Application of Multi-Perspectives in Tea Breeding and the Main Directions
Abstract
1. Introduction
2. Status of Germplasm Resources and Utilization
3. Application of Multidimensional Omics in Tea Plants Genetic Breeding
3.1. Genome
3.2. Transcriptome and Metabolome
| Genes | Function Description | References |
|---|---|---|
| Secondary metabolism | ||
| CsAlaDC | Theanine biosynthesis | [73] |
| CsTS | Theanine biosynthesis | [74] |
| CsMYB5 | Regulating anthocyanin and proanthocyanidin biosynthesis | [75] |
| Negatively regulating phenylpropane and shikimic pathway | ||
| CsMYB4a | Regulating flavonoid 3′-hydroxylase | [76] |
| Participating in flavonoid biosynthesis | ||
| CsF3′H | Biosynthesis of terpenes in MVA pathway | [77] |
| CsF3Ha, CsF3Hb | Regulating Cinnamate 4-hydroxylase biosynthesis | [78] |
| Cstps1 | Regulating of catechin production in phenylpropanoid pathway | [79] |
| Regulation of epicatechin content | ||
| CsC4H | Participating in flavan-3-ol biosynthesis | [80] |
| Catalyzing caffeine biosynthesis pathway | ||
| Regulating Jasmonic acid biosynthesis | ||
| Cs4CL | [81] | |
| CsANR | Converting volatile compounds into β-primeverosides | [82] |
| CsLAR | Formation of the volatile component indole in oolong tea | [83] |
| CsTCS | Participating in aroma quality regulation | [84] |
| Biosynthesis of linalool and nerolidol | ||
| CsAOC | Regulating salicylic acid carboxyl methyltransferase and salicylic acid to produce methyl salicylate | [85] |
| Aroma synthesis | Insect-induced defense response | |
| CsGT1 | Involved in herbivore defense | [86] |
| Participating in the response of low temperature, high temperature, osmosis and hormone stress | ||
| CsTSA, CsTSB2 | Respond to abiotic stress | [87] |
| Response to most abiotic stresses (including salinity, heavy metal toxicity, drought, high temperature and phytohormones) | ||
| CsTPS08, CsTPS10 | Participating in pest defense | [88] |
| Significantly improving the cold tolerance of plant | ||
| CsLIS/NES | Exogenous application of SPM to improve tea plants drought tolerance | [89] |
| CsSAMT | Low temperature induced during dormancy | [90] |
| Directing the gene expression of tea somatic embryo nucleus | ||
| Regulating the absorption of ammonium from soil by tea plants roots | ||
| Abiotic and biotic stresses | ||
| Cstps1 | Regulating nitrogen absorption by tea plants root system | [79] |
| CsCPI3 | Regulating tea plants dormancy | [91] |
| CsVQ | [92] | |
| CsAQP | [93] | |
| CsGPX2 | [94] | |
| CsTPS08, CsTPS10 | [88] | |
| CsPPO | [95] | |
| CsSPMS | [96] | |
| CsTUA | [97] | |
| Others | ||
| CsH1 | [98] | |
| CsAMT1.2 | [99] | |
| CsNRT2.4 | [100] | |
| CsLAX2 | [101] |
3.3. Whole Genome Resequencing
4. Molecular Markers
5. Major Directions and Measures for Tea Plants Breeding
5.1. High Productivity
5.2. High Quality
| Metabolite | Genes | Biosynthetic Pathway | References |
|---|---|---|---|
| L-theanine | TS GS ADC GOGAT GDH | Nitrogen metabolism pathway | [202,203] |
| Galloylated catechins | ECGT UGGT | Shikimate pathway | [204,205] |
| phenylpropane pathway | |||
| Caffeine | SAM TCS MXMT | S-Adenosyl-L-methionine (SAM) pathway, nicotinamide adenine dinucleotide pathway, | [84] |
| adenosine 5-monophosphate pathway, guanosine 5-monophosphate pathway | |||
| (E)-Nerolidol | LIS/NES | Terpene synthesis pathway | [89] |
| Linalool | LIS/NES | Terpene synthesis pathway | [89] |
| α-Farnesene | AFS | Terpene synthesis pathway | [206] |
| Anthocyanin | PAL C4H 4CL CHS | Phenylpropane pathway, | [207] |
| CHI F3′H F3′5′H | flavonoid pathway | ||
| F3H DFR ANS LAR | |||
| Indole | CsTSA CsTSB2 | Shikimic acid-derived pathway | [87] |
5.3. High Resistance
6. Conclusions and Prospects
6.1. Single Cell Multiomic
6.2. Pan-Genome
6.3. Metagenomics
6.4. Epigenetics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Wang, D.; Zhang, S.; Zhao, H. Global Expansion Strategy of Chinese Herbal Tea Beverage. Adv. J. Food Sci. Technol. 2015, 7, 739–745. [Google Scholar] [CrossRef]
- Drew, L.J. The growth of tea. Nature 2019, 566, S2–S4. [Google Scholar] [CrossRef]
- Koch, W. Theaflavins, Thearubigins, and Theasinensins. In Handbook of Dietary Phytochemicals; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–29. [Google Scholar]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Antoniewicz, J.; Mruk, H.; Janda, K. Health Benefits and Chemical Composition of Matcha Green Tea: A Review. Molecules 2020, 26, 85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ho, C.T.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and Biological Activities of Processed Camellia sinensis Teas: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S. Potential Bioactive Components and Health Promotional Benefits of Tea (Camellia sinensis). J. Am. Nutr. Assoc. 2022, 41, 65–93. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, R.; Carimi, F. Management and Utilization of Plant Genetic Resources for a Sustainable Agriculture. Plants 2022, 11, 2038. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Zhang, M.; Zhang, Z.; Liang, S.; Fan, L.; Yang, X.; Yuan, Y.; Pan, Y.; Zhou, G.; Liu, S.; et al. Natural allelic variation of GmST05 controlling seed size and quality in soybean. Plant. Biotechnol. J. 2022, 20, 1807–1818. [Google Scholar] [CrossRef]
- Bazhenov, M.S.; Chernook, A.G.; Goncharov, N.P.; Chikida, N.N.; Belousova, M.K.; Karlov, G.I.; Divashuk, M.G. The Allelic Diversity of the Gibberellin Signaling Pathway Genes in Aegilops tauschii Coss. Plants 2020, 9, 1696. [Google Scholar] [CrossRef]
- Zenda, T.; Liu, S.; Dong, A.; Li, J.; Wang, Y.; Liu, X.; Wang, N.; Duan, H. Omics-Facilitated Crop Improvement for Climate Resilience and Superior Nutritive Value. Front. Plant. Sci. 2021, 12, 774994. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, D.; Mathur, S.; Singh, A.; Ranjan, R. Upcoming progress of transcriptomics studies on plants: An overview. Front. Plant. Sci. 2022, 13, 1030890. [Google Scholar] [CrossRef]
- Muhammad, I.I.; Kong, S.L.; Akmar Abdullah, S.N.; Munusamy, U. RNA-seq and ChIP-seq as Complementary Approaches for Comprehension of Plant Transcriptional Regulatory Mechanism. Int. J. Mol. Sci. 2019, 21, 167. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Yang, L.; Zhang, D.; Shi, J. Plant Metabolomics: An Indispensable System Biology Tool for Plant Science. Int. J. Mol. Sci. 2016, 17, 767. [Google Scholar] [CrossRef]
- Patel, M.K.; Pandey, S.; Kumar, M.; Haque, M.I.; Pal, S.; Yadav, N.S. Plants Metabolome Study: Emerging Tools and Techniques. Plants 2021, 10, 2409. [Google Scholar] [CrossRef]
- Franchina, F.A.; Zanella, D.; Dubois, L.M.; Focant, J.F. The role of sample preparation in multidimensional gas chromatographic separations for non-targeted analysis with the focus on recent biomedical, food, and plant applications. J. Sep. Sci. 2021, 44, 188–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, X.; Xia, Y.; Ban, Q.; Cao, L.; Li, S.; Li, Y. CsCBF5 depletion impairs cold tolerance in tea plants. Plant. Sci. 2022, 325, 111463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yi, X.; Zhou, F.; Gao, X.; Wang, M.; Chen, J.; Huang, J.; Shen, C. Comprehensive transcriptome profiling of tea leaves (Camellia sinensis) in response to simulated acid rain. Sci. Hortic. 2020, 272, 109491. [Google Scholar] [CrossRef]
- Gong, A.D.; Lian, S.B.; Wu, N.N.; Zhou, Y.J.; Zhao, S.Q.; Zhang, L.M.; Cheng, L.; Yuan, H.Y. Integrated transcriptomics and metabolomics analysis of catechins, caffeine and theanine biosynthesis in tea plant (Camellia sinensis) over the course of seasons. BMC Plant. Biol. 2020, 20, 294. [Google Scholar] [CrossRef]
- Fang, K.; Xia, Z.; Li, H.; Jiang, X.; Qin, D.; Wang, Q.; Wang, Q.; Pan, C.; Li, B.; Wu, H. Genome-wide association analysis identified molecular markers associated with important tea flavor-related metabolites. Hortic. Res. 2021, 8, 42. [Google Scholar] [CrossRef]
- An, Y.; Chen, L.; Tao, L.; Liu, S.; Wei, C. QTL Mapping for Leaf Area of Tea Plants (Camellia sinensis) Based on a High-Quality Genetic Map Constructed by Whole Genome Resequencing. Front. Plant. Sci. 2021, 12, 705285. [Google Scholar] [CrossRef]
- Xia, E.; Tong, W.; Hou, Y.; An, Y.; Chen, L.; Wu, Q.; Liu, Y.; Yu, J.; Li, F.; Li, R.; et al. The Reference Genome of Tea Plant and Resequencing of 81 Diverse Accessions Provide Insights into Its Genome Evolution and Adaptation. Mol. Plant. 2020, 13, 1013–1026. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Chen, X.; Sun, W.; Li, J. Genetic diversity and structure of tea plant in Qinba area in China by three types of molecular markers. Hereditas 2018, 155, 22. [Google Scholar] [CrossRef]
- Priyanka, V.; Kumar, R.; Dhaliwal, I.; Kaushik, P. Germplasm Conservation: Instrumental in Agricultural Biodiversity—A Review. Sustainability 2021, 13, 6743. [Google Scholar] [CrossRef]
- Kottawa-Arachchi, J.D.; Gunasekare, M.T.K.; Ranatunga, M.A.B. Biochemical diversity of global tea [Camellia sinensis (L.) O. Kuntze] germplasm and its exploitation: A review. Genet. Resour. Crop. Evol. 2018, 66, 259–273. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Mondal, T.K.; Chand, P.K. Biotechnological advances in tea (Camellia sinensis [L.] O. Kuntze): A review. Plant. Cell. Rep. 2016, 35, 255–287. [Google Scholar] [CrossRef]
- Rawal, H.C.; Borchetia, S.; Bera, B.; Soundararajan, S.; Ilango, R.V.J.; Barooah, A.K.; Sharma, T.R.; Singh, N.K.; Mondal, T.K. Comparative analysis of chloroplast genomes indicated different origin for Indian tea (Camellia assamica cv TV1) as compared to Chinese tea. Sci. Rep. 2021, 11, 110. [Google Scholar] [CrossRef]
- Li, J.; Xiao, Y.; Zhou, X.; Liao, Y.; Wu, S.; Chen, J.; Qian, J.; Yan, Y.; Tang, J.; Zeng, L. Characterizing the cultivar-specific mechanisms underlying the accumulation of quality-related metabolites in specific Chinese tea (Camellia sinensis) germplasms to diversify tea products. Food Res. Int. 2022, 161, 111824. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Ban, Q.; Zhu, X.; Jiang, C.; Wei, C.; Bennetzen, J.L. Comparative transcriptomic analysis reveals gene expression associated with cold adaptation in the tea plant Camellia sinensis. BMC Genom. 2019, 20, 624. [Google Scholar] [CrossRef]
- Hao, X.; Tang, H.; Wang, B.; Yue, C.; Wang, L.; Zeng, J.; Yang, Y.; Wang, X. Integrative transcriptional and metabolic analyses provide insights into cold spell response mechanisms in young shoots of the tea plant. Tree Physiol. 2018, 38, 1655–1671. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, F.; Chen, B.; Su, E.; Chen, Y.; Cao, F. Composition, bioactive substances, extraction technologies and the influences on characteristics of Camellia oleifera oil: A review. Food Res. Int. 2022, 156, 111159. [Google Scholar] [CrossRef]
- Dong, L.; Yin, X.; Huang, B.; Li, T.; Huang, J.J.; Wen, Q. The complete chloroplast genome of Camellia semiserrata Chi. (Theaceae), an excellent woody edible oil and landscaping species in South China. Mitochondrial DNA B Resour. 2021, 6, 3013–3015. [Google Scholar] [CrossRef]
- Liu, L.Q.; Gu, Z.J. Genomic in situ hybridization identifies genome donors of Camellia reticulata (Theaceae). Plant. Sci. 2011, 180, 554–559. [Google Scholar] [CrossRef]
- Fan, M.; Li, X.; Zhang, Y.; Yang, M.; Wu, S.; Yin, H.; Liu, W.; Fan, Z.; Li, J. Novel insight into anthocyanin metabolism and molecular characterization of its key regulators in Camellia sasanqua. Plant. Mol. Biol. 2023, 111, 249–262. [Google Scholar] [CrossRef]
- Widhianata, H.; Basunanda, P.; Supriyadi, S.; Taryono, T. Transcriptional Comparison of New Hybrid Progenies and Clone-Cultivars of Tea (Camellia sinensis L.) Associated to Catechins Content. Plants 2022, 11, 1972. [Google Scholar] [CrossRef]
- Meegahakumbura, M.K.; Wambulwa, M.C.; Li, M.M.; Thapa, K.K.; Sun, Y.S.; Moller, M.; Xu, J.C.; Yang, J.B.; Liu, J.; Liu, B.Y.; et al. Domestication Origin and Breeding History of the Tea Plant (Camellia sinensis) in China and India Based on Nuclear Microsatellites and cpDNA Sequence Data. Front. Plant. Sci. 2017, 8, 2270. [Google Scholar] [CrossRef]
- Wang, P.; Yu, J.; Jin, S.; Chen, S.; Yue, C.; Wang, W.; Gao, S.; Cao, H.; Zheng, Y.; Gu, M.; et al. Genetic basis of high aroma and stress tolerance in the oolong tea cultivar genome. Hortic. Res. 2021, 8, 107. [Google Scholar] [CrossRef]
- Wang, P.; Gu, M.; Yu, X.; Shao, S.; Du, J.; Wang, Y.; Wang, F.; Chen, S.; Liao, Z.; Ye, N.; et al. Allele-specific expression and chromatin accessibility contribute to heterosis in tea plants (Camellia sinensis). Plant. J. 2022, 112, 1194–1211. [Google Scholar] [CrossRef]
- Singh, S.; Sud, R.K.; Gulati, A.; Joshi, R.; Yadav, A.K.; Sharma, R.K. Germplasm appraisal of western Himalayan tea: A breeding strategy for yield and quality improvement. Genet. Resour. Crop. Evol. 2012, 60, 1501–1513. [Google Scholar] [CrossRef]
- Pang, D.; Liu, Y.; Sun, Y.; Tian, Y.; Chen, L. Menghai Huangye, a novel albino tea germplasm with high theanine content and a high catechin index. Plant. Sci. 2021, 311, 110997. [Google Scholar] [CrossRef]
- Palmgren, M.G.; Edenbrandt, A.K.; Vedel, S.E.; Andersen, M.M.; Landes, X.; Osterberg, J.T.; Falhof, J.; Olsen, L.I.; Christensen, S.B.; Sandoe, P.; et al. Are we ready for back-to-nature crop breeding? Trends Plant. Sci. 2015, 20, 155–164. [Google Scholar] [CrossRef]
- Gallusci, P.; Dai, Z.; Genard, M.; Gauffretau, A.; Leblanc-Fournier, N.; Richard-Molard, C.; Vile, D.; Brunel-Muguet, S. Epigenetics for Plant Improvement: Current Knowledge and Modeling Avenues. Trends Plant. Sci. 2017, 22, 610–623. [Google Scholar] [CrossRef]
- Goodwin, S.; McPherson, J.D.; McCombie, W.R. Coming of age: Ten years of next-generation sequencing technologies. Nat. Rev. Genet. 2016, 17, 333–351. [Google Scholar] [CrossRef]
- Initiative, T.A.G. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar] [CrossRef]
- Sun, Y.; Shang, L.; Zhu, Q.H.; Fan, L.; Guo, L. Twenty years of plant genome sequencing: Achievements and challenges. Trends Plant. Sci. 2022, 27, 391–401. [Google Scholar] [CrossRef]
- Hu, L.; Xu, Z.; Fan, R.; Wang, G.; Wang, F.; Qin, X.; Yan, L.; Ji, X.; Meng, M.; Sim, S.; et al. The complex genome and adaptive evolution of polyploid Chinese pepper (Zanthoxylum armatum and Zanthoxylum bungeanum). Plant. Biotechnol. J. 2023, 21, 78–96. [Google Scholar] [CrossRef]
- Tang, D.; Jia, Y.; Zhang, J.; Li, H.; Cheng, L.; Wang, P.; Bao, Z.; Liu, Z.; Feng, S.; Zhu, X.; et al. Genome evolution and diversity of wild and cultivated potatoes. Nature 2022, 606, 535–541. [Google Scholar] [CrossRef]
- Xia, E.H.; Zhang, H.B.; Sheng, J.; Li, K.; Zhang, Q.J.; Kim, C.; Zhang, Y.; Liu, Y.; Zhu, T.; Li, W.; et al. The Tea Tree Genome Provides Insights into Tea Flavor and Independent Evolution of Caffeine Biosynthesis. Mol. Plant. 2017, 10, 866–877. [Google Scholar] [CrossRef]
- Wei, C.; Yang, H.; Wang, S.; Zhao, J.; Liu, C.; Gao, L.; Xia, E.; Lu, Y.; Tai, Y.; She, G.; et al. Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality. Proc. Natl. Acad. Sci. USA 2018, 115, E4151–E4158. [Google Scholar] [CrossRef]
- Wang, X.; Feng, H.; Chang, Y.; Ma, C.; Wang, L.; Hao, X.; Li, A.; Cheng, H.; Wang, L.; Cui, P.; et al. Population sequencing enhances understanding of tea plant evolution. Nat. Commun. 2020, 11, 4447. [Google Scholar] [CrossRef]
- Chen, J.D.; Zheng, C.; Ma, J.Q.; Jiang, C.K.; Ercisli, S.; Yao, M.Z.; Chen, L. The chromosome-scale genome reveals the evolution and diversification after the recent tetraploidization event in tea plant. Hortic. Res. 2020, 7, 63. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Qiu, H.; Guo, Y.; Wan, H.; Zhang, X.; Scossa, F.; Alseekh, S.; Zhang, Q.; Wang, P.; et al. Genome assembly of wild tea tree DASZ reveals pedigree and selection history of tea varieties. Nat. Commun. 2020, 11, 3719. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Li, W.; Li, K.; Nan, H.; Shi, C.; Zhang, Y.; Dai, Z.Y.; Lin, Y.L.; Yang, X.L.; Tong, Y.; et al. The Chromosome-Level Reference Genome of Tea Tree Unveils Recent Bursts of Non-autonomous LTR Retrotransposons in Driving Genome Size Evolution. Mol. Plant. 2020, 13, 935–938. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, S.; Shi, L.; Gong, D.; Zhang, S.; Zhao, Q.; Zhan, D.; Vasseur, L.; Wang, Y.; Yu, J.; et al. Haplotype-resolved genome assembly provides insights into evolutionary history of the tea plant Camellia sinensis. Nat. Genet. 2021, 53, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, B.; Wen, D.; Liu, R.; Yao, X.; Chen, Z.; Mu, R.; Pei, H.; Liu, M.; Song, B.; et al. Chromosome-scale genome assembly of Camellia sinensis combined with multi-omics provides insights into its responses to infestation with green leafhoppers. Front. Plant. Sci. 2022, 13, 1004387. [Google Scholar] [CrossRef]
- Lin, P.; Wang, K.; Wang, Y.; Hu, Z.; Yan, C.; Huang, H.; Ma, X.; Cao, Y.; Long, W.; Liu, W.; et al. The genome of oil-Camellia and population genomics analysis provide insights into seed oil domestication. Genome Biol. 2022, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Xiang, P.; Zhu, Q.; Zhang, L.; Xu, P.; Liu, L.; Li, Y.; Cheng, B.; Wang, X.; Liu, J.; Shi, Y.; et al. Integrative analyses of transcriptome and metabolome reveal comprehensive mechanisms of Epigallocatechin-3-gallate (EGCG) biosynthesis in response to ecological factors in tea plant (Camellia sinensis). Food Res. Int. 2023, 166, 112591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, J.; Zhou, Q.; Li, F.; Shen, Y.; Ye, Z.; Tang, D.; Chi, N.; Li, L.; Ma, S.; et al. Metabolite Profiling and Transcriptome Analysis Revealed the Conserved Transcriptional Regulation Mechanism of Caffeine Biosynthesis in Tea and Coffee Plants. J. Agric. Food Chem. 2022, 70, 3239–3251. [Google Scholar] [CrossRef]
- Song, S.; Tao, Y.; Gao, L.; Liang, H.; Tang, D.; Lin, J.; Wang, Y.; Gmitter, F.G., Jr.; Li, C. An Integrated Metabolome and Transcriptome Analysis Reveal the Regulation Mechanisms of Flavonoid Biosynthesis in a Purple Tea Plant Cultivar. Front. Plant. Sci. 2022, 13, 880227. [Google Scholar] [CrossRef]
- Cai, J.; Lv, L.; Zeng, X.; Zhang, F.; Chen, Y.; Tian, W.; Li, J.; Li, X.; Li, Y. Integrative Analysis of Metabolomics and Transcriptomics Reveals Molecular Mechanisms of Anthocyanin Metabolism in the Zikui Tea Plant (Camellia sinensis cv. Zikui). Int. J. Mol. Sci. 2022, 23, 4780. [Google Scholar] [CrossRef]
- Mei, Y.; Xie, H.; Liu, S.; Zhu, J.; Zhao, S.; Wei, C. Metabolites and Transcriptional Profiling Analysis Reveal the Molecular Mechanisms of the Anthocyanin Metabolism in the “Zijuan” Tea Plant (Camellia sinensis var. assamica). J. Agric. Food Chem. 2021, 69, 414–427. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, P.; Chen, X.; Yue, C.; Guo, Y.; Yang, J.; Sun, Y.; Ye, N. Integrated transcriptomics and metabolomics provide novel insight into changes in specialized metabolites in an albino tea cultivar (Camellia sinensis (L.) O. Kuntz). Plant. Physiol. Biochem. 2021, 160, 27–36. [Google Scholar] [CrossRef]
- Wu, H.; Wu, Z.; Wang, Y.; Ding, J.; Zheng, Y.; Tang, H.; Yang, L. Transcriptome and Metabolome Analysis Revealed the Freezing Resistance Mechanism in 60-Year-Old Overwintering Camellia sinensis. Biology 2021, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ban, Q.; Mao, J.; Lin, M.; Zhu, X.; Xia, Y.; Cao, X.; Zhang, X.; Li, Y. Integrated Metabolomic and Transcriptomic Analysis Reveals That Amino Acid Biosynthesis May Determine Differences in Cold-Tolerant and Cold-Sensitive Tea Cultivars. Int. J. Mol. Sci. 2023, 24, 1907. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, N.; Gao, T.; Jin, J.; Jing, T.; Wang, J.; Wu, Y.; Wan, X.; Schwab, W.; Song, C. Sesquiterpene glucosylation mediated by glucosyltransferase UGT91Q2 is involved in the modulation of cold stress tolerance in tea plants. New. Phytol. 2020, 226, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Shang, X.; Liu, G.; Zou, Z.; Zhu, X.; Ma, Y.; Li, F.; Fang, W. The effects of tea plants-soybean intercropping on the secondary metabolites of tea plants by metabolomics analysis. BMC Plant. Biol. 2021, 21, 482. [Google Scholar] [CrossRef]
- Ni, T.; Xu, S.; Wei, Y.; Li, T.; Jin, G.; Deng, W.-W.; Ning, J. Understanding the promotion of withering treatment on quality of postharvest tea leaves using UHPLC-orbitrap-MS metabolomics integrated with TMT-Based proteomics. Lwt 2021, 147, 111614. [Google Scholar] [CrossRef]
- Xue, J.; Liu, P.; Guo, G.; Wang, W.; Zhang, J.; Wang, W.; Le, T.; Yin, J.; Ni, D.; Jiang, H. Profiling of dynamic changes in non-volatile metabolites of shaken black tea during the manufacturing process using targeted and non-targeted metabolomics analysis. Lwt 2022, 156, 113010. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Wu, J.; Wen, J.; Yu, Y.; An, K.; Zou, B. GC-IMS and olfactometry analysis on the tea aroma of Yingde black teas harvested in different seasons. Food Res. Int. 2021, 150, 110784. [Google Scholar] [CrossRef]
- Shen, J.; Wang, H.; Sun, L.; Fan, K.; Zhang, X.; Huang, Q.; Ding, S.; Wang, Y.; Ding, Z. Metabolic Variations among Three New Tea Varieties Cultivated in Shandong, China. Foods 2023, 12, 1299. [Google Scholar] [CrossRef]
- Wei, K.; Liu, M.; Shi, Y.; Zhang, H.; Ruan, J.; Zhang, Q.; Cao, M. Metabolomics Reveal That the High Application of Phosphorus and Potassium in Tea Plantation Inhibited Amino-Acid Accumulation but Promoted Metabolism of Flavonoid. Agronomy 2022, 12, 1086. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, B.; Liu, K.; Li, R.; Chen, L.; Liu, Z.; Xiang, G.; An, J.; Luo, H.; Wu, J.; et al. Multi-omics analysis of the metabolism of phenolic compounds in tea leaves by Aspergillus luchuensis during fermentation of pu-erh tea. Food Res. Int. 2022, 162, 111981. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Wei, K.; Wang, L.; Zhang, F.; Ruan, L.; Li, H.; Wu, L.; Cheng, H. Identification of a Novel Gene Encoding the Specialized Alanine Decarboxylase in Tea (Camellia sinensis) Plants. Molecules 2019, 24, 540. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Ling, C.; Wang, H.; Yang, L.; She, G.; Wang, C.; Yu, S.; Chen, W.; Liu, C.; Wan, X. Comparative Transcriptomic Analysis Reveals Regulatory Mechanisms of Theanine Synthesis in Tea (Camellia sinensis) and Oil Tea (Camellia oleifera) Plants. J. Agric. Food Chem. 2019, 67, 10235–10244. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Huang, K.; Zheng, G.; Hou, H.; Wang, P.; Jiang, H.; Zhao, X.; Li, M.; Zhang, S.; Liu, Y.; et al. CsMYB5a and CsMYB5e from Camellia sinensis differentially regulate anthocyanin and proanthocyanidin biosynthesis. Plant. Sci. 2018, 270, 209–220. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Guo, L.; Gong, N.; Pang, Y.; Jiang, W.; Liu, Y.; Jiang, X.; Zhao, L.; Wang, Y.; et al. Functional Characterization of Tea (Camellia sinensis) MYB4a Transcription Factor Using an Integrative Approach. Front. Plant. Sci. 2017, 8, 943. [Google Scholar] [CrossRef]
- Zhou, T.S.; Zhou, R.; Yu, Y.B.; Xiao, Y.; Li, D.H.; Xiao, B.; Yu, O.; Yang, Y.J. Cloning and Characterization of a Flavonoid 3’-Hydroxylase Gene from Tea Plant (Camellia sinensis). Int. J. Mol. Sci. 2016, 17, 261. [Google Scholar] [CrossRef]
- Han, Y.; Huang, K.; Liu, Y.; Jiao, T.; Ma, G.; Qian, Y.; Wang, P.; Dai, X.; Gao, L.; Xia, T. Functional Analysis of Two Flavanone-3-Hydroxylase Genes from Camellia sinensis: A Critical Role in Flavonoid Accumulation. Genes 2017, 8, 300. [Google Scholar] [CrossRef]
- Fu, J.-y. Molecular cloning and expression analysis of a putative sesquiterpene synthase gene from tea plant (Camellia sinensis). Acta Physiol. Plant. 2012, 35, 289–293. [Google Scholar] [CrossRef]
- Xia, J.; Liu, Y.; Yao, S.; Li, M.; Zhu, M.; Huang, K.; Gao, L.; Xia, T. Characterization and Expression Profiling of Camellia sinensis Cinnamate 4-hydroxylase Genes in Phenylpropanoid Pathways. Genes 2017, 8, 193. [Google Scholar] [CrossRef]
- Rani, A.; Singh, K.; Sood, P.; Kumar, S.; Ahuja, P.S. p-Coumarate:CoA ligase as a key gene in the yield of catechins in tea [Camellia sinensis (L.) O. Kuntze]. Funct. Integr. Genom. 2009, 9, 271–275. [Google Scholar] [CrossRef]
- Singh, K.; Rani, A.; Paul, A.; Dutt, S.; Joshi, R.; Gulati, A.; Ahuja, P.S.; Kumar, S. Differential display mediated cloning of anthocyanidin reductase gene from tea (Camellia sinensis) and its relationship with the concentration of epicatechins. Tree Physiol. 2009, 29, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Abeysinghe, I.S.; He, J.; He, X.; Huhman, D.; Mewan, K.M.; Sumner, L.W.; Yun, J.; Dixon, R.A. Functional characterization of proanthocyanidin pathway enzymes from tea and their application for metabolic engineering. Plant. Physiol. 2013, 161, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Mizuno, K.; Crozier, A.; Fujimura, T.; Ashihara, H. Plant biotechnology: Caffeine synthase gene from tea leaves. Nature 2000, 406, 956–957. [Google Scholar] [CrossRef]
- Wang, M.-x.; Ma, Q.-p.; Han, B.-y.; Li, X.-h. Molecular cloning and expression of a jasmonate biosynthetic gene allene oxide cyclase from Camellia sinensis. Can. J. Plant. Sci. 2016, 96, 109–116. [Google Scholar] [CrossRef]
- Ohgami, S.; Ono, E.; Horikawa, M.; Murata, J.; Totsuka, K.; Toyonaga, H.; Ohba, Y.; Dohra, H.; Asai, T.; Matsui, K.; et al. Volatile Glycosylation in Tea Plants: Sequential Glycosylations for the Biosynthesis of Aroma beta-Primeverosides Are Catalyzed by Two Camellia sinensis Glycosyltransferases. Plant. Physiol. 2015, 168, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhou, Y.; Gui, J.; Fu, X.; Mei, X.; Zhen, Y.; Ye, T.; Du, B.; Dong, F.; Watanabe, N.; et al. Formation of Volatile Tea Constituent Indole During the Oolong Tea Manufacturing Process. J. Agric. Food Chem. 2016, 64, 5011–5019. [Google Scholar] [CrossRef]
- Qiao, D.; Tang, M.; Jin, L.; Mi, X.; Chen, H.; Zhu, J.; Liu, S.; Wei, C. A monoterpene synthase gene cluster of tea plant (Camellia sinensis) potentially involved in constitutive and herbivore-induced terpene formation. Plant. Physiol. Biochem. 2022, 184, 1–13. [Google Scholar] [CrossRef]
- Liu, G.F.; Liu, J.J.; He, Z.R.; Wang, F.M.; Yang, H.; Yan, Y.F.; Gao, M.J.; Gruber, M.Y.; Wan, X.C.; Wei, S. Implementation of CsLIS/NES in linalool biosynthesis involves transcript splicing regulation in Camellia sinensis. Plant. Cell. Env. 2018, 41, 176–186. [Google Scholar] [CrossRef]
- Deng, W.W.; Wang, R.; Yang, T.; Jiang, L.; Zhang, Z.Z. Functional Characterization of Salicylic Acid Carboxyl Methyltransferase from Camellia sinensis, Providing the Aroma Compound of Methyl Salicylate during the Withering Process of White Tea. J. Agric. Food Chem. 2017, 65, 11036–11045. [Google Scholar] [CrossRef]
- Zhang, X.; Ran, W.; Liu, F.; Li, X.; Hao, W.; Sun, X. Cloning, expression and enzymatic characterization of a cystatin gene involved in herbivore defense in tea plant (Camellia sinensis). Chemoecology 2020, 30, 233–244. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Y.-X.; Mao, J.; Hao, W.-J.; Liu, Y.-F.; Ni, D.-J.; Chen, L. Cloning and expression patterns of VQ-motif-containing proteins under abiotic stress in tea plant. Plant. Growth Regul. 2019, 87, 277–286. [Google Scholar] [CrossRef]
- Yue, C.; Cao, H.; Wang, L.; Zhou, Y.; Hao, X.; Zeng, J.; Wang, X.; Yang, Y. Molecular cloning and expression analysis of tea plant aquaporin (AQP) gene family. Plant. Physiol. Biochem. 2014, 83, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Fu, J. Cloning of a new glutathione peroxidase gene from tea plant (Camellia sinensis) and expression analysis under biotic and abiotic stresses. Bot. Stud. 2014, 55, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Wan, C.; Li, Y.F.; Zhao, D. Cloning and Functional Identification of Yunwu Tribute Tea CsPPO Gene. Russ. J. Plant. Physiol. 2023, 70, 109. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Q.; Hu, J.; Wang, M.; Li, X. Molecular Cloning and Characterization of Spermine Synthesis Gene Associated with Cold Tolerance in Tea Plant (Camellia sinensis). Appl. Biochem. Biotechnol. 2015, 177, 1055–1068. [Google Scholar] [CrossRef]
- Paul, A.; Lal, L.; Ahuja, P.S.; Kumar, S. Alpha-tubulin (CsTUA) up-regulated during winter dormancy is a low temperature inducible gene in tea [Camellia sinensis (L.) O. Kuntze]. Mol. Biol. Rep. 2012, 39, 3485–3490. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, Y.; Zhou, L.; Wang, W.; Li, X. Isolation and characterization of Histone1 gene and its promoter from tea plant (Camellia sinensis). Mol. Biol. Rep. 2013, 40, 3641–3648. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Wang, L.; Bai, P.; Ruan, L.; Zhang, C.; Wei, K.; Cheng, H. Molecular cloning and expression analysis of ammonium transporters in tea plants (Camellia sinensis (L.) O. Kuntze) under different nitrogen treatments. Gene 2018, 658, 136–145. [Google Scholar] [CrossRef]
- Zhang, F.; He, W.; Yuan, Q.; Wei, K.; Ruan, L.; Wang, L.; Cheng, H. Transcriptome analysis identifies CsNRT genes involved in nitrogen uptake in tea plants, with a major role of CsNRT2.4. Plant. Physiol. Biochem. 2021, 167, 970–979. [Google Scholar] [CrossRef]
- Hao, X.; Tang, H.; Wang, B.; Wang, L.; Cao, H.; Wang, Y.; Zeng, J.; Fang, S.; Chu, J.; Yang, Y.; et al. Gene Characterization and Expression Analysis Reveal the Importance of Auxin Signaling in Bud Dormancy Regulation in Tea Plant. J. Plant. Growth Regul. 2018, 38, 225–240. [Google Scholar] [CrossRef]
- Li, P.; Fu, J.; Xu, Y.; Shen, Y.; Zhang, Y.; Ye, Z.; Tong, W.; Zeng, X.; Yang, J.; Tang, D.; et al. CsMYB1 integrates the regulation of trichome development and catechins biosynthesis in tea plant domestication. New. Phytol. 2022, 234, 902–917. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen, H.; Wang, X.; Zhao, Y.; Yao, X.; Xiong, B.; Deng, Y.; Zhao, D. Genome-level diversification of eight ancient tea populations in the Guizhou and Yunnan regions identifies candidate genes for core agronomic traits. Hortic. Res. 2021, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Mi, X.; Zhao, S.; Guo, R.; Xia, X.; Liu, S.; Wei, C. Revealing Distinctions in Genetic Diversity and Adaptive Evolution Between Two Varieties of Camellia sinensis by Whole-Genome Resequencing. Front. Plant. Sci. 2020, 11, 603819. [Google Scholar] [CrossRef]
- Kage, U.; Kumar, A.; Dhokane, D.; Karre, S.; Kushalappa, A.C. Functional molecular markers for crop improvement. Crit. Rev. Biotechnol. 2016, 36, 917–930. [Google Scholar] [CrossRef]
- Ramesh, P.; Mallikarjuna, G.; Sameena, S.; Kumar, A.; Gurulakshmi, K.; Reddy, B.V.; Reddy, P.C.O.; Sekhar, A.C. Advancements in molecular marker technologies and their applications in diversity studies. J. Biosci. 2020, 45, 123. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, A.; Rajpal, V.R.; Singh, A.; Babbar, S.; Raina, S.N. Evaluation of genetic diversity and population structure in elite south Indian tea [Camellia sinensis (L.) Kuntze] using RAPD and ISSR markers. Genet. Resour. Crop. Evol. 2022, 70, 381–398. [Google Scholar] [CrossRef]
- Dong, B.; Deng, Z.; Liu, W.; Rehman, F.; Yang, T.-J.; Huang, Y.; Gong, H. Development of expressed sequence tag simple sequence repeat (EST-SSR) markers and genetic resource analysis of tea oil plants (Camellia spp.). Conserv. Genet. Resour. 2022, 14, 41–45. [Google Scholar] [CrossRef]
- Fan, K.; Zhang, J.; Wang, M.; Qian, W.; Sun, L.; Shen, J.; Ding, Z.; Wang, Y. Development and Application of SNP-KASP Markers Based on Genes Related to Nitrogen Uptake, Assimilation and Allocation in Tea Plant (Camellia sinensis L.). Agronomy 2022, 12, 2534. [Google Scholar] [CrossRef]
- Chang, Y.; Oh, E.U.; Lee, M.S.; Kim, H.B.; Moon, D.-G.; Song, K.J. Construction of a genetic linkage map based on RAPD, AFLP, and SSR markers for tea plant (Camellia sinensis). Euphytica 2017, 213, 190. [Google Scholar] [CrossRef]
- Rohilla, M.; Mazumder, A.; Saha, D.; Pal, T.; Begam, S.; Mondal, T.K. Genome-wide identification and development of miniature inverted-repeat transposable elements and intron length polymorphic markers in tea plant (Camellia sinensis). Sci. Rep. 2022, 12, 16233. [Google Scholar] [CrossRef]
- Ujihara, T.; Taniguchi, F.; Tanaka, J.; Hayashi, N. Development of Expressed Sequence Tag (EST)-based Cleaved Amplified Polymorphic Sequence (CAPS) markers of tea plant and their application to cultivar identification. J. Agric. Food Chem. 2011, 59, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Qi, H.; Li, Y.; Wu, Y.; Wang, Y.; Chen, J.; Yu, J. Assessment of the Genetic Relationship and Population Structure in Oil-Tea Camellia Species Using Simple Sequence Repeat (SSR) Markers. Genes 2022, 13, 2162. [Google Scholar] [CrossRef] [PubMed]
- Parmar, R.; Seth, R.; Sharma, R.K. Genome-wide identification and characterization of functionally relevant microsatellite markers from transcription factor genes of Tea (Camellia sinensis (L.) O. Kuntze). Sci. Rep. 2022, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; An, Y.; Li, F.; Li, S.; Liu, L.; Zhou, Q.; Zhao, S.; Wei, C. Genome-wide identification of simple sequence repeats and development of polymorphic SSR markers for genetic studies in tea plant (Camellia sinensis). Mol. Breed. 2018, 38, 59. [Google Scholar] [CrossRef]
- Wang, L.; Xun, H.; Aktar, S.; Zhang, R.; Wu, L.; Ni, D.; Wei, K.; Wang, L. Development of SNP Markers for Original Analysis and Germplasm Identification in Camellia sinensis. Plants 2022, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yu, W.; Zhou, L.; Fan, X.; Wang, F.; Wang, P.; Fang, W.; Cai, C.; Ye, N. Genetic diversity of oolong tea (Camellia sinensis) germplasms based on the nanofluidic array of single-nucleotide polymorphism (SNP) markers. Tree Genet. Genomes 2019, 16, 3. [Google Scholar] [CrossRef]
- Xu, L.Y.; Wang, L.Y.; Wei, K.; Tan, L.Q.; Su, J.J.; Cheng, H. High-density SNP linkage map construction and QTL mapping for flavonoid-related traits in a tea plant (Camellia sinensis) using 2b-RAD sequencing. BMC Genom. 2018, 19, 955. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Niu, S.; Deng, X.; Song, Q.; He, L.; Bai, D.; He, Y. Genome-wide association study of leaf-related traits in tea plant in Guizhou based on genotyping-by-sequencing. BMC Plant. Biol. 2023, 23, 196. [Google Scholar] [CrossRef] [PubMed]
- Janni, M.; Gulli, M.; Maestri, E.; Marmiroli, M.; Valliyodan, B.; Nguyen, H.T.; Marmiroli, N. Molecular and genetic bases of heat stress responses in crop plants and breeding for increased resilience and productivity. J. Exp. Bot. 2020, 71, 3780–3802. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, P.; Yamaji, N.; Ma, J.F. Plant Nutrition for Human Nutrition: Hints from Rice Research and Future Perspectives. Mol. Plant. 2020, 13, 825–835. [Google Scholar] [CrossRef]
- Villalobos-López, M.Á.; Arroyo-Becerra, A.; Quintero-Jiménez, A.; Iturriaga, G. Biotechnological Advances to Improve Abiotic Stress Tolerance in Crops. Int. J. Mol. Sci. 2022, 23, 12053. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Deng, Y.; Ning, Y.; He, Z.; Wang, G.L. Exploiting Broad-Spectrum Disease Resistance in Crops: From Molecular Dissection to Breeding. Annu. Rev. Plant. Biol. 2020, 71, 575–603. [Google Scholar] [CrossRef]
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Leal-Bertioli, S.C.M.; Tester, M.; Gao, C.; Godwin, I.D.; Hayes, B.J.; Wulff, B.B.H. Breeding crops to feed 10 billion. Nat. Biotechnol. 2019, 37, 744–754. [Google Scholar] [CrossRef]
- Steinwand, M.A.; Ronald, P.C. Crop biotechnology and the future of food. Nat. Food 2020, 1, 273–283. [Google Scholar] [CrossRef]
- Ma, J.F.; Ryan, P.R.; Delhaize, E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant. Sci. 2001, 6, 273–278. [Google Scholar] [CrossRef]
- Yan, P.; Wu, L.; Wang, D.; Fu, J.; Shen, C.; Li, X.; Zhang, L.; Zhang, L.; Fan, L.; Wenyan, H. Soil acidification in Chinese tea plantations. Sci. Total. Env. 2020, 715, 136963. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Bahrami Rad, S.; Barceló, J.; Poschenrieder, C. Mechanisms of aluminum-induced growth stimulation in tea (Camellia sinensis). J. Plant. Nutr. Soil. Sci. 2013, 176, 616–625. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Ding, Z.; Song, L.; Li, Y.; Ma, D.; Wang, Y.; Shen, J.; Jia, S.; Sun, H.; et al. Aluminum induced metabolic responses in two tea cultivars. Plant. Physiol. Biochem. 2016, 101, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cui, S.; Ma, J.; Wu, D.; Ye, Z.; Liu, D. Effects of Shellfish and Organic Fertilizer Amendments on Soil Nutrients and Tea Yield and Quality. Toxics 2023, 11, 262. [Google Scholar] [CrossRef]
- Ding, Z.J.; Shi, Y.Z.; Li, G.X.; Harberd, N.P.; Zheng, S.J. Tease out the future: How tea research might enable crop breeding for acid soil tolerance. Plant. Commun. 2021, 2, 100182. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X. Hormonal regulation of health-promoting compounds in tea (Camellia sinensis L.). Plant. Physiol. Biochem. 2022, 185, 390–400. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, M.; Yang, Y.; Xuan, W.; Zou, Z.; Arkorful, E.; Chen, Y.; Ma, Q.; Jeyaraj, A.; Chen, X.; et al. A novel insight into nitrogen and auxin signaling in lateral root formation in tea plant [Camellia sinensis (L.) O. Kuntze]. BMC Plant. Biol. 2020, 20, 232. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.W.; Chen, G.H.; Chen, C.T.; Tzen, J.T.C.; Yang, C.Y. Ethylene signaling modulates contents of catechin and ability of antioxidant in Camellia sinensis. Bot. Stud. 2018, 59, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, B.; Zhang, X.; Wang, C.; Zhang, Z.; Sun, P. Exogenous application of ethephon regulates flower abscission, shoot growth, and secondary metabolites in Camellia sinensis. Sci. Hortic. 2022, 304, 111333. [Google Scholar] [CrossRef]
- Ding, P.; Ding, Y. Stories of Salicylic Acid: A Plant Defense Hormone. Trends Plant. Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, Y.; Li, K.; Li, C.; Liu, B.; Zhao, L.; Zhang, X.; Qu, F.; Gao, L.; Xia, T.; et al. Transcriptional Analysis of Tea Plants (Camellia sinensis) in Response to Salicylic Acid Treatment. J. Agric. Food Chem. 2023, 71, 2377–2389. [Google Scholar] [CrossRef]
- Shi, Y.L.; Sheng, Y.Y.; Cai, Z.Y.; Yang, R.; Li, Q.S.; Li, X.M.; Li, D.; Guo, X.Y.; Lu, J.L.; Ye, J.H.; et al. Involvement of Salicylic Acid in Anthracnose Infection in Tea Plants Revealed by Transcriptome Profiling. Int. J. Mol. Sci. 2019, 20, 2439. [Google Scholar] [CrossRef]
- Li, J.; Xiao, Y.; Fan, Q.; Liao, Y.; Wang, X.; Fu, X.; Gu, D.; Chen, Y.; Zhou, B.; Tang, J.; et al. Transformation of Salicylic Acid and Its Distribution in Tea Plants (Camellia sinensis) at the Tissue and Subcellular Levels. Plants 2021, 10, 282. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Ye, M.; Li, X.W.; Lin, S.B.; Sun, X.L. The Jasmonic Acid Pathway Positively Regulates the Polyphenol Oxidase-Based Defense against Tea Geometrid Caterpillars in the Tea Plant (Camellia sinensis). J. Chem. Ecol. 2020, 46, 308–316. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Xia, X.; Li, X.; Shi, K.; Yu, J.; Zhou, Y. Role of brassinosteroid in plant adaptation to abiotic stresses and its interplay with other hormones. Curr. Protein Pept. Sci. 2015, 16, 462–473. [Google Scholar] [CrossRef]
- Jin, Q.; Wang, Z.; Chen, Y.; Luo, Y.; Tian, N.; Liu, Z.; Huang, J.; Liu, S. Transcriptomics analysis reveals the signal transduction mechanism of brassinolides in tea leaves and its regulation on the growth and development of Camellia sinensis. BMC Genom. 2022, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, M.H.; Deng, W.W.; Ahammed, G.J.; Wei, J.P.; Yan, P.; Zhang, L.P.; Fu, J.Y.; Han, W.Y. Exogenous melatonin improves tea quality under moderate high temperatures by increasing epigallocatechin-3-gallate and theanine biosynthesis in Camellia sinensis L. J. Plant. Physiol. 2020, 253, 153273. [Google Scholar] [CrossRef] [PubMed]
- Di, T.; Zhao, L.; Chen, H.; Qian, W.; Wang, P.; Zhang, X.; Xia, T. Transcriptomic and Metabolic Insights into the Distinctive Effects of Exogenous Melatonin and Gibberellin on Terpenoid Synthesis and Plant Hormone Signal Transduction Pathway in Camellia sinensis. J. Agric. Food Chem. 2019, 67, 4689–4699. [Google Scholar] [CrossRef]
- Vaistij, F.E.; Barros-Galvao, T.; Cole, A.F.; Gilday, A.D.; He, Z.; Li, Y.; Harvey, D.; Larson, T.R.; Graham, I.A. MOTHER-OF-FT-AND-TFL1 represses seed germination under far-red light by modulating phytohormone responses in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2018, 115, 8442–8447. [Google Scholar] [CrossRef]
- Miyamoto, T.; DeRose, R.; Suarez, A.; Ueno, T.; Chen, M.; Sun, T.P.; Wolfgang, M.J.; Mukherjee, C.; Meyers, D.J.; Inoue, T. Rapid and orthogonal logic gating with a gibberellin-induced dimerization system. Nat. Chem. Biol. 2012, 8, 465–470. [Google Scholar] [CrossRef]
- Li, W.; Xiang, F.; Su, Y.; Luo, Z.; Luo, W.; Zhou, L.; Liu, H.; Xiao, L. Gibberellin Increases the Bud Yield and Theanine Accumulation in Camellia sinensis (L.) Kuntze. Molecules 2021, 26, 3290. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Ou, L.; Ren, X.; Yang, X.; Tan, Y.; Zhou, X.; Jin, L. Transcriptomic and Metabolomic Analysis Reveal Possible Molecular Mechanisms Regulating Tea Plant Growth Elicited by Chitosan Oligosaccharide. Int. J. Mol. Sci. 2022, 23, 5469. [Google Scholar] [CrossRef]
- Zhang, L.; Li, M.; Li, X.; Yan, P.; Zhang, L.; Han, W. Summer pruning improves the branch growth and tea quality of tea trees (Camellia sinensis). Acta Physiol. Plant. 2021, 43, 65. [Google Scholar] [CrossRef]
- Xi, S.; Chu, H.; Zhou, Z.; Li, T.; Zhang, S.; Xu, X.; Pu, Y.; Wang, G.; Jia, Y.; Liu, X. Effect of potassium fertilizer on tea yield and quality: A meta-analysis. Eur. J. Agron. 2023, 144, 126767. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, F.; Wu, Z.; Jiang, F.; Yu, W.; Yang, J.; Chen, J.; Jian, G.; You, Z.; Zeng, L. Effects of Long-Term Nitrogen Fertilization on the Formation of Metabolites Related to Tea Quality in Subtropical China. Metabolites 2021, 11, 146. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, D.; Yang, X.; Geng, S.; He, Y.; Chen, Y.; Yi, X.; Ni, K.; Liu, M.; Ruan, J. Plant Availability of Magnesium in Typical Tea Plantation Soils. Front. Plant. Sci. 2021, 12, 641501. [Google Scholar] [CrossRef]
- Yang, W.; Li, C.; Wang, S.; Zhou, B.; Mao, Y.; Rensing, C.; Xing, S. Influence of biochar and biochar-based fertilizer on yield, quality of tea and microbial community in an acid tea orchard soil. Appl. Soil. Ecol. 2021, 166, 104005. [Google Scholar] [CrossRef]
- Ma, L.; Yang, X.; Shi, Y.; Yi, X.; Ji, L.; Cheng, Y.; Ni, K.; Ruan, J. Response of tea yield, quality and soil bacterial characteristics to long-term nitrogen fertilization in an eleven-year field experiment. Appl. Soil. Ecol. 2021, 166, 103976. [Google Scholar] [CrossRef]
- Shao, Y.-D.; Zhang, D.-J.; Hu, X.-C.; Wu, Q.-S.; Jiang, C.-J.; Gao, X.-B.; KuČA, K. Arbuscular Mycorrhiza Improves Leaf Food Quality of Tea Plants. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 608–614. [Google Scholar] [CrossRef]
- Dutta, J.; Handique, P.J.; Thakur, D. Assessment of Culturable Tea Rhizobacteria Isolated from Tea Estates of Assam, India for Growth Promotion in Commercial Tea Cultivars. Front. Microbiol. 2015, 6, 1252. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Feng, X.; Wang, M.; Wang, Y.; Kumar Awasthi, M.; Xu, P. Implications of endophytic microbiota in Camellia sinensis: A review on current understanding and future insights. Bioengineered 2020, 11, 1001–1015. [Google Scholar] [CrossRef]
- Tshikhudo, P.; Ntushelo, K.; Mudau, F.; Salehi, B.; Sharifi-Rad, M.; Martins, N.; Martorell, M.; Sharifi-Rad, J. Understanding Camellia sinensis using Omics Technologies along with Endophytic Bacteria and Environmental Roles on Metabolism: A Review. Appl. Sci. 2019, 9, 281. [Google Scholar] [CrossRef]
- Zhu, M.; Li, N.; Zhao, M.; Yu, W.; Wu, J.L. Metabolomic profiling delineate taste qualities of tea leaf pubescence. Food Res. Int. 2017, 94, 36–44. [Google Scholar] [CrossRef]
- Li, J.; Yao, Y.; Wang, J.; Hua, J.; Wang, J.; Yang, Y.; Dong, C.; Zhou, Q.; Jiang, Y.; Deng, Y.; et al. Rutin, gamma-Aminobutyric Acid, Gallic Acid, and Caffeine Negatively Affect the Sweet-Mellow Taste of Congou Black Tea Infusions. Molecules 2019, 24, 4221. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.P.; Yin, J.F.; Chen, G.S.; Wang, F.; Xu, Y.Q. Flavor characteristics and chemical compositions of oolong tea processed using different semi-fermentation times. J. Food Sci. Technol. 2018, 55, 1185–1195. [Google Scholar] [CrossRef]
- Zhao, J.; Li, P.; Xia, T.; Wan, X. Exploring plant metabolic genomics: Chemical diversity, metabolic complexity in the biosynthesis and transport of specialized metabolites with the tea plant as a model. Crit. Rev. Biotechnol. 2020, 40, 667–688. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Cheng, H.; Xu, P.; Wang, Y. Regulation of biosynthesis of the main flavor-contributing metabolites in tea plant (Camellia sinensis): A review. Crit. Rev. Food Sci. Nutr. 2022, 1–16. [Google Scholar] [CrossRef]
- Sharma, E.; Joshi, R.; Gulati, A. l-Theanine: An astounding sui generis integrant in tea. Food Chem. 2018, 242, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Ma, J.; Sun, Y.; Tian, L.; Liu, L.; Chen, Q.; Zhang, Z.; Wan, X.; Sun, J. gamma-Glutamyl-transpeptidase CsGGT2 functions as light-activated theanine hydrolase in tea plant (Camellia sinensis L.). Plant. Cell. Env. 2023, 46, 1596–1609. [Google Scholar] [CrossRef]
- Deng, W.; Ogita, S.; Ashihara, H. Ethylamine Content and Theanine Biosynthesis in Different Organs of Camellia sinensis Seedlings. Z. Für Nat. C. 2009, 64, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.V.; Bowyer, M.C.; Roach, P.D. L-Theanine: Properties, synthesis and isolation from tea. J. Sci. Food Agric. 2011, 91, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Cheng, S.; Liao, Y.; Xu, X.; Wang, X.; Hao, X.; Xu, P.; Dong, F.; Yang, Z. Characterization of l-Theanine Hydrolase in Vitro and Subcellular Distribution of Its Specific Product Ethylamine in Tea (Camellia sinensis). J. Agric. Food Chem. 2020, 68, 10842–10851. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Q.; Zhao, S.; Xia, X.; Yan, X.; An, Y.; Mi, X.; Guo, L.; Samarina, L.; Wei, C. Comprehensive co-expression analysis provides novel insights into temporal variation of flavonoids in fresh leaves of the tea plant (Camellia sinensis). Plant. Sci. 2020, 290, 110306. [Google Scholar] [CrossRef]
- Wang, W.; Xin, H.; Wang, M.; Ma, Q.; Wang, L.; Kaleri, N.A.; Wang, Y.; Li, X. Transcriptomic Analysis Reveals the Molecular Mechanisms of Drought-Stress-Induced Decreases in Camellia sinensis Leaf Quality. Front. Plant. Sci. 2016, 7, 385. [Google Scholar] [CrossRef]
- Li, L.; Li, T.; Jiang, Y.; Yang, Y.; Zhang, L.; Jiang, Z.; Wei, C.; Wan, X.; Yang, H. Alteration of local and systemic amino acids metabolism for the inducible defense in tea plant (Camellia sinensis) in response to leaf herbivory by Ectropis oblique. Arch. Biochem. Biophys. 2020, 683, 108301. [Google Scholar] [CrossRef]
- Huang, X.; Tang, Q.; Chen, C.; Li, Q.; Lin, H.; Bai, S.; Zhao, J.; Li, J.; Wang, K.; Zhu, M. Combined analysis of transcriptome and metabolome provides insights into nano-selenium foliar applications to improve summer tea quality (Camellia sinensis). Lwt 2023, 175, 114496. [Google Scholar] [CrossRef]
- Li, D.; Zhou, C.; Zou, N.; Wu, Y.; Zhang, J.; An, Q.; Li, J.Q.; Pan, C. Nanoselenium foliar application enhances biosynthesis of tea leaves in metabolic cycles and associated responsive pathways. Env. Pollut. 2021, 273, 116503. [Google Scholar] [CrossRef]
- Li, F.; Yao, X.; Lu, L.; Jiao, Y. Preparation of Zn-Gly and Se-Gly and Their Effects on the Nutritional Quality of Tea (Camellia sinensis). Plants 2023, 12, 1049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, Y.; Hu, H.; Shi, Y.; Tang, D.; Ruan, J.; Fernie, A.R.; Liu, M.Y. Magnesium promotes tea plant growth via enhanced glutamine synthetase-mediated nitrogen assimilation. Plant. Physiol. 2023, 192, 1321–1337. [Google Scholar] [CrossRef]
- Ye, J.; Wang, Y.; Wang, Y.; Hong, L.; Jia, X.; Kang, J.; Lin, S.; Wu, Z.; Wang, H. Improvement of soil acidification in tea plantations by long-term use of organic fertilizers and its effect on tea yield and quality. Front. Plant. Sci. 2022, 13, 1055900. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Tang, D.; Shi, Y.; Ma, L.; Zhang, Q.; Ruan, J. Foliar N Application on Tea Plant at Its Dormancy Stage Increases the N Concentration of Mature Leaves and Improves the Quality and Yield of Spring Tea. Front. Plant. Sci. 2021, 12, 753086. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; He, Y.; Lang, Z.; Zhao, Y.; Tao, H.; Li, Q.; Hong, G. The CsHSFA-CsJAZ6 module-mediated high temperature regulates flavonoid metabolism in Camellia sinensis. Plant. Cell. Env. 2023, 46, 2401–2418. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, L.; Lu, X.; Du, X.; Xiang, P.; Cheng, B.; Tan, M.; Huang, J.; Wu, L.; Kong, W.; et al. The biosynthesis of EGCG, theanine and caffeine in response to temperature is mediated by hormone signal transduction factors in tea plant (Camellia sinensis L.). Front. Plant. Sci. 2023, 14, 1149182. [Google Scholar] [CrossRef]
- Tan, X.; Li, H.; Zhang, Z.; Yang, Y.; Jin, Z.; Chen, W.; Tang, D.; Wei, C.; Tang, Q. Characterization of the Difference between Day and Night Temperatures on the Growth, Photosynthesis, and Metabolite Accumulation of Tea Seedlings. Int. J. Mol. Sci. 2023, 24, 6718. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, K.; Liang, Z.; Luo, H.; Wang, T.; An, J.; Wang, Q.; Li, X.; Guan, Y.; Xiao, Y.; et al. Unpruning improvement the quality of tea through increasing the levels of amino acids and reducing contents of flavonoids and caffeine. Front. Nutr. 2022, 9, 1017693. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, Y.; Zhang, S.; Wu, T. Induction of callus and establishment of cell suspension and effects on tea polyphenols in the cell suspension system of tea plants by PGRs. Sci. Hortic. 2023, 310, 111770. [Google Scholar] [CrossRef]
- Chen, X.; Ye, K.; Xu, Y.; Zhao, Y.; Zhao, D. Effect of Shading on the Morphological, Physiological, and Biochemical Characteristics as Well as the Transcriptome of Matcha Green Tea. Int. J. Mol. Sci. 2022, 23, 14169. [Google Scholar] [CrossRef]
- Yang, T.; Xie, Y.; Lu, X.; Yan, X.; Wang, Y.; Ma, J.; Cheng, X.; Lin, S.; Bao, S.; Wan, X.; et al. Shading Promoted Theanine Biosynthesis in the Roots and Allocation in the Shoots of the Tea Plant (Camellia sinensis L.) Cultivar Shuchazao. J. Agric. Food Chem. 2021, 69, 4795–4803. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Zhang, Y.Z.; Zhang, Z.Y.; Zhang, Y.H.; Ren, Q.Q.; Jin, S. Differences in transcriptomic and metabolomic analyses of metabolites of shoots on tea plants of different ages and relevant regulatory network. Front. Plant. Sci. 2022, 13, 910895. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Suh, J.H. Multi-omics approach in tea polyphenol research regarding tea plant growth, development and tea processing: Current technologies and perspectives. Food Sci. Hum. Wellness 2022, 11, 524–536. [Google Scholar] [CrossRef]
- Li, J.; Yuan, H.; Rong, Y.; Qian, M.C.; Liu, F.; Hua, J.; Zhou, Q.; Deng, Y.; Zeng, J.; Jiang, Y. Lipid metabolic characteristics and marker compounds of ripened Pu-erh tea during pile fermentation revealed by LC-MS-based lipidomics. Food Chem. 2023, 404, 134665. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Meng, Q.; Tong, H. Non-volatile metabolites profiling analysis reveals the tea flavor of “Zijuan” in different tea plantations. Food Chem. 2023, 412, 135534. [Google Scholar] [CrossRef]
- Cai, H.; Zhong, Z.; Li, Z.; Zhang, X.; Fu, H.; Yang, B.; Zhang, L. Metabolomics in quality formation and characterisation of tea products: A review. Int. J. Food Sci. Technol. 2022, 57, 4001–4014. [Google Scholar] [CrossRef]
- Farag, M.A.; Elmetwally, F.; Elghanam, R.; Kamal, N.; Hellal, K.; Hamezah, H.S.; Zhao, C.; Mediani, A. Metabolomics in tea products; a compile of applications for enhancing agricultural traits and quality control analysis of Camellia sinensis. Food Chem. 2023, 404, 134628. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Y.; Li, M.; Wang, Y.; Zhang, L.; Wan, X.; Yang, X. Tea aroma formation from six model manufacturing processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Kim, M.; Park, S.K. Effects of enzyme treatment on volatile and non-volatile compounds in dried green tea leaves. Food Sci. Biotechnol. 2022, 31, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Q.; Li, Q.S.; Xiang, L.P.; Liang, Y.R. Recent Advances in Volatiles of Teas. Molecules 2016, 21, 338. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Watanabe, N.; Yang, Z. Understanding the biosyntheses and stress response mechanisms of aroma compounds in tea (Camellia sinensis) to safely and effectively improve tea aroma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Liu, Y.; Xiao, J.; Ma, C.; Huang, Y. GC-MS and LC-MS/MS metabolomics revealed dynamic changes of volatile and non-volatile compounds during withering process of black tea. Food Chem. 2023, 410, 135396. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhu, Y.; Dai, W.; Lv, H.; Mu, B.; Li, P.; Tan, J.; Ni, D.; Lin, Z. Aroma formation and dynamic changes during white tea processing. Food Chem. 2019, 274, 915–924. [Google Scholar] [CrossRef]
- Guo, X.; Ho, C.T.; Schwab, W.; Song, C.; Wan, X. Aroma compositions of large-leaf yellow tea and potential effect of theanine on volatile formation in tea. Food Chem. 2019, 280, 73–82. [Google Scholar] [CrossRef]
- Qiu, A.; Wu, S.; Chen, Y.; Yu, Z.; Zhang, D.; Ni, D. Dynamic changes of color, volatile, and non-volatile components during mechanized processing of green tea. J. Food Process. Preserv. 2022, 46, e16797. [Google Scholar] [CrossRef]
- Chen, S.; Liu, H.; Zhao, X.; Li, X.; Shan, W.; Wang, X.; Wang, S.; Yu, W.; Yang, Z.; Yu, X. Non-targeted metabolomics analysis reveals dynamic changes of volatile and non-volatile metabolites during oolong tea manufacture. Food Res. Int. 2020, 128, 108778. [Google Scholar] [CrossRef]
- Mei, X.; Wan, S.; Lin, C.; Zhou, C.; Hu, L.; Deng, C.; Zhang, L. Integration of Metabolome and Transcriptome Reveals the Relationship of Benzenoid-Phenylpropanoid Pigment and Aroma in Purple Tea Flowers. Front. Plant. Sci. 2021, 12, 762330. [Google Scholar] [CrossRef]
- Liu, L.; Lin, N.; Liu, X.; Yang, S.; Wang, W.; Wan, X. From Chloroplast Biogenesis to Chlorophyll Accumulation: The Interplay of Light and Hormones on Gene Expression in Camellia sinensis cv. Shuchazao Leaves. Front. Plant. Sci. 2020, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Liu, M.Y.; Zhang, Q.; Ma, L.; Shi, Y.; Ruan, J. Preferential assimilation of NH(4)(+) over NO(3)(−) in tea plant associated with genes involved in nitrogen transportation, utilization and catechins biosynthesis. Plant. Sci. 2020, 291, 110369. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, L.; Jiang, X.; Dai, X.; Xu, L.; Li, T.; Xing, D.; Li, Y.; Li, M.; Gao, L.; et al. Evolutionary and functional characterization of leucoanthocyanidin reductases from Camellia sinensis. Planta 2018, 247, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, X.; Shulaev, V.; Dixon, R.A. A role for leucoanthocyanidin reductase in the extension of proanthocyanidins. Nat. Plants 2016, 2, 16182. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, L.; Liao, Y.; Li, J.; Tang, J.; Yang, Z. Formation of alpha-Farnesene in Tea (Camellia sinensis) Leaves Induced by Herbivore-Derived Wounding and Its Effect on Neighboring Tea Plants. Int. J. Mol. Sci. 2019, 20, 4151. [Google Scholar] [CrossRef]
- Wei, K.; Wang, L.; Zhang, Y.; Ruan, L.; Li, H.; Wu, L.; Xu, L.; Zhang, C.; Zhou, X.; Cheng, H.; et al. A coupled role for CsMYB75 and CsGSTF1 in anthocyanin hyperaccumulation in purple tea. Plant. J. 2019, 97, 825–840. [Google Scholar] [CrossRef]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.A.; Baenziger, P.S.; Borner, A. Drought Stress Tolerance in Wheat and Barley: Advances in Physiology, Breeding and Genetics Research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R. Plant responses to multifactorial stress combination. New. Phytol. 2022, 234, 1161–1167. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell. Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Zhang, C.; Yi, X.; Gao, X.; Wang, M.; Shao, C.; Lv, Z.; Chen, J.; Liu, Z.; Shen, C. Physiological and biochemical responses of tea seedlings (Camellia sinensis) to simulated acid rain conditions. Ecotoxicol. Env. Saf. 2020, 192, 110315. [Google Scholar] [CrossRef]
- Liu, K.; Li, S.; Han, J.; Zeng, X.; Ling, M.; Mao, J.; Li, Y.; Jiang, J. Effect of selenium on tea (Camellia sinensis) under low temperature: Changes in physiological and biochemical responses and quality. Environ. Exp. Bot. 2021, 188, 104475. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, C.; Zhang, H.; Duan, Y.; Zou, Z.; Zhou, L.; Zhu, X.; Fang, W.; Ma, Y. CsMYB Transcription Factors Participate in Jasmonic Acid Signal Transduction in Response to Cold Stress in Tea Plant (Camellia sinensis). Plants 2022, 11, 2869. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Qu, D.; Chen, X.; Zeng, H.; Li, X.; Hu, C.Y. Metabolomics Reveals 5-Aminolevulinic Acid Improved the Ability of Tea Leaves (Camellia sinensis L.) against Cold Stress. Metabolites 2022, 12, 392. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.P.; Scott, E.R.; Liu, J.W.; Guo, S.; Li, Y.; Zhang, L.; Han, W.Y. Exogenous Melatonin Alleviates Cold Stress by Promoting Antioxidant Defense and Redox Homeostasis in Camellia sinensis L. Molecules 2018, 23, 165. [Google Scholar] [CrossRef]
- Thomashow, M.F. PLANT COLD ACCLIMATION: Freezing Tolerance Genes and Regulatory Mechanisms. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Q.; Ma, C.; Zhang, Z.-H.; Cao, H.; Kong, Y.; Yue, C.; Hao, X.; Chen, L.; Ma, J.; et al. Global transcriptome profiles of Camellia sinensis during cold acclimation. BMC Genom. 2013, 14, 415. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Ma, Q.-P.; Zhu, Z.-X.; Cui, Q.-Y.; Chen, C.-S.; Chen, X.; Fang, W.-P.; Li, X.-H. Functional analysis of CsCBF3 transcription factor in tea plant (Camellia sinensis) under cold stress. Plant. Growth Regul. 2016, 80, 335–343. [Google Scholar] [CrossRef]
- Pi, X.; Chang, N.; Zhou, Z.; Li, Y.; Zhang, X. CsFAD2 and CsFAD5 are key genes for C18:2 fatty acid pathway-mediated cold tolerance in tea (Camellia sinensis). Environ. Exp. Bot. 2023, 210, 105317. [Google Scholar] [CrossRef]
- Chen, S.; Gao, Y.; Fan, K.; Shi, Y.; Luo, D.; Shen, J.; Ding, Z.; Wang, Y. Prediction of Drought-Induced Components and Evaluation of Drought Damage of Tea Plants Based on Hyperspectral Imaging. Front. Plant. Sci. 2021, 12, 695102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, H.; Chen, L.; Wang, N.; Wei, C.; Wan, X. Mesophyll cells’ ability to maintain potassium is correlated with drought tolerance in tea (Camellia sinensis). Plant. Physiol. Biochem. 2019, 136, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Sun, J.; Shen, J.; Zhang, S.; Ding, Y.; Gai, Z.; Fan, K.; Song, L.; Chen, B.; Ding, Z.; et al. Fulvic acid enhances drought resistance in tea plants by regulating the starch and sucrose metabolism and certain secondary metabolism. J. Proteom. 2021, 247, 104337. [Google Scholar] [CrossRef]
- Sun, J.; Qiu, C.; Ding, Y.; Wang, Y.; Sun, L.; Fan, K.; Gai, Z.; Dong, G.; Wang, J.; Li, X.; et al. Fulvic acid ameliorates drought stress-induced damage in tea plants by regulating the ascorbate metabolism and flavonoids biosynthesis. BMC Genom. 2020, 21, 411. [Google Scholar] [CrossRef]
- Malyukova, L.S.; Koninskaya, N.G.; Orlov, Y.L.; Samarina, L.S. Effects of exogenous calcium on the drought response of the tea plant (Camellia sinensis (L.) Kuntze). PeerJ 2022, 10, e13997. [Google Scholar] [CrossRef]
- Na, C.; Ziwen, Z.; Yeyun, L.; Xianchen, Z. Exogenously applied Spd and Spm enhance drought tolerance in tea plants by increasing fatty acid desaturation and plasma membrane H(+)-ATPase activity. Plant. Physiol. Biochem. 2022, 170, 225–233. [Google Scholar] [CrossRef]
- Zhao, M.; Jin, J.; Wang, J.; Gao, T.; Luo, Y.; Jing, T.; Hu, Y.; Pan, Y.; Lu, M.; Schwab, W.; et al. Eugenol functions as a signal mediating cold and drought tolerance via UGT71A59-mediated glucosylation in tea plants. Plant. J. 2022, 109, 1489–1506. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Y.; Zhang, Y.; Dong, Y.; Liu, Y.; Liu, L.; Wan, S.; He, J.; Yu, Y. Accumulation of Galactinol and ABA Is Involved in Exogenous EBR-Induced Drought Tolerance in Tea Plants. J. Agric. Food Chem. 2022, 70, 13391–13403. [Google Scholar] [CrossRef]
- Lu, J.; Du, J.; Tian, L.; Li, M.; Zhang, X.; Zhang, S.; Wan, X.; Chen, Q. Divergent Response Strategies of CsABF Facing Abiotic Stress in Tea Plant: Perspectives From Drought-Tolerance Studies. Front. Plant. Sci. 2021, 12, 763843. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Hao, X.Y.; Wang, L.; Bin, X.; Wang, X.C.; Yang, Y.J. Diverse Colletotrichum species cause anthracnose of tea plants (Camellia sinensis (L.) O. Kuntze) in China. Sci. Rep. 2016, 6, 35287. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Yang, Z.; Baldermann, S.; Sato, Y.; Asai, T.; Watanabe, N. Herbivore-Induced Volatiles from Tea (Camellia sinensis) Plants and Their Involvement in Intraplant Communication and Changes in Endogenous Nonvolatile Metabolites. J. Agric. Food Chem. 2011, 59, 13131–13135. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, L.; Cai, X.; Li, X.; Bian, L.; Luo, Z.; Li, Z.; Chen, Z.; Xin, Z. (E)-Nerolidol is a volatile signal that induces defenses against insects and pathogens in tea plants. Hortic. Res. 2020, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Ge, L.; Chen, S.; Sun, X. Enhanced transcriptome responses in herbivore-infested tea plants by the green leaf volatile (Z)-3-hexenol. J. Plant. Res. 2019, 132, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Liu, M.; Erb, M.; Glauser, G.; Zhang, J.; Li, X.; Sun, X. Indole primes defence signalling and increases herbivore resistance in tea plants. Plant. Cell. Env. 2021, 44, 1165–1177. [Google Scholar] [CrossRef]
- Liu, L.; Chen, H.; Zhu, J.; Tao, L.; Wei, C. miR319a targeting of CsTCP10 plays an important role in defense against gray blight disease in tea plant (Camellia sinensis). Tree Physiol. 2022, 42, 1450–1462. [Google Scholar] [CrossRef]
- Bag, S.; Mondal, A.; Banik, A. Exploring tea (Camellia sinensis) microbiome: Insights into the functional characteristics and their impact on tea growth promotion. Microbiol. Res. 2022, 254, 126890. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lin, S.; Li, J.; Chen, T.; Gu, Q.; Yang, T.; Zhang, Z. Theanine Improves Salt Stress Tolerance via Modulating Redox Homeostasis in Tea Plants (Camellia sinensis L.). Front. Plant. Sci. 2021, 12, 770398. [Google Scholar] [CrossRef]
- Guo, S.; Wang, Q.; Tang, L.; Zhang, T.; Li, J.; Xiao, Y.; Gao, Y.; Bai, J.; Xiao, B.; Gong, C. Inoculation with Arbuscular Mycorrhizal Fungi Reinforces Tea Plant’s Tolerance to Salinity. J. Plant. Growth Regul. 2021, 41, 3498–3517. [Google Scholar] [CrossRef]
- Li, Y.-W.; Tong, C.-L.; Sun, M.-F. Effects and Molecular Mechanism of Mycorrhiza on the Growth, Nutrient Absorption, Quality of Fresh Leaves, and Antioxidant System of Tea Seedlings Suffering from Salt Stress. Agronomy 2022, 12, 2163. [Google Scholar] [CrossRef]
- Wan, S.; Zhang, Y.; Duan, M.; Huang, L.; Wang, W.; Xu, Q.; Yang, Y.; Yu, Y. Integrated Analysis of Long Non-coding RNAs (lncRNAs) and mRNAs Reveals the Regulatory Role of lncRNAs Associated with Salt Resistance in Camellia sinensis. Front. Plant. Sci. 2020, 11, 218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, W.; Ni, D.; Wang, M.; Guo, G. Genome-wide characterization of tea plant (Camellia sinensis) Hsf transcription factor family and role of CsHsfA2 in heat tolerance. BMC Plant. Biol. 2020, 20, 244. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.P.; Ahammed, G.J.; Zhang, L.; Li, Y.; Yan, P.; Zhang, L.P.; Han, W.Y. Brassinosteroids Attenuate Moderate High Temperature-Caused Decline in Tea Quality by Enhancing Theanine Biosynthesis in Camellia sinensis L. Front. Plant. Sci. 2018, 9, 1016. [Google Scholar] [CrossRef]
- Wang, M.; Li, Q.; Sun, K.; Chen, X.; Zhou, Q.; Li, H.; Zhang, X.; Li, X. Involvement of CsCDPK20 and CsCDPK26 in Regulation of Thermotolerance in Tea Plant (Camellia sinensis). Plant. Mol. Biol. Report. 2018, 36, 176–187. [Google Scholar] [CrossRef]
- Seth, R.; Maritim, T.K.; Parmar, R.; Sharma, R.K. Underpinning the molecular programming attributing heat stress associated thermotolerance in tea (Camellia sinensis (L.) O. Kuntze). Hortic. Res. 2021, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zou, Z.; Li, Q.; Sun, K.; Chen, X.; Li, X. The CsHSP17.2 molecular chaperone is essential for thermotolerance in Camellia sinensis. Sci. Rep. 2017, 7, 1237. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, J.; Ruan, Q.; Zeng, X.; Wu, L.; Cai, L.; Wang, X.; Yang, C. Single-Cell Sequencing Methodologies: From Transcriptome to Multi-Dimensional Measurement. Small Methods 2021, 5, e2100111. [Google Scholar] [CrossRef]
- Giacomello, S. A new era for plant science: Spatial single-cell transcriptomics. Curr. Opin. Plant. Biol. 2021, 60, 102041. [Google Scholar] [CrossRef]
- Mo, Y.; Jiao, Y. Advances and applications of single-cell omics technologies in plant research. Plant. J. 2022, 110, 1551–1563. [Google Scholar] [CrossRef]
- Denyer, T.; Ma, X.; Klesen, S.; Scacchi, E.; Nieselt, K.; Timmermans, M.C.P. Spatiotemporal Developmental Trajectories in the Arabidopsis Root Revealed Using High-Throughput Single-Cell RNA Sequencing. Dev. Cell. 2019, 48, 840–852.e845. [Google Scholar] [CrossRef]
- Jean-Baptiste, K.; McFaline-Figueroa, J.L.; Alexandre, C.M.; Dorrity, M.W.; Saunders, L.; Bubb, K.L.; Trapnell, C.; Fields, S.; Queitsch, C.; Cuperus, J.T. Dynamics of Gene Expression in Single Root Cells of Arabidopsis thaliana. Plant. Cell. 2019, 31, 993–1011. [Google Scholar] [CrossRef] [PubMed]
- Bezrutczyk, M.; Zollner, N.R.; Kruse, C.P.S.; Hartwig, T.; Lautwein, T.; Kohrer, K.; Frommer, W.B.; Kim, J.Y. Evidence for phloem loading via the abaxial bundle sheath cells in maize leaves. Plant. Cell. 2021, 33, 531–547. [Google Scholar] [CrossRef]
- Liu, Q.; Liang, Z.; Feng, D.; Jiang, S.; Wang, Y.; Du, Z.; Li, R.; Hu, G.; Zhang, P.; Ma, Y.; et al. Transcriptional landscape of rice roots at the single-cell resolution. Mol. Plant. 2021, 14, 384–394. [Google Scholar] [CrossRef]
- Satterlee, J.W.; Strable, J.; Scanlon, M.J. Plant stem-cell organization and differentiation at single-cell resolution. Proc. Natl. Acad. Sci. USA 2020, 117, 33689–33699. [Google Scholar] [CrossRef]
- Omary, M.; Gil-Yarom, N.; Yahav, C.; Steiner, E.; Hendelman, A.; Efroni, I. A conserved superlocus regulates above- and belowground root initiation. Science 2022, 375, eabf4368. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Du, Q.; Xu, M.; Du, F.; Jiao, Y. Single-nucleus RNA-seq resolves spatiotemporal developmental trajectories in the tomato shoot apex. bioRxiv 2020, 2020, 305029. [Google Scholar]
- Wang, Q.; Wu, Y.; Peng, A.; Cui, J.; Zhao, M.; Pan, Y.; Zhang, M.; Tian, K.; Schwab, W.; Song, C. Single-cell transcriptome atlas reveals developmental trajectories and a novel metabolic pathway of catechin esters in tea leaves. Plant. Biotechnol. J. 2022, 20, 2089–2106. [Google Scholar] [CrossRef]
- Shaw, R.; Tian, X.; Xu, J. Single-Cell Transcriptome Analysis in Plants: Advances and Challenges. Mol. Plant. 2021, 14, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Jha, P.; Ochatt, S.J.; Kumar, V. Single-cell transcriptomics is revolutionizing the improvement of plant biotechnology research: Recent advances and future opportunities. Crit. Rev. Biotechnol. 2023, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Seyfferth, C.; Renema, J.; Wendrich, J.R.; Eekhout, T.; Seurinck, R.; Vandamme, N.; Blob, B.; Saeys, Y.; Helariutta, Y.; Birnbaum, K.D.; et al. Advances and Opportunities in Single-Cell Transcriptomics for Plant Research. Annu. Rev. Plant. Biol. 2021, 72, 847–866. [Google Scholar] [CrossRef]
- Jovic, D.; Liang, X.; Zeng, H.; Lin, L.; Xu, F.; Luo, Y. Single-cell RNA sequencing technologies and applications: A brief overview. Clin. Transl. Med. 2022, 12, e694. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Li, R.; Bai, F. Single cell sequencing: Technique, application, and future development. Sci. Bull. 2015, 60, 33–42. [Google Scholar] [CrossRef]
- Evrony, G.D.; Hinch, A.G.; Luo, C. Applications of Single-Cell DNA Sequencing. Annu. Rev. Genom. Hum. Genet. 2021, 22, 171–197. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Zhang, H.; Liu, Z.; Wang, Y.; Xing, L.; He, Q.; Du, H. Plant pan-genomics: Recent advances, new challenges, and roads ahead. J. Genet. Genom. 2022, 49, 833–846. [Google Scholar] [CrossRef]
- Shi, J.; Tian, Z.; Lai, J.; Huang, X. Plant pan-genomics and its applications. Mol. Plant. 2023, 16, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Goltsman, E.; Goodstein, D.; Wu, G.A.; Rokhsar, D.S.; Vogel, J.P. Plant Pan-Genomics Comes of Age. Annu. Rev. Plant. Biol. 2021, 72, 411–435. [Google Scholar] [CrossRef]
- Bayer, P.E.; Golicz, A.A.; Scheben, A.; Batley, J.; Edwards, D. Plant pan-genomes are the new reference. Nat. Plants 2020, 6, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Luo, H.; Xu, J.; Cruickshank, A.; Zhao, X.; Teng, F.; Hathorn, A.; Wu, X.; Liu, Y.; Shatte, T.; et al. Extensive variation within the pan-genome of cultivated and wild sorghum. Nat. Plants 2021, 7, 766–773. [Google Scholar] [CrossRef]
- Yang, T.; Liu, R.; Luo, Y.; Hu, S.; Wang, D.; Wang, C.; Pandey, M.K.; Ge, S.; Xu, Q.; Li, N.; et al. Improved pea reference genome and pan-genome highlight genomic features and evolutionary characteristics. Nat. Genet. 2022, 54, 1553–1563. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Li, P.; Shen, Y.; Peng, H.; Liu, S.; Zhou, G.A.; Zhang, H.; Liu, Z.; Shi, M.; et al. Pan-Genome of Wild and Cultivated Soybeans. Cell 2020, 182, 162–176.e113. [Google Scholar] [CrossRef] [PubMed]
- Torkamaneh, D.; Lemay, M.A.; Belzile, F. The pan-genome of the cultivated soybean (PanSoy) reveals an extraordinarily conserved gene content. Plant. Biotechnol. J. 2021, 19, 1852–1862. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Wang, J.; Wang, P.; Qiu, Y.; Zhao, W.; Pang, S.; Li, X.; Wang, H.; Song, J.; et al. Pan-genome of Raphanus highlights genetic variation and introgression among domesticated, wild, and weedy radishes. Mol. Plant. 2021, 14, 2032–2055. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Chai, S.; Yang, Z.; Zhang, Q.; Xin, H.; Xu, Y.; Lin, S.; Chen, X.; Yao, Z.; et al. Graph-based pan-genome reveals structural and sequence variations related to agronomic traits and domestication in cucumber. Nat. Commun. 2022, 13, 682. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Peng, J.; Fan, B.; Xu, D.; Wu, J.; Cao, Z.; Gao, Y.; Wang, X.; Li, S.; et al. High-quality genome assembly and pan-genome studies facilitate genetic discovery in mung bean and its improvement. Plant. Commun. 2022, 3, 100352. [Google Scholar] [CrossRef]
- Li, J.; Yuan, D.; Wang, P.; Wang, Q.; Sun, M.; Liu, Z.; Si, H.; Xu, Z.; Ma, Y.; Zhang, B.; et al. Cotton pan-genome retrieves the lost sequences and genes during domestication and selection. Genome Biol. 2021, 22, 119. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Edger, P.P.; Xue, L.; Qiong, L.; Lu, J.; Zhang, Y.; Cao, Q.; Yocca, A.E.; Platts, A.E.; Knapp, S.J.; et al. Evolutionary history and pan-genome dynamics of strawberry (Fragaria spp.). Proc. Natl. Acad. Sci. USA 2021, 118, e2105431118. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Jousset, A. Plant Breeding Goes Microbial. Trends Plant. Sci. 2017, 22, 555–558. [Google Scholar] [CrossRef]
- Nerva, L.; Sandrini, M.; Moffa, L.; Velasco, R.; Balestrini, R.; Chitarra, W. Breeding toward improved ecological plant-microbiome interactions. Trends Plant. Sci. 2022, 27, 1134–1143. [Google Scholar] [CrossRef]
- Nwachukwu, B.C.; Babalola, O.O. Metagenomics: A Tool for Exploring Key Microbiome With the Potentials for Improving Sustainable Agriculture. Front. Sustain. Food Syst. 2022, 6, 886987. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition-Current Knowledge and Future Directions. Front. Plant. Sci. 2017, 8, 1617. [Google Scholar] [CrossRef]
- Hestrin, R.; Hammer, E.C.; Mueller, C.W.; Lehmann, J. Synergies between mycorrhizal fungi and soil microbial communities increase plant nitrogen acquisition. Commun. Biol. 2019, 2, 233. [Google Scholar] [CrossRef]
- Conway, J.M.; Walton, W.G.; Salas-Gonzalez, I.; Law, T.F.; Lindberg, C.A.; Crook, L.E.; Kosina, S.M.; Fitzpatrick, C.R.; Lietzan, A.D.; Northen, T.R.; et al. Diverse MarR bacterial regulators of auxin catabolism in the plant microbiome. Nat. Microbiol. 2022, 7, 1817–1833. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Vismans, G.; Yu, K.; Song, Y.; de Jonge, R.; Burgman, W.P.; Burmolle, M.; Herschend, J.; Bakker, P.; Pieterse, C.M.J. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018, 12, 1496–1507. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, L.; Yan, Z.; Schneijderberg, M.; de Roij, M.; Pijnenburg, R.; Zheng, Q.; Franken, C.; Dechesne, A.; Trindade, L.M.; van Velzen, R.; et al. Synthetic bacterial community derived from a desert rhizosphere confers salt stress resilience to tomato in the presence of a soil microbiome. ISME J. 2022, 16, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Kanagendran, A.; Samaddar, S.; Pazouki, L.; Sa, T.M.; Niinemets, U. Inoculation of Brevibacterium linens RS16 in Oryza sativa genotypes enhanced salinity resistance: Impacts on photosynthetic traits and foliar volatile emissions. Sci. Total. Env. 2018, 645, 721–732. [Google Scholar] [CrossRef]
- Kwak, M.J.; Kong, H.G.; Choi, K.; Kwon, S.K.; Song, J.Y.; Lee, J.; Lee, P.A.; Choi, S.Y.; Seo, M.; Lee, H.J.; et al. Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat. Biotechnol. 2018, 36, 1100–1109. [Google Scholar] [CrossRef]
- Mueller, U.G.; Linksvayer, T.A. Microbiome breeding: Conceptual and practical issues. Trends Microbiol. 2022, 30, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Miryeganeh, M. Plants’ Epigenetic Mechanisms and Abiotic Stress. Genes 2021, 12, 1106. [Google Scholar] [CrossRef]
- Dalakouras, A.; Vlachostergios, D. Epigenetic approaches to crop breeding: Current status and perspectives. J. Exp. Bot. 2021, 72, 5356–5371. [Google Scholar] [CrossRef] [PubMed]
- Dar, F.A.; Mushtaq, N.U.; Saleem, S.; Rehman, R.U.; Dar, T.U.H.; Hakeem, K.R. Role of Epigenetics in Modulating Phenotypic Plasticity against Abiotic Stresses in Plants. Int. J. Genom. 2022, 2022, 1092894. [Google Scholar] [CrossRef]
- Sun, M.; Yang, Z.; Liu, L.; Duan, L. DNA Methylation in Plant Responses and Adaption to Abiotic Stresses. Int. J. Mol. Sci. 2022, 23, 6910. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, Z.; Zhu, J.K. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell. Biol. 2018, 19, 489–506. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, S.; Dong, X.; Zhao, X.; Zeng, B.; Chen, J.; Li, H.; Yang, W.; Zhao, H.; Wang, G.; et al. Genome-wide high resolution parental-specific DNA and histone methylation maps uncover patterns of imprinting regulation in maize. Genome Res. 2014, 24, 167–176. [Google Scholar] [CrossRef]
- Zhang, B.; Tieman, D.M.; Jiao, C.; Xu, Y.; Chen, K.; Fei, Z.; Giovannoni, J.J.; Klee, H.J. Chilling-induced tomato flavor loss is associated with altered volatile synthesis and transient changes in DNA methylation. Proc. Natl. Acad. Sci. USA 2016, 113, 12580–12585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhu, C.; Geng, Y.; Wang, Y.; Yang, Y.; Liu, Q.; Guo, W.; Chachar, S.; Riaz, A.; Yan, S.; et al. Rice and Arabidopsis homologs of yeast CHROMOSOME TRANSMISSION FIDELITY PROTEIN 4 commonly interact with Polycomb complexes but exert divergent regulatory functions. Plant. Cell. 2021, 33, 1417–1429. [Google Scholar] [CrossRef]
- Wang, L.; Xie, J.; Hu, J.; Lan, B.; You, C.; Li, F.; Wang, Z.; Wang, H. Comparative epigenomics reveals evolution of duplicated genes in potato and tomato. Plant. J. 2018, 93, 460–471. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Cao, H.; Xu, H.; Xu, Q.; Deng, X. Dynamic changes in methylome and transcriptome patterns in response to methyltransferase inhibitor 5-azacytidine treatment in citrus. DNA Res. 2017, 24, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, X.; Chen, X.; Shu, N.; Wang, J.; Wang, D.; Wang, S.; Fan, W.; Guo, L.; Guo, X.; et al. Single-base resolution methylomes of upland cotton (Gossypium hirsutum L.) reveal epigenome modifications in response to drought stress. BMC Genom. 2017, 18, 297. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, S.; Zhou, C.; Chen, L.; Fu, H.; Li, X.; Lin, Y.; Lai, Z.; Guo, Y. Genome-wide investigation and transcriptional analysis of cytosine-5 DNA methyltransferase and DNA demethylase gene families in tea plant (Camellia sinensis) under abiotic stress and withering processing. PeerJ 2020, 8, e8432. [Google Scholar] [CrossRef]
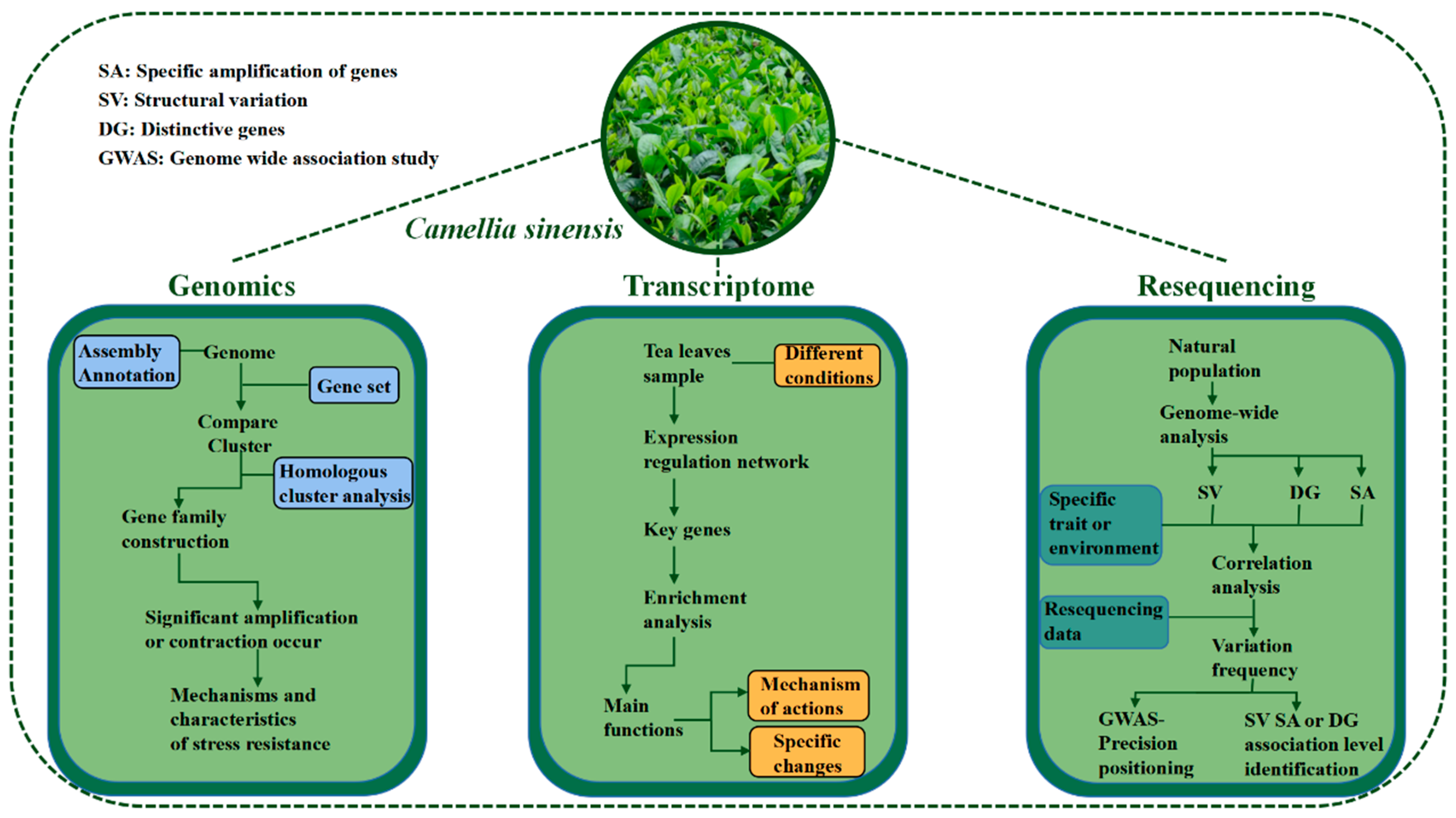
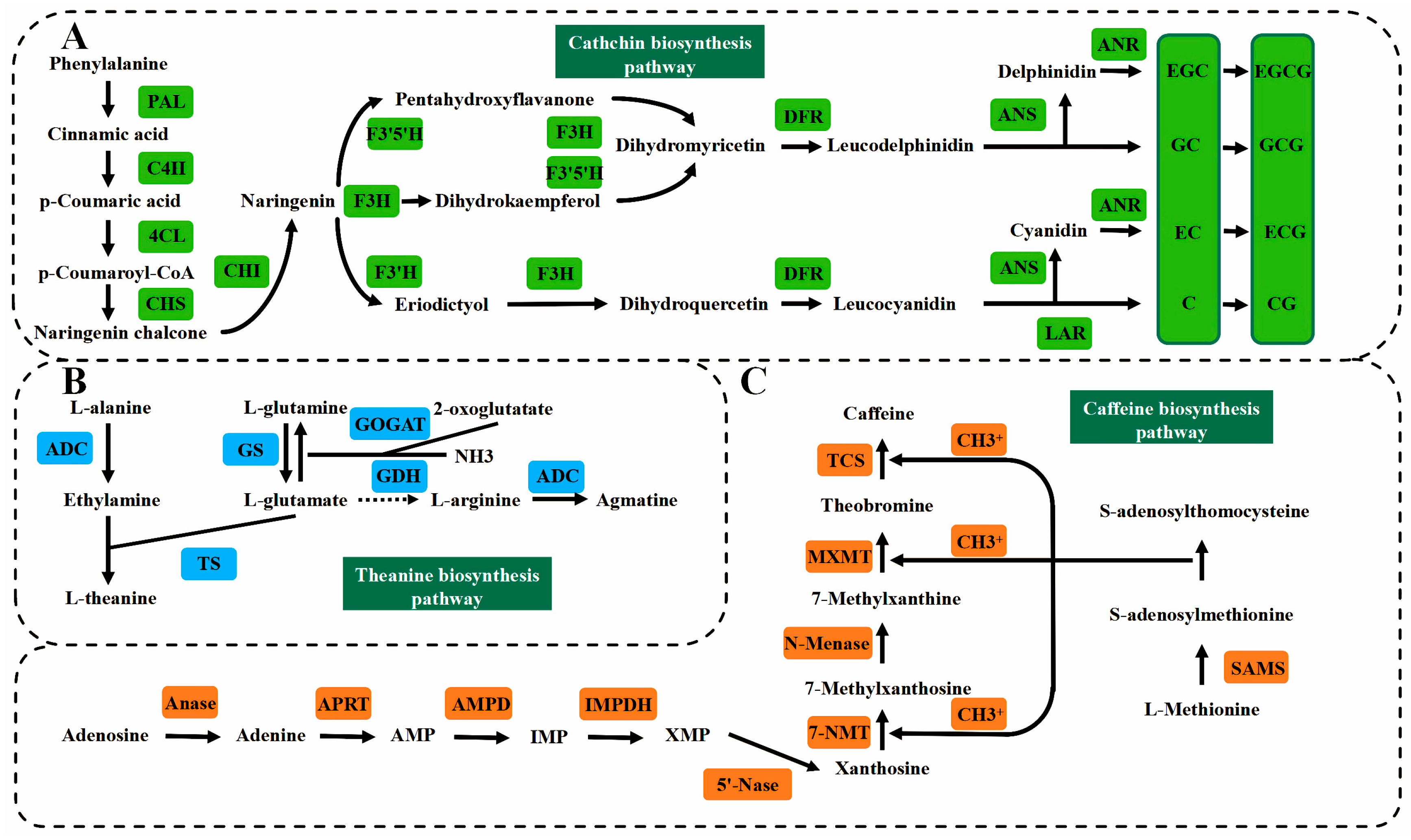
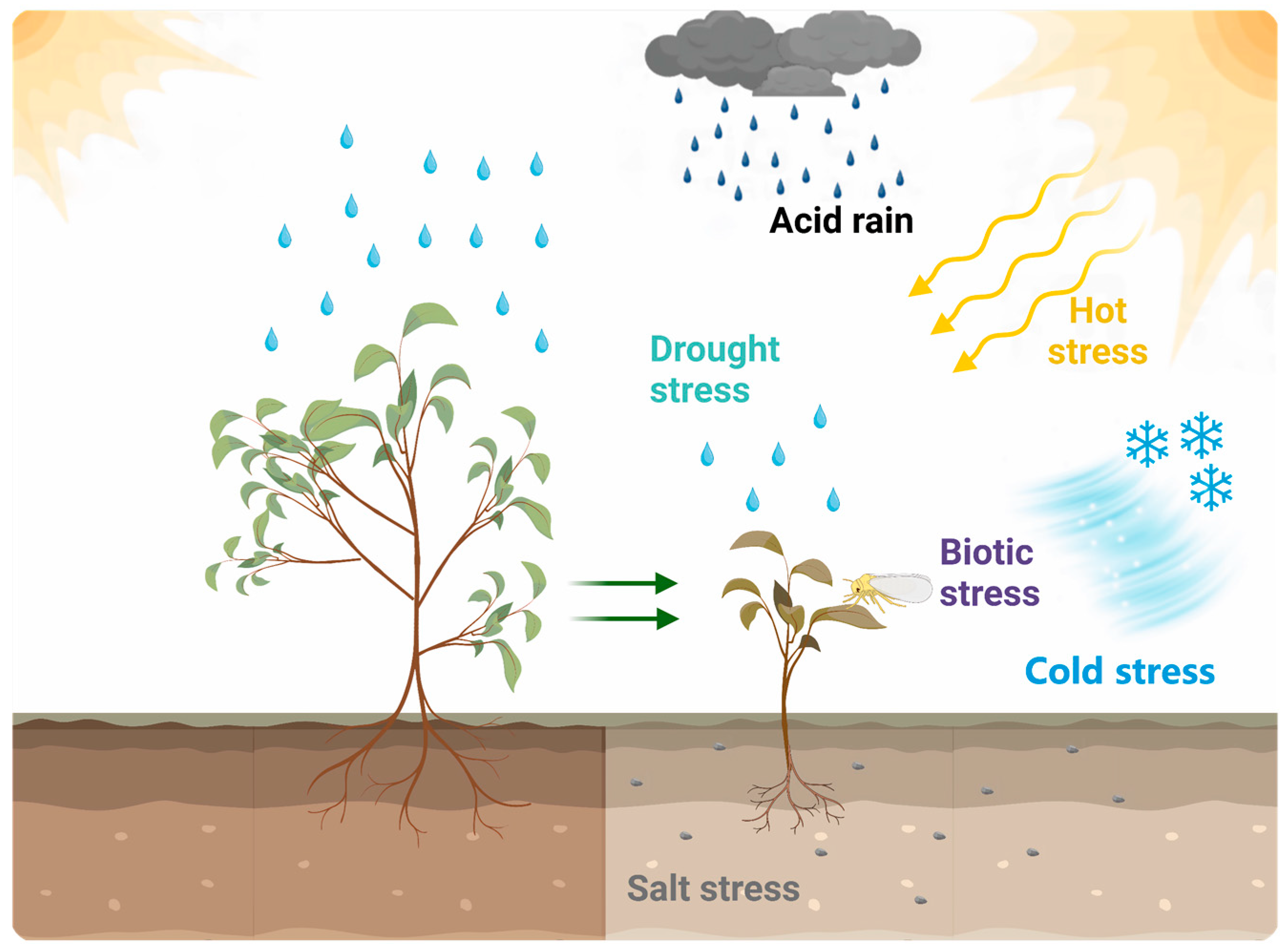
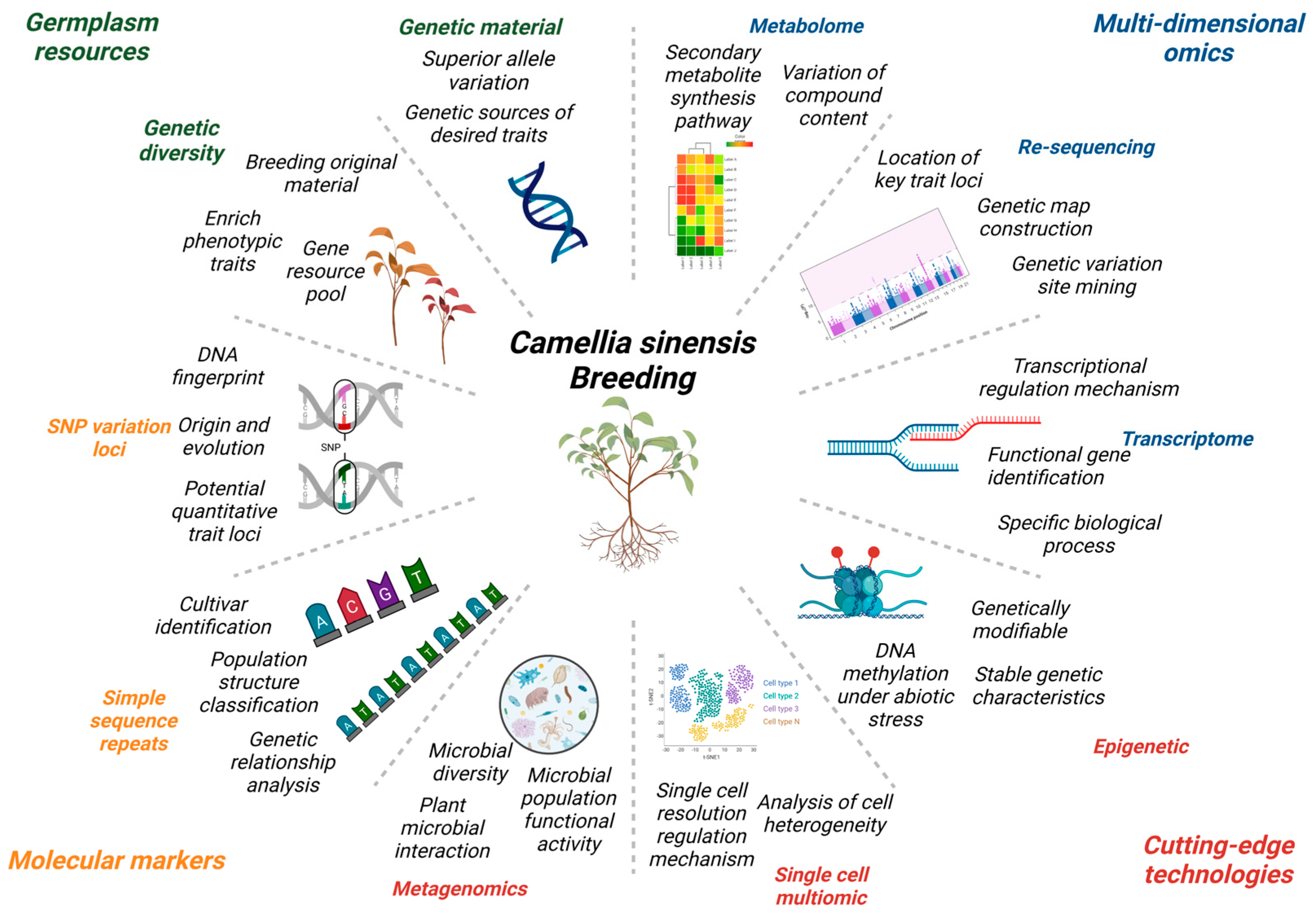
| Cultivar | Species | Genome Size/Gb | Reference |
|---|---|---|---|
| Yunkang10 | C.sinensis var. assamica | 3.02 | [48] |
| Shuchazao | C.sinensis var. sinensis | 3.14 | [49] |
| Shuchazao | C.sinensis var. sinensis | 2.94 | [22] |
| Shuchazao | C.sinensis var. sinensis | 3.20 | [51] |
| DASZ | Wild tea tree | 3.11 | [52] |
| Biyun | C.sinensis var. sinensis | 2.92 | [53] |
| Longjing43 | C.sinensis var. sinensis | 3.26 | [50] |
| Tieguanyin | C.sinensis var. sinensis | 3.06 | [54] |
| Huangdan | C.sinensis var. sinensis | 2.94 | [37] |
| Huangdan | C.sinensis var. sinensis | 5.53 | [37] |
| Duyunmaojian | C.sinensis var. sinensis | 2.97 | [55] |
| Camellia | C.oleifera Abel. var “Nanyongensis” oleifera | 2.89 | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Song, K.; Zhang, X.; Wang, D.; Dong, S.; Liu, Y.; Yang, L. Application of Multi-Perspectives in Tea Breeding and the Main Directions. Int. J. Mol. Sci. 2023, 24, 12643. https://doi.org/10.3390/ijms241612643
Li H, Song K, Zhang X, Wang D, Dong S, Liu Y, Yang L. Application of Multi-Perspectives in Tea Breeding and the Main Directions. International Journal of Molecular Sciences. 2023; 24(16):12643. https://doi.org/10.3390/ijms241612643
Chicago/Turabian StyleLi, Haozhen, Kangkang Song, Xiaohua Zhang, Di Wang, Shaolin Dong, Ying Liu, and Long Yang. 2023. "Application of Multi-Perspectives in Tea Breeding and the Main Directions" International Journal of Molecular Sciences 24, no. 16: 12643. https://doi.org/10.3390/ijms241612643
APA StyleLi, H., Song, K., Zhang, X., Wang, D., Dong, S., Liu, Y., & Yang, L. (2023). Application of Multi-Perspectives in Tea Breeding and the Main Directions. International Journal of Molecular Sciences, 24(16), 12643. https://doi.org/10.3390/ijms241612643







