Nanoparticles in Medicine: Current Status in Cancer Treatment
Abstract
1. Introduction
2. Types of Nanoparticles
2.1. Inorganic Nanoparticles
2.1.1. Quantum Dots (QDs)
2.1.2. Metallic Nanoparticles
2.1.3. Inorganic Porous Nanomaterials
2.1.4. Magnetic Nanoparticles
2.1.5. Calcium Phosphate (CaP)-Based Mineral Systems
2.1.6. Carbon Nanoparticles
2.2. Organic Nanoparticles
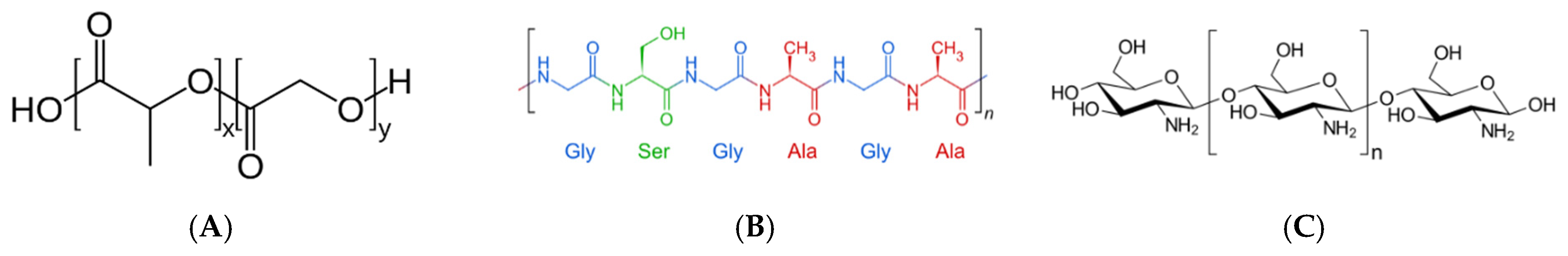
2.2.1. Polymer, Nanogel and Nanofiber Nanoparticles
2.2.2. Liposomes
2.2.3. Micelles
2.2.4. Extracellular Vesicles
3. Intracellular Transport of Nanoparticles
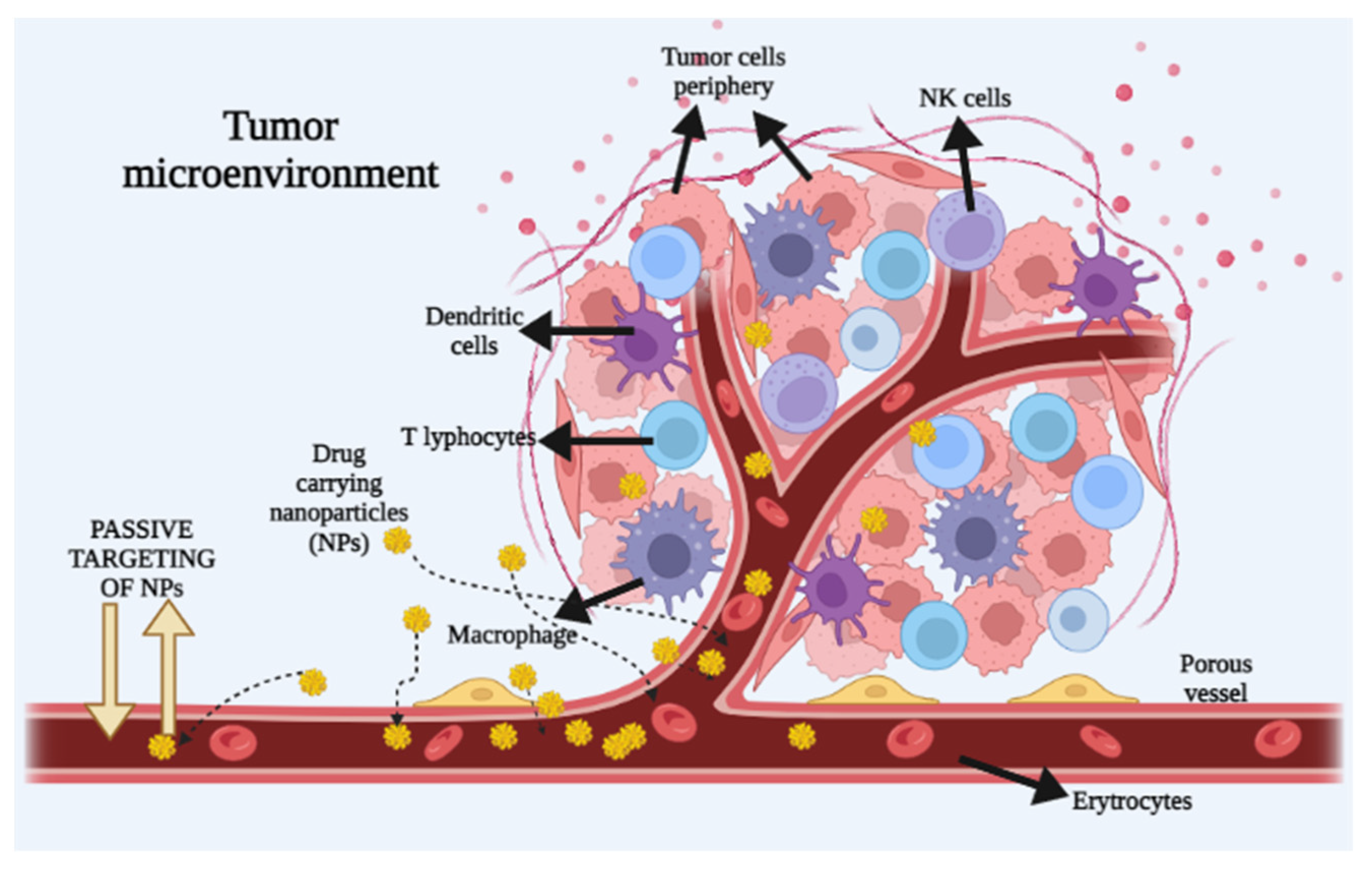
3.1. Passive Transport of Nanoparticles into the Tumor
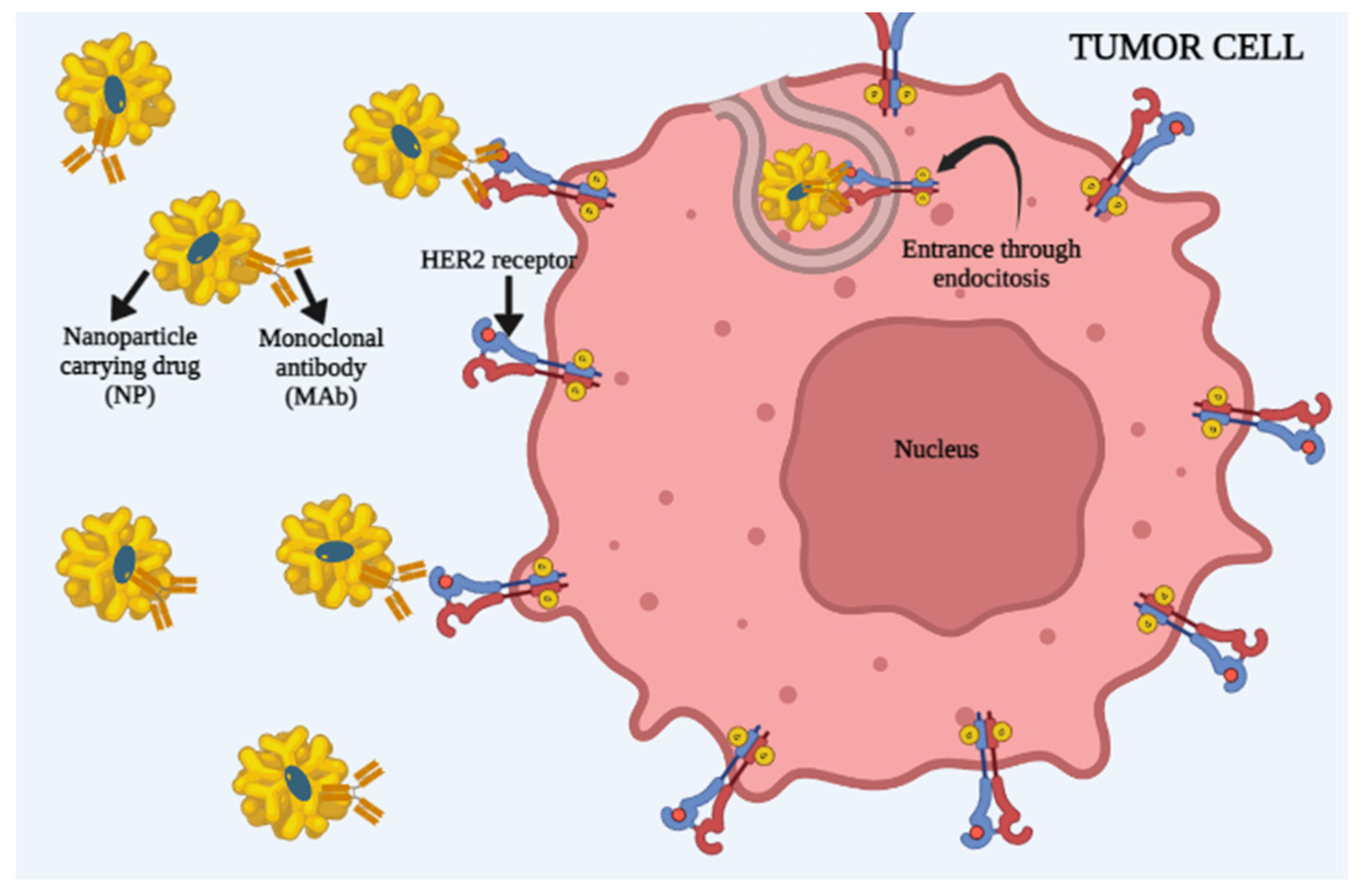
3.2. Active Cell Targeting by Nanoparticles
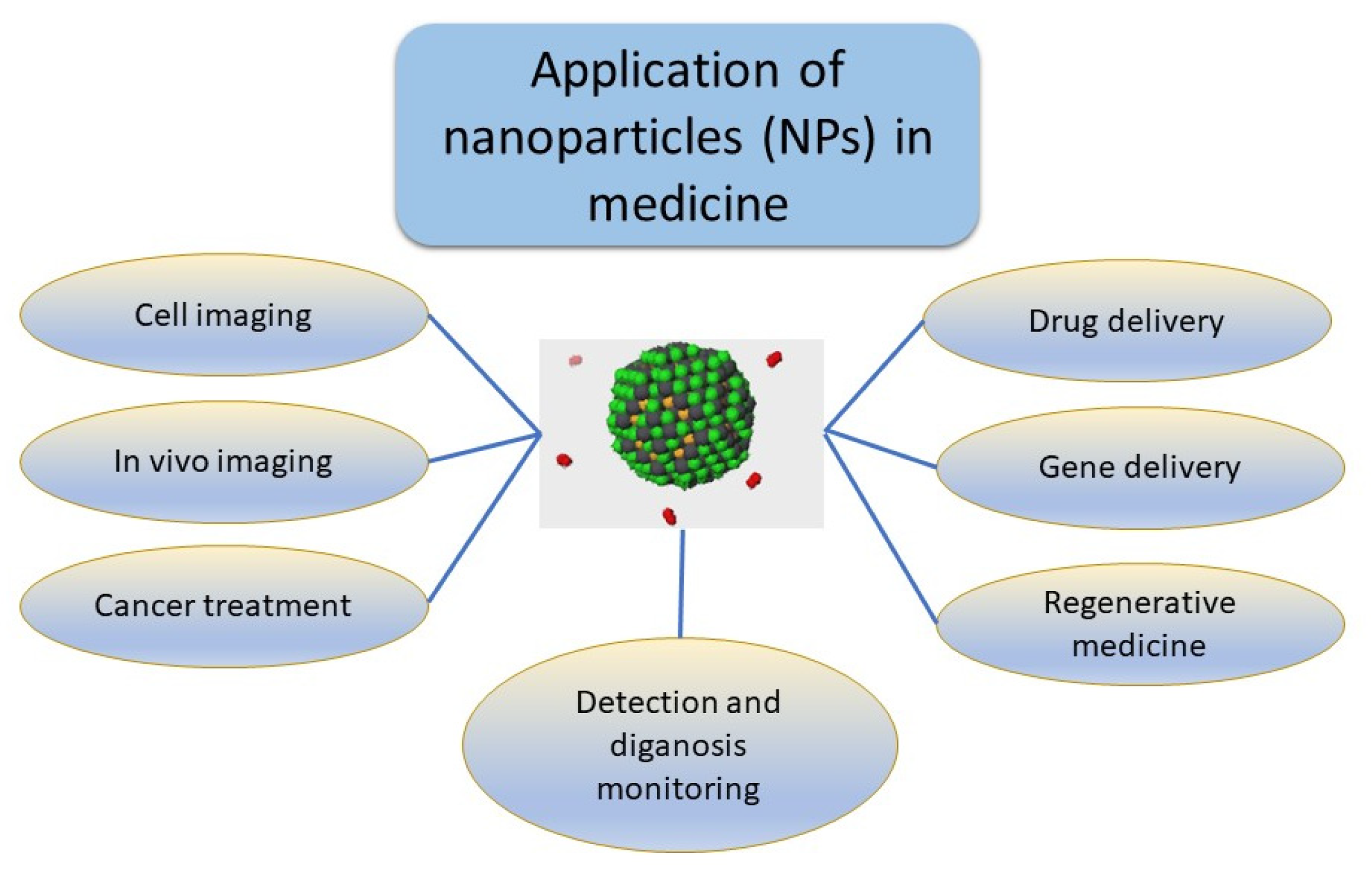
4. Current Usage of Nanoparticles
5. Nanotechnology-Mediated Cancer Treatment
6. New Developments in Nanomedicine: Nanobubbles
6.1. Fundamental Studies and Generation of Nanobubbles
6.2. Application of “Naked” Nanobubbles of Hydrogen in Cancer Treatment and Side Effects Reduction
6.3. Mechanism of Anticancer Action of “Naked” Hydrogen Nanobubbles
6.4. Armored Oxygen Nanobubbles [191]
7. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NP | nanoparticle |
| QD | quantum dot |
| ROS | reactive oxygen species |
| P-gp | P-glycoprotein |
| MDR | multidrug resistance |
| PEG | polyethylene glycol |
| PVA | polyvinyl alcohol |
| PLA | poly(lactic acid) |
| PLGA | poly(lactic-co-glycolic acid) |
| PAA | poly(aspartic acid) |
| PCL | poly(caprolactone) |
| PAMAM | Polyamidoamine |
| ZIF | nano-zeolitic imidazolate framework |
| CNT | carbon nanotube |
| AML | acute myeloid leukemia |
| EPR | enhanced permeability and retention |
| VPF | vascular permeability factor |
| VEGF | vascular endothelial growth factor |
| EGFR | epidermal growth factor |
| VCAM-1 | vascular cell adhesion molecule-1 |
| FR-β | folate receptor beta |
| TME | tumor microenvironment |
| BBB | blood–brain barrier |
| PDT | photodynamic therapy |
| PTT | photothermal therapy |
References
- World Health Organisation. World Cancer Report: Cancer Research for Cancer Prevention, Questions and Answers; World Health Organisation: Geneva, Switzerland, 2020; pp. 1–5.
- National Cancer Institute Cancer Treatment. Cancer Treatment. Available online: https://www.cancer.gov/about-cancer/treatment (accessed on 15 May 2023).
- Tímár, J.; Uhlyarik, A. On-Target Side Effects of Targeted Therapeutics of Cancer. Pathol. Oncol. Res. 2022, 28, 1610694. [Google Scholar] [CrossRef] [PubMed]
- Cone, E.B.; Marchese, M.; Paciotti, M.; Nguyen, D.D.; Nabi, J.; Cole, A.P.; Molina, G.; Molina, R.L.; Minami, C.A.; Mucci, L.A.; et al. Assessment of Time-to-Treatment Initiation and Survival in a Cohort of Patients With Common Cancers. JAMA Netw. Open 2020, 3, e2030072. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA. Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46 Pt 1, 6387–6392. [Google Scholar]
- Li, Z.; Di, C.; Li, S.; Yang, X.; Nie, G. Smart Nanotherapeutic Targeting of Tumor Vasculature. Acc. Chem. Res. 2019, 52, 2703–2712. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020, 157, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.W.; Bae, Y.H. EPR: Evidence and fallacy. J. Control. Release 2014, 190, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Levantini, E.; Maroni, G.; Del Re, M.; Tenen, D.G. EGFR signaling pathway as therapeutic target in human cancers. Semin. Cancer Biol. 2022, 85, 253–275. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, T.; Xia, R.; Wei, Y.; Wei, X. Targeting the tumor stroma for cancer therapy. Mol. Cancer 2022, 21, 208. [Google Scholar] [CrossRef]
- Wu, G.; Ma, Z.; Cheng, Y.; Hu, W.; Deng, C.; Jiang, S.; Li, T.; Chen, F.; Yang, Y. Targeting Gas6/TAM in cancer cells and tumor microenvironment. Mol. Cancer 2018, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Straehla, J.P.; Hajal, C.; Safford, H.C.; Offeddu, G.S.; Boehnke, N.; Dacoba, T.G.; Wyckoff, J.; Kamm, R.D.; Hammond, P.T. A predictive microfluidic model of human glioblastoma to assess trafficking of blood-brain barrier-penetrant nanoparticles. Proc. Natl. Acad. Sci. USA 2022, 119, e2118697119. [Google Scholar] [CrossRef]
- Ravindran, S.; Suthar, J.K.; Rokade, R.; Deshpande, P.; Singh, P.; Pratinidhi, A.; Khambadkhar, R.; Utekar, S. Pharmacokinetics, Metabolism, Distribution and Permeability of Nanomedicine. Curr. Drug Metab. 2018, 19, 327–334. [Google Scholar] [CrossRef]
- Nittayacharn, P.; Yuan, H.-X.; Hernandez, C.; Bielecki, P.; Zhou, H.; Exner, A.A. Enhancing Tumor Drug Distribution With Ultrasound-Triggered Nanobubbles. J. Pharm. Sci. 2019, 108, 3091–3098. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Kudgus, R.A.; Bhattacharya, R.; Mukherjee, P. Inorganic nanoparticles in cancer therapy. Pharm. Res. 2011, 28, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, M.J.; Kizhuveetil, U.; Johnson, A.; Balaji, G.; Nagarajan, R.; Muthuvijayan, V. Cancer nanomedicine: A review of nano-therapeutics and challenges ahead. RSC Adv. 2023, 13, 8606–8629. [Google Scholar] [CrossRef]
- Russell, E.; Dunne, V.; Russell, B.; Mohamud, H.; Ghita, M.; McMahon, S.J.; Butterworth, K.T.; Schettino, G.; McGarry, C.K.; Prise, K.M. Impact of superparamagnetic iron oxide nanoparticles on in vitro and in vivo radiosensitisation of cancer cells. Radiat. Oncol. 2021, 16, 104. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef]
- Tu, Z.; Li, S.; Xu, J.; Chu, W.; Jones, L.A.; Luedtke, R.R.; Mach, R.H. Effect of cyclosporin A on the uptake of D3-selective PET radiotracers in rat brain. Nucl. Med. Biol. 2011, 38, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Fulton, M.D.; Najahi-Missaoui, W. Liposomes in Cancer Therapy: How Did We Start and Where Are We Now. Int. J. Mol. Sci. 2023, 24, 6615. [Google Scholar] [CrossRef] [PubMed]
- Jehn, C.F.; Boulikas, T.; Kourvetaris, A.; Possinger, K.; Lüftner, D. Pharmacokinetics of liposomal cisplatin (lipoplatin) in combination with 5-FU in patients with advanced head and neck cancer: First results of a phase III study. Anticancer Res. 2007, 27, 471–475. [Google Scholar] [PubMed]
- Seetharamu, N.; Kim, E.; Hochster, H.; Martin, F.; Muggia, F. Phase II study of liposomal cisplatin (SPI-77) in platinum-sensitive recurrences of ovarian cancer. Anticancer Res. 2010, 30, 541–545. [Google Scholar]
- Hardman, R. A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef]
- Ozkan, M. Quantum dots and other nanoparticles: What can they offer to drug discovery? Drug Discov. Today 2004, 9, 1065–1071. [Google Scholar] [CrossRef]
- Winnik, F.M.; Maysinger, D. Quantum dot cytotoxicity and ways to reduce it. Acc. Chem. Res. 2013, 46, 672–680. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, M. Dysfunction of various organelles provokes multiple cell death after quantum dot exposure. Int. J. Nanomed. 2018, 13, 2729–2742. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, T.; Tang, M. Toxicity of quantum dots on target organs and immune system. J. Appl. Toxicol. 2022, 42, 17–40. [Google Scholar] [CrossRef]
- Pleskova, S.; Mikheeva, E.; Gornostaeva, E. Using of Quantum Dots in Biology and Medicine. Adv. Exp. Med. Biol. 2018, 1048, 323–334. [Google Scholar] [CrossRef]
- Strugari, A.F.G.; Stan, M.S.; Gharbia, S.; Hermenean, A.; Dinischiotu, A. Characterization of Nanoparticle Intestinal Transport Using an In Vitro Co-Culture Model. Nanomaterials 2019, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Darbandi, M.; Thomann, R.; Nann, T. Single Quantum Dots in Silica Spheres by Microemulsion Synthesis. Chem. Mater. 2005, 17, 5720–5725. [Google Scholar] [CrossRef]
- Yildiz, I.; McCaughan, B.; Cruickshank, S.F.; Callan, J.F.; Raymo, F.M. Biocompatible CdSe-ZnS core-shell quantum dots coated with hydrophilic polythiols. Langmuir 2009, 25, 7090–7096. [Google Scholar] [CrossRef]
- Qi, L.; Pan, T.; Ou, L.; Ye, Z.; Yu, C.; Bao, B.; Wu, Z.; Cao, D.; Dai, L. Biocompatible nucleus-targeted graphene quantum dots for selective killing of cancer cells via DNA damage. Commun. Biol. 2021, 4, 214. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Wang, L.; Rehberg, M.; Stoeger, T.; Zhang, J.; Chen, S. Applications and Immunological Effects of Quantum Dots on Respiratory System. Front. Immunol. 2021, 12, 795232. [Google Scholar] [CrossRef] [PubMed]
- Leung, K. Quantum dot-trastuzumab. In Molecular Imaging and Contrast Agent Database (MICAD); Bethesda: Rockville, MD, USA; National Center for Biotechnology Information: Bethesda, MD, USA, 2007; pp. 2004–2013. [Google Scholar]
- Ruzycka-Ayoush, M.; Kowalik, P.; Kowalczyk, A.; Bujak, P.; Nowicka, A.M.; Wojewodzka, M.; Kruszewski, M.; Grudzinski, I.P. Quantum dots as targeted doxorubicin drug delivery nanosystems in human lung cancer cells. Cancer Nanotechnol. 2021, 12, 8. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Yin, H.; Yin, J. MicroRNA-mediated suppression of P-glycoprotein by quantum dots in lung cancer cells. J. Appl. Toxicol. 2020, 40, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Li, Y.; Guo, L.; Zhang, F.; Liu, H.; Zhang, J.; Zheng, J.; Zhang, J.; Guo, S. Graphene Quantum Dots Downregulate Multiple Multidrug-Resistant Genes via Interacting with Their C-Rich Promoters. Adv. Healthc. Mater. 2017, 6, 8. [Google Scholar] [CrossRef]
- Huang, G.; Wang, L.; Zhang, X. Involvement of ABC transporters in the efflux and toxicity of MPA-COOH-CdTe quantum dots in human breast cancer SK-BR-3 cells. J. Biochem. Mol. Toxicol. 2019, 33, e22343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Shi, L.; Selke, M.; Wang, X. CdTe quantum dots with daunorubicin induce apoptosis of multidrug-resistant human hepatoma HepG2/ADM cells: In vitro and in vivo evaluation. Nanoscale Res. Lett. 2011, 6, 418. [Google Scholar] [CrossRef]
- Li, W.; Cao, Z.; Liu, R.; Liu, L.; Li, H.; Li, X.; Chen, Y.; Lu, C.; Liu, Y. AuNPs as an important inorganic nanoparticle applied in drug carrier systems. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4222–4233. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, G.; Ma, J.; Wang, X.; Zhou, L.; Li, X. Drug nanocrystals: In vivo performances. J. Control. Release 2012, 160, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Mandal, D.; Maran, A.; Yaszemski, M.J.; Bolander, M.E.; Sarkar, G. Cellular uptake of gold nanoparticles directly cross-linked with carrier peptides by osteosarcoma cells. J. Mater. Sci. Mater. Med. 2009, 20, 347–350. [Google Scholar] [CrossRef][Green Version]
- Yih, T.C.; Al-Fandi, M. Engineered nanoparticles as precise drug delivery systems. J. Cell. Biochem. 2006, 97, 1184–1190. [Google Scholar] [CrossRef]
- Penon, O.; Marín, M.J.; Russell, D.A.; Pérez-García, L. Water soluble, multifunctional antibody-porphyrin gold nanoparticles for targeted photodynamic therapy. J. Colloid Interface Sci. 2017, 496, 100–110. [Google Scholar] [CrossRef]
- Barabadi, H.; Hoseini, O.; Kaveh; Kamali, D.; Fereshteh; Shoushtari, J.; Rashedi, M.; Haghi-Aminjan, H.; Muthupandian, S. Emerging Theranostic Silver Nanomaterials to Combat Lung Cancer: A Systematic Review. J. Clust. Sci. 2020, 31, 1–10. [Google Scholar] [CrossRef]
- Guo, D.; Zhu, L.; Huang, Z.; Zhou, H.; Ge, Y.; Ma, W.; Wu, J.; Zhang, X.; Zhou, X.; Zhang, Y. Anti-leukemia activity of PVP-coated silver nanoparticles via generation of reactive oxygen species and release of silver ions. Biomaterials 2013, 34, 7884–7894. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Raman, J.; Abd Malek, S.N.; John, P.A.; Vikineswary, S. Green synthesis of silver nanoparticles using Ganoderma neo-japonicum Imazeki: A potential cytotoxic agent against breast cancer cells. Int. J. Nanomed. 2013, 8, 4399–4413. [Google Scholar] [CrossRef]
- Sahu, S.C.; Zheng, J.; Graham, L.; Chen, L.; Ihrie, J.; Yourick, J.J.; Sprando, R.L. Comparative cytotoxicity of nanosilver in human liver HepG2 and colon Caco2 cells in culture. J. Appl. Toxicol. 2014, 34, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Khutale, G.V.; Casey, A. Synthesis and characterization of a multifunctional gold-doxorubicin nanoparticle system for pH triggered intracellular anticancer drug release. Eur. J. Pharm. Biopharm. 2017, 119, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Li, S.R.; Huo, F.Y.; Wang, H.Q.; Wang, J.; Xu, C.; Liu, B.; Bu, L.L. Recent advances in porous nanomaterials-based drug delivery systems for cancer immunotherapy. J. Nanobiotechnol. 2022, 20, 277. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Jafari, S.; Sarvari, S.; Barzegari, E.; Moakedi, F.; Ghorbani, M.; Shiri Varnamkhasti, B.; Jaymand, M.; Izadi, Z.; Tayebi, L. Biomedical Applications of Zeolitic Nanoparticles, with an Emphasis on Medical Interventions. Int. J. Nanomed. 2020, 15, 363–386. [Google Scholar] [CrossRef]
- Cho, J.; Ishida, Y. Macroscopically Oriented Porous Materials with Periodic Ordered Structures: From Zeolites and Metal-Organic Frameworks to Liquid-Crystal-Templated Mesoporous Materials. Adv. Mater. 2017, 29, 1605974. [Google Scholar] [CrossRef]
- Kraljević Pavelić, S.; Micek, V.; Bobinac, D.; Bazdulj, E.; Gianoncelli, A.; Krpan, D.; Žuvić, M.; Eisenwagen, S.; Stambrook, P.J.; Pavelić, K. Treatment of osteoporosis with a modified zeolite shows beneficial effects in an osteoporotic rat model and a human clinical trial. Exp. Biol. Med. (Maywood) 2021, 246, 529–537. [Google Scholar] [CrossRef]
- Mallette, A.J.; Seo, S.; Rimer, J.D. Synthesis strategies and design principles for nanosized and hierarchical zeolites. Nat. Synth. 2022, 1, 521–534. [Google Scholar] [CrossRef]
- Mintova, S.; Grand, J.; Valtchev, V. Nanosized zeolites: Quo Vadis? Comptes Rendus Chim. 2016, 19, 183–191. [Google Scholar] [CrossRef]
- Helaine, C.; Özçelik, H.; Komaty, S.; Amedlous, A.; Ghojavand, S.; Goux, D.; Retoux, R.; Mintova, S.; Valable, S. Internalization study of nanosized zeolite crystals in human glioblastoma cells. Colloids Surf. B. Biointerfaces 2022, 218, 112732. [Google Scholar] [CrossRef]
- Kihara, T.; Zhang, Y.; Hu, Y.; Mao, Q.; Tang, Y.; Miyake, J. Effect of composition, morphology and size of nanozeolite on its in vitro cytotoxicity. J. Biosci. Bioeng. 2011, 111, 725–730. [Google Scholar] [CrossRef]
- Sağir, T.; Huysal, M.; Durmus, Z.; Kurt, B.Z.; Senel, M.; Isık, S. Preparation and in vitro evaluation of 5-flourouracil loaded magnetite-zeolite nanocomposite (5-FU-MZNC) for cancer drug delivery applications. Biomed. Pharmacother. 2016, 77, 182–190. [Google Scholar] [CrossRef]
- Mostafa, M.; El-Meligy, M.A.; Sharaf, M.; Soliman, A.T.; AbuKhadra, M.R. Insight into chitosan/zeolite-A nanocomposite as an advanced carrier for levofloxacin and its anti-inflammatory properties; loading, release, and anti-inflammatory studies. Int. J. Biol. Macromol. 2021, 179, 206–216. [Google Scholar] [CrossRef]
- Dutta, S. Immunotherapy of tumors by tailored nano-zeolitic imidazolate framework protected biopharmaceuticals. Biomater. Sci. 2021, 9, 6391–6402. [Google Scholar] [CrossRef] [PubMed]
- Alsaiari, S.K.; Qutub, S.S.; Sun, S.; Baslyman, W.; Aldehaiman, M.; Alyami, M.; Almalik, A.; Halwani, R.; Merzaban, J.; Mao, Z.; et al. Sustained and targeted delivery of checkpoint inhibitors by metal-organic frameworks for cancer immunotherapy. Sci. Adv. 2021, 7, eabe7174. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhang, L.; Xu, X.; Qi, M.; Zhang, J.; He, S.; Tian, Q.; Song, S. Engineering a Smart Agent for Enhanced Immunotherapy Effect by Simultaneously Blocking PD-L1 and CTLA-4. Adv. Sci. 2021, 8, 2102500. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, Y.; Tan, L.; Zheng, T.; Hou, Y.; Hong, X.; Du, G.; Chen, X.; Zhang, Y.; Sun, X. An aluminum adjuvant-integrated nano-MOF as antigen delivery system to induce strong humoral and cellular immune responses. J. Control. Release 2019, 300, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, L.; Hu, Q.; Dou, H. Monodisperse ZIF-8@dextran nanoparticles co-loaded with hydrophilic and hydrophobic functional cargos for combined near-infrared fluorescence imaging and photothermal therapy. Acta Biomater. 2022, 137, 290–304. [Google Scholar] [CrossRef]
- Chen, P.; Cui, B.; Bu, Y.; Yang, Z.; Wang, Y. Synthesis and characterization of mesoporous and hollow-mesoporous MxFe3-xO4 (M=Mg, Mn, Fe, Co, Ni, Cu, Zn) microspheres for microwave-triggered controllable drug delivery. J. Nanoparticle Res. 2017, 19, 398. [Google Scholar] [CrossRef]
- Price, P.M.; Mahmoud, W.E.; Al-Ghamdi, A.A.; Bronstein, L.M. Magnetic Drug Delivery: Where the Field Is Going. Front. Chem. 2018, 6, 619. [Google Scholar] [CrossRef]
- Surpi, A.; Shelyakova, T.; Murgia, M.; Rivas, J.; Piñeiro, Y.; Greco, P.; Fini, M.; Dediu, V.A. Versatile magnetic configuration for the control and manipulation of superparamagnetic nanoparticles. Sci. Rep. 2023, 13, 5301. [Google Scholar] [CrossRef]
- Wei, H.; Hu, Y.; Wang, J.; Gao, X.; Qian, X.; Tang, M. Superparamagnetic Iron Oxide Nanoparticles: Cytotoxicity, Metabolism, and Cellular Behavior in Biomedicine Applications. Int. J. Nanomed. 2021, 16, 6097–6113. [Google Scholar] [CrossRef]
- Price, D.N.; Stromberg, L.R.; Kunda, N.K.; Muttil, P. In Vivo Pulmonary Delivery and Magnetic-Targeting of Dry Powder Nano-in-Microparticles. Mol. Pharm. 2017, 14, 4741–4750. [Google Scholar] [CrossRef] [PubMed]
- Jurgons, R.; Seliger, C.; Hilpert, A.; Trahms, L.; Odenbach, S.; Alexiou, C. Drug loaded magnetic nanoparticles for cancer therapy. J. Phys. Condens. Matter 2006, 18, S2893. [Google Scholar] [CrossRef]
- Taratula, O.; Dani, R.K.; Schumann, C.; Xu, H.; Wang, A.; Song, H.; Dhagat, P.; Taratula, O. Multifunctional nanomedicine platform for concurrent delivery of chemotherapeutic drugs and mild hyperthermia to ovarian cancer cells. Int. J. Pharm. 2013, 458, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Vitol, E.A.; Liu, J.; Balasubramanian, S.; Gosztola, D.J.; Cohen, E.E.; Novosad, V.; Rozhkova, E.A. Stimuli-responsive magnetic nanomicelles as multifunctional heat and cargo delivery vehicles. Langmuir 2013, 29, 7425–7432. [Google Scholar] [CrossRef]
- Hu, S.-H.; Liao, B.-J.; Chiang, C.-S.; Chen, P.-J.; Chen, I.-W.; Chen, S.-Y. Core-shell nanocapsules stabilized by single-component polymer and nanoparticles for magneto-chemotherapy/hyperthermia with multiple drugs. Adv. Mater. 2012, 24, 3627–3632. [Google Scholar] [CrossRef] [PubMed]
- Kievit, F.M.; Stephen, Z.R.; Wang, K.; Dayringer, C.J.; Sham, J.G.; Ellenbogen, R.G.; Silber, J.R.; Zhang, M. Nanoparticle mediated silencing of DNA repair sensitizes pediatric brain tumor cells to γ-irradiation. Mol. Oncol. 2015, 9, 1071–1080. [Google Scholar] [CrossRef]
- Khalifehzadeh, R.; Arami, H. Biodegradable calcium phosphate nanoparticles for cancer therapy. Adv. Colloid Interface Sci. 2020, 279, 102157. [Google Scholar] [CrossRef]
- Tang, J.; Howard, C.B.; Mahler, S.M.; Thurecht, K.J.; Huang, L.; Xu, Z.P. Enhanced delivery of siRNA to triple negative breast cancer cells in vitro and in vivo through functionalizing lipid-coated calcium phosphate nanoparticles with dual target ligands. Nanoscale 2018, 10, 4258–4266. [Google Scholar] [CrossRef]
- Xu, L.; Tong, G.; Song, Q.; Zhu, C.; Zhang, H.; Shi, J.; Zhang, Z. Enhanced Intracellular Ca2+ Nanogenerator for Tumor-Specific Synergistic Therapy via Disruption of Mitochondrial Ca2+ Homeostasis and Photothermal Therapy. ACS Nano 2018, 12, 6806–6818. [Google Scholar] [CrossRef]
- Loh, K.P.; Ho, D.; Chiu, G.N.C.; Leong, D.T.; Pastorin, G.; Chow, E.K.-H. Clinical Applications of Carbon Nanomaterials in Diagnostics and Therapy. Adv. Mater. 2018, 30, e1802368. [Google Scholar] [CrossRef]
- Negri, V.; Pacheco-Torres, J.; Calle, D.; López-Larrubia, P. Carbon Nanotubes in Biomedicine. Top. Curr. Chem. 2020, 378, 15. [Google Scholar] [CrossRef]
- Watt, M.M.; Moitra, P.; Sheffield, Z.; Ostadhossein, F.; Maxwell, E.A.; Pan, D. A narrative review on the role of carbon nanoparticles in oncology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1845. [Google Scholar] [CrossRef] [PubMed]
- Itoo, A.M.; Vemula, S.L.; Gupta, M.T.; Giram, M.V.; Kumar, S.A.; Ghosh, B.; Biswas, S. Multifunctional graphene oxide nanoparticles for drug delivery in cancer. J. Control. Release 2022, 350, 26–59. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, D.; Zeng, K.; Li, D.; Qin, L.; Cai, Y.; Jin, J. Simultaneous Delivery of antimiR-21 and Doxorubicin by Graphene Oxide for Reducing Toxicity in Cancer Therapy. ACS Omega 2020, 5, 14437–14443. [Google Scholar] [CrossRef]
- Chai, D.; Hao, B.; Hu, R.; Zhang, F.; Yan, J.; Sun, Y.; Huang, X.; Zhang, Q.; Jiang, H. Delivery of Oridonin and Methotrexate via PEGylated Graphene Oxide. ACS Appl. Mater. Interfaces 2019, 11, 22915–22924. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, M.; Verre, A.F.; Iliut, M.; Peiris-Pagés, M.; Ozsvari, B.; Gandara, R.; Cappello, A.R.; Sotgia, F.; Vijayaraghavan, A.; Lisanti, M.P. Graphene oxide selectively targets cancer stem cells, across multiple tumor types: Implications for non-toxic cancer treatment, via “differentiation-based nano-therapy”. Oncotarget 2015, 6, 3553–3562. [Google Scholar] [CrossRef] [PubMed]
- Tabish, T.A.; Narayan, R.J. Mitochondria-targeted graphene for advanced cancer therapeutics. Acta Biomater. 2021, 129, 43–56. [Google Scholar] [CrossRef]
- Fernandes, N.B.; Shenoy, R.U.K.; Kajampady, M.K.; DCruz, C.E.M.; Shirodkar, R.K.; Kumar, L.; Verma, R. Fullerenes for the treatment of cancer: An emerging tool. Environ. Sci. Pollut. Res. Int. 2022, 29, 58607–58627. [Google Scholar] [CrossRef]
- Liu, J.; Tabata, Y. Photodynamic Antitumor Activity of Fullerene Modified with Poly(Ethylene Glycol) with Different Molecular Weights and Terminal Structures. J. Biomater. Sci. Polym. Ed. 2011, 22, 297–312. [Google Scholar] [CrossRef]
- Ji, Z.; Sun, H.; Wang, H.; Xie, Q.; Liu, Y.; Wang, Z. Biodistribution and tumor uptake of C60(OH) x in mice. J. Nanoparticle Res. 2006, 8, 53–63. [Google Scholar] [CrossRef]
- Liang, X.-J.; Meng, H.; Wang, Y.; He, H.; Meng, J.; Lu, J.; Wang, P.C.; Zhao, Y.; Gao, X.; Sun, B.; et al. Metallofullerene nanoparticles circumvent tumor resistance to cisplatin by reactivating endocytosis. Proc. Natl. Acad. Sci. USA 2010, 107, 7449–7454. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Park, S.J.; Park, J.Y.; Kim, S.-H.; Kwon, S.; Jung, Y.; Khang, D. Unfolded Protein Corona Surrounding Nanotubes Influence the Innate and Adaptive Immune System. Adv. Sci. 2021, 8, 2004979. [Google Scholar] [CrossRef]
- Nivethaa, E.A.K.; Dhanavel, S.; Rebekah, A.; Narayanan, V.; Stephen, A. A comparative study of 5-Fluorouracil release from chitosan/silver and chitosan/silver/MWCNT nanocomposites and their cytotoxicity towards MCF-7. Mater. Sci. Eng. C. Mater. Biol. Appl. 2016, 66, 244–250. [Google Scholar] [CrossRef]
- Aghebati-Maleki, A.; Dolati, S.; Ahmadi, M.; Baghbanzhadeh, A.; Asadi, M.; Fotouhi, A.; Yousefi, M.; Aghebati-Maleki, L. Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers. J. Cell. Physiol. 2020, 235, 1962–1972. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Zu, Y.-J.; Nie, S.-F.; Cao, J.; Wang, Q.; Nie, S.-P.; Deng, Z.-Y.; Xie, M.-Y.; Wang, S. Biocompatible and biodegradable nanoparticles for enhancement of anti-cancer activities of phytochemicals. Chin. J. Nat. Med. 2015, 13, 641–652. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Park, J.; Fong, P.M.; Lu, J.; Russell, K.S.; Booth, C.J.; Saltzman, W.M.; Fahmy, T.M. PEGylated PLGA nanoparticles for the improved delivery of doxorubicin. Nanomedicine 2009, 5, 410–418. [Google Scholar] [CrossRef]
- Shah, S.R.; Kim, J.; Schiapparelli, P.; Vazquez-Ramos, C.A.; Martinez-Gutierrez, J.C.; Ruiz-Valls, A.; Inman, K.; Shamul, J.G.; Green, J.J.; Quinones-Hinojosa, A. Verteporfin-Loaded Polymeric Microparticles for Intratumoral Treatment of Brain Cancer. Mol. Pharm. 2019, 16, 1433–1443. [Google Scholar] [CrossRef]
- Puluhulawa, L.E.; Joni, I.M.; Elamin, K.M.; Mohammed, A.F.A.; Muchtaridi, M.; Wathoni, N. Chitosan-Hyaluronic Acid Nanoparticles for Active Targeting in Cancer Therapy. Polymers 2022, 14, 3410. [Google Scholar] [CrossRef]
- Deng, X.; Cao, M.; Zhang, J.; Hu, K.; Yin, Z.; Zhou, Z.; Xiao, X.; Yang, Y.; Sheng, W.; Wu, Y.; et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials 2014, 35, 4333–4344. [Google Scholar] [CrossRef]
- Maya, S.; Sarmento, B.; Lakshmanan, V.-K.; Menon, D.; Seabra, V.; Jayakumar, R. Chitosan cross-linked docetaxel loaded EGF receptor targeted nanoparticles for lung cancer cells. Int. J. Biol. Macromol. 2014, 69, 532–541. [Google Scholar] [CrossRef]
- Venkatesan, P.; Puvvada, N.; Dash, R.; Prashanth Kumar, B.N.; Sarkar, D.; Azab, B.; Pathak, A.; Kundu, S.C.; Fisher, P.B.; Mandal, M. The potential of celecoxib-loaded hydroxyapatite-chitosan nanocomposite for the treatment of colon cancer. Biomaterials 2011, 32, 3794–3806. [Google Scholar] [CrossRef]
- Romero, G.; Moya, S. Synthesis of Organic Nanoparticles. Front. Nanosci. 2012, 4, 115–141. [Google Scholar] [CrossRef]
- Andreiuk, B.; Reisch, A.; Lindecker, M.; Follain, G.; Peyriéras, N.; Goetz, J.G.; Klymchenko, A.S. Fluorescent Polymer Nanoparticles for Cell Barcoding In Vitro and In Vivo. Small 2017, 13, 1701582. [Google Scholar] [CrossRef]
- Thakkar, D.; Gupta, R.; Mohan, P.; Monson, K.; Rapoport, N. Overcoming Biological Barriers with Ultrasound. AIP Conf. Proc. 2012, 1481, 381–387. [Google Scholar] [CrossRef]
- Tharkar, P.; Varanasi, R.; Wong, W.S.F.; Jin, C.T.; Chrzanowski, W. Nano-Enhanced Drug Delivery and Therapeutic Ultrasound for Cancer Treatment and Beyond. Front. Bioeng. Biotechnol. 2019, 7, 324. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Li, J.; Kong, M.; Liu, Y.; Cheng, X.J.; Li, Y.; Park, H.J.; Chen, X.G. Surface charge effect on mucoadhesion of chitosan based nanogels for local anti-colorectal cancer drug delivery. Colloids Surf. B. Biointerfaces 2015, 128, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, D.; Harada, N.; Shiku, H.; Akiyoshi, K. Self-assembled polysaccharide nanogel delivery system for overcoming tumor immune resistance. J. Control. Release 2022, 347, 175–182. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Khodadadi, M.; Alijani, S.; Montazeri, M.; Esmaeilizadeh, N.; Sadeghi-Soureh, S.; Pilehvar-Soltanahmadi, Y. Recent advances in electrospun nanofiber-mediated drug delivery strategies for localized cancer chemotherapy. J. Biomed. Mater. Res. A 2020, 108, 1444–1458. [Google Scholar] [CrossRef]
- Kataria, K.; Gupta, A.; Rath, G.; Mathur, R.B.; Dhakate, S.R. In vivo wound healing performance of drug loaded electrospun composite nanofibers transdermal patch. Int. J. Pharm. 2014, 469, 102–110. [Google Scholar] [CrossRef]
- Norouzi, M. Recent advances in brain tumor therapy: Application of electrospun nanofibers. Drug Discov. Today 2018, 23, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. P T 2017, 42, 742–755. [Google Scholar] [PubMed]
- Yadav, P.; Ambudkar, S.V.; Rajendra Prasad, N. Emerging nanotechnology-based therapeutics to combat multidrug-resistant cancer. J. Nanobiotechnol. 2022, 20, 423. [Google Scholar] [CrossRef]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, Biomedical Applications, and Stability Parameters With Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 705886. [Google Scholar] [CrossRef]
- Yang, B.; Song, B.-P.; Shankar, S.; Guller, A.; Deng, W. Recent advances in liposome formulations for breast cancer therapeutics. Cell. Mol. Life Sci. 2021, 78, 5225–5243. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, L.; Gao, H.; Liu, Y.; Zhang, Q.; Ran, R.; Zhang, Z.; He, Q. Co-delivery of doxorubicin and P-gp inhibitor by a reduction-sensitive liposome to overcome multidrug resistance, enhance anti-tumor efficiency and reduce toxicity. Drug Deliv. 2016, 23, 1130–1143. [Google Scholar] [CrossRef]
- Lancet, J.E.; Cortes, J.E.; Hogge, D.E.; Tallman, M.S.; Kovacsovics, T.J.; Damon, L.E.; Komrokji, R.; Solomon, S.R.; Kolitz, J.E.; Cooper, M.; et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood 2014, 123, 3239–3246. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Kim, D.-W.; Chung, J.-Y.; Shin, S.G.; Kim, S.-C.; Heo, D.S.; Kim, N.K.; Bang, Y.-J. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin. Cancer Res. 2004, 10, 3708–3716. [Google Scholar] [CrossRef]
- Lv, L.; Qiu, K.; Yu, X.; Chen, C.; Qin, F.; Shi, Y.; Ou, J.; Zhang, T.; Zhu, H.; Wu, J.; et al. Amphiphilic Copolymeric Micelles for Doxorubicin and Curcumin Co-Delivery to Reverse Multidrug Resistance in Breast Cancer. J. Biomed. Nanotechnol. 2016, 12, 973–985. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef]
- Tan, Z.; Jiang, Y.; Zhang, W.; Karls, L.; Lodge, T.P.; Reineke, T.M. Polycation Architecture and Assembly Direct Successful Gene Delivery: Micelleplexes Outperform Polyplexes via Optimal DNA Packaging. J. Am. Chem. Soc. 2019, 141, 15804–15817. [Google Scholar] [CrossRef] [PubMed]
- Dirisala, A.; Osada, K.; Chen, Q.; Tockary, T.A.; Machitani, K.; Osawa, S.; Liu, X.; Ishii, T.; Miyata, K.; Oba, M.; et al. Optimized rod length of polyplex micelles for maximizing transfection efficiency and their performance in systemic gene therapy against stroma-rich pancreatic tumors. Biomaterials 2014, 35, 5359–5368. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 2015, 219, 396–405. [Google Scholar] [CrossRef]
- Hadla, M.; Palazzolo, S.; Corona, G.; Caligiuri, I.; Canzonieri, V.; Toffoli, G.; Rizzolio, F. Exosomes increase the therapeutic index of doxorubicin in breast and ovarian cancer mouse models. Nanomedicine 2016, 11, 2431–2441. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ao, Q.; Wang, X.; Cao, Y.; Liu, Y.; Zheng, S.G.; Tian, X. Mesenchymal Stem Cell-Derived Exosomes: A Promising Biological Tool in Nanomedicine. Front. Pharmacol. 2020, 11, 590470. [Google Scholar] [CrossRef]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®--the first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Prakash, S.S.; Nagarkar, R.V.; Puligundla, K.C.; Lokesh, K.N.; Boya, R.R.; Patel, A.B.; Goyal, L.; Thoke, A.; Patel, J.G.; Mehta, A.O.; et al. Bioequivalence of a hybrid pegylated liposomal doxorubicin hydrochloride injection and Caelyx®: A single-dose, randomized, multicenter, open-label, two-period crossover study in patients with advanced ovarian cancer. Eur. J. Pharm. Sci. 2022, 176, 106248. [Google Scholar] [CrossRef]
- Cheng, R.; Santos, H.A. Smart Nanoparticle-Based Platforms for Regulating Tumor Microenvironment and Cancer Immunotherapy. Adv. Healthc. Mater. 2023, 12, e2202063. [Google Scholar] [CrossRef]
- Roscigno, G.; Scognamiglio, I.; Ingenito, F.; Chianese, R.V.; Palma, F.; Chan, A.; Condorelli, G. Modulating the Crosstalk between the Tumor and the Microenvironment Using SiRNA: A Flexible Strategy for Breast Cancer Treatment. Cancers 2020, 12, 3744. [Google Scholar] [CrossRef] [PubMed]
- Arap, W.; Pasqualini, R.; Ruoslahti, E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 1998, 279, 377–380. [Google Scholar] [CrossRef]
- Heldin, C.-H.; Rubin, K.; Pietras, K.; Ostman, A. High interstitial fluid pressure—An obstacle in cancer therapy. Nat. Rev. Cancer 2004, 4, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef]
- Tarudji, A.W.; Kievit, F.M. Active targeting and transport. In Nanoparticles for Biomedical Applications; Chung, E.J., Leon, L., Rinaldi, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 19–36. ISBN 978-0-12-816662-8. [Google Scholar]
- Durfee, P.N.; Lin, Y.-S.; Dunphy, D.R.; Muñiz, A.J.; Butler, K.S.; Humphrey, K.R.; Lokke, A.J.; Agola, J.O.; Chou, S.S.; Chen, I.-M.; et al. Mesoporous Silica Nanoparticle-Supported Lipid Bilayers (Protocells) for Active Targeting and Delivery to Individual Leukemia Cells. ACS Nano 2016, 10, 8325–8345. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Kitov, P.I.; Bundle, D.R. On the nature of the multivalency effect: A thermodynamic model. J. Am. Chem. Soc. 2003, 125, 16271–16284. [Google Scholar] [CrossRef]
- Fan, Z.; Fu, P.P.; Yu, H.; Ray, P.C. Theranostic nanomedicine for cancer detection and treatment. J. food drug Anal. 2014, 22, 3–17. [Google Scholar] [CrossRef] [PubMed]
- SynerGene Therapeutics Inc. Cell and Gene Therapy Library; PerkinElmer: Hongkong, China, 1998–2023. [Google Scholar]
- Palui, G.; Aldeek, F.; Wang, W.; Mattoussi, H. Strategies for interfacing inorganic nanocrystals with biological systems based on polymer-coating. Chem. Soc. Rev. 2015, 44, 193–227. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Wang, Y.; Cho, K.J.; Kim, G.; Gjyrezi, A.; Koenig, L.; Giannakakou, P.; Shin, H.J.C.; Tighiouart, M.; et al. HFT-T, a targeting nanoparticle, enhances specific delivery of paclitaxel to folate receptor-positive tumors. ACS Nano 2009, 3, 3165–3174. [Google Scholar] [CrossRef] [PubMed]
- de la Puente, P.; Azab, A.K. Nanoparticle delivery systems, general approaches, and their implementation in multiple myeloma. Eur. J. Haematol. 2017, 98, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Su, J.; Meng, Q.; Yin, Q.; Chen, L.; Gu, W.; Zhang, P.; Zhang, Z.; Yu, H.; Wang, S.; et al. Cancer-Cell-Biomimetic Nanoparticles for Targeted Therapy of Homotypic Tumors. Adv. Mater. 2016, 28, 9581–9588. [Google Scholar] [CrossRef]
- Li, J.; Sharkey, C.C.; Huang, D.; King, M.R. Nanobiotechnology for the Therapeutic Targeting of Cancer Cells in Blood. Cell. Mol. Bioeng. 2015, 8, 137–150. [Google Scholar] [CrossRef]
- Kievit, F.M.; Stephen, Z.R.; Veiseh, O.; Arami, H.; Wang, T.; Lai, V.P.; Park, J.O.; Ellenbogen, R.G.; Disis, M.L.; Zhang, M. Targeting of primary breast cancers and metastases in a transgenic mouse model using rationally designed multifunctional SPIONs. ACS Nano 2012, 6, 2591–2601. [Google Scholar] [CrossRef]
- Mandal, T.; Beck, M.; Kirsten, N.; Lindén, M.; Buske, C. Targeting murine leukemic stem cells by antibody functionalized mesoporous silica nanoparticles. Sci. Rep. 2018, 8, 989. [Google Scholar] [CrossRef]
- Amreddy, N.; Muralidharan, R.; Babu, A.; Mehta, M.; Johnson, E.V.; Zhao, Y.D.; Munshi, A.; Ramesh, R. Tumor-targeted and pH-controlled delivery of doxorubicin using gold nanorods for lung cancer therapy. Int. J. Nanomed. 2015, 10, 6773–6788. [Google Scholar] [CrossRef]
- Zhu, L.; Mu, Q.; Yu, J.; Griffin, J.I.; Xu, X.; Ho, R.J.Y. ICAM-1 Targeted Drug Combination Nanoparticles Enhanced Gemcitabine-Paclitaxel Exposure and Breast Cancer Suppression in Mouse Models. Pharmaceutics 2021, 14, 89. [Google Scholar] [CrossRef]
- Shen, J.; Putt, K.S.; Visscher, D.W.; Murphy, L.; Cohen, C.; Singhal, S.; Sandusky, G.; Feng, Y.; Dimitrov, D.S.; Low, P.S. Assessment of folate receptor-β expression in human neoplastic tissues. Oncotarget 2015, 6, 14700–14709. [Google Scholar] [CrossRef]
- Jeanson, A.; Tomasini, P.; Souquet-Bressand, M.; Brandone, N.; Boucekine, M.; Grangeon, M.; Chaleat, S.; Khobta, N.; Milia, J.; Mhanna, L.; et al. Efficacy of Immune Checkpoint Inhibitors in KRAS-Mutant Non-Small Cell Lung Cancer (NSCLC). J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2019, 14, 1095–1101. [Google Scholar] [CrossRef]
- Ryman-Rasmussen, J.P.; Riviere, J.E.; Monteiro-Riviere, N.A. Penetration of intact skin by quantum dots with diverse physicochemical properties. Toxicol. Sci. 2006, 91, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Er, S.; Laraib, U.; Arshad, R.; Sargazi, S.; Rahdar, A.; Pandey, S.; Thakur, V.K.; Díez-Pascual, A.M. Amino Acids, Peptides, and Proteins: Implications for Nanotechnological Applications in Biosensing and Drug/Gene Delivery. Nanomater. 2021, 11, 3002. [Google Scholar] [CrossRef]
- Sriraman, S.K.; Geraldo, V.; Luther, E.; Degterev, A.; Torchilin, V. Cytotoxicity of PEGylated liposomes co-loaded with novel pro-apoptotic drug NCL-240 and the MEK inhibitor cobimetinib against colon carcinoma in vitro. J. Control. Release 2015, 220 Pt A, 160–168. [Google Scholar] [CrossRef]
- Batist, G.; Gelmon, K.A.; Chi, K.N.; Miller, W.H.J.; Chia, S.K.L.; Mayer, L.D.; Swenson, C.E.; Janoff, A.S.; Louie, A.C. Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection in patients with advanced solid tumors. Clin. Cancer Res. 2009, 15, 692–700. [Google Scholar] [CrossRef]
- Deng, Z.J.; Morton, S.W.; Ben-Akiva, E.; Dreaden, E.C.; Shopsowitz, K.E.; Hammond, P.T. Layer-by-layer nanoparticles for systemic codelivery of an anticancer drug and siRNA for potential triple-negative breast cancer treatment. ACS Nano 2013, 7, 9571–9584. [Google Scholar] [CrossRef]
- Smith, D.M.; Simon, J.K.; Baker, J.R.J. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 2013, 13, 592–605. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, W.; Xin, H.; Liu, R.; Wang, Q.; Cai, W.; Peng, X.; Yang, F.; Xin, H. Nanoparticles advanced from preclinical studies to clinical trials for lung cancer therapy. Cancer Nanotechnol. 2023, 14, 28. [Google Scholar] [CrossRef]
- Liu, J.; Miao, L.; Sui, J.; Hao, Y.; Huang, G. Nanoparticle cancer vaccines: Design considerations and recent advances. Asian J. Pharm. Sci. 2020, 15, 576–590. [Google Scholar] [CrossRef]
- Gonçalves, A.; Machado, R.; Gomes, A.C.; Costa, A.D. Nanotechnology Solutions for Controlled Cytokine Delivery: An Applied Perspective. Appl. Sci. 2020, 10, 7098. [Google Scholar] [CrossRef]
- Sanaei, M.-J.; Pourbagheri-Sigaroodi, A.; Kaveh, V.; Sheikholeslami, S.A.; Salari, S.; Bashash, D. The application of nano-medicine to overcome the challenges related to immune checkpoint blockades in cancer immunotherapy: Recent advances and opportunities. Crit. Rev. Oncol. Hematol. 2021, 157, 103160. [Google Scholar] [CrossRef]
- Shang, N.; Figini, M.; Shangguan, J.; Wang, B.; Sun, C.; Pan, L.; Ma, Q.; Zhang, Z. Dendritic cells based immunotherapy. Am. J. Cancer Res. 2017, 7, 2091–2102. [Google Scholar]
- Choi, J.; Kim, G.; Cho, S.B.; Im, H.-J. Radiosensitizing high-Z metal nanoparticles for enhanced radiotherapy of glioblastoma multiforme. J. Nanobiotechnol. 2020, 18, 122. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Rivas-García, L.; Baptista, P.V.; Fernandes, A.R. Gene Therapy in Cancer Treatment: Why Go Nano? Pharmaceutics 2020, 12, 223. [Google Scholar] [CrossRef]
- Conde, J.; Tian, F.; Hernandez, Y.; Bao, C.; Baptista, P.V.; Cui, D.; Stoeger, T.; de la Fuente, J.M. RNAi-based glyconanoparticles trigger apoptotic pathways for in vitro and in vivo enhanced cancer-cell killing. Nanoscale 2015, 7, 9083–9091. [Google Scholar] [CrossRef]
- Guo, J.; O’Driscoll, C.M.; Holmes, J.D.; Rahme, K. Bioconjugated gold nanoparticles enhance cellular uptake: A proof of concept study for siRNA delivery in prostate cancer cells. Int. J. Pharm. 2016, 509, 16–27. [Google Scholar] [CrossRef]
- Hu, Q.L.; Jiang, Q.Y.; Jin, X.; Shen, J.; Wang, K.; Li, Y.B.; Xu, F.J.; Tang, G.P.; Li, Z.H. Cationic microRNA-delivering nanovectors with bifunctional peptides for efficient treatment of PANC-1 xenograft model. Biomaterials 2013, 34, 2265–2276. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Li, J.; Zhang, Z.; Huang, C.; Lian, G.; Yang, K.; Chen, S.; Lin, Y.; Wang, L.; et al. Co-delivery of microRNA-21 antisense oligonucleotides and gemcitabine using nanomedicine for pancreatic cancer therapy. Cancer Sci. 2017, 108, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- SynerGene Therapeutics Inc. Phase II Study of Combined SGT-53 Plus Gemcitabine/Nab-Paclitaxel for Metastatic Pancreatic Cancer; ClinicalTrials.gov: Bethesda, MD, USA, 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02340117 (accessed on 20 April 2023).
- Nieth, C.; Priebsch, A.; Stege, A.; Lage, H. Modulation of the classical multidrug resistance (MDR) phenotype by RNA interference (RNAi). FEBS Lett. 2003, 545, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Li, X. Liposomal delivery of CRISPR/Cas9. Cancer Gene Ther. 2020, 27, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Li, H.; Wang, L.; Gu, H.; Fan, C. DNA Nanotechnology-Enabled Drug Delivery Systems. Chem. Rev. 2019, 119, 6459–6506. [Google Scholar] [CrossRef]
- Jin, J.-O.; Kim, G.; Hwang, J.; Han, K.H.; Kwak, M.; Lee, P.C.W. Nucleic acid nanotechnology for cancer treatment. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188377. [Google Scholar] [CrossRef]
- Sabir, F.; Zeeshan, M.; Laraib, U.; Barani, M.; Rahdar, A.; Cucchiarini, M.; Pandey, S. DNA Based and Stimuli-Responsive Smart Nanocarrier for Diagnosis and Treatment of Cancer: Applications and Challenges. Cancers 2021, 13, 3396. [Google Scholar] [CrossRef]
- Juliano, R.; Alam, M.R.; Dixit, V.; Kang, H. Mechanisms and strategies for effective delivery of antisense and siRNA oligonucleotides. Nucleic Acids Res. 2008, 36, 4158–4171. [Google Scholar] [CrossRef]
- Ho, B.N.; Pfeffer, C.M.; Singh, A.T.K. Update on Nanotechnology-based Drug Delivery Systems in Cancer Treatment. Anticancer Res. 2017, 37, 5975–5981. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, M.; Shen, W.; Du, B.; Yang, J.; Zhang, Q. A Polycationic Brush Mediated Co-Delivery of Doxorubicin and Gene for Combination Therapy. Polymers 2019, 11, 60. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, S.; Gao, W. What Went Wrong with Anticancer Nanomedicine Design and How to Make It Right. ACS Nano 2020, 14, 12281–12290. [Google Scholar] [CrossRef]
- Foudas, A.W.; Kosheleva, R.I.; Favvas, E.P.; Kostoglou, M.; Mitropoulos, A.C.; Kyzas, G.Z. Fundamentals and applications of nanobubbles: A review. Chem. Eng. Res. Des. 2023, 189, 64–86. [Google Scholar] [CrossRef]
- Hirano, S.-I.; Yamamoto, H.; Ichikawa, Y.; Sato, B.; Takefuji, Y.; Satoh, F. Molecular Hydrogen as a Novel Antitumor Agent: Possible Mechanisms Underlying Gene Expression. Int. J. Mol. Sci. 2021, 22, 8724. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, S.; Zhang, L.; Hu, J. Generation and stability of bulk nanobubbles: A review and perspective. Curr. Opin. Colloid Interface Sci. 2021, 53, 101439. [Google Scholar] [CrossRef]
- Colic, M.; Morse, D. Influence of Resonant rf Radiation on Gas/Liquid Interface: Can It Be a Quantum Vacuum Radiation? Phys. Rev. Lett. 1998, 80, 2465–2468. [Google Scholar] [CrossRef]
- Khan, M.S.; Hwang, J.; Lee, K.; Choi, Y.; Kim, K.; Koo, H.-J.; Hong, J.W.; Choi, J. Oxygen-Carrying Micro/Nanobubbles: Composition, Synthesis Techniques and Potential Prospects in Photo-Triggered Theranostics. Molecules 2018, 23, 2210. [Google Scholar] [CrossRef]
- Takahashi, M.; Shirai, Y.; Sugawa, S. Free-Radical Generation from Bulk Nanobubbles in Aqueous Electrolyte Solutions: ESR Spin-Trap Observation of Microbubble-Treated Water. Langmuir 2021, 37, 5005–5011. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, W.; Li, X.; Wang, W.-X.; Liu, S. Enhanced Removal of Free Radicals by Aqueous Hydrogen Nanobubbles and Their Role in Oxidative Stress. Environ. Sci. Technol. 2022, 56, 15096–15107. [Google Scholar] [CrossRef]
- Liu, S.; Li, J.; Oshita, S.; Kamruzzaman, M.; Cui, M.; Fan, W. Formation of a Hydrogen Radical in Hydrogen Nanobubble Water and Its Effect on Copper Toxicity in Chlorella. ACS Sustain. Chem. Eng. 2021, 9, 11100–11109. [Google Scholar] [CrossRef]
- Colic, M. Prophylactic, Therapeutic and Industrial Antioxidant Compositions Enhanced with Stabilized Atomic Hydrogen/Free Electrons and Methods to Prepare and Use Such Compositions. U.S. Patent CA002377232A, 9 June 2000. [Google Scholar]
- Owen, J.; McEwan, C.; Nesbitt, H.; Bovornchutichai, P.; Averre, R.; Borden, M.; McHale, A.P.; Callan, J.F.; Stride, E. Reducing Tumour Hypoxia via Oral Administration of Oxygen Nanobubbles. PLoS ONE 2016, 11, e0168088. [Google Scholar] [CrossRef]
- Gao, R.; Luo, Q.; Li, Y.; Song, L.; Cai, J.S.; Xiong, Y.; Yan, F.; Liu, J. Biosynthetic Nanobubble-Mediated CRISPR/Cas9 Gene Editing of Cdh2 Inhibits Breast Cancer Metastasis. Pharmaceutics 2022, 14, 1382. [Google Scholar] [CrossRef]
- Su, C.; Ren, X.; Yang, F.; Li, B.; Wu, H.; Li, H.; Nie, F. Ultrasound-sensitive siRNA-loaded nanobubbles fabrication and antagonism in drug resistance for NSCLC. Drug Deliv. 2022, 29, 99–110. [Google Scholar] [CrossRef]
- Capolla, S.; Argenziano, M.; Bozzer, S.; D’Agaro, T.; Bittolo, T.; De Leo, L.; Not, T.; Busato, D.; Dal Bo, M.; Toffoli, G.; et al. Targeted chitosan nanobubbles as a strategy to down-regulate microRNA-17 into B-cell lymphoma models. Front. Immunol. 2023, 14, 1200310. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, Q.; Ma, Y.; Lin, C.; Li, J.; Hu, B.; Liu, C.; Zhao, Y. Nanobubbles containing PD-L1 Ab and miR-424 mediated PD-L1 blockade, and its expression inhibition to enable and potentiate hepatocellular carcinoma immunotherapy in mice. Int. J. Pharm. 2022, 629, 122352. [Google Scholar] [CrossRef]
- Lu, S.; Zhao, P.; Deng, Y.; Liu, Y. Mechanistic Insights and Therapeutic Delivery through Micro/Nanobubble-Assisted Ultrasound. Pharmaceutics 2022, 14, 480. [Google Scholar] [CrossRef]
- Qin, Y.; Geng, X.; Sun, Y.; Zhao, Y.; Chai, W.; Wang, X.; Wang, P. Ultrasound nanotheranostics: Toward precision medicine. J. Control. Release 2023, 353, 105–124. [Google Scholar] [CrossRef]
- Cai, W.; Lv, W.; Feng, Y.; Yang, H.; Zhang, Y.; Yang, G.; Duan, Y.; Wang, J. The therapeutic effect in gliomas of nanobubbles carrying siRNA combined with ultrasound-targeted destruction. Int. J. Nanomed. 2018, 13, 6791–6807. [Google Scholar] [CrossRef]

| Product | Drug | Nanotechnology Platform | Cancer Type | Approval | Advantages | Toxicity |
|---|---|---|---|---|---|---|
| NANOTHERM | Fe2O3 | Nanoparticles of superparamagnetic iron oxide coated with amino silane | Glioblastoma, prostate and pancreatic cancers | 2013 (European medicine agency, EMA) | Upon interstitial administration, high blood circulation time and tumor uptake (EPR), heat production under stimulation with EMF and theranostic properties | Moderate adverse effect |
| NBTXR3 (HENSIFY) | Hafnium oxide nanoparticles stimulated with external radiation to enhance tumor cell death via electron production | Hafnium oxide nanoparticles | Locally advanced squamous cell carcinoma | 2019 (CE Mark) | Radiotherapy enhancer | Injection site pain, hypotension and radiation skin injury |
| Name of Drug | Active Ingredients/Drugs Used | Nanocarrier/Formulation Type | Cancer Type | Properties/Objectives | Status |
|---|---|---|---|---|---|
| CARBON NANOPARTICLES | Carbon nanoparticle | Carbon nanoparticle | Advanced gastric cancer | Harvest lymph nodes after surgery | Phase 3 |
| MAGNETIC NANOPARTICLES | Iron nanoparticle | Nanoparticle | Prostate cancer | Magnetic thermo-ablation | Early phase 1 (completed) |
| AGUIX GADOLINIUM-BASED NANOPARTICLES | AGuIX | Gadolinium-based nanoparticle | Centrally located lung tumors and pancreatic cancer | Safety and efficacy, stereotactic magnetic resonance-guided adaptive radiation therapy | Phase 1 & 2 |
| MR-LINAC-SPION | Ferumoxytol | Iron oxide nanoparticles (SPION) | Primary and metastatic hepatic cancers | Radiotherapy | Not mentioned |
| CD24-GOLD NANOCOMPOSITE | CD24 primer and gold nanoparticle | Gold nanoparticles | Salivary gland tumors | Diagnostic tool, biomarker | Not mentioned |
| MAGNETIC PARTICLE-ICG | Magnetic tracers (FerroTrace) and indocyanine green (ICG) | Magnetic nanoparticles | Colorectal cancer | Feasibility of sentinel lymph node (SLN) mapping and safety | Phase 1 and 2 |
| NANOTHERM® | Iron nanoparticles | Iron nanoparticles | Intermediate-risk prostate cancer | NanoTherm ablation | Not applicable |
| CARBON NANOPARTICLES | Carbon nanoparticles and indocyanine green | Carbon nanoparticles | Colorectal cancer | Lymph node tracers | Phase 2 and 3 |
| NBTXR3 | Hafnium oxide | Nanoparticles | Locally advanced or borderline-resectable pancreatic cancer | Particle activation by radiation therapy | Phase 1 |
| SILICON INCORPORATED WITH QUATERNARY AMMONIUM POLYETHYLENIMINE NANOPARTICLES | Silicon | Quaternary ammonium poly-ethylenimine nanoparticles | Carcinoma of head and neck | Antibacterial activity | Phase 1 |
| NBTXR3 | Hafnium oxide | Hafnium oxide-containing nanoparticles | Esophageal cancer | Radiation therapy with concurrent chemotherapy | Phase 1 |
| SILICA NANOPARTICLES | Silica nanoparticles | Silica nanoparticles | Head and neck melanoma | Bioimaging | Phase 1 and 2 |
| POLYMERIC NANOPARTICLES | Polymeric nanoparticles | Polymeric nanoparticles | Colorectal cancer | Targeting somatostatin receptors | Phase 1 |
| NANOPARTICLE | Inorganic nanoparticle | Nanoparticle | Advanced breast cancer | Pharmacokinetic profile | Phase 1 |
| PLGA-PEG NANOPARTICLES | Amphiphilic polymer | PLGA nanoparticles | Squamous cell carcinoma | Therapeutic efficacy | Phase 2 |
| SUPERPARAMAGNETIC IRON OXIDE NANOPARTICLE | SPIONs | Iron nanoparticle | Breast and colon cancer cells | Radio-sensitization of cancer cells Hyperthermia effect on cancer cells | Not mentioned |
| CRIPEC NANOPARTICLES | Cisplatin, carboplatin and oxaliplatin nanoparticles | Nanoparticle | Platinum resistant ovarian cancer | Chemotherapeutic eradication of cancer | Phase 2 (completed) |
| Clinical Products | Active Agent | Lipid/Lipid:Drug | Indication |
|---|---|---|---|
| DOXIL | Doxorubicin | HSPC:Cholesterol:PEG 2000-DSPE | Ovarian, breast cancer, Kaposi’s sarcoma |
| DAUNOXOME | Daunorubicin | DSPC and Cholesterol | AIDS-related Kaposi’s sarcoma |
| DEPOCYT | Cytarabine/Ara-C | DOPC, DPPG, Cholesterol and Triolein | Neoplastic meningitis |
| MYOCET | Doxorubicin | EPC:Cholesterol | Combination therapy with cyclophosphamide in metastatic breast cancer |
| MEPACT | Mifamurtide | DOPS:POPC | High-grade, resectable, non-metastatic osteosarcoma |
| MARQIBO | Vincristine | SM:Cholesterol | Acute lymphoblastic leukemia |
| ONIVYDE | Irinotecan | DSPC:MPEG-2000:DSPE | Combination therapy with fluorouracil and leucovorin in metastatic adenocarcinoma of the pancreas |
| VYXEOS/CPX-351 | Cytarabine: daunorubicin | DSPG:DSPC:CL | Newly diagnosed therapy–related acute myeloid leukemia, acute myeloid leukemia |
| ZOLSKETIL | Doxorubicin | HSPC:CL:MPEG | Metastatic breast cancer, advanced ovarian cancer, multiple myeloma, AIDS-related Kaposi’s sarcoma |
| LIPO-DOX | Doxorubicin | DSPC:CL:MPEG | Kaposi’s sarcoma, ovarian cancer, breast cancer, multiple myeloma |
| LIPOPLATIN | Cisplatin | SPC-3:DPPG:CL: | Malignant pleural effusions |
| SPI-077 | Cisplatin | HSPC:CL:MPEG2000-DSPE | Ovarian cancer |
| Drug | Type | Status in Clinical Practice |
|---|---|---|
| ABRAXANE® | Protein-bound paclitaxel, also known as nanoparticle albumin–bound | 2005 FDA, 2008 EMA approval for solid cancers, 2012 and 2013 widened approvals |
| DAUNOXOME® | NonPEGylated daunorubicin citrate liposome injection | 1996 FDA approval for HIV-related Kaposi’s sarcoma. The permanent discontinuation was purely a business decision. |
| DEPOCYT® | Liposomal cytarabine | 1999 FDA approval for Lyposomatous meningitis 2017 Permanent discontinuation due to persistent technical issues in manufacturing process. |
| DOXIL® | Doxorubicin enclosed in uni-lamellar liposome coated with PEG | 1995 FDA approved as first nanodrug used to treat metastatic ovarian cancer and AIDS-related Kaposi’s sarcoma |
| ELIGARD® | Leuprolide acetate | FDA approval for prostate cancer |
| GENEXOL-PM® | Paclitaxel-loaded polymeric micelle | 2007 EMA and Korea for breast and lung cancer |
| LIPUSU® | (Liposomal paclitaxel) | 2006 China for solid cancers |
| HENSIFY® (NBTXR3) | Radio-enhancer composed of hafnium oxide nanoparticles | FDA 2019 locally advanced soft tissue sarcoma |
| MARQIBO® | vincristine sulfate liposome injection | 2012 FDA Philadelphia chromosome-negative acute lymphoblastic leukemia FDA withdraws approval clinical trial failed to verify the clinical benefit of the drug. |
| MEPACT® | muramyl tripeptide phosphatidylethanolamine, encapsulated into liposomes (L-MTP-PE). | FDA 2001 and EMA 2009 for osteosarcoma; FDA 2007 and EMA 2009 denied approval |
| MYOCET® | Liposome-encapsulated doxorubicin (nonPEGylated) | 2000 EMA for breast cancer |
| NANOTHERM® | Magnetic nanoparticles of iron oxide implanted into the tumor or cavity wall and heated by alternating magnetic field | EMA 2010 and FDA 2018 for glioblastoma and prostate Cancer |
| ONCASPAR® | PEGasparaginase | FDA 1994 for acute lymphocytic leukemia |
| ONIVYDE® | Topoisomerase I inhibitor with irinotecan contained within a liposomal sphere. | FDA 2015 metastatic adenocarcinoma |
| ONTAC® | (Engineered protein combining interleukin-2 and diphtheria toxin) | Cutaneous T-cell lymphoma FDA (1999) |
| SMANCS® | Poly(styrene-co-maleic acid)-conjugated neo-carzinostatin | Hepatoma Japan (1997) |
| VYXEOS® | (Daunorubicin/cytarabine)fixed-dose chemotherapy combination | FDA 2017, EMA 2018 newly-diagnosed therapy-related acute myeloid leukemia (t-AML) or AML with myelodysplasia-related changes (AML-MRC) in people aged one year of age and older. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavelić, K.; Kraljević Pavelić, S.; Bulog, A.; Agaj, A.; Rojnić, B.; Čolić, M.; Trivanović, D. Nanoparticles in Medicine: Current Status in Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 12827. https://doi.org/10.3390/ijms241612827
Pavelić K, Kraljević Pavelić S, Bulog A, Agaj A, Rojnić B, Čolić M, Trivanović D. Nanoparticles in Medicine: Current Status in Cancer Treatment. International Journal of Molecular Sciences. 2023; 24(16):12827. https://doi.org/10.3390/ijms241612827
Chicago/Turabian StylePavelić, Krešimir, Sandra Kraljević Pavelić, Aleksandar Bulog, Andrea Agaj, Barbara Rojnić, Miroslav Čolić, and Dragan Trivanović. 2023. "Nanoparticles in Medicine: Current Status in Cancer Treatment" International Journal of Molecular Sciences 24, no. 16: 12827. https://doi.org/10.3390/ijms241612827
APA StylePavelić, K., Kraljević Pavelić, S., Bulog, A., Agaj, A., Rojnić, B., Čolić, M., & Trivanović, D. (2023). Nanoparticles in Medicine: Current Status in Cancer Treatment. International Journal of Molecular Sciences, 24(16), 12827. https://doi.org/10.3390/ijms241612827








