Abstract
Thrombocytes are numerous in the blood of aves (birds) and ichthyoids (fish). The origin of this cell type is a common hematopoietic stem cell giving rise to a cell that is active in blood coagulation, inflammatory functions, and the immune response in general. It has been well documented that thrombocytes can phagocytize small particles and bacteria. While phagocytosis with an associated oxidative burst has been reported for chicken thrombocytes, some questions remain as to the degradation capacity of phagosomes in ichthyoids. As innate cells, thrombocytes can be stimulated by bacterial, viral, and fungal pathogens to express altered gene expression. Furthermore, there have been observations that led researchers to state that platelets/thrombocytes are capable of serving as “professional antigen presenting cells” expressing CD40, CD80/86, MHC I, and MHC II. This indeed may be the case or, more likely at this time, provide supporting evidence that these cells aid and assist in the role of professional antigen-presenting cells to initiate adaptive immune responses.
1. Overview
The immune system of birds and fish consists of innate and adaptive immunity, which includes cellular and non-cellular (or humoral) components. The cellular components of the non-specific, innate immune response include cells such as macrophages, granulocytes (polymorphonuclear cells such as heterophils), thrombocytes, basophils, eosinophils, and natural killer cells. Since innate cells express various pattern recognition receptors (PRRs), these cells play an important role in the earlier phases of pathogenic invasion. PRRs serve to recognize microbial invasion by detecting pathogen-associated molecular patterns (PAMPs) and harmful products produced by the host body through danger-associated molecular patterns (DAMPs). Upon detection of danger, the cells of the innate immune system respond by producing or releasing molecules such as defensins, cytokines, and chemokines for effector function (i.e., coordinating the recruitment and action of a series of specialized cell populations that fight invading pathogens via phagocytosis and lytic functions). The PRRs on innate cells are also important for initiating the competency of select cells to present antigens to lymphocytes. The lymphocytes (T and B cells) are adaptive immune cells that provide a second layer of protection in a host. Cells such as macrophages and dendritic cells are known to process the pathogenic peptides and present antigens in the context of the major histocompatibility complex (MHC) to lymphocytes to initiate a more specific adaptive immune response to clear infection.
This review focuses on the role of thrombocytes in immune response in birds and fish. Thrombocytes in lower vertebrates such as birds, reptiles, amphibians, and fish are homologous to enucleated platelets in mammals [1]. Nucleated hemocytes found in the hemolymph of invertebrate species perform functions comparable to thrombocytes or platelets [2,3]. In most vertebrate circulation, thrombocytes are the most abundant blood cells after erythrocytes [4,5,6]. Thrombopoiesis occurs in different tissues in adult animals, depending on the vertebrate group and species. In mammals, platelets develop from megakaryocytes [7]. Thrombocytes in birds originate from cells that resemble multipotent hematopoietic progenitors and are produced in the region where the earliest intraembryonic hematopoietic cells develop [8]. Thrombopoiesis occurs in the lymphomyeloid and lymphoid tissues such as the spleen, kidney, and liver in fish [9,10]. Thrombocytes are similar in size to lymphocytes and appear as round, oval, spindle, or spiked cells with long cell processes [1,5,6,11,12,13,14]. The cytoplasm of thrombocytes appears to have a surface-connected canalicular system [15]. Thrombocytes are also capable of producing and releasing a vast array of bioactive proteins that are inflammatory, antimicrobial, and immune-modulating molecules [16,17,18,19,20,21,22,23]. In addition, large, acid phosphatase-positive granules, similar to mammalian lysosomal granules, have been observed in avian thrombocytes [24]. When activated, thrombocytes can release into the circulation numerous intracellular secretory granules (e.g., α granules, dense granules, and lysosomes) that these cells possess.
2. Immune Receptors
Although platelets and thrombocytes have been known primarily to be involved in thrombosis and hemostasis, these cells have been studied in the last two decades to demonstrate a role in infection, inflammatory functions, and the immune response in general. The detection of PRRs such as toll-like receptors (TLRs) on thrombocytes has led to the discovery of the role of these cells in various innate immune responses. For birds, TLR1, 2, 3, 4, 5, 7, and 21 [25,26] have been identified, and TRL1, 2, 4, 5, 7, 8, 9, 20, and 21 [23,27] are functional for ichthyoid thrombocytes (Figure 1). TLRs 1, 2, 4, and 5 are generally associated with the recognition of patterns associated with bacteria, while TLRs 3 and 7–9 recognize double-stranded and single-stranded RNA often associated with viruses in fish and chicken [28,29]. Subtypes of TLR 1 and 2 are also associated with the recognition of mycoplasma and fungal particles [30]. TLR 20 is detected in fish only and is associated with the recognition of protozoan parasites [31]. TLR 21 is associated with recognizing the CpG dinucleotide sequences in DNA [28,32]. These cells exhibit other PRRs and associated genes such as nucleotide-binding oligomerization domain (NOD)-like receptors (NLR) Family Member X1 (NLRX1), NLR Family CARD Domain Containing 3 and 5 (NLRC3, NLRC5) [33], and C-type lectin receptor (CLR) [34]. The discovery of these molecules associated with pathogen recognition on thrombocytes reinforces the notion that these cells may be playing direct roles in protecting the host from infection like other leukocytes.
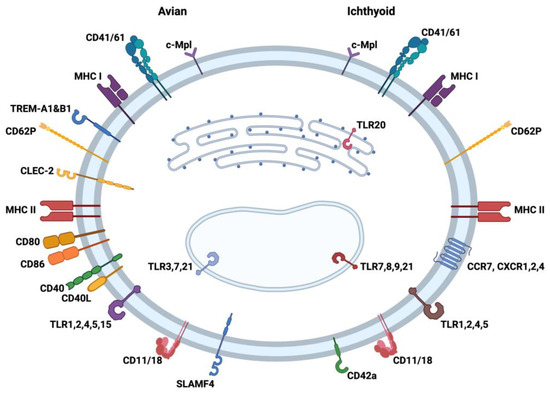
Figure 1.
Comparison of immune molecules reported to be present in/on avian (left) and ichthyoid (right) thrombocytes, which lend support to the unique role of this cell type in innate and adaptive immunity. The figure is divided into left (avian) and right (ichthyoid) sides with several receptors and molecules associated with antigen presentation on the cell surface and in the cytoplasm of thrombocytes. The markers in both bird and fish thrombocytes have been compiled from several refereed publications examining the immune characteristics of this cell type. TLR: toll-like receptor; CD: cluster of differentiation; MHC: major histocompatibility complex; TREM: Triggering Receptors Expressed on Myeloid cells; CLEC: C-type lectin-like receptors; SLAM: signaling lymphocytic activating molecule; CCR: C-C chemokine receptor; CXCR: C-X-C receptor; c-Mpl: thrombopoietin receptor. Created with BioRender.com (accessed on 26 July 2023).
In addition to the PRRs, both avian and ichthyoid thrombocytes express markers such as CD41/61 (glycoprotein IIb/IIIa) [35]. This molecule is known to be associated with the activation and aggregation of mammalian platelets [36,37]. The thrombopoietin receptor (c-Mpl) has been identified in birds and fish as a unique marker of thrombocytes related to the proliferation of immature stages of the cell. Thrombocytes additionally express CD62 (P-selectin), which is important cell adhesion molecule for the rolling of platelets and leukocytes on activated endothelial cells [38,39]. The presence of CD11/18 (Complement receptor 3) provides these cells with the ability to attract complement complexes engaged in immune capture by thrombocytes. The chicken thrombocyte exhibits immunoregulatory Ig-like receptors CLEC-2 (C-type lectin) [34], SLAMF4 (CD244, 2B4) [40], and TREM-A1 and -B1 [41,42], whereas CD42a (GPIX) [35] has been observed on the fish thrombocyte. Also, fish thrombocytes have been found to display chemokine receptors (CCR7, CXCR 1 and 2, and CXCR4) [35], while none have yet been identified on the avian cell. The interaction of platelets with neutrophils and other leukocytes is mainly mediated through CD62P and integrins (e.g., CD11b/CD18, CD41/CD61) [43,44]. Since a combination of these adhesion molecules is also observed on thrombocytes (Figure 1), these most likely are important for the interaction between thrombocytes and other immune cells. These interactions emphasize the possible role of thrombocytes as a link between innate and adaptive immunity.
3. Immunoregulatory Molecules
Thrombocytes have been shown to express, produce, or release a variety of mediators of inflammation, antimicrobial activity, and other immune-modulating activities. PRRs on thrombocytes play an important role in inflammation following microbial infections. Several transcripts associated with signaling downstream of TLRs in chicken thrombocytes were detected in a transcriptome study by our group [33]. In Figure 2, the transcripts detected in chicken thrombocytes were used to compose standard TLR signaling pathways. In response to stimulation of these PRRs, avian thrombocytes are able to produce a variety of bioactive compounds, chemotactic factors (e.g., Macrophage Inflammatory Protein-1 and nitric oxide), and other mediators of inflammation such as inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), prostaglandin D2 (PGD2), PGE2, and thromboxane A2 synthases [16,19,45,46]. Chicken thrombocytes express transcripts of anti-inflammatory cytokine transforming growth factor (TGF) and IL-10 [25], and pro-inflammatory cytokines/chemokines (IL-1, IL-6, IL-8, and IL-12) [16,18,19,45]. Chicken thrombocytes have been shown to respond to PAMPs associated with bacterial infection (lipopolysaccharide (LPS) and lipoteichoic acid (LTA)) and viral infection (thymidine homopolymer phosphorothioate oligonucleotide [Poly(dT)] and polyinosinic-polycytidylic acid [Poly(I:C)]) [16,18,47]. Additionally, these cells can induce a pro-inflammatory response to unmethylated CpG oligodeoxynucleotides (ODN) associated with nucleic acids of certain bacteria and viruses [25]. LPS-stimulated chicken thrombocytes have been shown to upregulate transcripts associated with the production of inflammatory cytokines and chemokines, apoptosis, activation of T and B lymphocytes, MAPK activation, IFN activation, and JAK/STAT signaling [33]. Winkler et al. [20] were able to map pathways associated with the response of chicken thrombocytes to LPS stimulation by using inhibitors for kinases such as extracellular-signal-regulated kinase (ERK), p38, mitogen-activated protein kinase kinase (MEK)1/2, and inhibitor of nuclear factor kappa-B kinase (IKK). Chicken thrombocytes have also been shown to have transcripts for platelet-derived growth factors that may have important roles in the vascular system and healing damaged tissue [48,49,50].
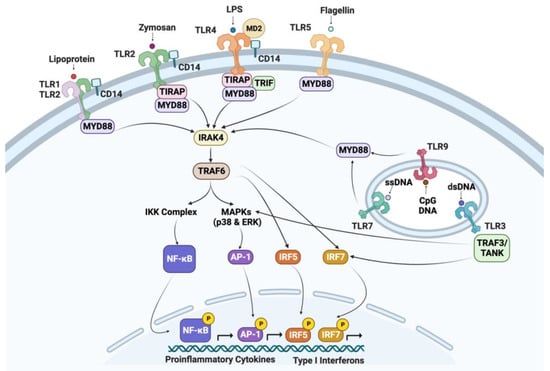
Figure 2.
Genes of the TLR signaling pathway observed in chicken thrombocytes. TLR: toll-like receptor; MD2: myeloid differentiation protein 2; CD14: cluster of differentiation 14; LPS: lipopolysaccharide; MyD88: myeloid differentiation primary-response protein 88; TIRAP: Toll/Il-1 receptor domain-containing adaptor protein; TRIF: Toll/Il-1 receptor domain-containing adaptor inducing IFN-β; IRAK: IL-1 receptor-associated protein kinase; TRAF: tumor necrosis factor receptor-associated factor; NF-κB-nuclear factor κB; TANK-TRAF-family-member-associated NF-κB activator; MAPKs: mitogen-activated protein kinases; AP-1: activator protein; IRF: interferon regulatory factor; IKK-IκB kinase; and ERK: extracellular signal-regulated kinase. Created with BioRender.com (Accessed on 26 July 2023).
Fish thrombocytes also express many genes associated with inflammation including CD62P, IL-1, IL-6, tumor necrosis factor (TNF)α, IFNγ, IL-11, iNOS, TGF, the interleukin receptor common chain, as well as CXC and CC chemokines [21,22,23,51]. The pro-inflammatory cytokines released by leukocytes in response to injury and tissue damage stimulate hepatocytes to produce acute-phase proteins (e.g., C-reactive protein (CRP), serum amyloid A (SAA), metal-binding protein, lysozyme, lectin, etc.) [52]. These proteins are responsible for a variety of defense-related activities such as the inactivation of proteolytic enzymes, preventing the distribution of infectious agents (i.e., either by the destruction of microorganisms or making microbial cells suitable for cell response by modifying surface targets), and the restoration of damaged tissue to a healthy condition. In a study performed by He et al. [22], it was demonstrated that grass carp thrombocytes significantly upregulated the mRNA expressions of some innate immune genes when challenged with bacteria (Aeromonas hydrophila) and reovirus (grass carp reovirus). In addition, they determined that the number of thrombocytes in peripheral blood increased after viral and bacterial stimulation, indicating that the increase in the number of thrombocytes in the body may be involved in immune regulation during pathogen invasion.
4. Response to Pathogens
Various pathogens that impact the poultry and aquaculture industry have been shown to infect or interact with thrombocytes. According to a study carried out by Schat et al. [53], highly pathogenic (HP) H5N1 avian influenza viruses (AIVs) can infect chicken thrombocytes, which plays a major role in the pathogenesis of this disease. In another study, it has been demonstrated that the AIV strain A (fowl plague virus) is able to replicate in chicken thrombocytes [54]. Another economically important virus, the Newcastle Disease Virus, has also been shown to not only infect chicken thrombocytes but also impair the ability of these cells to perform phagocytosis and migration [55]. On the mammalian side, platelets have been shown to interact with different viruses in different capacities through the various surface receptors for integrins, surface lectins, and TLRs [56,57,58]. Several studies have indicated that during infection with human immunodeficiency virus (HIV), there is a direct interaction of HIV with megakaryocytes and platelets [59]. Viral infections can lead to thrombocytopenia via decreased platelet production or increased platelet destruction [58]. Platelets can also provide protective and pathophysiologic responses during certain viral infections using mediators originating from platelets and the interaction of these cells with other vascular and immune cells [59,60,61,62].
In addition to viruses, parasites have been shown to interact with thrombocytes. Gametocytes of leucocytozoon parasites infect and develop within chicken thrombocytes [63]. In common carp, an infection with the protozoan parasite Trypanoplasma borreli leads to severe thrombocytopenia due to nitric-oxide-mediated apoptosis [23]. Nitric-oxide-mediated apoptosis may be the reason for thrombocytopenia in chickens infected with Plasmodium gallinaceum [64]. Thrombocytopenia is also observed with infection with most Plasmodium species in humans [65]. However, the mechanisms leading to thrombocytopenia during malaria are not fully understood [65]. Platelets are able to directly interact with and kill circulating plasmodium parasites and infected erythrocytes in patients with malaria. In a study where trout were infected with Candida albicans, thrombocytes were observed to interact with erythrocytes, macrophages, other polymorphonuclear cells, and lymphocytes [66]. The thrombocytes appear to form cellular aggregates as rosettes that interact with erythrocytes and macrophages [65]. This phenomenon can also be observed with platelets interacting with erythrocytes, neutrophils, or other cells. Kho et al. [65] have demonstrated that the major parasites associated with human malaria can be killed by platelets.
5. Phagocytosis
Phagocytic cells are essential components of the immune system. These cells are responsible for the ingestion and destruction of pathogens, cellular debris, and other foreign elements. PRRs such as TLRs play an essential role in the recognition of pathogens. They trigger both the degradation of pathogens mediated by the release of bioactive, cytotoxic contents from large granules and the subsequent presentation of pathogen-derived peptide antigens. Mammalian platelets are able to bind circulating bacteria and microbial products and present those to neutrophils and other phagocytic cells [67]. In addition to binding and internalizing microorganisms, contact with certain bacteria causes an aggregation and degranulation of platelets. The α-granules of platelets contain and release cationic antibacterial/microbicidal proteins referred to as thrombocidins [68,69] and reactive oxygen species [70]. Thrombocidins support the killing of adherent bacteria [71] and also have been shown to have fungicidal properties in vitro [71].
The phagocytic ability of circulating thrombocytes in chickens was shown first by Glick et al. [72]. Avian thrombocytes are capable of phagocytosing dye particles [24] and several bacterial species including various strains of Salmonella, Escherichia coli, Pseudomonas aeruginosa, and Burkholderia cepaciao [24,72,73]. Chicken thrombocytes have been shown to phagocytize about three times as rapidly as heterophils and monocytes, and circulating thrombocytes engulfed nearly twice as many bacteria as the heterophil and monocyte together [4]. The acid-phosphatase-positive granules in avian thrombocytes are considered lysosomal structures associated with phagocytic activity [24].
The phagocytic ability of thrombocytes from other lower vertebrates has also been demonstrated using teleost (Paralichthys olivaceus) and amphibian (Xenopuslaevis) models [51]. Among peripheral leukocytes in common carp, thrombocytes represent nearly half of the phagocyte population [51]. The process of triggering phagocytosis in carp thrombocytes is closely linked to the presence and activity of factors that are released by LPS from Escherichia coli O55- and phorbol 12-myristate 13-acetate (PMA)-activated leukocytes [74]. Phagocytic particles have been detected in the cytoplasm of thrombocytes of various fish species [35]. In addition, carp thrombocytes exhibit genes for several lysozymes [74] and possess bactericidal activity [75].
Osteichthyoid thrombocytes are considered phagocytes that are fully capable of recognizing opsonized antigens, kill pathogens, and are able to influence the development of both innate and adaptive immune responses [35,51,74]. The detection of phagocytic and antigen-burdened thrombocytes in the spleen and kidneys of common carp indicates a possible role of thrombocytes in the transport of antigens to lymphoid tissues and a contribution to the initiation of adaptive immune response [51]. According to a study carried out by Nagasawa et al. [74], carp thrombocytes efficiently recognized antigens in the presence of inflammatory signals originating from stimulated leukocytes.
The capacity for thrombocytes to express genes encoding various immune factors has been well documented in birds and fish. Several cytokines, chemokines, and immune regulators are produced and secreted to influence other cell types in the immediate vicinity of thrombocyte activation [16,17,20]. These effects range from pro-inflammatory responses and cell migration to an activation/differentiation of effector cells [15,35,76]. The spectrum of gene activation events observed for thrombocytes includes inducible expression as a result of Gram-negative and -positive bacterial ligands to RNA and DNA viral nucleotide sequences [46,47]. The differentiated responses have been reported and result from the engagement of TLRs found on thrombocyte cell surfaces and vesicles [20,25,47]. The soluble factor production of these activation treatments has been shown through bioassays, ELISAs, and Western blotting [16,17,20].
6. Participation in Adaptive Immunity
Reports of platelets participating in adaptive immunity number more than a few in the literature [77,78,79,80,81,82]. Ali et al. [78] provided a comprehensive review of the mammalian platelet and its roles in immunity. Discussions of their involvement in adaptive immunity describe platelets as merely assisting APCs through the expression of CD40L (CD154) to engage in the full scope of antigen processing and presentation with the formation of co-stimulatory binding via B7 [83]. Platelets aid DC maturation via CD40L and have the capability to deliver antigens to APCs. More importantly, platelets are able to process and present antigens through MHC I and have been observed to present antigens with MHC II during certain diseases. Class I processing is accomplished in platelets with the cellular mechanism associated with APCs, and the set of co-stimulatory molecules for T-cell activation are present on platelets. Furthermore, platelets appear to enhance T-dependent B-cell responses via CD40:CD40L linkages of these lymphocytes [78,83].
Professional APCs have been well characterized with regard to the properties that define the roles played by dendritic cells (DC), macrophages, and B cells. When implicating a platelet or thrombocyte in APC activity, the comparison will be based upon cellular activity as well as the cell expression of essential surface receptors. Fundamentally, APCs are capable of antigen uptake, express MHC II, provide co-stimulatory activity via B7 (CD80/86), and participate in T-cell activation. All three cell types engulf antigens via endocytosis and/or phagocytosis. With regard to MHC II expression, DCs and B cells constitutively express this molecule, while it is necessary to induce class II on macrophages. B7 molecules are constitutively expressed on DCs but must be induced for expression on both B cells and macrophages. Only DCs and activated B cells stimulate naïve T cells, and effector and memory T cells are activated when the three APC types are expressing MHC II and B7.
Work conducted by our group [33,76] led us to postulate that chicken thrombocytes may indeed support the APC process, if not participate in the induction of adaptive immunity. Our current evidence for this lies in gene expression studies and some initial attempts to observe thrombocyte aggregation at sites of adaptive immune genesis in the spleen [19]. The gene expression profile for the antigen processing and presentation of a chicken thrombocyte is presented in Figure 3. Although not a mammalian platelet, the chicken thrombocyte exhibits immune characteristics, particularly MHC processes, nearly identical to the mammalian platelet [78].
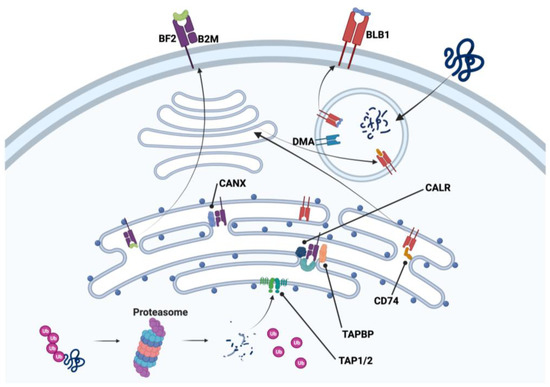
Figure 3.
Constitutively expressed genes of class I and II major histocompatibility complex processes of endogenous and exogenous antigen processing pathways found in chicken thrombocytes. B2M: β2-microglobulin; BF2: MCH I alpha chain 2; BLB1: MHC II beta chain; CALR: calrecticulin; CANX: calnexin; CD74: invariant chain of CLIP; DMA: MHC II DM alpha chain; TAPBP: tapasin; TAP1/2: transporter 1/2. Created with BioRender.com (Accessed on 27 July 2023).
Thrombocytes, like other cells of the body, express MHC I on the cell surface. This imparts individual histocompatibility to the host’s cells, and in the case of thrombocytes, there appears to be cellular connectivity with the MHC I antigen processing mechanisms associated with the endoplasmic reticulum as seen in Figure 3 from our transcriptomic data [33,76]. Table S2 of our publication [33] lists MHC I α-chain (BF2), β2-microglobulin (B2M), and tapasin (TAPBP) as examples of antigen processing and presentation gene expression found in chicken thrombocytes. Transcriptomic results from our other study [66] revealed both MHC I and II expression in chicken thrombocytes that included the MHC I processing/presenting molecules calnexin (CANX), calrecticulin (CALR), and transporter 1 and 2 (TAP1 and 2), and the MHC II processing/presenting molecules MHC II beta chain (BLB2), MHC II DM alpha chain (DMA), and invariant chain of CLIP (CD74). Therefore, foreign proteins can be digested through the proteasome and the resulting antigenic peptides can be bound to the MHC I molecule prior to transport to the cell surface via the Golgi apparatus. Likewise, MHC II produced in the endoplasmic reticulum relocates to the Golgi apparatus and then moves to an endosome to digest the invariant chain of CLIP. This is then followed by CLIP removal and antigen placement in the peptide binding groove with the assistance of DM. This then expands the role of thrombocytes beyond a unique cell to an accessory cell. It not only responds to foreign antigens via a release of soluble factors but also interacts with other immune cells to assist in the orchestration of crucial aspects of immunity [23,25,33,35,51,76].
Equally interesting is the gene expression of MHC II in carp thrombocytes [23,35], lending these cells a potential role in the induction of humoral immune responses like a professional APC. Carp thrombocytes act as phagocytic cells [51,75] and express IL-1β and MHC II [51]. It is apparent from gene expression in chicken thrombocytes that MHC II proteins could be produced and participate in antigen processing per the mechanisms of foreign peptide sequence trafficking in our model (Figure 3). As stated above, Table S2 [33] also provides a list of MHC II gene expressions associated with antigen processing and presentation (e.g., DM). Various roles for chicken thrombocytes in adaptive immunity have been covered by Astill et al. [84], who characterized the avian thrombocyte as a central cell in the immune system. Considering the full range of functions identified for avian and fish thrombocytes, it is apropos to consider this cell as a major component of innate and adaptive immunities in a context greater than blood coagulation and phagocytosis. Indeed, the array of thrombocyte cytokines, chemokines, APC molecules, and co-stimulatory ligands/receptors places it firmly among important immune cells.
The reactivity and subsequent responses to foreign organisms have been mentioned previously in this article. The responses have a typical innate-type component leading to phagocytosis with antigen degradation and the production/release of immune factors (e.g., cytokines and chemokines). The role of these cells in assisting/enhancing DC, macrophages, B cells, and T cells is unquestioned, and their capacity to engage in APC function cannot be denied when several reported studies have revealed strong evidence for antigen processing and presentation. Clearly, the argument for this status is convincing for avian thrombocytes (left side of Figure 1, and Figure 3). Though less documented for the ichthyoid thrombocyte, there is more than sufficient evidence for APC functionality in these non-mammalian species since MHC I and II molecules are present as well as molecules for the associated processing mechanisms. Non-mammalian thrombocytes have MHC I and II molecules on the cell membranes along with co-stimulatory markers CD40, CD40L, CD80, and CD86 on avian thrombocytes. Intracellularly, ichthyoid thrombocytes express LMP and TAP1/2 of MHC I antigen processing [35]. Taking this together with functional evidence for the degradation of foreign proteins strengthens the case for including thrombocytes among other antigen-processing and -presenting cell types.
7. Final Thoughts
To fully understand the role of thrombocytes in antigen presentation and interaction with other lymphocytes and APCs, further investigations by many other immunologists are needed. Defining the role of these cells in immune responses will be useful for designing effective vaccines and prophylactics for economically important agriculture species such as poultry and fish. In this new era of mRNA vaccines and our growing knowledge of the cellular activity of the thrombocyte, an improved approach to designing effective vaccines can be undertaken. We are gaining a deeper understanding of the mechanisms employed by thrombocytes as vital participants in both innate and adaptive immunity. In addition to the importance of these cells in blood coagulation, we now have evidence of thrombocyte participation in the activation of sustained immunity for long-term protection.
Author Contributions
Conceptualization, F.F. and T.S.; writing—original draft preparation, F.F. and T.S.; writing—review and editing, F.F. and T.S.; visualization, F.F. and T.S.; supervision, F.F. and T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Acknowledgments
We would like to acknowledge the Department of Biological Sciences and the J Murrey Atkins Library of University of North Carolina at Charlotte, and the Department of Animal and Veterinary Sciences of Clemson University for the partial contribution towards the article processing fee for this manuscript publication.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lucas, C. Atlas of avian hematology. In Agriculture Monograph; US Department of Agriculture: Washington, DC, USA, 1961. [Google Scholar]
- Levin, J. 1—The Evolution of Mammalian Platelets. In Platelets, 4th ed.; Michelson, A.D., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–23. [Google Scholar]
- Loof, T.G.; Schmidt, O.; Herwald, H.; Theopold, U. Coagulation Systems of Invertebrates and Vertebrates and Their Roles in Innate Immunity: The Same Side of Two Coins? J. Innate Immun. 2010, 3, 34–40. [Google Scholar] [CrossRef]
- Chang, C.F.; Hamilton, P.B. The thrombocyte as the primary circulating phagocyte in chickens. J. Reticuloendothel. Soc. 1979, 25, 585–590. [Google Scholar]
- Vázquez, G.R.; Guerrero, G. Characterization of blood cells and hematological parameters in Cichlasoma dimerus (Teleostei, Perciformes). Tissue Cell 2007, 39, 151–160. [Google Scholar] [CrossRef]
- Ueda, I.K.; Egami, M.I.; Sassp, W.D.S.; Matushima, E.R. Estudos hematológicos em Oreochromis niloticus (Linnaeus, 1758) (Cichlidae, Teleostei)—Parte I. Braz. J. Vet. Res. Anim. Sci. 1997, 34, 270. [Google Scholar] [CrossRef][Green Version]
- Patel, S.R.; Hartwig, J.H.; Italiano, J.E. The biogenesis of platelets from megakaryocyte proplatelets. J. Clin. Investig. 2005, 115, 3348–3354. [Google Scholar] [CrossRef]
- McNagny, K.M.; Pettersson, I.; Rossi, F.; Flamme, I.; Shevchenko, A.; Mann, M.; Graf, T. Thrombomucin, a Novel Cell Surface Protein that Defines Thrombocytes and Multipotent Hematopoietic Progenitors. J. Cell Biol. 1997, 138, 1395–1407. [Google Scholar] [CrossRef]
- Esteban, M.A.; Meseguer, J.; Ayala, A.G.; Agulleiro, B. Erythropoiesis and thrombopoiesis in the head-kidney of the sea bass (Dicentrarchus labrax L.): An ultrastructural study. Arch. Histol. Cytol. 1989, 52, 407–419. [Google Scholar] [CrossRef]
- Zapata, A.; Gomariz, R.P.; Garrido, E.; Cooper, E.L. Lymphoid Organs and Blood Cells of the Caecilian Ichthyophis kohtaoensis. Acta Zool. 1982, 63, 11–16. [Google Scholar] [CrossRef]
- Jagadeeswaran, P.; Liu, Y.C.; Sheehan, J.P. Chapter 18 Analysis of Hemostasis in the Zebrafish. In Methods in Cell Biology; Detrich, H.W., Westerfield, M., Zon, L.I., Eds.; Academic Press: Cambridge, MA, USA, 1998; Volume 59, pp. 337–357. [Google Scholar]
- Daimon, T.; Mizuhira, V.; Takahashi, I.; Uchida, K. The surface connected canalicular system of carp (Cyprinus carpio) thrombocytes: Its fine structure and three-dimensional architecture. Cell Tissue Res. 1979, 203, 355–365. [Google Scholar] [CrossRef]
- Sweeny, P.R.; Carlson, H.C. Electron Microscopy and Histochemical Demonstration of Lysosomal Structures in Chicken Thrombocytes. Avian Dis. 1968, 12, 636–644. [Google Scholar] [CrossRef]
- Esteban, M.A.; Muñoz, J.; Meseguer, J. Blood cells of sea bass (Dicentrarchus labrax L.). Flow cytometric and microscopic studies. Anat Rec. 2000, 258, 80–89. [Google Scholar] [CrossRef]
- Khandekar, G.; Kim, S.; Jagadeeswaran, P. Zebrafish Thrombocytes: Functions and Origins. Adv. Hematol. 2012, 2012, 857058. [Google Scholar] [CrossRef]
- Scott, T.; Owens, M.D. Thrombocytes respond to lipopolysaccharide through Toll-like receptor-4, and MAP kinase and NF-κB pathways leading to expression of interleukin-6 and cyclooxygenase-2 with production of prostaglandin E2. Mol. Immunol. 2008, 45, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, F.; Scott, T. A comparative examination of thrombocyte/platelet immunity. Immunol. Lett. 2015, 163, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, F.; Maurice, D.; Scott, T. Broiler Chick Thrombocyte Response to Lipopolysaccharide. Poult. Sci. 2008, 87, 61–63. [Google Scholar] [CrossRef]
- Ferdous, F. The Avian Thrombocyte Is a Specialized Immune Cell. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2014. [Google Scholar]
- Winkler, C.; Ferdous, F.; Dimmick, M.; Scott, T. Lipopolysaccharide induced Interleukin-6 production is mediated through activation of ERK 1/2, p38 MAPK, MEK, and NFκB in chicken thrombocytes. Dev. Comp. Immunol. 2017, 73, 124–130. [Google Scholar] [CrossRef]
- Köllner, B.; Fischer, U.; Rombout, J.; Taverne-Thiele, J.; Hansen, J. Potential involvement of rainbow trout thrombocytes in immune functions: A study using a panel of monoclonal antibodies and RT-PCR. Dev. Comp. Immunol. 2004, 28, 1049–1062. [Google Scholar] [CrossRef]
- He, Y.; Zhu, W.; Xu, T.; Liao, Z.; Su, J. Identification and immune responses of thrombocytes in bacterial and viral infections in grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2022, 123, 314–323. [Google Scholar] [CrossRef]
- Fink, I.R.; Ribeiro, C.M.; Forlenza, M.; Taverne-Thiele, A.; Rombout, J.H.; Savelkoul, H.F.; Wiegertjes, G.F. Immune-relevant thrombocytes of common carp undergo parasite-induced nitric oxide-mediated apoptosis. Dev. Comp. Immunol. 2015, 50, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Carlson, H.C.; Sweeny, P.R.; Tokaryk, J.M. Demonstration of Phagocytic and Trephocytic Activities of Chicken Thrombocytes by Microscopy and Vital Staining Techniques. Avian Dis. 1968, 12, 700–715. [Google Scholar] [CrossRef]
- Paul, M.S.; Paolucci, S.; Barjesteh, N.; Wood, R.D.; Schat, K.A.; Sharif, S. Characterization of Chicken Thrombocyte Responses to Toll-Like Receptor Ligands. PLoS ONE 2012, 7, e43381. [Google Scholar] [CrossRef]
- Iqbal, M.; Philbin, V.J.; Smith, A.L. Expression patterns of chicken Toll-like receptor mRNA in tissues, immune cell subsets and cell lines. Vet. Immunol. Immunopathol. 2005, 104, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Pietretti, D.; Spaink, H.P.; Falco, A.; Forlenza, M.; Wiegertjes, G.F. Accessory molecules for Toll-like receptors in Teleost fish. Identification of TLR4 interactor with leucine-rich repeats (TRIL). Mol. Immunol. 2013, 56, 745–756. [Google Scholar] [CrossRef]
- Keestra, A.M.; de Zoete, M.R.; Bouwman, L.I.; Vaezirad, M.M.; van Putten, J.P.M. Unique features of chicken Toll-like receptors. Dev. Comp. Immunol. 2013, 41, 316–323. [Google Scholar] [CrossRef]
- Sahoo, B.R. Structure of fish Toll-like receptors (TLR) and NOD-like receptors (NLR). Int. J. Biol. Macromol. 2020, 161, 1602–1617. [Google Scholar] [CrossRef]
- Rehman, M.S.-U.; Rehman, S.U.; Yousaf, W.; Hassan, F.-U.; Ahmad, W.; Liu, Q.; Pan, H. The Potential of Toll-Like Receptors to Modulate Avian Immune System: Exploring the Effects of Genetic Variants and Phytonutrients. Front. Genet. 2021, 12, 671235. [Google Scholar] [CrossRef]
- Pietretti, D.; Scheer, M.; Fink, I.R.; Taverne, N.; Savelkoul, H.F.J.; Spaink, H.P.; Forlenza, M.; Wiegertjes, G.F. Identification and functional characterization of nonmammalian Toll-like receptor 20. Immunogenetics 2014, 66, 123–141. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, X.; Zhou, C.; Li, L.; Nie, G.; Li, X. Toll-like receptor recognition of bacteria in fish: Ligand specificity and signal pathways. Fish Shellfish Immunol. 2014, 41, 380–388. [Google Scholar] [CrossRef]
- Ferdous, F.; Saski, C.; Bridges, W.; Burns, M.; Dunn, H.; Elliott, K.; Scott, T.R. Transcriptome Profile of the Chicken Thrombocyte: New Implications as an Advanced Immune Effector Cell. PLoS ONE 2016, 11, e0163890. [Google Scholar] [CrossRef]
- Neulen, M.-L.; Göbel, T.W. Identification of a chicken CLEC-2 homologue, an activating C-type lectin expressed by thrombocytes. Immunogenetics 2012, 64, 389–397. [Google Scholar] [CrossRef]
- Stosik, M.; Tokarz-Deptuła, B.; Deptuła, W. Characterisation of thrombocytes in Osteichthyes. J. Vet. Res. 2019, 63, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Nieswandt, B.; Varga-Szabo, D.; Elvers, M. Integrins in platelet activation. J. Thromb. Haemost. 2009, 7 (Suppl. S1), 206–209. [Google Scholar] [CrossRef] [PubMed]
- Semple, J.W.; Kapur, R. Platelet immunology from the inside out. ISBT Sci. Ser. 2020, 15, 315–319. [Google Scholar] [CrossRef]
- Stolla, M.C.; Leyens, K.; Catherman, S.C.; McGrath, K.E.; Palis, J. P-Selectin Expression and Platelet Function Are Developmentally Regulated. Blood 2014, 124, 1439. [Google Scholar] [CrossRef]
- Merten, M.; Thiagarajan, P. P-Selectin Expression on Platelets Determines Size and Stability of Platelet Aggregates. Circulation 2000, 102, 1931–1936. [Google Scholar] [CrossRef]
- Straub, C.; Neulen, M.-L.; Viertlboeck, B.C.; Göbel, T.W. Chicken SLAMF4 (CD244, 2B4), a receptor expressed on thrombocytes, monocytes, NK cells, and subsets of αβ-, γδ-T cells and B cells binds to SLAMF2. Dev. Comp. Immunol. 2014, 42, 159–168. [Google Scholar] [CrossRef]
- Viertlboeck, B.C.; Hanczaruk, M.A.; Amann, B.; Bader, S.R.; Schmitt, R.; Sperling, B.; Schwarz, S.C.; Schmahl, W.; Deeg, C.A.; Göbel, T.W. Chicken immunoregulatory Ig-like receptor families: An overview and expression details on ggTREM-A1. Dev. Comp. Immunol. 2013, 41, 403–412. [Google Scholar] [CrossRef]
- Turowski, V.; Sperling, B.; Hanczaruk, M.A.; Göbel, T.W.; Viertlboeck, B.C. Chicken TREM-B1, an Inhibitory Ig-Like Receptor Expressed on Chicken Thrombocytes. PLoS ONE 2016, 11, e0151513. [Google Scholar] [CrossRef]
- Peters, M.J.; Dixon, G.; Kotowicz, K.T.; Hatch, D.J.; Heyderman, R.S.; Klein, N.J. Circulating platelet-neutrophil complexes represent a subpopulation of activated neutrophils primed for adhesion, phagocytosis and intracellular killing. Br. J. Haematol. 1999, 106, 391–399. [Google Scholar] [CrossRef]
- Zarbock, A.; Polanowska-Grabowska, R.K.; Ley, K. Platelet-neutrophil-interactions: Linking hemostasis and inflammation. Blood Rev. 2007, 21, 99–111. [Google Scholar] [CrossRef]
- Hitchcock, C. ERK1/2 and p38 MAPK Pathways Are Both Involved in the Expression of Interleukin-6 and Cyclooxygenase-2 in Thrombocytes Stimulated Withlipopolysaccharide. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2009. [Google Scholar]
- Lam, K. The macrophage inflammatory protein-1β in the supernatants of Mycoplasma gallisepticum-infected chicken leukocytes attracts the migration of chicken heterophils and lymphocytes. Dev. Comp. Immunol. 2002, 26, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, F.; Scott, T. Bacterial and viral induction of chicken thrombocyte inflammatory responses. Dev. Comp. Immunol. 2015, 49, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, H.; Inoue, T.; Furusawa, S.; Matsuda, H. Characterization and expression of three forms of cDNA encoding chicken platelet-derived growth factor-A chain. Gene 2001, 272, 181–190. [Google Scholar] [CrossRef]
- Horiuchi, H.; Inoue, T.; Furusawa, S.; Matsuda, H. Cloning and characterization of a chicken platelet-derived growth factor B-chain cDNA. Dev. Comp. Immunol. 2002, 26, 73–83. [Google Scholar] [CrossRef]
- Horiuchi, H.; Matsuda, H.; Murata, M. Preliminary evidence of growth factor(s) from chicken thrombocytes. Growth effects on chicken embryo fibroblasts culture. Jpn. J. Vet. Sci. 1990, 52, 559–565. [Google Scholar] [CrossRef]
- Nagasawa, T.; Nakayasu, C.; Rieger, A.M.; Barreda, D.R.; Somamoto, T.; Nakao, M. Phagocytosis by Thrombocytes is a Conserved Innate Immune Mechanism in Lower Vertebrates. Front. Immunol. 2014, 5, 445. [Google Scholar] [CrossRef]
- Roy, S.; Kumar, V.; Behera, B. Acute Phase Proteins and their Potential Role as an Indicator for Fish Health and in Diagnosis of Fish Diseases. Protein Pept. Lett. 2017, 24, 78–89. [Google Scholar] [CrossRef]
- Schat, K.A.; Bingham, J.; Butler, J.M.; Chen, L.-M.; Lowther, S.; Crowley, T.M.; Moore, R.J.; Donis, R.O.; Lowenthal, J.W. Role of Position 627 of PB2 and the Multibasic Cleavage Site of the Hemagglutinin in the Virulence of H5N1 Avian Influenza Virus in Chickens and Ducks. PLoS ONE 2012, 7, e30960. [Google Scholar] [CrossRef]
- Sterz, I.; Weiss, E. Electron microscopical and virological studies of chicken thrombocytesin vitro infected with fowl plague virus (FPV). Med. Microbiol. Immunol. 1974, 159, 151–160. [Google Scholar] [CrossRef]
- Lam, K.M. Activation, adhesion, migration and death of chicken thrombocytes. Comp. Clin. Pathol. 1997, 7, 81–87. [Google Scholar] [CrossRef]
- Hottz, E.D.; Bozza, F.A.; Bozza, P.T. Platelets in Immune Response to Virus and Immunopathology of Viral Infections. Front. Med. 2018, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Chabert, A.; Hamzeh-Cognasse, H.; Pozzetto, B.; Cognasse, F.; Schattner, M.; Gomez, R.M.; Garraud, O. Human platelets and their capacity of binding viruses: Meaning and challenges? BMC Immunol. 2015, 16, 26. [Google Scholar] [CrossRef] [PubMed]
- Assinger, A. Platelets and Infection—An Emerging Role of Platelets in Viral Infection. Front. Immunol. 2014, 5, 649. [Google Scholar] [CrossRef] [PubMed]
- Youssefian, T.; Drouin, A.; Massé, J.-M.; Guichard, J.; Cramer, E.M. Host defense role of platelets: Engulfment of HIV andStaphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood J. Am. Soc. Hematol. 2002, 99, 4021–4029. [Google Scholar] [CrossRef]
- Simon, A.Y.; Sutherland, M.R.; Pryzdial, E.L.G. Dengue virus binding and replication by platelets. Blood 2015, 126, 378–385. [Google Scholar] [CrossRef]
- Zucker-Franklin, D.; Seremetis, S.; Zheng, Z.Y. Internalization of human immunodeficiency virus type I and other retroviruses by megakaryocytes and platelets. Blood 1990, 75, 1920–1923. [Google Scholar] [CrossRef]
- Lê, V.B.; Schneider, J.G.; Boergeling, Y.; Berri, F.; Ducatez, M.; Guerin, J.-L.; Adrian, I.; Errazuriz-Cerda, E.; Frasquilho, S.; Antunes, L.; et al. Platelet Activation and Aggregation Promote Lung Inflammation and Influenza Virus Pathogenesis. Am. J. Respir. Crit. Care Med. 2015, 191, 804–819. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, J.; Xu, R.; Zhang, C.; Pang, Q.; Chen, X.; Liu, S.; Hong, L.; Yuan, J.; Li, X.; et al. The Gametocytes of Leucocytozoon sabrazesi Infect Chicken Thrombocytes, Not Other Blood Cells. PLoS ONE 2015, 10, e0133478. [Google Scholar] [CrossRef]
- de Macchi, B.M.; Miranda, F.J.B.; de Souza, F.S.; de Carvalho, E.C.Q.; Albernaz, A.P.; Nascimento, J.L.M.D.; DaMatta, R.A. Chickens treated with a nitric oxide inhibitor became more resistant to Plasmodium gallinaceum infection due to reduced anemia, thrombocytopenia and inflammation. Vet. Res. 2013, 44, 8. [Google Scholar] [CrossRef]
- Kho, S.; Barber, B.E.; Johar, E.; Andries, B.; Poespoprodjo, J.R.; Kenangalem, E.; Piera, K.A.; Ehmann, A.; Price, R.N.; William, T.; et al. Platelets kill circulating parasites of all major Plasmodium species in human malaria. Blood 2018, 132, 1332–1344. [Google Scholar] [CrossRef]
- Passantino, L.; Cianciotta, A.; Patruno, R.; Ribaud, M.R.; Jirillo, E.; Passantino, G.F. Do Fish Thrombocytes Play an Immunological Role? Their Cytoenzymatic Profiles and Function During an Accidental Piscine Candidiasis in Aquarium. Immunopharmacol. Immunotoxicol. 2005, 27, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Aslam, R.; Speck, E.R.; Kim, M.; Crow, A.R.; Bang, K.W.A.; Nestel, F.P.; Ni, H.; Lazarus, A.H.; Freedman, J.; Semple, J.W. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-α production in vivo. Blood 2006, 107, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Puentes, S.M.; Norman, D.C.; Bayer, A.S. Partial characterization and staphylocidal activity of thrombin-induced platelet microbicidal protein. Infect. Immun. 1992, 60, 1202–1209. [Google Scholar] [CrossRef]
- Yeaman, M.R. The Role of Platelets in Antimicrobial Host Defense. Clin. Infect. Dis. 1997, 25, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Varghese, S.; Vitseva, O.; Tanriverdi, K.; Freedman, J.E. CD40 Ligand Influences Platelet Release of Reactive Oxygen Intermediates. Arter. Thromb. Vasc. Biol. 2005, 25, 2428–2434. [Google Scholar] [CrossRef]
- Dankert, J.; van der Werff, J.; Zaat, S.A.; Joldersma, W.; Klein, D.; Hess, J. Involvement of bactericidal factors from thrombin-stimulated platelets in clearance of adherent viridans streptococci in experimental infective endocarditis. Infect. Immun. 1995, 63, 663–671. [Google Scholar] [CrossRef]
- Glick, B.; Sato, K.; Cohenour, F. Comparison of the phagocytic ability of normal and bursectomized birds. J. Reticuloendothel. Soc. 1964, 1, 442–449. [Google Scholar]
- Wigley, P.; Hulme, S.D.; Barrow, P.A. Phagocytic and oxidative burst activity of chicken thrombocytes to Salmonella, Escherichia coli and other bacteria. Avian Pathol. 1999, 28, 567–572. [Google Scholar] [CrossRef]
- Nagasawa, T.; Somamoto, T.; Nakao, M. Carp thrombocyte phagocytosis requires activation factors secreted from other leukocytes. Dev. Comp. Immunol. 2015, 52, 107–111. [Google Scholar] [CrossRef]
- Stosik, M.; Deptuła, W.; Trávniček, M.; Baldy-Chudzik, K. Phagocytic and bactericidal activity of blood thrombocytes in carps (Cyprinus carpio). Veterinární Medicína 2002, 47, 21–25. [Google Scholar] [CrossRef]
- Ferdous, F.; Saski, C.; Bridges, W.; Burns, M.; Dunn, H.; Elliott, K.; Scott, T.R. Bacterial and Viral Products Affect Differential Pattern Recognition Receptor Activation of Chicken Thrombocytes Evidenced through RNA Sequencing. J. Immunol. 2017, 199, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R.; Ibrahim, A.S.; Edwards, J.E.; Bayer, A.S.; A Ghannoum, M. Thrombin-induced rabbit platelet microbicidal protein is fungicidal in vitro. Antimicrob. Agents Chemother. 1993, 37, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.A.; Wuescher, L.M.; Worth, R.G. Platelets: Essential components of the immune system. Curr. Trends Immunol. 2015, 16, 65–78. [Google Scholar] [PubMed]
- Kapur, R.; Semple, J.W. Platelet immunobiology: Platelets as prey and predator. ISBT Sci. Ser. 2017, 13, 87–92. [Google Scholar] [CrossRef]
- Nishat, S.; Wuescher, L.M.; Worth, R.G. Platelets Enhance Dendritic Cell Responses against Staphylococcus aureus through CD40-CD40L. Infect. Immun. 2018, 86, 10–1128. [Google Scholar] [CrossRef]
- Gautam, I.; Storad, Z.; Filipiak, L.; Huss, C.; Meikle, C.K.; Worth, R.G.; Wuescher, L.M. From Classical to Unconventional: The Immune Receptors Facilitating Platelet Responses to Infection and Inflammation. Biology 2020, 9, 343. [Google Scholar] [CrossRef]
- Gautam, I.; Huss, C.W.; Storad, Z.A.; Krebs, M.; Bassiouni, O.; Ramesh, R.; Wuescher, L.M.; Worth, R.G. Activated Platelets Mediate Monocyte Killing of Klebsiella pneumoniae. Infect. Immun. 2023, 91, e0055622. [Google Scholar] [CrossRef]
- Koupenova, M.; Livada, A.C.; Morrell, C.N. Platelet and Megakaryocyte Roles in Innate and Adaptive Immunity. Circ. Res. 2022, 130, 288–308. [Google Scholar] [CrossRef]
- Astill, J.; Wood, R.D.; Sharif, S. Chapter 8.3—Thrombocyte functions in the avian immune system. In Avian Immunology, 3rd ed.; Kaspers, B., Schat, K.A., Göbel, T.W., Vervelde, L., Eds.; Academic Press: Boston, MA, USA, 2022; pp. 205–212. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).