Bioactive Polyetheretherketone with Gelatin Hydrogel Leads to Sustained Release of Bone Morphogenetic Protein-2 and Promotes Osteogenic Differentiation
Abstract
1. Introduction
2. Results
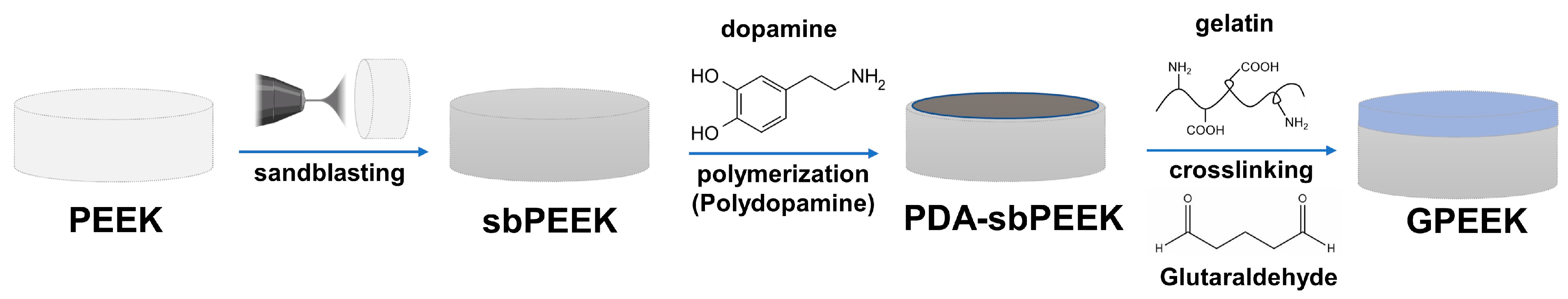
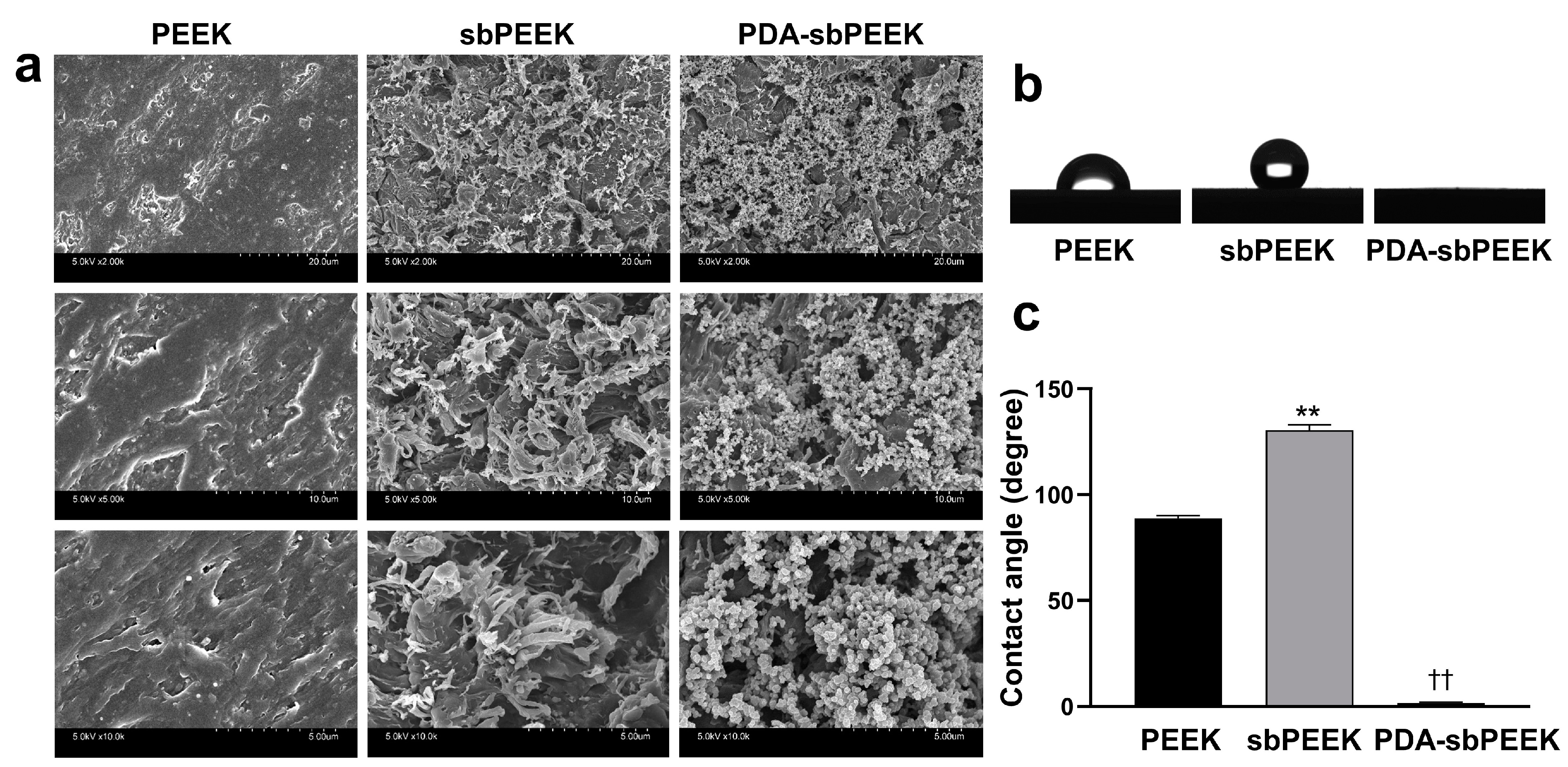
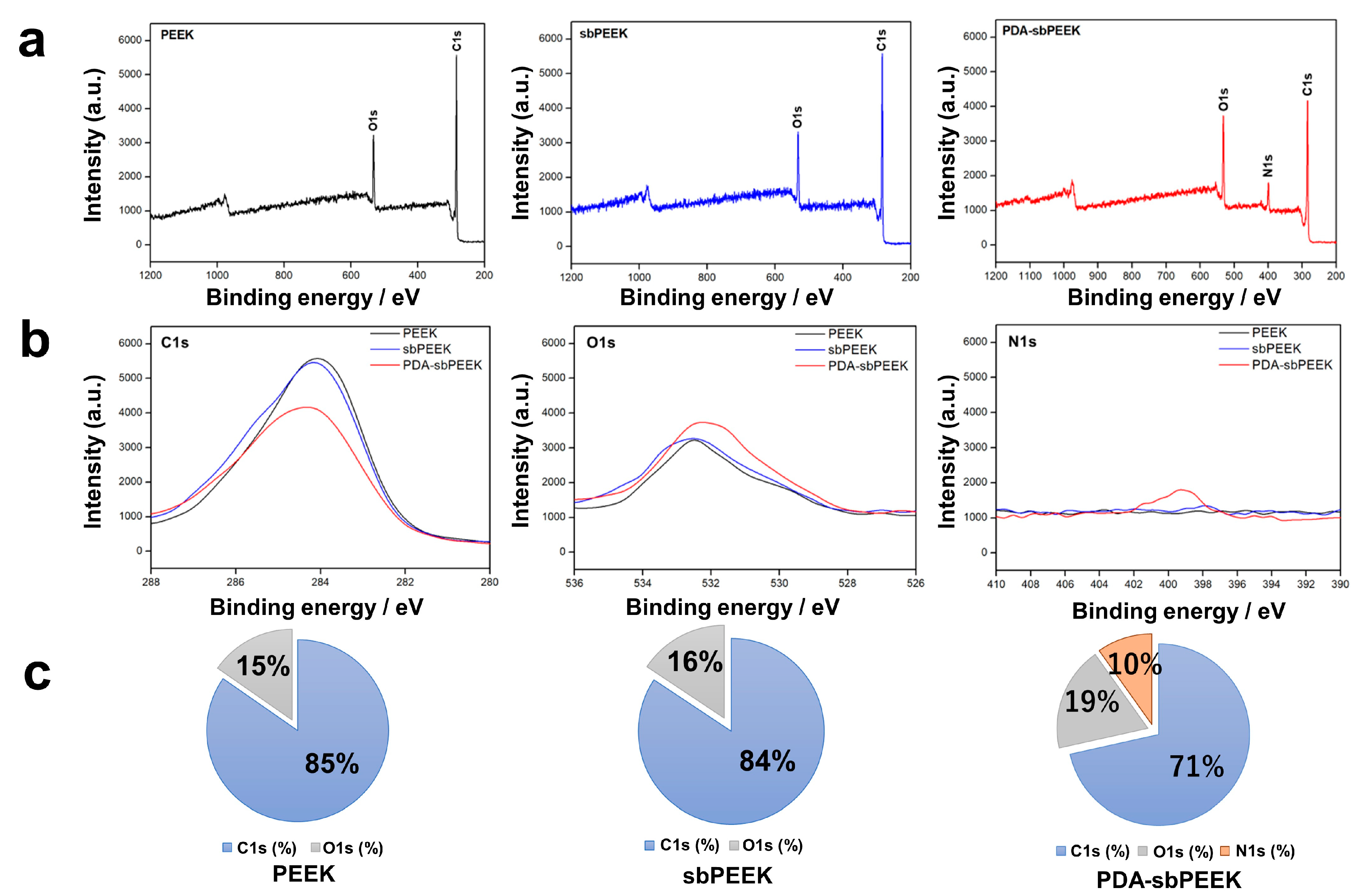
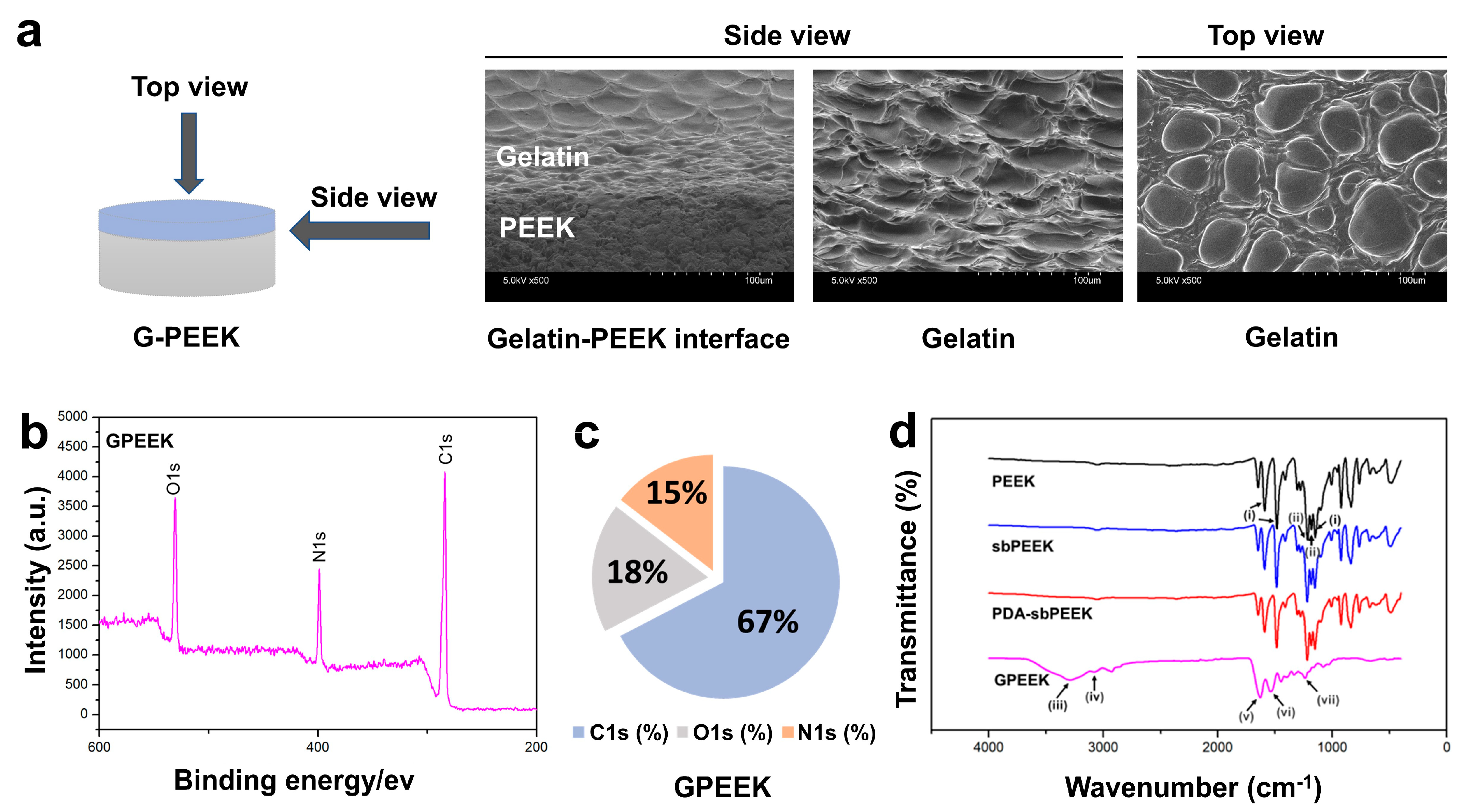
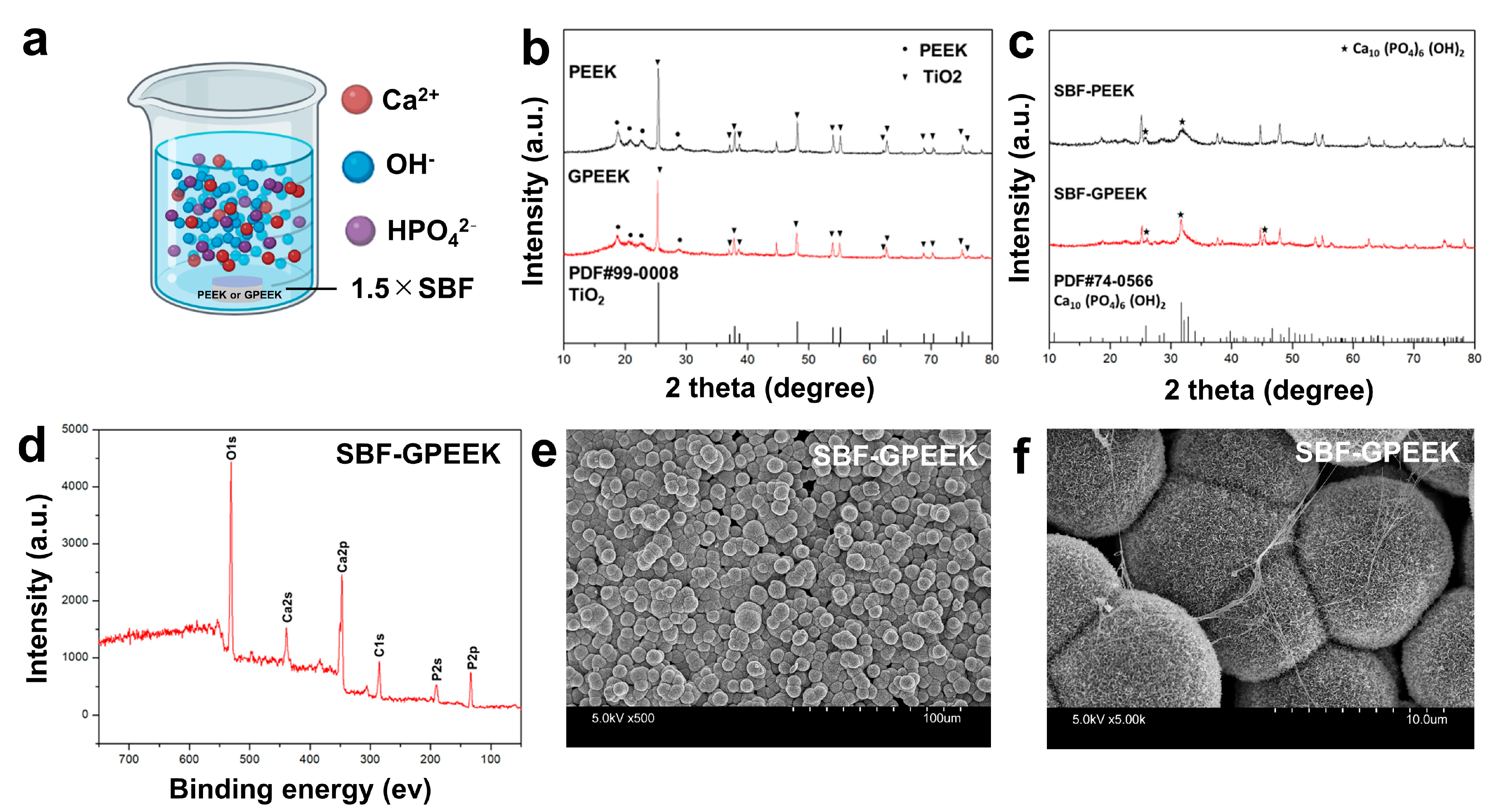
2.1. Preparation and Physicochemical Characterization of PEEK with Gelatin Hydrogel through Polydopamine (PDA) Chemistry
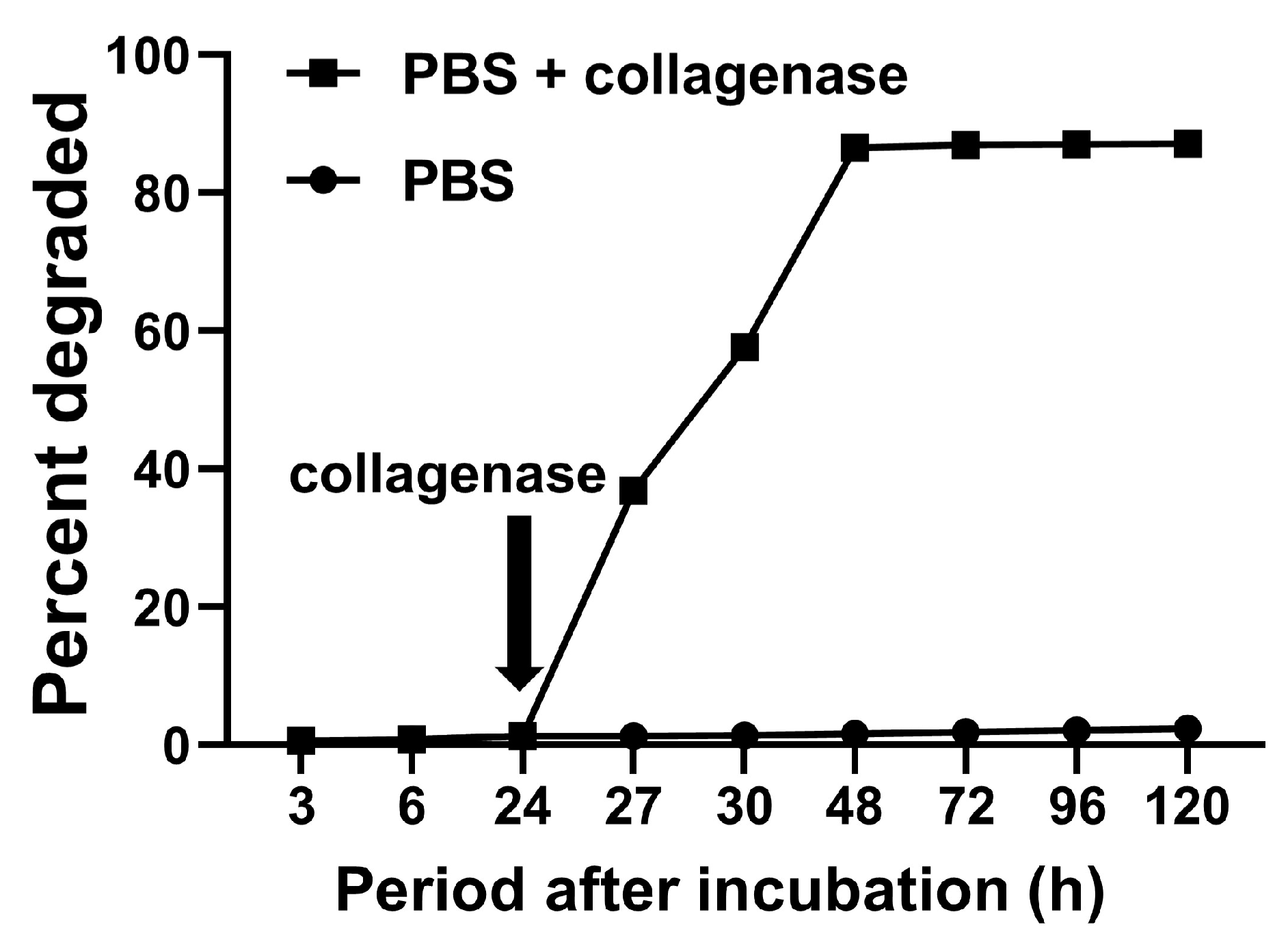
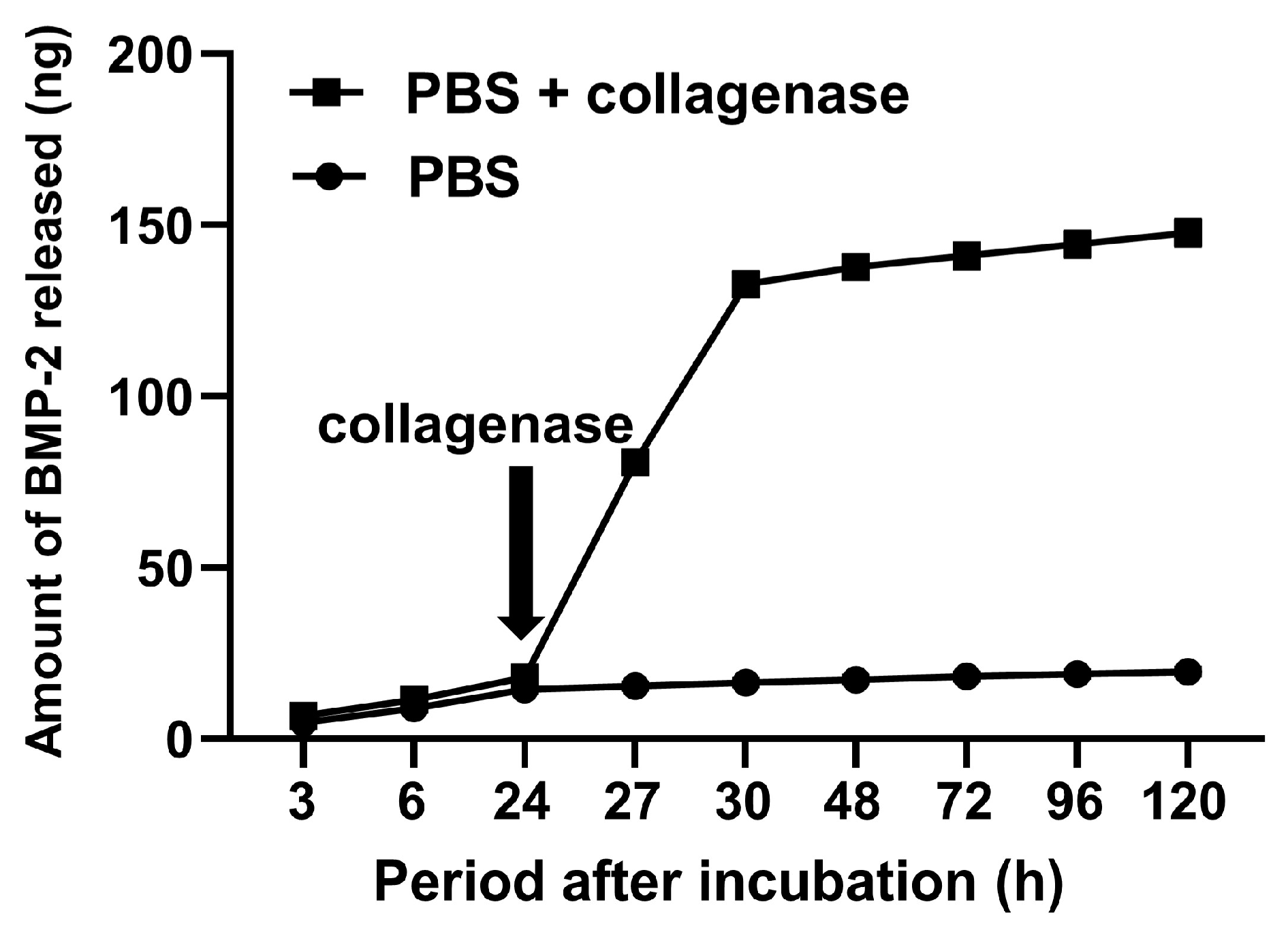
2.2. Biological Potentials of GPEEK
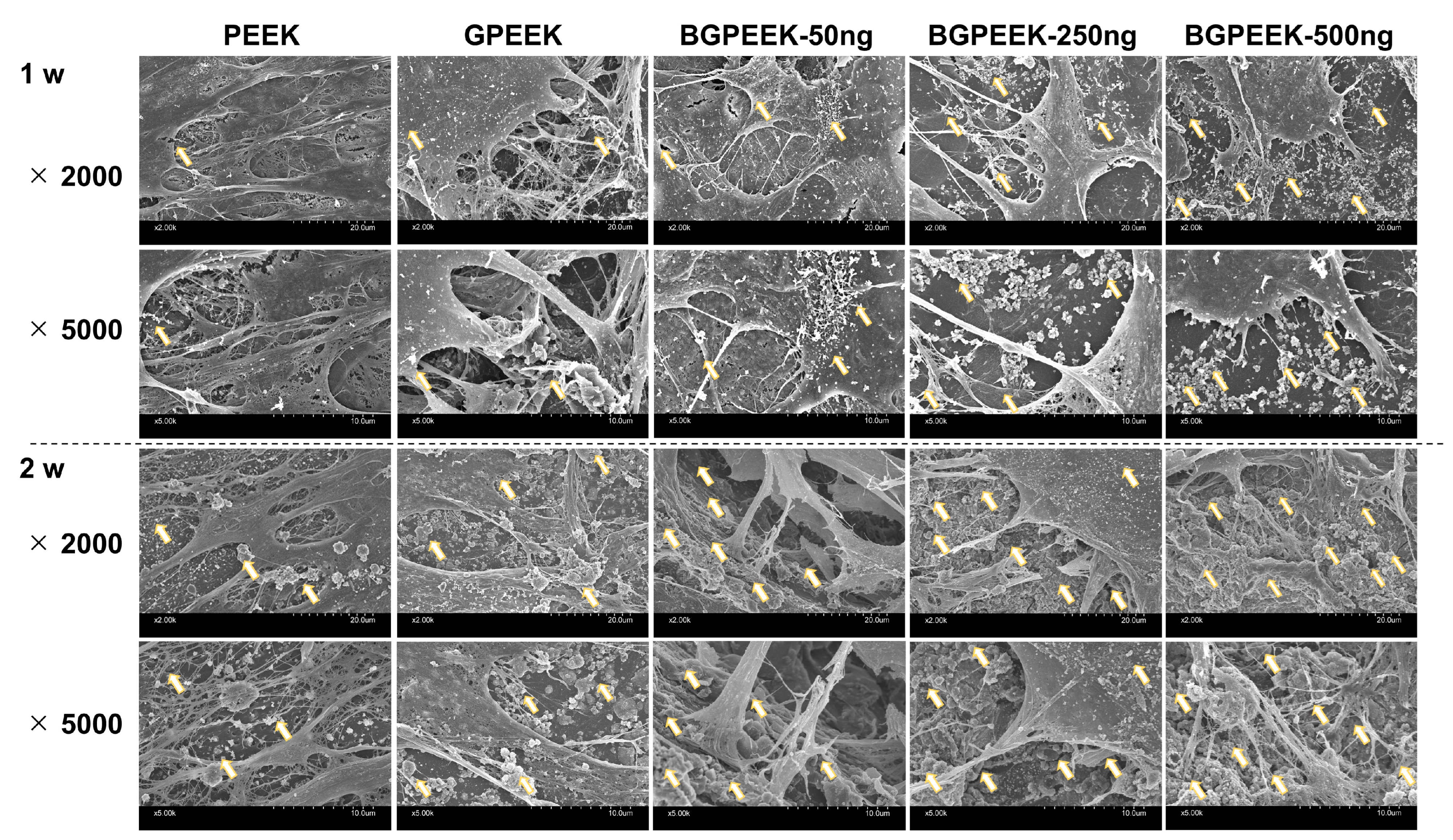
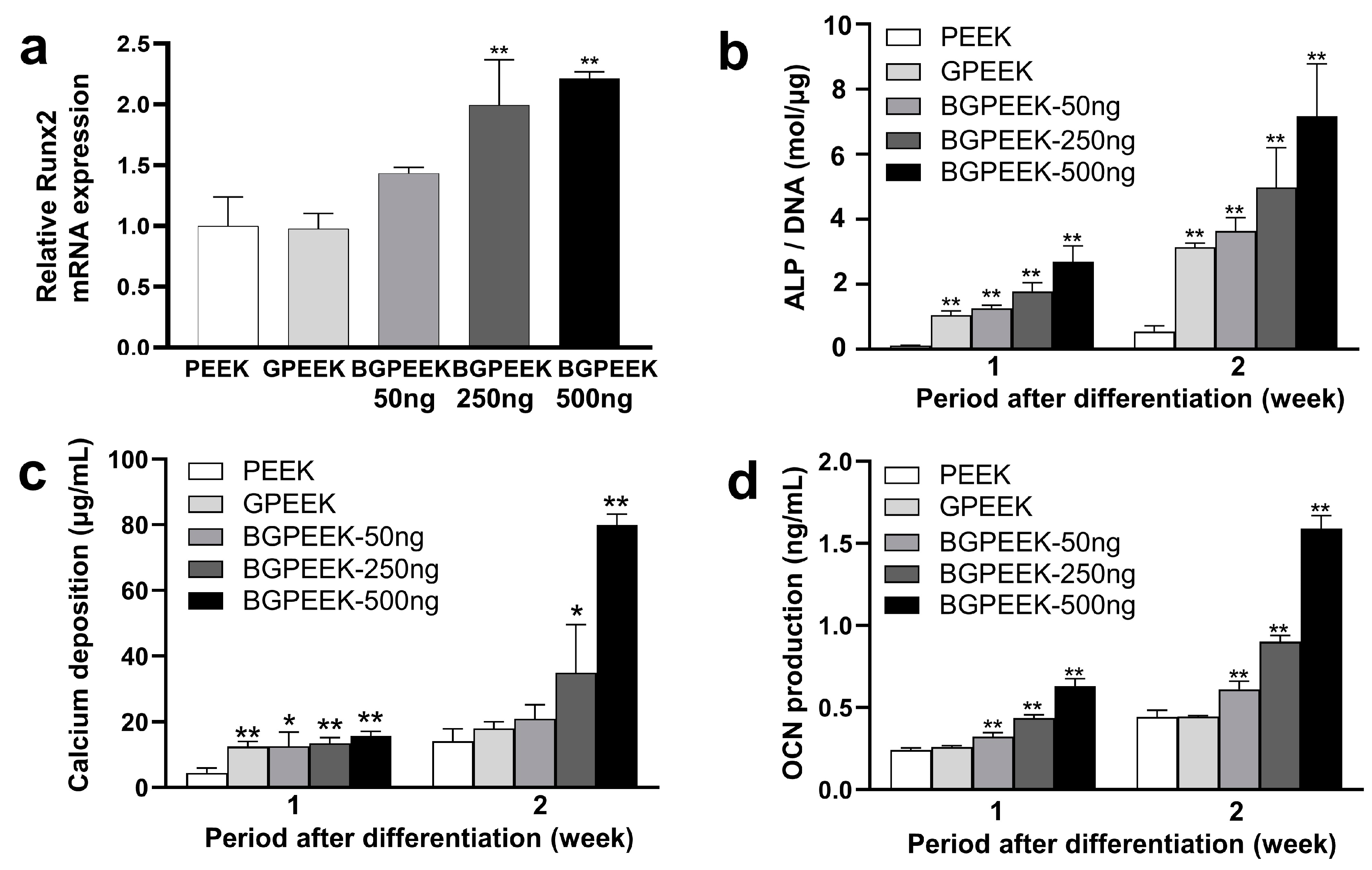
2.3. Osteogenic Differentiation of Stem Cells on PEEK Substrates
3. Discussion
4. Materials and Methods
4.1. Modification of PEEK with PDA
4.2. Binding of Gelatin Hydrogel onto PDA-sbPEEK
4.3. Degradation Behavior of Gelatin Hydrogel in GPEEK
4.4. In Vitro Mineralization Assay for PEEK Substrates
4.5. Incorporation of Bone Morphogenetic Protein (BMP)-2 into GPEEK
4.6. Osteogenic Differentiation for Human Mesenchymal Stem Cells Cultured on Various PEEK Substrates
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blumenfeld, I.; Srouji, S.; Lanir, Y.; Laufer, D.; Livne, E. Enhancement of Bone Defect Healing in Old Rats by TGF-β and IGF-1. Exp. Gerontol. 2002, 37, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Takahashi, Y.; Tabata, Y. Controlled Release by Biodegradable Hydrogels Enhances the Ec-topic Bone Formation of Bone Morphogenetic Protein. Biomaterials 2003, 24, 4375–4383. [Google Scholar] [CrossRef]
- Perry, C.R. Bone Repair Techniques, Bone Graft, and Bone Graft Substitutes. Clin. Orthop. Relat. Res. 1999, 360, 71–86. [Google Scholar] [CrossRef]
- Clements, J.R.; Carpenter, B.B.; Pourciau, J.K. Treating Segmental Bone Defects: A New Technique. J. Foot Ankle Surg. 2008, 47, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhao, L.; Zhang, W.; Cui, L.; Liu, W.; Cao, Y. Repair of Goat Tibial Defects with Bone Marrow Stromal Cells and β-Tricalcium Phosphate. J. Mater. Sci. Mater. Med. 2008, 19, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Theos, C.; Koulouvaris, P.; Kottakis, S.; Demertzis, N. Reconstruction of Tibia Defects by Ipsilateral Vascu-larized Fibula Transposition. Arch. Orthop. Trauma Surg. 2008, 128, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Oest, M.E.; Dupont, K.M.; Kong, H.-J.; Mooney, D.J.; Guldberg, R.E. Quantitative Assessment of Scaffold and Growth Factor-Mediated Repair of Critically Sized Bone Defects. J. Orthop. Res. 2007, 25, 941–950. [Google Scholar] [CrossRef]
- den Boer, F.C.; Wippermann, B.W.; Blokhuis, T.J.; Patka, P.; Bakker, F.C.; Haarman, H.J.T.M. Healing of Segmen tal Bone Defects with Granular Porous Hydroxyapatite Augmented with Recombinant Human Os-teogenic Protein-I or Autologous Bone Marrow. J. Orthop. Res. 2003, 21, 521–528. [Google Scholar] [CrossRef]
- Mankin, H.J.; Hornicek, F.J.; Raskin, K.A. Infection in Massive Bone Allografts. Clin. Orthop. Relat. Res. 2005, 432, 210–216. [Google Scholar] [CrossRef]
- Hinsenkamp, M.; Muylle, L.; Eastlund, T.; Fehily, D.; Noël, L.; Strong, D.M. Adverse Reactions and Events Related to Musculoskeletal Allografts: Reviewed by the World Health Organisation Project NOTIFY. Int. Or-Thop. 2012, 36, 633–641. [Google Scholar] [CrossRef]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Sub-stitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef]
- Yu, D.; Lei, X.; Zhu, H. Modification of Polyetheretherketone (PEEK) Physical Features to Improve Osteoin-tegra tion. J. Zhejiang Univ.-Sci. B 2022, 23, 189–203. [Google Scholar] [CrossRef]
- Gültan, T.; Yurtsever, M.Ç.; Gümüşderelioğlu, M. NaOH-Etched/Boron-Doped Nanohydroxyapatite-Coated PEEK Implants Enhance the Proliferation and Differentiation of Osteogenic Cells. Biomed. Mater. 2020, 15, 035019. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, Y.; Gao, H.; Ge, P.; Ren, K.; Gao, J.; Cao, Y.; Han, D.; Zhang, J. One-Step Fabrication of Function-alized Poly(Etheretherketone) Surfaces with Enhanced Biocompatibility and Osteogenic Activity. Mater. Sci. Eng. C 2018, 88, 70–78. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, F.S.F.; Vieira, M.; da Silva, H.N.; Tomás, H.; Fook, M.V.L. Surface Bioactivation of Polyether Ether Ketone (PEEK) by Sulfuric Acid and Piranha Solution: Influence of the Modification Route in Capacity for Inducing Cell Growth. Biomolecules 2021, 11, 1260. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Jiao, Z.; Guo, M.; Wang, Z.; Wan, Y.; Lin, K.; Liu, Q.; Zhang, P. Gaseous Sulfur Trioxide Induced Con trollable Sulfonation Promoting Biomineralization and Osseointegration of Polyetheretherketone Im-plants. Bioact. Mater. 2020, 5, 1004–1017. [Google Scholar] [CrossRef]
- Zhao, Y.; Wong, H.M.; Lui, S.C.; Chong, E.Y.W.; Wu, G.; Zhao, X.; Wang, C.; Pan, H.; Cheung, K.M.C.; Wu, S.; et al. Plasma Surface Functionalized Polyetheretherketone for Enhanced Osseo-Integration at Bone-Implant Interface. ACS Appl. Mater. Interfaces 2016, 8, 3901–3911. [Google Scholar] [CrossRef]
- Czwartos, J.; Budner, B.; Bartnik, A.; Wachulak, P.; Butruk-Raszeja, B.A.; Lech, A.; Ciach, T.; Fiedorowicz, H. Effect of Extreme Ultraviolet (EUV) Radiation and EUV Induced, N2 and O2 Based Plasmas on a PEEK Sur-face’s Physico-Chemical Properties and MG63 Cell Adhesion. Int. J. Mol. Sci. 2021, 22, 8455. [Google Scholar] [CrossRef]
- Panayotov, I.V.; Orti, V.; Cuisinier, F.; Yachouh, J. Polyetheretherketone (PEEK) for Medical Applications. J. Mater. Sci. Mater. Med. 2016, 27, 118. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, X.; Wei, J.; Ma, J.; Deng, F.; Wei, S. Nano-TiO2/PEEK Bioactive Composite as a Bone Substitute Mate rial: In Vitro and in Vivo Studies. Int. J. Nanomed. 2012, 7, 1215–1225. [Google Scholar] [CrossRef]
- Bostrom, M.P.G. Expression of Bone Morphogenetic Proteins in Fracture Healing. Clin. Orthop. Relat. Res. 1998, 355, S116–S123. [Google Scholar] [CrossRef] [PubMed]
- Neffe, A.T.; Wischke, C.; Racheva, M.; Lendlein, A. Progress in Biopolymer-Based Biomaterials and Their Appli cation in Controlled Drug Delivery. Expert Rev. Med. Devices 2013, 10, 813–833. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zi, Y.; Peng, J.; Shi, C.; Zheng, Y.; Zhong, J. Gelatin as a Bioactive Nanodelivery System for Functional Food Applications. Food Chem. 2023, 423, 136265. [Google Scholar] [CrossRef] [PubMed]
- Madkhali, O.; Mekhail, G.; Wettig, S.D. Modified Gelatin Nanoparticles for Gene Delivery. Int. J. Pharm. 2019, 554, 224–234. [Google Scholar] [CrossRef]
- Salahuddin, B.; Wang, S.; Sangian, D.; Aziz, S.; Gu, Q. Hybrid Gelatin Hydrogels in Nanomedicine Applica-tions. ACS Appl. Bio Mater. 2021, 4, 2886–2906. [Google Scholar] [CrossRef]
- Ozekp, M.; Ishii, T.; Hirano, Y.; Tabata, Y. Controlled Release of Hepatocyte Growth Factor from Gelatin Hy-drogels Based on Hydrogel Degradation. J. Drug. Target 2001, 9, 461–471. [Google Scholar] [CrossRef]
- Tabata, Y.; Ikada, Y. Vascularization Effect of Basic Fibroblast Growth Factor Released from Gelatin Hydro-gels with Different Biodegradabilities. Biomaterials 1999, 20, 2169–2175. [Google Scholar] [CrossRef]
- Patel, Z.S.; Yamamoto, M.; Ueda, H.; Tabata, Y.; Mikos, A.G. Biodegradable Gelatin Microparticles as Delivery Systems for the Controlled Release of Bone Morphogenetic Protein-2. Acta Biomater. 2008, 4, 1126–1138. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Tabata, Y. Dual-Controlled Release System of Drugs for Bone Regeneration. Adv. Drug Deliv. Rev. 2015, 94, 28–40. [Google Scholar] [CrossRef]
- Kim, H.; Yang, G.H.; Choi, C.H.; Cho, Y.S.; Kim, G. Gelatin/PVA Scaffolds Fabricated Using a 3D-Printing Process Employed with a Low-Temperature Plate for Hard Tissue Regeneration: Fabrication and Characteri-zations. Int. J. Biol. Macromol. 2018, 120, 119–127. [Google Scholar] [CrossRef]
- Jalaja, K.; Naskar, D.; Kundu, S.C.; James, N.R. Fabrication of Cationized Gelatin Nanofibers by Electrospinning for Tissue Regeneration. RSC Adv. 2015, 5, 89521–89530. [Google Scholar] [CrossRef]
- Zhao, X.; Xiong, D.; Wang, K.; Wang, N. Improved Biotribological Properties of PEEK by Photo-Induced Graft Polymerization of Acrylic Acid. Mater. Sci. Eng. C 2017, 75, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, K.; Zhou, W.; Gu, J.; Liu, Y.; Han, C.C.; Xu, S. Factors Influencing the Interactions in Gela-tin/Hy droxyapatite Hybrid Materials. Front. Chem. 2020, 8, 489. [Google Scholar] [CrossRef]
- Zhao, X.; Xiong, D.; Liu, Y. Improving Surface Wettability and Lubrication of Polyetheretherketone (PEEK) by Combining with Polyvinyl Alcohol (PVA) Hydrogel. J. Mech. Behav. Biomed. Mater. 2018, 82, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Karthik, N.; Xiong, D.; Liu, Y. Bio-Inspired Surface Modification of PEEK through the Dual Cross-Linked Hydrogel Layers. J. Mech. Behav. Biomed. Mater. 2020, 112, 104032. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Wang, N.; Cheng, Y.; Zhao, Y.; Meng, L.; Yue, X.; She, P.; Gao, H. Application of Porous Polyether-ether ketone Scaffold/Vancomycin-Loaded Thermosensitive Hydrogel Composites for Antibacterial Therapy in Bone Repair. Macromol. Biosci. 2022, 22, 2200114. [Google Scholar] [CrossRef]
- Ferroni, L.; D’Amora, U.; Leo, S.; Tremoli, E.; Raucci, M.G.; Ronca, A.; Ambrosio, L.; Zavan, B. PEEK and Hy-aluronan-Based 3D Printed Structures: Promising Combination to Improve Bone Regeneration. Molecules 2022, 27, 8749. [Google Scholar] [CrossRef]
- He, X.; Deng, Y.; Yu, Y.; Lyu, H.; Liao, L. Drug-Loaded/Grafted Peptide-Modified Porous PEEK to Promote Bone Tissue Repair and Eliminate Bacteria. Colloids Surf. B Biointerfaces 2019, 181, 767–777. [Google Scholar] [CrossRef]
- Deng, L.-J.; Wu, Y.-L.; He, X.-H.; Xie, K.-N.; Xie, L.; Deng, Y. Simvastatin Delivery on PEEK for Bioactivity and Osteogenesis Enhancements. J. Biomater. Sci. Polym. Ed. 2018, 29, 2237–2251. [Google Scholar] [CrossRef]
- Han, X.; Gao, W.; Zhou, Z.; Yang, S.; Wang, J.; Shi, R.; Li, Y.; Jiao, J.; Qi, Y.; Zhao, J. Application of Biomole-cules Modification Strategies on PEEK and Its Composites for Osteogenesis and Antibacterial Properties. Colloids. Surf. B 2022, 215, 112492. [Google Scholar] [CrossRef]
- Waser-Althaus, J.; Salamon, A.; Waser, M.; Padeste, C.; Kreutzer, M.; Pieles, U.; Müller, B.; Peters, K. Differ-entia tion of Human Mesenchymal Stem Cells on Plasma-Treated Polyetheretherketone. J. Mater. Sci. Mater. Med. 2014, 25, 515–525. [Google Scholar] [CrossRef]
- Zheng, Y.; Xiong, C.; Zhang, S.; Li, X.; Zhang, L. Bone-like Apatite Coating on Functionalized Poly(Etherether ketone) Surface via Tailored Silanization Layers Technique. Mater. Sci. Eng. C 2015, 55, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Noiset, O.; Schneider, Y.-J.; Marchand-Brynaert, J. Adhesion and Growth of CaCo2 Cells on Surface-Modified PEEK Substrata. J. Biomater. Sci. Polym. Ed. 2000, 11, 767–786. [Google Scholar] [CrossRef] [PubMed]
- Kunomura, S.; Iwasaki, Y. Immobilization of Polyphosphoesters on Poly(Ether Ether Ketone) (PEEK) for Fa-cili tating Mineral Coating. J. Biomater. Sci. Polym. Ed. 2019, 30, 861–876. [Google Scholar] [CrossRef] [PubMed]
- Miyagaki, A.; Kamaya, Y.; Matsumoto, T.; Honda, K.; Shibahara, M.; Hongo, C.; Nishino, T. Surface Modifica-tion of Poly(Ether Ether Ketone) through Friedel–Crafts Reaction for High Adhesion Strength. Langmuir 2019, 35, 9761–9768. [Google Scholar] [CrossRef]
- Awad, K.R.; Ahuja, N.; Shah, A.; Tran, H.; Aswath, P.B.; Brotto, M.; Varanasi, V. Silicon Nitride Enhances Oste oprogenitor Cell Growth and Differentiation via Increased Surface Energy and Formation of Amide and Nanocrystalline HA for Craniofacial Reconstruction. Med. Devices Sens. 2019, 2, e10032. [Google Scholar] [CrossRef]
- Salazar, P.; Martín, M.; González-Mora, J.L. Polydopamine-Modified Surfaces in Biosensor Applications. In Polymer Science: Research Advances, Practical Applications and Educational Aspects; Formatex Research Center: Badajoz, Spain, 2016; pp. 385–396. [Google Scholar]
- Chen, T.; Chen, Q.; Fu, H.; Wang, D.; Gao, Y.; Zhang, M.; Liu, H. Construction and Performance Evaluation of a Sustained Release Implant Material Polyetheretherketone with Antibacterial Properties. Mater. Sci. Eng. C 2021, 126, 112109. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Im plants. Biomed. Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef]
- Sunarso; Tsuchiya, A.; Fukuda, N.; Toita, R.; Tsuru, K.; Ishikawa, K. Effect of Micro-Roughening of Poly(Ether Ether Ketone) on Bone Marrow Derived Stem Cell and Macrophage Responses, and Osseointegration. J. Bio-Mater. Sci. Polym. Ed. 2018, 29, 1375–1388. [Google Scholar] [CrossRef]
- Li, Y.; Jongberg, S.; Andersen, M.L.; Davies, M.J.; Lund, M.N. Quinone-Induced Protein Modifications: Kinet-ic Preference for Reaction of 1,2-Benzoquinones with Thiol Groups in Proteins. Free Radic. Biol. Med. 2016, 97, 148–157. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X.; Zhang, J. Assessment of the Characteristics and Bio-com patibility of Gelatin Sponge Scaffolds Prepared by Various Crosslinking Methods. Sci. Rep. 2018, 8, 1616. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, S.; Xiao, X. Fabrication and Characterization of Poly (Ethylenimine) Modified Poly (l-Lactic Acid) Nanofibrous Scaffolds. J. Biomater. Sci. Polym. Ed. 2019, 30, 1523–1541. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, J.; Chen, F.; Bai, D.; Shao, C.; Wang, J.; Xi, P.; Zeng, Z. Gelatin Functionalized Graphene Oxide for Mineralization of Hydroxyapatite: Biomimetic and in Vitro Evaluation. Nanoscale 2014, 6, 5315–5322. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gasga, J.; Martínez-Piñeiro, E.L.; Rodríguez-Álvarez, G.; Tiznado-Orozco, G.E.; García-García, R.; Brès, E.F. XRD and FTIR Crystallinity Indices in Sound Human Tooth Enamel and Synthetic Hydroxyapatite. Mater. Sci. Eng. C 2013, 33, 4568–4574. [Google Scholar] [CrossRef]
- Nakaguchi, K.; Jinnou, H.; Kaneko, N.; Sawada, M.; Hikita, T.; Saitoh, S.; Tabata, Y.; Sawamoto, K. Growth Factors Released from Gelatin Hydrogel Microspheres Increase New Neurons in the Adult Mouse Brain. Stem. Cells Int. 2012, 2012, 915160. [Google Scholar] [CrossRef]
- Komatsu, K.; Shibata, T.; Shimada, A.; Ideno, H.; Nakashima, K.; Tabata, Y.; Nifuji, A. Cationized Gelatin Hydro gels Mixed with Plasmid DNA Induce Stronger and More Sustained Gene Expression than Atelocol-lagen at Calvarial Bone Defects in Vivo. J. Biomater. Sci. Polym. Ed. 2016, 27, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Wang, Y.; Cai, R.; Chang, H.; Song, K.; Zuo, H.; Zhao, P.; Xia, Q.; He, H. Design and Performance of Sericin/Poly(Vinyl Alcohol) Hydrogel as a Drug Delivery Carrier for Potential Wound Dressing Application. Mater. Sci. Eng. C 2019, 101, 341–351. [Google Scholar] [CrossRef]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A. Controlled Drug Release from Hydrogel-Based Ma trices: Experiments and modeling. Int. J. Pharm. 2015, 486, 144–152. [Google Scholar] [CrossRef]
- Sheth, S.; Barnard, E.; Hyatt, B.; Rathinam, M.; Zustiak, S.P. Predicting Drug Release From Degradable Hy-drogels Using Fluorescence Correlation Spectroscopy and Mathematical Modeling. Front. Bioeng. Biotechnol. 2019, 7, 410. [Google Scholar] [CrossRef]
- Lei, L.; Bai, Y.; Qin, X.; Liu, J.; Huang, W.; Lv, Q. Current Understanding of Hydrogel for Drug Release and Tissue Engineering. Gels 2022, 8, 301. [Google Scholar] [CrossRef]
- Chinnasami, H.; Dey, M.K.; Devireddy, R. Three-Dimensional Scaffolds for Bone Tissue Engineering. Bioengineering 2023, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, M.; Yao, T.; Kokubo, T.; Minoda, M.; Miyamoto, T.; Nakamura, T.; Yamamuro, T. Apatite Coated on Organic Polymers by Biomimetic Process: Improvement in Its Adhesion to Substrate by NaOH Treatment. J. Appl. Biomater. Func. 1994, 5, 339–347. [Google Scholar] [CrossRef] [PubMed]
| Substrate | Roughness (μm) |
|---|---|
| PEEK | 0.67 ± 0.01 |
| sbPEEK | 0.82 ± 0.04 |
| PDA-sbPEEK | 0.85 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Jo, J.-I.; Kanda, R.; Nishiura, A.; Hashimoto, Y.; Matsumoto, N. Bioactive Polyetheretherketone with Gelatin Hydrogel Leads to Sustained Release of Bone Morphogenetic Protein-2 and Promotes Osteogenic Differentiation. Int. J. Mol. Sci. 2023, 24, 12741. https://doi.org/10.3390/ijms241612741
Zhang R, Jo J-I, Kanda R, Nishiura A, Hashimoto Y, Matsumoto N. Bioactive Polyetheretherketone with Gelatin Hydrogel Leads to Sustained Release of Bone Morphogenetic Protein-2 and Promotes Osteogenic Differentiation. International Journal of Molecular Sciences. 2023; 24(16):12741. https://doi.org/10.3390/ijms241612741
Chicago/Turabian StyleZhang, Ruonan, Jun-Ichiro Jo, Ryuhei Kanda, Aki Nishiura, Yoshiya Hashimoto, and Naoyuki Matsumoto. 2023. "Bioactive Polyetheretherketone with Gelatin Hydrogel Leads to Sustained Release of Bone Morphogenetic Protein-2 and Promotes Osteogenic Differentiation" International Journal of Molecular Sciences 24, no. 16: 12741. https://doi.org/10.3390/ijms241612741
APA StyleZhang, R., Jo, J.-I., Kanda, R., Nishiura, A., Hashimoto, Y., & Matsumoto, N. (2023). Bioactive Polyetheretherketone with Gelatin Hydrogel Leads to Sustained Release of Bone Morphogenetic Protein-2 and Promotes Osteogenic Differentiation. International Journal of Molecular Sciences, 24(16), 12741. https://doi.org/10.3390/ijms241612741





