Y.; Li, Q. Chondroitin sulfate-functionalized polyamidoamine as a tumor-targeted carrier for miR-34a delivery. Acta Biomater. 2017, 57, 238–250. [Google Scholar]
- Liang, L.; Cen, H.; Huang, J.; Qin, A.; Xu, W.; Wang, S.; Chen, Z.; Tan, L.; Zhang, Q.; Yu, X.; et al. The reversion of DNA methylation-induced miRNA silence via biomimetic nanoparticles-mediated gene delivery for efficient lung adenocarcinoma therapy. Mol. Cancer 2022, 21, 186. [Google Scholar]
- Monroig-Bosque Pdel, C.; Rivera, C.A.; Calin, G.A. MicroRNAs in cancer therapeutics: “from the bench to the bedside”. Expert Opin. Biol. Ther. 2015, 15, 1381–1385. [Google Scholar] [PubMed]
- Zhang, X.; Zhu, J.; Xing, R.; Tie, Y.; Fu, H.; Zheng, X.; Yu, B. miR-513a-3p sensitizes human lung adenocarcinoma cells to chemotherapy by targeting GSTP1. Lung Cancer 2012, 77, 488–494. [Google Scholar]
- Zhu, W.; Shan, X.; Wang, T.; Shu, Y.; Liu, P. miR-181b modulates multidrug resistance by targeting BCL2 in human cancer cell lines. Int. J. Cancer 2010, 127, 2520–2529. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Song, Y.; Fu, Z.; Yu, W. miR-27a regulates cisplatin resistance and metastasis by targeting RKIP in human lung adenocarcinoma cells. Mol. Cancer 2014, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Wang, R.; Chen, L.B. MiR-100 resensitizes docetaxel-resistant human lung adenocarcinoma cells (SPC-A1) to docetaxel by targeting Plk1. Cancer Lett. 2012, 317, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Wang, R.; Song, H.Z.; Chen, L.B. MicroRNA-200b reverses chemoresistance of docetaxel-resistant human lung adenocarcinoma cells by targeting E2F3. Cancer 2012, 118, 3365–3376. [Google Scholar] [CrossRef]
- Shi, S.B.; Wang, M.; Tian, J.; Li, R.; Chang, C.X.; Qi, J.L. MicroRNA 25, microRNA 145, and microRNA 210 as biomarkers for predicting the efficacy of maintenance treatment with pemetrexed in lung adenocarcinoma patients who are negative for epidermal growth factor receptor mutations or anaplastic lymphoma kinase translocations. Transl. Res. 2016, 170, 1–7. [Google Scholar]
- Yuan, C.; Bai, R.; Gao, Y.; Jiang, X.; Li, S.; Sun, W.; Li, Y.; Huang, Z.; Gong, Y.; Xie, C. Effects of MicroRNA-195-5p on Biological Behaviors and Radiosensitivity of Lung Adenocarcinoma Cells via Targeting HOXA10. Oxid. Med. Cell. Longev. 2021, 2021, 4522210. [Google Scholar] [CrossRef]
- Zhang, H.H.; Pang, M.; Dong, W.; Xin, J.X.; Li, Y.J.; Zhang, Z.C.; Yu, L.; Wang, P.Y.; Li, B.S.; Xie, S.Y. miR-511 induces the apoptosis of radioresistant lung adenocarcinoma cells by triggering BAX. Oncol. Rep. 2014, 31, 1473–1479. [Google Scholar] [CrossRef]
- He, H.; Song, X.; Yang, Z.; Mao, Y.; Zhang, K.; Wang, Y.; Su, B.; Li, Q.; Chen, H.; Li, Y. Upregulation of KCNQ1OT1 promotes resistance to stereotactic body radiotherapy in lung adenocarcinoma by inducing ATG5/ATG12-mediated autophagy via miR-372-3p. Cell Death Dis. 2020, 11, 883. [Google Scholar] [CrossRef]
- Hao, C.C.; Xu, C.Y.; Zhao, X.Y.; Luo, J.N.; Wang, G.; Zhao, L.H.; Ge, X.; Ge, X.F. Up-regulation of VANGL1 by IGF2BPs and miR-29b-3p attenuates the detrimental effect of irradiation on lung adenocarcinoma. J. Exp. Clin. Cancer Res. 2020, 39, 256. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Huang, D.; Meng, J.; Chu, J.; Wang, M.; Chen, S. miR-126-5p enhances radiosensitivity of lung adenocarcinoma cells by inhibiting EZH2 via the KLF2/BIRC axis. J. Cell Mol. Med. 2022, 26, 2529–2542. [Google Scholar] [CrossRef]
- Rosell, R.; Moran, T.; Queralt, C.; Porta, R.; Cardenal, F.; Camps, C.; Majem, M.; Lopez-Vivanco, G.; Isla, D.; Provencio, M.; et al. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med. 2009, 361, 958–967. [Google Scholar] [CrossRef]
- Gao, Y.; Fan, X.; Li, W.; Ping, W.; Deng, Y.; Fu, X. miR-138-5p reverses gefitinib resistance in non-small cell lung cancer cells via negatively regulating G protein-coupled receptor 124. Biochem. Biophys Res Commun 2014, 446, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.; Takigawa, N.; Ito, S.; Kashihara, H.; Ichihara, E.; Yasuda, T.; Shimizu, K.; Tanimoto, M.; Kiura, K. Liposomal delivery of MicroRNA-7-expressing plasmid overcomes epidermal growth factor receptor tyrosine kinase inhibitor-resistance in lung cancer cells. Mol. Cancer Ther. 2011, 10, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, Y.; Zheng, Y.; Zhang, L.; Pan, Y.; Yu, J.; Yang, M. miR-608 and miR-4513 significantly contribute to the prognosis of lung adenocarcinoma treated with EGFR-TKIs. Lab. Investig. 2019, 99, 568–576. [Google Scholar] [CrossRef]
- Fife, B.T.; Bluestone, J.A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008, 224, 166–182. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, L.; Giordano, M.; Niccoli, C.; Melfi, F.; Lucchi, M.; Mussi, A.; Fontanini, G. Role of microRNA-33a in regulating the expression of PD-1 in lung adenocarcinoma. Cancer Cell Int. 2017, 17, 105. [Google Scholar] [CrossRef] [PubMed]
- Cortez, M.A.; Ivan, C.; Valdecanas, D.; Wang, X.; Peltier, H.J.; Ye, Y.; Araujo, L.; Carbone, D.P.; Shilo, K.; Giri, D.K.; et al. PDL1 Regulation by p53 via miR-34. J. Natl. Cancer Inst. 2016, 108, djv303. [Google Scholar] [CrossRef]
- Hua, X.; Chu, H.; Wang, C.; Shi, X.; Wang, A.; Zhang, Z. Targeting USP22 with miR-30-5p to inhibit the hypoxia-induced expression of PD-L1 in lung adenocarcinoma cells. Oncol. Rep. 2021, 46, 215. [Google Scholar] [CrossRef]
- Yang, Q.; Li, J.; Hu, Y.; Tang, X.; Yu, L.; Dong, L.; Chen, D. MiR-218-5p Suppresses the Killing Effect of Natural Killer Cell to Lung Adenocarcinoma by Targeting SHMT1. Yonsei Med. J. 2019, 60, 500–508. [Google Scholar] [CrossRef]
- Guo, J.; Jin, H.; Xi, Y.; Guo, J.; Jin, Y.; Jiang, D. The miR-582/CD1B Axis Is Involved in Regulation of Dendritic Cells and Is Associated with Clinical Outcomes in Advanced Lung Adenocarcinoma. Biomed. Res. Int. 2020, 2020, 4360930. [Google Scholar] [CrossRef]
- Tang, L.; Yuan, Y.; Zhai, H.; Wang, J.; Zhang, D.; Liang, H.; Shi, Y.; Duan, L.; Jiang, X. MicroRNA-125b-5p Correlates With Prognosis and Lung Adenocarcinoma Progression. Front. Mol. Biosci. 2021, 8, 788690. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yuan, Y.; Tang, L.; Wang, J.; Zhang, D.; Cho, W.C.; Duan, L. Identification and Validation Prognostic Impact of MiRNA-30a-5p in Lung Adenocarcinoma. Front. Oncol. 2022, 12, 831997. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Jiang, X.; Tang, L.; Wang, J.; Liu, Q.; Zou, X.; Duan, L. SNX20AR/MiRNA-301a-3p/SNX20 Axis Associated With Cell Proliferation and Immune Infiltration in Lung Adenocarcinoma. Front. Mol. Biosci. 2021, 8, 744363. [Google Scholar] [CrossRef] [PubMed]
| MiRNA | Possible Target | Identified Effects | References (PMID and Link accessed on 22 August 2023) |
|---|---|---|---|
| miR-1 | FAM83A | suppress A549 cell growth and motility | 33266425 (https://www.mdpi.com/1422-0067/21/22/8833) |
| miR-7 | BCL-2 | inhibit A549 cell proliferation, migration and induce apoptosis | 21750649 (https://www.ijbs.com/v07p0805.htm) |
| miR-22 | ErbB3 | exhibit excellent anticancer activity both in vitro and in vivo | 22484852 (https://link.springer.com/article/10.1007/s00432-012-1194-2) |
| miR-23b | cyclin D1 | inhibit the proliferation and migration | 28976503 (https://pubs.rsc.org/en/content/articlelanding/2017/BM/C7BM00599G) |
| miR-98 | TGFBR1 | inhibit proliferation and metastasis in A549 cell | 30387848 (https://www.spandidos-publications.com/ijo/54/1/128) |
| miR-125a | STAT3 | inhibit the proliferation, invasion and metastasis | 31930562 (https://onlinelibrary.wiley.com/doi/10.1002/jcb.29586) |
| miR-126 | ADAM9 | inhibit lung adenocarcinoma (LUAD) development and progression | 36171576 (https://molecular-cancer.biomedcentral.com/articles/10.1186/s12943-022-01651-4) |
| miR-142 | NR2F6 | suppress the proliferation, migration and invasion | 31168689 (https://link.springer.com/article/10.1007/s13577-019-00258-0) |
| miR-144 | EZH2 | contribute to progression of LUAD | 30280514 (https://onlinelibrary.wiley.com/doi/10.1002/cam4.1714) |
| miR-145 | EGFR/NUDT1 | inhibit cell proliferation of human LUAD | 21289483 (https://www.tandfonline.com/doi/abs/10.4161/rna.8.1.14259) |
| miR-149 | RAP1B | inhibit the progression of LUAD | 32432747 (https://www.europeanreview.org/article/21173) |
| miR-150 | TNS4 | inhibit LUAD cell malignancy | 31052206 (https://www.mdpi.com/2072-6694/11/5/601) |
| MiRNA | Possible Target | Identified Effects | References (PMID and Link accessed on 22 August 2023) |
|---|---|---|---|
| miR-9 | ID4 | promotes LUAD cell progression | 34723712 (https://journals.sagepub.com/doi/full/10.1177/15330338211048592) |
| miR-10b | KLF4 | promotes A549 cell proliferation and invasion | 24216130 (https://eurjmedres.biomedcentral.com/articles/10.1186/2047-783X-18-41) |
| miR-19 | PTEN | triggers EMT of LUAD cells accompanied by growth inhibition | 26098000 (https://www.laboratoryinvestigation.org/article/S0023-6837(22)01359-9/fulltext) |
| miR-21 | SET/TAF-Iα | promotes LUAD progression | 31176779 (https://www.sciencedirect.com/science/article/abs/pii/S002432051930459X) |
| miR-93 | PTEN, RB1 | plays an oncogenic role by inhibiting PTEN and RB1 | 29309884 (https://www.sciencedirect.com/science/article/abs/pii/S0378111918300313) |
| miR-96 | ARHGAP6 | an oncogene in LUAD and facilitate tumor progression | 34338998 (https://link.springer.com/article/10.1007/s13353-021-00652-1) |
| miR-183 | PECAM1 | positive influence on LUAD cell viability and proliferation | 29749535 (https://www.spandidos-publications.com/or/40/1/83) |
| miR-196a | ANXA1 | promotes migration and invasion | 33775710 (https://www.sciencedirect.com/science/article/abs/pii/S0304383521001324) |
| miR-196b | RSPO2 | promotes proliferation, migration and invasion | 33402849 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7778444/) |
| MiRNA | Possible Target | Identified Effects | References (PMID and Link accessed on 22 August 2023) |
|---|---|---|---|
| miR-7 | IRS2 | circFAT1 promotes tumorigenesis through sequestering miR-7 | 35844799 (https://www.ijbs.com/v18p3944.htm) |
| miR-9 | CPEB3 | linc00968/miR-9/CPEB3 regulatory axis plays a critical role in LUAD | 33159015 (https://www.aging-us.com/article/103833) |
| miR-17 | QKI-5 | circ-MTO1/miR-17/QKI-5 feedback loop inhibits LUAD progression | 30975029 (https://www.tandfonline.com/doi/full/10.1080/15384047.2019.1598762) |
| miR-18b | VMA21 | lncRNA ZFPM2-AS1 promotes proliferation via miR-18b/VMA21 axis in LUAD | 31297866 (https://onlinelibrary.wiley.com/doi/10.1002/jcb.29176) |
| miR-20a | SLC7A5 | circRNA LDLRAD3 enhances the malignant behaviors of LUAD cells via the miR-20a-5p-SLC7A5 axis | 35035814 (https://www.hindawi.com/journals/jhe/2022/2373580/) |
| miR-20b | CCND1 | linc00467 promotes LUAD proliferation via sponging miR-20b-5p | 31686834 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6709798/) |
| miR-22 | BCL2 | lncRNA DGCR5 promotes LUAD progression via inhibiting miR-22 | 29030962 (https://onlinelibrary.wiley.com/doi/10.1002/jcp.26215) |
| miR-26a | E2F7 | SNHG6 may act as an oncogenic lncRNA in LUAD carcinogenesis by regulating the miR-26a-5p/E2F7 axis | 30257360 (https://www.sciencedirect.com/science/article/abs/pii/S0753332218341921) |
| miR-29b | STAT3 | lncRNA H19 promotes viability and EMT of LUAD cells by targeting miR-29b-3p and modifying STAT3 | 30747209 (https://www.spandidos-publications.com/ijo/54/3/929) |
| miR-33b | GPAM | lncRNA MSC-AS1 facilitates LUAD through sponging miR-33b-5p to upregulate GPAM | 33821667 (https://cdnsciencepub.com/doi/full/10.1139/bcb-2020-0239) |
| miR-34 | PDL1 | has-circRNA-002178 could enhance PDL1 expression via sponging miR-34 in LUAD cells to induce T-cell exhaustion | 31949130 (https://www.nature.com/articles/s41419-020-2230-9) |
| miR-96 | CYLD | lncRNA GMDS-AS1 inhibits LUAD development by regulating miR-96-5p/CYLD signaling | 31860169 (https://onlinelibrary.wiley.com/doi/10.1002/cam4.2776) |
| miR-98 | AKR1B10-ERK | linc00665 promotes LUAD progression and functions as ceRNA to regulate AKR1B10-ERK signaling by sponging miR-98 | 30692511 (https://www.nature.com/articles/s41419-019-1361-3) |
| miR-100 | SMARCA5 | lncRNA HAGLROS facilitates the malignant phenotypes via repressing miR-100 and upregulating SMARCA5 | 35307327 (https://www.sciencedirect.com/science/article/pii/S2319417020302365) |
| MiRNA | Sample Source and Identification Effects | Statistics | Patient Stage, n (%) | References (PMID and Link accessed on 22 August 2023) |
|---|---|---|---|---|
| miR-10b | MiR-10b in extracellular vesicles may be a potential diagnostic biomarker for LUAD | AUC = 0.998, sensitivity = 98.75%, specificity = 98.55% | I 58 (72.5%) II 16 (20.0%) III 5 (6.3%) Unknown 1(1.2%) | 34257722 (https://www.spandidos-publications.com/10.3892/ol.2021.12875) |
| miR-126 | Bronchoalveolar lavage fluid exosomal miR-126 could serve as diagnostic biomarkers in early-stage LUAD | / | IA 8 (61.5%), IB 2 (15.4%), IIA 3 (23.1%) | 29806739 (https://onlinelibrary.wiley.com/doi/10.1111/1759-7714.12756) |
| miR-130a | MiR-130A as a diagnostic marker to differentiate malignant mesothelioma from LUAD in pleural effusion cytology | AUC = 0.70, sensitivity = 77%, specificity = 67% | / | 28449331 (https://acsjournals.onlinelibrary.wiley.com/doi/10.1002/cncy.21869) |
| miR-505 | Extracellular vesicle-delivered miR-505-5p as a diagnostic biomarker for early-stage LUAD | AUC = 0.899, sensitivity = 83.3%, specificity = 93.3% | / | 30864684 (https://www.spandidos-publications.com/ijo/54/5/1821) |
| miR-19b, miR-183 | Plasma-derived miR-19b, miR-183 can be used to identify lung cancer and miR-183 was more effective in discriminating LUAD from healthy individuals | AUC = 0.990, sensitivity = 94.7%, specificity = 95.2% | I - II 24 (32.0%) III 47 (62.7%) IV 4 (5.3%) | 27768748 (https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0165261) |
| miR-339, miR-21 | Plasma-based miR-339 and miR-21 evaluation can serve as the tumor markers for LUAD screening | AUC = 0.963, sensitivity = 92.9%, specificity = 92.9% | IA 12 (42.9%), IB 3 (10.7%), IIA 2 (7.1%), IIB 5 (17.9%), IIIA 4 (14.3%), IIIB 2 (7.1%) | 29103767 (https://www.sciencedirect.com/science/article/abs/pii/S0344033817308737) |
| miR-4529, miR-8075, miR-7704 | Three miRNA integrations in Exhaled Breath Condensate differentiated LUAD and LUSC with high accuracy | AUC = 0.98, sensitivity = 100%, specificity = 88.0% | I 1 (4.8%) II 3 (14.3%) III 5 (23.8%) IV 12 (57.1%) | 33572343 (https://www.mdpi.com/2075-4426/11/2/111) |
| MiRNA | Sample Source and Identification Effects | Statistics | Patient Stage, n (%) | References (PMID and Link accessed on 22 August 2023) |
|---|---|---|---|---|
| miR-125b | MiR-125b is decreased in LUAD tissues and correlates with poor prognosis | p = 0.001 | / | 35187068 (https://www.frontiersin.org/articles/10.3389/fmolb.2021.788690/full) |
| miR-142 | Serum miR-142-3p is associated with early relapse in operable LUAD patients | p = 0.007 | / | 23410826 (https://www.lungcancerjournal.info/article/S0169-5002(13)00023-8/fulltext) |
| miR-145 | MiR-145 level in LUAD tissues is an independent risk factor for both OS and DFS in LUAD | p = 0.004 | I 34 (37.0%) II 25 (27.1%) III 33 (35.9%) | 26582602 (https://www.nature.com/articles/srep16901) |
| miR-210 | MiR-210 expression in LUAD tissues is a prognostic factor for OS in patients | p = 0.001 | I 54 (67.5%) II-III 26 (32.5%) | 25733977 (https://www.hindawi.com/journals/jo/2015/316745/) |
| miR-324 | The combination of TP53 mutations and high miR-324-5p expression in LUAD tissues can predict poor prognosis | p = 0.052 | / | 34257080 (https://aacrjournals.org/mcr/article/19/10/1635/665704/MicroRNA-324-5p-CUEDC2-Axis-Mediates-Gain-of) |
| miR-650 | MiR-650 expression level in LUAD tissues is significantly correlated with lymph node metastasis and clinical stage | p = 0.019 | I-II 53 (55.2%) III-IV 43 (44.8%) | 23991130 (https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0072615) |
| miR-940 | Decreased miR-940 expression in LUAD tissues can predict a negative prognosis in early-stage female patients | p = 0.011 | IA 8 (65.7%), IB 4 (33.3%) | 35004257 (https://tlcr.amegroups.org/article/view/58742/html) |
| miR-126 | Both miRNAs within LUAD tissues exhibit the capability to predict pathological stage, tumor diameter, and lymph node metastasis. | AUC = 0.715, sensitivity = 64%, specificity = 75% | I 25 (51%) II-III 24 (49%) | 27277197 (https://www.spandidos-publications.com/or/36/2/909) |
| miR-451a | AUC = 0.742, sensitivity = 84%, specificity = 67% | |||
| miR-141 | High miR-141 and miR-200c expression in LUAD tissues are associated with shorter OS through MET and angiogenesis | p = 0.009 | I 94 (60.6%) II 34 (22%) III 27 (17.4%) | 25003366 (https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0101899) |
| miR-200c | p < 0.001 |
| MiRNA | Identified Mechanism | References (PMID and Link accessed on 22 August 2023) |
|---|---|---|
| Tyrosine kinase inhibitors | ||
| miR-1 | decrease sensitivity to EGFR-TKI by changing tumor immune microenvironment | 33305905 (https://onlinelibrary.wiley.com/doi/10.1002/cam4.3639) |
| miR-7 | reduce EGFR expression in LUAD cell lines with acquired EGFR-TKI resistance | 21712475 (https://aacrjournals.org/mct/article/10/9/1720/91084/Liposomal-Delivery-of-MicroRNA-7-Expressing) |
| miR-16 | restoring sensitivity to erlotinib in KRAS-mutated LUAD in vitro and in vivo | 34948154 (https://www.mdpi.com/1422-0067/22/24/13357) |
| miR-17 | inhibit the EZH1 enhancer that contributes to EGFR-TKI resistance in cancer | 27633093 (https://www.tandfonline.com/doi/full/10.1080/1061186X.2016.1207647) |
| miR-21 | correlate with progression of EML4-ALK-translocated LUAD in patients prescribed ALK-TKI treatment | 30658414 (https://www.mdpi.com/2072-6694/11/1/104) |
| miR-23a | inhibition of miR-23a increases the sensitivity of LUAD stem cells to erlotinib | 28901474 (https://www.spandidos-publications.com/or/38/5/3064) |
| Chemotherapy—Cisplatin | ||
| miR-10a | increase the cisplatin resistance of LUAD circulating tumor cells via targeting PIK3CA | 32186774 (https://www.spandidos-publications.com/10.3892/or.2020.7547) |
| miR-15b | increase cisplatin resistance and metastasis by targeting PEBP4 in LUAD cells | 25721211 (https://www.nature.com/articles/cgt201473) |
| miR-20a | suppress the PTEN/PI3K-AKT pathway to promote chemoresistance to cisplatin of LUAD cells | 35857905 (https://onlinelibrary.wiley.com/doi/10.1002/ctm2.989) |
| miR-26a | responsible for A549 cell sensitivity in the treatment of cisplatin through E2F1-Akt pathway | 26492332 (https://www.tandfonline.com/doi/full/10.1080/15384047.2015.1095405) |
| miR-30b | inhibit cancer progression and enhance cisplatin sensitivity in LUAD through targeting LRP8 | 33779882 (https://link.springer.com/article/10.1007/s10495-021-01665-1) |
| miR-31 | inhibit cisplatin-induced apoptosis in LUAD cells by regulating the drug transporter ABCB9 | 24099915 (https://www.sciencedirect.com/science/article/abs/pii/S0304383513007039) |
| miR-32, miR-548a | promote sensitivity of LUAD cells to cisplatin by targeting ROBO1 and inhibiting the activation of Wnt/β-catenin axis | 33854371 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8039019/) |
| Chemotherapy—Celastrol | ||
| miR-33a | enhance the sensitivity of LUAD cells to celastrol by regulating mTOR signaling | 29484434 (https://www.spandidos-publications.com/ijo/52/4/1328) |
| Chemotherapy—Docetaxel | ||
| miR-100 | resensitize docetaxel-resistant LUAD cells (SPC-A1) by targeting Plk1 | 22120675 (https://www.sciencedirect.com/science/article/abs/pii/S0304383511007257) |
| Radiotherapy | ||
| miR-15a/16 | enhance radiation sensitivity of A549 cell by targeting the TLR1/NF-κB signaling | 25442346 (https://www.redjournal.org/article/S0360-3016(14)04127-3/fulltext) |
| miR-18a | increase the radiosensitivity in LUAD cells via downregulating ATM and HIF-1α expressions | 29860718 (https://onlinelibrary.wiley.com/doi/10.1002/cam4.1527) |
| miR-26b | downregulate ATF2 to enhance radiosensitivity of LUAD cells | 32476275 (https://onlinelibrary.wiley.com/doi/10.1111/jcmm.15402) |
| miR-29b | upregulation of VANGL1 by IGF2BPs and miR-29b attenuates the detrimental effect of irradiation on LUAD | 33228740 (https://jeccr.biomedcentral.com/articles/10.1186/s13046-020-01772-y) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Kelava, L.; Kiss, I. MiRNAs in Lung Adenocarcinoma: Role, Diagnosis, Prognosis, and Therapy. Int. J. Mol. Sci. 2023, 24, 13302. https://doi.org/10.3390/ijms241713302
Song Y, Kelava L, Kiss I. MiRNAs in Lung Adenocarcinoma: Role, Diagnosis, Prognosis, and Therapy. International Journal of Molecular Sciences. 2023; 24(17):13302. https://doi.org/10.3390/ijms241713302
Chicago/Turabian StyleSong, Yongan, Leonardo Kelava, and István Kiss. 2023. "MiRNAs in Lung Adenocarcinoma: Role, Diagnosis, Prognosis, and Therapy" International Journal of Molecular Sciences 24, no. 17: 13302. https://doi.org/10.3390/ijms241713302
APA StyleSong, Y., Kelava, L., & Kiss, I. (2023). MiRNAs in Lung Adenocarcinoma: Role, Diagnosis, Prognosis, and Therapy. International Journal of Molecular Sciences, 24(17), 13302. https://doi.org/10.3390/ijms241713302






