New Ideas for the Prevention and Treatment of Preeclampsia and Their Molecular Inspirations
Abstract
1. Introduction
2. The Possible Pathomechanism of Preeclampsia
3. The Preventive and Therapeutic Strategies Recommended by Obstetricians and Gynaecological Societies
4. Novel Therapies for the Prevention and Treatment of Preeclampsia
4.1. Statins—The Old Friends of the Cardiovascular System Offering New Perspectives in Preventing Preeclampsia
4.2. Anti-Inflammatory Agents in the Treatment of Preeclampsia
4.3. Therapies Targeting sFtl1 and Its Signalling
4.3.1. Apheresis—Scavenger of sFlt1
4.3.2. Inhibition of Synthesis of sFlt1
4.3.3. Therapeutic Peptides Interfering with sFlt1 Signalling
4.4. Novel Therapies Targeting the Production of Nitric Oxide
5. Other Agents for the Prevention or Treatment of Preeclampsia Currently under Investigation
5.1. Proton Pump Inhibitors
5.2. Metformin
5.3. Antithrombin III
5.4. Natural Products, and Their Power to Reduce the Risk of Preeclampsia
6. Medicine of the Future: The Transplantation of Mesenchymal Cells and Their Derivatives for Treatment of Preeclampsia
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collier, A.; Ris, Y.; Smith, L.A.; Karumanchi, S.A. Review of the immune mechanisms of preeclampsia and the potential of immune modulating therapy. Hum. Immunol. 2021, 82, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.S.; Davey, M.A.; Mol, B.W.; Rolnik, D.L. The evolution of the diagnostic criteria of preeclampsia-eclampsia. Am. J. Obstet. Gynecol. 2022, 226, S835–S843. [Google Scholar] [CrossRef] [PubMed]
- Robillard, P.-Y.; Boukerrou, M.; Dekker, G.; Scioscia, M.; Bonsante, F.; Boumahni, B.; Iacobelli, S. Risk Factors for Early and Late Onset Preeclampsia in Reunion Island: Multivariate Analysis of Singleton and Twin Pregnancies. A 20-Year Population-Based Cohort of 2120 Preeclampsia Cases. Reprod. Med. 2021, 2, 131–143. [Google Scholar] [CrossRef]
- ACOG. Practice Bulletin no. 222. Clinical Management Guidelines for Obstetrician–Gynecologists Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar] [CrossRef]
- González-Ramos, R.; Rocco, J.; Rojas, C.; Sovino, H.; Poch, A.; Kohen, P.; Alvarado-Díaz, C.; Devoto, L. Physiologic activation of nuclear factor kappa-B in the endometrium during the menstrual cycle is altered in endometriosis patients. Fertil. Steril. 2012, 97, 645–651. [Google Scholar] [CrossRef]
- Yan, C.; Boyd, D.D. Regulation of Matrix Metalloproteinase Gene Expression. J. Cell Physiol. 2007, 211, 19–26. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, e17023. [Google Scholar] [CrossRef]
- Allport, V.C.; Slater, D.M.; Newton, R.; Bennett, P.R. NF-κB and AP-1 are required for cyclo-oxygenase 2 gene expression in amnion epithelial cell line (WISH). Mol. Hum. Reprod. 2000, 6, 561–565. [Google Scholar] [CrossRef]
- Gupta, S.C.; Sundaram, C.; Reuter, S.; Aggarwal, B.B. Inhibiting NF-κB Activation by Small Molecules As a Therapeutic Strategy. Biochim. Biophys. Acta 2010, 1799, 775–787. [Google Scholar] [CrossRef]
- Walsh, S.W. Prostaglandins in Pregnancy. Glob. Libr. Womens Med. 2011, 10315. [Google Scholar] [CrossRef]
- Tenório, M.B.; Ferreira, R.C.; Moura, F.A.; Bueno, N.B.; De Oliveira, A.C.M.; Goulart, M.O.F. Cross-Talk between Oxidative Stress and Inflammation in Preeclampsia. Oxid. Med. Cell. Longev. 2019, 2019, 8238727. [Google Scholar] [CrossRef]
- Ye, Y.; Kong, Y.; Zhang, Y. Complement Split Products C3a/C5a and Receptors: Are They Regulated by Circulating Angiotensin II Type 1 Receptor Autoantibody in Severe Preeclampsia? Gynecol. Obstet. Investig. 2016, 81, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Pierik, E.; Prins, J.R.; van Goor, H.; Dekker, G.A.; Daha, M.R.; Seelen, M.A.J.; Scherjon, S.A. Dysregulation of Complement Activation and Placental Dysfunction: A Potential Target to Treat Preeclampsia? Front. Immunol. 2020, 10, 3098. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Kong, L.R.; Ge, Q.; Lu, Y.Y.; Hong, M.N.; Zhang, Y.; Ruan, C.C.; Gao, P.J. Complement 5a-mediated trophoblasts dysfunction is involved in the development of pre-eclampsia. J. Cell Mol. Med. 2018, 22, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Buurma, A.; Cohen, D.; Veraar, K.; Schonkeren, D.; Claas, F.H.; Bruijn, J.A.; Bloemenkamp, K.W.; Baelde, H.J. Preeclampsia is characterized by placental complement dysregulation. Hypertension 2012, 60, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Wells, M.; Faulk, P.W. Immunological studies of human placentae: Complement components in immature and mature chorionic villi. Clin. Exp. Immunol. 1984, 56, 175–184. [Google Scholar]
- Choi, S.Y.; Kim, K.H.; Lee, M.; Yeo, M.K.; Kim, J.; Suh, K.S. Complement component C4d deposition in the placenta of preeclampsia patients and renal glomeruli in 1 postpartum renal biopsy. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 139–145. [Google Scholar] [CrossRef]
- RǍdulescu, C.; Bacârea, A.; Huanu, A.; Gabor, R.; Dobreanu, M. Placental Growth Factor, Soluble fms-Like Tyrosine Kinase 1, Soluble Endoglin, IL-6, and IL-16 as Biomarkers in Preeclampsia. Mediat. Inflamm. 2016, 2016, 3027363. [Google Scholar] [CrossRef]
- Di Marco, G.S.; Reuter, S.; Hillebrand, U.; Amler, S.; König, M.; Larger, E.; Oberleithner, H.; Brand, E.; Pavenstädt, H.; Brand, M. The soluble VEGF receptor sFlt1 contributes to endothelial dysfunction in CKD. J. Am. Soc. Nephrol. 2009, 20, 2235–2245. [Google Scholar] [CrossRef]
- Cindrova-Davies, T.; Sanders, D.A.; Burton, G.J.; Charnock-Jones, D.S. Soluble FLT1 sensitizes endothelial cells to inflammatory cytokines by antagonizing VEGF receptor-mediated signalling. Cardiovasc. Res. 2011, 89, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Rolnik, D.L.; Nicolaides, K.H.; Poon, L.C. Prevention of preeclampsia with aspirin. Am. J. Obstet. Gynecol. 2022, 226, S1108–S1119. [Google Scholar] [CrossRef]
- Roberge, S.; Nicolaides, K.; Demers, S.; Hyett, J.; Chaillet, N.; Bujold, E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: Systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017, 216, 110–120.e6. [Google Scholar] [CrossRef]
- Roberge, S.; Nicolaides, K.H.; Demers, S.; Villa, P.; Bujold, E. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: A meta-analysis. Ultrasound Obstet. Gynecol. 2013, 41, 491–499. [Google Scholar] [CrossRef]
- Atallah, A.; Lecarpentier, E.; Goffinet, F.; Doret-Dion, M.; Gaucherand, P.; Tsatsaris, V. Aspirin for Prevention of Preeclampsia. Drugs 2017, 77, 1819–1831. [Google Scholar] [CrossRef]
- Thorp, J.A.; Walsh, S.W.; Brath, P.C. Low-dose aspirin inhibits thromboxane, but not prostacyclin, production by human placental arteries. Am. J. Obstet. Gynecol. 1988, 159, 1381–1384. [Google Scholar] [CrossRef]
- Bitko, V.; Velazquez, A.; Yang, L.; Yang, Y.C.; Barik, S. Transcriptional induction of multiple cytokines by human respiratory syncytial virus requires activation of NF-κB and is inhibited by sodium salicylate and aspirin. Virology 1997, 232, 369–378. [Google Scholar] [CrossRef]
- Sakowicz, A. The Targeting of Nuclear Factor Kappa B by Drugs Adopted for the Prevention and Treatment of Preeclampsia. Int. J. Mol. Sci. 2022, 23, 2881. [Google Scholar] [CrossRef]
- Dimitrova, N.; Zamudio, J.R.; Jong, R.M.; Soukup, D.; Resnick, R.; Sarma, K.; Ward, A.J.; Raj, A.; Lee, J.; Sharp, P.A.; et al. Histone Deacetylase-3 antagonizes Aspirin-stimulated Endothelial Nitric Oxide production by reversing Aspirin- induced lysine acetylation of Endothelial Nitric Oxide Synthase. Circ. Res. 2010, 107, 877–887. [Google Scholar] [CrossRef]
- Ai, G.; Dachineni, R.; Kumar, D.R.; Marimuthu, S.; Alfonso, L.F.; Bhat, G.J. Aspirin acetylates wild type and mutant p53 in colon cancer cells: Identification of aspirin acetylated sites on recombinant p53. Tumor. Biol. 2016, 37, 6007–6016. [Google Scholar] [CrossRef]
- ACOG. Practice Biulletin no 203. Clinical Management Guidelines for Obstetrician: Chronic Hypertension in Pregnancy. Obstet. Gynecol. 2019, 133, 168–186. [Google Scholar]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.; Magee, L.A.; Verlohren, S.; Shennan, A.; von Dadelszen, P.; Sheiner, E.; Hadar, E.; Visser, G.; Da Silva Costa, F.; Kapur, A.; et al. A literature review and best practice advice for second and third trimester risk stratification, monitoring, and management of pre-eclampsia: Compiled by the Pregnancy and Non-Communicable Diseases Committee of FIGO (the International Federation of Gyneco. Int. J. Gynecol. Obstet. 2021, 154, 3–31. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [CrossRef]
- Webster, K.; Fishburn, S.; Maresh, M.; Findlay, S.C.; Chappell, L.C. Diagnosis and management of hypertension in pregnancy: Summary of updated NICE guidance. BMJ 2019, 366, l5119. [Google Scholar] [CrossRef]
- Prejbisz, A.; Dobrowolski, P.; Kosiński, P.; Bomba-Opoń, D.; Adamczyk, M.; Bekiesińska-Figatowska, M.; Kądziela, J.; Konopka, A.; Kostka-Jeziorny, K.; Kurnatowska, I. Management of hypertension in pregnancy—prevention, diagnosis, treatment and long-term prognosis. A position statement based on expert consensus of the Polish Society of Hypertension, Polish Cardiac Society and Polish Society of Gynecologists and Obstet. Arter. Hypertens 2019, 3, 117–182. [Google Scholar] [CrossRef]
- Lowe, S.A.; Bowyer, L.; Lust, K.; McMahon, L.; Morton, M.; North, R.A.; Paech, M.; Said, J.; Paech, M.; Walters, B. SOMANZ Guidelines for the Management of Hypertensive Disorders of Pregnancy, 2014. Aust. N. Z. J. Obstet. Gynaecol. 2015, 55, e1–e29. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Malinowski, B.; Puk, O.; Socha, M.; Słupski, M. Methyldopa as an inductor of postpartum depression and maternal blues: A review. Biomed. Pharmacother. 2020, 127, 110196. [Google Scholar] [CrossRef]
- Khalil, A.; Muttukrishna, S.; Harrington, K.; Jauniaux, E. Effect of antihypertensive therapy with alpha methyldopa on levels of angiogenic factors in pregnancies with hypertensive disorders. PLoS ONE 2008, 3, e2766. [Google Scholar] [CrossRef]
- Xu, B.; Charlton, F.; Makris, A.; Hennessy, A. Antihypertensive drugs methyldopa, labetalol, hydralazine, and clonidine improve trophoblast interaction with endothelial cellular networks in vitro. J. Hypertens. 2014, 32, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Herwati, T.W.; Yulistiani, Y.; Eddy Zarkaty, M. Analysis of methyldopa therapy on sFlt-1 antiangiogenic levels in patients with severe preeclampsia. Folia Med. Indones. 2018, 54, 46–52. [Google Scholar] [CrossRef]
- Xu, B.; Bobek, G.; Hennessy, A. Antihypertensive methyldopa, labetalol, hydralazine, and clonidine reversed TNF-α inhibited eNOS expression in endothelial-trophoblast cellular networks. Clin. Exp. Pharmacol. Physiol. 2017, 44, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Juwita, D.R.; Yulistiani, Y.; Eddy Zarkaty, M. Effects of Methyldopa on Vegf Levels As Proangiogenic Factor in Severe Pre-Eclampsia At Haji Hospital, Surabaya. Folia Med. Indones. 2017, 53, 267. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, Z.; Yin, X.; Tang, L.; Luo, H.; Li, H.; Zhang, Y.; Luo, W. In vitro and in vivo study of hydralazine, a potential anti-angiogenic agent. Eur. J. Pharmacol. 2016, 779, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Knowles, H.J.; Tian, Y.M.; Mole, D.R.; Harris, A.L. Novel mechanism of action for hydralazine: Induction of hypoxia-inducible factor-1 α, vascular endothelial growth factor, and angiogenesis by inhibition of prolyl hydroxylases. Circ. Res. 2004, 95, 162–169. [Google Scholar] [CrossRef]
- Xu, B.; Charlton, F.; Makris, A.; Hennessy, A. PP042. Anti-hypertensive drugs hydralazine, clonidine and labetalol improve trophoblast integration into endothelial cellular networks in vitro. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2012, 2, 264. [Google Scholar] [CrossRef]
- Xu, B.; Makris, A.; Thornton, C.; Ogle, R.; Horvath, J.S.; Hennessy, A. Antihypertensive drugs clonidine, diazoxide, hydralazine and furosemide regulate the production of cytokines by placentas and peripheral blood mononuclear cells in normal pregnancy. J. Hypertens. 2006, 24, 915–922. [Google Scholar] [CrossRef]
- Xu, B.; Thornton, C.; Makris, A.; Ogle, R.; Hennessy, A. Anti-hypertensive drugs alter cytokine production from preeclamptic placentas and peripheral blood mononuclear cells. Hypertens. Pregnancy 2007, 26, 343–356. [Google Scholar] [CrossRef]
- Chang, T.T.; Chen, J.W. Hydralazine improves ischemia-induced neovasculogenesis via xanthine-oxidase inhibition in chronic renal insufficiency. Pharmacol. Res. 2020, 151, 104509. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Long, H.; Zhao, J.; Zhong, G.; Li, J. Nifedipine inhibits oxidative stress and ameliorates osteoarthritis by activating the nuclear factor erythroid-2-related factor 2 pathway. Life Sci. 2020, 253, 117292. [Google Scholar] [CrossRef]
- Chou, T.C. New mechanisms of antiplatelet activity of nifedipine, an L-type calcium channel blocker. BioMedicine 2014, 4, 17–24. [Google Scholar] [CrossRef]
- Gao, X.-Y.; Yu, Q.; Liu, S.-J.; Lu, F.-Q.; Zhou, S.-M.; Zhang, S.-T. Nifedipine attenuates vascular inflammation via inhibin NF-κB activity. Zhonghua Xin Xue Guan Bing Za Zhi 2010, 38, 1025–1030. (In Chinese) [Google Scholar]
- Matsumori, A.; Nunokawa, Y.; Sasayama, S. Nifedipine inhibits activation of transcription factor NF-κB. Life Sci. 2000, 67, 2655–2661. [Google Scholar] [CrossRef] [PubMed]
- Agabiti Rosei, E.; Morelli, P.; Rizzoni, D. Effects of nifedipine GITS 20 mg or enalapril 20 mg on blood pressure and inflammatory markers in patients with mild-moderate hypertension. Blood Press. 2005, 14, 14–22. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, T.; Kureishi-Bando, Y.; Numaguchi, Y.; Yoshida, O.; Dohi, Y.; Kimura, G.; Ueda, R.; Rabelink, T.J.; Murohara, T. Nifedipine improves endothelial function: Role of endothelial progenitor cells. Hypertension 2008, 52, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, M.J.; Knight, B.P.; Martinez, F.J.; Rubenfire, M. Inhaled nitric oxide in primary pulmonary hypertension: A safe and effective agent for predicting response to nifedipine. J. Am. Coll. Cardiol. 1998, 32, 1068–1073. [Google Scholar] [CrossRef]
- Berkels, R.; Egink, G.; Marsen, T.A.; Bartels, H.; Roesen, R.; Klaus, W. Nifedipine increases endothelial nitric oxide bioavailability by antioxidative mechanisms. Hypertension 2001, 37, 240–245. [Google Scholar] [CrossRef]
- Cífková, R. Hypertension in Pregnancy: A Diagnostic and Therapeutic Overview. High Blood Press. Cardiovasc. Prev. 2023. ahead of print. [Google Scholar] [CrossRef]
- Kouoh, F.; Gressier, B.; Dine, T.; Luyckx, M.; Brunet, C.; Ballester, L.; Cazin, J.C. In vitro and ex vivo antioxidant activities of labetalol on rabbit neutrophil respiratory burst. Adv. Ther. 2004, 21, 178–185. [Google Scholar] [CrossRef]
- Xia, Z.; Irwin, M.G. Esmolol may abolish volatile anesthetic-induced postconditioning by scavenging reactive oxygen species. Anesthesiology 2009, 111, 924–925. [Google Scholar] [CrossRef]
- Roth, E.; Torok, B. Effect of the ultrashort-acting β-blocker Brevibloc on free- radical-mediated injuries dining the early reperfusion state. Basic Res. Cardiol. 1991, 86, 422–433. [Google Scholar] [CrossRef]
- Röth, E.; Matos, G.; Guarnieri, C.; Papp, B.; Varga, V. Influence of the beta-blocker therapy on neutrophil superoxide generation and platelet aggregation in experimental myocardial ischemia and reflow. Acta Physiol. Hung. 1995, 83, 163–170. [Google Scholar]
- Wang, Y.; Zhang, Y.; Canzoneri, B.J.; Gu, Y.; Philibert, L.; Lewis, D.F. Prostacyclin and thromboxane levels in women with severe preeclampsia undergoing magnesium sulfate therapy during antepartum and postpartum periods. Hypertens. Pregnancy 2008, 27, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V.; Whitehead, C.; Hyett, J.; da Silva Costa, F.; Nicolaides, K.; et al. Pre-eclampsia. Nat. Rev. Dis. Prim. 2023, 9, 8. [Google Scholar] [CrossRef]
- Smith, D.D.; Costantine, M.M. The role of statins in the prevention of preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S1171–S1181. [Google Scholar] [CrossRef]
- Yilmaz, A.; Reiss, C.; Weng, A.; Cicha, I.; Stumpf, C.; Steinkasserer, A.; Daniel, W.G.; Garlichs, C.D. Differential effects of statins on relevant functions of human monocyte-derived dendritic cells. J. Leukoc. Biol. 2005, 79, 529–538. [Google Scholar] [CrossRef]
- Cimato, T.R.; Palka, B.A. Effects of statins on TH1 modulating cytokines in human subjects. PeerJ 2015, 2015, e764. [Google Scholar] [CrossRef]
- Hilgendorff, A.; Muth, H.; Parviz, B.; Staubitz, A.; Haberbosch, W.; Tillmanns, H.; Hölschermann, H. Statins differ in their ability to block NF-kappaB activation in human blood monocytes. Int. J. Clin. Pharmacol. Ther. 2003, 41, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Hölschermann, H.; Schuster, D.; Parviz, B.; Haberbosch, W.; Tillmanns, H.; Muth, H. Statins prevent NF-κB transactivation independently of the IKK-pathway in human endothelial cells. Atherosclerosis 2006, 185, 240–245. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Phosphoinositide 3-Kinase/Akt signaling and redox metabolism in cancer. Front. Oncol. 2018, 8, 2–10. [Google Scholar] [CrossRef]
- Rozas-Villanueva, M.F.; Casanello, P.; Retamal, M.A. Role of ROS/RNS in preeclampsia: Are connexins the missing piece? Int. J. Mol. Sci. 2020, 21, 4698. [Google Scholar] [CrossRef] [PubMed]
- Brownfoot, F.C.; Tong, S.; Hannan, N.J.; Hastie, R.; Cannon, P.; Kaitu’u-Lino, T.J. Effects of simvastatin, rosuvastatin and pravastatin on soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin (sENG) secretion from human umbilical vein endothelial cells, primary trophoblast cells and placenta. BMC Pregnancy Childbirth 2016, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Putra, R.A.; Effendi, J.S.; Permadi, W.; Bandiara, R.; Fauziah, P.N. Role of statin as inducer of Hmox-1 system in treatment of preeclampsia. Cell. Mol. Biol. 2018, 64, 1–4. [Google Scholar] [CrossRef]
- Saad, A.F.; Kechichian, T.; Yin, H.; Sbrana, E.; Longo, M.; Wen, M.; Tamayo, E.; Hankins, G.D.V.; Saade, G.R.; Costantine, M.M. Effects of pravastatin on angiogenic and placental hypoxic imbalance in a mouse model of preeclampsia. Reprod. Sci. 2014, 21, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.J.; Banek, C.T.; Needham, K.; Gillham, H.; Capoccia, S.; Regal, J.F.; Gilbert, J.S. Pravastatin attenuates hypertension, oxidative stress, and angiogenic imbalance in rat model of placental ischemia-induced hypertension. Hypertension 2013, 61, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Ahmed, A.; Girardi, G. Role of complement component C1q in the onset of preeclampsia in mice. Hypertension 2011, 58, 716–724. [Google Scholar] [CrossRef]
- Katsi, V.; Georgountzos, G.; Kallistratos, M.S.; Zerdes, I.; Makris, T.; Manolis, A.J.; Nihoyannopoulos, P.; Tousoulis, D. The role of statins in prevention of preeclampsia: A promise for the future? Front. Pharmacol. 2017, 8, 247. [Google Scholar] [CrossRef]
- Brownfoot, F.C.; Tong, S.; Hannan, N.J.; Binder, N.K.; Walker, S.P.; Cannon, P.; Hastie, R.; Onda, K.; Kaitu’U-Lino, T.J. Effects of Pravastatin on Human Placenta, Endothelium, and Women with Severe Preeclampsia. Hypertension 2015, 66, 687–697. [Google Scholar] [CrossRef]
- Cudmore, M.; Ahmad, S.; Al-Ani, B.; Fujisawa, T.; Coxall, H.; Chudasama, K.; Devey, L.R.; Wigmore, S.J.; Abbas, A.; Hewett, P.W.; et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation 2007, 115, 1789–1797. [Google Scholar] [CrossRef]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai, J.; Mammoto, T.; Kim, Y.M.; Bdolah, Y.; Lim, K.; Yuan, H.; Libermann, T.A.; et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006, 12, 642–650. [Google Scholar] [CrossRef]
- Omori, H.; Nagashima, H.; Tsurumi, Y.; Takagi, A.; Ishizuka, N.; Hagiwara, N.; Kawana, M.; Kasanuki, H. Direct in vivo evidence of a vascular statin: A single dose of cerivastatin rapidly increases vascular endothelial responsiveness in healthy normocholesterolaemic subjects. Br. J. Clin. Pharmacol. 2002, 54, 395–399. [Google Scholar] [CrossRef]
- Wolfrum, S.; Jensen, K.S.; Liao, J.K. Endothelium-dependent effects of statins. Arter. Thromb. Vasc. Biol. 2003, 23, 729–736. [Google Scholar] [CrossRef]
- Costantine, M.M.; West, H.; Wisner, K.L.; Caritis, S.; Clark, S.; Venkataramanan, R.; Stika, C.S.; Rytting, E.; Wang, X.; Ahmed, M.S.; et al. A randomized pilot clinical trial of pravastatin versus placebo in pregnant patients at high risk of preeclampsia. Am. J. Obstet. Gynecol. 2021, 225, 666.e1–666.e15. [Google Scholar] [CrossRef]
- Costantine, M.M.; Cleary, K.; Hebert, M.F.; Ahmed, M.S.; Brown, L.M.; Ren, Z.; Easterling, T.R.; Haas, D.M.; Haneline, L.S.; Caritis, S.N.; et al. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: A pilot randomized controlled trial. Am. J. Obstet. Gynecol. 2016, 214, 720.e1–720.e17. [Google Scholar] [CrossRef]
- Akbar, M.I.A.; Azis, M.A.; Riu, D.S.; Wawengkang, E.; Ernawati, E.; Bachnas, M.A.; Sulistyowati, S.; Dachlan, E.G.; Mose, J.C.; Dekker, G. INOVASIA Study: A Multicenter Randomized Clinical Trial of Pravastatin to Prevent Preeclampsia in High-Risk Patients. Am. J. Perinatol. 2022. [Google Scholar] [CrossRef]
- Akbar, M.I.A.; Yosediputra, A.; Pratama, R.E.; Fadhilah, N.L.; Sulistyowati, S.; Amani, F.Z.; Ernawati, E.; Dachlan, E.G.; Angsar, M.D.; Dekker, G. INOVASIA Study: A Randomized Open Controlled Trial to Evaluate Pravastatin to Prevent Preeclampsia and Its Effects on sFlt1/PlGF Levels. Am. J. Perinatol. 2021. [Google Scholar] [CrossRef]
- Ahmed, A.; Williams, D.J.; Cheed, V.; Middleton, L.J.; Ahmad, S.; Wang, K.; Vince, A.T.; Hewett, P.; Spencer, K.; Khan, K.S.; et al. Pravastatin for early-onset pre-eclampsia: A randomised, blinded, placebo-controlled trial. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 478–488. [Google Scholar] [CrossRef]
- Emami, F.; Baharak, M.A.; Seydi, E.; Zargar, M.; Naserzadeh, P.; Pourahmad, J. Embryotoxic effects of atorvastatin on mouse fetus. Iran. J. Pharm. Sci. 2014, 9, 13–23. [Google Scholar]
- Edison, R.J.; Muenke, M. Central Nervous System and Limb Anomalies in Case Reports of First-Trimester Statin Exposure. N. Engl. J. Med. 2004, 350, 1579–1582. [Google Scholar] [CrossRef]
- Godfrey, L.M.; Erramouspe, J.; Cleveland, K.W. Teratogenic Risk of Statins in Pregnancy. Ann. Pharmacother. 2012, 46, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Poornima, I.G.; Pulipati, V.P.; Brinton, E.A.; Wild, R.A. Update on Statin Use in Pregnancy. Am. J. Med. 2023, 136, 12–14. [Google Scholar] [CrossRef]
- Girardi, G.; Lingo, J.J.; Fleming, S.D.; Regal, J.F. Essential Role of Complement in Pregnancy: From Implantation to Parturition and Beyond. Front. Immunol. 2020, 11, 1681. [Google Scholar] [CrossRef] [PubMed]
- Girardi, G.; Yarilin, D.; Thurman, J.M.; Holers, V.M.; Salmon, J.E. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J. Exp. Med. 2006, 203, 2165–2175. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wu, K.; Liang, X.; Li, R.; Lai, K.P. Clinical Efficacy and Safety of Eculizumab for Treating Myasthenia Gravis. Front. Immunol. 2021, 12, 715036. [Google Scholar] [CrossRef] [PubMed]
- Lonze, B.E.; Zachary, A.A.; Magro, C.M.; Desai, N.M.; Orandi, B.J.; Dagher, N.N.; Singer, A.L.; Carter-Monroe, N.; Nazarian, S.M.; Segev, D.L.; et al. Eculizumab prevents recurrent antiphospholipid antibody syndrome and enables successful renal transplantation. Am. J. Transpl. 2014, 14, 459–465. [Google Scholar] [CrossRef]
- Wright, R.D.; Bannerman, F.; Beresford, M.W.; Oni, L. A systematic review of the role of eculizumab in systemic lupus erythematosus-associated thrombotic microangiopathy. BMC Nephrol. 2020, 21, 248. [Google Scholar] [CrossRef]
- Phillips, J.; Nathan, E.; Graham, D. Preeclampsia in women with lupus-Influence of aspirin and hydroxychloroquine on pregnancy outcome. Pregnancy Hypertens. 2023, 31, 14–16. [Google Scholar] [CrossRef]
- Clark, E.A.S.; Silver, R.M.; Branch, D.W. Do antiphospholipid antibodies cause preeclampsia and HELLP syndrome? Curr. Rheumatol. Rep. 2007, 9, 219–225. [Google Scholar] [CrossRef]
- Kelly, R.; Arnold, L.; Richards, S.; Hill, A.; Bomken, C.; Hanley, J.; Loughney, A.; Beauchamp, J.; Khursigara, G.; Rother, R.P.; et al. The management of pregnancy in paroxysmal nocturnal haemoglobinuria on long term eculizumab. Br. J. Haematol. 2010, 149, 446–450. [Google Scholar] [CrossRef]
- Hallstensen, R.F.; Bergseth, G.; Foss, S.; Jæger, S.; Gedde-Dahl, T.; Holt, J.; Christiansen, D.; Lau, C.; Brekke, O.L.; Armstrong, E.; et al. Eculizumab treatment during pregnancy does not affect the complement system activity of the newborn. Immunobiology 2015, 220, 452–459. [Google Scholar] [CrossRef]
- U. S. Department of Health and Human Services Food and Drug Administration. In Soliris (Eculizumab) Medication Guide; FDA: Silver Spring, MD, USA, 2007; Volume March, pp. 8–11. [Google Scholar]
- Burwick, R.M.; Feinberg, B.B. Eculizumab for the treatment of preeclampsia/HELLP syndrome. Placenta 2013, 34, 201–203. [Google Scholar] [CrossRef]
- Morales, E.; Galindo, A.; García, L.; Villalaín, C.; Alonso, M.; Gutiérrez, E.; Rodríguez-Almaraz, M.E.; Praga, M.; Herraiz, I. Eculizumab in Early-Stage Pregnancy. Kidney Int. Rep. 2020, 5, 2383–2387. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, N.S.; Thomson, S.E.; Heffernan, S.J.; Lim, S.; Thompson, J.; Ogle, R.; McKenzie, P.; Kirwan, P.J.; Makris, A.; Hennessy, A. Tumor necrosis factor α induces a model of preeclampsia in pregnant baboons (Papio hamadryas). Cytokine 2011, 56, 192–199. [Google Scholar] [CrossRef] [PubMed]
- LaMarca, B.; Wallukat, G.; Llinas, M.; Herse, F.; Dechend, R.; Granger, J.P. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor α in pregnant rats. Hypertension 2008, 52, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Small, H.Y.; Nosalski, R.; Morgan, H.; Beattie, E.; Guzik, T.J.; Graham, D.; Delles, C. Role of tumor necrosis factor-α and natural killer cells in uterine artery function and pregnancy outcome in the stroke-prone spontaneously hypertensive rat. Hypertension 2016, 68, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, A.; Cunnigham, M.W.; Ibrahim, T.; Amaral, L.; Cornelius, D.; Ramana Vaka, V.; LaMarca, B. 109: Etanercept improves natural killer cell activation and hypertension in a preclinical rat model of pre-eclampsia. Am. J. Obstet. Gynecol. 2019, 220, S86–S87. [Google Scholar] [CrossRef]
- Murphy, S.R.; LaMarca, B.B.D.; Parrish, M.; Cockrell, K.; Granger, J.P. Control of soluble fms-like tyrosine-1 (sFlt-1) production response to placental ischemia/hypoxia: Role of tumor necrosis factor-α. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2013, 304, 130–135. [Google Scholar] [CrossRef]
- Cunningham, M.W.; Jayaram, A.; Deer, E.; Amaral, L.M.; Vaka, V.R.; Ibrahim, T.; Cornelius, D.C.; LaMarca, B. Tumor necrosis factor alpha (TNF-α) blockade improves natural killer cell (NK) activation, hypertension, and mitochondrial oxidative stress in a preclinical rat model of preeclampsia. Hypertens. Pregnancy 2020, 39, 399–404. [Google Scholar] [CrossRef]
- Berthelsen, B.G.; Fjeldsøe-Nielsen, H.; Nielsen, C.T.; Hellmuth, E. Etanercept concentrations in maternal serum, umbilical cord serum, breast milk and child serum during breastfeeding. Rheumatology 2010, 49, 2225–2227. [Google Scholar] [CrossRef]
- Eliesen, G.A.M.; van Drongelen, J.; van Hove, H.; Kooijman, N.I.; van den Broek, P.; de Vries, A.; Roeleveld, N.; Russel, F.G.M.; Greupink, R. Assessment of Placental Disposition of Infliximab and Etanercept in Women With Autoimmune Diseases and in the Ex Vivo Perfused Placenta. Clin. Pharmacol. Ther. 2020, 108, 99–106. [Google Scholar] [CrossRef]
- Araujo, G.G.; dos Passos Junior, R.R.; Lunardi, R.R.; Volpato, G.T.; Soares, T.S.; Giachini, F.R.; Lima, V.V. Maternal and Fetal-Placental Effects of Etanercept Treatment During Rats’ Pregnancy. Front. Physiol. 2022, 12, 787369. [Google Scholar] [CrossRef]
- Bröms, G.; Kieler, H.; Ekbom, A.; Gissler, M.; Hellgren, K.; Lahesmaa-Korpinen, A.M.; Pedersen, L.; Schmitt-Egenolf, M.; Sørensen, H.T.; Granath, F. Anti-TNF treatment during pregnancy and birth outcomes: A population-based study from Denmark, Finland, and Sweden. Pharmacoepidemiol. Drug Saf. 2020, 29, 316–327. [Google Scholar] [CrossRef]
- Fu, J.; Li, L.; Qi, L.; Zhao, L. A randomized controlled trial of etanercept in the treatment of refractory recurrent spontaneous abortion with innate immune disorders. Taiwan J. Obstet. Gynecol. 2019, 58, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Carman, W.J.; Accortt, N.A.; Anthony, M.S.; Iles, J.; Enger, C. Pregnancy and infant outcomes including major congenital malformations among women with chronic inflammatory arthritis or psoriasis, with and without etanercept use. Pharmacoepidemiol. Drug Saf. 2017, 26, 1109–1118. [Google Scholar] [CrossRef]
- Drechsel, P.; Stüdemann, K.; Niewerth, M.; Horneff, G.; Fischer-Betz, R.; Seipelt, E.; Spähtling-Mestekemper, S.; Aries, P.; Zink, A.; Klotsche, J.; et al. Pregnancy outcomes in DMARD-exposed patients with juvenile idiopathic arthritis-results from a JIA biologic registry. Rheumatology 2020, 59, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Kattah, A.; Becker, B.; Kane, S. Increased Risk of Preeclampsia in Women With Inflammatory Bowel Disease on Anti-TNF Therapy. Am. J. Gastroenterol. 2019, 114, S443–S444. [Google Scholar] [CrossRef]
- Wahl, C.; Liptay, S.; Adler, G.; Schmid, R.M. Sulfasalazine: A potent and specific inhibitor of nuclear factor kappa B. J. Clin. Investig. 1998, 101, 1163–1174. [Google Scholar] [CrossRef]
- Weber, C.K.; Liptay, S.; Wirth, T.; Adler, G.; Schmid, R.M. Suppression of NF-kappaB activity by sulfasalazine is mediated by direct inhibition of IkappaB kinases alpha and beta. Gastroenterology 2000, 119, 1209–1218. [Google Scholar] [CrossRef]
- Sykes, L.; Thomson, K.R.; Boyce, E.J.; Lee, Y.S.; Rasheed, Z.B.M.; Macintyre, D.A.; Teoh, T.G.; Bennett, P.R. Sulfasalazine augments a pro-inflammatory response in interleukin-1β-stimulated amniocytes and myocytes. Immunology 2015, 146, 630–644. [Google Scholar] [CrossRef]
- Binder, N.K.; de Alwis, N.; Beard, S.; Kadife, E.; Harper, A.; Kaitu’u-Lino, T.J.; Brownfoot, F.C.; Hannan, N.J. Sulfasalazine for the treatment of preeclampsia in a nitric oxide synthase antagonist mouse model. Placenta 2023, 132, 20–26. [Google Scholar] [CrossRef]
- Sonmez, M.I.; Shahzadi, A.; Kose, C.; Sonmez, H.; Ozyazgan, S.; Akkan, A.G. Effect of sulfasalazine on endothelium-dependent vascular response by the activation of Nrf2 signalling pathway. Front. Pharmacol. 2022, 13, 979300. [Google Scholar] [CrossRef]
- Brownfoot, F.C.; Hannan, N.J.; Cannon, P.; Nguyen, V.; Hastie, R.; Parry, L.J.; Senadheera, S.; Tuohey, L.; Tong, S.; Kaitu’u-Lino, T.J. Sulfasalazine reduces placental secretion of antiangiogenic factors, up-regulates the secretion of placental growth factor and rescues endothelial dysfunction. EBioMedicine 2019, 41, 636–648. [Google Scholar] [CrossRef]
- Brownfoot, F.C.; Tong, S.; Hannan, N.; Hastie, R.; Cannon, P.; Kaitu’u-Lino, T.J. Sulfasalazine reduces the toxins of preeclampsia soluble Flt1 and soluble endoglin and quenches endothelial dys- function in primary human tissues: A novel potential therapeutic [273-Pos]. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2015, 5, 136–137. [Google Scholar] [CrossRef]
- Hastie, R.; Brownfoot, F.C.; Cannon, P.; Nguyen, V.; Tuohey, L.; Hannan, N.J.; Tong, S.; Kaitu’u-Lino, T.J. Sulfasalazine decreases soluble fms-like tyrosine kinase-1 secretion potentially via inhibition of upstream placental epidermal growth factor receptor signalling. Placenta 2019, 87, 53–57. [Google Scholar] [CrossRef]
- Nuri, E.; Taraborelli, M.; Andreoli, L.; Tonello, M.; Gerosa, M.; Calligaro, A.; Argolini, L.M.; Kumar, R.; Pengo, V.; Meroni, P.L.; et al. Long-term use of hydroxychloroquine reduces antiphospholipid antibodies levels in patients with primary antiphospholipid syndrome. Immunol. Res. 2017, 65, 17–24. [Google Scholar] [CrossRef]
- Shippey, E.A.; Wagler, V.D.; Collamer, A.N. Hydroxychloroquine: An old drug with new relevance. Clevel. Clin. J. Med. 2018, 85, 459–467. [Google Scholar] [CrossRef]
- Hariharan, A.; Hakeem, A.R.; Radhakrishnan, S.; Reddy, M.S.; Rela, M. The Role and Therapeutic Potential of NF-kappa-B Pathway in Severe COVID-19 Patients. Inflammopharmacology 2021, 29, 91–100. [Google Scholar] [CrossRef]
- Rahman, R.; Murthi, P.; Singh, H.; Gurusinghe, S.; Mockler, J.C.; Lim, R.; Wallace, E.M. The effects of hydroxychloroquine on endothelial dysfunction. Pregnancy Hypertens. 2016, 6, 259–262. [Google Scholar] [CrossRef]
- Kadife, E.; Hannan, N.; Harper, A.; Binder, N.; Beard, S.; Brownfoot, F.C. Hydroxychloroquine reduces soluble Flt-1 secretion from human cytotrophoblast, but does not mitigate markers of endothelial dysfunction in vitro. PLoS ONE 2022, 17, e0271560. [Google Scholar] [CrossRef]
- De Moreuil, C.; Alavi, Z.; Pasquier, E. Hydroxychloroquine may be beneficial in preeclampsia and recurrent miscarriage. Br. J. Clin. Pharmacol. 2020, 86, 39–49. [Google Scholar] [CrossRef]
- Bonnar, J.; McNicol, G.P.; Douglas, A.S. Coagulation and fibrinolytic systems in pre-eclampsia and eclampsia. Obstet. Gynecol. Surv. 1971, 26, 702–704. [Google Scholar] [CrossRef]
- Do, S.C.; Rizk, N.M.; Druzin, M.L.; Simard, J.F. Does Hydroxychloroquine Protect against Preeclampsia and Preterm Delivery in Systemic Lupus Erythematosus Pregnancies? Am. J. Perinatol. 2020, 37, 873–880. [Google Scholar] [CrossRef]
- Schreiber, K.; Breen, K.; Cohen, H.; Jacobsen, S.; Middeldorp, S.; Pavord, S.; Regan, L.; Roccatello, D.; Robinson, S.E.; Sciascia, S.; et al. Hydroxychloroquine to Improve Pregnancy Outcome in Women with Antiphospholipid Antibodies (HYPATIA) Protocol: A Multinational Randomized Controlled Trial of Hydroxychloroquine versus Placebo in Addition to Standard Treatment in Pregnant Women with Antipho. Semin. Thromb. Hemost. 2017, 43, 562–571. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Y.; Zhang, Y.; Yang, H. Hydroxychloroquine significantly decreases the risk of preeclampsia in pregnant women with autoimmune disorders: A systematic review and meta-analysis. Clin. Rheumatol. 2023, 42, 1223–1235. [Google Scholar] [CrossRef]
- Turanov, A.A.; Lo, A.; Hassler, M.R.; Makris, A.; Ashar-patel, A.; Alterman, J.F.; Coles, A.H.; Haraszti, R.A.; Roux, L.; Godinho, B.M.D.C.; et al. Articles RNAi modulation of placental sFLT1 for the treatment of preeclampsia. Nat. Biotechnol. 2018, 36, 1164–1175. [Google Scholar] [CrossRef]
- Bian, Z.; Shixia, C.; Duan, T. First-trimester maternal serum levels of sFLT1, PGF and ADMA predict preeclampsia. PLoS ONE 2015, 10, e0124684. [Google Scholar] [CrossRef]
- Amraoui, F.; Spijkers, L.; Lahsinoui, H.H.; Vogt, L.; Van Der Post, J.; Peters, S.; Afink, G.; Ris-Stalpers, C.; Van Den Born, B.J. SFlt-1 elevates blood pressure by augmenting endothelin-1-mediated vasoconstriction in mice. PLoS ONE 2014, 9, e91897. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef]
- Wind, M.; Gaasbeek, A.G.A.; Oosten, L.E.M.; Rabelink, T.J.; van Lith, J.M.M.; Sueters, M.; Teng, Y.K.O. Therapeutic plasma exchange in pregnancy: A literature review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 260, 29–36. [Google Scholar] [CrossRef]
- Colpo, A.; Marson, P.; Pavanello, F.; Tison, T.; Gervasi, M.T.; Zambon, A.; Ruffatti, A.; De Silvestro, G.; Hoxha, A. Therapeutic apheresis during pregnancy: A single center experience. Transfus. Apher. Sci. 2019, 58, 652–658. [Google Scholar] [CrossRef]
- Winkler, K.; Hoffmann, M.M.; Pütz, G. Letter by Winkler et al regarding article, “Pilot study of extracorporeal removal of soluble Fms-Like tyrosine kinase 1 in preeclampsia”. Circulation 2012, 125, 60954. [Google Scholar] [CrossRef][Green Version]
- Winkler, K.; Contini, C.; König, B.; Krumrey, B.; Pütz, G.; Zschiedrich, S.; Pecks, U.; Stavropoulou, D.; Prömpeler, H.; Kunze, M.; et al. Treatment of very preterm preeclampsia via heparin-mediated extracorporeal LDL-precipitation (H.E.L.P.) apheresis: The Freiburg preeclampsia H.E.L.P.-Apheresis study. Pregnancy Hypertens. 2018, 12, 136–143. [Google Scholar] [CrossRef]
- Thadhani, R.; Kisner, T.; Hagmann, H.; Bossung, V.; Noack, S.; Schaarschmidt, W.; Jank, A.; Kribs, A.; Cornely, O.A.; Kreyssig, C.; et al. Pilot study of extracorporeal removal of soluble Fms-like tyrosine kinase 1 in preeclampsia. Circulation 2011, 124, 940–950. [Google Scholar] [CrossRef]
- Nakakita, B.; Mogami, H.; Kondoh, E.; Tsukamoto, T.; Yanagita, M.; Konishi, I. Case of soluble fms-like tyrosine kinase 1 apheresis in severe pre-eclampsia developed at 15 weeks’ gestation. J. Obstet. Gynaecol. Res. 2015, 41, 1661–1663. [Google Scholar] [CrossRef] [PubMed]
- Thadhani, R.; Hagmann, H.; Schaarschmidt, W.; Roth, B.; Cingoez, T.; Karumanchi, S.A.; Wenger, J.; Lucchesi, K.J.; Tamez, H.; Lindner, T.; et al. Removal of soluble fms-like tyrosine kinase-1 by dextran sulfate apheresis in preeclampsia. J. Am. Soc. Nephrol. 2016, 27, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Gubenšek, J.; Ponikvar, R.; Sršen, T.P.; Vodušek, V.F.; Moertl, M.G.; Lučovnik, M. Treatment of preeclampsia at extremely preterm gestation with therapeutic plasma exchange. Clin. Nephrol. 2021, 96, S101–S106. [Google Scholar] [CrossRef]
- Haddad, B.; Lefevre, G.; Rousseau, A.; Robert, T.; Saheb, S.; Rafat, C.; Bornes, M.; Tsatsaris, V.; Petit-Hoang, C.; Rondeau, E.; et al. LDL aphérèse dans la pré-éclampsie précoce et sévère: L’étude ADENA. Néphrologie Thérapeutique 2016, 12, 380–381. [Google Scholar] [CrossRef]
- Haddad, B.; Lefèvre, G.; Rousseau, A.; Robert, T.; Saheb, S.; Rafat, C.; Bornes, M.; Petit-Hoang, C.; Richard, F.; Lecarpentier, E.; et al. LDL-apheresis to decrease sFlt-1 during early severe preeclampsia: Report of two cases from a discontinued phase II trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 231, 70–74. [Google Scholar] [CrossRef]
- Pietro, L.; Daher, S.; Rudge, M.V.C.; Calderon, I.M.P.; Damasceno, D.C.; Sinzato, Y.K.; Bandeira, C.; Bevilacqua, E. Vascular endothelial growth factor (VEGF) and VEGF-receptor expression in placenta of hyperglycemic pregnant women. Placenta 2010, 31, 770–780. [Google Scholar] [CrossRef]
- Yu, J.; Jia, J.; Guo, X.; Chen, R.; Feng, L. Modulating circulating sFlt1 in an animal model of preeclampsia using PAMAM nanoparticles for siRNA delivery. Placenta 2017, 58, 1–8. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal. Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Bidwell, G.L. Novel protein therapeutics created using the elastin-like polypeptide platform. Physiology 2021, 36, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Logue, O.C.; Mahdi, F.; Chapman, H.; George, E.M.; Bidwell, G.L. A maternally sequestered, biopolymer-stabilized Vascular Endothelial Growth Factor (VEGF) chimera for treatment of preeclampsia. J. Am. Heart Assoc. 2017, 6, e007216. [Google Scholar] [CrossRef] [PubMed]
- Waller, J.P.; Howell, J.A.; Peterson, H.; George, E.M.; Bidwell, G.L., 3rd. Elastin-Like Polypeptide: VEGF-B Fusion Protein for Treatment of Preeclampsia. Hypertension 2021, 78, 1888–1901. [Google Scholar] [CrossRef]
- Mould, A.W.; Scotney, P.; Greco, S.A.; Hayward, N.K.; Nash, A.; Kay, G.F. Prophylactic but not therapeutic activity of a monoclonal antibody that neutralizes the binding of VEGF-B to VEGFR-1 in a murine collagen-induced arthritis model. Rheumatology 2008, 47, 263–266. [Google Scholar] [CrossRef]
- Bidwell, G.L.; George, E.M. Maternally sequestered polypeptide-based therapeutics to treat preeclampsia [276-Pos]. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2015, 5, 138. [Google Scholar] [CrossRef]
- Choi, J.W.; Im, M.W.; Pai, S.H. Nitric oxide production increases during normal pregnancy and decreases in preeclampsia. Ann. Clin. Lab. Sci. 2002, 32, 257–263. [Google Scholar]
- Hodžić, J.; Izetbegović, S.; Muračević, B.; Iriškić, R.; Jović, H.Š. Nitric oxide biosynthesis during normal pregnancy and pregnancy complicated by preeclampsia. Med. Glas. 2017, 14, 211–217. [Google Scholar] [CrossRef]
- Tashie, W.; Fondjo, L.A.; Owiredu, W.K.B.A.; Ephraim, R.K.D.; Asare, L.; Adu-Gyamfi, E.A.; Seidu, L. Altered bioavailability of nitric oxide and L-arginine is a key determinant of endothelial dysfunction in preeclampsia. Biomed. Res. Int. 2020, 2020, 3251956. [Google Scholar] [CrossRef] [PubMed]
- Oludare, G.O.; Jinadu, H.D.; Aro, O.O. L-arginine attenuates blood pressure and reverses the suppression of angiogenic risk factors in a rat model of preeclampsia. Pathophysiology 2018, 25, 389–395. [Google Scholar] [CrossRef]
- Vadillo-Ortega, F.; Perichart-Perera, O.; Espino, S.; Avila-Vergara, M.A.; Ibarra, I.; Ahued, R.; Godines, M.; Parry, S.; Macones, G.; Yanow, M.; et al. Effect of supplementation during pregnancy with L-arginine and antioxidant vitamins in medical food on pre-eclampsia in high risk population: Randomised controlled trial. BMJ 2011, 342, d2901. [Google Scholar] [CrossRef]
- Neri, I.; Jasonni, V.M.; Gori, G.F.; Blasi, I.; Facchinetti, F. Effect of L-arginine on blood pressure in pregnancy-induced hypertension: A randomized placebo-controlled trial. J. Matern. Neonatal. Med. 2006, 19, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Rytlewski, K.; Olszanecki, R.; Korbut, R.; Zdebski, Z. Effects of prolonged oral supplementation with L-arginine on blood pressure and nitric oxide synthesis in preeclampsia. Eur. J. Clin. Investig. 2005, 35, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Gemmel, M.; Sutton, E.F.; Brands, J.; Burnette, L.; Gallaher, M.J.; Powers, R.W. L-Citrulline supplementation during pregnancy improves perinatal and postpartum maternal vascular function in a mouse model of preeclampsia. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2021, 321, R3364–R3376. [Google Scholar] [CrossRef]
- Man, A.W.C.; Zhou, Y.; Lam, U.D.P.; Reifenberg, G.; Werner, A.; Habermeier, A.; Closs, E.I.; Daiber, A.; Münzel, T.; Xia, N.; et al. l-Citrulline ameliorates pathophysiology in a rat model of superimposed preeclampsia. Br. J. Pharmacol. 2022, 179, 3007–3023. [Google Scholar] [CrossRef]
- Atwa, K.A.; Ibrahim, Z.M.; Elshaer, M.; Taha, O.T.; Aboelroose, A.A. Role of Esomeprazole in Early Preeclampsia: A Randomized Controlled Trial. Austin J. Obstet. Gynecol. 2021, 8, 1189. [Google Scholar] [CrossRef]
- Cluver, C.A.; Hannan, N.J.; van Papendorp, E.; Hiscock, R.; Beard, S.; Mol, B.W.; Theron, G.B.; Hall, D.R.; Decloedt, E.H.; Stander, M.; et al. Esomeprazole to treat women with preterm preeclampsia: A randomized placebo controlled trial. Am. J. Obstet. Gynecol. 2018, 219, 388.e1–388.e17. [Google Scholar] [CrossRef]
- Saleh, L.; Samantar, R.; Garrelds, I.M.; Van Den Meiracker, A.H.; Visser, W.; Danser, A.H.J. Low Soluble Fms-Like Tyrosine Kinase-1, Endoglin, and Endothelin-1 Levels in Women with Confirmed or Suspected Preeclampsia Using Proton Pump Inhibitors. Hypertension 2017, 70, 594–600. [Google Scholar] [CrossRef]
- Onda, K.; Tong, S.; Beard, S.; Binder, N.; Muto, M.; Senadheera, S.N.; Parry, L.; Dilworth, M.; Renshall, L.; Brownfoot, F.; et al. Proton pump inhibitors decrease soluble fms-like tyrosine kinase-1 and soluble endoglin secretion, decrease hypertension, and rescue endothelial dysfunction. Hypertension 2017, 69, 457–468. [Google Scholar] [CrossRef]
- George, E.M.; Arany, M.; Cockrell, K.; Storm, M.V.; Stec, D.E.; Granger, J.P. Induction of heme oxygenase-1 attenuates sFlt-1-induced hypertension in pregnant rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 301, R1495–R1500. [Google Scholar] [CrossRef]
- Kedika, R.R.; Souza, R.F.; Spechler, S.J. Potential anti-inflammatory effects of proton pump inhibitors: A review and discussion of the clinical implications. Dig. Dis. Sci. 2009, 54, 2312–2317. [Google Scholar] [CrossRef]
- Tanigawa, T.; Watanabe, T.; Higuchi, K.; Machida, H.; Okazaki, H.; Yamagami, H.; Watanabe, K.; Tominaga, K.; Fujiwara, Y.; Oshitani, N.; et al. Lansoprazole, a proton pump inhibitor, suppresses production of tumor necrosis factor-α and interleukin-1β induced by lipopolysaccharide and Helicobacter pylori bacterial components in human monocytic cells via inhibition of activation of nuclear factor-κ. J. Clin. Biochem. Nutr. 2009, 45, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Song, X.P.; Zhang, Y.M.; Sui, S.A.; Li, X.Y.; Huang, Y. Activation of the ERK1/2 signaling pathway enhances proliferation and apoptosis of trophoblast in preeclampsia rats. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Arakawa, T. Lansoprazole decreases peripheral blood monocytes and intercellular adhesion molecule-1-positive mononuclear cells. Dig. Dis. Sci. 1999, 44, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Yoshikawa, T.; Tanaka, Y.; Fujita, N.; Kassai, K.; Naito, Y.; Kondo, M. A new mechanism for anti-inflammatory actions of proton pump inhibitors-Inhibitory effects on neutrophil-endothelial cell interactions. Aliment. Pharmacol. Ther. Suppl. 2000, 14, 74–81. [Google Scholar] [CrossRef]
- Ghebremariam, Y.T.; Lependu, P.; Lee, J.C.; Erlanson, D.A.; Slaviero, A.; Shah, N.H.; Leiper, J.; Cooke, J.P. Unexpected effect of proton pump inhibitors: Elevation of the cardiovascular risk factor asymmetric dimethylarginine. Circulation 2013, 128, 845–853. [Google Scholar] [CrossRef]
- Németh, B.; Murányi, E.; Hegyi, P.; Mátrai, P.; Szakács, Z.; Varjú, P.; Hamvas, S.; Tinusz, B.; Budán, F.; Czimmer, J.; et al. Asymmetric dimethylarginine levels in preeclampsia–Systematic review and meta-analysis. Placenta 2018, 69, 57–63. [Google Scholar] [CrossRef]
- Giusti, M.; Sidoti, M.; Augeri, C.; Rabitti, C.; Minuto, F. Effect of short-term treatment with low dosages ot the proton-pump inhibitor omeprazole on serum chromogranin A levels in man. Eur. J. Endocrinol. 2004, 150, 299–303. [Google Scholar] [CrossRef]
- Bralewska, M.; Pietrucha, T.; Sakowicz, A. Reduction in CgA-Derived CST Protein Level in HTR-8/SVneo and BeWo Trophoblastic Cell Lines Caused by the Preeclamptic Environment. Int. J. Mol. Sci. 2023, 24, 7124. [Google Scholar] [CrossRef]
- Bralewska, M.; Biesiada, L.; Grzesiak, M.; Rybak-Krzyszkowska, M.; Huras, H.; Gach, A.; Pietrucha, T.; Sakowicz, A. Chromogranin A demonstrates higher expression in preeclamptic placentas than in normal pregnancy. BMC Pregnancy Childbirth 2021, 21, 680. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Ye, S.; Wang, S.; Sun, W.; Hu, Y. Metformin inhibits nuclear factor-κB activation and inflammatory cytokines expression induced by high glucose via adenosine monophosphate-activated protein kinase activation in rat glomerular mesangial cells in vitro. Chin. Med. J. 2014, 127, 1755–1760. [Google Scholar] [CrossRef]
- Hattori, Y.; Suzuki, K.; Hattori, S.; Kasai, K. Metformin inhibits cytokine-induced nuclear factor κB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension 2006, 47, 1183–1188. [Google Scholar] [CrossRef]
- Cluver, C.A.; Hiscock, R.; Decloedt, E.H.; Hall, D.R.; Schell, S.; Mol, B.W.; Brownfoot, F.; Kaitu’u-Lino, T.J.; Walker, S.P.; Tong, S. Use of metformin to prolong gestation in preterm pre-eclampsia: Randomised, double blind, placebo controlled trial. BMJ 2021, 374, n2103. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.E.L.; Sadler, L.; Rowan, J. Metformin for gestational diabetes in routine clinical practice. Diabet. Med. 2011, 28, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Alqudah, A.; McKinley, M.C.; McNally, R.; Graham, U.; Watson, C.J.; Lyons, T.J.; McClements, L. Risk of pre-eclampsia in women taking metformin: A systematic review and meta-analysis. Diabet. Med. 2018, 35, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Syngelaki, A.; Nicolaides, K.H.; Balani, J.; Hyer, S.; Akolekar, R.; Kotecha, R.; Pastides, A.; Shehata, H. Metformin Versus Placebo in Obese Pregnant Women Without Diabetes Mellitus. Obstet. Gynecol. Surv. 2016, 71, 324–326. [Google Scholar] [CrossRef]
- Leiberman, J.R.; Hagay, Z.J.; Mazor, M.; Wiznitzer, A.; Aharon, M.; Nathan, I.; Dvilansky, A. Plasma antithrombin III levels in pre-eclampsia and chronic hypertension. Int. J. Gynecol. Obstet. 1988, 27, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Maki, M.; Kobayashi, T.; Terao, T.; Ikenoue, T.; Satoh, K.; Nakabayashi, M.; Sagara, Y.; Kajiwara, Y.; Urata, M. Antithrombin therapy for severe preeclampsia: Results of a double-blind, randomized, placebo-controlled trial. Thromb. Haemost. 2000, 84, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Terao, T.; Ikenoue, T.; Sameshima, H.; Nakabayashi, M.; Kajiwara, Y.; Maki, M. Treatment of Severe Preeclampsia with Antithrombin Concentrate: Results of a Prospective Feasibility Study. Semin. Thromb. Hemost. 2003, 29, 645–652. [Google Scholar] [CrossRef]
- Paidas, M.J.; Tita, A.T.N.; Macones, G.A.; Saade, G.A.; Ehrenkranz, R.A.; Triche, E.W.; Streisand, J.B.; Lam, G.K.; Magann, E.F.; Lewis, D.F.; et al. Prospective, randomized, double-blind, placebo-controlled evaluation of the Pharmacokinetics, Safety and Efficacy of Recombinant Antithrombin Versus Placebo in Preterm Preeclampsia. Am. J. Obstet. Gynecol. 2020, 223, 739.e1–739.e13. [Google Scholar] [CrossRef]
- Mallott, E.K.; Borries, C.; Koenig, A.; Amato, K.R.; Lu, A. Reproductive hormones mediate changes in the gut microbiome during pregnancy and lactation in Phayre’s leaf monkeys. Sci. Rep. 2020, 10, 9961. [Google Scholar] [CrossRef]
- Amir, M.; Brown, J.A.; Rager, S.L.; Sanidad, K.Z.; Ananthanarayanan, A.; Zeng, M.Y. Maternal microbiome and infections in pregnancy. Microorganisms 2020, 8, 1996. [Google Scholar] [CrossRef]
- Campbell, C.; Kandalgaonkar, M.R.; Golonka, R.M.; Yeoh, B.S.; Vijay-Kumar, M.; Saha, P. Crosstalk between Gut Microbiota and Host Immunity: Impact on Inflammation and Immunotherapy. Biomedicines 2023, 11, 294. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Feng, L.; Zhang, J. Interactions between gut microbiota and metabolites modulate cytokine network imbalances in women with unexplained miscarriage. Npj Biofilms Microbiomes 2021, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, H.; Guo, L.; Gou, X.; Chen, G.; Lin, D.; Fan, D.; Guo, X.; Liu, Z. Association between gut microbiota and preeclampsia-eclampsia: A two-sample Mendelian randomization study. BMC Med. 2022, 20, 443. [Google Scholar] [CrossRef]
- Panzer, J.J.; Romero, R.; Greenberg, J.M.; Winters, A.D.; Galaz, J.; Gomez-Lopez, N.; Theis, K.R. Is there a placental microbiota? A critical review and re-analysis of published placental microbiota datasets. BMC Microbiol. 2023, 23, 76. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, R.; Jayasekara, R.W.; Senanayake, H.; Dissanayake, V.H.W. Microbiome of the placenta in pre-eclampsia supports the role of bacteria in the multifactorial cause of pre-eclampsia. J. Obstet. Gynaecol. Res. 2015, 41, 662–669. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lin, C.Y.; Yeh, Y.M.; Yang, L.Y.; Lee, Y.S.; Chao, A.; Chin, C.Y.; Chao, A.S.; Yang, C.Y. Severe preeclampsia is associated with a higher relative abundance of Prevotella bivia in the vaginal microbiota. Sci. Rep. 2020, 10, 18249. [Google Scholar] [CrossRef]
- Li, X.; Tian, Z.; Cui, R.; Lv, J.; Yang, X.; Qin, L.; Liu, Z.; Zhang, C.; Jin, C.; Xu, Y.; et al. Association between Pregestational Vaginal Dysbiosis and Incident Hypertensive Disorders of Pregnancy Risk: A Nested Case-Control Study. Msphere 2023, 8, e00096-23. [Google Scholar] [CrossRef]
- Huang, L.; Cai, M.; Li, L.; Zhang, X.; Xu, Y.; Xiao, J.; Huang, Q.; Luo, G.; Zeng, Z.; Jin, C.; et al. Gut microbiota changes in preeclampsia, abnormal placental growth and healthy pregnant women. BMC Microbiol. 2021, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Brantsæter, A.L.; Myhre, R.; Haugen, M.; Myking, S.; Sengpiel, V.; Magnus, P.; Jacobsson, B.; Meltzer, H.M. Intake of probiotic food and risk of preeclampsia in primiparous women. Am. J. Epidemiol. 2011, 174, 807–815. [Google Scholar] [CrossRef]
- Nordqvist, M.; Jacobsson, B.; Brantsæter, A.L.; Myhre, R.; Nilsson, S.; Sengpiel, V. Timing of probiotic milk consumption during pregnancy and effects on the incidence of preeclampsia and preterm delivery: A prospective observational cohort study in Norway. BMJ Open 2018, 8, e018021. [Google Scholar] [CrossRef] [PubMed]
- Raia-Barjat, T.; Sarkis, C.; Rancon, F.; Thibaudin, L.; Gris, J.C.; Alfaidy, N.; Chauleur, C. Vitamin D deficiency during late pregnancy mediates placenta-associated complications. Sci. Rep. 2021, 11, 20708. [Google Scholar] [CrossRef] [PubMed]
- Dahma, G.; Neamtu, R.; Nitu, R.; Gluhovschi, A.; Bratosin, F.; Grigoras, M.L.; Silaghi, C.; Citu, C.; Orlu, I.N.; Bhattarai, S.; et al. The Influence of Maternal Vitamin D Supplementation in Pregnancies Associated with Preeclampsia: A Case-Control Study. Nutrients 2022, 14, 3008. [Google Scholar] [CrossRef]
- Behjat Sasan, S.; Zandvakili, F.; Soufizadeh, N.; Baybordi, E. The Effects of Vitamin D Supplement on Prevention of Recurrence of Preeclampsia in Pregnant Women with a History of Preeclampsia. Obstet. Gynecol. Int. 2017, 2017, 8249264. [Google Scholar] [CrossRef] [PubMed]
- Fogacci, S.; Fogacci, F.; Banach, M.; Michos, E.D.; Hernandez, A.V.; Lip, G.Y.H.; Blaha, M.J.; Toth, P.P.; Borghi, C.; Cicero, A.F.G. Vitamin D supplementation and incident preeclampsia: A systematic review and meta-analysis of randomized clinical trials. Clin. Nutr. 2020, 39, 1742–1752. [Google Scholar] [CrossRef]
- Jamilian, M.; Amirani, E.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 2098–2105. [Google Scholar] [CrossRef]
- Rumbold, A.R.; Crowther, C.A.; Haslam, R.R.; Dekker, G.A.; Robinson, J.S. Vitamins C and E and the risks of preeclampsia and perinatal complications. N. Engl. J. Med. 2006, 354, 1796–1806. [Google Scholar] [CrossRef]
- Bastani, P.; Hamdi, K.; Abasalizadeh, F.; Navali, N. Effects of vitamin E supplementation on some pregnancy health indices: A randomized clinical trial. Int. J. Gen. Med. 2011, 4, 461. [Google Scholar] [CrossRef]
- Kiondo, P.; Wamuyu-Maina, G.; Wandabwa, J.; Bimenya, G.S.; Tumwesigye, N.M.; Okong, P. The effects of vitamin C supplementation on pre-eclampsia in Mulago Hospital, Kampala, Uganda: A randomized placebo controlled clinical trial. BMC Pregnancy Childbirth 2014, 14, 283. [Google Scholar] [CrossRef]
- Rahimi, R.; Nikfar, S.; Rezaie, A.; Abdollahi, M. A meta-analysis on the efficacy and safety of combined vitamin C and e supplementation in preeclamptic women vitamin C and e supplements in preeclamptic women. Hypertens. Pregnancy 2009, 28, 417–434. [Google Scholar] [CrossRef]
- Nacka-Aleksić, M.; Pirković, A.; Vilotić, A.; Bojić-Trbojević, Ž.; Jovanović Krivokuća, M.; Giampieri, F.; Battino, M.; Dekanski, D. The Role of Dietary Polyphenols in Pregnancy and Pregnancy-Related Disorders. Nutrients 2022, 14, 5246. [Google Scholar] [CrossRef]
- Gong, P.; Liu, M.; Hong, G.; Li, Y.; Xue, P.; Zheng, M.; Wu, M.; Shen, L.; Yang, M.; Diao, Z.; et al. Curcumin improves LPS-induced preeclampsia-like phenotype in rat by inhibiting the TLR4 signaling pathway. Placenta 2016, 41, 45–52. [Google Scholar] [CrossRef]
- Zhou, J.; Miao, H.; Li, X.; Hu, Y.; Sun, H.; Hou, Y. Curcumin inhibits placental inflammation to ameliorate LPS-induced adverse pregnancy outcomes in mice via upregulation of phosphorylated Akt. Inflamm. Res. 2017, 66, 177–185. [Google Scholar] [CrossRef]
- Li, Q.; Yin, L.; Si, Y.; Zhang, C.; Meng, Y.; Yang, W. The bioflavonoid quercetin improves pathophysiology in a rat model of preeclampsia. Biomed. Pharmacother. 2020, 127, 110122. [Google Scholar] [CrossRef]
- Liu, J.; Yao, L.; Wang, Y. Resveratrol alleviates preeclampsia-like symptoms in rats through a mechanism involving the miR-363-3p/PEDF/VEGF axis. Microvasc. Res. 2023, 146, 104451. [Google Scholar] [CrossRef]
- Saftlas, A.F.; Triche, E.W.; Beydoun, H.; Bracken, M.B. Does Chocolate Intake During Pregnancy Reduce the Risks of Preeclampsia and Gestational Hypertension? Ann. Epidemiol. 2010, 20, 584–591. [Google Scholar] [CrossRef][Green Version]
- Triche, E.W.; Grosso, L.M.; Belanger, K.; Darefsky, A.S.; Benowitz, N.L.; Bracken, M.B. Chocolate consumption in pregnancy and reduced likelihood of preeclampsia. Epidemiology 2008, 19, 459–464. [Google Scholar] [CrossRef]
- Wei, S.Q.; Xu, H.; Xiong, X.; Luo, Z.C.; Audibert, F.; Fraser, W.D. Tea consumption during pregnancy and the risk of pre-eclampsia. Int. J. Gynecol. Obstet. 2009, 105, 123–126. [Google Scholar] [CrossRef]
- Li, L.; Tan, J.; Dai, W.; Wang, B.; Bai, Y.; Ren, Y.; Liu, Q.; Zhang, Y. The association between maternal tea consumption and the risk of pregnancy induced hypertension: A retrospective cohort study in Lanzhou, China. Pregnancy Hypertens. 2022, 30, 44–50. [Google Scholar] [CrossRef]
- Kawanishi, Y.; Kakigano, A.; Kimura, T.; Ikehara, S.; Sato, T.; Tomimatsu, T.; Kimura, T.; Iso, H. Hypertensive disorders of pregnancy in relation to coffee and tea consumption: The Japan environment and children’s study. Nutrients 2021, 13, 343. [Google Scholar] [CrossRef]
- Jorquera, G.; Fornes, R.; Cruz, G.; Thomas-Valdés, S. Association of Polyphenols Consumption with Risk for Gestational Diabetes Mellitus and Preeclampsia: A Systematic Review and Meta-Analysis. Antioxidants 2022, 11, 2294. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Q.; Zhu, J.; Guo, H.; Zhai, Q.; Li, B.; Jin, Y.; He, X.; Jin, F. Comparison of therapeutic effects of different mesenchymal stem cells on rheumatoid arthritis in mice. PeerJ 2019, 2019, e7023. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, R.; Liu, T.; Yang, L.; Yin, G.; Xie, Q. Immunomodulatory Effects of Mesenchymal Stem Cells and Mesenchymal Stem Cell-Derived Extracellular Vesicles in Rheumatoid Arthritis. Front. Immunol. 2020, 11, 1912. [Google Scholar] [CrossRef]
- Baharlooi, H.; Azimi, M.; Salehi, Z.; Izad, M. Mesenchymal stem cell-derived exosomes: A promising therapeutic ace card to address autoimmune diseases. Int. J. Stem Cells 2020, 13, 13–23. [Google Scholar] [CrossRef]
- Sarsenova, M.; Issabekova, A.; Abisheva, S.; Rutskaya-Moroshan, K.; Ogay, V.; Saparov, A. Mesenchymal stem cell-based therapy for rheumatoid arthritis. Int. J. Mol. Sci. 2021, 22, 11592. [Google Scholar] [CrossRef]
- Krampera, M.; Le Blanc, K. Mesenchymal stromal cells: Putative microenvironmental modulators become cell therapy. Cell Stem Cell 2021, 28, 1708–1725. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Lee, M.J.; Seok, O.S.; Paek, Y.C.; Cho, G.J.; Seol, H.J.; Lee, J.K.; Oh, M.J. Cytokine expression in placenta-derived mesenchymal stem cells in patients with pre-eclampsia and normal pregnancies. Cytokine 2010, 49, 95–101. [Google Scholar] [CrossRef]
- Rolfo, A.; Giuffrida, D.; Nuzzo, A.M.; Pierobon, D.; Cardaropoli, S.; Piccoli, E.; Giovarelli, M.; Todros, T. Pro-Inflammatory Profile of Preeclamptic Placental Mesenchymal Stromal Cells: New Insights into the Etiopathogenesis of Preeclampsia. PLoS ONE 2013, 8, e59403. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Liu, Y.; Zhang, D.; Xie, J.; Guan, H.; Shang, T. Beneficial effect of human umbilical cord-derived mesenchymal stem cells on an endotoxin-induced rat model of preeclampsia. Exp. Ther. Med. 2015, 10, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, A.M.; Moretti, L.; Mele, P.; Todros, T.; Eva, C.; Rolfo, A. Effect of Placenta-Derived Mesenchymal Stromal Cells Conditioned Media on an LPS-Induced Mouse Model of Preeclampsia. Int. J. Mol. Sci. 2022, 23, 1674. [Google Scholar] [CrossRef]
- Taglauer, E.S.; Fernandez-Gonzalez, A.; Willis, G.R.; Reis, M.; Yeung, V.; Liu, X.; Prince, L.S.; Mitsialis, S.A.; Kourembanas, S. Antenatal Mesenchymal Stromal Cell Extracellular Vesicle Therapy Prevents Preeclamptic Lung Injury in Mice. Am. J. Respir. Cell Mol. Biol. 2022, 66, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, Y.; Fard, J.K.; Ghafoor, D.; Eid, A.H.; Sahebkar, A. Paradoxical effects of statins on endothelial and cancer cells: The impact of concentrations. Cancer Cell Int. 2023, 23, 43. [Google Scholar] [CrossRef]
 process activation;
process activation;  process inhibition;
process inhibition;  the direction of common relationships between the analysed factors.
the direction of common relationships between the analysed factors.
 process activation;
process activation;  process inhibition;
process inhibition;  the direction of common relationships between the analysed factors.
the direction of common relationships between the analysed factors.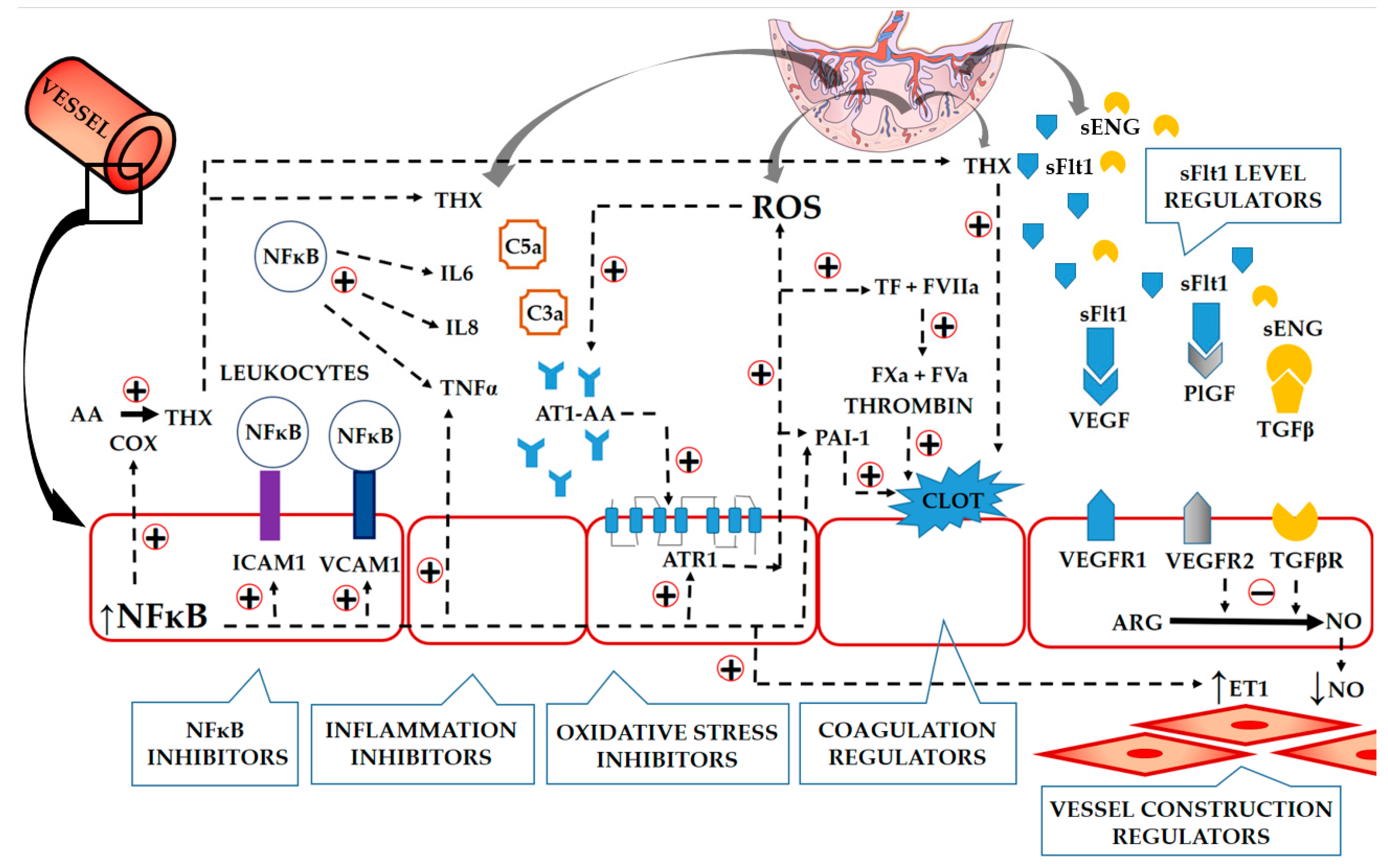
| Society | Drug | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Nonsevere hypertension (HA) > 140/90 mmHg | |||||||||
| Nifedip. | Labet. | Hydral. | mDopa | Oxypren. | Prazos. | Urapid. | nGlic. | Diazo | |
| ACOG | + | + | + | ||||||
| ESC | + (IR) | + | + | ||||||
| FIGO | + | + | + | ||||||
| ISSHP | + | + | + II | + | + | + II | |||
| NICE | + | + | + | ||||||
| PSH/PCS/PSGO | + | + | + | ||||||
| SOMANZ | + (ER) II | + | + II | + | + | + II | |||
| Severe hypertension (HA) > 160/100 mmHg or >170/110 mmHg | |||||||||
| ACOG | + (o) | + (iv) | +(iv, im) | ||||||
| ESC | + (IR, o) | + (iv) | + (iv) II | + (o) | |||||
| FIGO | + (o) | + (iv, o) | + (iv) | ||||||
| ISSHP | + (IR, o) | + (iv, o) | + (iv) | + (o) II | |||||
| NICE | + (o) | + (iv, o) | + (iv) | ||||||
| PSH/PCS/PSGO | + (o) | + (iv, o) | + (iv) | + (iv) II | |||||
| SOMANZ | + (o) | + (iv) | + (iv) | + (iv) II | + (iv) | ||||
| Study | No. of Recruited Women * | Gest. Age (Weeks) | Statin/ Dose per Day | Observed Results |
|---|---|---|---|---|
| Constantine et al., 2021 [82] (NCT01717586) | 10/10 | 12–16 | Pravastatin (20 mg) |
|
| Constantine et al., 2016 [83] (NCT01717586) | 10/10 | 12–16 | Pravastatin (10 mg) |
|
| Akbar et al., 2022 [84] (NCT03648970) | 87/86 | 14–20 | Pravastatin (20 mg) |
|
| Akbar et al., 2021 [85] (NCT03648970) | 40/40 | 14–20 | Pravastatin (20 mg) |
|
| Ahemed et al., 2020 [86] (NCT23410175) | 30/32 in both groups PE women | 24–37 | Pravastatin (40 mg) |
|
| Brownfood 2015 [77] | 4 PE women | 20–30 | Pravastatin (40 mg) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakowicz, A.; Bralewska, M.; Rybak-Krzyszkowska, M.; Grzesiak, M.; Pietrucha, T. New Ideas for the Prevention and Treatment of Preeclampsia and Their Molecular Inspirations. Int. J. Mol. Sci. 2023, 24, 12100. https://doi.org/10.3390/ijms241512100
Sakowicz A, Bralewska M, Rybak-Krzyszkowska M, Grzesiak M, Pietrucha T. New Ideas for the Prevention and Treatment of Preeclampsia and Their Molecular Inspirations. International Journal of Molecular Sciences. 2023; 24(15):12100. https://doi.org/10.3390/ijms241512100
Chicago/Turabian StyleSakowicz, Agata, Michalina Bralewska, Magda Rybak-Krzyszkowska, Mariusz Grzesiak, and Tadeusz Pietrucha. 2023. "New Ideas for the Prevention and Treatment of Preeclampsia and Their Molecular Inspirations" International Journal of Molecular Sciences 24, no. 15: 12100. https://doi.org/10.3390/ijms241512100
APA StyleSakowicz, A., Bralewska, M., Rybak-Krzyszkowska, M., Grzesiak, M., & Pietrucha, T. (2023). New Ideas for the Prevention and Treatment of Preeclampsia and Their Molecular Inspirations. International Journal of Molecular Sciences, 24(15), 12100. https://doi.org/10.3390/ijms241512100






