Al-Doped Octahedral Cu2O Nanocrystal for Electrocatalytic CO2 Reduction to Produce Ethylene
Abstract
1. Introduction
2. Results and Discussion
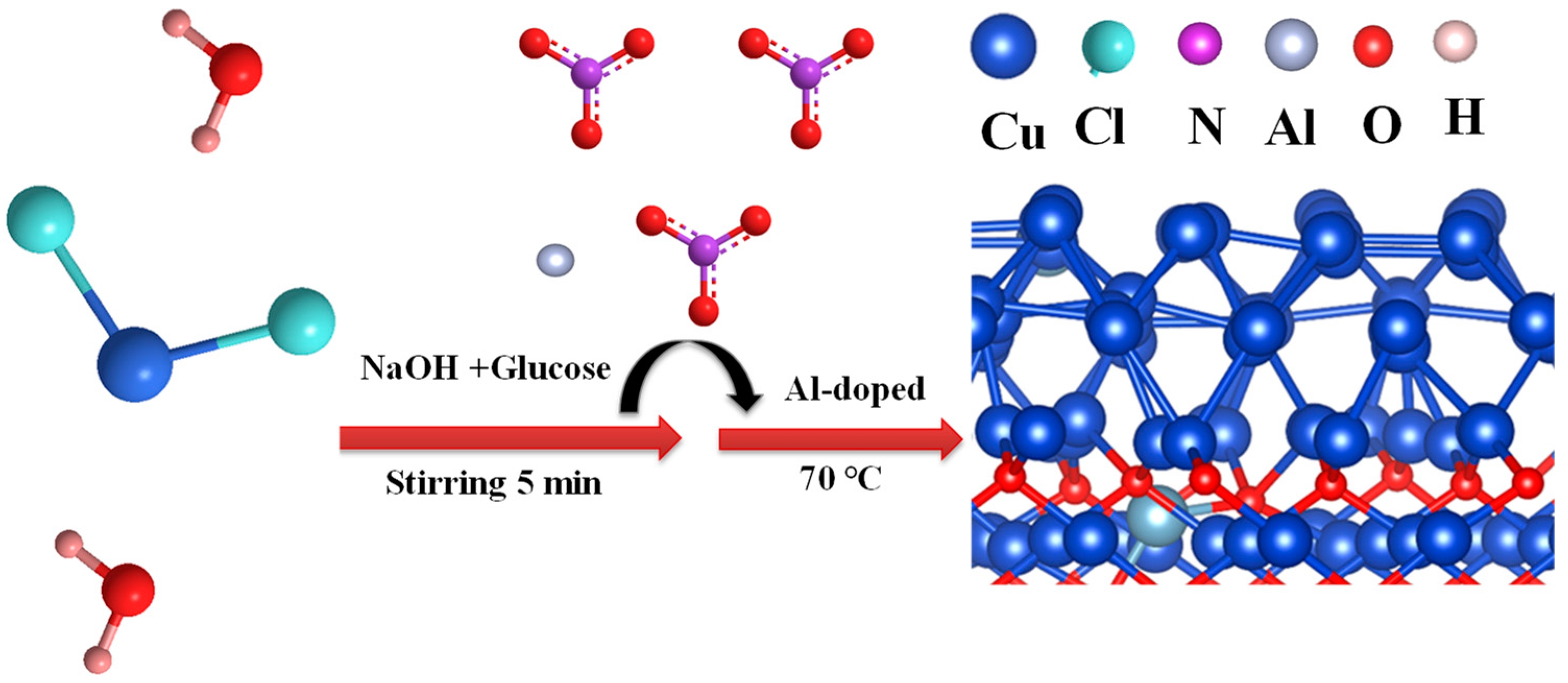
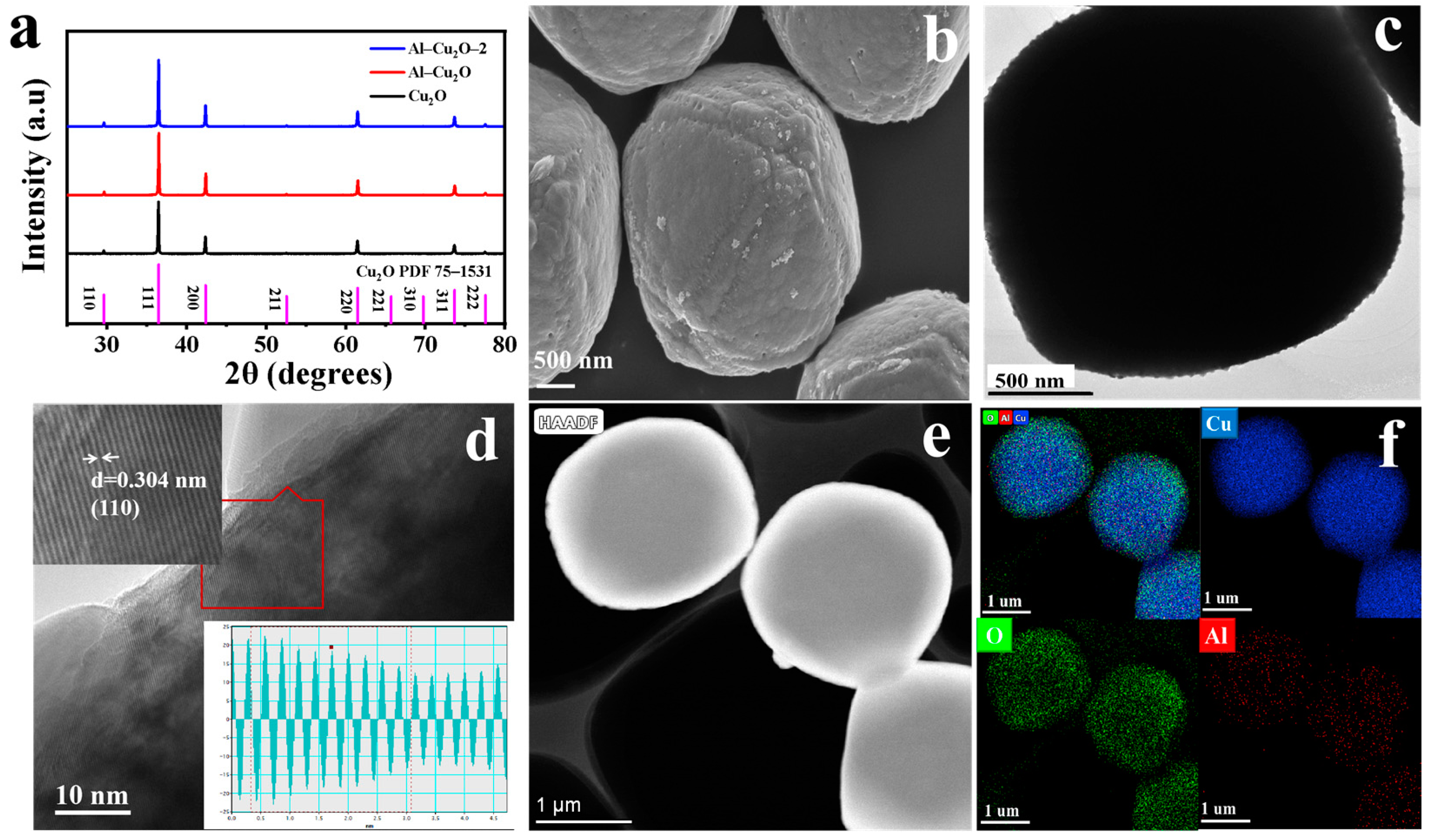
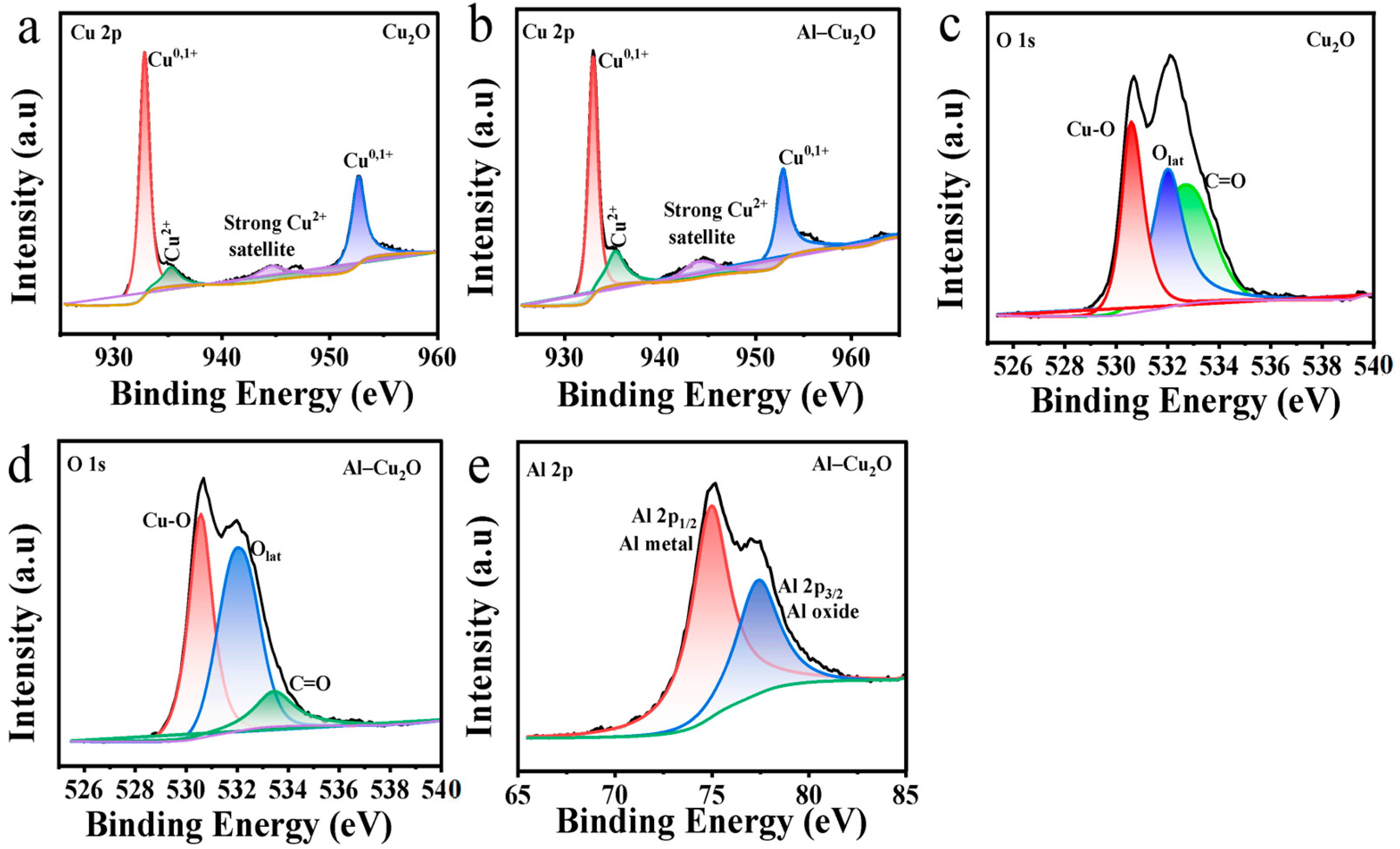
2.1. Morphology and Structure Analysis
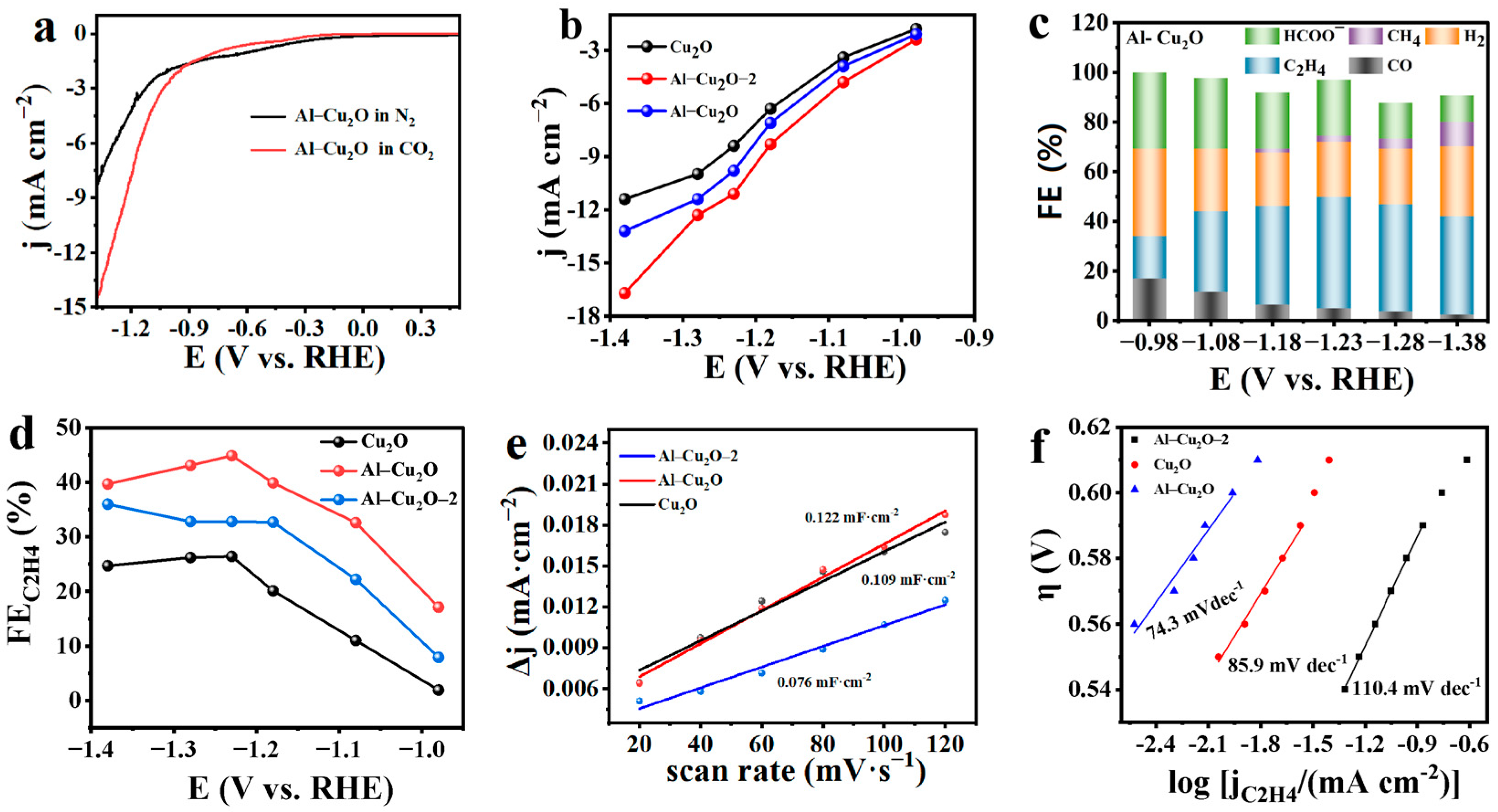
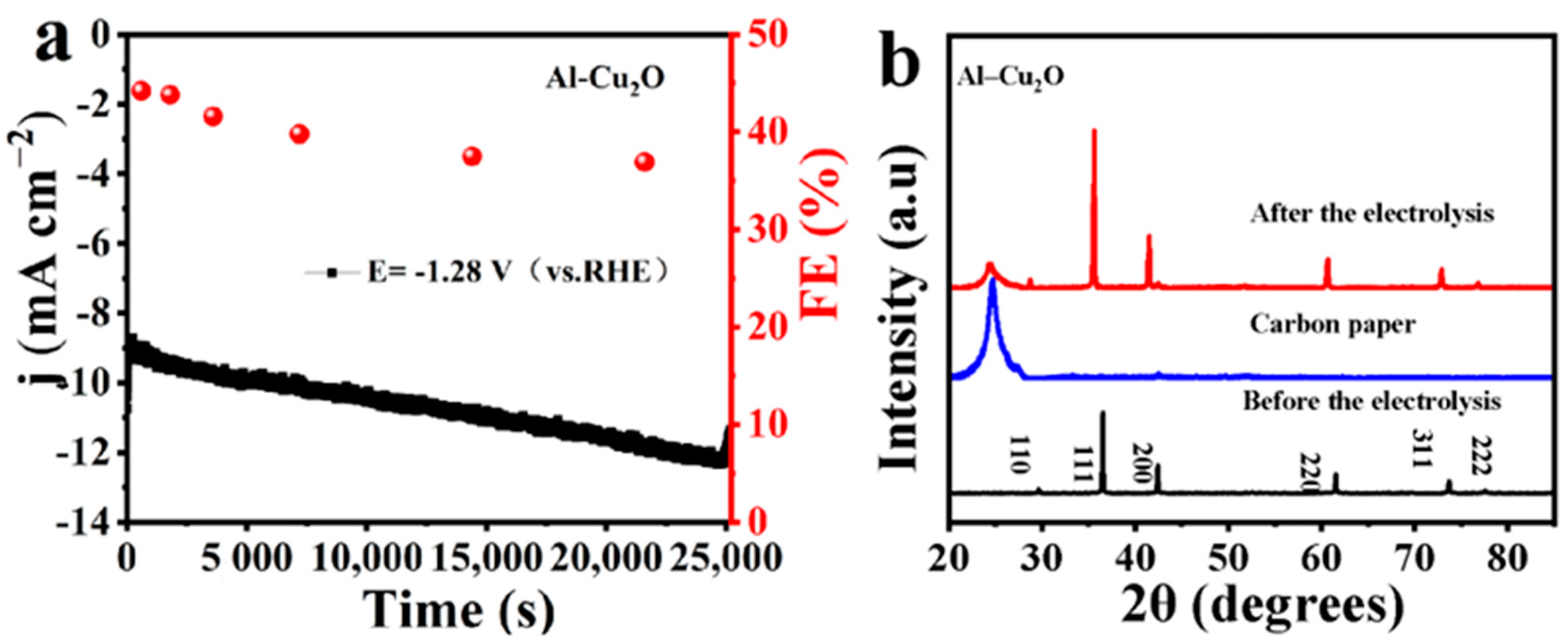
2.2. Electrocatalytic CO2RR Performances
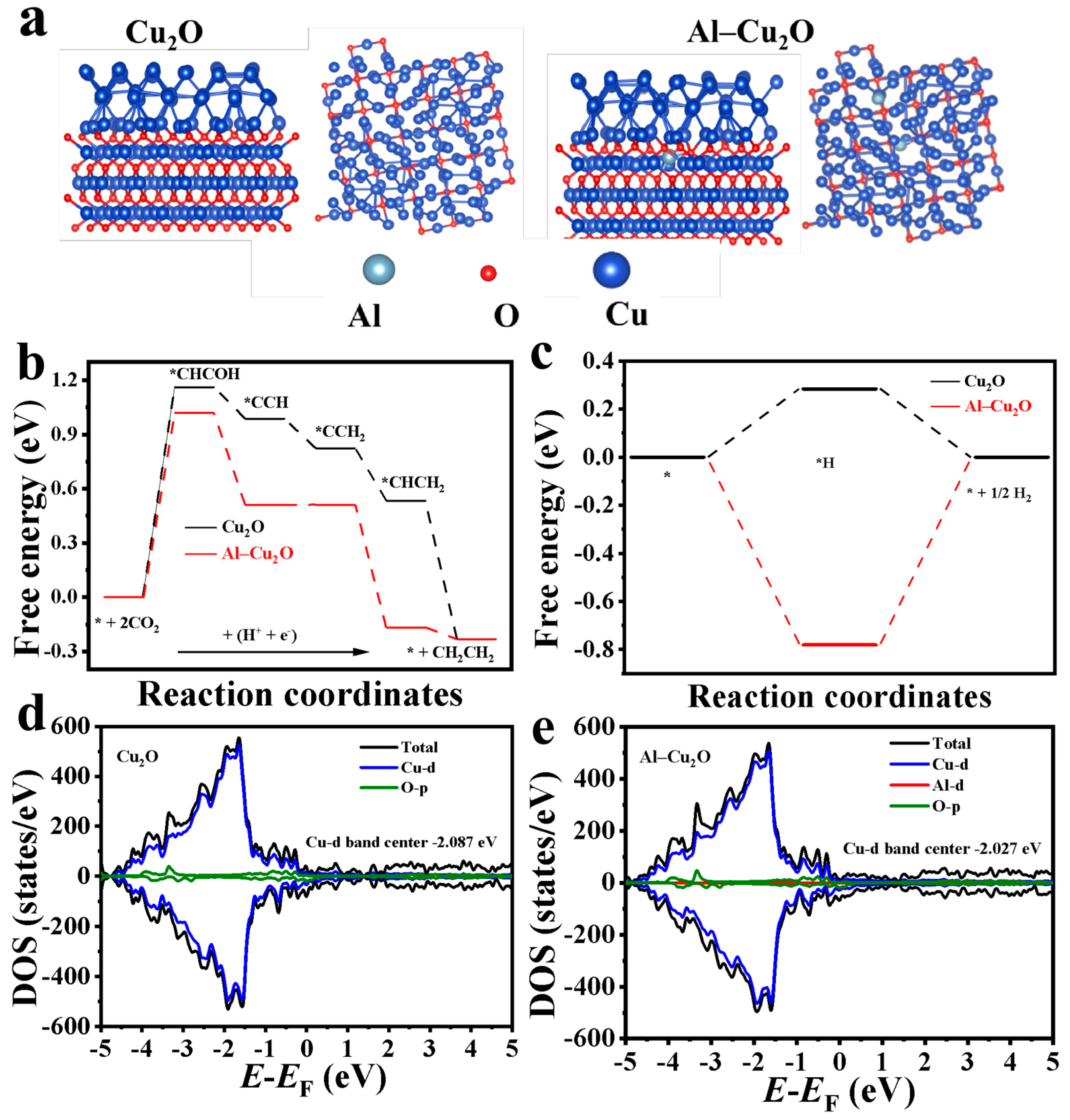
2.3. DFT Computations
3. Materials and Methods
3.1. Preparation of Al–Cu2O Nanocrystals
3.2. Preparation of Al–Cu2O Coated Carbon Paper Electrode
3.3. Electrochemical Measurements
3.4. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alli, Y.A.; Oladoye, P.O.; Ejeromedoghene, O.; Bankole, O.M.; Alimi, O.A.; Omotola, E.O.; Olanrewaju, C.A.; Philippot, K.; Adeleye, A.S.; Ogunlaja, A.S. Nanomaterials as catalysts for CO2 transformation into value-added products: A review. Sci. Total Environ. 2023, 868, 161547. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Gu, Y.; Gao, W.; Cui, P.; Tang, H.; Wei, X.; Zhu, H.; Li, G.; Yan, S.; Zhang, X.; et al. Atom vacancies induced electron-rich surface of ultrathin Bi nanosheet for efficient electrochemical CO2 reduction. Appl. Catal. B Environ. 2020, 266, 118625. [Google Scholar] [CrossRef]
- Feng, X.; Zou, H.; Zheng, R.; Wei, W.; Wang, R.; Zou, W.; Lim, G.; Hong, J.; Duan, L.; Chen, H. Bi2O3/BiO2 Nanoheterojunction for Highly Efficient Electrocatalytic CO2 Reduction to Formate. Nano Lett. 2022, 22, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, A.; Yang, M.; Zou, Q.; Li, H.; Liu, Z.; Tao, C.; Du, J. Selective electroreduction of CO2 to ethanol over a highly stable catalyst derived from polyaniline/CuBi2O4. Catal. Sci. Technol. 2021, 11, 5908–5916. [Google Scholar] [CrossRef]
- Sakamoto, N.; Nishimura, Y.F.; Nonaka, T.; Ohashi, M.; Ishida, N.; Kitazumi, K.; Kato, Y.; Sekizawa, K.; Morikawa, T.; Arai, T. Self-assembled Cuprous Coordination Polymer as a Catalyst for CO2 Electrochemical Reduction into C2 Products. ACS Catal. 2020, 10, 10412–10419. [Google Scholar] [CrossRef]
- Liu, B.; Yao, X.; Zhang, Z.; Li, C.; Zhang, J.; Wang, P.; Zhao, J.; Guo, Y.; Sun, J.; Zhao, C. Synthesis of Cu2O Nanostructures with Tunable Crystal Facets for Electrochemical CO2 Reduction to Alcohols. ACS Appl. Mater. Interfaces 2021, 13, 39165–39177. [Google Scholar] [CrossRef]
- Zhou, X.; Shan, J.; Chen, L.; Xia, B.Y.; Ling, T.; Duan, J.; Jiao, Y.; Zheng, Y.; Qiao, S.Z. Stabilizing Cu2+ Ions by Solid Solutions to Promote CO2 Electroreduction to Methane. J. Am. Chem. Soc. 2022, 144, 2079–2084. [Google Scholar] [CrossRef]
- Jiang, R.; Gao, M.; Mao, X.; Wang, D. Advancements and potentials of molten salt CO2 capture and electrochemical transformation (MSCC-ET) process. Curr. Opin. Electrochem. 2019, 17, 38–46. [Google Scholar] [CrossRef]
- Küngas, R. Review—Electrochemical CO2 Reduction for CO Production: Comparison of Low- and High-Temperature Electrolysis Technologies. J. Electrochem. Soc. 2020, 167, 044508. [Google Scholar] [CrossRef]
- Lacarbonara, G.; Chini, S.; Ratso, S.; Kruusenberg, I.; Arbizzani, C. A MnOx–graphitic carbon composite from CO2 for sustainable Li-ion battery anodes. Mater. Adv. 2022, 3, 7087–7097. [Google Scholar] [CrossRef]
- Remmel, A.-L.; Ratso, S.; Divitini, G.; Danilson, M.; Mikli, V.; Uibu, M.; Aruväli, J.; Kruusenberg, I. Nickel and Nitrogen-Doped Bifunctional ORR and HER Electrocatalysts Derived from CO2. ACS Sustain. Chem. Eng. 2021, 10, 134–145. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, N.; Li, S.; Min, S.; Peng, J.; Liu, W.; Zhan, D.; Bai, H. A Mn single atom catalyst with Mn–N2O2 sites integrated into carbon nanosheets for efficient electrocatalytic CO2 reduction. J. Mater. Chem. A 2022, 10, 10892–10901. [Google Scholar] [CrossRef]
- Clark, E.L.; Ringe, S.; Tang, M.; Walton, A.; Hahn, C.; Jaramillo, T.F.; Chan, K.; Bell, A.T. Influence of Atomic Surface Structure on the Activity of Ag for the Electrochemical Reduction of CO2 to CO. ACS Catal. 2019, 9, 4006–4014. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, K.; Hu, K.; Cai, C.; Li, H.; Li, H.; Herran, M.; Lu, Y.-R.; Chan, T.-S.; Ma, C. Attenuating metal-substrate conjugation in atomically dispersed nickel catalysts for electroreduction of CO2 to CO. Nat. Commun. 2022, 13, 6082. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.X.; Zhou, Y.T.; Yu, Z.; Liu, D.X.; Wen, Z.; Yan, J.M.; Jiang, Q. Boosting production of HCOOH from CO2 electroreduction via Bi/CeOx. Angew. Chem. Int. Ed. 2021, 60, 8798–8802. [Google Scholar] [CrossRef]
- Koh, J.H.; Won, D.H.; Eom, T.; Kim, N.-K.; Jung, K.D.; Kim, H.; Hwang, Y.J.; Min, B.K. Facile CO2 electro-reduction to formate via oxygen bidentate intermediate stabilized by high-index planes of Bi dendrite catalyst. ACS Catal. 2017, 7, 5071–5077. [Google Scholar] [CrossRef]
- Li, D.; Huang, L.; Tian, Y.; Liu, T.; Zhen, L.; Feng, Y. Facile synthesis of porous Cu-Sn alloy electrode with prior selectivity of formate in a wide potential range for CO2 electrochemical reduction. Appl. Catal. B Environ. 2021, 292, 120119. [Google Scholar] [CrossRef]
- Grace, A.N.; Choi, S.Y.; Vinoba, M.; Bhagiyalakshmi, M.; Chu, D.H.; Yoon, Y.; Nam, S.C.; Jeong, S.K. Electrochemical reduction of carbon dioxide at low overpotential on a polyaniline/Cu2O nanocomposite based electrode. Appl. Energy 2014, 120, 85–94. [Google Scholar] [CrossRef]
- Boutin, E.; Wang, M.; Lin, J.C.; Mesnage, M.; Mendoza, D.; Lassalle-Kaiser, B.; Hahn, C.; Jaramillo, T.F.; Robert, M. Aqueous electrochemical reduction of carbon dioxide and carbon monoxide into methanol with cobalt phthalocyanine. Angew. Chem. Int. Ed. 2019, 58, 16172–16176. [Google Scholar] [CrossRef]
- Ren, D.; Deng, Y.; Handoko, A.D.; Chen, C.S.; Malkhandi, S.; Yeo, B.S. Selective electrochemical reduction of carbon dioxide to ethylene and ethanol on copper (I) oxide catalysts. ACS Catal. 2015, 5, 2814–2821. [Google Scholar] [CrossRef]
- Yuan, J.; Yang, M.-P.; Zhi, W.-Y.; Wang, H.; Wang, H.; Lu, J.-X. Efficient electrochemical reduction of CO2 to ethanol on Cu nanoparticles decorated on N-doped graphene oxide catalysts. J. CO2 Util. 2019, 33, 452–460. [Google Scholar] [CrossRef]
- Iyengar, P.; Huang, J.; De Gregorio, G.L.; Gadiyar, C.; Buonsanti, R. Size dependent selectivity of Cu nano-octahedra catalysts for the electrochemical reduction of CO2 to CH4. Chem. Commun. 2019, 55, 8796–8799. [Google Scholar] [CrossRef]
- Chu, S.; Kang, C.; Park, W.; Han, Y.; Hong, S.; Hao, L.; Zhang, H.; Lo, T.W.B.; Robertson, A.W.; Jung, Y. Single atom and defect engineering of CuO for efficient electrochemical reduction of CO2 to C2H4. SmartMat 2022, 3, 194–205. [Google Scholar] [CrossRef]
- Luo, H.; Li, B.; Ma, J.G.; Cheng, P. Surface Modification of Nano-Cu2O for Controlling CO2 Electrochemical Reduction to Ethylene and Syngas. Angew. Chem. Int. Ed. 2022, 61, e202116736. [Google Scholar] [CrossRef]
- Tan, X.; Yu, C.; Zhao, C.; Huang, H.; Yao, X.; Han, X.; Guo, W.; Cui, S.; Huang, H.; Qiu, J. Interfaces, Restructuring of Cu2O to Cu2O@Cu-metal–organic frameworks for selective electrochemical reduction of CO2. ACS Appl. Mater. Interfaces 2019, 11, 9904–9910. [Google Scholar] [CrossRef]
- Cao, S.; Chen, H.; Han, T.; Zhao, C.; Peng, L. Rose-like Cu2O nanoflowers via hydrothermal synthesis and their gas sensing properties. Mater. Lett. 2016, 180, 135–139. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Q.; Liang, X.; Wang, Z.; Zheng, Z.; Wang, P.; Liu, Y.; Dai, Y.; Whangbo, M.H.; Huang, B. Cu2O Nanoparticles with Both 100 and 111 Facets for Enhancing the Selectivity and Activity of CO2 Electroreduction to Ethylene. Adv. Sci. 2020, 7, 1902820. [Google Scholar] [CrossRef]
- Fu, W.; Liu, Z.; Wang, T.; Liang, J.; Duan, S.; Xie, L.; Han, J.; Li, Q. Engineering, Promoting C2+ production from electrochemical CO2 reduction on shape-controlled cuprous oxide nanocrystals with high-index facets. ACS Sustain. Chem. Eng. 2020, 8, 15223–15229. [Google Scholar] [CrossRef]
- Luo, T.; Liu, K.; Fu, J.; Chen, S.; Li, H.; Hu, J.; Liu, M. Tandem catalysis on adjacent active motifs of copper grain boundary for efficient CO2 electroreduction toward C2 products. J. Energy Chem. 2022, 70, 219–223. [Google Scholar] [CrossRef]
- Wang, Y.; Lou, Z.; Niu, W.; Ye, Z.; Zhu, L. Optimization of photoelectrochemical performance in Pt-modified p-Cu2O/n-Cu2O nanocomposite. Nanotechnology 2018, 29, 145402. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.; Liu, H.; Dong, C.; Wang, J.; Kulinich, S.A.; Du, X. Laser-prepared CuZn alloy catalyst for selective electrochemical reduction of CO2 to ethylene. Langmuir 2018, 34, 13544–13549. [Google Scholar] [CrossRef]
- Chen, C.; Sun, X.; Yan, X.; Wu, Y.; Liu, M.; Liu, S.; Zhao, Z.; Han, B. A strategy to control the grain boundary density and Cu+/Cu0 ratio of Cu-based catalysts for efficient electroreduction of CO2 to C2 products. Green Chem. 2020, 22, 1572–1576. [Google Scholar] [CrossRef]
- Shang, L.; Lv, X.; Shen, H.; Shao, Z.; Zheng, G. Selective carbon dioxide electroreduction to ethylene and ethanol by core-shell copper/cuprous oxide. J. Colloid Interface Sci. 2019, 552, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Wang, X.; Wang, W.; Mao, Q.; Yang, Z.; Zhao, Q.; Song, Y.; Wu, M. Cubic Cu2O on nitrogen-doped carbon shells for electrocatalytic CO2 reduction to C2H4. Carbon 2019, 146, 218–223. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Zhao, Z.J.; Yang, P.; Greeley, J.; Mu, R.; Zhang, G.; Gong, Z.; Luo, Z.; Chen, J. Tuning Cu/Cu2O interfaces for the reduction of carbon dioxide to methanol in aqueous solutions. Angew. Chem. Int. Ed. 2018, 57, 15415–15419. [Google Scholar] [CrossRef]
- Qiu, Y.-L.; Zhong, H.-X.; Zhang, T.-T.; Xu, W.-B.; Li, X.-F.; Zhang, H.-M. Copper Electrode Fabricated via Pulse Electrodeposition: Toward High Methane Selectivity and Activity for CO2 Electroreduction. ACS Catal. 2017, 7, 6302–6310. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, L.; Guan, J.; Qian, S.; Zhang, Z.; Ngaw, C.K.; Wan, S.; Wang, S.; Lin, J.; Wang, Y.; et al. Engineering, Controlled synthesis of Cu0/Cu2O for efficient photothermal catalytic conversion of CO2 and H2O. ACS Sustain. Chem. Eng. 2021, 9, 1754–1761. [Google Scholar] [CrossRef]
- Wang, S.; Kou, T.; Varley, J.B.; Akhade, S.A.; Weitzner, S.E.; Baker, S.E.; Duoss, E.B.; Li, Y. Cu2O/CuS nanocomposites show excellent selectivity and stability for formate generation via electrochemical reduction of carbon dioxide. ACS Mater. Lett. 2020, 3, 100–109. [Google Scholar] [CrossRef]
- Lv, X.-W.; Liu, Y.; Hao, R.; Tian, W.; Yuan, Z.-Y. Urchin-like Al-doped Co3O4 nanospheres rich in surface oxygen vacancies enable efficient ammonia electrosynthesis. ACS Appl. Mater. Interfaces 2020, 12, 17502–17508. [Google Scholar] [CrossRef]
- Sun, B.; Dai, M.; Cai, S.; Cheng, H.; Song, K.; Yu, Y.; Hu, H. Challenges and strategies towards copper-based catalysts for enhanced electrochemical CO2 reduction to multi-carbon products. Fuel 2023, 332, 126114. [Google Scholar] [CrossRef]
- Li, Y.C.; Wang, Z.; Yuan, T.; Nam, D.-H.; Luo, M.; Wicks, J.; Chen, B.; Li, J.; Li, F.; de Arquer, F.P.G.; et al. Binding Site Diversity Promotes CO2 Electroreduction to Ethanol. J. Am. Chem. Soc. 2019, 141, 8584–8591. [Google Scholar] [CrossRef]
- Yang, R.; Wu, Z.; Tang, D.; Xing, Y.; Ren, Y.; Li, F.; Li, X. Synthesis of Cu2O crystals with negative surface curvature at various positions via Al3+ ions inducing. Mater. Lett. 2014, 117, 211–213. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J.J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M.J. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Hammer, B.; Hansen, L.B.; Nørskov, J.K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 1999, 59, 7413. [Google Scholar] [CrossRef]
- Grimme, S.J. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Sha, X.; Gao, X.; Peng, J. Al-Doped Octahedral Cu2O Nanocrystal for Electrocatalytic CO2 Reduction to Produce Ethylene. Int. J. Mol. Sci. 2023, 24, 12680. https://doi.org/10.3390/ijms241612680
Li S, Sha X, Gao X, Peng J. Al-Doped Octahedral Cu2O Nanocrystal for Electrocatalytic CO2 Reduction to Produce Ethylene. International Journal of Molecular Sciences. 2023; 24(16):12680. https://doi.org/10.3390/ijms241612680
Chicago/Turabian StyleLi, Sanxiu, Xuelan Sha, Xiafei Gao, and Juan Peng. 2023. "Al-Doped Octahedral Cu2O Nanocrystal for Electrocatalytic CO2 Reduction to Produce Ethylene" International Journal of Molecular Sciences 24, no. 16: 12680. https://doi.org/10.3390/ijms241612680
APA StyleLi, S., Sha, X., Gao, X., & Peng, J. (2023). Al-Doped Octahedral Cu2O Nanocrystal for Electrocatalytic CO2 Reduction to Produce Ethylene. International Journal of Molecular Sciences, 24(16), 12680. https://doi.org/10.3390/ijms241612680





