Abstract
Cancer is one of the most difficult diseases for human beings to overcome. Its development is closely related to a variety of factors, and its specific mechanisms have been a hot research topic in the field of scientific research. The tropomyosin family (Tpm) is a group of proteins closely related to the cytoskeleton and actin, and recent studies have shown that they play an important role in various cancers, participating in a variety of biological activities, including cell proliferation, invasion, and migration, and have been used as biomarkers for various cancers. The purpose of this review is to explore the research progress of the Tpm family in tumorigenesis development, focusing on the molecular pathways associated with them and their relevant activities involved in tumors. PubMed and Web of Science databases were searched for relevant studies on the role of Tpms in tumorigenesis and development and the activities of Tpms involved in tumors. Data from the literature suggest that the Tpm family is involved in tumor cell proliferation and growth, tumor cell invasion and migration, tumor angiogenesis, tumor cell apoptosis, and immune infiltration of the tumor microenvironment, among other correlations. It can be used as a potential biomarker for early diagnosis, follow-up, and therapeutic response of some tumors. The Tpm family is involved in cancer in a close relationship with miRNAs and LncRNAs. Tpms are involved in tumor tissue invasion and migration as a key link. On this basis, TPM is frequently used as a biomarker for various cancers. However, the specific molecular mechanism of its involvement in cancer progression has not been explained clearly, which remains an important direction for future research.
Keywords:
tropomyosin; TPM; cancer; migration; miRNA; epithelial–mesenchymal transition; proliferation; biomarker; apoptosis 1. Introduction
The tropomyosin family (Tpm) is a two-chained α-helical coiled-coil actin-binding protein that is widely expressed in muscle and non-muscle cells [1]. Tpm localizes laterally along actin filaments and regulates actin interactions; at the same time, it regulates actin dynamics by binding and modulating the activity of other actin-binding proteins [2]. The role of Tpm in actin is inseparable from Ca2+. In skeletal muscles, in the absence of Ca2+, Tpm is located above the outer domain of actin, and its position spatially blocks most sites of myosin binding. However, in the presence of Ca2+, Tpms move to the internal domain of actin, and Tpm prompts actin to expose most of the previously blocked myosin-binding sites. Based on the continuous influence of actin dynamics in the above ways, spatial segregation and non-redundant functional diversity of the Tpm also regulate and influence the function of the actin cytoskeleton [3,4]. In addition to the direct contribution of actin structures to physical stability and mechanical properties, actin fibers are actively involved in contractile force generation and cell adhesion. Some researchers have found that cancer cells have higher contractility than normal cells [5,6,7]. There is a direct relationship between the invasion of cancer cells and the traction force. The improved traction force, due to the rearrangement of actin structure, production of more stress fibers, and enhanced ATP hydrolysis, allows cancer cells to better penetrate into the extracellular matrix (ECM) through thin, long filamentous processes [8]. The occurrence and progression of cancer are significantly related to cell–cell and cell–ECM adhesion, which provides suitable conditions for the migration, invasion, and proliferation of cancer cells. In addition to this, alterations in actin structure not only enhance the deformability of cancer cells but also the higher traction force and alterations in cell adhesion that are crucial during invasion [9]. Tpms have a role in stabilizing the cytoskeleton and wrapping actin filaments and play a decisive role in the fine motility of almost all actin filament structures [10]. In addition, studies have confirmed that Tpms are also involved in other physiological processes, including cell division, cell motility, apoptosis, and signal transduction [2]. At present, members of the Tpm family have been detected in mammals with four main genetic components, namely, TPM1, TPM2, TPM3, and TPM4 [11]. These four TPM genes can produce more than 40 Tpm subtypes through selective splicing [12]. Direct differences between TPM1-4 coding regions and alternate use of variant exons 1, 2, 6, and 9 contribute to the diversity of Tpm isoforms [13]. Products encoded by different Tpm isoforms can be divided into high-molecular-weight (HMW) and low-molecular-weight (LMW) subtypes. The HMW isoform binds to seven consecutive actin subunits, whereas the LMW binds to six actin subunits [14]. Numerous studies have shown that HMW Tpms are expressed at down-regulated levels in cancer, while LMW Tpms are overexpressed in tumor tissues, such as TPM3 in esophageal squamous cell carcinoma (ESCC), which was shown to be associated with malignant transformation-related invasion and poor survival of malignant cell lines in breast cancer [15]. Altered expression levels of TPM genes also promote changes in other genes. Overexpression of Tpm isoforms in undifferentiated B35 adult neuroblastoma cells results in differential expression of a large number of genes. Tpm isoforms modulate gene expression patterns in a subtype-specific manner that is consistent with their ability to control actin filament function [16]. The different isoforms play their unique roles while at the same time being related to each other.
1.1. TPM1
The products of the TPM1 gene (Tpm1.1, Tpm1.3, and Tpm1.4 isoforms earlier designated as α-tropomyosin) are widely expressed actin-binding proteins that are involved in molecular communication on the cell surface and proliferation signaling between normal cells [17]. The TPM1 gene and its products are strongly associated with cancer, which is usually considered as tumor suppressors, and TPM1 overexpression induces apoptosis in cancer cells during cancer progression [18]. After bioinformatic and statistical analysis, the differentially expressed genes (DEGs) of ribosomal, RNA, and ubiquitin-related functional pathways resulting from Tpm1.12 overexpression in undifferentiated cells had the highest statistical significance and were also associated with a variety of cancers. Using B35 neuroblastoma as a study subject, changes in TPM expression levels can induce changes in the expression levels of a considerable number of genes. For example, overexpression of Tpm1.12 can lead to changes in the expression levels of more than 4000 genes. The regulation of other genes is not similar between different isoforms of Tpm and has a more pronounced homozygous specificity. This feature was more pronounced in differentiated versus undifferentiated cells. In differentiated cells, the number of DEGs was greatly reduced compared to that in undifferentiated cells [16]. For example, in bladder cancer, TPM1 has a role in inhibiting bladder cancer cell proliferation and promoting apoptosis [19]. It has been reported that TPM1 overexpression combined with radiotherapy can significantly inhibit the growth of U251 xenografts, suggesting that TPM1 may be the mechanism of radiation resistance in glioma [20]. In a study on lung cancer, the TPM1 gene was confirmed to inhibit the proliferation and invasion of lung cancer cells and enhance cell apoptosis through the regulatory effect of circ-RNAs [21]. Similar to these findings, EZH2 is a specific H3K27me3 histone methyltransferase, and EZH2 promotes the expression of H3K27mp3.35. EZH2 has oncogenic effects in human malignancies. TPM1 is one of the downstream targets of EZH2, and LINC01116 binds and recruits EZH2 to downregulate the expression of TPM1, which in turn enhances CRC proliferation and angiogenesis. The mechanism may be that overexpression of LINC01116 in CRC leads to a significant increase in the EZH2-rich TPM1 promoter and regulates EZH2 in CRC at the post-transcriptional level, which is a transcription factor of TPM1. EZH2 in CRC is negatively correlated with TPM1, and blocking TPM1 promotes CRC cell proliferation [22]. It is not the only case; the lncRNA MEG3 regulates both miR-96 and TPM1. Its overexpression downregulates miR-96 expression level and upregulates TPM1 expression, which inhibits cell proliferation and promotes apoptosis in bladder uroepithelial carcinoma cells. When lncRNA MEG3 is lowly expressed, it promotes bladder uroepithelial carcinoma cell proliferation and inhibits apoptosis by co-regulating miR-96 with TPM1 [19]. TPM1, a target gene of miR-96, is also thought to be associated with oxaliplatin resistance in CRC. All indications suggest that miR-96 is a tumor promoter. The miR-96 inhibits the expression level of TPM1 by targeting its 3’-UTR, based on which CRC cells show significant oxaliplatin resistance. This is another manifestation of TPM1 as a tumor-suppressive factor [23]. This further supports the tumor-suppressive role of TPM1. Notably, regarding the lncRNA-regulatory TPM1 mechanism, some scholars reported that the alternative splicing mechanism of endogenous TPM1 exon 2a or 2b was found to be different in ESCC cells than in non-cancerous cells, and they identified a previously unlabeled nuclear lncRNA TPM1-AS in human cancer cells (reverse transcribed from the fourth intron region of TPM1) and demonstrated that it is involved in alternative splicing and cell motility of TPM1 mRNA precursor. A novel mechanism of lncRNA involvement in alternative splicing of target genes was proposed by the interaction of TPM1-AS lncRNA with RNA-binding motif protein 4 (RBM4). That is, lncRNA interacts with the alternative splicing factor SR to promote the generation of variants [24].
On the other hand, there is a lot of evidence that miRNA and TPM1 are closely related. miR-558 is upregulated in neuroblastoma, gastric cancer, and other tumors and promotes tumor invasion and peritumor blood supply formation. It targets and inhibits the expression of TNFAIP1 and TPM1, which are considered to be tumor-suppressive factors and are often used as targets of miRNAs including miR-558 and miR-224, thereby allowing tumor progression [21]. The overexpression of miR-21 can promote the invasion and migration of ESCC by inhibiting TPM1 [25]. Consistent with previous findings, miRNA-21 is overexpressed in renal cell carcinoma (RCC) tissues and regulates the growth, apoptosis, and cell cycle progression of RCC cells, as well as the expression of programmed cell death 4 (PDCD4) and TPM1 [26]. Further studies have shown that overexpression of TPM1 in RCC inhibits tumor cell proliferation and promotes tumor cell apoptosis. The reason is that overexpression of TPM1 may lead to DNA damage in tumor cells, which activates p53 expression, subsequently regulating Bcl-2 family members and finally promoting apoptosis of RCC cells through the mitochondrial pathway [27]. Similar findings were found that miRNA-183-5p.1 promotes the migration and invasion of gastric cancer AGS cells by targeting TPM1 [28]. miR-107 promotes the survival, migration, and invasion of human osteosarcoma cells by regulating TPM1 [29] (Figure 1).
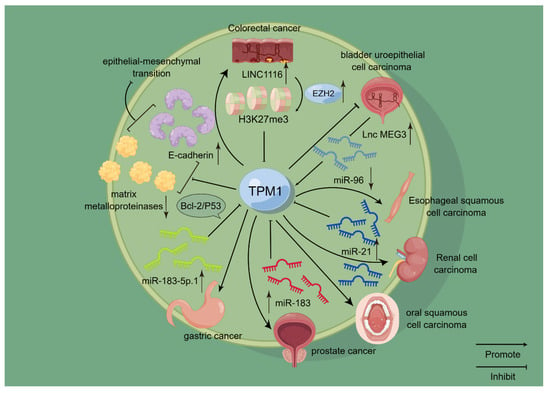
Figure 1.
Schematic representation of the mode of action of TPM1 in selected cancers. TPM1 has been confirmed to play a role in a variety of cancers, including kidney cancer, oral cancer, bladder cancer, and gastric cancer. Among them, it is closely related to the miRNA family. For example, TPM1 is negatively correlated with miR-21 and miR-183, and it is positively correlated with miR-96, thereby affecting the occurrence and development of related cancers. On this basis, TPM1 is also closely related to EMT and acts as a tumor suppressor to some extent.
However, it has also been reported that the misexpression of TPM1 can lead to the fracture of stress fibers, thus changing the morphology and vitality of cells, and eventually leading to the malignant transformation of normal cells [30]. But such results do not seem to affect the widely held belief that TPM1 plays a positive role in tumor tissues.
1.2. TPM2
Among the four Tpm isoforms expressed from the TPM2 gene, Tpm2.1 and Tpm2.2 were designated as β-tropomyosin in previous works. They are widely expressed in fibroblasts, smooth muscle cells, and skeletal muscle cells which are mainly involved in cell motility and muscle contraction regulation [31]. Research hotspots show that Tpm2.1 is crucial for sensing changes in substrate stiffness [32]. Studies on the abnormal expression levels of TPM2 in cancer cells are common [33]. It is well known that the occurrence and development of hepatocellular carcinoma (HCC) are closely related to the infection of patients with HBV virus. Studies have found that overexpression of TPM2 increases the production of HBV, and in HBV-related HCC or acute liver failure, TPM2 expression increases about 4-fold to over 6-fold, respectively [34,35]. These phenomena suggest that HBV may upregulate TPM2 expression and regulate the actin cytoskeleton for efficient propagation and/or replication in the liver, thereby promoting the progression of HCC [36].
The study of TPM2 as a biomarker of tumor tissue is also one of the hot research topics. TPM2 was identified as one of the fibroblast-specific biomarkers of poor prognosis in CRC [37].
In addition, it is worth noting that TPM2 also has a particular contribution to promote apoptosis in cancer cells. The exploration of the mechanism has also been added by scholars. Tpm2.1 has been shown to increase the sensitivity of cells to apoptosis by dissociating extracellular matrix (apoptosis) and modulating apoptosis-inducing proteins [38].
1.3. TPM3
Among the 10 Tpm isoforms expressed from the TPM3 gene, only Tpm3.12 (slow skeletal muscle Tpm isoform) was designated as γ-tropomyosin in previous works. Tpm3 mediates the response of myosin to calcium ions and maintains the stability of cytoskeletal microfilaments in cells [39]. There is more and more evidence that overexpression of TPM3 is strongly associated with cancer occurrence and progression. Similarly, Tpm3.1 has also been shown to be highly specifically upregulated in all cancer cell lines tested to date [10]. For example, Tpm3.1 is abundant in epithelial ovarian cancer tumors of all tissue types [40]. In addition, its combined targeting with microtubules has a strong anti-tumor synergistic effect [41,42]. The potential mechanisms of its aberrant expression in tumor tissues and the resulting effects have been illustrated by a number of studies. Overexpression of Tpm3.1 in undifferentiated cells up-regulated the expression levels of an integrin subunit gene α4 (Itga4) [16]. Fbln5 enhances cell adhesion and reduces proliferation through integrin binding [43,44]. Meanwhile, Fbln5 is reported to have context-dependent oncogenic and tumor-suppressing roles [45,46]. Itga4 can regulate cell migration by forming dimers with b1 subunits and binding to actin IIa [47]. Overexpression of Tpm3.1 may act synergistically with Fbln5, Itga4, and myosin IIa to enhance cell stability and adhesion.
Part of the reason why TPM3 is considered an oncogene is that its abnormal expression drives changes in other genes that allow tumor tissues to progress. E-cadherin has long been regarded as a tumor-metastasis-suppressor gene and a key gene in the process of EMT, and its abnormal expression is the molecular basis of cell division [48]. Down-regulation of E-cadherin expression promotes the occurrence of EMT and cell carcinogenesis. Down-regulation of TPM3 gene expression leads to abnormal activation of E-cadherin and vimentin genes, resulting in morphological changes of pancreatic cancer (PC) cells, increasing the degree of malignancy, and promoting PC metastasis through EMT [49].
In addition, one of the main research directions of TPM3 is oncogene fusion and gene rearrangement. TPM3 is frequently involved in gene rearrangements leading to fusion with the neurotrophic tyrosine kinase receptor type 1 (NTRK1) gene, which then acts as an oncogene [50,51]. The “TPM3-TRK” fusion oncogene is the result of an intra-chromosomal rearrangement that results in the fusion of the TPM3 gene with sequences encoding the transmembrane and intracellular domains of the transmembrane tyrosine kinase known as tropomyosin receptor kinase (TRK) [52,53]. Similar studies have reported that anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase. TPM3-ALK and TPM4-ALK, a fusion protein consisting of the N-terminal of TPM and the C-terminal kinase domain of ALK, have been reported in patients with inflammatory myofibroblastic tumors, with TPM3-ALK being the most commonly observed [54].
Targeted therapy for Tpm3.1 has become a hotspot. At present, three active compounds with selective anti-Tpm3.1 activity have been screened: TR100 [55], ATM1001 [56], and ATM350 [41]. Regarding the molecular mechanism of compound ATM-3507 acting on Tpm3.1, it has been shown that 3H-ATM-3507 is incorporated into filaments and saturates at about one molecule per Tpm3.1 dimer with an apparent binding affinity of about 2 µM when it is present during the co-polymerization of Tpm3.1 with actin. Meanwhile, ATM3507 may alter the lateral movement of Tpm3.1 on the actin surface, thereby altering the interaction of filaments with actin-binding proteins and myosin motors [57]. A study on the targeting of compound ATM reported that intervention with compounds (TR100 and ATM1001) in wild-type mice (WT) and Tpm3.1 knockout mice (KO) resulted in reduced glucose clearance (inhibition of glucose-stimulated insulin secretion (GSIS) from pancreatic islets). The results showed that the GSIS of Tpm3.1 knockout mice was significantly less affected than that of wild-type mice, indicating that the drug action was targeted. In cell experiments, it was found that the inhibition of GSIS by the drug was due to the destruction of the cortical actin cytoskeleton. Interestingly, both compounds inhibited insulin-stimulated glucose uptake in WT muscle, but in KO muscle, the drug had little effect [56]. These results indicate that ATM drugs affect glucose metabolism in vivo by inhibiting the function of Tpm3.1, with little off-target effect. This finding may provide new ideas for the research field of inhibiting glucose uptake in cancer cells to delay its development (Figure 2).
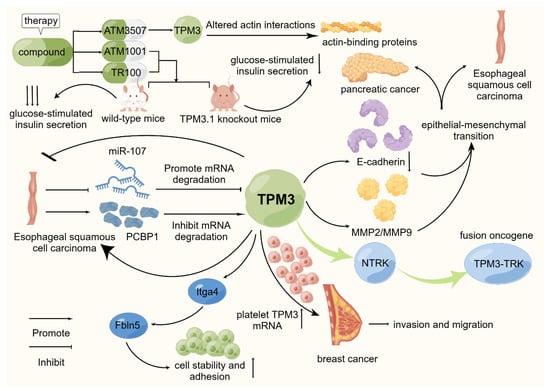
Figure 2.
Schematic representation of the mode of action of TPM3 in selected cancers. TPM3 is widely recognized as an oncogenic factor in existing studies. It is actively involved in promoting the proliferation, invasion, migration, and other biological behaviors of various cancers. On this basis, TPM3-TRK, as a fusion oncogene, is currently a hot topic in related research fields. Through existing studies, TPM3 has been confirmed to be involved in the occurrence and development of esophageal squamous cell carcinoma, breast cancer, and other tumors. At the same time, some scholars have studied related targeted drugs and achieved good results.
In summary, TPM3 research is relatively common, and its research direction tends to be multi-directional. There are also more and more mechanisms being elucidated by more studies.
1.4. TPM4
Among the two Tpm isoforms expressed from the TPM4 gene, only Tpm4.1 was designated as δ-Tpm in previous works. TPM4 regulates the contraction of skeletal muscle and smooth muscle cells or maintains the stability of the cytoskeleton in non-muscle cells [50,51]. In tumor tissues, TPM4 also plays an abnormal function. In undifferentiated rat B35 neuroblastoma, the expression of integrin subunit gene α7(Itga7) was up-regulated with the overexpression of Tpm4.2 [16]. Integrins play a role in cell surface adhesion and signaling and act as a mediator between the actin cytoskeleton and the extracellular matrix [58].
In addition, cancers with abnormal TPM4 expression have been confirmed, including lung cancer [59], breast cancer [60], esophageal cancer [61], ovarian cancer [59], cervical cancer [62], prostate cancer [63], and colon cancer [64].
2. Role of Tpm in Tumor Proliferation and Growth
The proliferation and growth of tumors are different from normal cells, but the mechanism is not very clear. More and more research tends to explore this aspect.
Interestingly, some researchers have found that cancer cells of different tissues can switch between transformed and rigidity-dependent growth states by the presence or absence of mechanosensory modules [65]. Tpm2.1 is involved in the formation of the cell stiffness sensing complex, sarcoma-like contractile units (CUs). The transformation and growth of cancer cells require the consumption of rigid sensing modules. The high level of TPM3 expression may inhibit CU formation and rigidity perception through competition of its gene products with Tpm2.1 [65]. There is a competitive relationship between Tpm2.1 and gene products of TPM3, where TPM3 overexpression leads to CU deletion. This may be a potential mechanism for TPM3 to act as an oncogene. It leads to the proliferation and migration of cancer cells by depleting the rigid-sensitive module, which leads to the transformed growth of cancer cells. This suggests that the transformation and growth of cancer cells is a mechanobiological phenomenon. Another study has also provided evidence supporting this notion. In tumor cells, when transformed by the loss of the rigid sensor protein (Tpm2.1), its behavior is similar to that of normal cells. Restoration of rigid sensing in tumor cells promotes rigid-dependent mechanical behavior, that is, cyclic stretching enhances growth on soft surfaces and reduces apoptosis. Thus, tumor cells can be selectively killed by mechanical perturbation while stimulating the growth of normal cells [66].
Actin filaments containing Tpm3.1 mediate nuclear translocation of extracellular signal-regulated kinases, thereby promoting cell growth and proliferation of mouse fibroblasts [67]. During mitosis, Tpm3.1 or Tpm3.1-containing actin filaments are enriched in the cell cortex [42], where stellate microtubules interact with cortical actin through protein complexes (such as NuMA-dynein-dynactin and LGN-gαi) required for mitotic spindle assembly and localization [68].
ATM analogs antagonize TPMs in tumor tissues with significant targeting properties. The anti-TPM3 compound ATM-3507 described in the part of the introduction was shown to inhibit tumor growth by targeting the C-terminus of Tpm3.1 [55,57]. Moreover, ATM-3507 does not impair muscle structure and function, suggesting that ATM-3507 may target the cytoskeleton of tumor cells but not muscle tissues [57].
Abnormal expression of TPMs at the level of TPMs is accompanied by aberrant regulation by other cellular regulators as well as mi-RNAs. They have a close association with TPMs. The inhibition of miR-107 leads to the elimination of the functional inhibition of its downstream genes, which further promotes the malignant phenotype and chemoresistance of cancer, and is regarded as a tumor-suppressor regulator [69,70]. Current evidence shows that miR-107 is repressed in ESCC tissues and cell lines, whereas TPM3 is upregulated, especially in advanced ESCC tissues [71]. Dual luciferase reporter analysis showed that miR-107 could directly target TPM3 mRNA and inhibit TPM3 protein expression in ESCC cells [72]. These results imply that miR-107 is a negative regulator of ESCC progression by inducing the degradation of TPM3 mRNA.
Regarding the mechanism of TPM3 action in ESCC, one line of evidence suggests that PCBP1 is an upstream regulator of TPM3 [73]. PCBP1 is an RNA-binding protein that is abnormally expressed in a variety of tumor tissues [74,75]. PCBP1 is responsible for the significant upregulation of TPM3 in esophageal squamous cell carcinoma. PCBP1 is an RNA-binding protein (RBP). PCBP1 is highly expressed in esophageal cancer tissues. Knockdown of PCBP1 significantly attenuates the proliferation, migration, and invasion of ESCC cells by binding directly to the 3′untranslated region (3′utr) of TPM3 mRNA and stabilizing mRNA degradation by binding directly to position 1317–1322 of TPM3 mRNA [73].
The specific mechanism of TPMs on tumor tissue growth and proliferation is not very completely elucidated. There is more evidence from a number of studies from various sources. Future studies need a main line of sight to connect these compelling pieces of evidence into a complete mechanism.
3. Role of Tpm in Tumor Invasion and Migration
It is well known that the invasion and migration of tumor tissues are often the main culprits in the refractory nature of cancer and poor prognosis. From the existing literature, the role played by TPMs in tumor invasion and migration cannot be underestimated. They may be involved in a number of physiological processes, and this mystery is slowly being unveiled by scholars.
The involvement of TPMs in this process has been shown in studies related to the mode and mechanism of tumor cell migration, and TPMs play a regulated role in this process. Stress fibers not only affect cell morphology and differentiation but also play an important role in the invasion and migration of tumor cells. In osteosarcoma cells, different formin isoforms determine the location of different Tpm isoforms in dorsal stress fibers. DAAM1 and FHOD1 are two subtypes of formins. DAAM1 specifically inhibited the dorsal stress fibers modified by Tpm3.1. Similarly, FHOD1 knockdown significantly changed the localization of Tpm3.1 in the dorsal stress fibers [76]. They do not affect other TPMs. This suggests that TPMs can be targeted and regulated by formins to change the migration and progression of tumors.
Likewise, mi-RNAs and TPMs have always maintained a close association. As the target genes of mi-RNAs, they are regulated and constantly act to change the state of tumor tissues. Conclusive evidence has shown that miRNA-183 has a high expression level in prostate cancer cells and contributes to the progression of malignant tumors and lymphatic metastasis [77]. Furthermore, miRNA-183 can target the TPM1 gene and down-regulate its expression, thereby promoting the progression of prostate cancer [78]. This is consistent with previous reports that miR-183-5p promotes tumor metastasis and growth of non-small cell lung cancer (NSCLC) by down-regulating PTEN [79]. There was a negative correlation between the expression of TPM1 and miR-21 in ESCC. The data indicate that miR-21 targets TPM1 in ESCC and affects ESCC migration and invasion. miR-21 inhibits TPM1 expression by binding to the 3 ‘untranslated region (3’ UTR) of TPM1 mRNA, thus promoting the migration and invasion of ESCC [25]. The underlying mechanism is that TPM is a class II tumor-suppressor gene, and its gene sequence structure is complete, but its expression is insufficient or not expressed due to downregulation or silencing in transcription or translation [80].
The expression of miR-107 is up-regulated in osteosarcoma cells U2OS, and its overexpression promotes the survival, migration, and invasion of U2OS cells by down-regulating the TPM1-stimulated MEK/ERK and NF-κB signaling pathways [29]. In clinical treatment, Skullcapflavone I was confirmed to affect the cell proliferation of CRC cells by blocking MEK/ERK and NF-κB by regulating TPM1 and miR-107 [81]. These findings all suggest that the signaling pathways with high relevance to TPMs are MEK/ERK and NF-κB.
SUSD2 is an NSCLC-associated gene, and it is under-expressed in HCC. Interestingly, SUSD2 abolished the regulatory effect of TPM4 on HCC cell behavior. Therefore, SUSD2 is responsible for TPM4-induced HCC exacerbation. The overexpression of TPM4 in HCC tissues aggravates the malignancy of HCC through the negative regulation of SUD-2 [82]. The tumor-suppressive effect of miR-133a may be related to the Mir-133a-dependent regulation of TPM4 expression. miR-133a → TPM4 and TAp63γ → TPM4 axis are key elements in muscle process. The low levels of miR-133a observed in CRC lead to increased TPM4 expression levels, which are responsible for altered cytoskeletal structure, high cell motility, migration, and metastasis, and may favor EMT [83] (Figure 3).
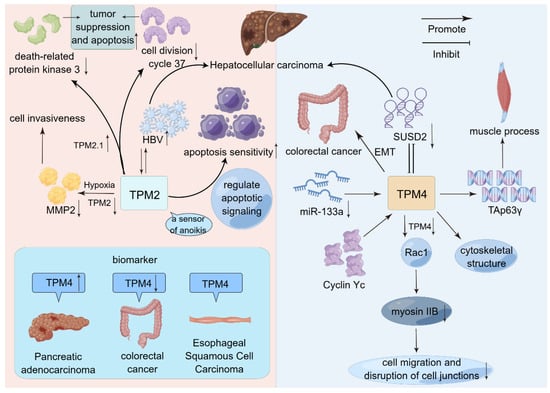
Figure 3.
Schematic representation of the mode of action of TPM2 and TPM4 in selected cancers. Both TPM2 and TPM4 promote HCC. However, TPM4 promotes HCC progression by interacting with SUSD2. TPM2 promotes HCC progression by interacting with HBV. On this basis, TPM2 is closely related to apoptosis. In addition to a series of biological behaviors, TPM4 has more appeared in the public eye as a tumor biomarker.
Another study on the involvement of TPMs in tumor tissue invasion and migration also claimed that they were closely associated with EMT. TPM1 is involved in EMT, a key process in tumor invasion and distant spread. EMT factor can promote tumor cell invasion by upregulating matrix metalloproteinases (MMPs) and downregulating E-cadherin [84]. These results suggest that TPM1 inhibits RCC cell migration by regulating these molecules [85]. This result also provides evidence for TPM1 as a tumor-suppressive factor. Down-regulation of MMP2/MMP9 expression can lead to different expressions of EMT markers and inhibit the progression of EMT. During EMT, cancer cells lose their epithelial phenotype and cell polarity and acquire a potent ability to degrade the ECM, resist apoptosis, invasion, and migration, and induce a mesenchymal cell phenotype [86]. TPM3 can up-regulate the expression of MMP2 and MMP9, induce the progression of EMT, and activate the proliferation, migration, and metastasis of ESCC cells [87].
The essential functions of TPMs seem to be reflected in the tumor tissue. A study suggests that the mechanism through which non-muscle TPMs interfere with tumor tissue migration in cancer cells may share the same regulatory pathway as myosin. In addition, TPMs were found to be closely related to the Rho protein family in the study. Loss of Tpm2.1 has been found to increase Rho-GTP levels and activate Rho-Rock-mediated regulation of actomyosin contractility [88]. Inhibition of Rho-associated kinase (ROCK) reversed the delay in collective cell migration caused by loss of Tpm2.1. In addition, another study found that loss of Tpm2.1 disrupted cell polarity at the leading edge [89]. These results suggest that Tpm2.1 regulates actomyosin contractility through striated muscles and is important for collective cell migration together with cell polarity modulators. Notably, in the study of multicellular invasion, some scholars reported that the expression of Tpm3.1 was negatively correlated with Rac GTPase-mediated cell invasion. Rac GTPases are known intracellular transducers that regulate multiple signaling pathways that control cytoskeletal organization, transcription, and cell proliferation [90]. Combination therapy with Rac inhibition and Tpm3.1 targeting inhibited multicellular invasion more than treatment alone. Data have shown that disruption of Tpm3.1 sensitized neuroblastoma cells to Rac inhibition of multicellular invasion [91].
Cyclin Y (CCNY) is a novel cyclin, and there are two subcellular subtypes of CCNY, namely, cytoplasmic subtype (CCNYc) and membrane subtype (CCNYm). CCNYc and CCNYm have also been shown to be expressed at high levels in lung cancer [59]. In addition, CCNY promotes the migration and invasion of various tumor cells, including liver cancer and ovarian cancer [92,93]. Meanwhile, CCNY’s potential CDK partner is PFTK1 [94,95]; in turn, CCNY c/PFTK1 regulates cytoskeletal structure through TPM4 and promotes cell migration and invasiveness through RoA remodeling in the Rho family of GTPases [59].
There were also significant differences in the expression levels of TPMs in tumor tissues with different degrees of metastasis. Its mechanism may be that the abnormal expression levels of related genes lead to protein alterations. Tpm4.1 expression was down-regulated in high metastatic breast cancer cell lines compared with low metastatic cells. TPM4 expression is reduced in invasive ductal breast cancer compared with ductal breast cancer in situ [60]. To invade the surrounding environment, cancer cells need to separate from the primary tumor, which requires breaking cell-to-cell adhesion. Actin reorganization and cadherin aggregation are important in the formation and maintenance of cell–cell junctions [96]. Loss of Tpm4.1 disrupts cuboidal morphology and actin organization in cell–cell junctions. The molecular mechanism involves the down-regulation of Tpm4.1 which induces Rac1-mediated changes in myosin IIB localization and loss of myosin IIB prevents increased cell migration and disruption of cell junctions after Tpm4.1 silencing [60].
Down-regulation of Tpm2.1 may play a key role in tumor progression by promoting the metastatic potential of tumor cells. In addition, studies not identical to the previous paper on the mechanism of cell growth in rigid substrates have been reported. Glioblastoma (GBM) brain tumors are highly invasive to surrounding healthy brain tissue, so the mechanism of invasion defined using a rigid matrix may not be applicable to GBM transmission. Tpm 2.1 is down-regulated in GBM grown on soft substrates and contributes to GBM colonization in the soft brain environment [97]. In addition, Tpm1.7 was also found to be down-regulated, suggesting that the effect of this isoform is matrix-dependent and that Tpm1.7 induces cell rounding in the 3D collagen gels set for the experiment [97]. These results suggest that Tpm 2.1 deletion in primary patient-derived GBM is associated with prolonged mesenchymal invasion.
It is worth noting that tumor tissues often metastasize through blood, and some scholars have discovered that platelets can be cultured by cancer cells, which are called tumor-cultured platelets (TEPs), and regulate their RNA content or absorb tumor RNA in response to signals from cancer cells, thus leading to changes in transcriptome profile and reflecting pathological progress [98]. In another study, the expression of TPM3 mRNA was significantly elevated in platelets from breast cancer patients compared with age-matched healthy controls. Interestingly, another study also reported that platelet TPM3 mRNA was delivered to breast cancer cells via microvesicles and resulted in an enhanced migratory phenotype of breast cancer cells [99]. These results suggest that the upregulation of TPM3 mRNA in platelets is significantly associated with metastasis in breast cancer patients.
An interesting point is that changes in tissue oxygen content can also indirectly affect the expression level of TPMs. TPM2 expression was down-regulated in breast cancer cells compared with normal breast cells. Hypoxia induces promoter methylation of TPM2, which is responsible for its low expression. Hypoxia may regulate cell invasiveness partly through changes in MMP2 expression mediated by TPM2 downregulation. Importantly, low TPM2 expression was associated with lymph node metastasis, tumor lymph node metastasis stage, histological grade, and shorter overall survival [100].
The Tpm family is often represented as a target gene or as a member of a signaling pathway in the invasion and metastasis of tumor tissue. Different Tpm isoforms play distinct roles, and the specific molecular mechanisms need to be further investigated.
4. Role of Tpm in Tumor Vasculogenesis
It is well known that the progression of tumors is closely related to the blood flow of surrounding tissues. It has been demonstrated that tropomyosin exists on the surface of proliferative-activated endothelial cells during the angiogenic transition and represents a variety of receptors for anti-angiogenic proteins [101]. It belongs to another evolutionarily and structurally relevant protein, high-molecular-weight kininogen (HKa). The histidine–proline-rich glycoprotein (HPRG/HRG) is a single chain (75 kDa) protein that has important functions in regulating immunity, angiogenesis, and coagulation system [102]. It is worth noting that HPRG has a high affinity for tropomyosin [103,104]. In addition to Zn2+, the metal complex TetraHPRG (the peptide fragment [Ac-(GHHPH)4-G-NH2] belonging to the H/P domain of HPRG) was formed when the Cu2+ level was increased in cancer cells [105,106], and its interaction with HKa enhanced the antiangiogenic properties [107].
With the rapid growth of malignant tumors, hypoxic stress appears inside the tumor, which initiates a variety of angiogenic signaling pathways to meet the increasing oxygen demand. TPM1 was found to be a mediator between 4’-acetamino-4-hydroxyl chalcone and its antitumor effects (including inhibition of angiogenesis) in glioma [108]. Upregulation of TPM1 inhibits RCC angiogenesis by decreasing vascular endothelial growth factor expression [85].
5. Role of Tpm in Tumor Apoptosis
Some researchers have found that overexpression of Tpm2.1 can up-regulate death-related protein kinase 3 (Dapk3) and down-regulate the expression of cell division cycle 37 (cdc37) [16]. These two genes are involved in apoptosis, among which Dapk3 has been shown to exert apoptotic function through the mitochondrial pathway [109] and has tumor-suppressor properties [109,110,111]. cdc37 and heat shock protein 90 can promote the proliferation and survival of cancer cells through synergistic interaction [112]. These findings suggest that Tpm2.1 can regulate the expression levels of these genes, thereby exerting the functions of tumor suppression and apoptosis promotion.
mi-RNA is also involved in this process. miRNA-183 is involved in the post-transcriptional process, is upregulated in gastric cancer, and acts as an oncogene in tumor migration. miR-183-5p.1 targets the 3′UTR of TPM1. Overexpression of miR-183-5p.1 promotes cell proliferation, migration, and invasion by down-regulating TPM1 and activating Bcl-2/P53 signaling pathway in gastric cancer. Further analysis of apoptotic signaling proteins showed that Bcl-2 and P53 were involved in the inhibitory effect of miR-183-5p.1 and TPM1 on AGS cells. These results suggest that the inhibitory effect of miR-183-5p.1 on the expression of TPM1 may be related to the activation of the apoptotic signaling pathway [28]. However, miR-183 expression was significantly reduced in GC cells and inhibited GC invasion, suggesting that although they belong to the same miRNA-183 family, it may be due to differences in targeting sites resulting in opposite effects on cancer cells. On the other hand, LncRNA-MEG3 is a miR-96 sponge in bladder cancer that adsorbs miR-96 expression and upregulates TPM1 expression, inhibits cell proliferation, delays the cell cycle, and promotes apoptosis. miR-96 directly targets TPM1 and downregulates its expression. This formed a regulatory network: lncRNA MEG3 sponge adsorbed miR-96 and directly increased the expression of tumor-suppressor TPM1 [19].
There are several similar studies. Tropomyosin subtype Tpm2.1 is an important regulator of cell exit apoptosis (anoikis) [32,33]. Caspase 3/7 activation and mitochondrial depolarization were specifically increased, indicating that overexpression of Tpm2.1 enhances sensitivity to apoptosis by regulating apoptotic signaling rather than by changes in actin filament dynamics [38]. Tpm2.1 loss in cancer cells may be part of the apoptosis evasion mechanism. In this study, Tpm2.1 was not only confirmed to be a sensor of anoikis but was also shown to enhance sensitivity to intrinsic apoptosis by enhancing the degradation of the pro-survival proteins, Mcl-1 and Bcl-2 [38].
6. The Relationship between Tpm and Immunity in Tumor Microenvironment
It has been documented that in HCC, due to up-regulation of TPM, B and T cells may be recruited to the tumor site. Among them, TPM3 is negatively correlated with M2 macrophages [113]. M2-like macrophages can promote the proliferation and invasion of HCC cells by activating TLR4/STAT3 signaling pathway [114]. This implies that TPMs may exert inhibitory effects on HCC cells by promoting the polarization of M2 macrophages.
The expression levels of TPM1, TPM2, and TPM4 genes were decreased in bladder cancer cells. However, they were in direct proportion to immune cells, including NK cells, macrophages, neutrophils, and Th1 cells. This may contribute to immune suppression in bladder cancer [115].
Through bioinformatics analysis, TPM2, as one of the six immune-related genes, is significantly associated with the prognosis of colon cancer and constitutes an immune-related prognosis model of colon cancer, which plays a key role in the tumor immune microenvironment [116].
In PC, high levels of TPM4 expression have been found to be strongly associated with poorer overall survival, disease-specific survival, disease-free survival, and progression-free survival [117]. These findings strongly suggest that TPM4 may be an oncogene and prognostic biomarker for PC. Some studies have found that pancreatic cancer patients with DNA mismatch repair defects (MMRDs) have specific clinical, pathological, and genomic features [118]. The expression of TPM4 was also confirmed to be significantly correlated with the mutation levels of five MMR genes in pancreatic cancer and the level of immune infiltration in the tumor microenvironment [117].
7. TPM as a Tumor Biomarker
As we all know, tumor biomarkers have a role that cannot be ignored in clinical diagnosis and have an important contribution to clinical therapy. Some scholars found that TPM1, TPM2, TPM3, and TPM4 genes are expressed in liver cancer tissues with an up-regulated expression level. The risk model showed that TPM1, TPM2, and TPM3 were used to assess the prognosis of HCC, and TPM1 was negatively correlated, which means that TPM1 may be a good prognostic factor for HCC. The high expression of TPM3 is associated with poor survival outcomes in patients with liver cancer [113]. However, another study showed that TPM1 expression was down-regulated in intrahepatic cholangiocarcinoma (ICC) compared with adjacent normal tissues. At the same time, TPM1 is also associated with the TNM stage of tumors. Patients with high TPM1 expression have a better survival rate. After univariate and multivariate analysis, TPM1 could be considered as an independent prognostic factor of ICC [119]. Similarly, TPM3 and TPM4 expression levels are also up-regulated in gliomas and are closely associated with poor prognosis. Among them, TPM3 can be used as a new independent prognostic factor for glioma [120].
It is worth noting that although TPM1 and TPM2 are highly expressed in normal urothelial tissues, the expression of TPM1 and TPM2 is decreased in the early stage of bladder cancer, which may be a marker event for the occurrence and development of bladder cancer [121]. Meanwhile, the expression levels of TPMs in low-grade bladder cancer were lower than those in high-grade bladder cancer [115]. Therefore, TPM1 and TPM2 are effective markers for the diagnosis of bladder cancer and are expected to be potential therapeutic targets for bladder cancer.
There is solid evidence that TPM1 can act as a tumor-suppressor gene in oral squamous cell carcinoma (OSCC). The expression level of TPM1 in adjacent normal tissues was significantly higher than that in OSCC lesions. At the same time, high expression of TPM1 can slightly inhibit cell proliferation, strongly inhibit cell mobility, and significantly promote cell apoptosis. This implies that down-regulated TPM1 is an important marker of poor prognosis in OSCC. It is worth noting that miR-21 has been studied in solid tumors and upregulated in tongue cancer [122,123], but luciferase detection did not reveal results that could be used to verify the association between miR-21 and TPM1. It may be that miR-21 regulates the expression of TPM1 while promoting OSCC [124]. Further studies are needed to elucidate the underlying molecular mechanisms.
Human tumor-associated stromal cells (TASCs) isolated from CRC tissues trigger EMT of tumor cells in vitro and promote metastasis and spread in vivo [125]. Expression of calponin 1 (CNN1) and TPM2 was significantly associated with adverse outcomes in independent databases. Recent studies based on single-cell gene expression analysis in colorectal cancer surgical specimens have consistently identified both CNN1 and TPM2 as TASC markers associated with poor prognosis [37,126]. It has been demonstrated that TASC overexpressing CNN1 and TPM2 at the protein level indeed directly supports cancer progression by enhancing the proliferation, migration, and metastatic potential of tumor cells [125].
In PC, high levels of TPM4 expression have been found to be strongly associated with poorer overall survival, disease-specific survival, disease-free survival, and progression-free survival. These findings strongly suggest that TPM4 may be an oncogene and prognostic biomarker for PC.
As we all know, alpha-fetoprotein (AFP) is very important for the diagnosis of liver cancer. However, for AFP-negative HCC patients, some scholars have proposed P53, MSH2, and the product of the TPM4 gene and inflammatory markers involved by TPM4 as diagnostic models [127]. Among them, P53 has high specificity for the diagnosis of primary liver cancer [128,129]. MSH2 is hardly expressed in normal hepatocytes, but its expression in HCC gradually increases with the progression of HCC [127]. The increase in the product of the TPM4 gene is mainly associated with an increase in ferritin source or clearance barrier [127]. Compared with the normal-liver-immortalized human urothelial cell line, the content of the product of the TPM4 gene in the three HCC cell lines was significantly up-regulated [130].
In a study of colon cancer, TPM4 expression was shown to be reduced in colon cancer tissues and cell lines. Low expression of TPM4 was significantly correlated with clinical stage, depth of invasion, lymph node metastasis, distant metastasis, and differentiation. In addition, up-regulation of TPM4 expression can inhibit the expression of genes related to migration, invasion, and metastasis of colon cancer cells [131]. Compared with normal colon tissues and colonic epithelial cell lines, the mRNA and protein expression of TPM4 in colon cancer tissues were decreased, respectively [64].
Tpm1.6 and Tpm1.7 proteins were significantly down-regulated in ESCC tumor tissues, but their expression levels were higher in adjacent normal tissues [132]. This is consistent with the downregulation of HMW Tpms reported in breast [133], bladder [115], colon [134], neuroblastoma [135], and prostate cancer [136] studies. However, it is worth noting that an interesting report is that a confounding concept of cellular identity (CCI) has been proposed for ESCC. The average number of genes detected in malignant cells was significantly higher than that in normal cells, and this new cellular identity relative to normal cells was termed CCI and used as an independent marker associated with poor prognosis in ESCC. On this basis, TPM4 was found to generate CCI by activating the Jak/STAT-SOX2 pathway, and thus, TPM4 was identified as a key CCI gene promoting ESCC invasiveness [137].
From the existing studies, TPMs have slowly become a research hotspot as biomarkers of tumors, which indicates that TPMs have significant clinical significance in the early diagnosis and prognosis of tumors. This also suggests that scholars in this field should shift more attention to TPMs.
8. Methods and Materials
PubMed and Web of Science were used to search for articles related to Tpm and cancer in the past 5 years and 694 articles were obtained. After screening, 564 articles were not related to the content of this study. Finally, 130 articles were obtained and included in the research scope, including 14 articles on Tpm and tumor proliferation and growth; 28 articles on Tpm and tumor invasion and migration; 9 articles on Tpm and tumor angiogenesis; 6 articles on Tpm and immune cell infiltration in tumor microenvironment; 10 articles on Tpm and tumor apoptosis; and 22 articles on TPM as a tumor biomarker. (There are a number of articles covering multiple aspects.)
9. Conclusions
In conclusion, Tpm is more involved in the invasion and migration of tumor tissues and has been studied mainly in tumor tissues, such as esophageal cancer and liver cancer. Meanwhile, the activities of Tpm in cancer are closely related to miRNAs and LncRNAs and are associated with epithelial–mesenchymal transition and apoptosis, etc. TPM is more often regulated as a downstream gene of oncogenes and can be regarded as a “dependent variable” of cancer development, while TPM3 is more specific and often appears as an oncogene. TPM3 is distinctive and often appears as an oncogene from the perspective of researchers. In general, the specific mechanism of Tpm in cancer is not clear, which will be an important direction for future Tpm research.
Author Contributions
Original draft preparation, Y.M., K.H. and M.S.; review—editing, Y.H. and L.H.; review—editing and supervision, Y.L. and B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the Natural Science Foundation of Gansu Province, China (Grant No. 20JR10RA691), the Open Subject Foundation of Key Laboratory of Dental Maxillofacial Reconstruction and Bio-logical Intelligence Manufacturing, School of Stomatology, Lanzhou University, Gansu Province (No. 20JR10RA653), and the Key Research and Development Program of Gansu Province (International Scientific and Technological Cooperation Category) (No. 23YFWA0003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
All figures presented in the manuscript were generated using Figdraw. We would like to express our sincere appreciation for the guidance and assistance provided by the HK_Potions_Lab WeChat official account during the course of this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brown, J.H.; Kim, K.H.; Jun, G.; Greenfield, N.J.; Dominguez, R.; Volkmann, N.; Hitchcock-DeGregori, S.E.; Cohen, C. Deciphering the design of the tropomyosin molecule. Proc. Natl. Acad. Sci. USA 2001, 98, 8496–8501. [Google Scholar] [CrossRef]
- Khaitlina, S.Y. Tropomyosin as a Regulator of Actin Dynamics. Int. Rev. Cell Mol. Biol. 2015, 318, 255–291. [Google Scholar] [CrossRef] [PubMed]
- Vibert, P.; Craig, R.; Lehman, W. Three-dimensional reconstruction of caldesmon-containing smooth muscle thin filaments. J. Cell Biol. 1993, 123, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Vibert, P.; Craig, R.; Lehman, W. Steric-model for activation of muscle thin filaments. J. Mol. Biol. 1997, 266, 8–14. [Google Scholar] [CrossRef]
- Koch, T.M.; Münster, S.; Bonakdar, N.; Butler, J.P.; Fabry, B. 3D Traction forces in cancer cell invasion. PLoS ONE 2012, 7, e33476. [Google Scholar] [CrossRef] [PubMed]
- Kraning-Rush, C.M.; Califano, J.P.; Reinhart-King, C.A. Cellular traction stresses increase with increasing metastatic potential. PLoS ONE 2012, 7, e32572. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Persson, H.; Adolfsson, K.; Abariute, L.; Borgström, M.T.; Hessman, D.; Åström, K.; Oredsson, S.; Prinz, C.N. Cellular traction forces: A useful parameter in cancer research. Nanoscale 2017, 9, 19039–19044. [Google Scholar] [CrossRef]
- Svitkina, T. The Actin Cytoskeleton and Actin-Based Motility. Cold Spring Harb. Perspect. Biol. 2018, 10, a018267. [Google Scholar] [CrossRef]
- Tafazzoli-Shadpour, M.; Mohammadi, E.; Torkashvand, E. Mechanics of actin filaments in cancer onset and progress. Int. Rev. Cell Mol. Biol. 2020, 355, 205–243. [Google Scholar] [CrossRef]
- Meiring, J.C.M.; Bryce, N.S.; Wang, Y.; Taft, M.H.; Manstein, D.J.; Liu Lau, S.; Stear, J.; Hardeman, E.C.; Gunning, P.W. Co-polymers of Actin and Tropomyosin Account for a Major Fraction of the Human Actin Cytoskeleton. Curr. Biol. 2018, 28, 2331–2337.e5. [Google Scholar] [CrossRef]
- Lees, J.G.; Bach, C.T.; O’Neill, G.M. Interior decoration: Tropomyosin in actin dynamics and cell migration. Cell Adhes. Migr. 2011, 5, 181–186. [Google Scholar] [CrossRef]
- Bryce, N.S.; Schevzov, G.; Ferguson, V.; Percival, J.M.; Lin, J.J.; Matsumura, F.; Bamburg, J.R.; Jeffrey, P.L.; Hardeman, E.C.; Gunning, P.; et al. Specification of actin filament function and molecular composition by tropomyosin isoforms. Mol. Biol. Cell 2003, 14, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Schevzov, G.; Whittaker, S.P.; Fath, T.; Lin, J.J.; Gunning, P.W. Tropomyosin isoforms and reagents. Bioarchitecture 2011, 1, 135–164. [Google Scholar] [CrossRef] [PubMed]
- Ampe, C.; Van Troys, M. Mammalian Actins: Isoform-Specific Functions and Diseases. Handb. Exp. Pharmacol. 2017, 235, 1–37. [Google Scholar] [CrossRef]
- Lin, W.; Lin, J.; Chen, B.; Tang, W.F.; Yu, S.B.; Chen, S.C.; Kang, M. Tropomyosin3 is associated with invasion, migration, and prognosis in esophageal squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2016, 9, 11313–11323. [Google Scholar]
- Stefen, H.; Suchowerska, A.K.; Chen, B.J.; Brettle, M.; Kuschelewski, J.; Gunning, P.W.; Janitz, M.; Fath, T. Tropomyosin isoforms have specific effects on the transcriptome of undifferentiated and differentiated B35 neuroblastoma cells. FEBS Open Bio. 2018, 8, 570–583. [Google Scholar] [CrossRef]
- Caldwell, B.J.; Lucas, C.; Kee, A.J.; Gaus, K.; Gunning, P.W.; Hardeman, E.C.; Yap, A.S.; Gomez, G.A. Tropomyosin isoforms support actomyosin biogenesis to generate contractile tension at the epithelial zonula adherens. Cytoskeleton 2014, 71, 663–676. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Prasad, G.L. Tropomyosin-1, a novel suppressor of cellular transformation is downregulated by promoter methylation in cancer cells. Cancer Lett. 2002, 183, 205–213. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, X.; Zhou, J.; Cheng, X.; Ye, Z.; Ji, Z. Long non-coding RNA MEG3 suppresses the development of bladder urothelial carcinoma by regulating miR-96 and TPM1. Cancer Biol. Ther. 2018, 19, 1039–1056. [Google Scholar] [CrossRef]
- Du, H.Q.; Wang, Y.; Jiang, Y.; Wang, C.H.; Zhou, T.; Liu, H.Y.; Xiao, H. Silencing of the TPM1 gene induces radioresistance of glioma U251 cells. Oncol. Rep. 2015, 33, 2807–2814. [Google Scholar] [CrossRef]
- Mao, Y.; He, J.X.; Zhu, M.; Dong, Y.Q.; He, J.X. Circ0001320 inhibits lung cancer cell growth and invasion by regulating TNFAIP1 and TPM1 expression through sponging miR-558. Hum. Cell 2021, 34, 468–477. [Google Scholar] [CrossRef]
- Liang, W.; Wu, J.; Qiu, X. LINC01116 facilitates colorectal cancer cell proliferation and angiogenesis through targeting EZH2-regulated TPM1. J. Transl. Med. 2021, 19, 45. [Google Scholar] [CrossRef] [PubMed]
- Ge, T.; Xiang, P.; Mao, H.; Tang, S.; Zhou, J.; Zhang, Y. Inhibition of miR-96 enhances the sensitivity of colorectal cancer cells to oxaliplatin by targeting TPM1. Exp. Ther. Med. 2020, 20, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.W.; Zhang, Y.L.; Liao, L.D.; Li, E.M.; Xu, L.Y. Natural antisense transcript TPM1-AS regulates the alternative splicing of tropomyosin I through an interaction with RNA-binding motif protein 4. Int. J. Biochem. Cell Biol. 2017, 90, 59–67. [Google Scholar] [CrossRef]
- Shen, Z.; Xu, X.; Lv, L.; Dai, H.; Chen, J.; Chen, B. miR-21 Overexpression Promotes Esophageal Squamous Cell Carcinoma Invasion and Migration by Repressing Tropomyosin 1. Gastroenterol. Res. Pract. 2020, 2020, 6478653. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Huang, F.; Mao, H.; Li, M.; Li, X.; Yang, M.; Yu, X. MicroRNA-21 is overexpressed in renal cell carcinoma. Int. J. Biol. Markers 2013, 28, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, J.; Wei, Q.; Du, Y.P.; Qiu, H.P.; Yang, C.; Hou, Y.C. Tropomyosin-1 promotes cancer cell apoptosis via the p53-mediated mitochondrial pathway in renal cell carcinoma. Oncol. Lett. 2018, 15, 7060–7068. [Google Scholar] [CrossRef]
- Lin, J.; Shen, J.; Yue, H.; Cao, Z. miRNA-183-5p.1 promotes the migration and invasion of gastric cancer AGS cells by targeting TPM1. Oncol. Rep. 2019, 42, 2371–2381. [Google Scholar] [CrossRef]
- Jiang, R.; Zhang, C.; Liu, G.; Gu, R.; Wu, H. MicroRNA-107 Promotes Proliferation, Migration, and Invasion of Osteosarcoma Cells by Targeting Tropomyosin 1. Oncol. Res. 2017, 25, 1409–1419. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Hitchcock-DeGregori, S.; Thorburn, A.; Prasad, G.L. N terminus is essential for tropomyosin functions: N-terminal modification disrupts stress fiber organization and abolishes anti-oncogenic effects of tropomyosin-1. J. Biol. Chem. 2004, 279, 14039–14048. [Google Scholar] [CrossRef]
- Ma, R.N.; Mabuchi, K.; Li, J.; Lu, Z.; Wang, C.L.; Li, X.D. Cooperation between the two heads of smooth muscle myosin is essential for full activation of the motor function by phosphorylation. Biochemistry 2013, 52, 6240–6248. [Google Scholar] [CrossRef]
- Wolfenson, H.; Meacci, G.; Liu, S.; Stachowiak, M.R.; Iskratsch, T.; Ghassemi, S.; Roca-Cusachs, P.; O’Shaughnessy, B.; Hone, J.; Sheetz, M.P. Tropomyosin controls sarcomere-like contractions for rigidity sensing and suppressing growth on soft matrices. Nat. Cell Biol. 2016, 18, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Raval, G.N.; Bharadwaj, S.; Levine, E.A.; Willingham, M.C.; Geary, R.L.; Kute, T.; Prasad, G.L. Loss of expression of tropomyosin-1, a novel class II tumor suppressor that induces anoikis, in primary breast tumors. Oncogene 2003, 22, 6194–6203. [Google Scholar] [CrossRef] [PubMed]
- Hass, H.G.; Vogel, U.; Scheurlen, M.; Jobst, J. Gene-expression Analysis Identifies Specific Patterns of Dysregulated Molecular Pathways and Genetic Subgroups of Human Hepatocellular Carcinoma. Anticancer Res. 2016, 36, 5087–5095. [Google Scholar] [CrossRef][Green Version]
- Chen, Z.; Diaz, G.; Pollicino, T.; Zhao, H.; Engle, R.E.; Schuck, P.; Shen, C.H.; Zamboni, F.; Long, Z.; Kabat, J.; et al. Role of humoral immunity against hepatitis B virus core antigen in the pathogenesis of acute liver failure. Proc. Natl. Acad. Sci. USA 2018, 115, E11369–E11378. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Ueda, K.; Honda, T. A Traditional Chinese Medicine, Maoto, Suppresses Hepatitis B Virus Production. Front. Cell Infect. Microbiol. 2021, 10, 581345. [Google Scholar] [CrossRef]
- Zhou, Y.; Bian, S.; Zhou, X.; Cui, Y.; Wang, W.; Wen, L.; Guo, L.; Fu, W.; Tang, F. Single-Cell Multiomics Sequencing Reveals Prevalent Genomic Alterations in Tumor Stromal Cells of Human Colorectal Cancer. Cancer Cell 2020, 38, 818–828.e5. [Google Scholar] [CrossRef]
- Desouza-Armstrong, M.; Gunning, P.W.; Stehn, J.R. Tumor suppressor tropomyosin Tpm2.1 regulates sensitivity to apoptosis beyond anoikis characterized by changes in the levels of intrinsic apoptosis proteins. Cytoskeleton 2017, 74, 233–248. [Google Scholar] [CrossRef]
- Pieples, K.; Arteaga, G.; Solaro, R.J.; Grupp, I.; Lorenz, J.N.; Boivin, G.P.; Jagatheesan, G.; Labitzke, E.; DeTombe, P.P.; Konhilas, J.P.; et al. Tropomyosin 3 expression leads to hypercontractility and attenuates myofilament length-dependent Ca(2+) activation. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1344–H1353. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Bryce, N.S.; Tang, K.; Meagher, N.S.; Kang, E.Y.; Kelemen, L.E.; Köbel, M.; Ramus, S.J.; Friedlander, M.; et al. Targeting the actin/tropomyosin cytoskeleton in epithelial ovarian cancer reveals multiple mechanisms of synergy with anti-microtubule agents. Br. J. Cancer 2021, 125, 265–276. [Google Scholar] [CrossRef]
- Currier, M.A.; Stehn, J.R.; Swain, A.; Chen, D.; Hook, J.; Eiffe, E.; Heaton, A.; Brown, D.; Nartker, B.A.; Eaves, D.W.; et al. Identification of Cancer-Targeted Tropomyosin Inhibitors and Their Synergy with Microtubule Drugs. Mol. Cancer Ther. 2017, 16, 1555–1565. [Google Scholar] [CrossRef]
- Wang, Y.; Stear, J.H.; Swain, A.; Xu, X.; Bryce, N.S.; Carnell, M.; Alieva, I.B.; Dugina, V.B.; Cripe, T.P.; Stehn, J.; et al. Drug Targeting the Actin Cytoskeleton Potentiates the Cytotoxicity of Low Dose Vincristine by Abrogating Actin-Mediated Repair of Spindle Defects. Mol. Cancer Res. 2020, 18, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.C.; Liu, J.H.; Liu, X.L.; Liang, X.; Cai, X.J. Effect of fibulin-5 on adhesion, migration and invasion of hepatocellular carcinoma cells via an integrin-dependent mechanism. World J. Gastroenterol. 2015, 21, 11127–11140. [Google Scholar] [CrossRef] [PubMed]
- Preis, M.; Cohen, T.; Sarnatzki, Y.; Ben Yosef, Y.; Schneiderman, J.; Gluzman, Z.; Koren, B.; Lewis, B.S.; Shaul, Y.; Flugelman, M.Y. Effects of fibulin-5 on attachment, adhesion, and proliferation of primary human endothelial cells. Biochem. Biophys. Res. Commun. 2006, 348, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Obaya, A.J.; Rua, S.; Moncada-Pazos, A.; Cal, S. The dual role of fibulins in tumorigenesis. Cancer Lett. 2012, 325, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Rivera Rosado, L.A.; Horn, T.A.; McGrath, S.C.; Cotter, R.J.; Yang, J.T. Association between α4 integrin cytoplasmic tail and non-muscle myosin IIA regulates cell migration. J. Cell Sci. 2011, 124 Pt 3, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Hsia, D.A.; Lim, S.T.; Bernard-Trifilo, J.A.; Mitra, S.K.; Tanaka, S.; den Hertog, J.; Streblow, D.N.; Ilic, D.; Ginsberg, M.H.; Schlaepfer, D.D. Integrin alpha4beta1 promotes focal adhesion kinase-independent cell motility via alpha4 cytoplasmic domain-specific activation of c-Src. Mol. Cell. Biol. 2005, 25, 9700–9712. [Google Scholar] [CrossRef]
- Tiwari, N.; Gheldof, A.; Tatari, M.; Christofori, G. EMT as the ultimate survival mechanism of cancer cells. Semin. Cancer Biol. 2012, 22, 194–207. [Google Scholar] [CrossRef]
- Zhou, S.; Ma, X.; Wang, Z.J.; Zhang, W.Y.; Jiang, H.; Li, S.D.; Zhang, T.Z.; Du, J.; Lu, Z. Research on the establishment of a TPM3 monoclonal stable transfected PANC-1 cell line and the experiment of the EMT occurrence in human pancreatic cancer. Onco Targets Ther. 2019, 12, 5577–5587. [Google Scholar] [CrossRef]
- Geeves, M.A.; Hitchcock-DeGregori, S.E.; Gunning, P.W. A systematic nomenclature for mammalian tropomyosin isoforms. J. Muscle Res. Cell Motil. 2015, 36, 147–153. [Google Scholar] [CrossRef]
- Gunning, P.W.; Hardeman, E.C.; Lappalainen, P.; Mulvihill, D.P. Tropomyosin—Master regulator of actin filament function in the cytoskeleton. J. Cell Sci. 2015, 128, 2965–2974. [Google Scholar] [CrossRef]
- Ardini, E.; Bosotti, R.; Borgia, A.L.; De Ponti, C.; Somaschini, A.; Cammarota, R.; Amboldi, N.; Raddrizzani, L.; Milani, A.; Magnaghi, P.; et al. The TPM3-NTRK1 rearrangement is a recurring event in colorectal carcinoma and is associated with tumor sensitivity to TRKA kinase inhibition. Mol. Oncol. 2014, 8, 1495–1507. [Google Scholar] [CrossRef] [PubMed]
- Martin-Zanca, D.; Hughes, S.H.; Barbacid, M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature 1986, 319, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.; Perez-Atayde, A.; Hibbard, M.K.; Rubin, B.P.; Dal Cin, P.; Pinkus, J.L.; Pinkus, G.S.; Xiao, S.; Yi, E.S.; Fletcher, C.D.; et al. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am. J. Pathol. 2000, 157, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Stehn, J.R.; Haass, N.K.; Bonello, T.; Desouza, M.; Kottyan, G.; Treutlein, H.; Zeng, J.; Nascimento, P.R.; Sequeira, V.B.; Butler, T.L.; et al. A novel class of anticancer compounds targets the actin cytoskeleton in tumor cells. Cancer Res. 2013, 73, 5169–5182. [Google Scholar] [CrossRef]
- Kee, A.J.; Chagan, J.; Chan, J.Y.; Bryce, N.S.; Lucas, C.A.; Zeng, J.; Hook, J.; Treutlein, H.; Laybutt, D.R.; Stehn, J.R.; et al. On-target action of anti-tropomyosin drugs regulates glucose metabolism. Sci. Rep. 2018, 8, 4604. [Google Scholar] [CrossRef]
- Janco, M.; Rynkiewicz, M.J.; Li, L.; Hook, J.; Eiffe, E.; Ghosh, A.; Böcking, T.; Lehman, W.J.; Hardeman, E.C.; Gunning, P.W. Molecular integration of the anti-tropomyosin compound ATM-3507 into the coiled coil overlap region of the cancer-associated Tpm3.1. Sci. Rep. 2019, 9, 11262. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, M.; Teng, Y.; Li, J.; Li, Z.; Hao, W.; Zhao, H.; Yin, C.; Yue, W. Cytoplasmic Localization Isoform of Cyclin Y Enhanced the Metastatic Ability of Lung Cancer via Regulating Tropomyosin 4. Front. Cell Dev. Biol. 2021, 9, 684819. [Google Scholar] [CrossRef]
- Jeong, S.; Lim, S.; Schevzov, G.; Gunning, P.W.; Helfman, D.M. Loss of Tpm4.1 leads to disruption of cell-cell adhesions and invasive behavior in breast epithelial cells via increased Rac1 signaling. Oncotarget 2017, 8, 33544–33559. [Google Scholar] [CrossRef]
- Du, X.L.; Hu, H.; Lin, D.C.; Xia, S.H.; Shen, X.M.; Zhang, Y.; Luo, M.L.; Feng, Y.B.; Cai, Y.; Xu, X.; et al. Proteomic profiling of proteins dysregulted in Chinese esophageal squamous cell carcinoma. J. Mol. Med. 2007, 85, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Lomnytska, M.I.; Becker, S.; Bodin, I.; Olsson, A.; Hellman, K.; Hellström, A.C.; Mints, M.; Hellman, U.; Auer, G.; Andersson, S. Differential expression of ANXA6, HSP27, PRDX2, NCF2, and TPM4 during uterine cervix carcinogenesis: Diagnostic and prognostic value. Br. J. Cancer 2011, 104, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Vasiljević, N.; Ahmad, A.S.; Carter, P.D.; Fisher, G.; Berney, D.M.; Foster, C.S.; Cuzick, J.; Lorincz, A.T. DNA methylation of PITX2 predicts poor survival in men with prostate cancer. Biomark. Med. 2014, 8, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zheng, G.; Ren, D.; Chen, C.; Zeng, C.; Lu, W.; Li, H. The clinical significance and biological function of tropomyosin 4 in colon cancer. Biomed. Pharmacother. 2018, 101, 1–7. [Google Scholar] [CrossRef]
- Yang, B.; Wolfenson, H.; Chung, V.Y.; Nakazawa, N.; Liu, S.; Hu, J.; Huang, R.Y.; Sheetz, M.P. Stopping transformed cancer cell growth by rigidity sensing. Nat. Mater. 2020, 19, 239–250. [Google Scholar] [CrossRef]
- Tijore, A.; Yao, M.; Wang, Y.H.; Hariharan, A.; Nematbakhsh, Y.; Lee Doss, B.; Lim, C.T.; Sheetz, M. Selective killing of transformed cells by mechanical stretch. Biomaterials 2021, 275, 120866. [Google Scholar] [CrossRef]
- Schevzov, G.; Kee, A.J.; Wang, B.; Sequeira, V.B.; Hook, J.; Coombes, J.D.; Lucas, C.A.; Stehn, J.R.; Musgrove, E.A.; Cretu, A.; et al. Regulation of cell proliferation by ERK and signal-dependent nuclear translocation of ERK is dependent on Tm5NM1-containing actin filaments. Mol. Biol. Cell 2015, 26, 2475–2490. [Google Scholar] [CrossRef]
- Di Pietro, F.; Echard, A.; Morin, X. Regulation of mitotic spindle orientation: An integrated view. EMBO Rep. 2016, 17, 1106–1130. [Google Scholar] [CrossRef]
- Chang, Z.; Fu, Y.; Jia, Y.; Gao, M.; Song, L.; Zhang, W.; Zhao, R.; Qin, Y. Circ-SFMBT2 drives the malignant phenotypes of esophageal cancer by the miR-107-dependent regulation of SLC1A5. Cancer Cell Int. 2021, 21, 495. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Z.; Zhao, J.; Zhai, X.; Li, J.; Zhang, Y.; Zong, L.; Peng, H.; Qi, J.; Kong, X.; et al. H19/miR-107/HMGB1 axis sensitizes laryngeal squamous cell carcinoma to cisplatin by suppressing autophagy in vitro and in vivo. Cell Biol. Int. 2021, 45, 674–685. [Google Scholar] [CrossRef]
- Yu, S.B.; Gao, Q.; Lin, W.W.; Kang, M.Q. Proteomic analysis indicates the importance of TPM3 in esophageal squamous cell carcinoma invasion and metastasis. Mol. Med. Rep. 2017, 15, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, W.; Jiang, J.; Shen, Z.; Chen, S.; Yu, S.; Kang, M. MiR-107 inhibits the malignant biological behavior of esophageal squamous cell carcinoma by targeting TPM3. J. Gastrointest. Oncol. 2022, 13, 1541–1555. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Chen, X.; Lin, A.; Tong, Z.; Lin, W. PolyC-RNA-binding protein 1 (PCBP1) enhances tropomyosin 3 (TPM3) mRNA stability to promote the progression of esophageal squamous cell carcinoma. Bioengineered 2022, 13, 8581–8592. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gu, M.; Cai, Z.K.; Zhao, H.; Sun, S.C.; Liu, C.; Zhan, M.; Chen, Y.B.; Wang, Z. TGF-β1 promotes epithelial-to-mesenchymal transition and stemness of prostate cancer cells by inducing PCBP1 degradation and alternative splicing of CD44. Cell. Mol. Life Sci. 2021, 78, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Li, H.; Yuan, R.; Guan, W.; Zhang, X.; Zhang, S.; Zhang, W.; Tong, F.; Li, L.; Song, Z.; et al. PCBP1 depletion promotes tumorigenesis through attenuation of p27Kip1 mRNA stability and translation. J. Exp. Clin. Cancer Res. 2018, 37, 187. [Google Scholar] [CrossRef]
- Zhao, S.; Cai, J.; Zhang, X.; Cui, J.; Jiu, Y. Different formins restrict localization of distinct tropomyosins on dorsal stress fibers in osteosarcoma cells. Cytoskeleton 2020, 77, 16–24. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, H.; Su, Y.; Kong, J.; Yu, H.; Qian, B. Up-regulation of microRNA-183-3p is a potent prognostic marker for lung adenocarcinoma of female non-smokers. Clin. Transl. Oncol. 2014, 16, 980–985. [Google Scholar] [CrossRef]
- Dai, Y.; Gao, X. Inhibition of cancer cell-derived exosomal microRNA-183 suppresses cell growth and metastasis in prostate cancer by upregulating TPM1. Cancer Cell Int. 2021, 21, 145. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Z.; Liu, X.; Zhang, C.; Hu, Y.; Ding, L.; Qi, P.; Wang, J.; Lu, S.; Li, Y. MiR-183-5p is required for non-small cell lung cancer progression by repressing PTEN. Biomed. Pharmacother. 2019, 111, 1103–1111. [Google Scholar] [CrossRef]
- Jones, P.A.; Laird, P.W. Cancer epigenetics comes of age. Nat. Genet. 1999, 21, 163–167. [Google Scholar] [CrossRef]
- Zhang, W.; Li, W.; Han, X. Skullcapflavone I inhibits proliferation of human colorectal cancer cells via down-regulation of miR-107 expression. Neoplasma 2019, 66, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.G.; Chen, M.H. TPM4 aggravates the malignant progression of hepatocellular carcinoma through negatively regulating SUSD2. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4756–4765. [Google Scholar] [CrossRef] [PubMed]
- Caporali, S.; Calabrese, C.; Minieri, M.; Pieri, M.; Tarantino, U.; Marini, M.; D’Ottavio, S.; Angeletti, S.; Mauriello, A.; Cortese, C.; et al. The miR-133a, TPM4 and TAp63γ Role in Myocyte Differentiation Microfilament Remodelling and Colon Cancer Progression. Int. J. Mol. Sci. 2021, 22, 9818. [Google Scholar] [CrossRef]
- Mikami, S.; Oya, M.; Mizuno, R.; Kosaka, T.; Ishida, M.; Kuroda, N.; Nagashima, Y.; Katsube, K.; Okada, Y. Recent advances in renal cell carcinoma from a pathological point of view. Pathol. Int. 2016, 66, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, C.; Yang, C.; Zheng, Q.; Hou, Y. Tropomyosin-1 Functions as a Tumor Suppressor with Respect to Cell Proliferation, Angiogenesis and Metastasis in Renal Cell Carcinoma. J. Cancer 2019, 10, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Chengyao, X.; Qingchang, L.; Qianze, D.; Enhua, W.; Yan, W. CRKL promotes lung cancer cell invasion through ERK-MMP9 pathway. Mol. Carcinog. 2015, 54 (Suppl. S1), E35–E44. [Google Scholar] [CrossRef]
- Chen, S.; Shen, Z.; Gao, L.; Yu, S.; Zhang, P.; Han, Z.; Kang, M. TPM3 mediates epithelial-mesenchymal transition in esophageal cancer via MMP2/MMP9. Ann. Transl. Med. 2021, 9, 1338. [Google Scholar] [CrossRef]
- Cui, J.; Cai, Y.; Hu, Y.; Huang, Z.; Luo, Y.; Kaz, A.M.; Yang, Z.; Chen, D.; Fan, X.; Grady, W.M.; et al. Epigenetic silencing of TPM2 contributes to colorectal cancer progression upon RhoA activation. Tumour Biol. 2016, 37, 12477–12483. [Google Scholar] [CrossRef]
- Shin, H.; Kim, D.; Helfman, D.M. Tropomyosin isoform Tpm2.1 regulates collective and amoeboid cell migration and cell aggregation in breast epithelial cells. Oncotarget 2017, 8, 95192–95205. [Google Scholar] [CrossRef]
- Bosco, E.E.; Mulloy, J.C.; Zheng, Y. Rac1 GTPase: A “Rac” of all trades. Cell. Mol. Life Sci. 2009, 66, 370–374. [Google Scholar] [CrossRef]
- Mitchell, C.B.; Stehn, J.R.; O’Neill, G.M. Small molecule targeting of the actin associating protein tropomyosin Tpm3.1 increases neuroblastoma cell response to inhibition of Rac-mediated multicellular invasion. Cytoskeleton 2018, 75, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shi, H.; Fan, Q.; Sun, X. Cyclin Y regulates the proliferation, migration, and invasion of ovarian cancer cells via Wnt signaling pathway. Tumour Biol. 2016, 37, 10161–10175. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Ru, Q.; Zhang, C.; Huang, J. Cyclin Y Modulates the Proliferation, Invasion, and Metastasis of Hepatocellular Carcinoma Cells. Med. Sci. Monit. 2018, 24, 1642–1653. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Guest, S.; Finley, R.L., Jr. Why cyclin Y? A highly conserved cyclin with essential functions. Fly 2010, 4, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Co, N.N.; Wong, N. PFTK1 interacts with cyclin Y to activate non-canonical Wnt signaling in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2014, 449, 163–168. [Google Scholar] [CrossRef]
- Cavey, M.; Lecuit, T. Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harb. Perspect. Biol. 2009, 1, a002998. [Google Scholar] [CrossRef]
- Mitchell, C.B.; Black, B.; Sun, F.; Chrzanowski, W.; Cooper-White, J.; Maisonneuve, B.; Stringer, B.; Day, B.; Biro, M.; O’Neill, G.M. Tropomyosin Tpm 2.1 loss induces glioblastoma spreading in soft brain-like environments. J. Neurooncol. 2019, 141, 303–313. [Google Scholar] [CrossRef]
- Sol, N.; Wurdinger, T. Platelet RNA signatures for the detection of cancer. Cancer Metastasis Rev. 2017, 36, 263–272. [Google Scholar] [CrossRef]
- Yao, B.; Qu, S.; Hu, R.; Gao, W.; Jin, S.; Ju, J.; Zhao, Q. Delivery of platelet TPM3 mRNA into breast cancer cells via microvesicles enhances metastasis. FEBS Open Bio 2019, 9, 2159–2169. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Xu, S.; Zhang, X.; Wang, P.; Wu, H.; Xia, B.; Zhang, G.; Lei, B.; Wan, L.; et al. Hypoxia-Induced TPM2 Methylation is Associated with Chemoresistance and Poor Prognosis in Breast Cancer. Cell Physiol. Biochem. 2018, 45, 692–705. [Google Scholar] [CrossRef]
- Gunning, P.; O’Neill, G.; Hardeman, E. Tropomyosin-based regulation of the actin cytoskeleton in time and space. Physiol. Rev. 2008, 88, 1–35. [Google Scholar] [CrossRef]
- Poon, I.K.; Patel, K.K.; Davis, D.S.; Parish, C.R.; Hulett, M.D. Histidine-rich glycoprotein: The Swiss Army knife of mammalian plasma. Blood 2011, 117, 2093–2101. [Google Scholar] [CrossRef]
- Jones, A.L.; Hulett, M.D.; Parish, C.R. Histidine-rich glycoprotein: A novel adaptor protein in plasma that modulates the immune, vascular and coagulation systems. Immunol. Cell Biol. 2005, 83, 106–118. [Google Scholar] [CrossRef]
- Doñate, F.; Juarez, J.C.; Guan, X.; Shipulina, N.V.; Plunkett, M.L.; Tel-Tsur, Z.; Shaw, D.E.; Morgan, W.T.; Mazar, A.P. Peptides derived from the histidine-proline domain of the histidine-proline-rich glycoprotein bind to tropomyosin and have antiangiogenic and antitumor activities. Cancer Res. 2004, 64, 5812–5817. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T. Anti-Cancer Effects of Zinc (II) Ion in Tumor Formation and Growth, Proliferation, Metastasis and DNA Damage. In Degenerative Intellectual & Developmental Disabilities; Crimson Publishers, LLC: New York, NY, USA, 2017; pp. 1–8. [Google Scholar]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: ‘Copper That Cancer’. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.M.; Zimbone, S.; Magrì, A.; La Mendola, D.; Grasso, G. The Role of Copper (II) on Kininogen Binding to Tropomyosin in the Presence of a Histidine-Proline-Rich Peptide. Int. J. Mol. Sci. 2020, 21, 9343. [Google Scholar] [CrossRef] [PubMed]
- Ku, B.M.; Ryu, H.W.; Lee, Y.K.; Ryu, J.; Jeong, J.Y.; Choi, J.; Cho, H.J.; Park, K.H.; Kang, S.S. 4’-Acetoamido-4-hydroxychalcone, a chalcone derivative, inhibits glioma growth and invasion through regulation of the tropomyosin 1 gene. Biochem. Biophys. Res. Commun. 2010, 402, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Kögel, D.; Reimertz, C.; Mech, P.; Poppe, M.; Frühwald, M.C.; Engemann, H.; Scheidtmann, K.H.; Prehn, J.H. Dlk/ZIP kinase-induced apoptosis in human medulloblastoma cells: Requirement of the mitochondrial apoptosis pathway. Br. J. Cancer 2001, 85, 1801–1808. [Google Scholar] [CrossRef]
- Das, T.P.; Suman, S.; Papu John, A.M.; Pal, D.; Edwards, A.; Alatassi, H.; Ankem, M.K.; Damodaran, C. Activation of AKT negatively regulates the pro-apoptotic function of death-associated protein kinase 3 (DAPK3) in prostate cancer. Cancer Lett. 2016, 377, 134–139. [Google Scholar] [CrossRef]
- Song, Y.; Que, T.; Long, H.; Zhang, X.S.A.; Fang, L.; Li, Z.; Qi, S. Downregulation of death-associated protein kinase 3 and caspase-3 correlate to the progression and poor prognosis of gliomas. Cancer Transl. Med. 2016, 2, 72–78. [Google Scholar]
- Pearl, L.H. Hsp90 and Cdc37—A chaperone cancer conspiracy. Curr. Opin. Genet. Dev. 2005, 15, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zhao, J.; Wang, Y. The prognostic value of TPM1-4 in hepatocellular carcinoma. Cancer Med. 2022, 11, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Ao, J.Y.; Zhu, X.D.; Chai, Z.T.; Cai, H.; Zhang, Y.Y.; Zhang, K.Z.; Kong, L.Q.; Zhang, N.; Ye, B.G.; Ma, D.N.; et al. Colony-Stimulating Factor 1 Receptor Blockade Inhibits Tumor Growth by Altering the Polarization of Tumor-Associated Macrophages in Hepatocellular Carcinoma. Mol. Cancer Ther. 2017, 16, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, J.; Ye, M.; Li, Z.; Li, S. Tropomyosin Is Potential Markers for the Diagnosis and Prognosis of Bladder Cancer. Dis. Markers 2022, 2022, 6936262. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Feng, H. An Immune-Related Prognostic Risk Model in Colon Cancer by Bioinformatics Analysis. Evid. Based Complement. Altern. Med. 2022, 2022, 3640589. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, X.; Yao, J.; Wang, X.; Wang, N. Comprehensive analysis of clinical prognosis and molecular immune characterization of tropomyosin 4 in pancreatic cancer. Investig. New Drugs 2021, 39, 1469–1483. [Google Scholar] [CrossRef]
- Grant, R.C.; Denroche, R.; Jang, G.H.; Nowak, K.M.; Zhang, A.; Borgida, A.; Holter, S.; Topham, J.T.; Wilson, J.; Dodd, A.; et al. Clinical and genomic characterisation of mismatch repair deficient pancreatic adenocarcinoma. Gut 2021, 70, 1894–1903. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, Z.; Lu, S.; Zhang, N.; Rong, G.; Chang, X.; Liu, Z.; Bai, W.; Dong, Z.; Gao, X.; et al. Downregulated Expression of Tropomyosin 1 in Intrahepatic Cholangiocarcinoma: A Predictor of Recurrence and Prognosis. Med. Sci. Monit. 2018, 24, 7875–7882. [Google Scholar] [CrossRef]
- Huang, K.; Wang, H.; Xu, J.; Xu, R.; Liu, Z.; Li, Y.; Xu, Z. The Tropomyosin Family as Novel Biomarkers in Relation to Poor Prognosis in Glioma. Biology 2022, 11, 1115. [Google Scholar] [CrossRef]
- Pawlak, G.; McGarvey, T.W.; Nguyen, T.B.; Tomaszewski, J.E.; Puthiyaveettil, R.; Malkowicz, S.B.; Helfman, D.M. Alterations in tropomyosin isoform expression in human transitional cell carcinoma of the urinary bladder. Int. J. Cancer 2004, 110, 368–373. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Sun, L.; Yang, M.; Pan, C.; Chen, W.; Wu, D.; Lin, Z.; Zeng, C.; Yao, Y.; et al. MiR-21 indicates poor prognosis in tongue squamous cell carcinomas as an apoptosis inhibitor. Clin. Cancer Res. 2009, 15, 3998–4008. [Google Scholar] [CrossRef]
- Zheng, G.; Li, N.; Jia, X.; Peng, C.; Luo, L.; Deng, Y.; Yin, J.; Song, Y.; Liu, H.; Lu, M.; et al. MYCN-mediated miR-21 overexpression enhances chemo-resistance via targeting CADM1 in tongue cancer. J. Mol. Med. 2016, 94, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Gu, L.; Liu, B.; Li, Y.; Wang, Y.; Bai, X.; Li, L.; Wang, B.; Peng, Q.; Yao, Z.; et al. Tropomyosin-1 acts as a potential tumor suppressor in human oral squamous cell carcinoma. PLoS ONE 2017, 12, e0168900. [Google Scholar] [CrossRef] [PubMed]
- Mele, V.; Basso, C.; Governa, V.; Glaus Garzon, J.F.; Muraro, M.G.; Däster, S.; Nebiker, C.A.; Mechera, R.; Bolli, M.; Schmidt, A.; et al. Identification of TPM2 and CNN1 as Novel Prognostic Markers in Functionally Characterized Human Colon Cancer-Associated Stromal Cells. Cancers 2022, 14, 2024. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.O.; Hong, Y.; Etlioglu, H.E.; Cho, Y.B.; Pomella, V.; Van den Bosch, B.; Vanhecke, J.; Verbandt, S.; Hong, H.; Min, J.W.; et al. Lineage-dependent gene expression programs influence the immune landscape of colorectal cancer. Nat. Genet. 2020, 52, 594–603. [Google Scholar] [CrossRef]
- Casper, M.; Weber, S.N.; Kloor, M.; Müllenbach, R.; Grobholz, R.; Lammert, F.; Zimmer, V. Hepatocellular carcinoma as extracolonic manifestation of Lynch syndrome indicates SEC63 as potential target gene in hepatocarcinogenesis. Scand. J. Gastroenterol. 2013, 48, 344–351. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, Z.; Huang, S.; Sun, C.; Zhao, J.; Shi, J.; Li, Z.; Wang, Z.; He, X.; Tam, N.L.; et al. SKA3 Promotes tumor growth by regulating CDK2/P53 phosphorylation in hepatocellular carcinoma. Cell Death Dis. 2019, 10, 929. [Google Scholar] [CrossRef]
- Yang, W.Y.; Rao, P.S.; Luo, Y.C.; Lin, H.K.; Huang, S.H.; Yang, J.M.; Yuh, C.H. Omics-based Investigation of Diet-induced Obesity Synergized with HBx, Src, and p53 Mutation Accelerating Hepatocarcinogenesis in Zebrafish Model. Cancers 2019, 11, 1899. [Google Scholar] [CrossRef]
- Li, L.; Ye, T.; Zhang, Q.; Li, X.; Ma, L.; Yan, J. The expression and clinical significance of TPM4 in hepatocellular carcinoma. Int. J. Med. Sci. 2021, 18, 169–175. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, P.; Zhou, X.; Xu, J.; Lu, S.; Chen, Y.; Zhang, Y. Differential proteomics mass spectrometry of melanosis coli. Am. J. Transl. Res. 2020, 12, 3133–3148. [Google Scholar]
- Zare, M.; Hadi, F.; Alivand, M.R. Considering the downregulation of Tpm1.6 and Tpm1.7 in squamous cell carcinoma of esophagus as a potent biomarker. Pers. Med. 2018, 15, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Franzén, B.; Linder, S.; Uryu, K.; Alaiya, A.A.; Hirano, T.; Kato, H.; Auer, G. Expression of tropomyosin isoforms in benign and malignant human breast lesions. Br. J. Cancer 1996, 73, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xiao, T.; Xu, Q.; Shao, X.; Wang, H. iTRAQ-based quantitative analysis of cancer-derived secretory proteome reveals TPM2 as a potential diagnostic biomarker of colorectal cancer. Front. Med. 2016, 10, 278–285. [Google Scholar] [CrossRef]
- Yager, M.L.; Hughes, J.A.; Lovicu, F.J.; Gunning, P.W.; Weinberger, R.P.; O’Neill, G.M. Functional analysis of the actin-binding protein, tropomyosin 1, in neuroblastoma. Br. J. Cancer 2003, 89, 860–863. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, F.L.; Wang, Y.; Wong, W.K.; Liu, Y.; Addivinola, F.J.; Liang, P.; Chen, L.B.; Kantoff, P.W.; Pardee, A.B. Two differentially expressed genes in normal human prostate tissue and in carcinoma. Cancer Res. 1996, 56, 3634–3637. [Google Scholar]
- Pan, X.; Wang, J.; Guo, L.; Na, F.; Du, J.; Chen, X.; Zhong, A.; Zhao, L.; Zhang, L.; Zhang, M.; et al. Identifying a confused cell identity for esophageal squamous cell carcinoma. Signal Transduct. Target. Ther. 2022, 7, 122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).