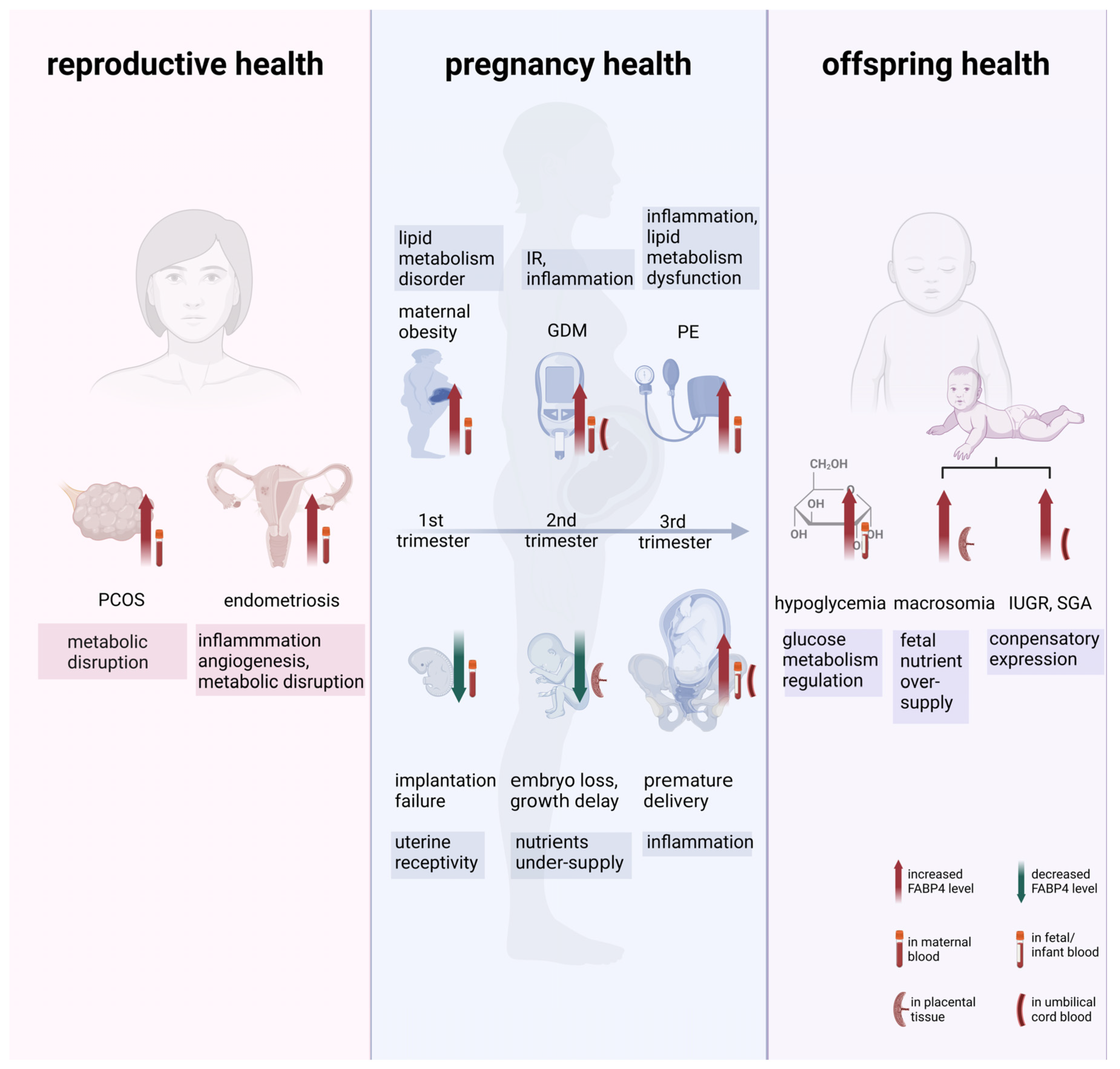
Pathophysiological Insight into Fatty Acid-Binding Protein-4: Multifaced Roles in Reproduction, Pregnancy, and Offspring Health
Abstract
1. Introduction
2. The Role of FABP4 on Women’s Reproductive Health
2.1. Polycystic Ovary Syndrome (PCOS)
2.2. Endometriosis
3. The Role of FABP4 on Pregnancy Health
3.1. The Molecular Mechanism of FABP4 Regulating Placental Function
3.2. Implantation Failure and Pregnancy Loss
3.3. Maternal Obesity
3.4. Gestational Diabetes Mellitus (GDM)
3.5. Preeclampsia (PE)
3.6. Preterm Birth
4. The Impact of FABP4 on Offspring’s Health
4.1. Neonatal Glucose Metabolism
4.2. Macrosomia and Low Birth Weight
| Year | Disease/ Condition | Sample Size (Patients vs. Control) | Region | Time of Sample Collection | FABP4 Levels (Patients vs. Control, ng/mL) | Measurement | Main Findings | Reference |
|---|---|---|---|---|---|---|---|---|
| 2021 | Preg-nancy loss | 78/79 | Xianyang, China | Serum; fasting, 8–12 wk of gestation | 28.96 ± 19.14/ 19.64 ± 10.59 p < 0.001 | ELISA | • Significantly elevated FABP4 levels in missed abortion than in control group independent of maternal age, BMI, or gestational age. • A discrimination ability of FABP4 levels with an AUROC of 0.70. • An improved distinguishing capacity (OR with 95% confidence intervals) of the FABP4 levels to 0.74 by cutoff value. | Yongkang Yang, et al. [77] |
| 2011 | GDM | 98/86 | Madrid, Spain | Serum; undescribed, <1 wk before delivery | 17.7 ± 0.8/19.9 ± 1.0 p < 0.001 | ELISA | • Significantly elevated FABP4 levels in GDM than in control group. • Positive correlations between FABP4 levels and neonatal fat mass, glycerol, and leptin in cord blood in GDM group (p < 0.05). | Henar Ortega- Senovilla, et al. [30] |
| 2015 | GDM | 30/30 | Hohhot, China | Plasma; fasting, 24–28 wk of gestation | 14.7 ± 2.5/12.0 ± 0.7 p < 0.01 | ELISA | • Elevated FABP4 levels in GDM than in control group. | Yuanyuan Li, et al. [31] |
| 2016 | GDM | 40/240 | Guangzhou, China | Serum; | ELISA | • Elevated FABP4 levels in GDM than in control group. • An increase of FABP4 levels from the second to third trimester in GDM group. • GDM was an independent factor of FABP4 levels (p < 0.05). | Ying Zhang, et al. [32] | |
| undescribed; 24–28 wk of gestation | 32.35 ± 3.06/ 22.01 ± 2.00 p < 0.01 | |||||||
| undescribed, ≥37 wk of gestation | 36.47 ± 4.00/21.79 ± 1.32 p < 0.01 | |||||||
| 2017 | GDM | 52/71 | multiple center, Australia | Blood; | multiple technology | • A positive correlation between FABP4 levels and IR in early pregnancy, but no longer significant at 28 wk of gestation. | Kym J Guelfi, et al. [34] | |
| undescribed, 12–14 wk of gestation | 19.4 (10.2–35.1) /20.3 (11.1–29.0) p < 0.05 | |||||||
| undescribed, 28 wk of gestation | 24.6 (16.4–41.5) /21.8 (15.5–28.8) n.s. | |||||||
| 2017 | GDM | 135/1015 | Haerbin and Beijing, China | Plasma; fasting, 24–28 wk of gestation, | 23.9 (17.6–32.2)/16.8 (12.3–23.1) n.s. | ELISA | • An optimal cutoff value of FABP4 level for the subsequent development of GDM at 18.5 ng/mL, with a sensitivity of 81.8% and a specificity of 71.2%. • An increased risk of GDM associated with FABP4 levels ≥ 18.6 ng/mL. | Wenjun Tu, et al. [35] |
| 2017 | GDM | 50/50 | Guangzhou, China | Serum; undescribed, ≥37 wk of gestation | 20 ± 10.38/10.50 ± 5.69 p < 0.05 | ELISA | • Elevated FABP4 levels in GDM than in control group | Ying Zhang, et al. [33] |
| 2020 | GDM | 54/55 | Zibo, China | Serum; undescribed, 24–30 wk of gestation | 5.46 ± 1.36/ 1.34 ± 0.44 p < 0.001 | ELISA | • Significantly elevated FABP4 levels in GDM than in control group. • Significantly positive correlations between TNF-α, IL-6 and FABP4 in GDM group (p < 0.001). | Bide Duan, et al. [36] |
| 2020 | GDM | 107/214 | multiple center, USA | Plasma; | (processed data) | ELISA | • A significantly positive correlation between GDM risk and FABP4 levels at 10–14 wk of gestation after adjusting (p < 0.0001). | Ellen C Francis, et al. [37] |
| undescribed, 10–14 wk of gestation | ||||||||
| fasting, 15–26 wk, 23–31 wk, 33–39 wk of gestation | ||||||||
| 2020 | GDM | 60/50 | Jinan, China | Serum; fasting, 24–28 wk of gestation | 35.14 ± 11.39/21.53 ± 8.89 p < 0.001 | ELISA | • Significantly elevated FABP4 levels in GDM than in control group. • Significantly positive correlations between TNF-α, IL-6 and FABP4 levels in GDM group (p < 0.001). | Xueling Wang, et al. [38] |
| 2021 | GDM | 135/135 | Beijing, China | Plasma; | ELISA | • Similar FABP4 levels in GDM and control groups. • A significantly positive correlation between FABP4 levels and IR (p < 0.001). | Chuyao Jin, et al. [39] | |
| undescribed, <14 wk of gestation | 53.3 (33.1–93.2)/42.4 (32.6–63.8) n.s. | |||||||
| undescribed, 25–28 wk of gestation | 53.8 (36.8–94.1)/41.6 (33.4–64.1) n.s. | |||||||
| 2021 | GDM | 22/18 | Israel | Serum of cord blood; at birth | 23.8 ± 16.9, umbilical artery; 22 ± 13.9, umbilical vein/15.2 ± 7.6 p < 0.05 | ELISA | • Elevated FABP4 levels in infants born from GDM mother than in control group. • A further rise of neonatal FABP4 levels within the first 6 h of birth in all infants. • No correlation between FABP4 levels and birth weight in all infants. • An inverse correlation between FABP4 levels and blood glucose in all infants (p = 0.022). | Idit Ron, et al. [108] |
| 2022 | GDM | 40/40 | Tartu, Estonia | Serum; Fasting; 25–28 wk of gestation | 80.30/94.03 n.s. | ELISA | • Similar FABP4 levels in GDM and control groups. • Significantly elevated FABP4 levels in women with BMI > 25 in GDM and control groups. (p < 0.001). • A significantly positive correlation between FABP4 levels and BMI in GDM and control groups (p < 0.001). | Tamara Vorobjova, et al. [29] |
| 2008 | PE | 16/20 | Leipzig, Germany | Serum; Fasting; <20 wk of gestation | 24.5 ± 9.7/ 14.8 ± 7.1 p < 0.05 | ELISA | • BMI and creatinine together explained 58% of the variation in FABP4 levels. • Elevated FABP4 levels in PE than in control group. • Elevated FABP4 level in patients with BMI > 25 than BMI < 25 (p < 0.05) in PE group. | Mathias Fasshaue, et al. [28] |
| 2012 | PE | 22/72 | Pittsburgh, PA, USA | Serum; | ELISA | • Elevated FABP4 levels in PE than in control group. • Similar magnitude of change of FABP4 levels in PE and control groups. | Christina M Scifres, et al. [40] | |
| Fasting; <13 wk of gestation | 20.4 ± 12.3/ 10.1 ± 4.7 p < 0.05 | |||||||
| Fasting; 24–28 wk of gestation | 20.7 ± 11.7/ 9.9 ± 4.5 p < 0.05 | |||||||
| 2014 | PE | 26/46 | Helsinki, Finland | Serum; | ELISA | • Similar FABP4 levels in PE and control groups. • An inverse correlation between FABP4 levels and weight gain during pregnancy in women with pregnancy-induced hypertension (p = 0.02). | A L Tuuri, et al. [42] | |
| Fasting; 24 wk of gestation | 20.1 (13.1–72.9)/ 19.2 (10.5–49.7) n.s. | |||||||
| Fasting; 32 wk of gestation | 25.9 (12.4–213.7)/ 23.0 (13.2–59.5) n.s. | |||||||
| 2017 | PE | 23/19 | Charleston, SC, USA | Plasma; | ELISA | • Elevated FABP4 levels in PE than in control group in 21.7 ± 1.4 wk and 31.4 ± 1.5 wk of gestation, both groups with GDM. • Progressive increase of FABP4 levels throughout pregnancy in all participates. | Clare B Kelly, et al. [41] | |
| Undescribed; 12.4 ± 1.8 wk of gestation | 9.4 (7.9, 11.1)/ 7.6 (6.5, 9.0) n.s. | |||||||
| Undescribed; 21.7 ± 1.4 wk of gestation | 11.1 (9.4, 13.1)/ 8.0 (6.8, 9.3) p < 0.01 | |||||||
| Undescribed; 31.4 ± 1.5 wk of gestation | 15.6 (13.4, 18.1)/ 10.2 (8.8, 11.7) p < 0.001 | |||||||
| 2010 | Pre-mature delivery | 55/23 | Athens, Greece | Serum; Undescribed; 31.9 ± 10.4 days of life | 29.2 (14.5)/ 26.2 (8.9) n.s. | ELISA | • Similar FABP4 levels in preterm and full-term infants. • A positive correlation between FABP4 levels and TC in preterm and full-term infants (p < 0.05) • An independently and positive correlation between FABP4 levels and weight gain in preterm infants (p = 0.01). | Tania Siahanidou, et al. [106] |
| 2017 | Pre-mature delivery | 144/217 | Boston, MA, USA | Plasma of cord blood; at birth | 48.2 (31.2~73.3)/ 35.8 (25.1~51.5) p < 0.01 | ELISA | • Elevated FABP4 levels in preterm than full-term infants. • Elevated FABP4 levels in all infants than adults (p < 0.01). • Lower FABP4 levels in SGA than AGA group in full-term neonates (p = 0.05). • Elevated FABP4 levels in infants born from mothers with gestational hypertension or PE than from healthy mothers after controlling (p = 0.003). | Kyoung Eun Joung, et al. [105] |
| 2021 | Neonatal size | 321 | multiple center, USA | Plasma; | processed data | ELISA | • No correlation between FABP4 levels and neonatal anthropometry. | Ellen C Francis, et al. [122] |
| Fasting; 15–26 wk of gestation | ||||||||
| Random; 10–14 wk, 23–31 wk, 33–39 wk of gestation | ||||||||
| 2019 | IUGR | 28/13 | Leipzig, Germany | Plasma of cord blood; at birth | 103.08 ± 80.61/ 49.63 ± 23.75 n.s. | ELISA | • Slightly elevated FABP4 levels in IUGR than in control group. • Elevated FABP4 levels in the smaller twins than in their larger co-twins (p = 0.028). • A significantly inverse correlation between FABP4 levels and birth weight and gestational age in all infants (p < 0.001). | Susanne Schrey-Petersen, et al. [123] |
| 2022 | Macro-somia | 38/39 | Wenzhou, China | Placental tissues; at birth | FABP4 mRNA relative expression | real-time PCR | • An increased mRNA expression of placental FABP4 in macrosomia than in control group (p < 0.05). | Li-Fang Ni, et al. [121] |
5. Influence of Environmental Factors on FABP4 during Pregnancy
6. The Potential Clinical Implications of FABP4 in Reproductive and Gestational Health
6.1. FABP4 Is a Potential Biomarker for Gestational Complications
6.2. A Potential Therapeutic Strategy for Pregnancy-Related Disorders by Targeting FABP4
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A-FABP | adipocyte fatty acid-binding protein |
| AGA | appropriate for gestational age |
| AP-1 | activator protein 1 |
| aP2 | adipocyte protein 2 |
| ATGL | adipose triglyceride lipase |
| AUROC | area under the roc curve |
| BMI | body mass index |
| DHA | docosahexaenoic acid |
| ELISA | enzyme linked immunosorbent assay |
| ER | endoplasmic reticulum |
| FA | fatty acid |
| FABP4 | fatty acid-binding protein 4 |
| FABPpm | plasma membrane-bound fatty acid binding proteins |
| FAT/CD36 | fatty acid translocase |
| FATP | fatty acid transport proteins |
| FFA | free fatty acid |
| GDM | gestational diabetes mellitus |
| HESCs | human endometrial stromal cell line |
| HOMA-IR | homeostasismodel assessment-insulin resistance |
| HSL | hormone-sensitive lipase |
| IADPSG | International Association of Diabetes and Pregnancy Study Group |
| IFN | interferon |
| IL-6 | Interleukin-6 |
| IR | insulin resistance |
| IUGR | intra-uterine growth restriction |
| JAK2 | Janus kinase 2 |
| JNK | c-Jun N-terminal kinase |
| LCPUFAs | long-chain polyunsaturated fatty acids |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| mTOR | mechanistic target of rapamycin |
| mtUPR | mitochondrial unfolded-protein response |
| NK | natural killer |
| OGTT | oral glucose tolerance test |
| OR | odds ratios |
| PCOS | polycystic ovary syndrome |
| PCR | polymerase chain reaction |
| PE | preeclampsia |
| PHT | primary human trophoblasts |
| PPAR | peroxisomeproliferator-activated receptor |
| PUFAs | polyunsaturated fatty acids |
| Rap1a | RAS-related protein-1a precursor |
| RBP4 | retinol-binding protein 4 |
| ROS | reactive oxygen species |
| SGA | small-for-gestational age |
| SIRT3 | recombinant sirtuin 3 |
| STAT2 | signal transducer and activator of the transcription 2 |
| TC | total cholesterol |
| TG | circulating triglyceride |
| TLR4 | lipopolysaccharide viatoll like receptor 4 |
| TNF-α | tumor necrosis factor-α |
| VD | vitamin D |
| VEGF | vascular endothelial growth factor |
| VEGFR2 | vascular endothelial growth factor receptor 2 |
References
- Bajpai, K.; Acharya, N.; Prasad, R.; Wanjari, M.B. Endometrial Receptivity During the Preimplantation Period: A Narrative Review. Cureus 2023, 15, e37753. [Google Scholar] [PubMed]
- Ryan, E.A. Hormones and insulin resistance during pregnancy. Lancet 2003, 362, 1777–1778. [Google Scholar] [CrossRef] [PubMed]
- Orefice, R. Immunology and the immunological response in pregnancy. Best. Pract. Res. Clin. Obs. Gynaecol. 2021, 76, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Elhaj, D.A.I.; Ibrahim, I.; Abdullahi, H.; Al Khodor, S. Maternal microbiota and gestational diabetes: Impact on infant health. J. Transl. Med. 2023, 21, 364. [Google Scholar]
- Wang, L.; O’Kane, A.M.; Zhang, Y.; Ren, J. Maternal obesity and offspring health: Adapting metabolic changes through autophagy and mitophagy. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2023, 24, e13567. [Google Scholar]
- Yang, L.; Huang, C.; Zhao, M.; Lee, P.M.Y.; Zhang, C.; Yu, Y.; Xi, B.; Li, J. Maternal hypertensive disorders during pregnancy and the risk of offspring diabetes mellitus in childhood, adolescence, and early adulthood: A nationwide population-based cohort study. BMC Med. 2023, 21, 59. [Google Scholar]
- Zhang, X.Z.; Tu, W.J.; Wang, H.; Zhao, Q.; Liu, Q.; Sun, L.; Yu, L. Circulating Serum Fatty Acid-Binding Protein 4 Levels Predict the Development of Diabetic Retinopathy in Type 2 Diabetic Patients. Am. J. Ophthalmol. 2018, 187, 71–79. [Google Scholar]
- Xu, H.; Diolintzi, A.; Storch, J. Fatty acid-binding proteins: Functional understanding and diagnostic implications. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 407–412. [Google Scholar]
- Guaita-Esteruelas, S.; Gumà, J.; Masana, L.; Borràs, J. The peritumoural adipose tissue microenvironment and cancer. The roles of fatty acid binding protein 4 and fatty acid binding protein 5. Mol. Cell Endocrinol. 2018, 462 Pt B, 107–118. [Google Scholar]
- Cao, H.; Sekiya, M.; Ertunc, M.E.; Burak, M.F.; Mayers, J.R.; White, A.; Inouye, K.; Rickey, L.M.; Ercal, B.C.; Furuhashi, M.; et al. Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab. 2013, 17, 768–778. [Google Scholar] [CrossRef]
- Guo, D.; Lin, C.; Lu, Y.; Guan, H.; Qi, W.; Zhang, H.; Shao, Y.; Zeng, C.; Zhang, R.; Zhang, H.; et al. FABP4 secreted by M1-polarized macrophages promotes synovitis and angiogenesis to exacerbate rheumatoid arthritis. Bone Res. 2022, 10, 45. [Google Scholar]
- St Louis, D.; Romero, R.; Plazyo, O.; Arenas-Hernandez, M.; Panaitescu, B.; Xu, Y.; Milovic, T.; Xu, Z.; Bhatti, G.; Mi, Q.S.; et al. Invariant NKT Cell Activation Induces Late Preterm Birth That Is Attenuated by Rosiglitazone. J. Immunol. 2016, 196, 1044–1059. [Google Scholar] [PubMed]
- Makkar, A.; Mishima, T.; Chang, G.; Scifres, C.; Sadovsky, Y. Fatty acid binding protein-4 is expressed in the mouse placental labyrinth, yet is dispensable for placental triglyceride accumulation and fetal growth. Placenta 2014, 35, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Scifres, C.M.; Chen, B.; Nelson, D.M.; Sadovsky, Y. Fatty acid binding protein 4 regulates intracellular lipid accumulation in human trophoblasts. J. Clin. Endocrinol. Metab. 2011, 96, E1083–E1091. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Bernlohr, D.A. Metabolic functions of FABPs—Mechanisms and therapeutic implications. Nat. Rev. Endocrinol. 2015, 11, 592–605. [Google Scholar] [CrossRef]
- Storch, J.; Thumser, A.E. The fatty acid transport function of fatty acid-binding proteins. Biochim. Biophys. Acta 2000, 1486, 28–44. [Google Scholar] [CrossRef]
- Tan, N.S.; Shaw, N.S.; Vinckenbosch, N.; Liu, P.; Yasmin, R.; Desvergne, B.; Wahli, W.; Noy, N. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol. Cell. Biol. 2002, 22, 5114–5127. [Google Scholar] [CrossRef] [PubMed]
- Boon Yin, K.; Najimudin, N.; Muhammad, T.S. The PPARgamma coding region and its role in visceral obesity. Biochem. Biophys. Res. Commun. 2008, 371, 177–179. [Google Scholar]
- Hofer, P.; Boeszoermenyi, A.; Jaeger, D.; Feiler, U.; Arthanari, H.; Mayer, N.; Zehender, F.; Rechberger, G.; Oberer, M.; Zimmermann, R.; et al. Fatty Acid-binding Proteins Interact with Comparative Gene Identification-58 Linking Lipolysis with Lipid Ligand Shuttling. J. Biol. Chem. 2015, 290, 18438–18453. [Google Scholar] [PubMed]
- Ge, X.N.; Bastan, I.; Dileepan, M.; Greenberg, Y.; Ha, S.G.; Steen, K.A.; Bernlohr, D.A.; Rao, S.P.; Sriramarao, P. FABP4 regulates eosinophil recruitment and activation in allergic airway inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L227–L240. [Google Scholar] [CrossRef]
- Hui, X.; Li, H.; Zhou, Z.; Lam, K.S.; Xiao, Y.; Wu, D.; Ding, K.; Wang, Y.; Vanhoutte, P.M.; Xu, A. Adipocyte fatty acid-binding protein modulates inflammatory responses in macrophages through a positive feedback loop involving c-Jun NH2-terminal kinases and activator protein-1. J. Biol. Chem. 2010, 285, 10273–10280. [Google Scholar] [PubMed]
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [PubMed]
- Jenkins-Kruchten, A.E.; Bennaars-Eiden, A.; Ross, J.R.; Shen, W.J.; Kraemer, F.B.; Bernlohr, D.A. Fatty acid-binding protein-hormone-sensitive lipase interaction. Fatty acid dependence on binding. J. Biol. Chem. 2003, 278, 47636–47643. [Google Scholar] [PubMed]
- Gugliucci, A. Biomarkers of dysfunctional visceral fat. Adv. Clin. Chem. 2022, 109, 1–30. [Google Scholar]
- Lee, C.H.; Cheung, C.Y.Y.; Woo, Y.C.; Lui, D.T.W.; Yuen, M.M.A.; Fong, C.H.Y.; Chow, W.S.; Xu, A.; Lam, K.S.L. Circulating Adipocyte Fatty Acid-Binding Protein Concentrations Predict Multiple Mortality Outcomes among Men and Women with Diabetes. Clin. Chem. 2018, 64, 1496–1504. [Google Scholar]
- Lee, C.H.; Lui, D.T.W.; Lam, K.S.L. Adipocyte Fatty Acid-Binding Protein, Cardiovascular Diseases and Mortality. Front. Immunol. 2021, 12, 589206. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Shao, Y.; Zhou, H.; Pang, L.; Zhu, W. Diagnostic, Prognostic, and Immunological Roles of FABP4 in Pancancer: A Bioinformatics Analysis. Comput. Math. Methods Med. 2022, 2022, 3764914. [Google Scholar]
- Fasshauer, M.; Seeger, J.; Waldeyer, T.; Schrey, S.; Ebert, T.; Kratzsch, J.; Lössner, U.; Blüher, M.; Stumvoll, M.; Faber, R.; et al. Serum levels of the adipokine adipocyte fatty acid-binding protein are increased in preeclampsia. Am. J. Hypertens. 2008, 21, 582–586. [Google Scholar] [CrossRef][Green Version]
- Vorobjova, T.; Tagoma, A.; Talja, I.; Janson, H.; Kirss, A.; Uibo, R. FABP4 and I-FABP Levels in Pregnant Women Are Associated with Body Mass Index but Not Gestational Diabetes. J. Diabetes Res. 2022, 2022, 1089434. [Google Scholar]
- Ortega-Senovilla, H.; Schaefer-Graf, U.; Meitzner, K.; Abou-Dakn, M.; Graf, K.; Kintscher, U.; Herrera, E. Gestational diabetes mellitus causes changes in the concentrations of adipocyte fatty acid-binding protein and other adipocytokines in cord blood. Diabetes Care 2011, 34, 2061–2066. [Google Scholar] [CrossRef]
- Li, Y.Y.; Xiao, R.; Li, C.P.; Huangfu, J.; Mao, J.F. Increased plasma levels of FABP4 and PTEN is associated with more severe insulin resistance in women with gestational diabetes mellitus. Med. Sci. Monit. 2015, 21, 426–431. [Google Scholar]
- Zhang, Y.; Zhang, H.H.; Lu, J.H.; Zheng, S.Y.; Long, T.; Li, Y.T.; Wu, W.Z.; Wang, F. Changes in serum adipocyte fatty acid-binding protein in women with gestational diabetes mellitus and normal pregnant women during mid- and late pregnancy. J. Diabetes Investig. 2016, 7, 797–804. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, J.H.; Zheng, S.Y.; Yan, J.H.; Chen, L.; Liu, X.; Wu, W.Z.; Wang, F. Serum levels of nesfatin-1 are increased in gestational diabetes mellitus. Gynecol. Endocrinol. 2017, 33, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Guelfi, K.J.; Ong, M.J.; Li, S.; Wallman, K.E.; Doherty, D.A.; Fournier, P.A.; Newnham, J.P.; Keelan, J.A. Maternal circulating adipokine profile and insulin resistance in women at high risk of developing gestational diabetes mellitus. Metabolism 2017, 75, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.J.; Guo, M.; Shi, X.D.; Cai, Y.; Liu, Q.; Fu, C.W. First-Trimester Serum Fatty Acid-Binding Protein 4 and Subsequent Gestational Diabetes Mellitus. Obs. Gynecol. 2017, 130, 1011–1016. [Google Scholar] [CrossRef]
- Duan, B.; Li, Y.; Dong, K.; Sun, Y.; Ma, A.; Yang, X. Regulative effect of maternal serum fatty acid-binding protein 4 on insulin resistance and the development of gestational diabetes mellitus. Prostaglandins Leukot. Essent. Fat. Acids 2020, 163, 102213. [Google Scholar] [CrossRef]
- Francis, E.C.; Li, M.; Hinkle, S.N.; Cao, Y.; Chen, J.; Wu, J.; Zhu, Y.; Cao, H.; Kemper, K.; Rennert, L.; et al. Adipokines in early and mid-pregnancy and subsequent risk of gestational diabetes: A longitudinal study in a multiracial cohort. BMJ Open Diabetes Res. Care 2020, 8, e001333. [Google Scholar]
- Wang, X.; Liu, J.; Wang, D.; Zhu, H.; Kang, L.; Jiang, J. Expression and correlation of Chemerin and FABP4 in peripheral blood of gestational diabetes mellitus patients. Exp. Ther. Med. 2020, 19, 710–716. [Google Scholar]
- Jin, C.; Lin, L.; Han, N.; Zhao, Z.; Xu, X.; Luo, S.; Liu, J.; Wang, H. Risk of Gestational Diabetes Mellitus in relation to Plasma Concentrations of Fatty Acid-Binding Protein 4: A Nested Case-Control Study in China. J. Diabetes Res. 2021, 2021, 6681432. [Google Scholar]
- Scifres, C.M.; Catov, J.M.; Simhan, H. Maternal serum fatty acid binding protein 4 (FABP4) and the development of preeclampsia. J. Clin. Endocrinol. Metab. 2012, 97, E349–E356. [Google Scholar] [CrossRef]
- Kelly, C.B.; Hookham, M.B.; Yu, J.Y.; Lockhart, S.M.; Du, M.; Jenkins, A.J.; Nankervis, A.; Hanssen, K.F.; Henriksen, T.; Garg, S.K.; et al. Circulating adipokines are associated with pre-eclampsia in women with type 1 diabetes. Diabetologia 2017, 60, 2514–2524. [Google Scholar] [PubMed]
- Tuuri, A.L.; Jauhiainen, M.S.; Tikkanen, M.J.; Kaaja, R.J. Systolic blood pressure and fatty acid-binding protein 4 predict pregnancy-induced hypertension in overweight nulliparous women. Placenta 2014, 35, 797–801. [Google Scholar] [PubMed]
- Wang, P.; Zhu, Q.; Peng, H.; Du, M.; Dong, M.; Wang, H. Fatty Acid-Binding Protein 4 in Endometrial Epithelium Is Involved in Embryonic Implantation. Cell Physiol. Biochem. 2017, 41, 501–509. [Google Scholar]
- Duttaroy, A.K.; Basak, S. Maternal Fatty Acid Metabolism in Pregnancy and Its Consequences in the Feto-Placental Development. Front. Physiol. 2021, 12, 787848. [Google Scholar]
- Duttaroy, A.K. Transport of fatty acids across the human placenta: A review. Prog. Lipid Res. 2009, 48, 52–61. [Google Scholar]
- Xiao, Y.; Shu, L.; Wu, X.; Liu, Y.; Cheong, L.Y.; Liao, B.; Xiao, X.; Hoo, R.L.; Zhou, Z.; Xu, A. Fatty acid binding protein 4 promotes autoimmune diabetes by recruitment and activation of pancreatic islet macrophages. JCI Insight 2021, 6, e141814. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Wang, Y.; Yang, A.; Dong, X.; Gu, L.; Liu, D.; Ding, N.; Jiang, Y. FABP4 activates the JAK2/STAT2 pathway via Rap1a in the homocysteine-induced macrophage inflammatory response in ApoE−/− mice atherosclerosis. Lab. Investig. J. Tech. Methods Pathol. 2022, 102, 25–37. [Google Scholar]
- Hertzel, A.V.; Xu, H.; Downey, M.; Kvalheim, N.; Bernlohr, D.A. Fatty acid binding protein 4/aP2-dependent BLT1R expression and signaling. J. Lipid Res. 2017, 58, 1354–1361. [Google Scholar] [CrossRef]
- Murphy, S.P.; Tayade, C.; Ashkar, A.A.; Hatta, K.; Zhang, J.; Croy, B.A. Interferon gamma in successful pregnancies. Biol. Reprod. 2009, 80, 848–859. [Google Scholar] [CrossRef]
- Wang, F.; Qualls, A.E.; Marques-Fernandez, L.; Colucci, F. Biology and pathology of the uterine microenvironment and its natural killer cells. Cell. Mol. Immunol. 2021, 18, 2101–2113. [Google Scholar]
- Mirza, F.G.; Tahlak, M.A.; Rjeili, R.B.; Hazari, K.; Ennab, F.; Hodgman, C.; Khamis, A.H.; Atiomo, W. Polycystic Ovarian Syndrome (PCOS): Does the Challenge End at Conception? Int. J. Environ. Res. Public Health 2022, 19, 14914. [Google Scholar]
- Deswal, R.; Narwal, V.; Dang, A.; Pundir, C.S. The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. J. Hum. Reprod. Sci. 2020, 13, 261–271. [Google Scholar] [PubMed]
- Wang, J.; Tang, J.; Wang, B.; Song, J.; Liu, J.; Wei, Z.; Zhang, F.; Ma, X.; Cao, Y. FABP4: A novel candidate gene for polycystic ovary syndrome. Endocrine 2009, 36, 392–396. [Google Scholar]
- Hu, W.; Qiao, J. Expression and regulation of adipocyte fatty acid binding protein in granulosa cells and its relation with clinical characteristics of polycystic ovary syndrome. Endocrine 2011, 40, 196–202. [Google Scholar]
- Möhlig, M.; Weickert, M.O.; Ghadamgadai, E.; Machlitt, A.; Pfüller, B.; Arafat, A.M.; Pfeiffer, A.F.; Schöfl, C. Adipocyte fatty acid-binding protein is associated with markers of obesity, but is an unlikely link between obesity, insulin resistance, and hyperandrogenism in polycystic ovary syndrome women. Eur. J. Endocrinol. 2007, 157, 195–200. [Google Scholar] [PubMed]
- Doğanay, M.; Ozyer, S.S.; Var, T.; Tonguc, E.; Gun Eryilmaz, O.; Ozer, I.; Guzel, A.I. Associations between adipocyte fatty acid-binding protein and clinical parameters in polycystic ovary syndrome. Arch. Gynecol. Obstet. 2015, 291, 447–450. [Google Scholar]
- Lazaro, I.; Diaz, M.; Cabre, A.; Masana, L.; Ibanez, L. Fatty acid-binding protein-4 plasma levels are associated to metabolic abnormalities and response to therapy in girls and young women with androgen excess. Gynecol. Endocrinol. 2011, 27, 935–939. [Google Scholar] [PubMed]
- Giudice, L.C. Clinical practice. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [PubMed]
- Eyster, K.M.; Klinkova, O.; Kennedy, V.; Hansen, K.A. Whole genome deoxyribonucleic acid microarray analysis of gene expression in ectopic versus eutopic endometrium. Fertil. Steril. 2007, 88, 1505–1533. [Google Scholar]
- Kubo, K.; Kamada, Y.; Hasegawa, T.; Sakamoto, A.; Nakatsuka, M.; Matsumoto, T.; Masuyama, H. Inflammation of the adipose tissue in the retroperitoneal cavity adjacent to pelvic endometriosis. J. Obstet. Gynaecol. Res. 2021, 47, 3598–3606. [Google Scholar]
- Tang, W.; Chen, O.; Yao, F.; Cui, L. miR-455 targets FABP4 to protect human endometrial stromal cells from cytotoxicity induced by hydrogen peroxide. Mol. Med. Rep. 2019, 20, 4781–4790. [Google Scholar]
- Huang, C.C.; Hsueh, Y.W.; Chang, C.W.; Hsu, H.C.; Yang, T.C.; Lin, W.C.; Chang, H.M. Establishment of the fetal-maternal interface: Developmental events in human implantation and placentation. Front. Cell Dev. Biol. 2023, 11, 1200330. [Google Scholar] [PubMed]
- Zhu, Q.; Jin, Y.; Wang, P.; Wang, H.; Lu, B.; Wang, Z.; Dong, M. Expression and function of fatty acid-binding protein 4 in epithelial cell of uterine endometrium. Cell Biol. Int. 2015, 39, 540–547. [Google Scholar] [PubMed]
- Herrera, E.; Amusquivar, E. Lipid metabolism in the fetus and the newborn. Diabetes Metab. Res. Rev. 2000, 16, 202–210. [Google Scholar] [PubMed]
- Pan, X.; Jin, X.; Wang, J.; Hu, Q.; Dai, B. Placenta inflammation is closely associated with gestational diabetes mellitus. Am. J. Transl. Res. 2021, 13, 4068–4079. [Google Scholar]
- Bizargity, P.; Bonney, E.A. Dendritic cells: A family portrait at mid-gestation. Immunology 2009, 126, 565–578. [Google Scholar]
- Xu, H.; Hertzel, A.V.; Steen, K.A.; Bernlohr, D.A. Loss of Fatty Acid Binding Protein 4/aP2 Reduces Macrophage Inflammation Through Activation of SIRT3. Mol. Endocrinol. 2016, 30, 325–334. [Google Scholar]
- Steen, K.A.; Xu, H.; Bernlohr, D.A. FABP4/aP2 Regulates Macrophage Redox Signaling and Inflammasome Activation via Control of UCP2. Mol. Cell. Biol. 2017, 37, e00282-16. [Google Scholar]
- Michelet, X.; Dyck, L.; Hogan, A.; Loftus, R.M.; Duquette, D.; Wei, K.; Beyaz, S.; Tavakkoli, A.; Foley, C.; Donnelly, R.; et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 2018, 19, 1330–1340. [Google Scholar]
- Kobayashi, T.; Lam, P.Y.; Jiang, H.; Bednarska, K.; Gloury, R.; Murigneux, V.; Tay, J.; Jacquelot, N.; Li, R.; Tuong, Z.K.; et al. Increased lipid metabolism impairs NK cell function and mediates adaptation to the lymphoma environment. Blood 2020, 136, 3004–3017. [Google Scholar]
- Yang, X.; Glazebrook, P.; Ranasinghe, G.C.; Haghiac, M.; Calabuig-Navarro, V.; Minium, J.; O’Tierney-Ginn, P. Fatty acid transporter expression and regulation is impaired in placental macrovascular endothelial cells in obese women. J. Matern. Fetal Neonatal Med. 2019, 32, 971–978. [Google Scholar] [PubMed]
- Gopalakrishnan, K.; Mishra, J.S.; Ross, J.R.; Abbott, D.H.; Kumar, S. Hyperandrogenism diminishes maternal-fetal fatty acid transport by increasing FABP4-mediated placental lipid accumulationdagger. Biol. Reprod. 2022, 107, 514–528. [Google Scholar] [PubMed]
- Elmasri, H.; Karaaslan, C.; Teper, Y.; Ghelfi, E.; Weng, M.; Ince, T.A.; Kozakewich, H.; Bischoff, J.; Cataltepe, S. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2009, 23, 3865–3873. [Google Scholar]
- Siemers, K.M.; Baack, M.L. The importance of placental lipid metabolism across gestation in obese and non-obese pregnancies. Clin. Sci. 2023, 137, 31–34. [Google Scholar]
- Murata, H.; Tanaka, S.; Okada, H. Immune Tolerance of the Human Decidua. J. Clin. Med. 2021, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Aldo, P.; Alvero, A.B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar]
- Yang, Y.; Wu, J.; Wang, X.; Yao, J.; Lao, K.S.; Qiao, Y.; Xu, Y.; Hu, Y.; Feng, Y.; Cui, Y.; et al. Circulating fibroblast growth factor 21 as a potential biomarker for missed abortion in humans. Fertil. Steril. 2021, 116, 1040–1049. [Google Scholar]
- Pandya, A.D.; Das, M.K.; Sarkar, A.; Vilasagaram, S.; Basak, S.; Duttaroy, A.K. Tube formation in the first trimester placental trophoblast cells: Differential effects of angiogenic growth factors and fatty acids. Cell Biol. Int. 2016, 40, 652–661. [Google Scholar]
- Filant, J.; Spencer, T.E. Uterine glands: Biological roles in conceptus implantation, uterine receptivity and decidualization. Int. J. Dev. Biol. 2014, 58, 107–116. [Google Scholar]
- Roberti, S.L.; Higa, R.; White, V.; Powell, T.L.; Jansson, T.; Jawerbaum, A. Critical role of mTOR, PPARγ and PPARδ signaling in regulating early pregnancy decidual function, embryo viability and feto-placental growth. Mol. Hum. Reprod. 2018, 24, 327–340. [Google Scholar]
- WHO. Physical Status: The Use and Interpretation of Anthropometry, Report of a WHO Expert Committee; World Health Organ Technical Report Series; WHO: Geneva, Switzerland, 1995; Volume 854, pp. 1–452. [Google Scholar]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol. Metab. 2018, 29, 743–754. [Google Scholar]
- Furigo, I.C.; Dearden, L. Mechanisms mediating the impact of maternal obesity on offspring hypothalamic development and later function. Front. Endocrinol. 2022, 13, 1078955. [Google Scholar]
- Kislal, S.; Shook, L.L.; Edlow, A.G. Perinatal exposure to maternal obesity: Lasting cardiometabolic impact on offspring. Prenat. Diagn. 2020, 40, 1109–1125. [Google Scholar] [PubMed]
- Perng, W.; Oken, E.; Dabelea, D. Developmental overnutrition and obesity and type 2 diabetes in offspring. Diabetologia 2019, 62, 1779–1788. [Google Scholar] [PubMed]
- Ishimura, S.; Furuhashi, M.; Watanabe, Y.; Hoshina, K.; Fuseya, T.; Mita, T.; Okazaki, Y.; Koyama, M.; Tanaka, M.; Akasaka, H.; et al. Circulating levels of fatty acid-binding protein family and metabolic phenotype in the general population. PLoS ONE 2013, 8, e81318. [Google Scholar]
- Wang, H.; Li, N.; Chivese, T.; Werfalli, M.; Sun, H.; Yuen, L.; Hoegfeldt, C.A.; Elise Powe, C.; Immanuel, J.; Karuranga, S.; et al. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes Res. Clin. Pract. 2022, 183, 109050. [Google Scholar] [PubMed]
- American Diabetes Association. 14. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42 (Suppl. S1), S165–S172. [Google Scholar]
- Liu, X.; Zheng, T.; Xu, Y.J.; Yang, M.N.; Wang, W.J.; Huang, R.; Zhang, G.H.; Guo, Y.N.; Zhang, J.; Ouyang, F.; et al. Sex Dimorphic Associations of Gestational Diabetes Mellitus with Cord Plasma Fatty Acid Binding Protein 4 and Estradiol. Front. Endocrinol. 2021, 12, 740902. [Google Scholar]
- Trojnar, M.; Patro-Malysza, J.; Kimber-Trojnar, Z.; Czuba, M.; Mosiewicz, J.; Leszczynska-Gorzelak, B. Vaspin in Serum and Urine of Post-Partum Women with Excessive Gestational Weight Gain. Medicina 2019, 55, 76. [Google Scholar]
- Dong, X.; Yang, L. Inhibition of fatty acid binding protein 4 attenuates gestational diabetes mellitus. Prostaglandins Leukot. Essent. Fat. Acids 2020, 161, 102179. [Google Scholar]
- Kinalski, M.; Kuzmicki, M.; Telejko, B.; Bachorzewski, R.; Buraczyk, M.; Kretowski, A.; Kinalska, I. Tumor necrosis factor-alpha system in patients with gestational diabetes. Przegl. Lek. 2006, 63, 173–175. [Google Scholar]
- Basak, S.; Das, M.K.; Srinivas, V.; Duttaroy, A.K. The interplay between glucose and fatty acids on tube formation and fatty acid uptake in the first trimester trophoblast cells, HTR8/SVneo. Mol. Cell Biochem. 2015, 401, 11–19. [Google Scholar]
- Sun, J.; Zhang, D.; Xu, J.; Chen, C.; Deng, D.; Pan, F.; Dong, L.; Li, S.; Ye, S. Circulating FABP4, nesfatin-1, and osteocalcin concentrations in women with gestational diabetes mellitus: A meta-analysis. Lipids Health Dis. 2020, 19, 199. [Google Scholar]
- Ramos, M.P.; Crespo-Solans, M.D.; del Campo, S.; Cacho, J.; Herrera, E. Fat accumulation in the rat during early pregnancy is modulated by enhanced insulin responsiveness. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E318–E328. [Google Scholar]
- Macedo, T.C.C.; Montagna, E.; Trevisan, C.M.; Zaia, V.; de Oliveira, R.; Barbosa, C.P.; Lagana, A.S.; Bianco, B. Prevalence of preeclampsia and eclampsia in adolescent pregnancy: A systematic review and meta-analysis of 291,247 adolescents worldwide since 1969. Eur. J. Obs. Gynecol. Reprod. Biol. 2020, 248, 177–186. [Google Scholar]
- Brown, M.A.; Lindheimer, M.D.; de Swiet, M.; Van Assche, A.; Moutquin, J.M. The classification and diagnosis of the hypertensive disorders of pregnancy: Statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens. Pregnancy 2001, 20, IX–XIV. [Google Scholar]
- Yang, A.; Zhang, H.; Sun, Y.; Wang, Y.; Yang, X.; Yang, X.; Zhang, H.; Guo, W.; Zhu, G.; Tian, J.; et al. Modulation of FABP4 hypomethylation by DNMT1 and its inverse interaction with miR-148a/152 in the placenta of preeclamptic rats and HTR-8 cells. Placenta 2016, 46, 49–62. [Google Scholar]
- Mora-Palazuelos, C.; Bermúdez, M.; Aguilar-Medina, M.; Ramos-Payan, R.; Ayala-Ham, A.; Romero-Quintana, J.G. Cytokine-polymorphisms associated with Preeclampsia: A review. Medicine 2022, 101, e30870. [Google Scholar]
- Lorentzen, B.; Endresen, M.J.; Hovig, T.; Haug, E.; Henriksen, T. Sera from preeclamptic women increase the content of triglycerides and reduce the release of prostacyclin in cultured endothelial cells. Thromb. Res. 1991, 63, 363–372. [Google Scholar]
- Slattery, M.M.; Morrison, J.J. Preterm delivery. Lancet 2002, 360, 1489–1497. [Google Scholar]
- Walani, S.R. Global burden of preterm birth. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2020, 150, 31–33. [Google Scholar]
- WHO. WHO Guidelines Approved by the Guidelines Review Committee. In WHO Recommendations for Care of the Preterm or Low-Birth-Weight Infant; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Locatelli, A.; Consonni, S.; Ghidini, A. Preterm labor: Approach to decreasing complications of prematurity. Obstet. Gynecol. Clin. N. Am. 2015, 42, 255–274. [Google Scholar]
- Joung, K.E.; Cataltepe, S.U.; Michael, Z.; Christou, H.; Mantzoros, C.S. Cord Blood Adipocyte Fatty Acid-Binding Protein Levels Correlate with Gestational Age and Birth Weight in Neonates. J. Clin. Endocrinol. Metab. 2017, 102, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Siahanidou, T.; Margeli, A.; Davradou, M.; Apostolakou, F.; Papassotiriou, I.; Roma, E.; Mandyla, H.; Chrousos, G. Circulating adipocyte fatty acid binding protein levels in healthy preterm infants: Positive correlation with weight gain and total-cholesterol levels. Early Hum. Dev. 2010, 86, 197–201. [Google Scholar]
- James, E.J.; Raye, J.R.; Gresham, E.L.; Makowski, E.L.; Meschia, G.; Battaglia, F.C. Fetal oxygen consumption, carbon dioxide production, and glucose uptake in a chronic sheep preparation. Pediatrics 1972, 50, 361–371. [Google Scholar] [CrossRef]
- Mitanchez, D. Glucose regulation in preterm newborn infants. Horm. Res. 2007, 68, 265–271. [Google Scholar]
- Ron, I.; Lerner, R.K.; Rathaus, M.; Livne, R.; Ron, S.; Barhod, E.; Hemi, R.; Tirosh, A.; Strauss, T.; Ofir, K.; et al. The adipokine FABP4 is a key regulator of neonatal glucose homeostasis. JCI Insight 2021, 6, e138288. [Google Scholar] [CrossRef]
- Menon, R.K.; Sperling, M.A. Carbohydrate metabolism. Semin. Perinatol. 1988, 12, 157–162. [Google Scholar]
- Cutland, C.L.; Lackritz, E.M.; Mallett-Moore, T.; Bardají, A.; Chandrasekaran, R.; Lahariya, C.; Nisar, M.I.; Tapia, M.D.; Pathirana, J.; Kochhar, S.; et al. Low birth weight: Case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine 2017, 35 Pt A, 6492–6500. [Google Scholar]
- Duryea, E.L.; Hawkins, J.S.; McIntire, D.D.; Casey, B.M.; Leveno, K.J. A revised birth weight reference for the United States. Obs. Gynecol. 2014, 124, 16–22. [Google Scholar]
- Macrosomia: ACOG Practice Bulletin, Number 216. Obs. Gynecol. 2020, 135, e18–e35. [CrossRef]
- Schaefer-Graf, U.M.; Graf, K.; Kulbacka, I.; Kjos, S.L.; Dudenhausen, J.; Vetter, K.; Herrera, E. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 2008, 31, 1858–1863. [Google Scholar] [CrossRef]
- Hammami, M.; Walters, J.C.; Hockman, E.M.; Koo, W.W. Disproportionate alterations in body composition of large for gestational age neonates. J. Pediatr. 2001, 138, 817–821. [Google Scholar] [CrossRef]
- Briana, D.D.; Malamitsi-Puchner, A. The role of adipocytokines in fetal growth. Ann. N. Y. Acad. Sci. 2010, 1205, 82–87. [Google Scholar] [CrossRef]
- Papathanasiou, A.E.; Briana, D.D.; Gavrili, S.; Georgantzi, S.; Papathoma, E.; Marmarinos, A.; Christou, C.; Voulgaris, K.; Gourgiotis, D.; Malamitsi-Puchner, A. Cord blood fatty acid-binding protein-4 levels are upregulated at both ends of the birthweight spectrum. Acta Paediatr. 2019, 108, 2083–2088. [Google Scholar] [CrossRef]
- Garin-Shkolnik, T.; Rudich, A.; Hotamisligil, G.S.; Rubinstein, M. FABP4 attenuates PPARgamma and adipogenesis and is inversely correlated with PPARgamma in adipose tissues. Diabetes 2014, 63, 900–911. [Google Scholar] [CrossRef]
- Malamitsi-Puchner, A.; Briana, D.D.; Boutsikou, M.; Kouskouni, E.; Hassiakos, D.; Gourgiotis, D. Perinatal circulating visfatin levels in intrauterine growth restriction. Pediatrics 2007, 119, e1314–e1318. [Google Scholar] [CrossRef]
- Yang, H.; He, B.; Yallampalli, C.; Gao, H. Fetal macrosomia in a Hispanic/Latinx predominant cohort and altered expressions of genes related to placental lipid transport and metabolism. Int. J. Obes. 2020, 44, 1743–1752. [Google Scholar] [CrossRef]
- Ni, L.F.; Han, Y.; Wang, C.C.; Ye, Y.; Ding, M.M.; Zheng, T.; Wang, Y.H.; Yan, H.T.; Yang, X.J. Relationships Between Placental Lipid Activated/Transport-Related Factors and Macrosomia in Healthy Pregnancy. Reprod. Sci. 2022, 29, 904–914. [Google Scholar] [CrossRef]
- Francis, E.C.; Li, M.; Hinkle, S.N.; Chen, J.; Wu, J.; Zhu, Y.; Cao, H.; Tsai, M.Y.; Chen, L.; Zhang, C. Maternal Proinflammatory Adipokines Throughout Pregnancy and Neonatal Size and Body Composition: A Prospective Study. Curr. Dev. Nutr. 2021, 5, nzab113. [Google Scholar]
- Schrey-Petersen, S.; Baumer, S.; Lossner, U.; Stepan, H. Adipocyte Fatty Acid-Binding Protein 4 is Altered in Growth Discordant Dichorionic, but not in Monochorionic Twins. J. Endocr. Soc. 2020, 4, bvz031. [Google Scholar]
- Njiru, H.; Njogu, E.; Gitahi, M.W.; Kabiru, E. Effectiveness of public health education on the uptake of iron and folic acid supplements among pregnant women: A stepped wedge cluster randomised trial. BMJ Open 2022, 12, e063615. [Google Scholar]
- Sairoz; Prabhu, K.; Poojari, V.G.; Shetty, S.; Rao, M.; Kamath, A. Maternal Serum Zinc, Copper, Magnesium, and Iron in Spontaneous Abortions. Indian J. Clin. Biochem. 2023, 38, 128–131. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Tuo, L.; Zhai, Q.; Cui, J.; Chen, D.; Xu, D. Relationship between Maternal Vitamin D Levels and Adverse Outcomes. Nutrients 2022, 14, 4230. [Google Scholar]
- Ohseto, H.; Ishikuro, M.; Obara, T.; Murakami, K.; Onuma, T.; Noda, A.; Takahashi, I.; Matsuzaki, F.; Ueno, F.; Iwama, N.; et al. Dietary calcium intake was related to the onset of pre-eclampsia: The TMM BirThree Cohort Study. J. Clin. Hypertens. 2023, 25, 61–70. [Google Scholar]
- Jadhav, A.; Jadhav, S.; Varma, A. Pregnancy- and Lactation-Associated Osteoporotic Vertebral Fracture: A Case Report. Cureus 2022, 14, e31322. [Google Scholar]
- Ibrahim, S.A.; Basak, S. Fats in maternal and child health: Regional ISSFAL congress in India. Prostaglandins Leukot. Essent. Fat. Acids 2020, 156, 102092. [Google Scholar] [CrossRef]
- Klevebro, S.; Juul, S.E.; Wood, T.R. A More Comprehensive Approach to the Neuroprotective Potential of Long-Chain Polyunsaturated Fatty Acids in Preterm Infants Is Needed-Should We Consider Maternal Diet and the n-6:n-3 Fatty Acid Ratio? Front. Pediatr. 2019, 7, 533. [Google Scholar]
- Hay, S.M.; McArdle, H.J.; Hayes, H.E.; Stevens, V.J.; Rees, W.D. The effect of iron deficiency on the temporal changes in the expression of genes associated with fat metabolism in the pregnant rat. Physiol. Rep. 2016, 4, e12908. [Google Scholar] [CrossRef]
- Li, P.; Chang, X.; Fan, X.; Fan, C.; Tang, T.; Wang, R.; Qi, K. Dietary calcium status during maternal pregnancy and lactation affects lipid metabolism in mouse offspring. Sci. Rep. 2018, 8, 16542. [Google Scholar]
- Li, P.; Wang, Y.; Li, P.; Liu, Y.L.; Liu, W.J.; Chen, X.Y.; Tang, T.T.; Qi, K.M.; Zhang, Y. Maternal inappropriate calcium intake aggravates dietary-induced obesity in male offspring by affecting the differentiation potential of mesenchymal stem cells. World J. Stem Cells 2022, 14, 756–776. [Google Scholar]
- Li, P.; Wang, Y.; Li, P.; Chen, X.; Liu, Y.; Zha, L.; Zhang, Y.; Qi, K. Maternal vitamin D deficiency aggravates the dysbiosis of gut microbiota by affecting intestinal barrier function and inflammation in obese male offspring mice. Nutrion 2023, 105, 111837. [Google Scholar]
- Srinivas, V.; Molangiri, A.; Mallepogu, A.; Kona, S.R.; Ibrahim, A.; Duttaroy, A.K.; Basak, S. Maternal n-3 PUFA deficiency alters uterine artery remodeling and placental epigenome in the mice. J. Nutr. Biochem. 2021, 96, 108784. [Google Scholar] [CrossRef] [PubMed]
- Gimpfl, M.; Rozman, J.; Dahlhoff, M.; Kübeck, R.; Blutke, A.; Rathkolb, B.; Klingenspor, M.; Hrabě de Angelis, M.; Öner-Sieben, S.; Seibt, A.; et al. Modification of the fatty acid composition of an obesogenic diet improves the maternal and placental metabolic environment in obese pregnant mice. Biochim. Biophys. Acta. Mol. Basis Dis. 2017, 1863, 1605–1614. [Google Scholar] [PubMed]
- Ibarretxe, D.; Girona, J.; Plana, N.; Cabré, A.; Heras, M.; Ferré, R.; Merino, J.; Vallvé, J.C.; Masana, L. FABP4 plasma concentrations are determined by acquired metabolic derangements rather than genetic determinants. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 875–880. [Google Scholar] [PubMed]
- Sulsky, R.; Magnin, D.R.; Huang, Y.; Simpkins, L.; Taunk, P.; Patel, M.; Zhu, Y.; Stouch, T.R.; Bassolino-Klimas, D.; Parker, R.; et al. Potent and selective biphenyl azole inhibitors of adipocyte fatty acid binding protein (aFABP). Bioorg Med. Chem. Lett. 2007, 17, 3511–3515. [Google Scholar] [CrossRef]
- Furuhashi, M.; Tuncman, G.; Gorgun, C.Z.; Makowski, L.; Atsumi, G.; Vaillancourt, E.; Kono, K.; Babaev, V.R.; Fazio, S.; Linton, M.F.; et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature 2007, 447, 959–965. [Google Scholar] [CrossRef]
- Hertzel, A.V.; Hellberg, K.; Reynolds, J.M.; Kruse, A.C.; Juhlmann, B.E.; Smith, A.J.; Sanders, M.A.; Ohlendorf, D.H.; Suttles, J.; Bernlohr, D.A. Identification and characterization of a small molecule inhibitor of Fatty Acid binding proteins. J. Med. Chem. 2009, 52, 6024–6031. [Google Scholar]
- Szatmari, I.; Gogolak, P.; Im, J.S.; Dezso, B.; Rajnavolgyi, E.; Nagy, L. Activation of PPARgamma specifies a dendritic cell subtype capable of enhanced induction of iNKT cell expansion. Immunity 2004, 21, 95–106. [Google Scholar]
- Barak, Y.; Nelson, M.C.; Ong, E.S.; Jones, Y.Z.; Ruiz-Lozano, P.; Chien, K.R.; Koder, A.; Evans, R.M. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 1999, 4, 585–595. [Google Scholar] [CrossRef]
- Basak, S.; Duttaroy, A.K. Leptin induces tube formation in first-trimester extravillous trophoblast cells. Eur. J. Obs. Gynecol. Reprod. Biol. 2012, 164, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Look, C.; Morano, I.; Ehrhart-Bornstein, M.; Bornstein, S.R.; Lamounier-Zepter, V. BMS309403 directly suppresses cardiac contractile function. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 384, 255–263. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Wang, C.-C.; Wu, L.; Zhang, Y.; Xu, A.; Wang, Y. Pathophysiological Insight into Fatty Acid-Binding Protein-4: Multifaced Roles in Reproduction, Pregnancy, and Offspring Health. Int. J. Mol. Sci. 2023, 24, 12655. https://doi.org/10.3390/ijms241612655
Shi Y, Wang C-C, Wu L, Zhang Y, Xu A, Wang Y. Pathophysiological Insight into Fatty Acid-Binding Protein-4: Multifaced Roles in Reproduction, Pregnancy, and Offspring Health. International Journal of Molecular Sciences. 2023; 24(16):12655. https://doi.org/10.3390/ijms241612655
Chicago/Turabian StyleShi, Yue, Chi-Chiu Wang, Liqun Wu, Yunqing Zhang, Aimin Xu, and Yao Wang. 2023. "Pathophysiological Insight into Fatty Acid-Binding Protein-4: Multifaced Roles in Reproduction, Pregnancy, and Offspring Health" International Journal of Molecular Sciences 24, no. 16: 12655. https://doi.org/10.3390/ijms241612655
APA StyleShi, Y., Wang, C.-C., Wu, L., Zhang, Y., Xu, A., & Wang, Y. (2023). Pathophysiological Insight into Fatty Acid-Binding Protein-4: Multifaced Roles in Reproduction, Pregnancy, and Offspring Health. International Journal of Molecular Sciences, 24(16), 12655. https://doi.org/10.3390/ijms241612655








