Endogenous Digitalis-like Factors as a Key Molecule in the Pathophysiology of Pregnancy-Induced Hypertension and a Potential Therapeutic Target in Preeclampsia
Abstract
1. Introduction
2. Pathogenesis of Preeclampsia
3. Na+/K+-ATPase- “Sodium Pump”
4. Endogenous Digitalis-Like Factors (EDLF)
4.1. General Information
4.2. Cardiotonic Steroids in Normal Pregnancy
4.3. Endogenous Digitalis-like Factors in Preeclampsia
4.3.1. Endogenous Digitalis-like Immunoreactivity in Preeclampsia
4.3.2. MBG as the Main Cardiotonic Steroid Implicated in the Pathogenesis of PE
4.4. EDLF and Pathophysiology of Preeclampsia
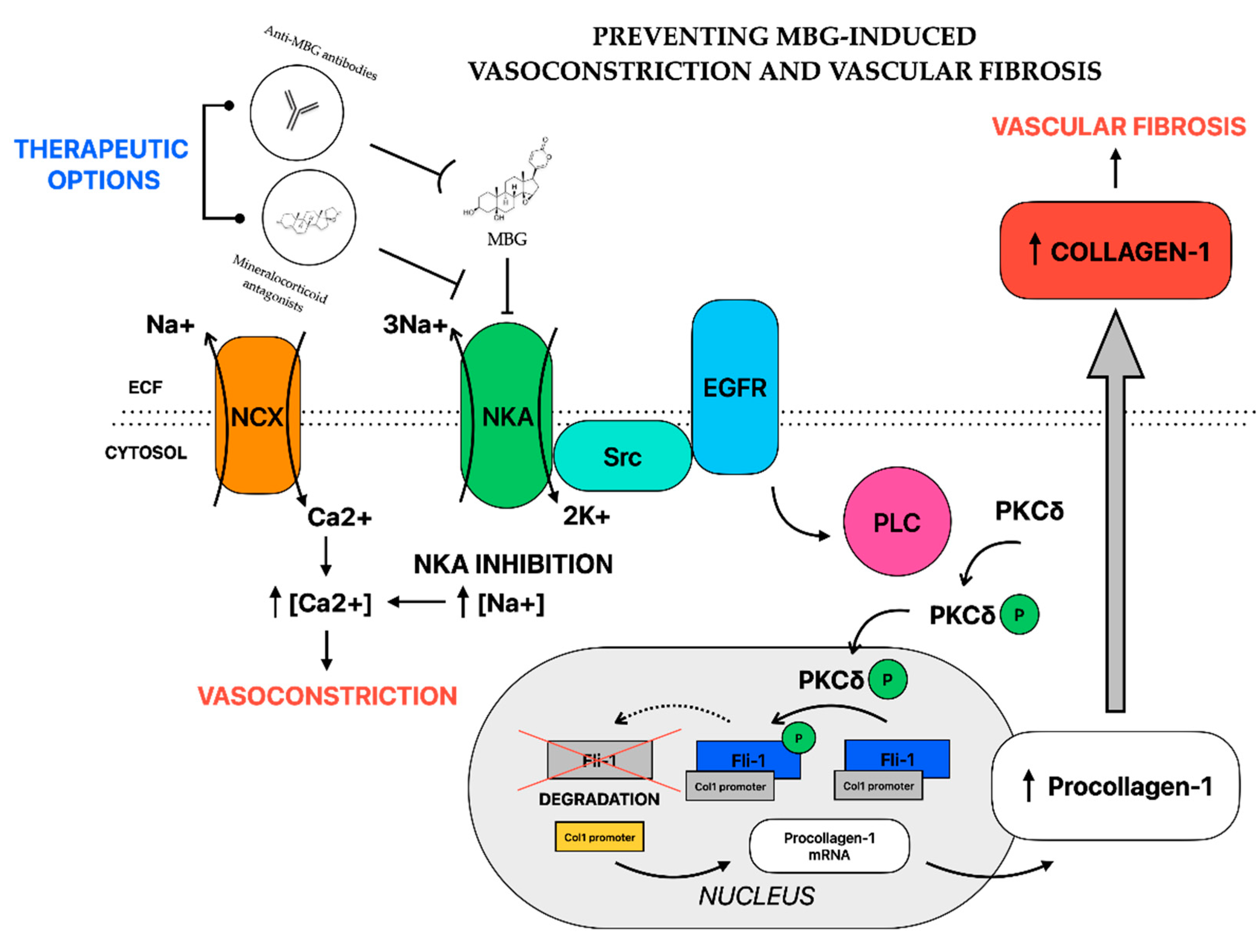
4.4.1. Vasoconstriction
4.4.2. Vascular Fibrosis
4.4.3. Other Mechanisms
4.5. EDLFs as a Potential Therapeutic Target in Preeclampsia
4.5.1. In Vitro and Animal Studies
4.5.2. Case Reports and Clinical Trails
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuklina, E.V.; Ayala, C.; Callaghan, W.M. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet. Gynecol. 2009, 113, 1299–1306. [Google Scholar] [CrossRef]
- Say, L.; Chou, D.; Gemmill, A.; Tuncalp, O.; Moller, A.B.; Daniels, J.; Gulmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef]
- Hypertension in Pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S.; et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Wu, P.; Haththotuwa, R.; Kwok, C.S.; Babu, A.; Kotronias, R.A.; Rushton, C.; Zaman, A.; Fryer, A.A.; Kadam, U.; Chew-Graham, C.A.; et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003497. [Google Scholar] [CrossRef] [PubMed]
- Mosca, L.; Benjamin, E.J.; Berra, K.; Bezanson, J.L.; Dolor, R.J.; Lloyd-Jones, D.M.; Newby, L.K.; Pina, I.L.; Roger, V.L.; Shaw, L.J.; et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: A guideline from the american heart association. Circulation 2011, 123, 1243–1262. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, C.; McCullough, L.D.; Awad, I.A.; Chireau, M.V.; Fedder, W.N.; Furie, K.L.; Howard, V.J.; Lichtman, J.H.; Lisabeth, L.D.; Pina, I.L.; et al. Guidelines for the prevention of stroke in women: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 1545–1588. [Google Scholar] [CrossRef]
- Poon, L.C.; Nguyen-Hoang, L.; Smith, G.N.; Bergman, L.; O’Brien, P.; Hod, M.; Okong, P.; Kapur, A.; Maxwell, C.V.; McIntyre, H.D.; et al. Hypertensive disorders of pregnancy and long-term cardiovascular health: FIGO Best Practice Advice. Int. J. Gynecol. Obstet. 2023, 160 (Suppl. S1), 22–34. [Google Scholar] [CrossRef]
- Nahum Sacks, K.; Friger, M.; Shoham-Vardi, I.; Spiegel, E.; Sergienko, R.; Landau, D.; Sheiner, E. Prenatal exposure to preeclampsia as an independent risk factor for long-term cardiovascular morbidity of the offspring. Pregnancy Hypertens. 2018, 13, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Karatza, A.A.; Dimitriou, G. Preeclampsia Emerging as a Novel Risk Factor for Cardiovascular Disease in the Offspring. Curr. Pediatr. Rev. 2020, 16, 194–199. [Google Scholar] [CrossRef]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. 2019, 145 (Suppl. S1), 1–33. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Wright, D.; Poon, L.C.Y.; Syngelaki, A.; O’Gorman, N.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. ASPRE trial: Performance of screening for preterm pre-eclampsia. Ultrasound Obstet. Gynecol. 2017, 50, 492–495. [Google Scholar] [CrossRef]
- Fetal Medicine Fundation—I Trimester Risk Assessment for Preeclampisa. Available online: https://fetalmedicine.org/research/assess/preeclampsia/first-trimester (accessed on 22 June 2023).
- Hofmeyr, G.J.; Lawrie, T.A.; Atallah, A.N.; Torloni, M.R. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst. Rev. 2018, 10, CD001059. [Google Scholar] [CrossRef]
- Verlohren, S.; Brennecke, S.P.; Galindo, A.; Karumanchi, S.A.; Mirkovic, L.B.; Schlembach, D.; Stepan, H.; Vatish, M.; Zeisler, H.; Rana, S. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hypertens. 2022, 27, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O.V.; Tapilskaya, N.I.; Bzhelyansky, A.M.; Frolova, E.V.; Nikitina, E.R.; Reznik, V.A.; Kashkin, V.A.; Bagrov, A.Y. Interaction of Digibind with endogenous cardiotonic steroids from preeclamptic placentae. J. Hypertens. 2010, 28, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Buckalew, V.M. Role of endogenous digitalis-like factors in the clinical manifestations of severe preeclampsia: A sytematic review. Clin. Sci. Lond. 2018, 132, 1215–1242. [Google Scholar] [CrossRef]
- Redman, C.W. Current topic: Pre-eclampsia and the placenta. Placenta 1991, 12, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Woods, A.W.; Jauniaux, E.; Kingdom, J.C. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009, 30, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Staff, A.C. The two-stage placental model of preeclampsia: An update. J. Reprod. Immunol. 2019, 134–135, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bakrania, B.A.; Spradley, F.T.; Drummond, H.A.; LaMarca, B.; Ryan, M.J.; Granger, J.P. Preeclampsia: Linking Placental Ischemia with Maternal Endothelial and Vascular Dysfunction. Compr. Physiol. 2020, 11, 1315–1349. [Google Scholar] [CrossRef]
- Ives, C.W.; Sinkey, R.; Rajapreyar, I.; Tita, A.T.N.; Oparil, S. Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1690–1702. [Google Scholar] [CrossRef]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, E.; Medcalf, K.E.; Park, A.L.; Ray, J.G.; High Risk of Pre-eclampsia Identification, G. Clinical risk factors for pre-eclampsia determined in early pregnancy: Systematic review and meta-analysis of large cohort studies. BMJ 2016, 353, i1753. [Google Scholar] [CrossRef] [PubMed]
- Bagrov, A.Y.; Shapiro, J.I.; Fedorova, O.V. Endogenous cardiotonic steroids: Physiology, pharmacology, and novel therapeutic targets. Pharmacol. Rev. 2009, 61, 9–38. [Google Scholar] [CrossRef] [PubMed]
- Skou, J.C.; Esmann, M. The Na,K-ATPase. J. Bioenerg. Biomembr. 1992, 24, 249–261. [Google Scholar] [CrossRef]
- Seflova, J.; Habibi, N.R.; Yap, J.Q.; Cleary, S.R.; Fang, X.; Kekenes-Huskey, P.M.; Espinoza-Fonseca, L.M.; Bossuyt, J.B.; Robia, S.L. Fluorescence lifetime imaging microscopy reveals sodium pump dimers in live cells. J. Biol. Chem. 2022, 298, 101865. [Google Scholar] [CrossRef]
- Clausen, M.V.; Hilbers, F.; Poulsen, H. The Structure and Function of the Na,K-ATPase Isoforms in Health and Disease. Front. Physiol. 2017, 8, 371. [Google Scholar] [CrossRef]
- Fedorova, O.V.; Bagrov, A.Y. Inhibition of Na/K ATPase from rat aorta by two Na/K pump inhibitors, ouabain and marinobufagenin: Evidence of interaction with different alpha-subunit isoforms. Am. J. Hypertens. 1997, 10, 929–935. [Google Scholar] [CrossRef]
- Aperia, A.; Akkuratov, E.E.; Fontana, J.M.; Brismar, H. Na+-K+-ATPase, a new class of plasma membrane receptors. Am. J. Physiol. Cell Physiol. 2016, 310, C491–C495. [Google Scholar] [CrossRef]
- Hamlyn, J.M.; Manunta, P. Endogenous cardiotonic steroids in kidney failure: A review and an hypothesis. Adv. Chronic Kidney Dis. 2015, 22, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.J.; Shrestha, K.; Sheehey, B.; Li, X.S.; Guggilam, A.; Wu, Y.; Finucan, M.; Gabi, A.; Medert, C.M.; Westfall, K.; et al. Elevated Plasma Marinobufagenin, An Endogenous Cardiotonic Steroid, Is Associated with Right Ventricular Dysfunction and Nitrative Stress in Heart Failure. Circ. Heart Fail. 2015, 8, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Hamlyn, J.M.; Blaustein, M.P. Endogenous Ouabain: Recent Advances and Controversies. Hypertension 2016, 68, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, F.K.; Dube, P.; Mohamed, A.; Tian, J.; Malhotra, D.; Haller, S.T.; Kennedy, D.J. Cardiotonic Steroids and the Sodium Trade Balance: New Insights into Trade-Off Mechanisms Mediated by the Na(+)/K(+)-ATPase. Int. J. Mol. Sci. 2018, 19, 2576. [Google Scholar] [CrossRef]
- Nesher, M.; Shpolansky, U.; Rosen, H.; Lichtstein, D. The digitalis-like steroid hormones: New mechanisms of action and biological significance. Life Sci. 2007, 80, 2093–2107. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Khalifa, S.A.M.; Taher, E.A.; Farag, M.A.; Saeed, A.; Gamal, M.; Hegazy, M.F.; Youssef, D.; Musharraf, S.G.; Alajlani, M.M.; et al. Cardenolides: Insights from chemical structure and pharmacological utility. Pharmacol. Res. 2019, 141, 123–175. [Google Scholar] [CrossRef]
- Hamlyn, J.M. Biosynthesis of endogenous cardiac glycosides by mammalian adrenocortical cells: Three steps forward. Clin. Chem. 2004, 50, 469–470. [Google Scholar] [CrossRef]
- Murrell, J.R.; Randall, J.D.; Rosoff, J.; Zhao, J.L.; Jensen, R.V.; Gullans, S.R.; Haupert, G.T., Jr. Endogenous ouabain: Upregulation of steroidogenic genes in hypertensive hypothalamus but not adrenal. Circulation 2005, 112, 1301–1308. [Google Scholar] [CrossRef]
- Bagrov, A.Y.; Bagrov, Y.Y.; Fedorova, O.V.; Kashkin, V.A.; Patkina, N.A.; Zvartau, E.E. Endogenous digitalis-like ligands of the sodium pump: Possible involvement in mood control and ethanol addiction. Eur. Neuropsychopharmacol. 2002, 12, 1–12. [Google Scholar] [CrossRef]
- Huang, B.S.; Amin, M.S.; Leenen, F.H. The central role of the brain in salt-sensitive hypertension. Curr. Opin. Cardiol. 2006, 21, 295–304. [Google Scholar] [CrossRef]
- Blaustein, M.P. The pump, the exchanger, and the holy spirit: Origins and 40-year evolution of ideas about the ouabain-Na(+) pump endocrine system. Am. J. Physiol. Cell Physiol. 2018, 314, C3–C26. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O.V.; Zernetkina, V.I.; Shilova, V.Y.; Grigorova, Y.N.; Juhasz, O.; Wei, W.; Marshall, C.A.; Lakatta, E.G.; Bagrov, A.Y. Synthesis of an Endogenous Steroidal Na Pump Inhibitor Marinobufagenin, Implicated in Human Cardiovascular Diseases, Is Initiated by CYP27A1 via Bile Acid Pathway. Circ. Cardiovasc. Genet. 2015, 8, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O.V.; Kolodkin, N.I.; Agalakova, N.I.; Lakatta, E.G.; Bagrov, A.Y. Marinobufagenin, an endogenous alpha-1 sodium pump ligand, in hypertensive Dahl salt-sensitive rats. Hypertension 2001, 37, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O.V.; Dorofeeva, N.A.; Lopatin, D.A.; Lakatta, E.G.; Bagrov, A.Y. Phorbol diacetate potentiates na(+)-k(+) ATPase inhibition by a putative endogenous ligand, marinobufagenin. Hypertension 2002, 39, 298–302. [Google Scholar] [CrossRef]
- Averina, I.V.; Tapilskaya, N.I.; Reznik, V.A.; Frolova, E.V.; Fedorova, O.V.; Lakatta, E.G.; Bagrov, A.Y. Endogenous Na/K-ATPase inhibitors in patients with preeclampsia. Cell. Mol. Biol. 2006, 52, 19–23. [Google Scholar] [PubMed]
- Valdes, R., Jr. Endogenous digoxin-like immunoreactive factors: Impact on digoxin measurements and potential physiological implications. Clin. Chem. 1985, 31, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Ishkaraeva-Yakovleva, V.V.; Fedorova, O.V.; Solodovnikova, N.G.; Frolova, E.V.; Bzhelyansky, A.M.; Emelyanov, I.V.; Adair, C.D.; Zazerskaya, I.E.; Bagrov, A.Y. DigiFab interacts with endogenous cardiotonic steroids and reverses preeclampsia-induced Na/K-ATPase inhibition. Reprod. Sci. 2012, 19, 1260–1267. [Google Scholar] [CrossRef]
- Goodlin, R.C. Antidigoxin antibodies in eclampsia. N. Engl. J. Med. 1988, 318, 518–519. [Google Scholar] [CrossRef]
- Mottelson, M.N.; Lundsgaard, C.C.; Moller, S. Mechanisms in fluid retention—Towards a mutual concept. Clin. Physiol. Funct. Imaging 2020, 40, 67–75. [Google Scholar] [CrossRef]
- Agalakova, N.I.; Kolodkin, N.I.; Adair, C.D.; Trashkov, A.P.; Bagrov, A.Y. Preeclampsia: Cardiotonic Steroids, Fibrosis, Fli1 and Hint to Carcinogenesis. Int. J. Mol. Sci. 2021, 22, 1941. [Google Scholar] [CrossRef] [PubMed]
- Graves, S.W.; Valdes, R., Jr.; Brown, B.A.; Knight, A.B.; Craig, H.R. Endogenous digoxin-immunoreactive substance in human pregnancies. J. Clin. Endocrinol. Metab. 1984, 58, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Gusdon, J.P., Jr.; Buckalew, V.M., Jr.; Hennessy, J.F. A digoxin-like immunoreactive substance in preeclampsia. Am. J. Obstet. Gynecol. 1984, 150, 83–85. [Google Scholar] [CrossRef]
- Graves, S.W.; Williams, G.H. An endogenous ouabain-like factor associated with hypertensive pregnant women. J. Clin. Endocrinol. Metab. 1984, 59, 1070–1074. [Google Scholar] [CrossRef]
- Gonzalez, A.R.; Phelps, S.J.; Cochran, E.B.; Sibai, B.M. Digoxin-like immunoreactive substance in pregnancy. Am. J. Obstet. Gynecol. 1987, 157, 660–664. [Google Scholar] [CrossRef]
- Clerico, A.; Strigini, F.; del Chicca, M.G.; Melis, G.B.; Balzan, S.; Fruzzetti, F.; Bernardini, G.; Fioretti, P. Endogenous digitalis-like factor in pregnant and non-pregnant women. J. Nucl. Med. Allied Sci. 1988, 32, 33–38. [Google Scholar]
- Phelps, S.J.; Cochran, E.B.; Gonzalez-Ruiz, A.; Tolley, E.A.; Hammond, K.D.; Sibai, B.M. The influence of gestational age and preeclampsia on the presence and magnitude of serum endogenous digoxin-like immunoreactive substance(s). Am. J. Obstet. Gynecol. 1988, 158, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Kerkez, S.A.; Poston, L.; Wolfe, C.D.; Quartero, H.W.; Carabelli, P.; Petruckevitch, A.; Hilton, P.J. A longitudinal study of maternal digoxin-like immunoreactive substances in normotensive pregnancy and pregnancy-induced hypertension. Am. J. Obstet. Gynecol. 1990, 162, 783–787. [Google Scholar] [CrossRef]
- Kaminski, K.; Rechberger, T. Concentration of digoxin-like immunoreactive substance in patients with preeclampsia and its relation to severity of pregnancy-induced hypertension. Am. J. Obstet. Gynecol. 1991, 165, 733–736. [Google Scholar] [CrossRef]
- Gilson, G.J.; Graves, S.W.; Qualls, C.R.; Curet, L.B. Digoxin-like immunoreactive substance and sodium-potassium-adenosine triphosphatase inhibition in normal pregnancy: A longitudinal study. Obstet. Gynecol. 1997, 89, 743–746. [Google Scholar] [CrossRef]
- Lopatin, D.A.; Ailamazian, E.K.; Dmitrieva, R.I.; Shpen, V.M.; Fedorova, O.V.; Doris, P.A.; Bagrov, A.Y. Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. J. Hypertens. 1999, 17, 1179–1187. [Google Scholar] [CrossRef]
- Vakkuri, O.; Arnason, S.S.; Pouta, A.; Vuolteenaho, O.; Leppaluoto, J. Radioimmunoassay of plasma ouabain in healthy and pregnant individuals. J. Endocrinol. 2000, 165, 669–677. [Google Scholar] [CrossRef][Green Version]
- Fedorova, O.V.; Simbirtsev, A.S.; Kolodkin, N.I.; Kotov, A.Y.; Agalakova, N.I.; Kashkin, V.A.; Tapilskaya, N.I.; Bzhelyansky, A.; Reznik, V.A.; Frolova, E.V.; et al. Monoclonal antibody to an endogenous bufadienolide, marinobufagenin, reverses preeclampsia-induced Na/K-ATPase inhibition and lowers blood pressure in NaCl-sensitive hypertension. J. Hypertens. 2008, 26, 2414–2425. [Google Scholar] [CrossRef] [PubMed]
- Dvela-Levitt, M.; Cohen-Ben Ami, H.; Rosen, H.; Ornoy, A.; Hochner-Celnikier, D.; Granat, M.; Lichtstein, D. Reduction in maternal circulating ouabain impairs offspring growth and kidney development. J. Am. Soc. Nephrol. 2015, 26, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Agunanne, E.; Horvat, D.; Harrison, R.; Uddin, M.N.; Jones, R.; Kuehl, T.J.; Ghanem, D.A.; Berghman, L.R.; Lai, X.; Li, J.; et al. Marinobufagenin levels in preeclamptic patients: A preliminary report. Am. J. Perinatol. 2011, 28, 509–514. [Google Scholar] [CrossRef]
- Nikitina, E.R.; Mikhailov, A.V.; Nikandrova, E.S.; Frolova, E.V.; Fadeev, A.V.; Shman, V.V.; Shilova, V.Y.; Tapilskaya, N.I.; Shapiro, J.I.; Fedorova, O.V.; et al. In preeclampsia endogenous cardiotonic steroids induce vascular fibrosis and impair relaxation of umbilical arteries. J. Hypertens. 2011, 29, 769–776. [Google Scholar] [CrossRef]
- Lenaerts, C.; Bond, L.; Tuytten, R.; Blankert, B. Revealing of endogenous Marinobufagin by an ultra-specific and sensitive UHPLC-MS/MS assay in pregnant women. Talanta 2018, 187, 193–199. [Google Scholar] [CrossRef]
- Ma, J.; Esplin, M.S.; Adair, C.D.; Mason, L.A.; Graves, S.W. Increasing evidence for and regulation of a human placental endogenous digitalis-like factor. Reprod. Sci. 2012, 19, 437–448. [Google Scholar] [CrossRef]
- Beyers, A.D.; Spruyt, L.L.; Seifart, H.I.; Kriegler, A.; Parkin, D.P.; Van Jaarsveld, P.P. Endogenous immunoreactive digitalis-like substance in neonatal serum and placental extracts. S. Afr. Med. J. 1984, 65, 878–882. [Google Scholar]
- Diamandis, E.P.; Papanastasiou-Diamandi, A.; Soldin, S.J. Digoxin immunoreactivity in cord and maternal serum and placental extracts. Partial characterization of immunoreactive substances by high-performance liquid chromatography and inhibition of Na+, K+-ATPase. Clin. Biochem. 1985, 18, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Hilton, P.J.; White, R.W.; Lord, G.A.; Garner, G.V.; Gordon, D.B.; Hilton, M.J.; Forni, L.G.; McKinnon, W.; Ismail, F.M.; Keenan, M.; et al. An inhibitor of the sodium pump obtained from human placenta. Lancet 1996, 348, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Hopoate-Sitake, M.L.; Adair, C.D.; Mason, L.A.; Torres, C.; Kipikasa, J.; Graves, S.W. Digibind reverses inhibition of cellular rb+ uptake caused by endogenous sodium pump inhibitors present in serum and placenta of women with preeclampsia. Reprod. Sci. 2011, 18, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Agalakova, N.I.; Grigorova, Y.N.; Ershov, I.A.; Reznik, V.A.; Mikhailova, E.V.; Nadei, O.V.; Samuilovskaya, L.; Romanova, L.A.; Adair, C.D.; Romanova, I.V.; et al. Canrenone Restores Vasorelaxation Impaired by Marinobufagenin in Human Preeclampsia. Int. J. Mol. Sci. 2022, 23, 3336. [Google Scholar] [CrossRef]
- Zimmer, E.Z.; Jakobi, P.; Weissman, A.; Cligher, J.; Krivoy, N. Maternal and fetal digoxin-like immunoreactive factor in elective cesarean sections and spontaneous vaginal delivery. Eur. J. Obstet. Gynecol. Reprod. Biol. 1990, 36, 53–58. [Google Scholar] [CrossRef]
- Valdes, R., Jr.; Graves, S.W.; Brown, B.A.; Landt, M. Endogenous substance in newborn infants causing false positive digoxin measurements. J. Pediatr. 1983, 102, 947–950. [Google Scholar] [CrossRef]
- Bagrov, A.Y.; Shapiro, J.I. Endogenous digitalis: Pathophysiologic roles and therapeutic applications. Nat. Clin. Pract. Nephrol. 2008, 4, 378–392. [Google Scholar] [CrossRef]
- West, C.A.; Sasser, J.M.; Baylis, C. The enigma of continual plasma volume expansion in pregnancy: Critical role of the renin-angiotensin-aldosterone system. Am. J. Physiol. Ren. Physiol. 2016, 311, F1125–F1134. [Google Scholar] [CrossRef]
- Fedorova, O.V.; Kolodkin, N.I.; Agalakova, N.I.; Namikas, A.R.; Bzhelyansky, A.; St-Louis, J.; Lakatta, E.G.; Bagrov, A.Y. Antibody to marinobufagenin lowers blood pressure in pregnant rats on a high NaCl intake. J. Hypertens. 2005, 23, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, N.; Dostanic-Larson, I.; Neumann, J.C.; Lingrel, J.B. The ouabain-binding site of the alpha2 isoform of Na,K-ATPase plays a role in blood pressure regulation during pregnancy. Am. J. Hypertens. 2010, 23, 1279–1285. [Google Scholar] [CrossRef]
- Peng, M.; Yang, M.; Ding, Y.; Yu, L.; Deng, Y.; Lai, W.; Hu, Y. Mechanism of endogenous digitalis-like factor-induced vascular endothelial cell damage in patients with severe preeclampsia. Int. J. Mol. Med. 2018, 41, 985–994. [Google Scholar] [CrossRef]
- Beyers, A.D.; Odendaal, H.J.; Spruyt, L.L.; Parkin, D.P. The possible role of endogenous digitalis-like substance in the causation of pre-eclampsia. S. Afr. Med. J. 1984, 65, 883–885. [Google Scholar] [PubMed]
- Schabort, I.; Odendaal, H.J.; Lombard, C.J.; Bredell, L. Comparison between umbilical artery and vein endogenous digoxin-like immuno-active factor levels in normal and pre-eclamptic patients. S. Afr. Med. J. 1991, 79, 197–199. [Google Scholar] [PubMed]
- Seely, E.W.; Williams, G.H.; Graves, S.W. Markers of sodium and volume homeostasis in pregnancy-induced hypertension. J. Clin. Endocrinol. Metab. 1992, 74, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Graves, S.W.; Lincoln, K.; Cook, S.L.; Seely, E.W. Digitalis-like factor and digoxin-like immunoreactive factor in diabetic women with preeclampsia, transient hypertension of pregnancy, and normotensive pregnancy. Am. J. Hypertens. 1995, 8, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Adair, C.D.; Haupert, G.T., Jr.; Koh, H.P.; Wang, Y.; Veille, J.C.; Buckalew, V. Erythrocyte sodium/potassium ATPase activity in severe preeclampsia. J. Perinatol. 2009, 29, 280–283. [Google Scholar] [CrossRef]
- Odendaal, H.J.; Beyers, A.D.; van Heyningen, C.F.; Spruyt, L.L.; Kotze, T.J.; van Jaarsveld, P.P. Immunoreactive digitalis-like substance in pre-eclampsia. S. Afr. Med. J. 1986, 70, 535–537. [Google Scholar]
- Amler, E.; Cester, N.; Salvolini, E.; Staffolani, R.; Burkhard, M.; Mazzanti, L.; Kotyk, A.; Romanini, C. Human hypertensive placenta contains an increased amount of Na,K-ATPase with higher affinity for cardiac glycosides. Cell Biol. Int. 1994, 18, 723–727. [Google Scholar] [CrossRef]
- Fedorova, O.V.; Ishkaraeva, V.V.; Grigorova, Y.N.; Reznik, V.A.; Kolodkin, N.I.; Zazerskaya, I.E.; Zernetkina, V.; Agalakova, N.I.; Tapilskaya, N.I.; Adair, C.D.; et al. Antibody to Marinobufagenin Reverses Placenta-Induced Fibrosis of Umbilical Arteries in Preeclampsia. Int. J. Mol. Sci. 2018, 19, 2377. [Google Scholar] [CrossRef]
- Juhaszova, M.; Blaustein, M.P. Na+ pump low and high ouabain affinity alpha subunit isoforms are differently distributed in cells. Proc. Natl. Acad. Sci. USA 1997, 94, 1800–1805. [Google Scholar] [CrossRef]
- Shelly, D.A.; He, S.; Moseley, A.; Weber, C.; Stegemeyer, M.; Lynch, R.M.; Lingrel, J.; Paul, R.J. Na(+) pump alpha 2-isoform specifically couples to contractility in vascular smooth muscle: Evidence from gene-targeted neonatal mice. Am. J. Physiol. Cell Physiol. 2004, 286, C813–C820. [Google Scholar] [CrossRef]
- Arnon, A.; Hamlyn, J.M.; Blaustein, M.P. Ouabain augments Ca(2+) transients in arterial smooth muscle without raising cytosolic Na(+). Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H679–H691. [Google Scholar] [CrossRef] [PubMed]
- Golovina, V.A.; Song, H.; James, P.F.; Lingrel, J.B.; Blaustein, M.P. Na+ pump alpha 2-subunit expression modulates Ca2+ signaling. Am. J. Physiol. Cell Physiol. 2003, 284, C475–C486. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, M.P.; Hamlyn, J.M. Role of a natriuretic factor in essential hypertension: An hypothesis. Ann. Intern. Med. 1983, 98, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Reznik, V.A.; Kashkin, V.A.; Agalakova, N.I.; Adair, C.D.; Bagrov, A.Y. Endogenous Bufadienolides, Fibrosis and Preeclampsia. Cardiol. Res. Pract. 2019, 2019, 5019287. [Google Scholar] [CrossRef]
- Fedorova, O.V.; Emelianov, I.V.; Bagrov, K.A.; Grigorova, Y.N.; Wei, W.; Juhasz, O.; Frolova, E.V.; Marshall, C.A.; Lakatta, E.G.; Konradi, A.O.; et al. Marinobufagenin-induced vascular fibrosis is a likely target for mineralocorticoid antagonists. J. Hypertens. 2015, 33, 1602–1610. [Google Scholar] [CrossRef]
- Elkareh, J.; Kennedy, D.J.; Yashaswi, B.; Vetteth, S.; Shidyak, A.; Kim, E.G.; Smaili, S.; Periyasamy, S.M.; Hariri, I.M.; Fedorova, L.; et al. Marinobufagenin stimulates fibroblast collagen production and causes fibrosis in experimental uremic cardiomyopathy. Hypertension 2007, 49, 215–224. [Google Scholar] [CrossRef]
- Strauss, M.; Smith, W.; Fedorova, O.V.; Schutte, A.E. The Na(+)K(+)-ATPase Inhibitor Marinobufagenin and Early Cardiovascular Risk in Humans: A Review of Recent Evidence. Curr. Hypertens. Rep. 2019, 21, 38. [Google Scholar] [CrossRef]
- Xie, Z. Molecular mechanisms of Na/K-ATPase-mediated signal transduction. Ann. N. Y. Acad. Sci. 2003, 986, 497–503. [Google Scholar] [CrossRef]
- Elkareh, J.; Periyasamy, S.M.; Shidyak, A.; Vetteth, S.; Schroeder, J.; Raju, V.; Hariri, I.M.; El-Okdi, N.; Gupta, S.; Fedorova, L.; et al. Marinobufagenin induces increases in procollagen expression in a process involving protein kinase C and Fli-1: Implications for uremic cardiomyopathy. Am. J. Physiol. Renal. Physiol. 2009, 296, F1219–F1226. [Google Scholar] [CrossRef]
- Agalakova, N.I.; Reznik, V.A.; Nadei, O.V.; Ershov, I.A.; Rassokha, O.S.; Vasyutina, M.L.; Tapilskaya, N.I.; Rukhliada, N.N.; Galagudza, M.M.; Bagrov, A.Y. Endogenous cardiotonic steroids and vascular fibrosis in preeclampsia. Arter. Gipertenz. Arter. Hypertens. 2018, 24, 684–692. (In Russian) [Google Scholar] [CrossRef]
- Fedorova, O.V.; Fadeev, A.V.; Grigorova, Y.N.; Marshall, C.A.; Zernetkina, V.; Kolodkin, N.I.; Agalakova, N.I.; Konradi, A.O.; Lakatta, E.G.; Bagrov, A.Y. Cardiotonic Steroids Induce Vascular Fibrosis Via Pressure-Independent Mechanism in NaCl-Loaded Diabetic Rats. J. Cardiovasc. Pharmacol. 2019, 74, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Agalakova, N.I.; Reznik, V.A.; Ershov, I.A.; Lupanova, E.A.; Nadei, O.V.; Ivanov, D.O.; David Adair, C.; Bagrov, A.Y. Silencing of Fli1 Gene Mimics Effects of Preeclampsia and Induces Collagen Synthesis in Human Umbilical Arteries. Am. J. Hypertens. 2022, 35, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Ohmaru-Nakanishi, T.; Asanoma, K.; Fujikawa, M.; Fujita, Y.; Yagi, H.; Onoyama, I.; Hidaka, N.; Sonoda, K.; Kato, K. Fibrosis in Preeclamptic Placentas Is Associated with Stromal Fibroblasts Activated by the Transforming Growth Factor-beta1 Signaling Pathway. Am. J. Pathol. 2018, 188, 683–695. [Google Scholar] [CrossRef]
- Orabona, R.; Sciatti, E.; Prefumo, F.; Vizzardi, E.; Bonadei, I.; Valcamonico, A.; Metra, M.; Frusca, T. Pre-eclampsia and heart failure: A close relationship. Ultrasound Obstet. Gynecol. 2018, 52, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, M.P.; Hamlyn, J.M. Signaling mechanisms that link salt retention to hypertension: Endogenous ouabain, the Na(+) pump, the Na(+)/Ca(2+) exchanger and TRPC proteins. Biochim. Biophys. Acta 2010, 1802, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H. Upregulation of the Renin-Angiotensin-aldosterone-ouabain system in the brain is the core mechanism in the genesis of all types of hypertension. Int. J. Hypertens. 2012, 2012, 242786. [Google Scholar] [CrossRef]
- Xavier, F.E.; Rossoni, L.V.; Alonso, M.J.; Balfagon, G.; Vassallo, D.V.; Salaices, M. Ouabain-induced hypertension alters the participation of endothelial factors in alpha-adrenergic responses differently in rat resistance and conductance mesenteric arteries. Br. J. Pharmacol. 2004, 143, 215–225. [Google Scholar] [CrossRef]
- Xavier, F.E.; Salaices, M.; Marquez-Rodas, I.; Alonso, M.J.; Rossoni, L.V.; Vassallo, D.V.; Balfagon, G. Neurogenic nitric oxide release increases in mesenteric arteries from ouabain hypertensive rats. J. Hypertens. 2004, 22, 949–957. [Google Scholar] [CrossRef]
- Fedorova, O.V.; Agalakova, N.I.; Talan, M.I.; Lakatta, E.G.; Bagrov, A.Y. Brain ouabain stimulates peripheral marinobufagenin via angiotensin II signalling in NaCl-loaded Dahl-S rats. J. Hypertens. 2005, 23, 1515–1523. [Google Scholar] [CrossRef]
- Kaur, G.; Kapoor, N.; Mohan, P.; Sri Nageswari, K.; Singh, M.J.; Prasad, R. Alteration in ouabain-sensitive sodium potassium pump of erythrocytes during pregnancy induced hypertension: A kinetic study. J. Biochem. Mol. Biol. Biophys. 2002, 6, 163–166. [Google Scholar] [CrossRef]
- Saunders, R.; Scheiner-Bobis, G. Ouabain stimulates endothelin release and expression in human endothelial cells without inhibiting the sodium pump. Eur. J. Biochem. 2004, 271, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Ehrig, J.C.; Horvat, D.; Allen, S.R.; Jones, R.O.; Kuehl, T.J.; Uddin, M.N. Cardiotonic steroids induce anti-angiogenic and anti-proliferative profiles in first trimester extravillous cytotrophoblast cells. Placenta 2014, 35, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Rajakumar, A.; Geahchan, C.; Salahuddin, S.; Cerdeira, A.S.; Burke, S.D.; George, E.M.; Granger, J.P.; Karumanchi, S.A. Ouabain inhibits placental sFlt1 production by repressing HSP27-dependent HIF-1alpha pathway. FASEB J. 2014, 28, 4324–4334. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Horvat, D.; Glaser, S.S.; Mitchell, B.M.; Puschett, J.B. Examination of the cellular mechanisms by which marinobufagenin inhibits cytotrophoblast function. J. Biol. Chem. 2008, 283, 17946–17953. [Google Scholar] [CrossRef] [PubMed]
- Gatford, K.L.; Andraweera, P.H.; Roberts, C.T.; Care, A.S. Animal Models of Preeclampsia: Causes, Consequences, and Interventions. Hypertension 2020, 75, 1363–1381. [Google Scholar] [CrossRef] [PubMed]
- Bakrania, B.A.; George, E.M.; Granger, J.P. Animal models of preeclampsia: Investigating pathophysiology and therapeutic targets. Am. J. Obstet. Gynecol. 2022, 226, S973–S987. [Google Scholar] [CrossRef]
- Taylor, E.B.; George, E.M. Animal Models of Preeclampsia: Mechanistic Insights and Promising Therapeutics. Endocrinology 2022, 163, bqac096. [Google Scholar] [CrossRef]
- Goodlin, R.C. Will treatment with digoxin antibody benefit pregnant patients with toxemia and elevated digoxin like factor? Med. Hypotheses 1987, 24, 107–110. [Google Scholar] [CrossRef]
- Di Grande, A.; Boura, A.L.; Read, M.A.; Malatino, L.S.; Walters, W.A. Release of a substance from the human placenta having digoxin-like immunoreactivity. Clin. Exp. Pharmacol. Physiol. 1993, 20, 603–607. [Google Scholar] [CrossRef]
- Uddin, M.N. 316: Novel anti-MBG antibodies protect cytotrophoblast cells from a marinobufagenin-induced preeclampsia phenotype. Am. J. Obstet. Gynecol. 2018, 218, S199. [Google Scholar] [CrossRef]
- Pullen, M.A.; Brooks, D.P.; Edwards, R.M. Characterization of the neutralizing activity of digoxin-specific Fab toward ouabain-like steroids. J. Pharmacol. Exp. Ther. 2004, 310, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Agalakova, N.I.; Reznik, V.A.; Nadei, O.V.; Ershov, I.A.; Rassokha, O.S.; Vasyutina, M.L.; Ivanov, D.O.; Adair, C.D.; Galagudza, M.M.; Bagrov, A.Y. Antibody against Na/K-ATPase Inhibitor Lowers Blood Pressure and Increases Vascular Fli1 in Experimental Preeclampsia. Am. J. Hypertens. 2020, 33, 514–519. [Google Scholar] [CrossRef]
- Balzan, S.; Nicolini, G.; Bellitto, L.; Ghione, S.; Biver, P.; Montali, U. Effect of canrenone on the digitalis site of Na+/K(+)-ATPase in human placental membranes and in erythrocytes. J. Cardiovasc. Pharmacol. 2003, 42, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Shidyak, A.; Periyasamy, S.M.; Haller, S.; Taleb, M.; El-Okdi, N.; Elkareh, J.; Gupta, S.; Gohara, S.; Fedorova, O.V.; et al. Spironolactone attenuates experimental uremic cardiomyopathy by antagonizing marinobufagenin. Hypertension 2009, 54, 1313–1320. [Google Scholar] [CrossRef]
- Hecker, A.; Hasan, S.H.; Neumann, F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol. Cph. 1980, 95, 540–545. [Google Scholar] [CrossRef]
- Adair, C.D.; Buckalew, V.; Taylor, K.; Ernest, J.M.; Frye, A.H.; Evans, C.; Veille, J.C. Elevated endoxin-like factor complicating a multifetal second trimester pregnancy: Treatment with digoxin-binding immunoglobulin. Am. J. Nephrol. 1996, 16, 529–531. [Google Scholar] [CrossRef]
- Adair, C.D.; Buckalew, V.M.; Kipikasa, J.; Torres, C.; Stallings, S.P.; Briery, C.M. Repeated dosing of digoxin-fragmented antibody in preterm eclampsia. J. Perinatol. 2009, 29, 163–165. [Google Scholar] [CrossRef][Green Version]
- Adair, C.D.; Luper, A.; Rose, J.C.; Russell, G.; Veille, J.C.; Buckalew, V.M. The hemodynamic effects of intravenous digoxin-binding fab immunoglobulin in severe preeclampsia: A double-blind, randomized, clinical trial. J. Perinatol. 2009, 29, 284–289. [Google Scholar] [CrossRef][Green Version]
- Sibai, B.M. Etiology and management of postpartum hypertension-preeclampsia. Am. J. Obstet. Gynecol. 2012, 206, 470–475. [Google Scholar] [CrossRef]
- ACOG Technical Bulletin. Management of Preeclampsia; No. 91; Committee on Technical Bulletins of the American College of Obstetricians and Gynecologists: Washington, DC, USA, 1986. [Google Scholar]
- Adair, C.D.; Buckalew, V.M.; Graves, S.W.; Lam, G.K.; Johnson, D.D.; Saade, G.; Lewis, D.F.; Robinson, C.; Danoff, T.M.; Chauhan, N.; et al. Digoxin immune fab treatment for severe preeclampsia. Am. J. Perinatol. 2010, 27, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Lam, G.K.; Hopoate-Sitake, M.; Adair, C.D.; Buckalew, V.M.; Johnson, D.D.; Lewis, D.F.; Robinson, C.J.; Saade, G.R.; Graves, S.W. Digoxin antibody fragment, antigen binding (Fab), treatment of preeclampsia in women with endogenous digitalis-like factor: A secondary analysis of the DEEP Trial. Am. J. Obstet. Gynecol. 2013, 209, e111–e116. [Google Scholar] [CrossRef] [PubMed]
| Cross-Reactant | Digibind (ADA-FAB) | DigiFab (ADA-FAB) | Anti-O Polyclonal Antibody | 3E9 Anti-MBG Monoclonal Antibody | 4G4 Anti-MBG Monoclonal Antibody |
|---|---|---|---|---|---|
| Digoxin | 100 | 100 | 1.8 | 1.8 | 0.03 |
| Digitoxin | 1.4 | 1.3 | 0.47 | 0.7 | <0.001 |
| Ouabain | 0.4 | 0.14 | 100 | 0.02 | 0.005 |
| MBG | 0.2 | 0.29 | 0.036 | 100 | 100 |
| Marinobufotoxin | 0.06 | 0.02 | 0.06 | 4 | 43 |
| Telocinobufagin | 1.0 | 0.34 | 0.02 | 7 | 14 |
| Bufalin | 2.7 | 0.9 | 0.10 | 0.3 | 0.08 |
| Cinobufagin | 0.02 | 0.03 | 0.02 | 1.4 | 0.07 |
| Resibufagenin | 1 | 0.02 | 0.15 | 0.5 | 0.5 |
| Proscillaridin-A | 1.46 | 0.2 | 0.03 | 3 | <0.001 |
| Prednisone | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 |
| Progesterone | <0.001 | <0.001 | 0.002 | <0.01 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Socha, M.W.; Chmielewski, J.; Pietrus, M.; Wartęga, M. Endogenous Digitalis-like Factors as a Key Molecule in the Pathophysiology of Pregnancy-Induced Hypertension and a Potential Therapeutic Target in Preeclampsia. Int. J. Mol. Sci. 2023, 24, 12743. https://doi.org/10.3390/ijms241612743
Socha MW, Chmielewski J, Pietrus M, Wartęga M. Endogenous Digitalis-like Factors as a Key Molecule in the Pathophysiology of Pregnancy-Induced Hypertension and a Potential Therapeutic Target in Preeclampsia. International Journal of Molecular Sciences. 2023; 24(16):12743. https://doi.org/10.3390/ijms241612743
Chicago/Turabian StyleSocha, Maciej W., Jakub Chmielewski, Miłosz Pietrus, and Mateusz Wartęga. 2023. "Endogenous Digitalis-like Factors as a Key Molecule in the Pathophysiology of Pregnancy-Induced Hypertension and a Potential Therapeutic Target in Preeclampsia" International Journal of Molecular Sciences 24, no. 16: 12743. https://doi.org/10.3390/ijms241612743
APA StyleSocha, M. W., Chmielewski, J., Pietrus, M., & Wartęga, M. (2023). Endogenous Digitalis-like Factors as a Key Molecule in the Pathophysiology of Pregnancy-Induced Hypertension and a Potential Therapeutic Target in Preeclampsia. International Journal of Molecular Sciences, 24(16), 12743. https://doi.org/10.3390/ijms241612743







