Adsorption of Ferritin at Nanofaceted Al2O3 Surfaces
Abstract
1. Introduction
2. Results
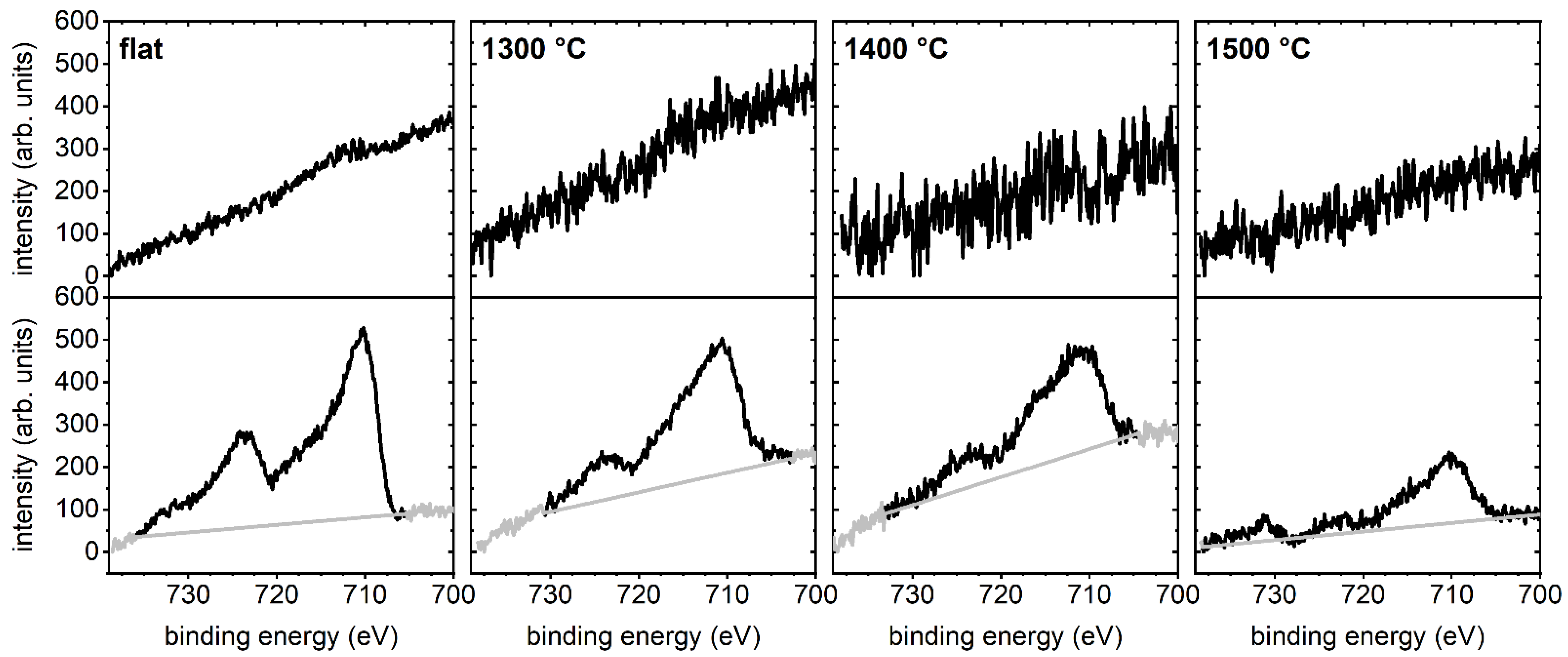
2.1. Substrate Surface Characterization
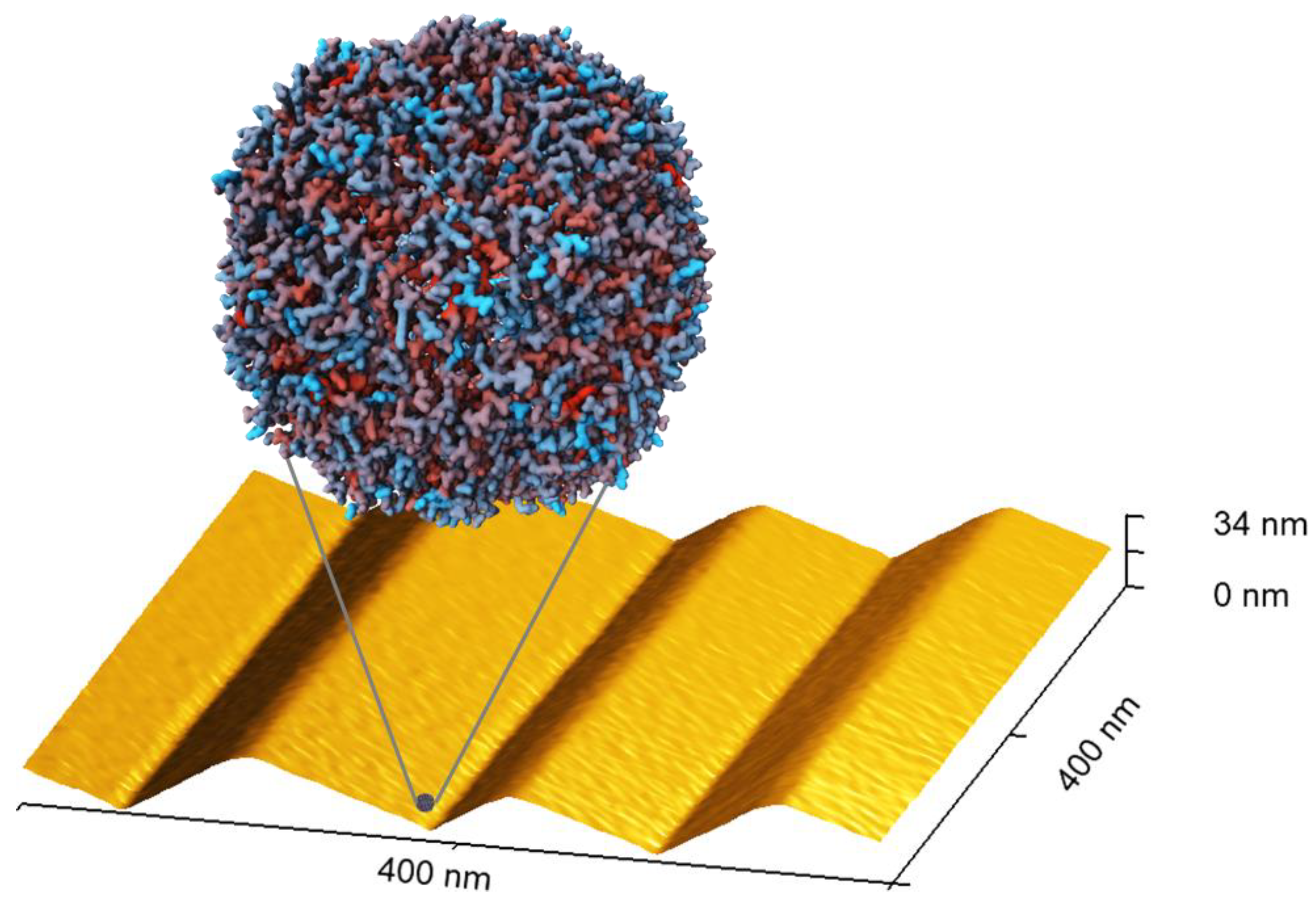
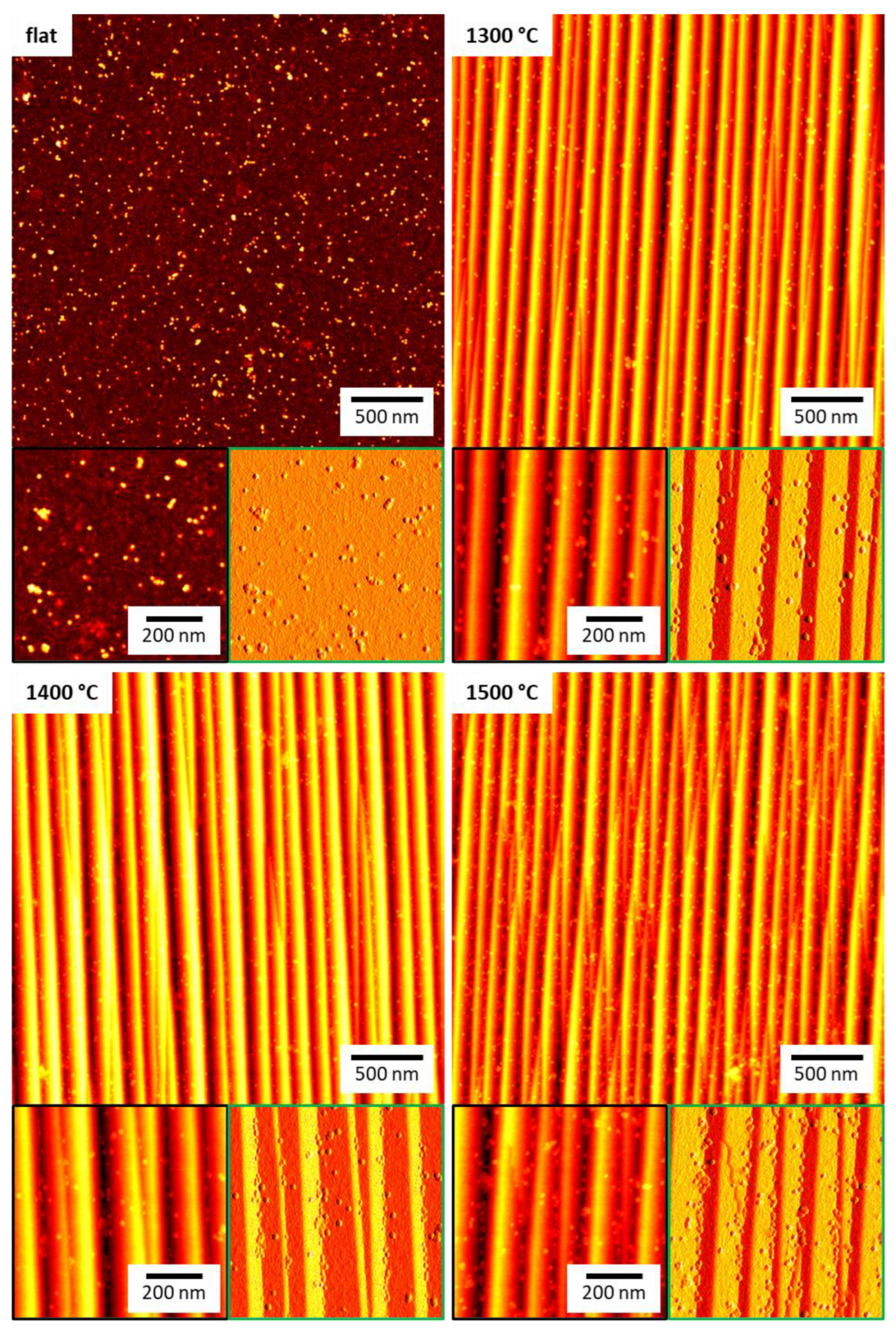
2.2. Ferritin Adsorption
3. Discussion
4. Materials and Methods
4.1. Fabrication of Nanofaceted Al2O3 Surfaces
4.2. Ferritin Adsorption
4.3. Atomic Force Microscopy (AFM)
4.4. X-ray Photoelectron Spectroscopy (XPS)
4.5. Quantification and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef]
- Jaffer, I.H.; Weitz, J.I. The blood compatibility challenge. Part 1: Blood-contacting medical devices: The scope of the problem. Acta Biomater. 2019, 94, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, J.Y.; Ling, Y.; Kim, S. Non-Specific Adsorption Reduction Methods in Biosensing. Sensors 2019, 19, 2488. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Schwiderek, S.; Grundmeier, G.; Keller, A. Strain-Dependent Adsorption of Pseudomonas aeruginosa-Derived Adhesin-Like Peptides at Abiotic Surfaces. Micro 2021, 1, 129–139. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, J.; Dornbusch, D.; Grundmeier, G.; Fahmy, K.; Keller, A.; Cheung, D.L. Effect of Surface Hydrophobicity on the Adsorption of a Pilus-Derived Adhesin-like Peptide. Langmuir 2022, 38, 9257–9265. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Grundmeier, G.; Keller, A. Adsorption of SARS-CoV-2 Spike Protein S1 at Oxide Surfaces Studied by High-Speed Atomic Force Microscopy. Adv. NanoBiomed Res. 2021, 1, 2000024. [Google Scholar] [CrossRef]
- Żeliszewska, P.; Wasilewska, M.; Batys, P.; Pogoda, K.; Deptuła, P.; Bucki, R.; Adamczyk, Z. SARS-CoV-2 Spike Protein (RBD) Subunit Adsorption at Abiotic Surfaces and Corona Formation at Polymer Particles. Int. J. Mol. Sci. 2022, 23, 12374. [Google Scholar] [CrossRef]
- Rabe, M.; Verdes, D.; Seeger, S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef]
- Latour, R. Biomaterials: Protein–Surface Interactions. In Encyclopedia of Biomaterials and Biomedical Engineering, Second Edition-Four Volume Set; Wnek, G., Bowlin, G., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 270–284. ISBN 978-1-4200-7802-2. [Google Scholar]
- Dee, K.C.; Puleo, D.A.; Bizios, R. (Eds.) Protein-Surface Interactions. In An Introduction To Tissue-Biomaterial Interactions; John Wiley & Sons, Inc.: New York, NY, USA, 2002; pp. 37–52. ISBN 9780471270591. [Google Scholar]
- Lord, M.S.; Foss, M.; Besenbacher, F. Influence of nanoscale surface topography on protein adsorption and cellular response. Nano Today 2010, 5, 66–78. [Google Scholar] [CrossRef]
- Firkowska-Boden, I.; Zhang, X.; Jandt, K.D. Controlling Protein Adsorption through Nanostructured Polymeric Surfaces. Adv. Healthc. Mater. 2018, 7, 1700995. [Google Scholar] [CrossRef]
- Barberi, J.; Spriano, S. Titanium and Protein Adsorption: An Overview of Mechanisms and Effects of Surface Features. Materials 2021, 14, 1590. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Keller, A. Ion Beam Nanopatterning of Biomaterial Surfaces. Appl. Sci. 2021, 11, 6575. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, M.; Böke, F.; Qin, Q.; Hübner, R.; Knust, S.; Schwiderek, S.; Grundmeier, G.; Fischer, H.; Keller, A. Effect of nanoscale surface topography on the adsorption of globular proteins. Appl. Surf. Sci. 2021, 535, 147671. [Google Scholar] [CrossRef]
- Yang, Y.; Knust, S.; Schwiderek, S.; Qin, Q.; Yun, Q.; Grundmeier, G.; Keller, A. Protein Adsorption at Nanorough Titanium Oxide Surfaces: The Importance of Surface Statistical Parameters beyond Surface Roughness. Nanomaterials 2021, 11, 357. [Google Scholar] [CrossRef] [PubMed]
- Hanke, M.; Yang, Y.; Ji, Y.; Grundmeier, G.; Keller, A. Nanoscale Surface Topography Modulates hIAPP Aggregation Pathways at Solid-Liquid Interfaces. Int. J. Mol. Sci. 2021, 22, 5142. [Google Scholar] [CrossRef]
- Denry, I.; Holloway, J. Ceramics for Dental Applications: A Review. Materials 2010, 3, 351–368. [Google Scholar] [CrossRef]
- Rahmati, M.; Mozafari, M. Biocompatibility of alumina-based biomaterials-A review. J. Cell. Physiol. 2019, 234, 3321–3335. [Google Scholar] [CrossRef]
- Salerno, M.; Caneva-Soumetz, F.; Pastorino, L.; Patra, N.; Diaspro, A.; Ruggiero, C. Adhesion and proliferation of osteoblast-like cells on anodic porous alumina substrates with different morphology. IEEE Trans. Nanobiosci. 2013, 12, 106–111. [Google Scholar] [CrossRef]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials 2000, 21, 1803–1810. [Google Scholar] [CrossRef]
- Wittenbrink, I.; Hausmann, A.; Schickle, K.; Lauria, I.; Davtalab, R.; Foss, M.; Keller, A.; Fischer, H. Low-aspect ratio nanopatterns on bioinert alumina influence the response and morphology of osteoblast-like cells. Biomaterials 2015, 62, 58–65. [Google Scholar] [CrossRef]
- Theil, E.C. Ferritin: Structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu. Rev. Biochem. 1987, 56, 289–315. [Google Scholar] [CrossRef]
- Wang, W.; Knovich, M.A.; Coffman, L.G.; Torti, F.M.; Torti, S.V. Serum ferritin: Past, present and future. Biochim. Biophys. Acta 2010, 1800, 760–769. [Google Scholar] [CrossRef]
- Ramírez-Carmona, W.; Díaz-Fabregat, B.; Yuri Yoshigae, A.; Musa de Aquino, A.; Scarano, W.R.; de Souza Castilho, A.C.; Avansini Marsicano, J.; Leal do Prado, R.; Pessan, J.P.; de Oliveira Mendes, L. Are Serum Ferritin Levels a Reliable Cancer Biomarker? A Systematic Review and Meta-Analysis. Nutr. Cancer 2022, 74, 1917–1926. [Google Scholar] [CrossRef]
- Kappert, K.; Jahić, A.; Tauber, R. Assessment of serum ferritin as a biomarker in COVID-19: Bystander or participant? Insights by comparison with other infectious and non-infectious diseases. Biomarkers 2020, 25, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, I.; Iwahori, K.; Kumagai, S. Ferritin in the field of nanodevices. Biochim. Biophys. Acta 2010, 1800, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Erb, D.J.; Perlich, J.; Roth, S.V.; Röhlsberger, R.; Schlage, K. Real-Time Observation of Temperature-Induced Surface Nanofaceting in M-Plane α-Al2O3. ACS Appl. Mater. Interfaces 2022, 14, 31373–31384. [Google Scholar] [CrossRef]
- Heffelfinger, J.R.; Carter, C. Mechanisms of surface faceting and coarsening. Surf. Sci. 1997, 389, 188–200. [Google Scholar] [CrossRef]
- de Val, N.; Declercq, J.-P.; Lim, C.K.; Crichton, R.R. Structural analysis of haemin demetallation by L-chain apoferritins. J. Inorg. Biochem. 2012, 112, 77–84. [Google Scholar] [CrossRef]
- Dietz, C.; Herruzo, E.T.; Lozano, J.R.; Garcia, R. Nanomechanical coupling enables detection and imaging of 5 nm superparamagnetic particles in liquid. Nanotechnology 2011, 22, 125708. [Google Scholar] [CrossRef]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef]
- Hikono, T.; Matsumura, T.; Miura, A.; Uraoka, Y.; Fuyuki, T.; Takeguchi, M.; Yoshii, S.; Yamashita, I. Electron confinement in a metal nanodot monolayer embedded in silicon dioxide produced using ferritin protein. Appl. Phys. Lett. 2006, 88, 23108. [Google Scholar] [CrossRef]
- Kubota, T.; Baba, T.; Saito, S.; Yamasaki, S.; Kumagai, S.; Matsui, T.; Uraoka, Y.; Fuyuki, T.; Yamashita, I.; Samukawa, S. Low-damage fabrication of high aspect nanocolumns by using neutral beams and ferritin-iron-core mask. J. Vac. Sci. Technol. B 2007, 25, 760. [Google Scholar] [CrossRef]
- Martin, K.C.; Villano, S.M.; McCurdy, P.R.; Zapien, D.C. Studies of Electrochemically Transformed Ferritin Adsorbed at Tin-Doped Indium Oxide Electrodes Using X-ray Photoelectron Spectroscopy. Langmuir 2003, 19, 5808–5812. [Google Scholar] [CrossRef]
- Yamazaki, K.; Ikeda, T.; Isono, T.; Ogino, T. Selective adsorption of protein molecules on phase-separated sapphire surfaces. J. Colloid Interface Sci. 2011, 361, 64–70. [Google Scholar] [CrossRef]
- Isono, T.; Ikeda, T.; Aoki, R.; Yamazaki, K.; Ogino, T. Structural- and chemical-phase-separation on single crystalline sapphire (0001) surfaces. Surf. Sci. 2010, 604, 2055–2063. [Google Scholar] [CrossRef]
- Gabai, R.; Ismach, A.; Joselevich, E. Nanofacet Lithography: A New Bottom-Up Approach to Nanopatterning and Nanofabrication by Soft Replication of Spontaneously Faceted Crystal Surfaces. Adv. Mater. 2007, 19, 1325–1330. [Google Scholar] [CrossRef]
- Franks, G.V.; Meagher, L. The isoelectric points of sapphire crystals and alpha-alumina powder. Colloids Surf. A 2003, 214, 99–110. [Google Scholar] [CrossRef]
- Cardier, J.; Romano, E.; Soyano, A. Effect of hepatic isoferritins from iron overloaded rats on lymphocyte proliferative response: Role of ferritin iron content. Immunopharmacol. Immunotoxicol. 1995, 17, 719–732. [Google Scholar] [CrossRef]
- Li, N.; Thomas, R.K.; Rennie, A.R. Adsorption of non-ionic surfactants to the sapphire/solution interface--effects of temperature and pH. J. Colloid Interface Sci. 2012, 369, 287–293. [Google Scholar] [CrossRef]
- Li, N.; Thomas, R.K.; Rennie, A.R. Effect of pH, surface charge and counter-ions on the adsorption of sodium dodecyl sulfate to the sapphire/solution interface. J. Colloid Interface Sci. 2012, 378, 152–158. [Google Scholar] [CrossRef]
- Taylor, C.E.; Schwartz, D.K. Octadecanoic Acid Self-Assembled Monolayer Growth at Sapphire Surfaces. Langmuir 2003, 19, 2665–2672. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 99. [Google Scholar] [CrossRef]
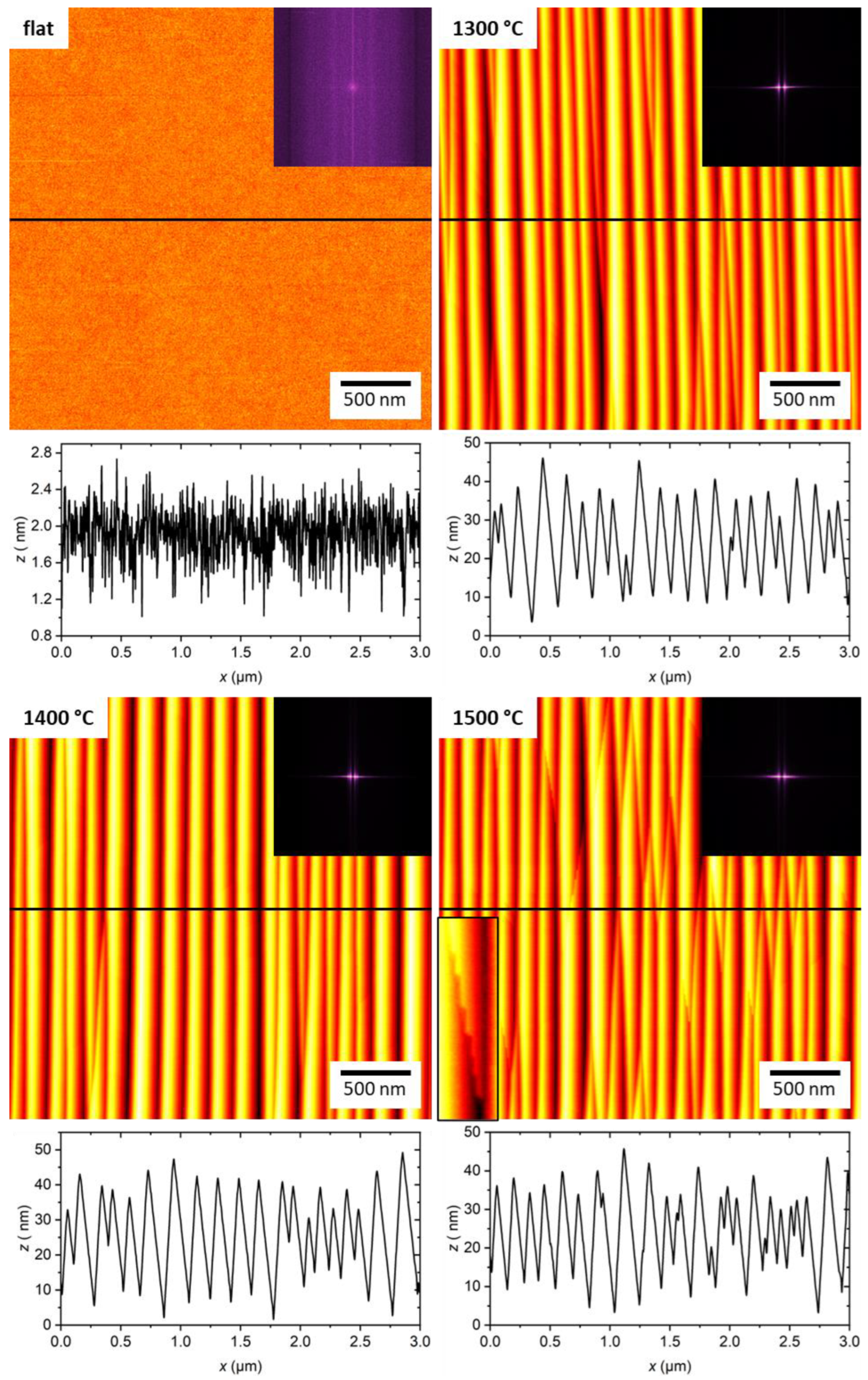


| Flat | 1300 °C | 1400 °C | 1500 °C | |
|---|---|---|---|---|
| Sq (nm) | 0.30 ± 0.02 | 8.43 ± 0.91 | 10.09 ± 0.26 | 7.98 ± 0.66 |
| r | 1.01 ± 0.01 | 1.08 ± 0.01 | 1.09 ± 0.01 | 1.09 ± 0.01 |
| λ (nm) | - | 162.3 ± 7.1 | 177.5 ± 13.2 | 145.9 ± 15.7 |
| Ap2p (nm) | - | 15.3 ± 1.5 | 18.4 ± 0.3 | 14.7 ± 1.1 |
| Ap2p/λ | - | 0.094 ± 0.014 | 0.104 ± 0.009 | 0.100 ± 0.018 |
| Flat | 1300 °C | 1400 °C | 1500 °C | |
|---|---|---|---|---|
| C 1s (at%) | 8.0 | 16.1 | 12.9 | 10.7 |
| O 1s (at%) | 53.5 | 48.1 | 49.5 | 51.5 |
| Al 2p (at%) | 38.5 | 35.8 | 37.6 | 37.8 |
| O 1s/Al 2p | 1.39 | 1.34 | 1.32 | 1.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pothineni, B.K.; Kollmann, S.; Li, X.; Grundmeier, G.; Erb, D.J.; Keller, A. Adsorption of Ferritin at Nanofaceted Al2O3 Surfaces. Int. J. Mol. Sci. 2023, 24, 12808. https://doi.org/10.3390/ijms241612808
Pothineni BK, Kollmann S, Li X, Grundmeier G, Erb DJ, Keller A. Adsorption of Ferritin at Nanofaceted Al2O3 Surfaces. International Journal of Molecular Sciences. 2023; 24(16):12808. https://doi.org/10.3390/ijms241612808
Chicago/Turabian StylePothineni, Bhanu K., Sabrina Kollmann, Xinyang Li, Guido Grundmeier, Denise J. Erb, and Adrian Keller. 2023. "Adsorption of Ferritin at Nanofaceted Al2O3 Surfaces" International Journal of Molecular Sciences 24, no. 16: 12808. https://doi.org/10.3390/ijms241612808
APA StylePothineni, B. K., Kollmann, S., Li, X., Grundmeier, G., Erb, D. J., & Keller, A. (2023). Adsorption of Ferritin at Nanofaceted Al2O3 Surfaces. International Journal of Molecular Sciences, 24(16), 12808. https://doi.org/10.3390/ijms241612808







