Enhancement of Neutralization Responses through Sequential Immunization of Stable Env Trimers Based on Consensus Sequences from Select Time Points by Mimicking Natural Infection
Abstract
1. Introduction
2. Results
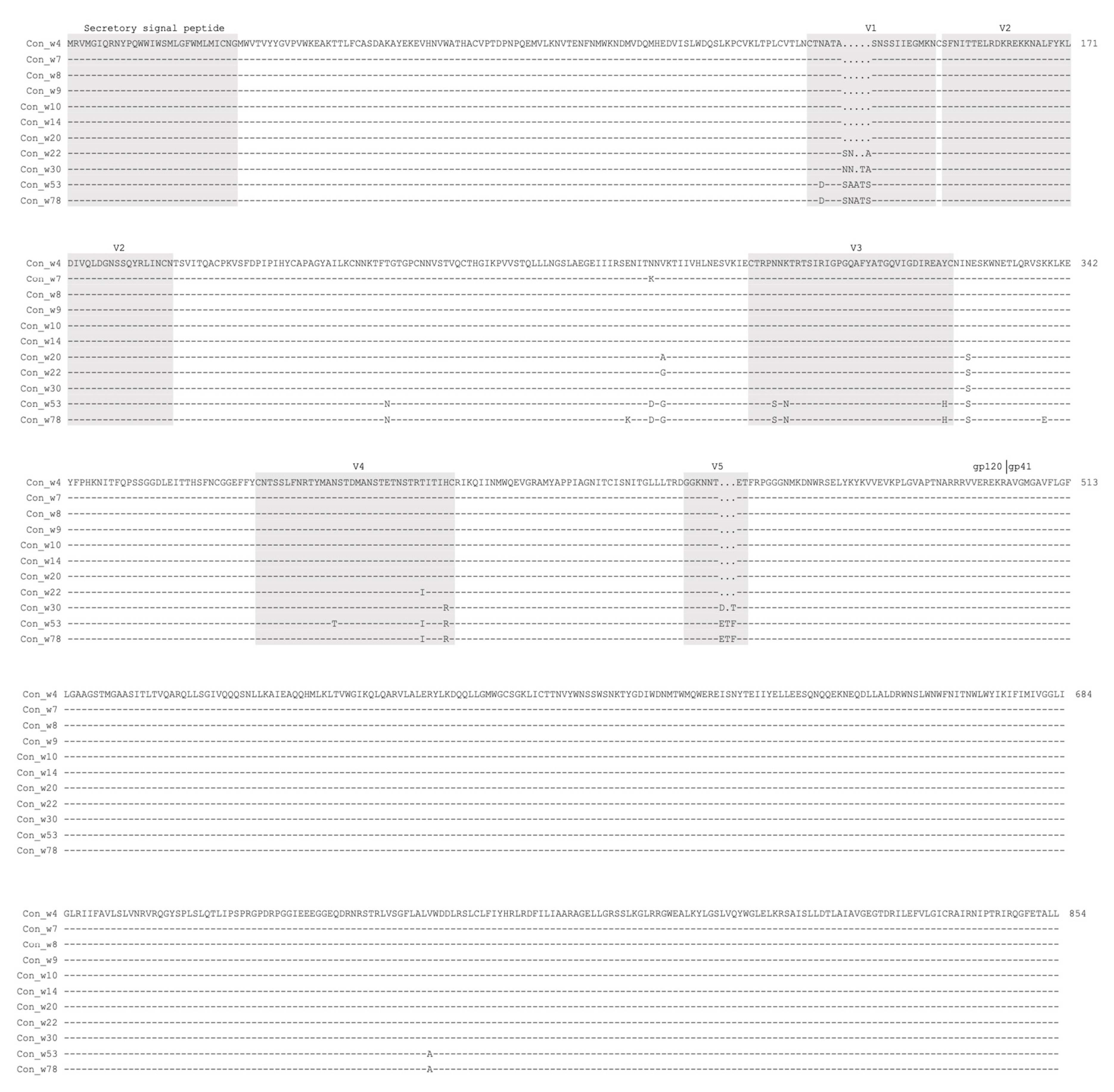
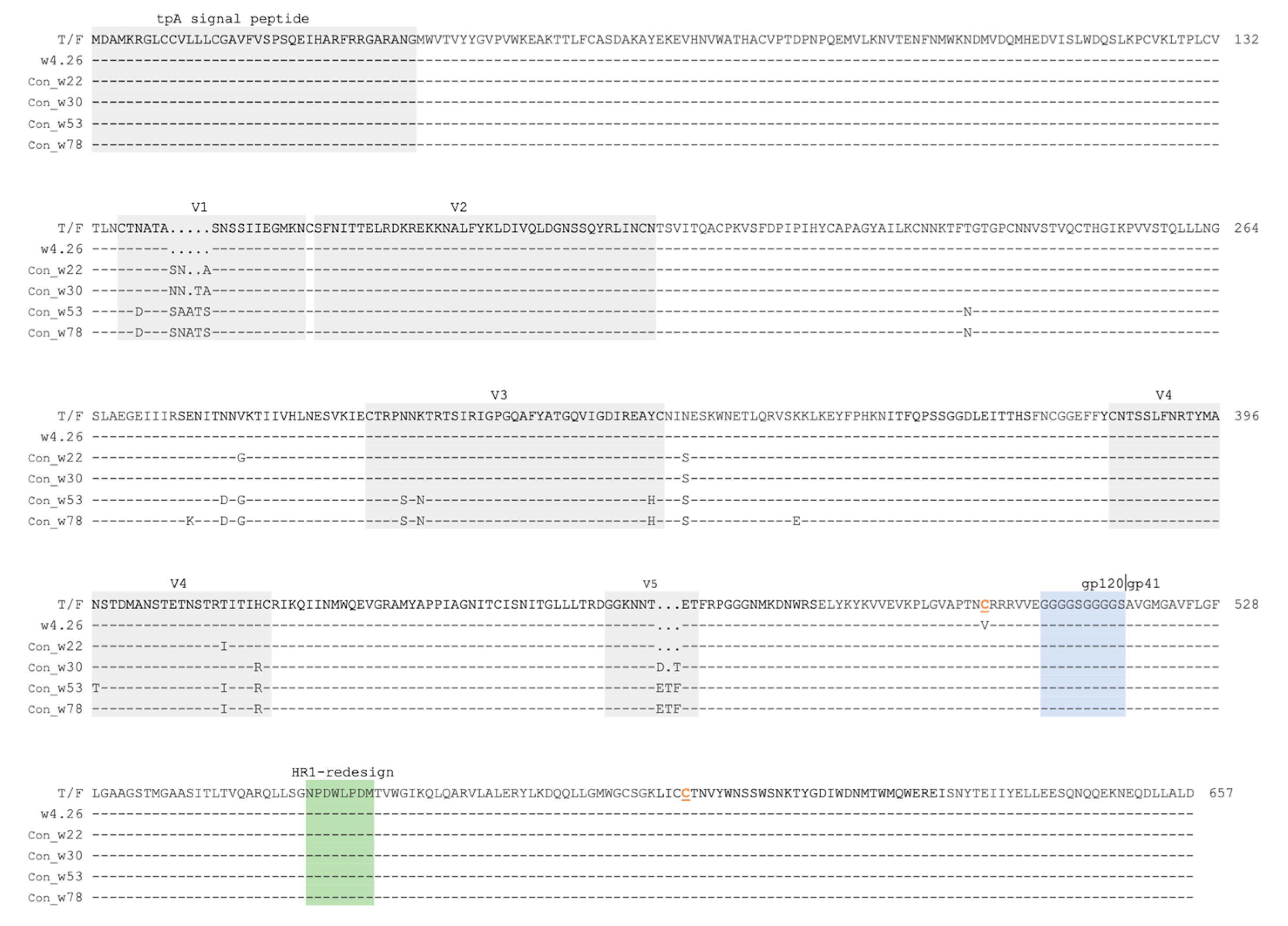
2.1. Generation of the Env Consensus Sequences for Various Time Points from Subject CH505
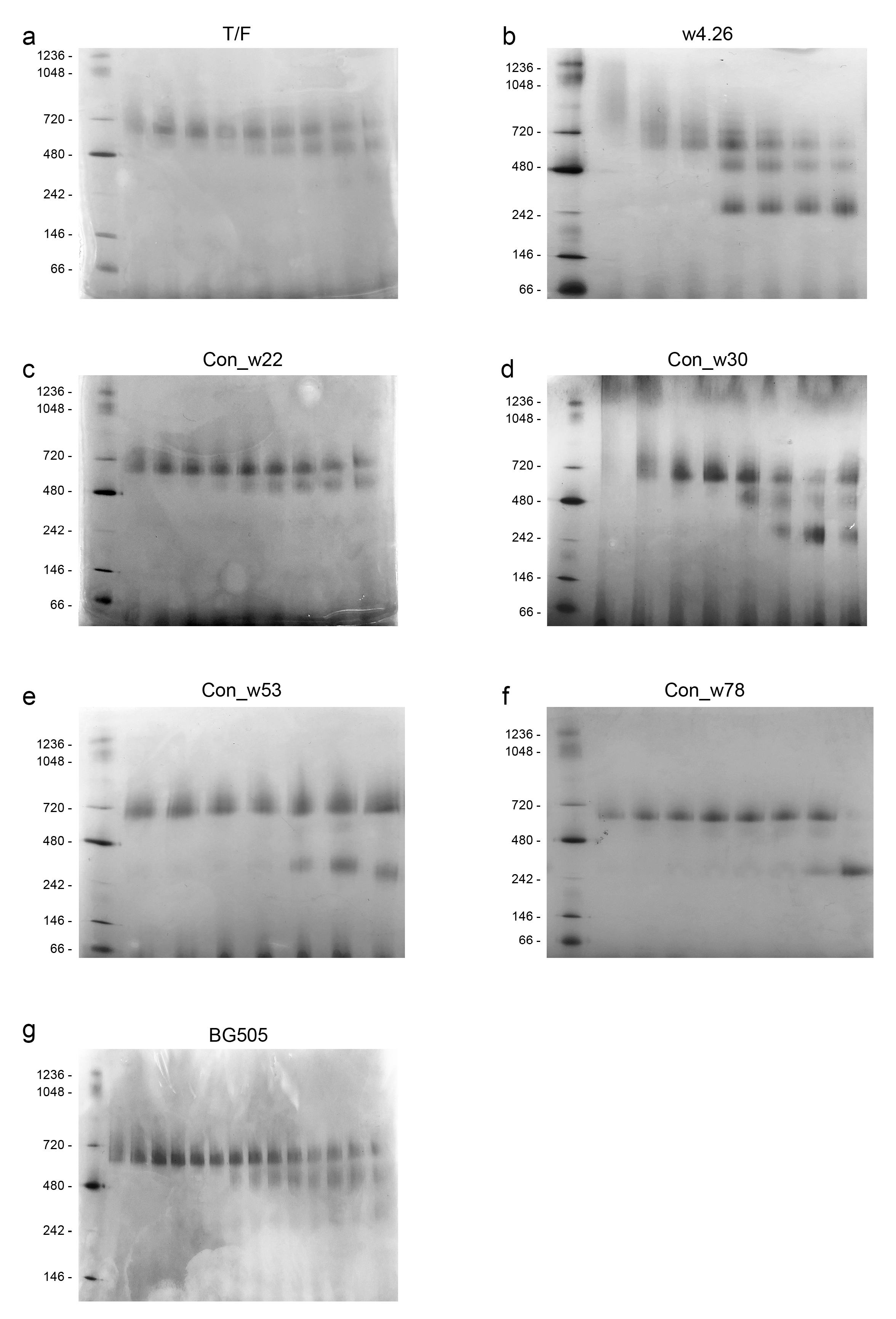
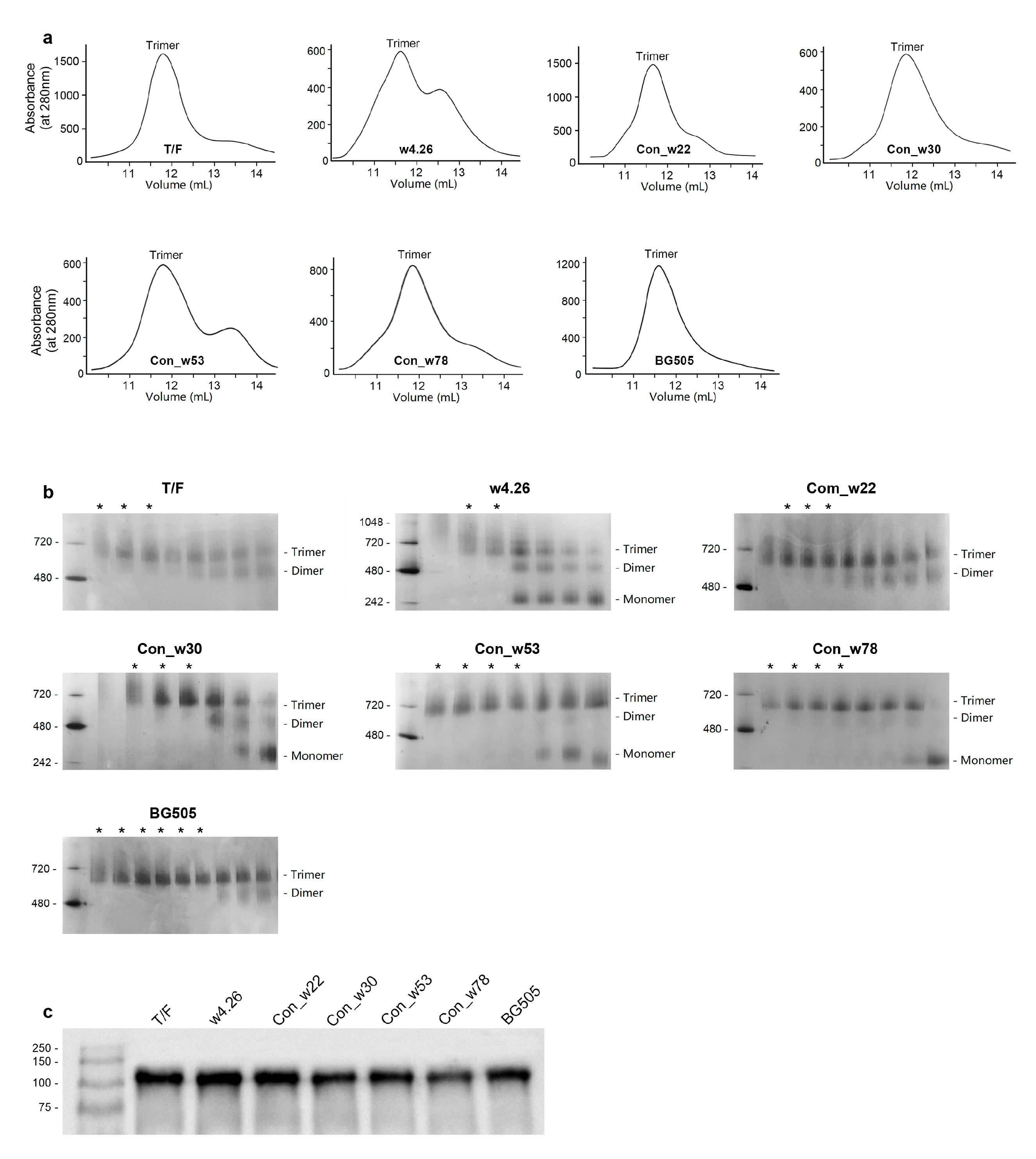
2.2. Characterization of Native-like UFO CH505 Env Trimers
2.3. Induction of HIV-1-specific Binding Abs
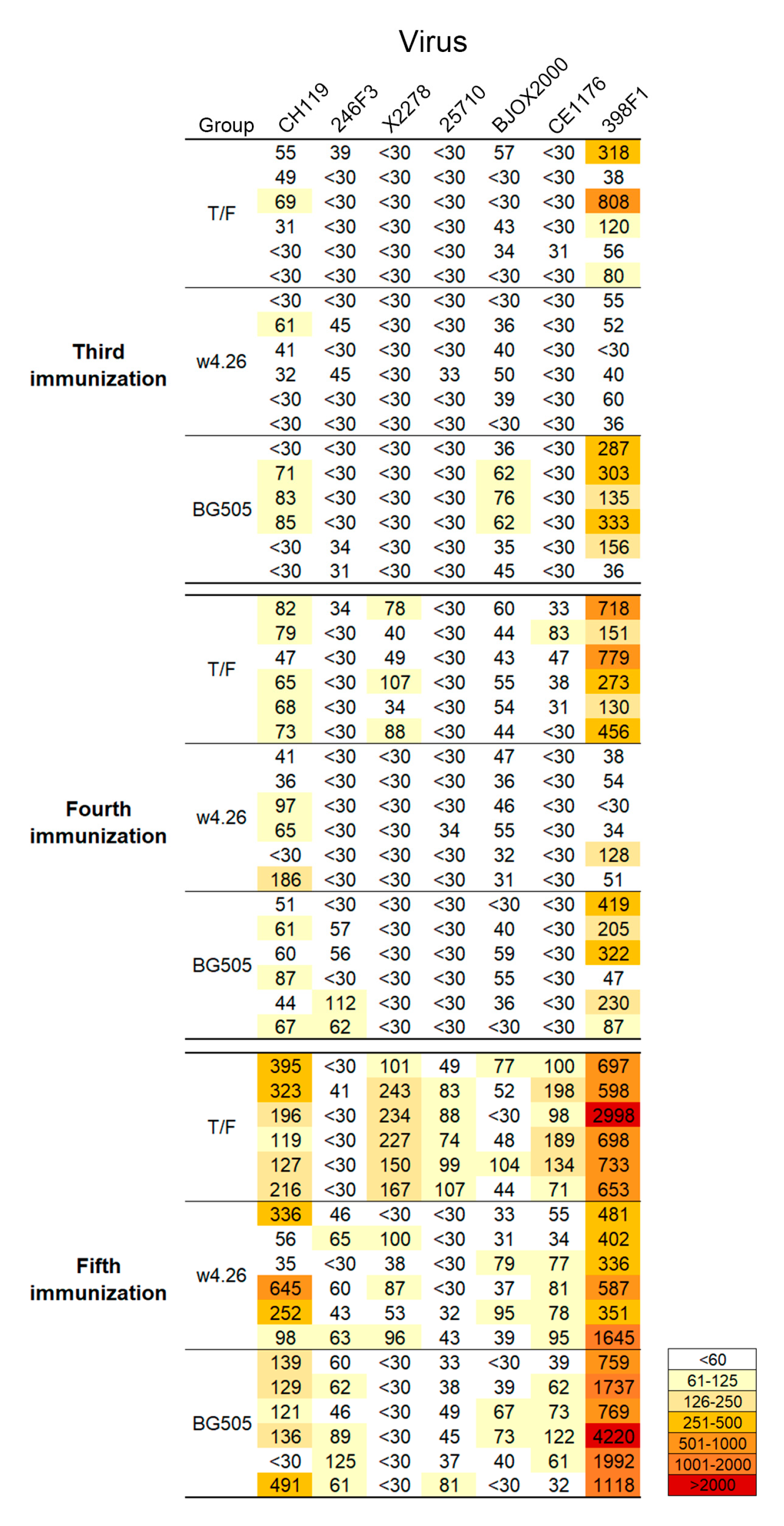
2.4. Elicitation of Broad Neutralization Activity
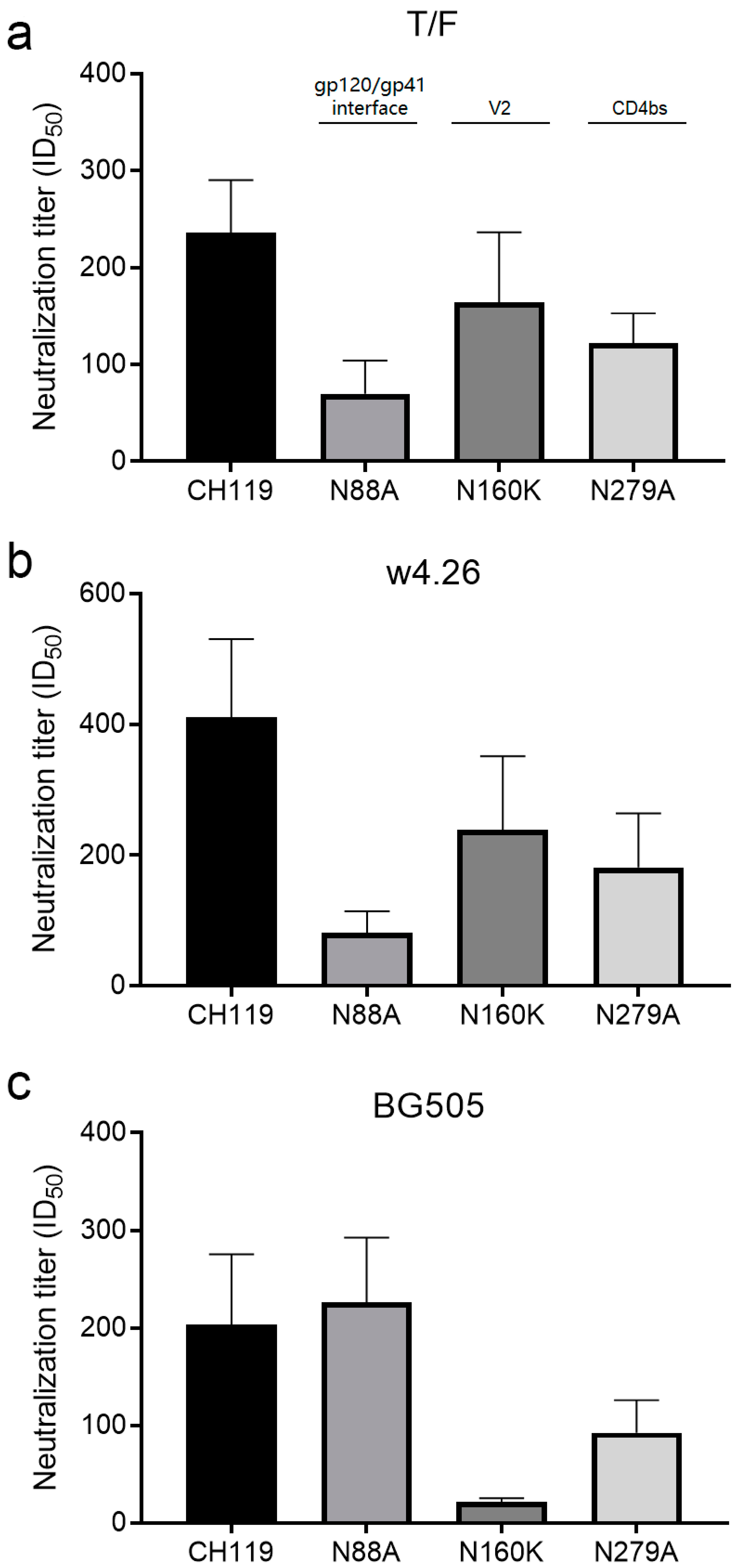
2.5. Different Neutralization Specificities among Immunogen Groups
3. Discussion
4. Materials and Methods
4.1. Generation of Env Consensus Sequences for Different Time Points from Subject CH505
4.2. Expression and Purification of UFO Env Trimer Proteins
4.3. Western Blot Analysis
4.4. Immunization
4.5. ELISA
4.6. Neutralization Assays
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Tier2 | Tier1 | MLV | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | X1632(G) | CH119(CRF07) | TRO.11(B) | 246F3(AC) | 398F1(A) | CNE8(CRF01) | CNE55(CRF01) | CE0217(C) | BJOX2000(CRF07) | CE1176(C) | X2278(B) | 25710(C) | MW965.26(C) | SF162.LS(B) | ||
| T/F | 1 | <30 | 395 | 56 | <30 | 697 | 63 | <30 | 52 | 77 | 100 | 101 | 49 | 17,427 | 508 | <30 |
| 2 | <30 | 323 | 43 | 41 | 598 | <30 | 41 | 44 | 52 | 198 | 243 | 83 | 12,300 | 489 | <30 | |
| 3 | <30 | 196 | <30 | <30 | 2998 | 82 | <30 | <30 | <30 | 98 | 234 | 88 | 26,325 | 615 | <30 | |
| 4 | <30 | 119 | <30 | <30 | 698 | 51 | 33 | 35 | 48 | 189 | 227 | 74 | 74,329 | 523 | <30 | |
| 5 | <30 | 127 | <30 | <30 | 733 | <30 | 34 | 70 | 104 | 134 | 150 | 99 | 5229 | 330 | <30 | |
| 6 | <30 | 216 | 90 | <30 | 653 | 31 | <30 | <30 | 44 | 71 | 167 | 107 | 13,277 | 447 | <30 | |
| w4.26 | 7 | <30 | 336 | 72 | 46 | 481 | 55 | <30 | 55 | 33 | 55 | <30 | <30 | 23,368 | 385 | <30 |
| 8 | <30 | 56 | 93 | 65 | 402 | 45 | 40 | <30 | 31 | 34 | 100 | <30 | 6604 | 303 | <30 | |
| 9 | <30 | 35 | <30 | <30 | 336 | <30 | <30 | 42 | 79 | 77 | 38 | <30 | 3833 | 413 | <30 | |
| 10 | <30 | 645 | 75 | 60 | 587 | 34 | <30 | 75 | 37 | 81 | 87 | 163 | 3831 | 511 | <30 | |
| 11 | <30 | 252 | 89 | 43 | 351 | <30 | 82 | 79 | 95 | 78 | 53 | 32 | 9926 | 376 | <30 | |
| 12 | <30 | 98 | <30 | 63 | 1645 | 40 | 75 | 68 | 39 | 95 | 96 | 43 | 8618 | 2263 | <30 | |
| BG505 | 13 | <30 | 139 | <30 | <30 | 759 | 39 | <30 | <30 | <30 | 39 | <30 | 33 | 13,220 | 401 | <30 |
| 14 | <30 | 129 | <30 | <30 | 1737 | <30 | <30 | <30 | 39 | 62 | <30 | 38 | 29,252 | 353 | <30 | |
| 15 | <30 | 121 | <30 | <30 | 769 | <30 | <30 | <30 | 67 | 73 | <30 | 49 | 15,007 | 418 | <30 | |
| 16 | <30 | 136 | <30 | <30 | 4220 | <30 | <30 | <30 | 73 | 122 | <30 | 45 | 8836 | 1444 | <30 | |
| 17 | 80 | <30 | <30 | 34 | 1992 | <30 | <30 | <30 | 40 | 61 | 31 | 37 | 10,940 | 605 | <30 | |
| 18 | 65 | 491 | <30 | 31 | 1118 | <30 | <30 | <30 | <30 | 32 | 38 | 81 | 15,634 | 1080 | <30 | |
| PBS | 19 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 |
| 20 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | |
| 21 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | |
| 22 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | 32 | <30 | 37 | <30 | <30 | <30 | <30 | |
| 23 | <30 | 43 | <30 | <30 | 31 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | |
| 24 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | <30 | 35 | <30 | <30 | <30 | <30 | <30 | |
References
- UNAIDS. Global HIV Statistics; Fact Sheet 2023. Ending the AIDS Epidemic. 2022. Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed on 30 June 2023).
- Cohen, M.S.; Chen, Y.Q.; McCauley, M.; Gamble, T.; Hosseinipour, M.C.; Kumarasamy, N.; Hakim, J.G.; Kumwenda, J.; Grinsztejn, B.; Pilotto, J.H.; et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N. Engl. J. Med. 2016, 375, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Saag, M.S.; Gandhi, R.T.; Hoy, J.F.; Landovitz, R.J.; Thompson, M.A.; Sax, P.E.; Smith, D.M.; Benson, C.A.; Buchbinder, S.P.; del Rio, C.; et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2020 Recommendations of the International Antiviral Society–USA Panel. JAMA 2020, 324, 1651–1669. [Google Scholar] [CrossRef]
- Haynes, B.F.; Wiehe, K.; Borrow, P.; Saunders, K.O.; Korber, B.; Wagh, K.; McMichael, A.J.; Kelsoe, G.; Hahn, B.H.; Alt, F.; et al. Strategies for HIV-1 vaccines that induce broadly neutralizing antibodies. Nat. Rev. Immunol. 2023, 23, 142–158. [Google Scholar] [CrossRef]
- Burton, D.R. Advancing an HIV vaccine; advancing vaccinology. Nat. Rev. Immunol. 2019, 19, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; de Souza, M.; Adams, E.; et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N. Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar] [CrossRef]
- Korber, B.; Hraber, P.; Wagh, K.; Hahn, B.H. Polyvalent vaccine approaches to combat HIV-1 diversity. Immunol. Rev. 2017, 275, 230–244. [Google Scholar] [CrossRef]
- Burton, D.; Poignard, P.; Stanfield, R.; Wilson, I. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science 2012, 337, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Mascola, J.; Haynes, B. HIV-1 neutralizing antibodies: Understanding nature’s pathways. Immunol. Rev. 2013, 254, 225–244. [Google Scholar] [CrossRef]
- Liu, J.; Bartesaghi, A.; Borgnia, M.J.; Sapiro, G.; Subramaniam, S. Molecular architecture of native HIV-1 gp120 trimers. Nature 2008, 455, 109–113. [Google Scholar] [CrossRef]
- Zolla-Pazner, S. Identifying epitopes of HIV-1 that induce protective antibodies. Nat. Rev. Immunol. 2004, 4, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Flynn, N.M.; Forthal, D.N.; Harro, C.D.; Judson, F.N.; Mayer, K.H.; Para, M.F.; rgp120 HIV Vaccine Study Group. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 2005, 191, 654–665. [Google Scholar] [CrossRef]
- Pitisuttithum, P.; Gilbert, P.; Gurwith, M.; Heyward, W.; Martin, M.; van Griensven, F.; Hu, D.; Tappero, J.W.; Choopanya, K.; Bangkok Vaccine Evaluation, G. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 2006, 194, 1661–1671. [Google Scholar] [CrossRef]
- Burton, D.; Desrosiers, R.; Doms, R.; Koff, W.; Kwong, P.; Moore, J.; Nabel, G.; Sodroski, J.; Wilson, I.; Wyatt, R. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 2004, 5, 233–236. [Google Scholar] [CrossRef]
- Sanders, R. Clinical evaluation of a soluble trimeric HIV-1 envelope glycoprotein vaccine. Expert Rev. Vaccines 2011, 10, 1117–1120. [Google Scholar] [CrossRef]
- Kovacs, J.; Nkolola, J.; Peng, H.; Cheung, A.; Perry, J.; Miller, C.; Seaman, M.; Barouch, D.; Chen, B. HIV-1 envelope trimer elicits more potent neutralizing antibody responses than monomeric gp120. Proc. Natl. Acad. Sci. USA 2012, 109, 12111–12116. [Google Scholar] [CrossRef] [PubMed]
- Sanders, R.W.; van Gils, M.J.; Derking, R.; Sok, D.; Ketas, T.J.; Burger, J.A.; Ozorowski, G.; Cupo, A.; Simonich, C.; Goo, L.; et al. HIV-1 VACCINES. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science 2015, 349, aac4223. [Google Scholar] [CrossRef]
- Zhang, P.; Narayanan, E.; Liu, Q.; Tsybovsky, Y.; Boswell, K.; Ding, S.; Hu, Z.; Follmann, D.; Lin, Y.; Miao, H.; et al. A multiclade env-gag VLP mRNA vaccine elicits tier-2 HIV-1-neutralizing antibodies and reduces the risk of heterologous SHIV infection in macaques. Nat. Med. 2021, 27, 2234–2245. [Google Scholar] [CrossRef]
- Kim, J.; Vasan, S.; Kim, J.H.; Ake, J.A. Current approaches to HIV vaccine development: A narrative review. J. Int. AIDS Soc. 2021, 24, e25793. [Google Scholar]
- Sharma, S.K.; de Val, N.; Bale, S.; Guenaga, J.; Tran, K.; Feng, Y.; Dubrovskaya, V.; Ward, A.B.; Wyatt, R.T. Cleavage-Independent HIV-1 Env Trimers Engineered as Soluble Native Spike Mimetics for Vaccine Design. Cell Rep. 2015, 11, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; He, L.; de Val, N.; Vora, N.; Morris, C.D.; Azadnia, P.; Sok, D.; Zhou, B.; Burton, D.R.; Ward, A.B.; et al. Uncleaved prefusion-optimized gp140 trimers derived from analysis of HIV-1 envelope metastability. Nat. Commun. 2016, 7, 12040. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Kumar, S.; Allen, J.; Huang, D.; Lin, X.; Mann, C.; Saye-Francisco, K.; Copps, J.; Sarkar, A.; Blizard, G.; et al. HIV-1 vaccine design through minimizing envelope metastability. Sci. Adv. 2018, 4, eaau6769. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Paynter, J.; Antanasijevic, A.; Allen, J.D.; Eldad, M.; Lee, Y.Z.; Copps, J.; Newby, M.L.; He, L.; Chavez, D.; et al. Single-component multilayered self-assembling protein nanoparticles presenting glycan-trimmed uncleaved prefusion optimized envelope trimmers as HIV-1 vaccine candidates. Nat. Commun. 2023, 14, 1985. [Google Scholar] [CrossRef]
- Liao, H.; Lynch, R.; Zhou, T.; Gao, F.; Alam, S.; Boyd, S.; Fire, A.; Roskin, K.; Schramm, C.; Zhang, Z.; et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 2013, 496, 469–476. [Google Scholar] [CrossRef]
- Gao, F.; Bonsignori, M.; Liao, H.; Kumar, A.; Xia, S.; Lu, X.; Cai, F.; Hwang, K.; Song, H.; Zhou, T.; et al. Cooperation of B cell lineages in induction of HIV-1-broadly neutralizing antibodies. Cell 2014, 158, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Doria-Rose, N.; Schramm, C.; Gorman, J.; Moore, P.; Bhiman, J.; DeKosky, B.; Ernandes, M.; Georgiev, I.; Kim, H.; Pancera, M.; et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature 2014, 509, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Wang, W.; Wang, C.; Gu, T.; Guo, R.; Yu, B.; Kong, W.; Qin, C.; Giorgi, E.E.; Chen, Z. Development of broad neutralization activity in SHIV-infected rhesus macaques after long-term infection. Aids 2017, 32, 1. [Google Scholar]
- Gao, N.; Gai, Y.; Meng, L.; Wang, C.; Wang, W.; Li, X.; Gu, T.; Louder, M.; Doria-Rose, N.; Wiehe, K.; et al. Development of Neutralization Breadth against Diverse HIV-1 by Increasing Ab-Ag Interface on V2. Adv. Sci. 2022, 9, e2200063. [Google Scholar] [CrossRef]
- Bonsignori, M.; Hwang, K.; Chen, X.; Tsao, C.; Morris, L.; Gray, E.; Marshall, D.; Crump, J.; Kapiga, S.; Sam, N.; et al. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J. Virol. 2011, 85, 9998–10009. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.S.; Madiga, M.C.; Hermanus, T.; Moore, P.L.; Wibmer, C.K.; Tumba, N.L.; Werner, L.; Mlisana, K.; Sibeko, S.; Williamson, C.; et al. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J. Virol. 2011, 85, 4828–4840. [Google Scholar] [CrossRef] [PubMed]
- Lynch, R.; Tran, L.; Louder, M.; Schmidt, S.; Cohen, M.; Dersimonian, R.; Euler, Z.; Gray, E.; Abdool Karim, S.; Kirchherr, J.; et al. The development of CD4 binding site antibodies during HIV-1 infection. J. Virol. 2012, 86, 7588–7595. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Huang, Y.; Yuan, S.; Li, Y.; Wu, S.; Xu, J.; Huang, R. Sequential immunization with consensus influenza hemagglutinins raises cross-reactive neutralizing antibodies against various heterologous HA strains. Vaccine 2017, 35, 305–312. [Google Scholar] [CrossRef]
- Isakova-Sivak, I.; Matyushenko, V.; Kotomina, T.; Kiseleva, I.; Krutikova, E.; Donina, S.; Rekstin, A.; Larionova, N.; Mezhenskaya, D.; Sivak, K.; et al. Sequential Immunization with Universal Live Attenuated Influenza Vaccine Candidates Protects Ferrets against a High-Dose Heterologous Virus Challenge. Vaccines 2019, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, X.; Liao, G.; Hu, M.; Xu, J.; Xu, X. Induction of cross-neutralizing antibodies by sequential immunization with heterologous papillomavirus L1VLPs and its implications for HPV prophylactic vaccines. J. Med. Virol. 2020, 92, 3750–3758. [Google Scholar] [CrossRef]
- Escolano, A.; Steichen, J.M.; Dosenovic, P.; Kulp, D.W.; Golijanin, J.; Sok, D.; Freund, N.T.; Gitlin, A.D.; Oliveira, T.; Araki, T.; et al. Sequential Immunization Elicits Broadly Neutralizing Anti-HIV-1 Antibodies in Ig Knockin Mice. Cell 2016, 166, 1445–1458.e12. [Google Scholar] [CrossRef] [PubMed]
- Malherbe, D.C.; Doria-Rose, N.A.; Misher, L.; Beckett, T.; Puryear, W.B.; Schuman, J.T.; Kraft, Z.; O’Malley, J.; Mori, M.; Srivastava, I.; et al. Sequential immunization with a subtype B HIV-1 envelope quasispecies partially mimics the in vivo development of neutralizing antibodies. J. Virol. 2011, 85, 5262–5274. [Google Scholar] [CrossRef]
- Klinman, D.M.; Higgins, K.W.; Conover, J. Sequential immunizations with rgp120s from independent isolates of human immunodeficiency virus type 1 induce the preferential expansion of broadly crossreactive B cells. J. Exp. Med. 1991, 173, 881–887. [Google Scholar] [CrossRef]
- Haynes, B.; Kelsoe, G.; Harrison, S.; Kepler, T. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nat. Biotechnol. 2012, 30, 423–433. [Google Scholar] [CrossRef]
- MacLeod, D.T.; Choi, N.M.; Briney, B.; Garces, F.; Ver, L.S.; Landais, E.; Murrell, B.; Wrin, T.; Kilembe, W.; Liang, C.-H.; et al. Early Antibody Lineage Diversification and Independent Limb Maturation Lead to Broad HIV-1 Neutralization Targeting the Env High-Mannose Patch. Immunity 2016, 44, 1215–1226. [Google Scholar] [CrossRef]
- Williams, W.B.; Zhang, J.; Jiang, C.; Nicely, N.I.; Fera, D.; Luo, K.; Moody, M.A.; Liao, H.X.; Alam, S.M.; Kepler, T.B.; et al. Initiation of HIV neutralizing B cell lineages with sequential envelope immunizations. Nat. Commun. 2017, 8, 1732. [Google Scholar] [CrossRef]
- Blasi, M.; Negri, D.; Saunders, K.O.; Baker, E.J.; Stadtler, H.; LaBranche, C.; Mildenberg, B.; Morton, G.; Ciarla, A.; Shen, X.; et al. Immunogenicity, safety, and efficacy of sequential immunizations with an SIV-based IDLV expressing CH505 Envs. npj Vaccines 2020, 5, 107. [Google Scholar] [CrossRef]
- Briney, B.; Sok, D.; Jardine, J.G.; Kulp, D.W.; Skog, P.; Menis, S.; Jacak, R.; Kalyuzhniy, O.; de Val, N.; Sesterhenn, F.; et al. Tailored Immunogens Direct Affinity Maturation toward HIV Neutralizing Antibodies. Cell 2016, 166, 1459–1470.e11. [Google Scholar] [CrossRef]
- Jardine, J.G.; Ota, T.; Sok, D.; Pauthner, M.; Kulp, D.W.; Kalyuzhniy, O.; Skog, P.D.; Thinnes, T.C.; Bhullar, D.; Briney, B.; et al. HIV-1 VACCINES. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science 2015, 349, 156–161. [Google Scholar] [CrossRef]
- Jardine, J.; Kulp, D.; Havenar-Daughton, C.; Sarkar, A.; Briney, B.; Sok, D.; Sesterhenn, F.; Ereño-Orbea, J.; Kalyuzhniy, O.; Deresa, I.; et al. HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science 2016, 351, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Leggat, D.J.; Cohen, K.W.; Willis, J.R.; Fulp, W.J.; deCamp, A.C.; Kalyuzhniy, O.; Cottrell, C.A.; Menis, S.; Finak, G.; Ballweber-Fleming, L.; et al. Vaccination induces HIV broadly neutralizing antibody precursors in humans. Science 2022, 378, eadd6502. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Bhattacharya, T.; Gaschen, B.; Taylor, J.; Korber, B. Consensus and Ancestral State HIV Vaccines: Response. Science 2003, 299, 1517–1518. [Google Scholar]
- Gaschen, B. Diversity consideration in HIV-1 vaccine selection. Science 2002, 296, 2354–2360. [Google Scholar] [CrossRef]
- Gao, F.; Korber, B.T.; Weaver, E.; Liao, H.X.; Hahn, B.H.; Haynes, B.F. Centralized immunogens as a vaccine strategy to overcome HIV-1 diversity. Expert Rev. Vaccines 2004, 3, S161–S168. [Google Scholar] [CrossRef]
- Weaver, E.A.; Lu, Z.; Camacho, Z.T.; Moukdar, F.; Liao, H.X.; Ma, B.J.; Muldoon, M.; Theiler, J.; Nabel, G.J.; Letvin, N.L.; et al. Cross-subtype T-cell immune responses induced by a human immunodeficiency virus type 1 group m consensus env immunogen. J. Virol. 2006, 80, 6745–6756. [Google Scholar] [CrossRef]
- Santra, S.; Korber, B.; Muldoon, M.; Barouch, D.; Nabel, G.; Gao, F.; Hahn, B.; Haynes, B.; Letvin, N. A centralized gene-based HIV-1 vaccine elicits broad cross-clade cellular immune responses in rhesus monkeys. Proc. Natl. Acad. Sci. USA 2008, 105, 10489–10494. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.X.; Sutherland, L.L.; Xia, S.M.; Brock, M.E.; Scearce, R.M.; Vanleeuwen, S.; Alam, S.M.; Mcadams, M.; Weaver, E.A.; Camacho, Z.T. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology 2006, 353, 268–282. [Google Scholar] [CrossRef]
- del Moral-Sánchez, I.; Russell, R.A.; Schermer, E.E.; Cottrell, C.A.; Allen, J.D.; Torrents de la Peña, A.; LaBranche, C.C.; Kumar, S.; Crispin, M.; Ward, A.B.; et al. High thermostability improves neutralizing antibody responses induced by native-like HIV-1 envelope trimers. npj Vaccines 2022, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Seaman, M.S.; Janes, H.; Hawkins, N.; Grandpre, L.E.; Devoy, C.; Giri, A.; Coffey, R.T.; Harris, L.; Wood, B.; Daniels, M.G.; et al. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J. Virol. 2010, 84, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Hodge, E.A.; LaBranche, C.C.; Styles, T.M.; Shen, X.; Cheedarla, N.; Shiferaw, A.; Ozorowski, G.; Lee, W.H.; Ward, A.B.; et al. Structure-guided changes at the V2 apex of HIV-1 clade C trimer enhance elicitation of autologous neutralizing and broad V1V2-scaffold antibodies. Cell Rep. 2022, 38, 110436. [Google Scholar] [CrossRef]
- Kumar, S.; Lin, X.; Ngo, T.; Shapero, B.; Sou, C.; Allen, J.D.; Copps, J.; Zhang, L.; Ozorowski, G.; He, L.; et al. Neutralizing Antibodies Induced by First-Generation gp41-Stabilized HIV-1 Envelope Trimers and Nanoparticles. mBio 2021, 12, e0042921. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Liu, Y.; Guo, L.; Wang, H.; Lu, D.; Chang, Y.; Wan, M.; Zhang, Y.; Shan, Y.; Zhang, Q.; et al. Improvement of B Cell Responses by an HIV-1 Amphiphilic Polymer Nanovaccine. Nano Lett. 2023, 23, 4090–4094. [Google Scholar] [CrossRef]
- Gouy, M.; Guindon, S.; Gascuel, O. SeaView Version 4: A Multiplatform Graphical User Interface for Sequence Alignment and Phylogenetic Tree Building. Mol. Biol. Evol. 2010, 27, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Gai, Y.; Meng, L.; Wang, C.; Zhang, X.; Wang, W.; Qin, C.; Yu, X.; Gao, F. Development of Antibodies with Broad Neutralization Specificities against HIV-1 after Long Term SHIV Infection in Macaques. Viruses 2020, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Montefiori, D.C. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol. Biol. 2009, 485, 395–405. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]


| Group | Week 0 | Week 3 | Week 6 | Week 9 | Week 12 |
|---|---|---|---|---|---|
| T/F | T/F | Con_w22 | Con_w30 | Con_w53 | Con_w78 |
| w4.26 | w4.26 | Con_w22 | Con_w30 | Con_w53 | Con_w78 |
| BG505 | BG505 | BG505 | BG505 | BG505 | BG505 |
| PBS | PBS | PBS | PBS | PBS | PBS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, M.; Yang, X.; Sun, J.; Giorgi, E.E.; Ding, X.; Zhou, Y.; Zhang, Y.; Su, W.; Jiang, C.; Shan, Y.; et al. Enhancement of Neutralization Responses through Sequential Immunization of Stable Env Trimers Based on Consensus Sequences from Select Time Points by Mimicking Natural Infection. Int. J. Mol. Sci. 2023, 24, 12642. https://doi.org/10.3390/ijms241612642
Wan M, Yang X, Sun J, Giorgi EE, Ding X, Zhou Y, Zhang Y, Su W, Jiang C, Shan Y, et al. Enhancement of Neutralization Responses through Sequential Immunization of Stable Env Trimers Based on Consensus Sequences from Select Time Points by Mimicking Natural Infection. International Journal of Molecular Sciences. 2023; 24(16):12642. https://doi.org/10.3390/ijms241612642
Chicago/Turabian StyleWan, Mingming, Xiao Yang, Jie Sun, Elena E. Giorgi, Xue Ding, Yan Zhou, Yong Zhang, Weiheng Su, Chunlai Jiang, Yaming Shan, and et al. 2023. "Enhancement of Neutralization Responses through Sequential Immunization of Stable Env Trimers Based on Consensus Sequences from Select Time Points by Mimicking Natural Infection" International Journal of Molecular Sciences 24, no. 16: 12642. https://doi.org/10.3390/ijms241612642
APA StyleWan, M., Yang, X., Sun, J., Giorgi, E. E., Ding, X., Zhou, Y., Zhang, Y., Su, W., Jiang, C., Shan, Y., & Gao, F. (2023). Enhancement of Neutralization Responses through Sequential Immunization of Stable Env Trimers Based on Consensus Sequences from Select Time Points by Mimicking Natural Infection. International Journal of Molecular Sciences, 24(16), 12642. https://doi.org/10.3390/ijms241612642





