An Overview of Lutein in the Lipid Membrane
Abstract
1. Introduction
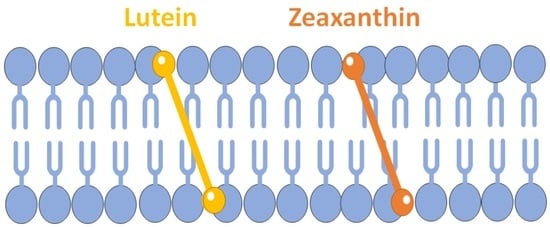
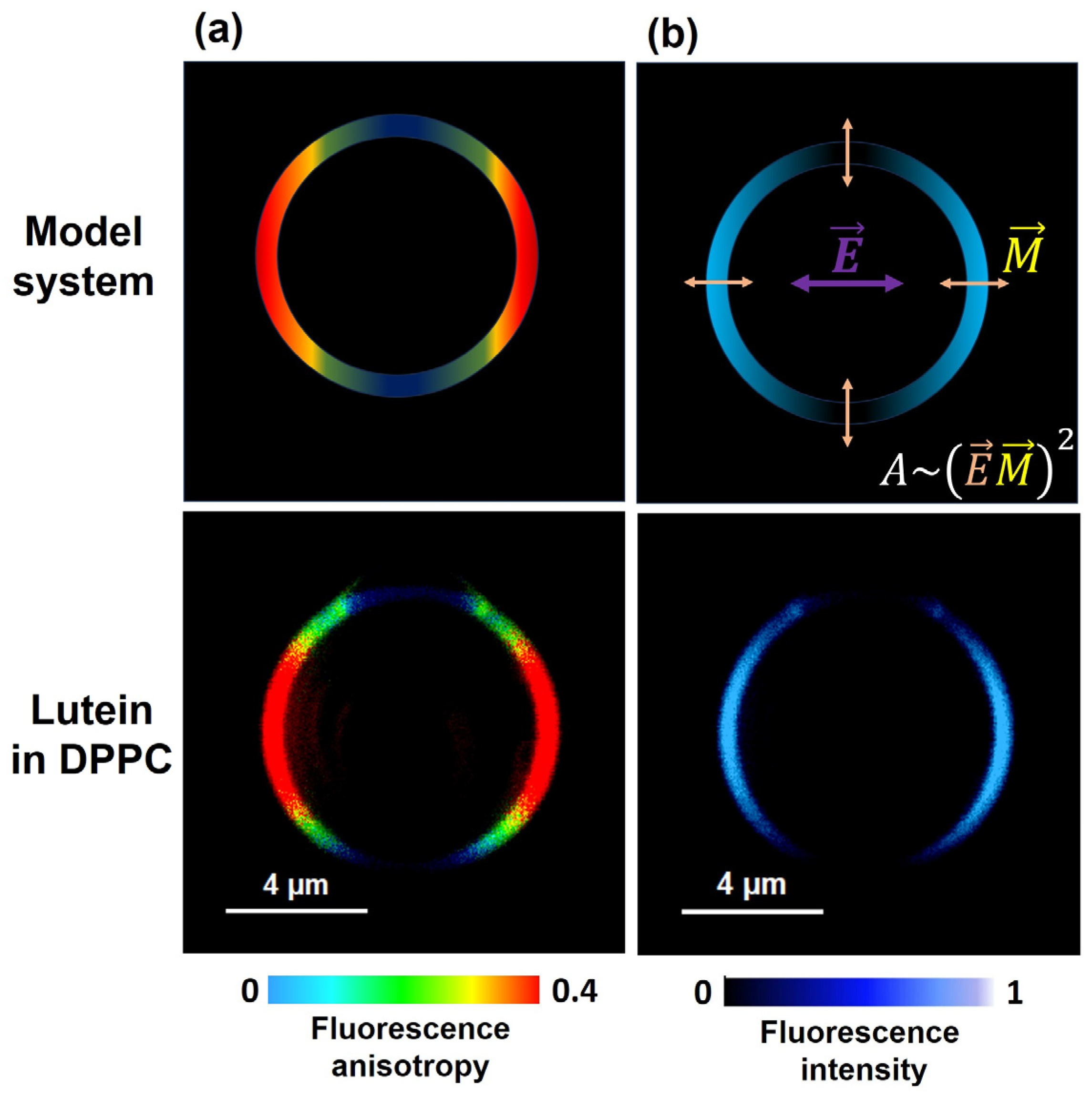
2. Horizontal or Vertical Orientation of Lut in the Lipid Bilayer?
3. Lutein as Modifier of Physical Properties of the Lipid Bilayers
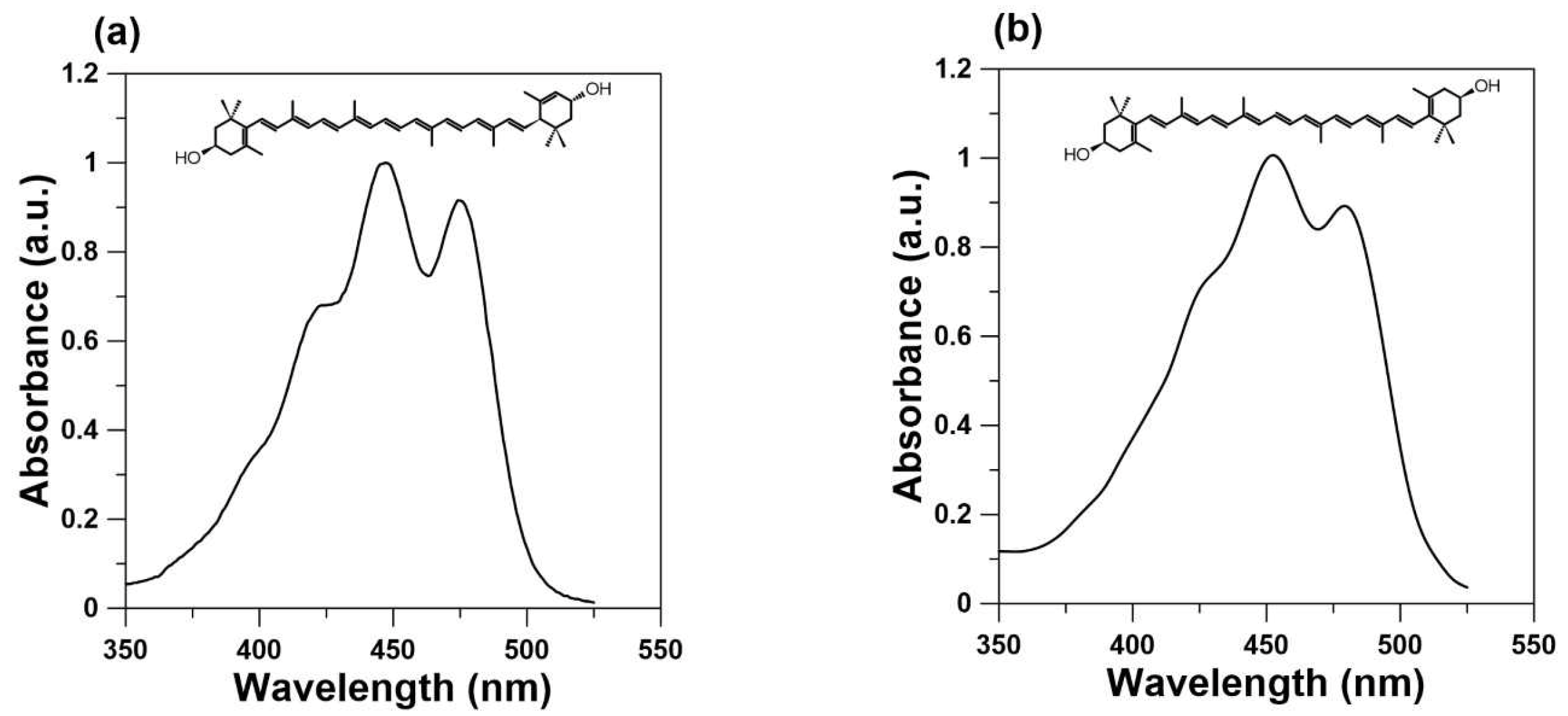
4. Lutein as a Blue Light Filter
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bone, R.A.; Landrum, J.T.; Hime, G.W.; Cains, A.; Zamor, J. Stereochemistry of the human macular carotenoids. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2033–2040. [Google Scholar]
- Bone, R.A.; Landrum, J.T.; Fernandez, L.; Tarsis, S.L. Analysis of the macular pigment by HPLC: Retinal distribution and age study. Investig. Ophthalmol. Vis. Sci. 1988, 29, 843–849. [Google Scholar]
- Arunkumar, R.; Gorusupudi, A.; Bernstein, P.S. The macular carotenoids: A biochemical overview. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158617. [Google Scholar] [CrossRef] [PubMed]
- Murillo, A.G.; Hu, S.; Fernandez, M.L. Zeaxanthin: Metabolism, Properties, and Antioxidant Protection of Eyes, Heart, Liver, and Skin. Antioxidants 2019, 8, 390. [Google Scholar] [CrossRef] [PubMed]
- Shyam, R.; Gorusupudi, A.; Nelson, K.; Horvath, M.P.; Bernstein, P.S. RPE65 has an additional function as the lutein to meso-zeaxanthin isomerase in the vertebrate eye. Proc. Natl. Acad. Sci. USA 2017, 114, 10882–10887. [Google Scholar] [CrossRef]
- Li, B.; George, E.W.; Rognon, G.T.; Gorusupudi, A.; Ranganathan, A.; Chang, F.Y.; Shi, L.; Frederick, J.M.; Bernstein, P.S. Imaging lutein and zeaxanthin in the human retina with confocal resonance Raman microscopy. Proc. Natl. Acad. Sci. USA 2020, 117, 12352–12358. [Google Scholar] [CrossRef]
- Beatty, S.; Boulton, M.; Henson, D.; Koh, H.H.; Murray, I.J. Macular pigment and age related macular degeneration. Br. J. Ophthalmol. 1999, 83, 867–877. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Arunkumar, R. The emerging roles of the macular pigment carotenoids throughout the lifespan and in prenatal supplementation. J. Lipid Res. 2021, 62, 100038. [Google Scholar] [CrossRef]
- Gass, J.D. Müller cell cone, an overlooked part of the anatomy of the fovea centralis: Hypotheses concerning its role in the pathogenesis of macular hole and foveomacualr retinoschisis. Arch. Ophthalmol. 1999, 117, 821–823. [Google Scholar] [CrossRef]
- Rapp, L.M.; Maple, S.S.; Choi, J.H. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1200–1209. [Google Scholar]
- Sommerburg, O.G.; Siems, W.G.; Hurst, J.S.; Lewis, J.W.; Kliger, D.S.; van Kuijk, F.J. Lutein and zeaxanthin are associated with photoreceptors in the human retina. Curr. Eye Res. 1999, 19, 491–495. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Friedes, L.M.; Gomez, C.M.; Kilburn, M.D.; Menendez, E.; Vidal, I.; Wang, W. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp. Eye Res. 1997, 64, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, R.; Kuchan, M.J.; Sen, S.; Johnson, E.J. Lutein and preterm infants with decreased concentrations of brain carotenoids. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J.; Vishwanathan, R.; Johnson, M.A.; Hausman, D.B.; Davey, A.; Scott, T.M.; Green, R.C.; Miller, L.S.; Gearing, M.; Woodard, J.; et al. Relationship between Serum and Brain Carotenoids, α-Tocopherol, and Retinol Concentrations and Cognitive Performance in the Oldest Old from the Georgia Centenarian Study. J. Aging Res. 2013, 2013, 951786. [Google Scholar] [CrossRef] [PubMed]
- Craft, N.E.; Haitema, T.B.; Garnett, K.M.; Fitch, K.A.; Dorey, C.K. Carotenoid, tocopherol, and retinol concentrations in elderly human brain. J. Nutr. Health Aging 2004, 8, 156–162. [Google Scholar]
- Widomska, J.; SanGiovanni, J.P.; Subczynski, W.K. Why is Zeaxanthin the Most Concentrated Xanthophyll in the Central Fovea? Nutrients 2020, 12, 1333. [Google Scholar] [CrossRef]
- Bhosale, P.; Larson, A.J.; Frederick, J.M.; Southwick, K.; Thulin, C.D.; Bernstein, P.S. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J. Biol. Chem. 2004, 279, 49447–49454. [Google Scholar] [CrossRef]
- Li, B.; Vachali, P.; Frederick, J.M.; Bernstein, P.S. Identification of StARD3 as a lutein-binding protein in the macula of the primate retina. Biochemistry 2011, 50, 2541–2549. [Google Scholar] [CrossRef]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2005, 1740, 108–115. [Google Scholar] [CrossRef]
- Gruszecki, W.I.; Sielewiesiuk, J. Orientation of xanthophylls in phosphatidylcholine multibilayers. Biochim. Biophys. Acta 1990, 1023, 405–412. [Google Scholar] [CrossRef]
- Sujak, A.; Gabrielska, J.; Grudziński, W.; Borc, R.; Mazurek, P.; Gruszecki, W.I. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: The structural aspects. Arch. Biochem. Biophys. 1999, 371, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Makuch, K.; Hryc, J.; Markiewicz, M.; Pasenkiewicz-Gierula, M. Lutein and Zeaxanthin in the Lipid Bilayer-Similarities and Differences Revealed by Computational Studies. Front. Mol. Biosci. 2021, 8, 768449. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I. Possible Biologic Mechanisms for a Protective Role of Xanthophylls. J. Nutr. 2002, 132, 540S–542S. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Markowska, E.; Sielewiesiuk, J. Spin-label studies on phosphatidylcholine-polar carotenoid membranes: Effects of alkyl-chain length and unsaturation. Biochim. Biophys. Acta 1993, 1150, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska, A.; Subczynski, W.K. Effects of polar carotenoids on the shape of the hydrophobic barrier of phospholipid bilayers. Biochim. Biophys. Acta 1998, 1368, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska, A.; Widomska, J.; Subczynski, W.K. Carotenoid-membrane interactions in liposomes: Effect of dipolar, monopolar, and nonpolar carotenoids. Acta Biochim. Pol. 2006, 53, 475–484. [Google Scholar] [CrossRef]
- Pasenkiewicz-Gierula, M.; Baczyński, K.; Murzyn, K.; Markiewicz, M. Orientation of lutein in a lipid bilayer: Revisited. Acta Biochim. Pol. 2012, 59, 115–118. [Google Scholar] [CrossRef]
- Makuch, K.; Markiewicz, M.; Pasenkiewicz-Gierula, M. Asymmetric Spontaneous Intercalation of Lutein into a Phospholipid Bilayer, a Computational Study. Comput. Struct. Biotechnol. J. 2019, 17, 516–526. [Google Scholar] [CrossRef]
- Grudzinski, W.; Nierzwicki, L.; Welc, R.; Reszczynska, E.; Luchowski, R.; Czub, J.; Gruszecki, W.I. Localization and Orientation of Xanthophylls in a Lipid Bilayer. Sci. Rep. 2017, 7, 9619. [Google Scholar] [CrossRef]
- Grudzinski, W.; Sagan, J.; Welc, R.; Luchowski, R.; Gruszecki, W.I. Molecular organization, localization and orientation of antifungal antibiotic amphotericin B in a single lipid bilayer. Sci. Rep. 2016, 6, 32780. [Google Scholar] [CrossRef]
- Lakowicz, J.R. (Ed.) Fluorescence Anisotropy. In Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; pp. 353–382. [Google Scholar] [CrossRef]
- Birge, R.R.; Zgierski, M.Z.; Serrano-Andres, L.; Hudson, B.S. Transition Dipole Orientation of Linear Polyenes: Semiempirical Models and Extrapolation to the Infinite Chain Limit. J. Phys. Chem. A 1999, 103, 2251–2255. [Google Scholar] [CrossRef]
- Luchowski, R.; Grudzinski, W.; Welc, R.; Mendes Pinto, M.M.; Sek, A.; Ostrowski, J.; Nierzwicki, L.; Chodnicki, P.; Wieczor, M.; Sowinski, K.; et al. Light-Modulated Sunscreen Mechanism in the Retina of the Human Eye. J. Phys. Chem. B 2021, 125, 6090–6102. [Google Scholar] [CrossRef] [PubMed]
- Subczynski, W.K.; Widomska, J.; Raguz, M.; Pasenkiewicz-Gierula, M. Molecular oxygen as a probe molecule in EPR spin-labeling studies of membrane structure and dynamics. Oxygen 2022, 2, 295–316. [Google Scholar] [CrossRef] [PubMed]
- Subczynski, W.; Widomska, J. EPR Spin Labeling in Carotenoid-Membrane Interaction. In Carotenoids: Physical, Chemical, and Biological Function and Properties; Landrum, J.T., Ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Subczynski, W.K.; Raguz, M.; Widomska, J. Studying Lipid Organization in Biological Membranes Using Liposomes and EPR Spin Labeling. In Liposomes: Methods and Protocols, Volume 2: Biological Membrane Models; Weissig, V., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 247–269. [Google Scholar] [CrossRef]
- Yin, J.J.; Subczynski, W.K. Effects of lutein and cholesterol on alkyl chain bending in lipid bilayers: A pulse electron spin resonance spin labeling study. Biophys. J. 1996, 71, 832–839. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Mainali, L.; Camenisch, T.G.; Froncisz, W.; Hyde, J.S. Spin-label oximetry at Q- and W-band. J. Magn. Reson. 2011, 209, 142–148. [Google Scholar] [CrossRef][Green Version]
- Hyde, J.S.; Subczynski, W.K. Spin-Label Oximetry. In Spin Labeling: Theory and Applications; Berliner, L.J., Reuben, J., Eds.; Springer: Boston, MA, USA, 1989; pp. 399–425. [Google Scholar]
- Subczynski, W.K.; Widomska, J.; Feix, J.B. Physical properties of lipid bilayers from EPR spin labeling and their influence on chemical reactions in a membrane environment. Free Radic. Biol. Med. 2009, 46, 707–718. [Google Scholar] [CrossRef]
- Johnson, Q.R.; Mostofian, B.; Fuente Gomez, G.; Smith, J.C.; Cheng, X. Effects of carotenoids on lipid bilayers. Phys. Chem. Chem. Phys. 2018, 20, 3795–3804. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 1998, 3, 147–151. [Google Scholar] [CrossRef]
- Mostofian, B.; Johnson, Q.R.; Smith, J.C.; Cheng, X. Carotenoids promote lateral packing and condensation of lipid membranes. Phys. Chem. Chem. Phys. 2020, 22, 12281–12293. [Google Scholar] [CrossRef]
- Ruban, A.V.; Johnson, M.P. Xanthophylls as modulators of membrane protein function. Arch. Biochem. Biophys. 2010, 504, 78–85. [Google Scholar] [CrossRef]
- Rohmer, M.; Bouvier, P.; Ourisson, G. Molecular evolution of biomembranes: Structural equivalents and phylogenetic precursors of sterols. Proc. Natl. Acad. Sci. USA 1979, 76, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Subczynski, W.K.; Wisniewska-Becker, A.; Widomska, J. Can macular xanthophylls replace cholesterol in formation of the liquid-ordered phase in lipid-bilayer membranes? Acta Biochim. Pol. 2012, 59, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Subczynski, W.K.; Wisniewska, A.; Hyde, J.S.; Kusumi, A. Three-Dimensional Dynamic Structure of the Liquid-Ordered Domain in Lipid Membranes as Examined by Pulse-EPR Oxygen Probing. Biophys. J. 2007, 92, 1573–1584. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Wisniewska, A.; Yin, J.-J.; Hyde, J.S.; Kusumi, A. Hydrophobic Barriers of Lipid Bilayer Membranes Formed by Reduction of Water Penetration by Alkyl Chain Unsaturation and Cholesterol. Biochemistry 1994, 33, 7670–7681. [Google Scholar] [CrossRef]
- Griffith, O.H.; Dehlinger, P.J.; Van, S.P. Shape of the hydrophobic barrier of phospholipid bilayers (Evidence for water penetration in biological membranes). J. Membr. Biol. 1974, 15, 159–192. [Google Scholar] [CrossRef]
- Junghans, A.; Sies, H.; Stahl, W. Macular pigments lutein and zeaxanthin as blue light filters studied in liposomes. Arch. Biochem. Biophys. 2001, 391, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Machida, N.; Kosehira, M.; Kitaichi, N. Clinical Effects of Dietary Supplementation of Lutein with High Bio-Accessibility on Macular Pigment Optical Density and Contrast Sensitivity: A Randomized Double-Blind Placebo-Controlled Parallel-Group Comparison Trial. Nutrients 2020, 12, 2966. [Google Scholar] [CrossRef]
- Hammond, B.R.; Wooten, B.R.; Engles, M.; Wong, J.C. The influence of filtering by the macular carotenoids on contrast sensitivity measured under simulated blue haze conditions. Vis. Res. 2012, 63, 58–62. [Google Scholar] [CrossRef]
- Maxwell, J.C. On the unequal sensibility of the Foramen Centrale to light of different colours. Athenaeum 1856, 1505, 1093. [Google Scholar]
- Isobe, K.; Motokawa, K. Functional Structure of the Retinal Fovea and Maxwell’s Spot. Nature 1955, 175, 306–307. [Google Scholar] [CrossRef]
- Temple, S.; McGregor, J.; Miles, C.; Graham, L.; Miller, J.; Buck, J.; Scott-Samuel, N.; Roberts, N. Perceiving polarization with the naked eye: Characterization of human polarization sensitivity. Proc. R. Soc. B 2015, 282, 20150338. [Google Scholar] [CrossRef] [PubMed]
- Landrum, J.T.; Bone, R.A.; Neuringer, M.; Cao, Y. Macular Pigment: From discovery to function. In Carotenoids and Retinal Disease; Landrum, J.T., Nolan, J., Eds.; CR Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Müller, P.L.; Müller, S.; Gliem, M.; Küpper, K.; Holz, F.G.; Harmening, W.M.; Charbel Issa, P. Perception of Haidinger Brushes in Macular Disease Depends on Macular Pigment Density and Visual Acuity. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.A.; Landrum, J.T. Macular pigment in Henle fiber membranes: A model for Haidinger’s brushes. Vis. Res. 1984, 24, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Widomska, J.; Gruszecki, W.I.; Subczynski, W.K. Factors Differentiating the Antioxidant Activity of Macular Xanthophylls in the Human Eye Retina. Antioxidants 2021, 10, 601. [Google Scholar] [CrossRef]
- Wisniewska-Becker, A.; Nawrocki, G.; Duda, M.; Subczynski, W.K. Structural aspects of the antioxidant activity of lutein in a model of photoreceptor membranes. Acta Biochim. Pol. 2012, 59, 119–124. [Google Scholar] [CrossRef]
- Wisniewska, A.; Subczynski, W.K. Distribution of macular xanthophylls between domains in a model of photoreceptor outer segment membranes. Free Radic. Biol. Med. 2006, 41, 1257–1265. [Google Scholar] [CrossRef]
- Wisniewska, A.; Subczynski, W.K. Accumulation of macular xanthophylls in unsaturated membrane domains. Free Radic. Biol. Med. 2006, 40, 1820–1826. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Wisniewska, A.; Widomska, J. Location of macular xanthophylls in the most vulnerable regions of photoreceptor outer-segment membranes. Arch Biochem. Biophys. 2010, 504, 61–66. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widomska, J.; Subczynski, W.K.; Welc-Stanowska, R.; Luchowski, R. An Overview of Lutein in the Lipid Membrane. Int. J. Mol. Sci. 2023, 24, 12948. https://doi.org/10.3390/ijms241612948
Widomska J, Subczynski WK, Welc-Stanowska R, Luchowski R. An Overview of Lutein in the Lipid Membrane. International Journal of Molecular Sciences. 2023; 24(16):12948. https://doi.org/10.3390/ijms241612948
Chicago/Turabian StyleWidomska, Justyna, Witold K. Subczynski, Renata Welc-Stanowska, and Rafal Luchowski. 2023. "An Overview of Lutein in the Lipid Membrane" International Journal of Molecular Sciences 24, no. 16: 12948. https://doi.org/10.3390/ijms241612948
APA StyleWidomska, J., Subczynski, W. K., Welc-Stanowska, R., & Luchowski, R. (2023). An Overview of Lutein in the Lipid Membrane. International Journal of Molecular Sciences, 24(16), 12948. https://doi.org/10.3390/ijms241612948